Submitted:
02 February 2023
Posted:
06 February 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of stock solutions
2.2. Cell lines and cell culture preparation and maintenance
2.3. Cell cytotoxicity measurements using Cell Counting Kit-8 (CCK-8) assay
2.4. Single dose treatment for the development of cisplatin-resistant HepG2cr cells
2.5. Cell migration assay
2.6. Inhibition of TG2 by cystamine
2.7. siRNA knockdown inhibition of TG2 expression
2.8. Reverse transcription quantitative real time-PCR (RT-qPCR)
| Transcript Name | Sequence |
|---|---|
| GAPDH | (F) – 5’-CACTAGGCGCTCACTGTTCTC-3’ (R) – 3’-GACTCCACGACGTACTCAGC-5’ |
| TG2 | (F) – 5’-CTGGGCCACTTCATTTTGC-3’ (R) – 3’-ACTCCTGCCGCTCCTCTTC-5’ |
2.9. Isolation of cell membrane proteins
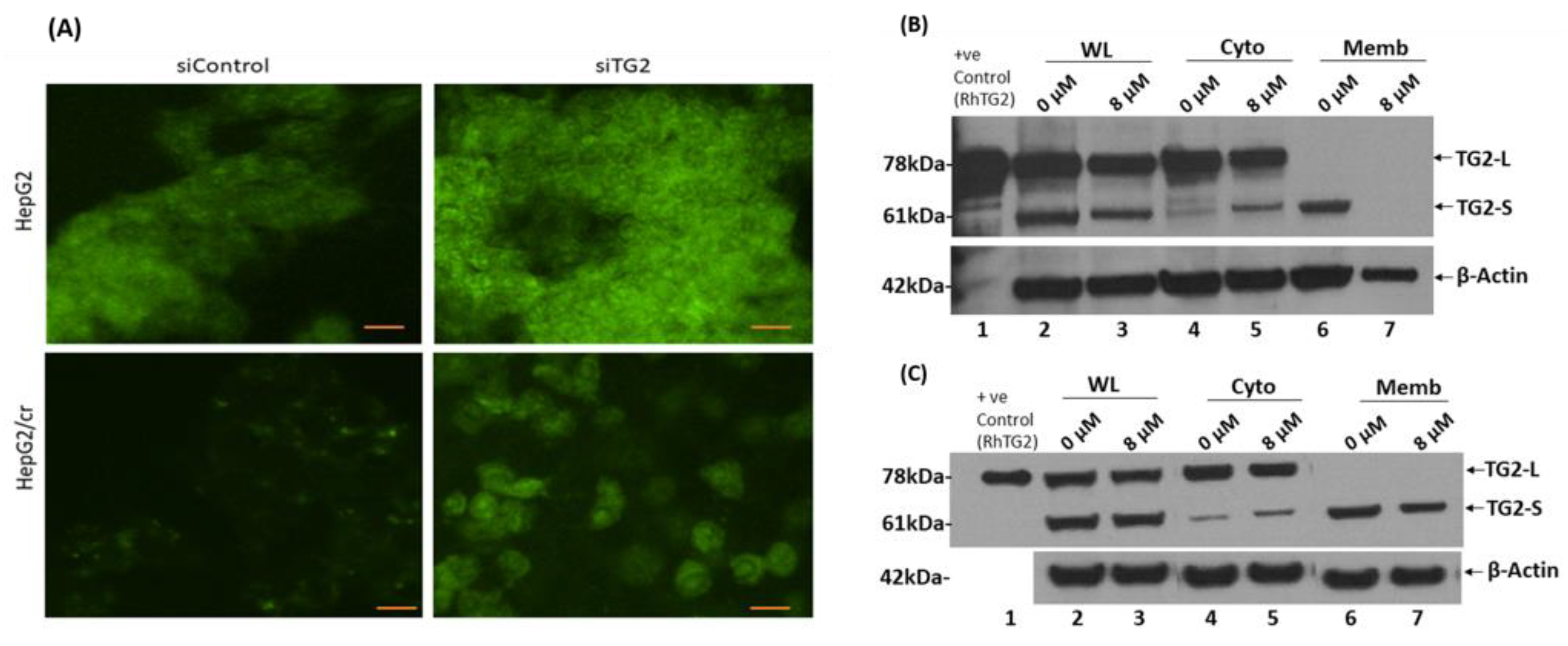
2.10. Measurement of TG2 isoform expression by Western blotting
2.11. In vitro specific TG2 colorimetric microassay (TG2-CovTest)
2.12. Confocal microscopic detection of uptake of Alexa fluor 546/488 labelled-cisplatin
2.13. Alexa fluor 546-labelled cisplatin uptake assay by flow cytometry
2.14. Statistical analysis
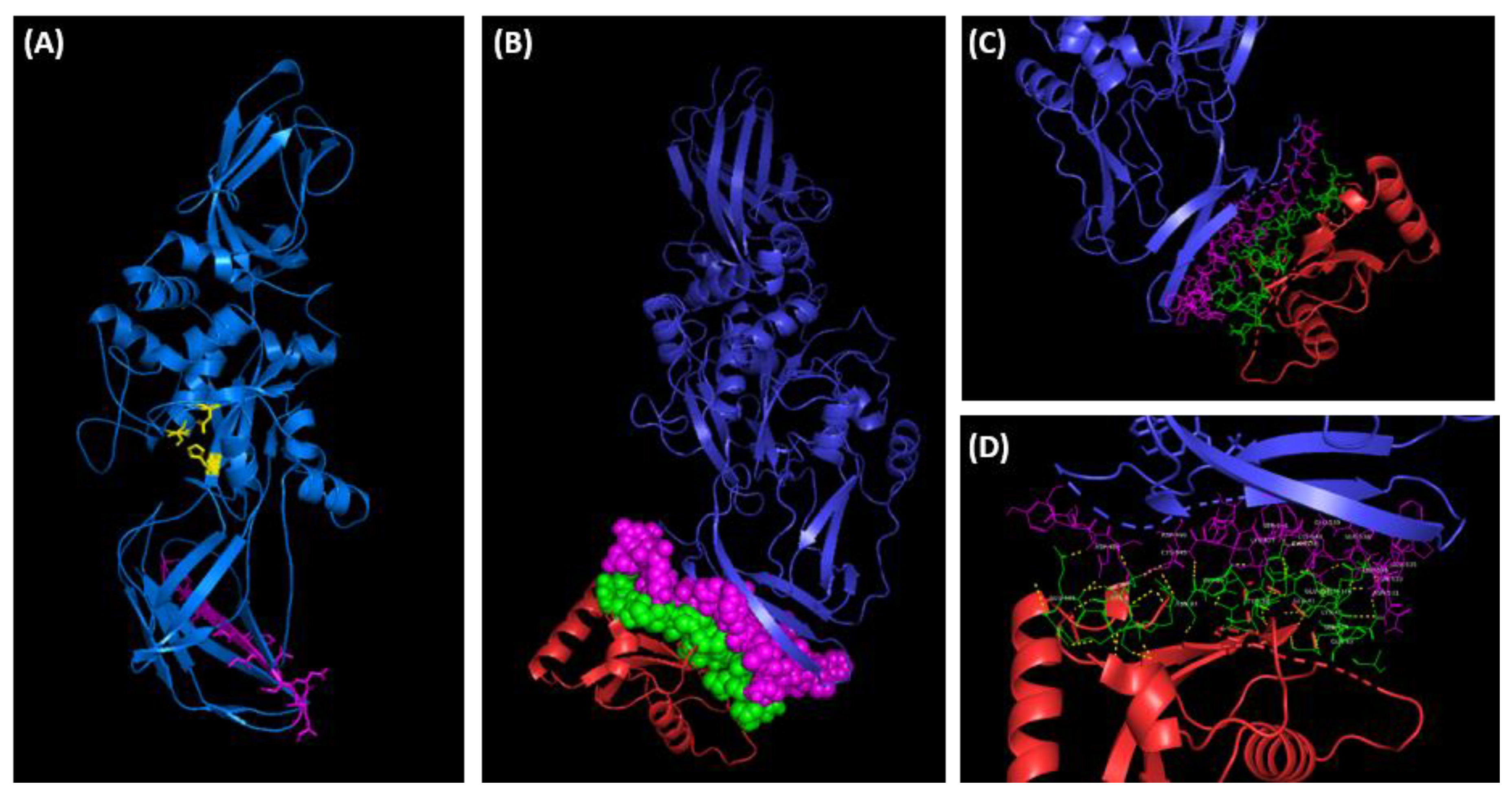
2.15. Structure analysis and molecular docking simulations
3. Results
3.1. Morphological and phenotypic characterisation of HepG2 and HepG2/cr cells
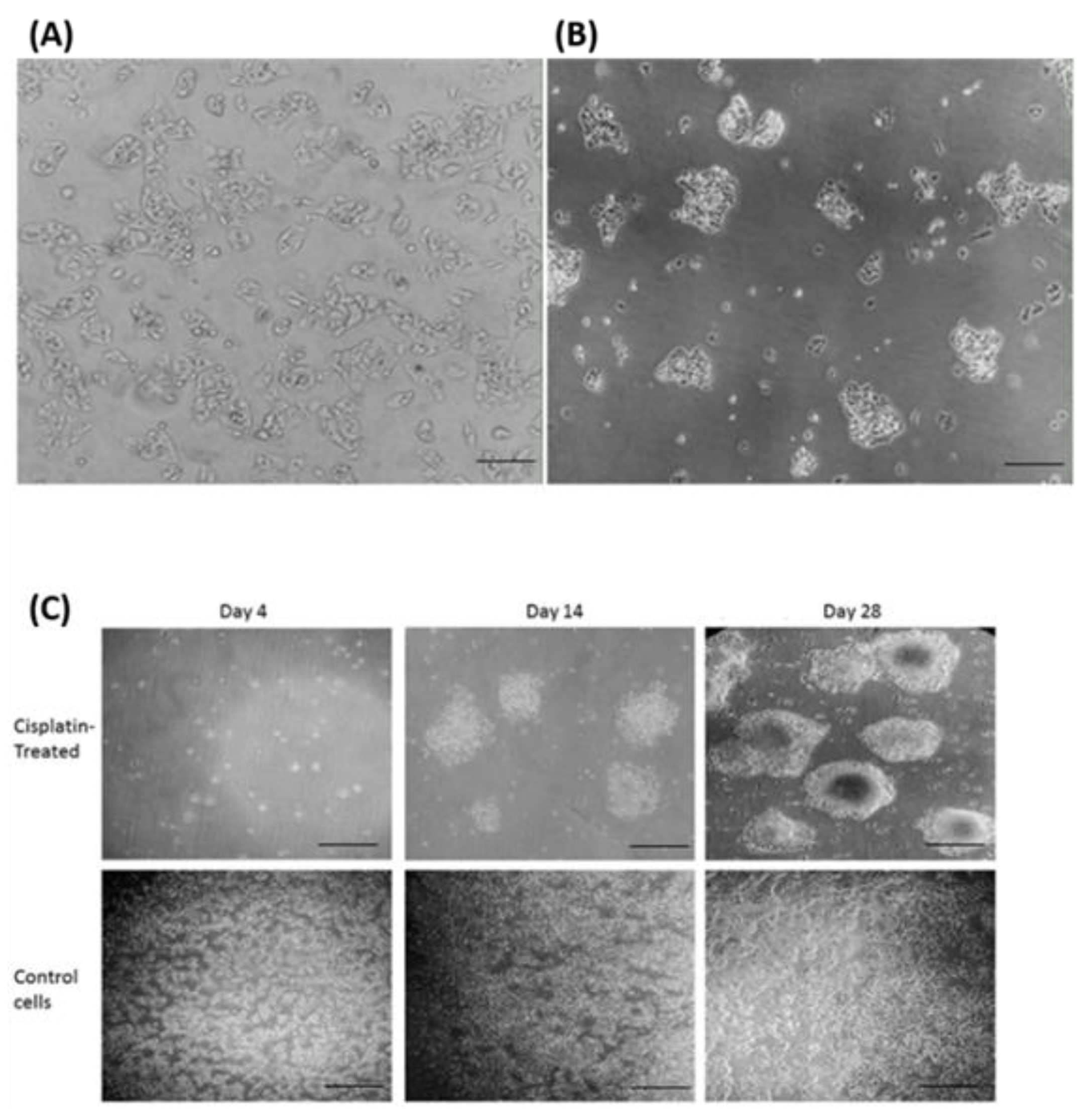
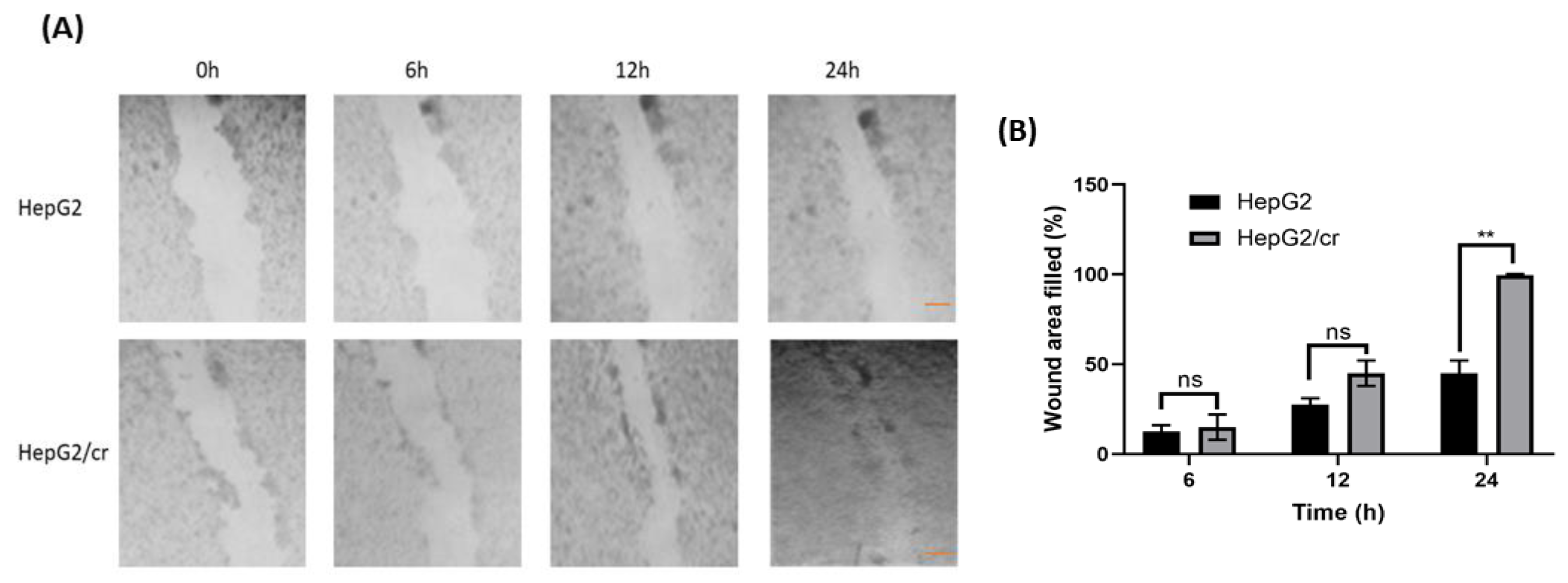
3.2. Effects of chemoresistance on cell migration capacity
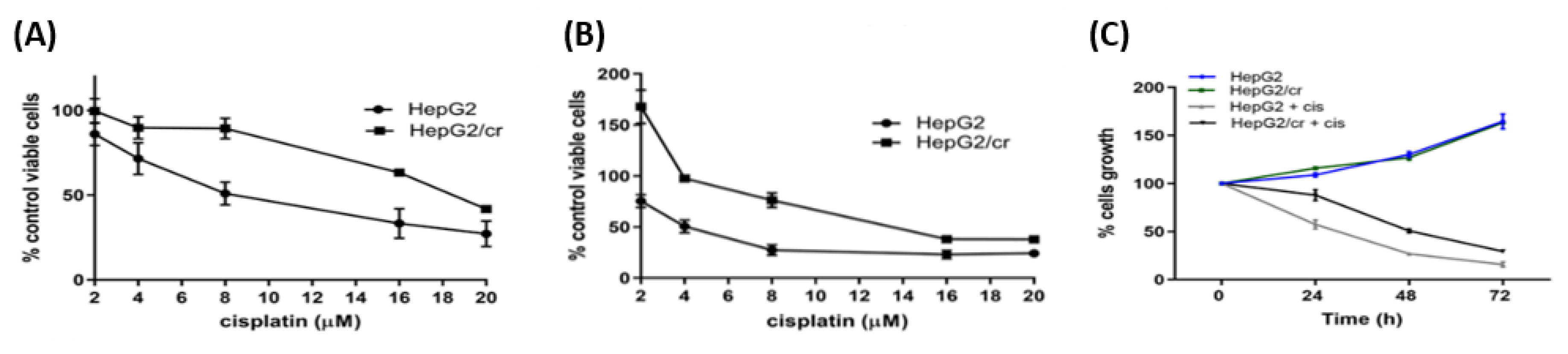
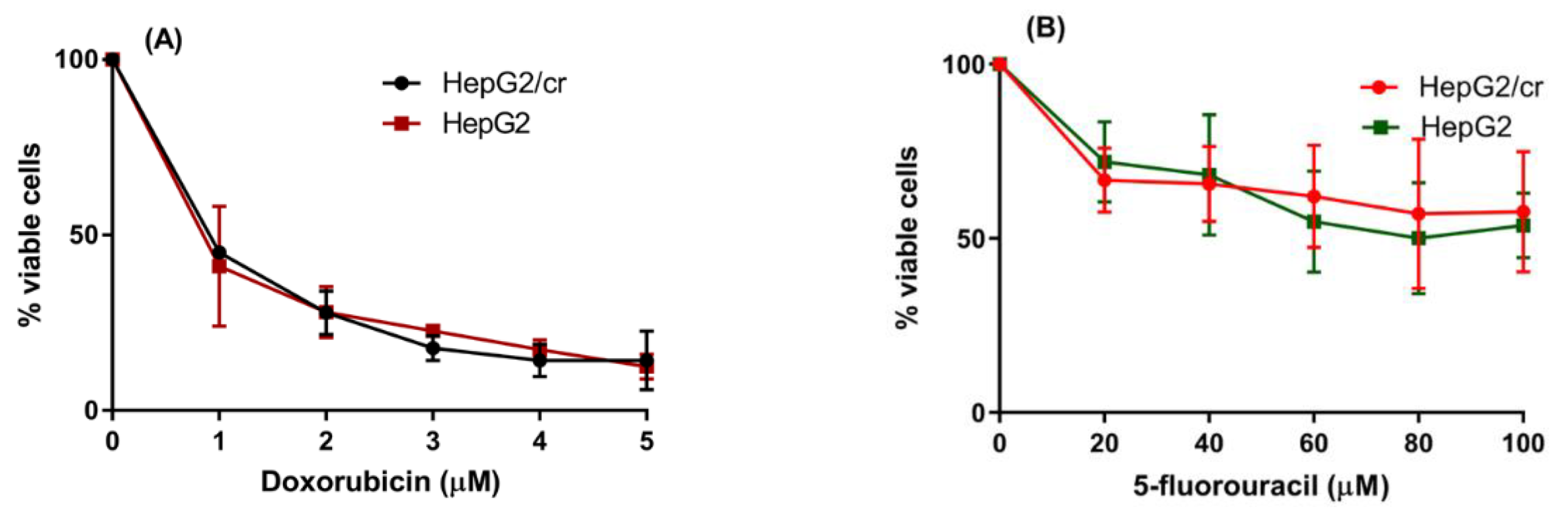
3.3. Cisplatin-resistant cells showed no cross-resistance to other anti-cancer drugs
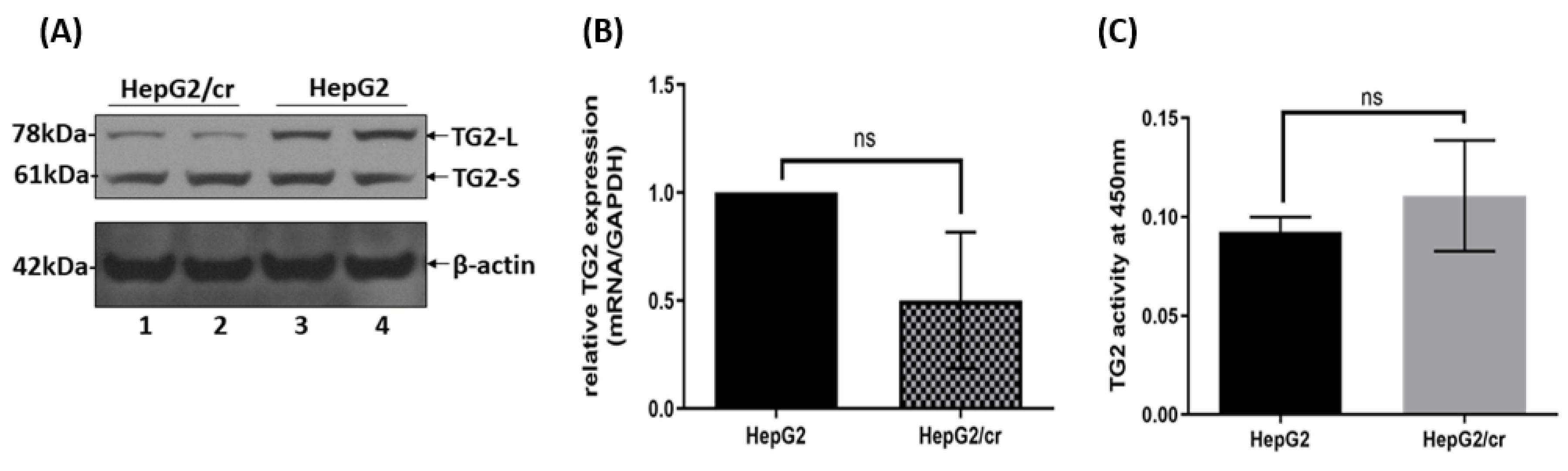
3.4. TG2 isoform expression and transaminating activity in HepG2 and HepG2/cr cell lines
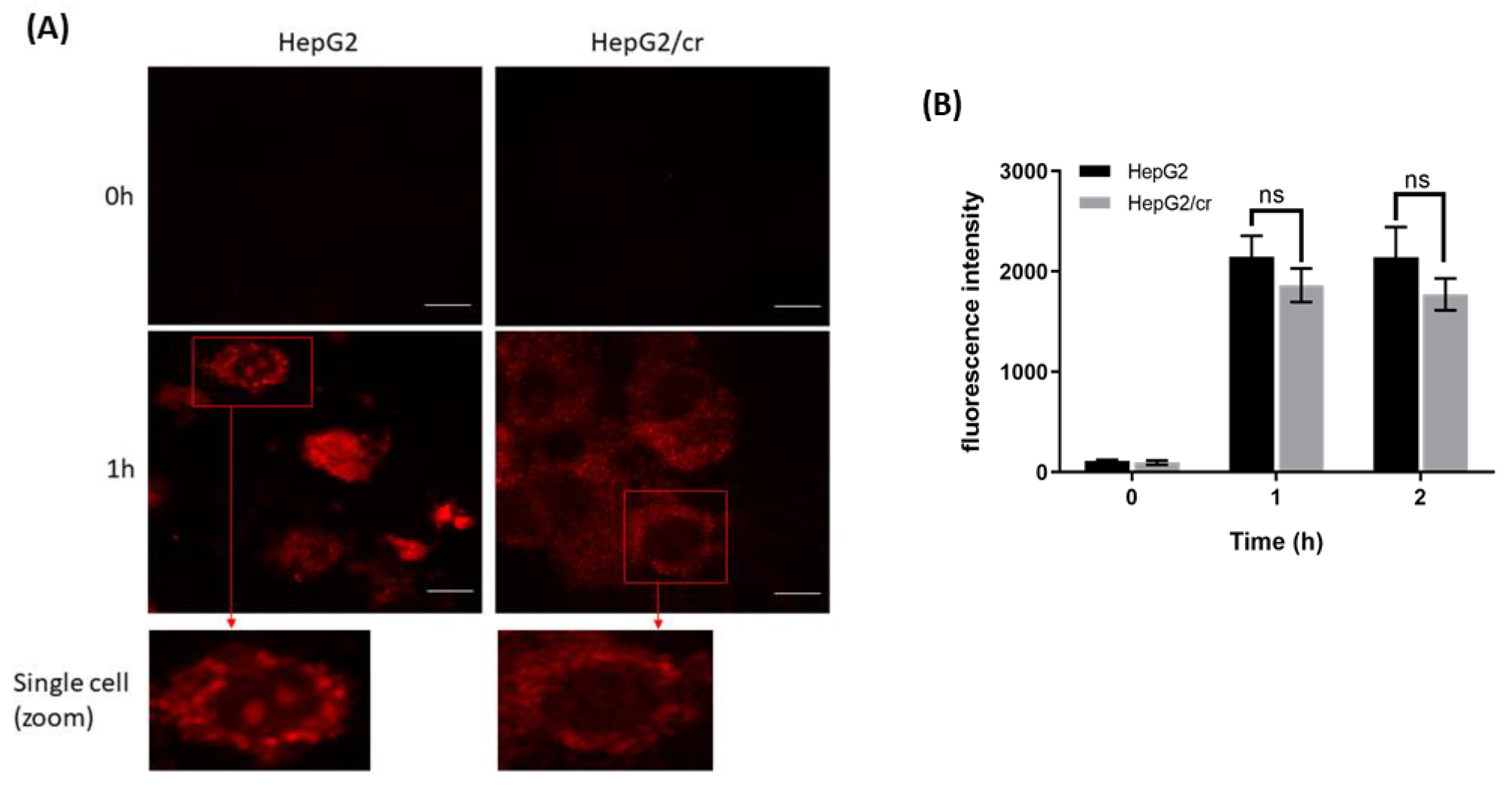
3.5. Alexa fluor 546-labelled cisplatin uptake by HepG2 and HepG2/cr cell lines
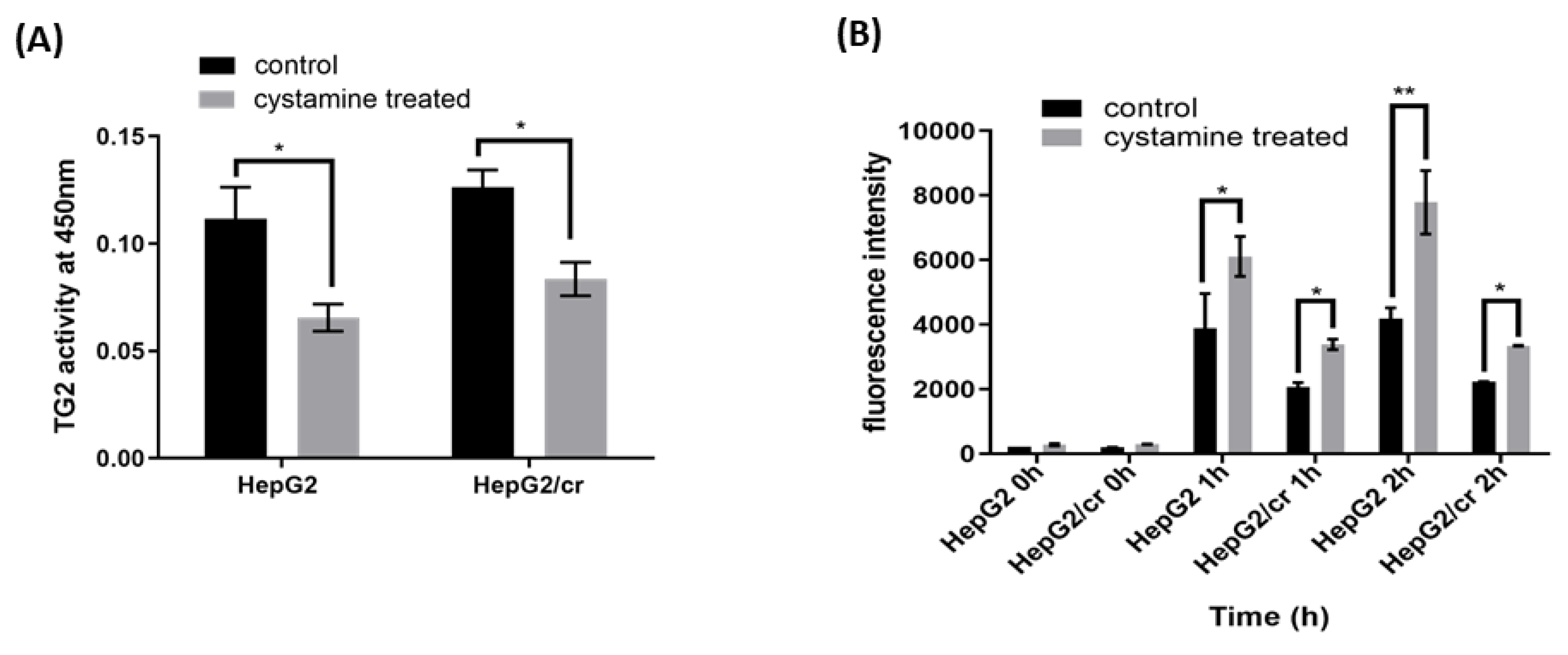
3.6. Effects of cystamine on the uptake of Alexa fluor 546-labelled cisplatin
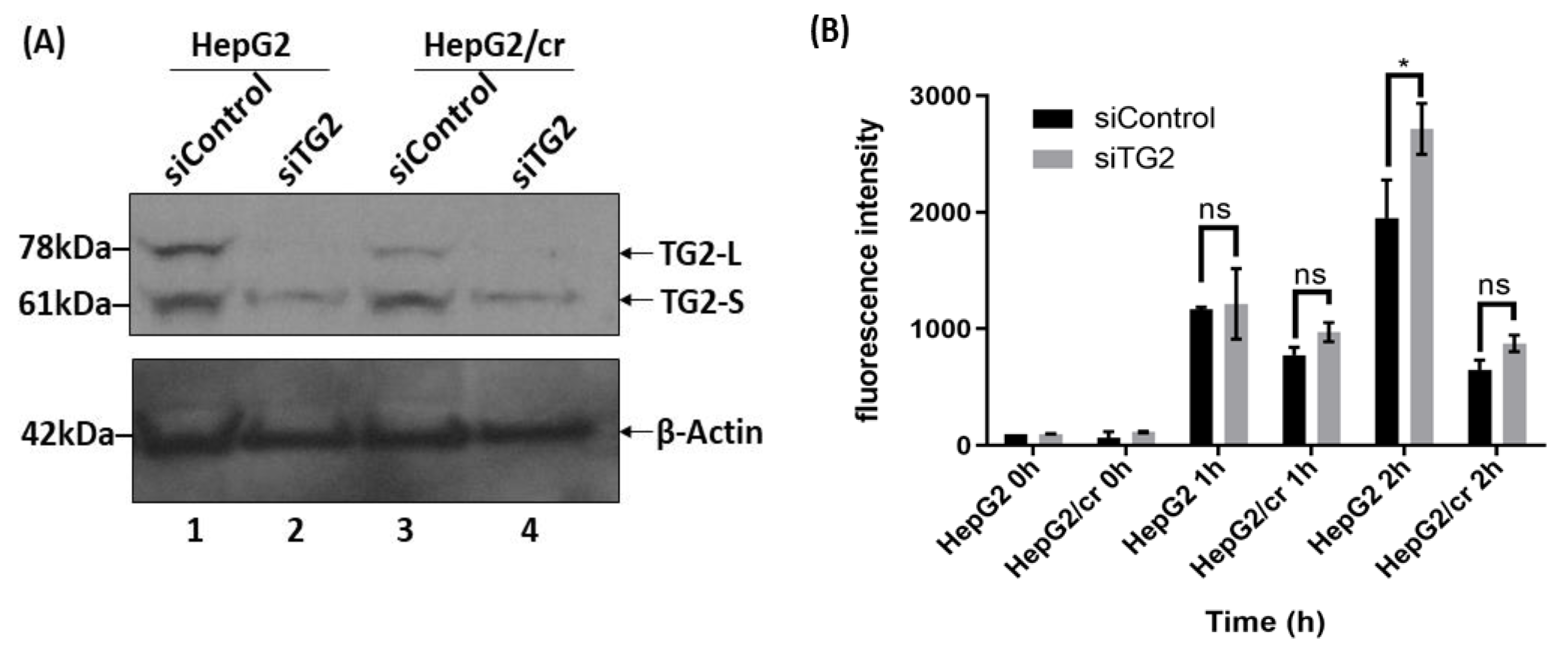
3.7. Effects of siRNA silencing of TG2 expression on the uptake of cisplatin
3.8. Changes in intracellular distribution of TG2-L and TG2-S accompany chemoresistance
3.9. Membrane protein-protein interaction and molecular docking simulation of TG2 isoforms
4. Discussion
4.1. Translocation of TG2 from the cytoplasm to the ECM may contribute to chemoresistance
4.2. Dual role performed by TG2-S in response to cisplatin cytotoxicity
4.3. TG2-S role in cell survival and chemoresistance following cisplatin treatment
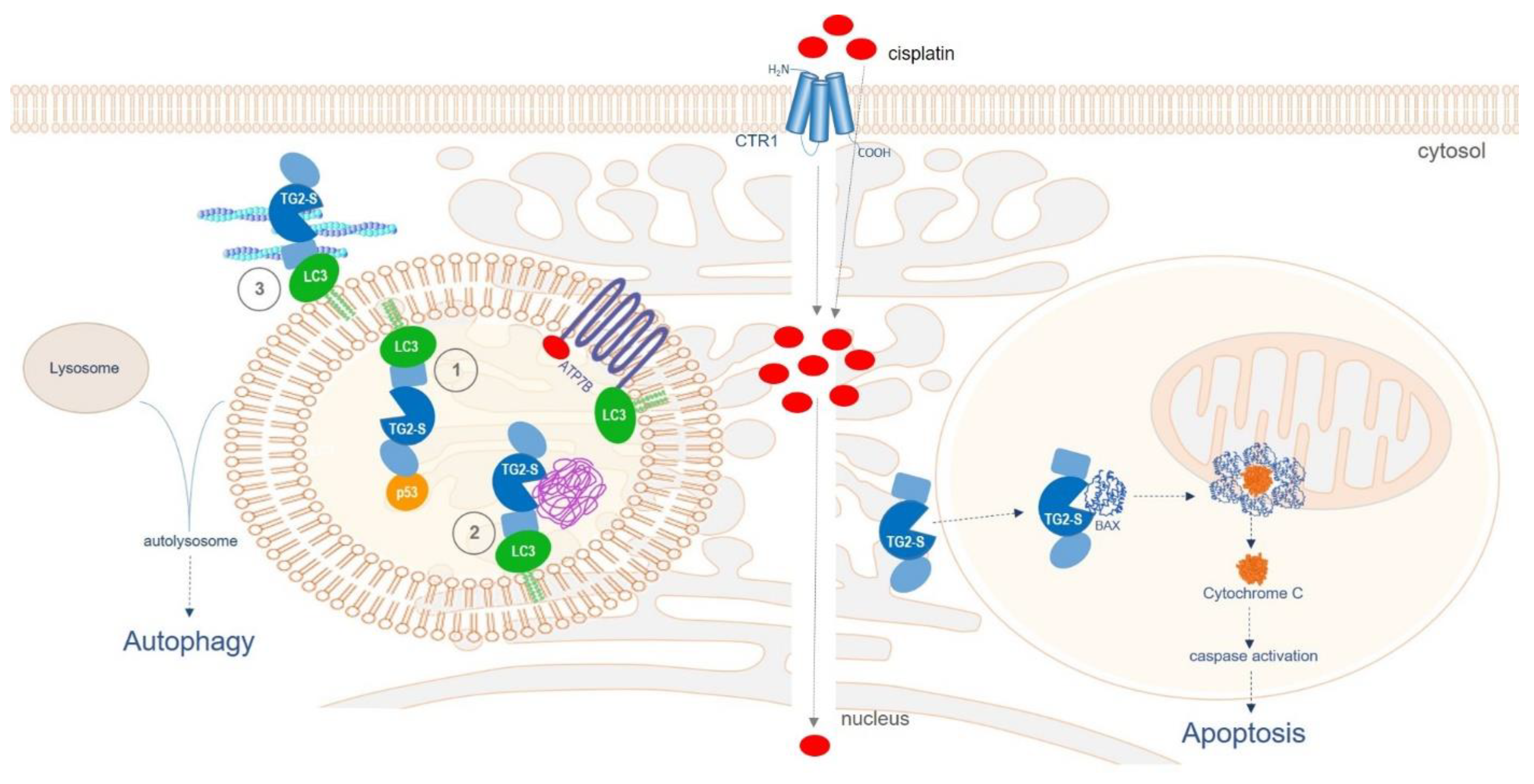
4.4. TG2-S role in apoptic cell death following cisplatin treatment
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TG2 | Transglutaminase type 2 |
| TG2-L | Full-length TG2 ‘long’ isoform |
| TG2-S | Truncated TG2 ‘short’ isoform |
| HepG2 | Hepatocellular carcinoma cell line |
| HepG2/cr | Cisplatin-resistant HepG2 |
| HCC | Hepatocellular carcinoma |
References
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. The Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; Fleming, T.; Forouzanfar, M.H.; Hancock, J.; Hay, R.J.; Hunter-Merrill, R.; Huynh, C.; Hosgood, H.D.; Johnson, C.O.; Jonas, J.B.; … Naghavi, M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA oncology 2017, 3(4), 524–548. [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.C.; Chen, B.H.; Huang, J.J.; Huang, W.S.; Cai, M.Y.; Zhou, J.W.; Guo, Y.J.; Zhu, K.S. Early prediction of survival in hepatocellular carcinoma patients treated with transarterial chemoembolization plus sorafenib. World J. Gastroenterol. 2018, 24(4), 484–493. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014, 0, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K. Recent advances in medical management of hepatocellular carcinoma. Hepatol. Res. 2019, 49, 14–32. [Google Scholar] [CrossRef]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef]
- Brown, S.K. Chemotherapy and other systemic therapies for hepatocellular carcinoma and liver metastases. Sem. Int. Radiol. 2006, 23, 99–108. [Google Scholar] [CrossRef]
- Jain, A.; Jahagirdar, D.; Nilendu, P.; Sharma, N.K. Molecular approaches to potentiate cisplatin responsiveness in carcinoma therapeutics. Expert. Rev. Anticancer Ther. 2017, 17, 815–825. [Google Scholar] [CrossRef]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, B.; Edirisinghe, M.; Knowles, J.C.; Stride, E.P.J. Drug delivery strategies for platinum-based chemotherapy. ACS Nano 2017, 11(9), 8560–8578. [Google Scholar] [CrossRef]
- Guindon, J.; Deng, L.; Fan, B.; Wager-Miller, J.; Hohmann, A.G. Optimization of a cisplatin model of chemotherapy-induced peripheral neuropathy in mice: use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol. Pain. 2014, 10, 56. [Google Scholar] [CrossRef]
- Dong, H.; Huang, J.; Zheng, K.; Tan, D.; Chang, Q.; Gong, G.; Zhang, Q.; Tang, H.; Sun, J.; Zhang, S. Metformin enhances the chemotherapy of hepatocarcinoma cell to cisplatin through AMPK pathway. Oncol Lett. 2017, 14, 7807–12. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef]
- Mann, A.P.; Verma, A.; Sethi, G.; Manavathi, B.; Wang, H.; Fok, J.Y.; Kunnumakkara, A.B.; Kumar, R.; Aggarwal, B.B.; Mehta, K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006, 66, 8788–95. [Google Scholar] [CrossRef]
- Gundemir, S.; Colak, G.; Tucholski, J.; Johnson, G. Transglutaminase 2: A molecular Swiss army knife. Biochim. Biophys. Acta. 2012, 1823, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Nurminskaya, M.V.; Belkin, A.M. Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 2012, 294, 1–97. [Google Scholar] [PubMed]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Fesus, L.; Piacentini, M. Transglutaminase 2; an enigmatic enzyme with diverse functions. Trends Biol. Sci. 2002, 27, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Eckert, R. Transglutaminases family of enzymes with diverse functions. Prog. Exp. Tum. Res. 2005, 38, 1–18. [Google Scholar] [CrossRef]
- Belkin, A. Extracellular TG2: emerging functions and regulation. FEBS J. 2011, 278, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Odii, B.; Coussons, P. Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family. TheScientificWorldJournal 2014, 2014, 714561. [Google Scholar] [CrossRef]
- Ling, D.; Marshall, G.; Liu, P.; Xu, N.; Nelson, C.; Iismaa, S.; Liu, T. Enhancing the anticancer effect of the histone deacetylase inhibitor by activating transglutaminase. Eur. J. Cancer. 2012, 48, 3278–87. [Google Scholar] [CrossRef]
- Lentini, A.; Abbruzzese, A.; Provenzano, B.; Tabolacci, C.; Beninati, S. Transglutaminases: key regulators of cancer metastasis. Amino Acids 2013, 44, 25–32. [Google Scholar] [CrossRef]
- Eckert, R.L.; Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Kerr, C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol Carcinog. 2015, 54, 947–58. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Furutani, Y.; Hitomi, K.; Kojima, S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016, 7, e2244. [Google Scholar] [CrossRef] [PubMed]
- Citron, B.A.; Suo, Z.; SantaCruz, K.; Davies, P.J.; Qin, F.; Festoff, B.W. Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem. Int. 2002, 40, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Liu, Y.; Li, W.; Greenberg, C.S. Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. Faseb J. 2007, 21, 4131–4143. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Greenberg, C.S. TGM2 and implications for human disease: role of alternative splicing. Front. Biosci. 2013, 18, 504–519. [Google Scholar] [CrossRef]
- Phatak, V.M.; Croft, S.M.; Rameshaiah Setty, S.G.; Scarpellini, A.; Hughes, D.C.; Rees, R.; McArdle, S.; Verderio, E.A. Expression of transglutaminase-2 isoforms in normal human tissues and cancer cell lines: dysregulation of alternative splicing in cancer. Amino Acids 2013, 44, 33–44. [Google Scholar] [CrossRef]
- Arbildi, P.; Séfiora, C.; Del Rio, N.; Marqués, J.M.; Hernandez, A. Alternative RNA splicing of leucocyte tissue transglutaminase in coeliac disease. Scand. J. Immunol. 2018, 87, el2659. [Google Scholar] [CrossRef]
- Bianchi, N.; Beninati, S.; Bergamini, C.M. Spotlight on the transglutaminase 2 gene: a focus on genomic and transcriptional aspects. Biochem. J. 2018, 475, 1643–1667. [Google Scholar] [CrossRef]
- Sestito, C.; Brevé, J.; Killestein, J.; Teunissen, C.E.; Wilhelmus, M.; Drukarch, B.; van Dam, A.M. Differential expression of tissue transglutaminase splice variants in peripheral blood mononuclear cells of primary progressive multiple sclerosis patients. Medical sciences (Basel, Switzerland) 2018, 6(4), 108. [Google Scholar] [CrossRef]
- Mellman, I.; Yarden, Y. Endocytosis and cancer. Cold Spring Harb Perspect Biol. 2013, 5(12):a016949. [CrossRef] [PubMed]
- Donaldson, J.G.; Johnson, D.L.; Dutta, D. Rab and Arf G proteins in endosomal trafficking and cell surface homeostasis. Small GTPases. 2016, 7(4), 247–251. [Google Scholar] [CrossRef] [PubMed]
- Peurois, F.; Peyroche, G.; Cherfils, J. Small GTPase peripheral binding to membranes: molecular determinants and supramolecular organization. Biochem. Soc. Trans. 2017, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ientile, R.; Caccamo, D.; Griffin, M. Tissue transglutaminase and the stress response. Amino Acids 2007, 33, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cerione, R.A.; Clardy, J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. 2002, 99, 2743–2747. [Google Scholar] [CrossRef]
- Jang, T.H.; Lee, D.S.; Choi, K.; Jeong, E.M.; Kim, I.G.; Kim, Y.W.; Chun, J.N.; Jeon, J.H.; Park, H.H. Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site. PloS one 2014, 9(9), e107005. [Google Scholar] [CrossRef]
- Kerr, C.; Szmacinski, H.; Fisher, M.L.; Nance, B.; Lakowicz, J.R.; Akbar, A.; Keillor, J.W.; Lok Wong, T.; Godoy-Ruiz, R.; Toth, E.A.; Weber, D.J.; Eckert, R.L. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene 2017, 36(21), 2981–2990. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Jansen, J.M.; Miller, A.M.; Ly, T.K.; Endo, M.; Cerione, R.A. Two isoforms of tissue transglutaminase mediate apposing cellular fates. Proc. Natl. Acad. Sci. 2006, 103(49), 18609–18614. [Google Scholar] [CrossRef]
- Verma, A.; Mehta, K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resistance Updates 2007, 10, 144–51. [Google Scholar] [CrossRef]
- Meshram, D.; Pike, C.; Coussons, P. Inhibition of transglutaminase 2 activity increases cisplatin cytotoxicity in a model of human hepatocarcinoma chemotherapy. Eur. J. Pharmacol. 2017, 815, 332–342. [Google Scholar] [CrossRef]
- Zhou, Y.; Ling, X.L.; Li, S.W.; Li, X.Q.; Yan, B. Establishment of a human hepatoma multidrug resistant cell line in vitro. World J Gastroenterol. 2010, 16, 2291–7. [Google Scholar] [CrossRef]
- McDermott, M.; Eustace, A.J.; Busschots, S.; Breen, L.; Crown, J.; Clynes, M.; O'Donovan, N.; Stordal, B. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: A practical guide with case studies. Front. Oncol. 2014, 4, 40. [Google Scholar] [CrossRef]
- van Zundert, G.C.P.; Rodrigues, J.P.G.LM.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J Mol Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L., Mezulis, S., Yates, C. et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10, 845–858 (2015). [CrossRef]
- Kumar, A.; Xu, J.; Brady, S.; Gao, H.; Yu, D.; Reuben, J.; Mehta, K. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PloS one 2010, 5(10), e13390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; McCarty, N. Tampering with cancer chemoresistance by targeting the TGM2-IL6-autophagy regulatory network. Autophagy 2017, 13, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, M.; D'Eletto, M.; Farrace, M.G.; Rodolfo, C.; Del Nonno, F.; Ippolito, G.; Falasca, L. Characterization of distinct sub-cellular location of transglutaminase type II: changes in intracellular distribution in physiological and pathological states. Cell and tissue research 2014, 358(3), 793–805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hu, J.; LaVoie, H.; Walsh, K.; DiPette, D.; Singh, U. Conformational changes and translocation of tissue-transglutaminase to the plasma membranes: role in cancer cell migration. BMC Cancer 2014, 14, 256. [Google Scholar] [CrossRef]
- Diaz-Hidalgo, L.; Altuntas, S.; Rossin, F.; D'Eletto, M.; Marsella, C.; Farrace, M.G.; Falasca, L.; Antonioli, M.; Fimia, G.M.; Piacentini, M. Transglutaminase type 2-dependent selective recruitment of proteins into exosomes under stressful cellular conditions. Biochim. Biophys. Acta. 2016, 1863(8), 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Gundemir, S.; Johnson, G.V. Intracellular localization and conformational state of transglutaminase 2: implications for cell death. PLoS ONE 2009, 4, e6123. [Google Scholar] [CrossRef]
- Kuo, T.F.; Tatsukawa, H.; Kojima, S. New insights into the functions and localization of nuclear transglutaminase 2. FEBS J. 2011, 278, 4756–4767. [Google Scholar] [CrossRef]
- Meshram, D.D.; Pike, C.V.S.; Coussons, P.J. Cystamine treatment unblocks uptake of dansylcadaverine by human kidney cancer cell line CAKI-2. Proceedings of the Gordon Research Conference “Transglutaminases in Human Disease Processes”, June 29 - July 4, 2014, Lucca (Barga), Italy. 29 June.
- Herman, J.; Mangala, L.; Mehta, K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006, 25, 3049–3058. [Google Scholar] [CrossRef]
- Fujisawa, T.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PloS one 2012, 7(4), e34437. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The drug-resistance mechanisms of five platinum-based antitumor agents. Front. Pharmacol. 2020, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.; Tew, K. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Y.A.; Yan, K.L.; Hussain, S.N. Immunohistochemical expression of pi class glutathione S-transferase and alpha-fetoprotein in hepatocellular carcinoma and chronic liver disease. Anal. Quent. Cytol. Histol. 2003, 25, 322–328. [Google Scholar]
- Peklak-Scott, C.; Smitherman, P.K.; Townsend, A.J.; Morrow, C.S. Role of glutathione S-transferase P1-1 in the cellular detoxification of cisplatin. Mol. Cancer Ther. 2008, 7, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Király, R.; Demény, M.; Fésüs, L. Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 2011, 278, 4717–4739. [Google Scholar] [CrossRef] [PubMed]
- Katt, W.P.; Antonyak, M.A.; Cerione, R. A. Opening up about tissue transglutaminase: when conformation matters more than enzymatic activity. Med One. 2018, 3, e180011. [Google Scholar] [CrossRef]
- Singh, G.; Zhang, J.; Ma, Y.; Cerione, R.A.; Antonyak, M.A. The different conformational states of tissue transglutaminase have opposing affects on cell viability. J Biol Chem. 2016, 22, 291, 9119-32. [CrossRef]
- D'Eletto, M.; Farrace, M.G.; Falasca, L.; Reali, V.; Oliverio, S.; Melino, G.; Griffin, M.; Fimia, G. M.; Piacentini, M. Transglutaminase 2 is involved in autophagosome maturation. Autophagy. 2009, 5, 1145–54. [Google Scholar] [CrossRef]
- D'Eletto, M.; Farrace, M.G.; Rossin, F.; Strappazzon, F.; Giacomo, G.D.; Cecconi, F.; Melino, G.; Sepe, S.; Moreno, S.; Fimia, G.M.; Falasca, L.; Nardacci, R.; Piacentini, M. Type 2 transglutaminase is involved in the autophagy-dependent clearance of ubiquitinated proteins. Cell Death Differ. 2012, 19, 1228–38. [Google Scholar] [CrossRef] [PubMed]
- D’Eletto, M.; Farrace, M.G.; Piacentini, M.; Rossin, F. Assessing the catalytic activity of transglutaminases in the context of autophagic responses. Meth. Enzymol. 2017, 587, 511–520. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, J.S.; Hong, D.; Lee, S.H.; Kim, N.; Lee, W.K.; Sung, T.W.; Gong, Y.D.; Kim, S.Y. Renal cell carcinoma escapes death by p53 depletion through transglutaminase 2-chaperoned autophagy. Cell Death Dis. 2016, 31, 7, e2163. [CrossRef] [PubMed]
- Shimizu, T.; Fujii, T.; Sakai, H. The relationship between actin cytoskeleton and membrane transporters in cisplatin resistance of cancer cells. Front. Cell Dev. Biol. 2020, 8, 597835. [Google Scholar] [CrossRef] [PubMed]
- Kast, D. J.; Dominguez, R. The Cytoskeleton-Autophagy Connection. Curr Biol. 2017, 27, R318–R326. [Google Scholar] [CrossRef] [PubMed]
- Mokady, D.; Meiri, D. RhoGTPases - A novel link between cytoskeleton organization and cisplatin resistance. Drug Resist Updat. 2015, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pantoom, S.; Pomorski, A.; Huth, K.; Hund, C.; Petters, J.; Krężel, A.; Hermann, A.; Lukas, J. Direct interaction of ATP7B and LC3B proteins suggests a cooperative role of copper transportation and autophagy. Cells. 2021, 10, 3118. [Google Scholar] [CrossRef]
- Pawlowski, J.; Kraft, A. S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. 2000, 97, 529–531. [Google Scholar] [CrossRef]
- Rodolfo, C.; Mormone, E.; Matarrese, P.; Ciccosanti, F.; Farrace, M.G.; Garofano, E.; Piredda, L.; Fimia, G.M.; Malorni, W.; Piacentini, M. Tissue transglutaminase is a multifunctional BH3-only protein. J. Biol. Chem. 2004, 279, 54783–54792. [Google Scholar] [CrossRef] [PubMed]
- Budillon, A.; Carbone, C.; D’Gennaro, E. Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino Acids. 2013, 44, 63–72. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




