1. Introduction
Cardiogenic shock (CS) is part of a clinical syndrome consisting of acute left ventricular failure causing severe hypotension leading to inadequate organ and tissue perfusion. Recovery is observed if the underlying cause is reversible and appropriate treatment is started promptly. CS may become irreversible if treatment is delayed leading to severe tissue damage and death even if blood pressure is restored [
1].
The most frequent cause of cardiogenic shock is heart failure (HF) secondary to acute myocardial infarction although other conditions such as arrhythmias, valve rupture, pulmonary embolus, pericardial tamponade and acute myocarditis may lead to its development. CS is characterized by the following hemodynamic parameter values: systolic aortic pressure (SAP <90 mmHg), cardiac index (CI <1.8 L/min/m2 without support or CI<2.2 L/min/m2 with support), pulmonary capillary wedge pressure (PCWP>15 mmHg), and elevated left ventricular end-diastolic pressure (LVEDP>18 mmHg) [
2].
Early pharmacological treatment may help avoid further worsening of the clinical picture and escalate to mechanical circulatory support. The most used drugs are positive inotropes (to increase the contractile force of the myocardium), anti-arrhythmics (to restore sinus rhythm and reduce the occurrence of further arrhythmias), anti-platelet agents (to prevent platelet aggregation and make the blood thinner), thrombolytics (to dissolve blood clots) and anti-coagulants (to slow down the blood clotting process).
The most commonly used devices to support patients affected by CS are the following:
- ✓
Intra-aortic balloon pump (IABP), consisting of a balloon positioned in the descending thoracic aorta that inflates (diastole) and deflates (systole) leading to an increase in coronary perfusion and a reduction in afterload;
- ✓
Impella 2.5 [
3], a coaxial pump that is retrogradely advanced in the aortic transvalvular position and works by aspirating blood from the left ventricle to expel it directly into the ascending aorta. This pump can deliver a flow of up to 2.5 liters per minute;
- ✓
Extracorporeal membrane oxygenation (ECMO), which can simultaneously provide mechanical support for the heart and oxygenation of the lungs.
The correct choice of the device, the timing of the implant, the duration of the support and the prevention of any complications represent the key management points in patients requiring mechanical circulatory support (MCS). The scientific evidence remains controversial and currently different centers follow local policy and experience in relation to decision-making and insertion/removal techniques.
The aim of this study is the comparison between Impella 2.5 and IABP using CARDIOSIM
© [
4,
5,
6,
7,
8,
9,
10,
11] software simulator of the cardiovascular system. Our study may contribute to fill the gap in the limited available data from other studies directly comparing Impella 2.5 with IABP.
For the purposes of this study we reproduced the CS status of a virtual patient using an upgraded version of CARDIOSIM©, which has been developed in the “Cardiovascular Numerical/Hybrid Modelling Lab” of the Institute of Clinical Physiology (IFC-CNR) based in Rome. Subsequently, assistance with IABP and Impella 2.5 pump was simulated to evaluate the effects induced on hemodynamic and energetic variables. Two new modules reproducing the behavior of IABP and Impella 2.5 were implemented in CARDIOSIM© platform to simulate the effects induced by the two devices in cooperation with the Faculty of Human Movement and Sport Sciences, “Foro Italico” University of Rome.
2. Materials and Methods
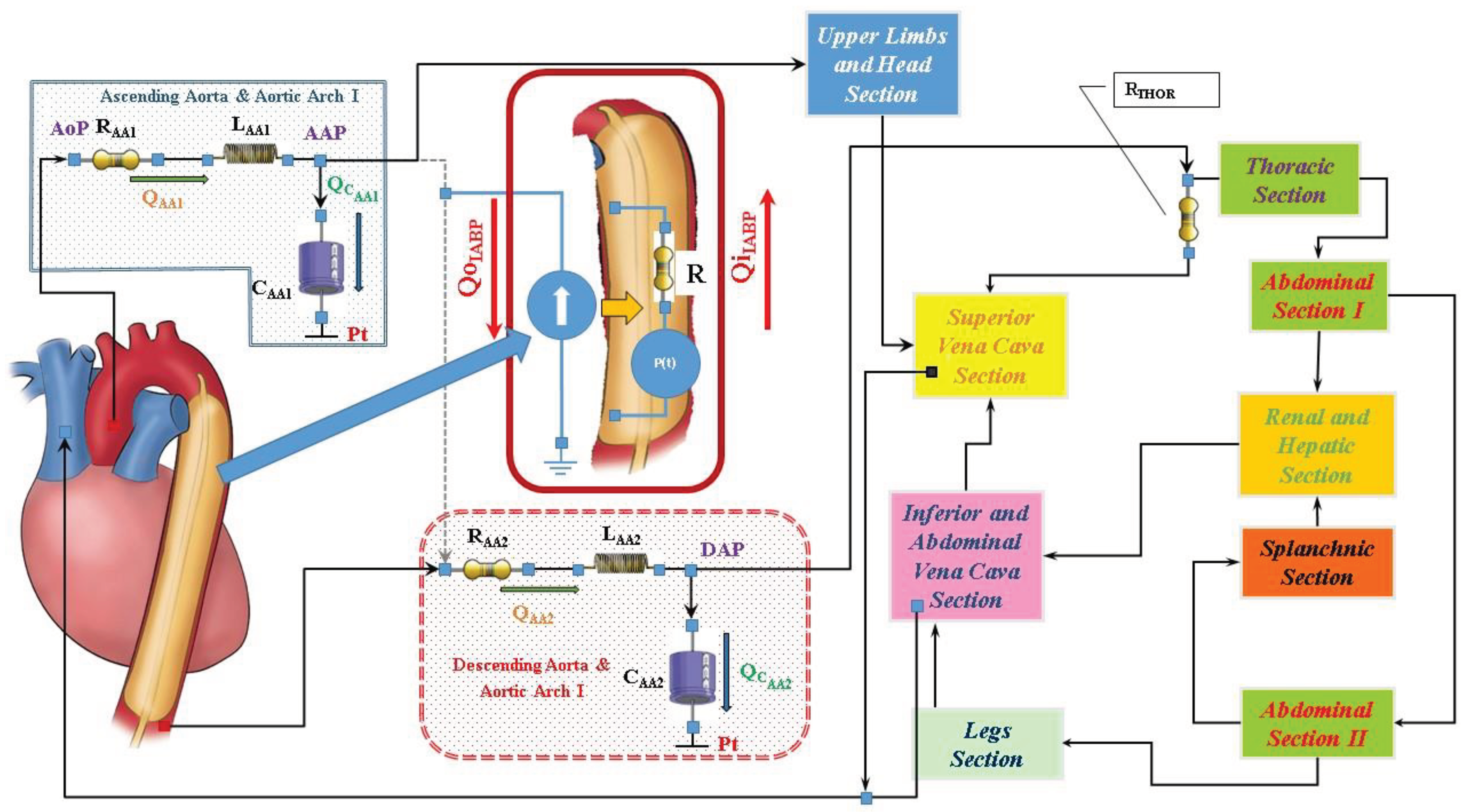
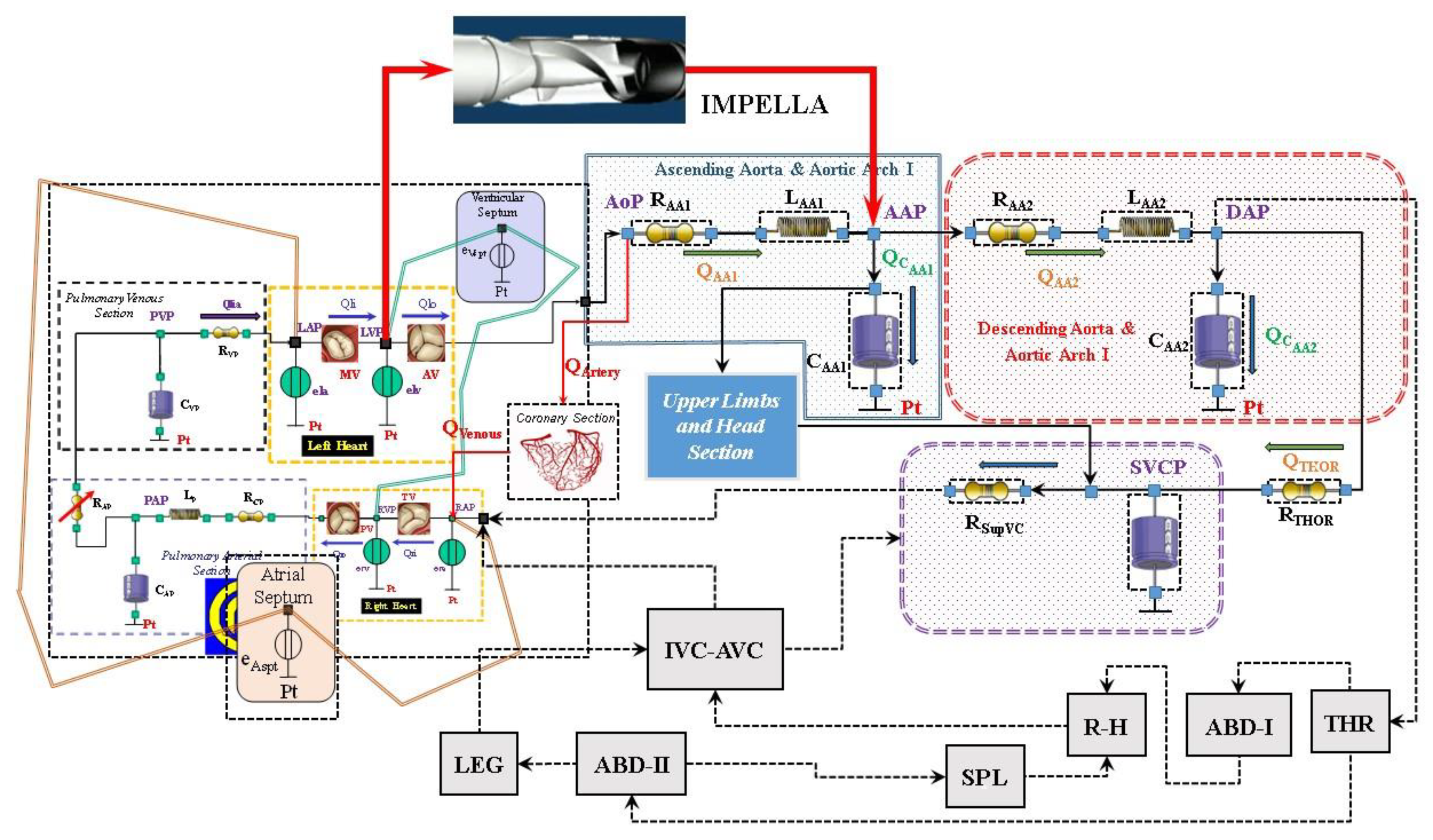
The cardiovascular and heart numerical models
The numerical model of the cardiovascular system used to perform our simulations has been previously described [
4,
5,
6,
7,
8]. The electric analogue of the cardiovascular network described in [
9] consists of the following compartments (
Figure 1): ascending and descending aorta with aortic arch, thoracic, upper limbs and head, superior and inferior vena cava, renal and hepatic, splanchnic, abdominal and lower limbs [
9]. All the compartments are developed using lumped parameter (0-D) models. Both atrial and ventricular septa are interdependent and they are modelled using the time-varying elastance approach [
5]. Mitral, tricuspid, pulmonary and aortic valves are modelled using resistance and diode. A model with inverse resistance is used to simulate pulmonary and tricuspid regurgitation [
4,
6]. The numerical model of the coronary circulation assembled in this configuration of the cardiovascular system is presented in [
10,
11].
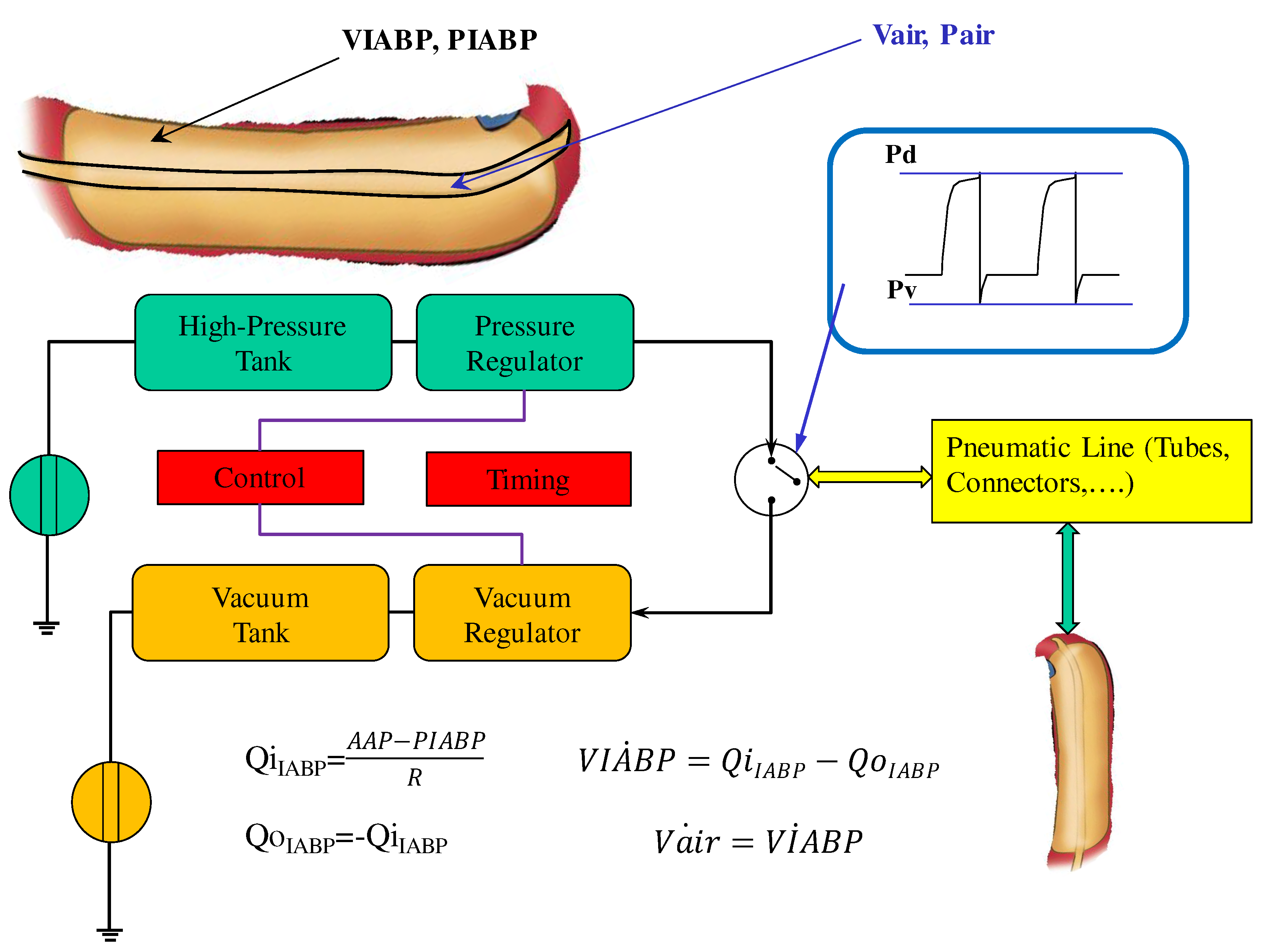
Intra-aortic balloon pump numerical model
Figure 1 shows the electric analogue of IABP inserted below the origin of the left subclavian artery and therefore placed after the ascending aorta and aortic arch compartment. The intra-aortic balloon pump is considered as a flow source Q
IABP(t) in the following way [
12,
13,
14,
15,
16]:

the balloon inflates in diastole and the flow is positive;

the balloon deflates in the following systole and the flow is negative.
The flow source QIABP(t) may be replaced by a pneumatic pressure source P(t), representing the compressed gas reservoir, and by resistance (R) representing the total gas delivery resistance of the system. The pneumatic source P(t) has been modelled describing the ejection and the filling phase separately as follows:

the air outflow from the high-pressure tank connected to the pressure source;

the air outflow from the lower-pressure tank connected to the vacuum source (
Figure 2).
IABP deflation is modelled by:
IABP inflation is described by:
where
Vair=Vt+Vmax-VIABP, Vt is the drive tube volume
Vt=160 [ml],
Vmax is the maximum balloon volume
Vmax= 195 [ml],
E1=3.5,
E2=1.42857,
E3=1.71428,
Ks=0.000799 and
Kd=0.00128.
The module implemented in the new configuration of CARDIOSIM© enables adjustment of the driving and vacuum pressures, the balloon volume and the timing of the IABP. The simulator allows also synchronization of the IABP timing with the QRS complex of the ECG signal or with the aortic pressure waveform. Weaning from IABP can be simulated by decreasing the balloon augmentation ratio from 1:1 to 1:2 or 1:4 or 1:8.
Impella 2.5 numerical model
Impella 2.5 is a catheter-based mechanical device designed to offer circulatory support through percutaneous insertion [
3]. This pump is connected as left ventricular assist device (LVAD) across the aortic valve, generating blood flow in the ascending aorta with direct pressure and volume unloading.
Figure 3 shows the schematic representation of the cardiovascular system assembled with the Impella 2.5 pump.
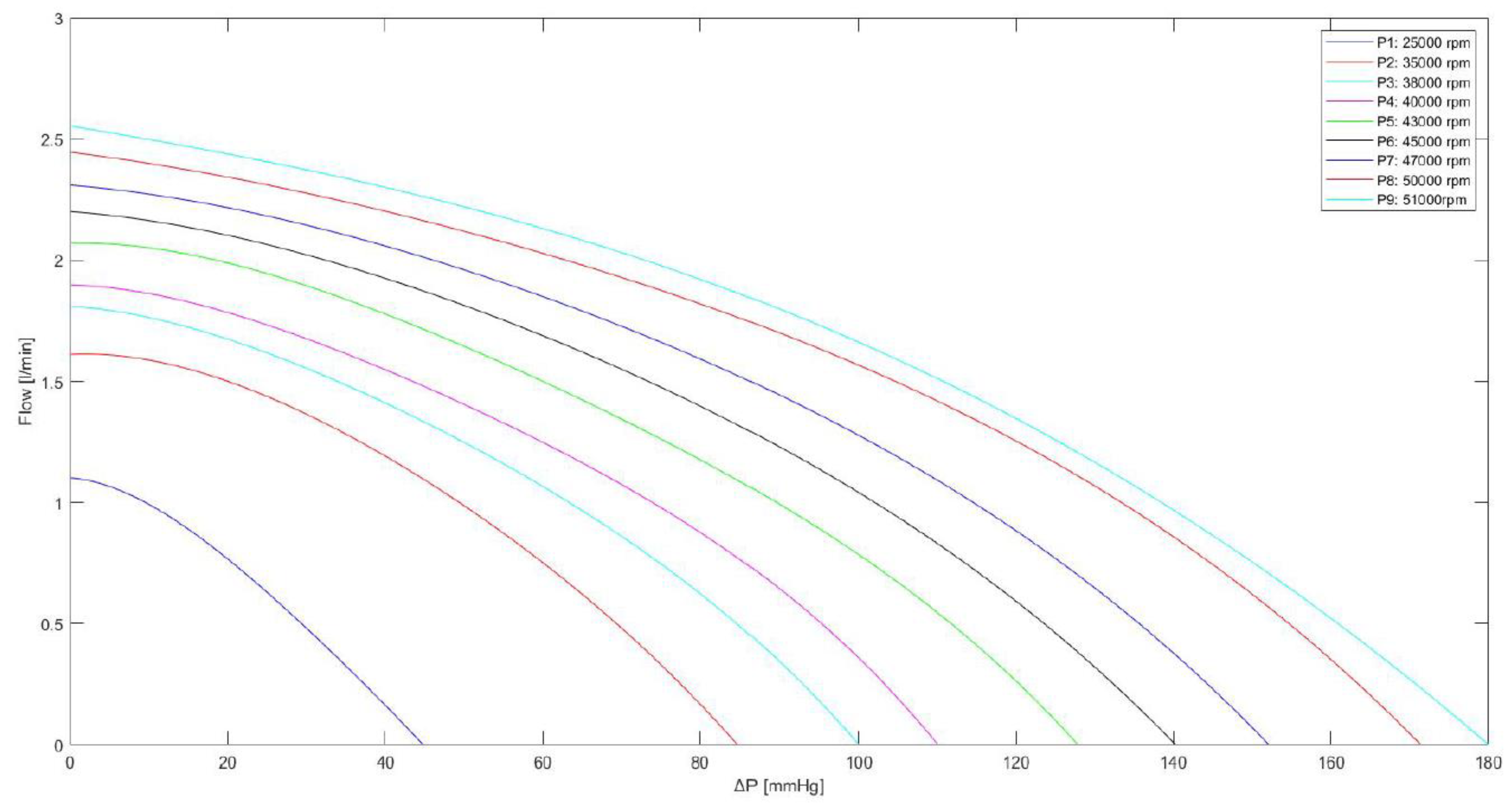
The Impella flow (
FIMP) obtained for different rotational speed is calculated using the following equation:
The values of
Ki(i=1,..,5) constants are listed in
Table 1; LVP is the left ventricular pressure.
Equation 3 is used to derive the curves in Figure 4, which are in a good agreement with the experimental data measured during the functioning of Impella 2.5 for different pump speeds ranging from 25000 to 51000 rpm [
3,
17].
Figure 4.
Relationship between the flow through the Impella 2.5 pump and the pressure difference for different rotational speeds. The curves were obtained using equation (3) with the values listed in
Table 1.
Figure 4.
Relationship between the flow through the Impella 2.5 pump and the pressure difference for different rotational speeds. The curves were obtained using equation (3) with the values listed in
Table 1.
Simulation Protocol
The benchmark for our simulations consisted of a virtual patient in cardiogenic shock whose baseline conditions included a systolic aortic pressure SAP=79.3 mmHg, heart rate HR=70 beat/min, mean left atrial pressure LAP=21.3 mmHg, mean pulmonary arterial pressure PAP= 25.7 mmHg, LVEDP=24 mmHg, cardiac output CO=3.29 l/min, mean coronary blood flow CBF=100.5 ml/min, cardiac index CI=1.73 L/min/m2, LVEDV=149.9 ml, LVESV= 103.0 ml, left ventricular ejection fraction EF%=31.3 and left (right) ventricular arterial coupling Ea/Ees=1.71 (Ees/Ea=1.43).
IABP support was initiated in synchronized mode at baseline conditions with a delay of 220 msec from the start of ventricular diastole for balloon inflation and timing of deflation before the next systole. IABP assist ratio was 1:1 (one inflation per cardiac cycle), driving pressure was set to 260 mmHg with vacuum pressure at -10, zero and +10 mmHg, respectively. The percentage variation with respect to baseline conditions was calculated during IABP assistance for the following parameters: left ventricular output (or cardiac output CO), total flow (CO + Impella flow), cerebral and renal flow, left ventricular external work (LVEW), left ventricular ejection fraction (LVEF), systolic aortic pressure (SAP), end-diastolic aortic pressure (DAP), mean aortic pressure (AoP), LAP, RAP, PAP, CBF, left ventricular end-diastolic (end-systolic) volume LVEDV (LVESV) and left ventricular-arterial coupling (Ea/Ees).
Subsequently, LVAD assistance with Impella 2.5 was initiated with different rotational speeds (35000, 45000 and 50000 rpm). The percentage variation with respect to baseline conditions was calculated for the above parameters.
3. Results
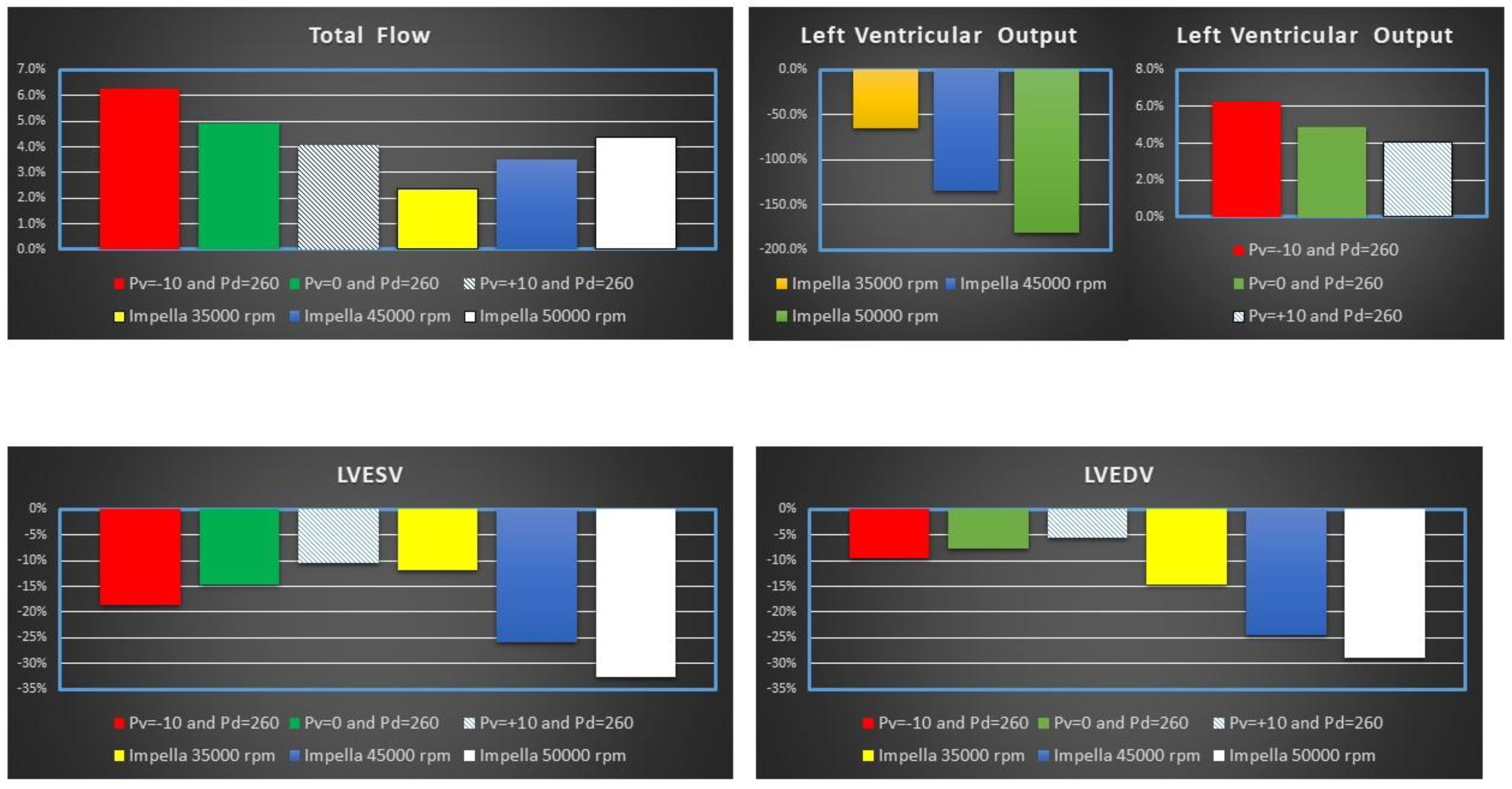
Figure 4 shows the percentage change of total flow (top left panel), LVOF (top right panel) LVESV (bottom left panel) and LVEDV (bottom right panel) calculated in comparison to baseline conditions for IABP and Impella 2.5 (LVAD) support. The simulation settings included LVAD rotational speed at 35000, 45000 and 50000 rpm and IABP support with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg.
Figure 4.
Percentage change calculated in comparison to baseline conditions for Total flow, left ventricular output (LVO) LVEDV and LVESV when IABP or Impella 2.5 (LVAD) support were simulated. The simulation settings included LVAD rotational speed at 35000 45000 and 50000 rpm and IABP support with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg. LVO (or CO) is the Total Flow under IABP support. The sum of CO and LVAD flow gives the Total Flow under Impella 2.5 support.
Figure 4.
Percentage change calculated in comparison to baseline conditions for Total flow, left ventricular output (LVO) LVEDV and LVESV when IABP or Impella 2.5 (LVAD) support were simulated. The simulation settings included LVAD rotational speed at 35000 45000 and 50000 rpm and IABP support with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg. LVO (or CO) is the Total Flow under IABP support. The sum of CO and LVAD flow gives the Total Flow under Impella 2.5 support.
Impella 2.5 support reduced LVO (or CO) by ≅180% (from 3.29 to 1.17 l/min) when the rotational speed was set to 50000 rpm (top left panel in Figure 4). Consequently, the pump increased the Total flow by 4.36% (from 3.29 to 3.44 l/min) with a pump flow of 2.27 l/min. The top left panel in Figure 4 shows that IABP assistance increased LVO from ≅4% (
Pv=+10 mmHg) to ≅6.3% (
Pv=-10 mmHg). Both Impella 2.5 and IABP reduced LVESV (bottom left panel) and LVEDV (bottom right panel). A reduction in LVEDV by ≅15% to ≅30% was observed on LVAD support. Volume unloading on IABP was only 5-10%. The bottom left panel in Figure 4 shows that a reduction in LVESV by ≅10% to ≅18% (≅12% to ≅33%) was observed on IABP (Impella) assistance [
18].
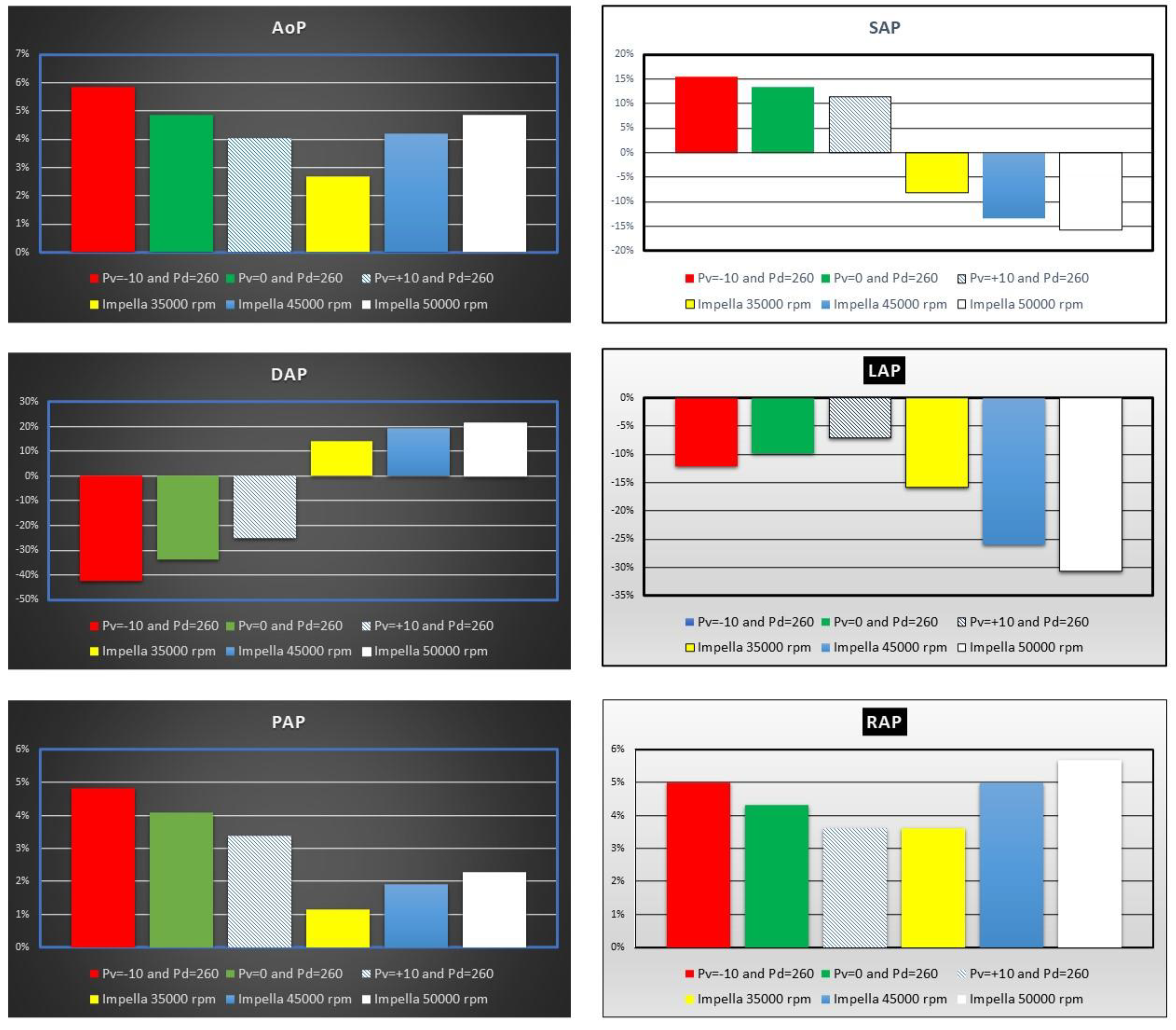
Figure 5 shows the effects induced by IABP and Impella assistance on aortic blood pressure and left atrial pressure (LAP).
Figure 5.
Percentage change calculated in comparison to baseline conditions for mean aortic pressure (AoP), systolic and end-diastolic aortic pressure (SAP and DAP), mean left atrial pressure (LAP), mean pulmonary arterial pressure (PAP) and mean right atrial pressure (RAP) when IABP or Impella 2.5 support were simulated. The simulation settings included LVAD rotational speed at 35000, 45000 and 50000 rpm and IABP with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg.
Figure 5.
Percentage change calculated in comparison to baseline conditions for mean aortic pressure (AoP), systolic and end-diastolic aortic pressure (SAP and DAP), mean left atrial pressure (LAP), mean pulmonary arterial pressure (PAP) and mean right atrial pressure (RAP) when IABP or Impella 2.5 support were simulated. The simulation settings included LVAD rotational speed at 35000, 45000 and 50000 rpm and IABP with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg.
The top left panel shows an increase in mean aortic pressure (AoP) by 4% to ≅6% when IABP was activated. The simulation settings based on different rotational speed for Impella 2.5 pump show an increase in AoP by ≅2.5% to ≅5% in line with current published literature [
19,
20,
21,
22]. SAP decreased by ≅15% compared to baseline conditions when the LVAD rotational speed was set to 50000 rpm (top right panel in
Figure 5), but increased up to ≅15% when driving and vacuum IABP pressures were set to 260 and −10 mmHg respectively. In contrast, DAP increased up to ≅22% when the Impella rotational speed was set to 50000 rpm (middle left panel) whilst decreased by ≅43% when driving and vacuum IABP pressures were set to 260 and −10 mmHg, respectively. The middle right panel (
Figure 5) shows that IABP support (
Pv=-10= and
Pd=260 mmHg) reduced LAP by ≅13% whilst Impella 2.5 assistance increased it by more than ≅30%. Mean pulmonary arterial (bottom left panel) and right atrial (bottom right panel) pressures showed similar percentage increase on IABP support. LVAD assistance increased mean PAP (RAP) up to ≅2.3% (≅5.7%) when the rotational speed was set to 50000 rpm.
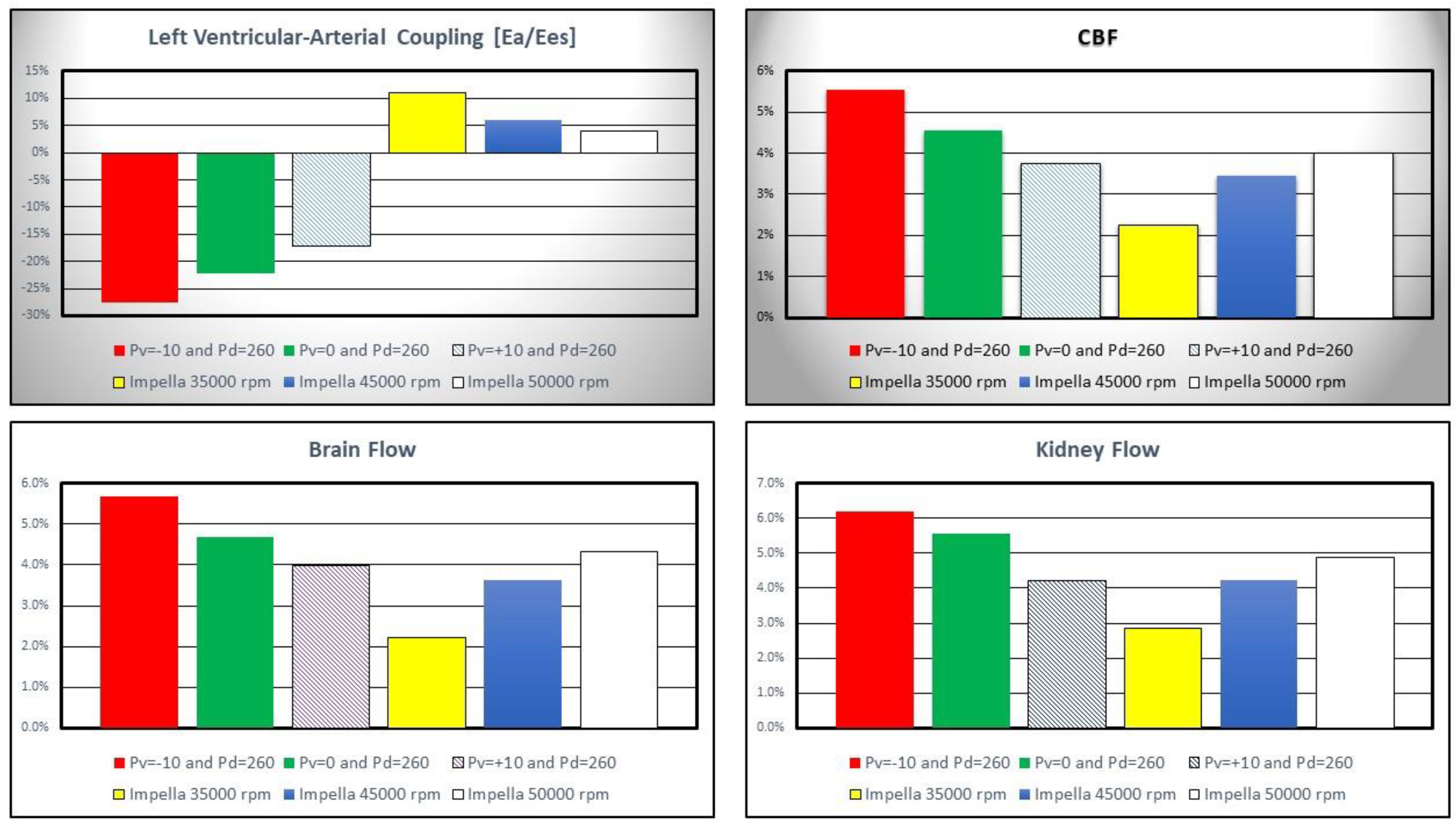
Figure 6 shows the percentage change in left ventricular-arterial coupling and coronary, cerebral and renal blood flow calculated in comparison to baseline conditions for IABP and Impella 2.5 assistance.
Figure 6.
Percentage change calculated in comparison to baseline conditions for left ventricular-arterial coupling, coronary, cerebral and renal blood flow when IABP or LVAD support were simulated. The simulation settings included Impella 2.5 rotational speed at 35000, 45000 and 50000 rpm and IABP support with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg. Ea is the arterial elastance and Ees is the left ventricular elastance.
Figure 6.
Percentage change calculated in comparison to baseline conditions for left ventricular-arterial coupling, coronary, cerebral and renal blood flow when IABP or LVAD support were simulated. The simulation settings included Impella 2.5 rotational speed at 35000, 45000 and 50000 rpm and IABP support with Pv=-10, Pv=0 and Pv=+10 mmHg and Pd= 260 mmHg. Ea is the arterial elastance and Ees is the left ventricular elastance.
IABP assistance reduced
Ea/Ees by more than 25% when
Pv=-10 and
Pd=260 mmHg whilst Impella pump increased left ventricular-arterial coupling although inversely related to pump rotational speed (top left panel). Coronary, cerebral and renal blood flow increased with both Impella and IABP support (top right and bottom left and right panel in
Figure 6) [
19,
23].
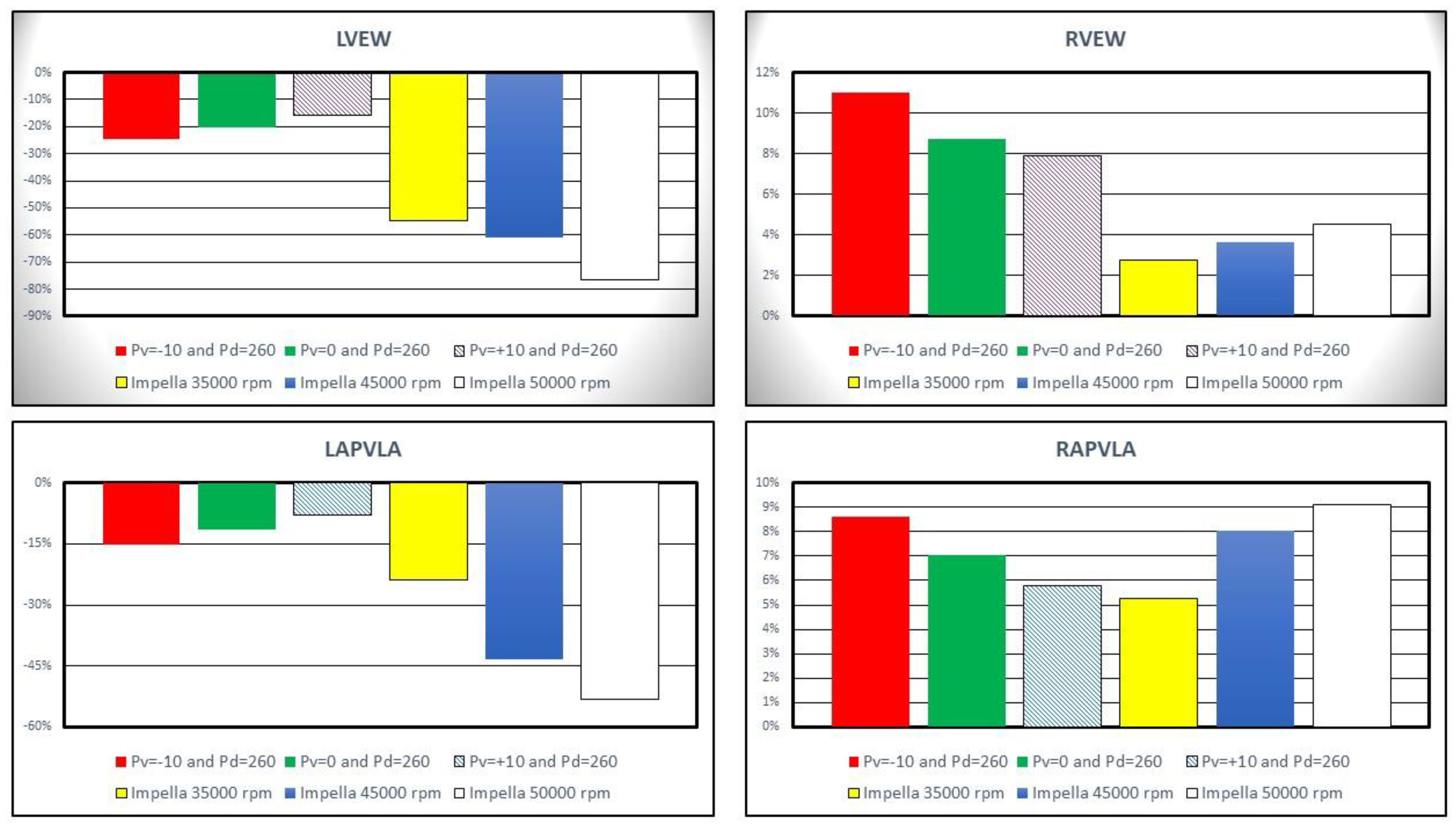
Left ventricular external work (LVEW) decreased by more than 20% compared to baseline conditions on IABP assistance with
Pd= 260 mmHg and
Pv=-10 (top left panel in
Figure 7). Impella 2.5 support reduced LVEW by more than 75% (55%) at 50000 (35000) rpm. Left ventricular pressure-volume area (LPVA) decreased by ≅33.7% at 35000 rpm and by ≅65.9% at 50000 rpm [
24,
25]. LVPA is an index of myocardial oxygen consumption; therefore, increased pump rotational speed was related to a decrease in myocardial oxygen consumption. The top right panel in
Figure 7 shows that the percentage change in right ventricular external work (RVEW) is highest when IABP support is turned on. The percentage reduction in left atrial pressure-volume loop area (LAPVLA) is negligible under IABP support whilst a percentage variation in LAPVLA ranging between ≅24% (35000 rpm) and ≅53.3% (50000 rpm) is observed during Impella assistance (bottom left panel). Finally, the bottom left panel in
Figure 7 shows that right atrial pressure-volume loop area (RAPVLA) is not significantly affected by the two devices.
Figure 7.
Percentage change calculated in comparison to baseline conditions for left ventricular external work (top left panel), right ventricular external work (top right panel) and left and right atrial pressure-volume loop area (bottom left and right panels). The above values were obtained when the Impella rotational speed was set to 35000 45000 and 50000 rpm. During the simulations with IABP support the driving and vacuum pressures were set to Pd= 260 mmHg and to Pv=-10, Pv=0 and Pv=+10 mmHg, respectively.
Figure 7.
Percentage change calculated in comparison to baseline conditions for left ventricular external work (top left panel), right ventricular external work (top right panel) and left and right atrial pressure-volume loop area (bottom left and right panels). The above values were obtained when the Impella rotational speed was set to 35000 45000 and 50000 rpm. During the simulations with IABP support the driving and vacuum pressures were set to Pd= 260 mmHg and to Pv=-10, Pv=0 and Pv=+10 mmHg, respectively.
Simulation data were stored in Excel file and analyzed with Excel software (
Figure 8) to plot pressure-volume loops and coronary blood flow waveforms.
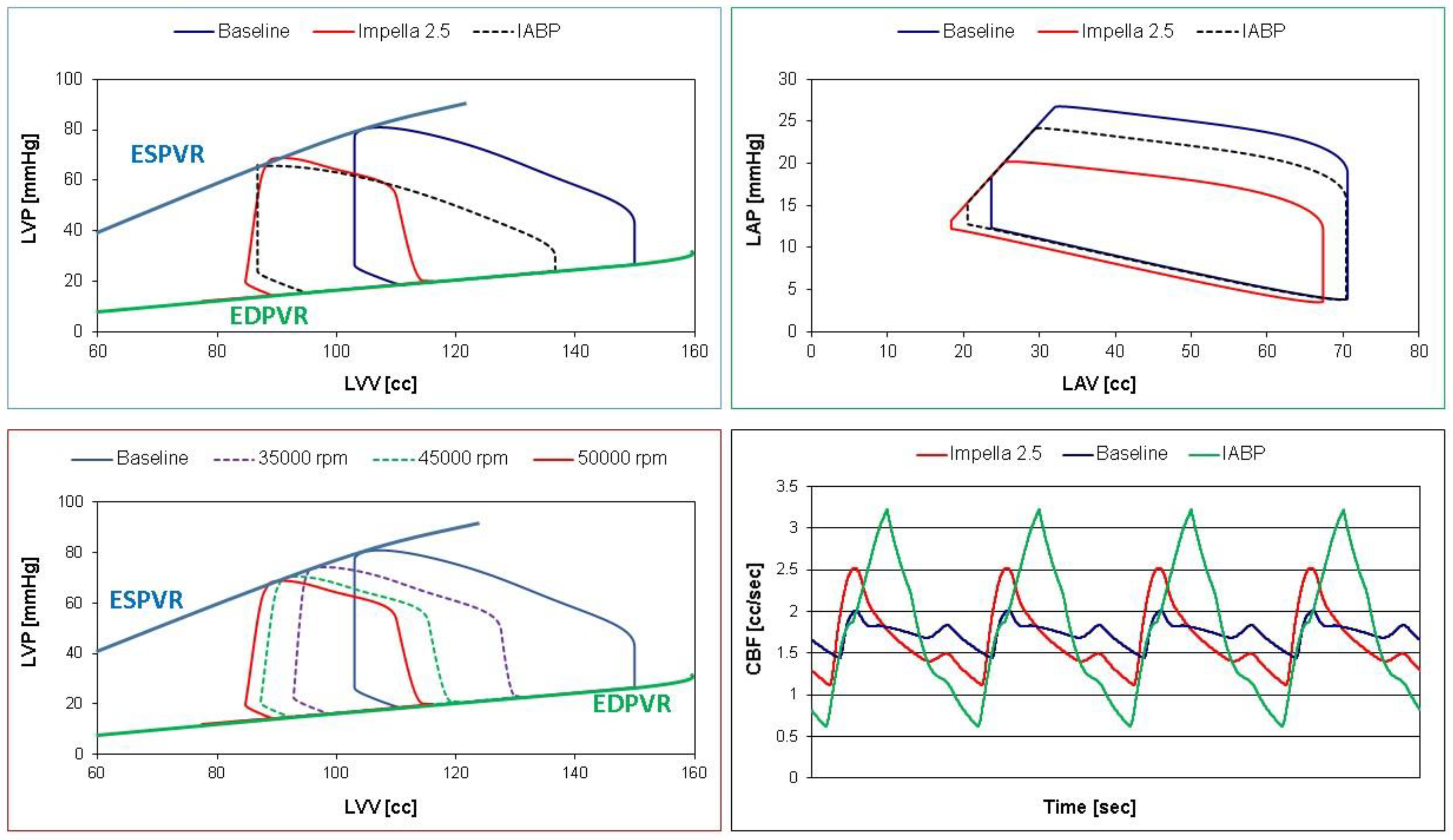
Figure 8.
Left ventricular pressure-volume loops (top and bottom left panels) and coronary blood flow waveforms (bottom right panel) obtained storing data in Excel file and subsequently processed with Excel software. The top left (right) panel shows the left ventricular (atrial) pressure-volume loops obtained in baseline (blue continuous lines) and assisted conditions with IABP (Pd= 260 and Pv=-10 mmHg – dashed black lines) and Impella 2.5 (red lines) at 50000 rpm. The bottom left panel shows the left ventricular pressure-volume loops obtained in baseline (blue continuous lines) and assisted conditions with LVAD rotational speed at 35000, 45000 and 50000 rpm.
Figure 8.
Left ventricular pressure-volume loops (top and bottom left panels) and coronary blood flow waveforms (bottom right panel) obtained storing data in Excel file and subsequently processed with Excel software. The top left (right) panel shows the left ventricular (atrial) pressure-volume loops obtained in baseline (blue continuous lines) and assisted conditions with IABP (Pd= 260 and Pv=-10 mmHg – dashed black lines) and Impella 2.5 (red lines) at 50000 rpm. The bottom left panel shows the left ventricular pressure-volume loops obtained in baseline (blue continuous lines) and assisted conditions with LVAD rotational speed at 35000, 45000 and 50000 rpm.
The top left (right) panel in
Figure 8 shows the left ventricular (atrial) pressure-volume loops obtained in baseline (blue line) and assisted conditions with Impella 2.5 (red line) and IABP (dashed black line). Both devices reduced LVEDV, LVESV (top left panel) and left atrial end-systolic and end-diastolic volume (top right panel). The bottom left panel (
Figure 8) shows the different left ventricular pressure-volume loops in baseline (blue line) and assisted conditions with LVAD rotational speed at 35000 (lilac dashed line), 45000 (green dashed line) and 50000 rpm (red line). Finally, the coronary blood flow waveforms (bottom right panel) in baseline (blue line) and assisted conditions with LVAD (red line) and IABP (green line) have been developed using Excel software.
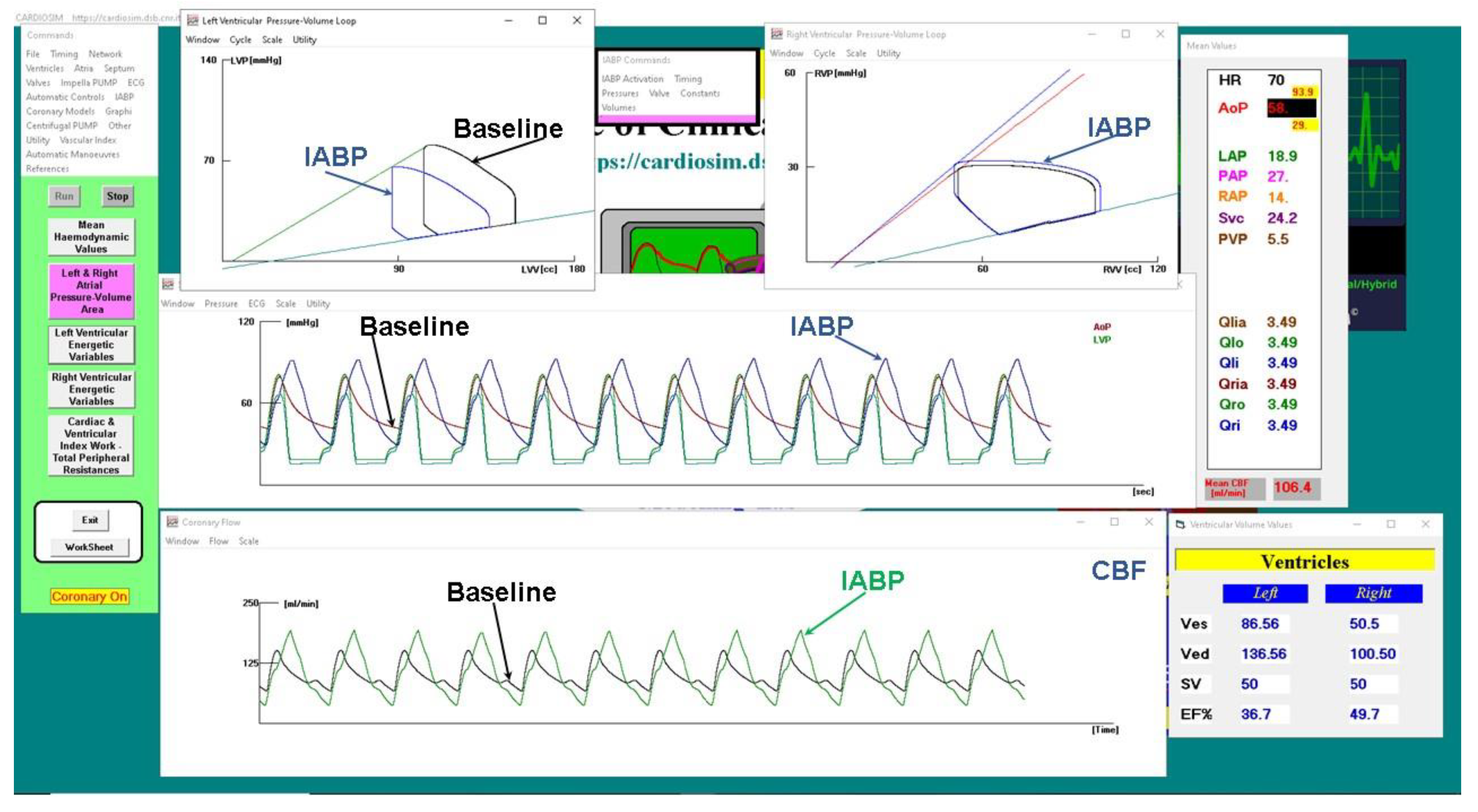
Figure 9 shows a screenshot of CARDIOSIM
© software simulator outlining baseline conditions and IABP support.
The driving and vacuum pressures were set to 260 mmHg and -10 mmHg, respectively, during IABP support. The green coronary blood flow waveforms are plotted in the bottom window (
Figure 9) whilst the black line is the CBF in baseline conditions.
Figure 9.
CARDIOSIM© screenshot showing baseline and assisted conditions with IABP. Proceeding from the top left to the right and then down, the left (top left side) and right (top right side) ventricular pressure-volume loops for baseline and IABP support are observed. The left ventricular and aortic pressure waveforms for baseline (red line) and IABP assistance (blue line) are plotted in the middle window. The bottom window shows the coronary blood flow waveforms.
Figure 9.
CARDIOSIM© screenshot showing baseline and assisted conditions with IABP. Proceeding from the top left to the right and then down, the left (top left side) and right (top right side) ventricular pressure-volume loops for baseline and IABP support are observed. The left ventricular and aortic pressure waveforms for baseline (red line) and IABP assistance (blue line) are plotted in the middle window. The bottom window shows the coronary blood flow waveforms.
4. Discussion
The intra-aortic balloon pump has been widely used as first-line circulatory support device since its first introduction in clinical practice. Despite its proven effects, there has been controversy about its role in cardiogenic shock following the questionable outcome of the SHOCK trial [
26,
27,
28] Alternatives have been proposed such as the Impella device. Each device has also been used combined with V-A ECMO [
29,
30]. A systematic review and meta-analysis of the combined use of V-A ECMO and IABP in cardiogenic shock has shown reduced in-hospital mortality without increased rate of complications [
31]. A combined use of IABP and Impella has been proposed as a potentially superior approach for refractory cardiogenic shock [
32,
33] based on initial experimental evidence of favorable hemodynamics following combined support with IABP and Impella p9 device in a sheep model of acute myocardial infarction [
34]. Another porcine model of acute myocardial infarction has shown that LV unloading with Impella CP decreases LV end-diastolic wall stress and increases microvascular perfusion of the infarcted area [
35]. A retrospective review of 128 patients undergoing V-A ECMO or Impella support because of refractory cardiogenic shock after acute MI showed significant reduction in adjusted 30-day mortality following V-A ECMO support. A higher rate of MCS escalation was observed in patients undergoing Impella device support [
2]. A review of 6290 patients sustaining acute myocardial infarction complicated by cardiogenic shock and requiring percutaneous coronary intervention (PCI) showed better out-come for patients receiving Impella support compared to those undergoing V-A ECMO insertion [
36]. An experimental model of porcine acute myocardial infarction has been used to compare peripheral V-A ECMO with Impella CP based on an open-labeled randomized setting. Impella CP resulted in a more effective volume unloading of the left ventricle. Both devices reduced myocardial oxygen consumption significantly. Impella CP shifted the pressure-volume loop to the left with a decreased pressure-volume-area (PVA) whilst V-A ECMO increased PVA and decreased heart rate [
37]. A systematic review of IABP versus Impella device in emergency revascularization following acute MI complicated by cardiogenic shock suggests that there is insufficient evidence to support superior survival in patients undergoing IABP or Impella insertion when compared to control group despite apparent superior hemodynamic support offered by Impella device [
38]. Therefore, given this contrasting evidence, our simulations may help understand the features of each device, their strengths and weaknesses and determine their potential in the context of a virtual patient in cardiogenic shock.
The outcome of IABP and Impella 2.5 support was compared with the use of numerical models describing blood flow rates and pressures in the cardiovascular system and IABP and Impella 2.5 features. Cardiogenic shock was simulated by tuning the parameters in the cardiovascular system model and the same settings were used to simulate the outcome of pump support. In addition, three different operating conditions were used in both IABP (Pv=-10 mmHg, 0 mmHg and 10 mmHg) and Impella 2.5 (n=35000 rpm, n=45000 rpm and n=50000 rpm) to evaluate hemodynamic variables on pump support.
IABP increased the total blood flow rate in the cardiovascular system more than Impella 2.5 at
Pv=-10 mmHg and
Pv=0 mmHg. Left ventricular output was reduced during Impella 2.5 support due to continuous unloading of the left ventricle whereas IABP support increased the left ventricular output. The simulation results show that Impella 2.5 provided better left ventricular unloading than IABP as the decrease in left ventricular end-systolic and end-diastolic volumes were relatively high at 45000 rpm and 50000 rpm operating speeds (Figure 4). The simulation results are consistent with the clinical findings for left ventricular unloading [
39].
Although both devices increased the mean aortic pressure with varying degree at different settings, the increase in the mean aortic pressure was higher on IABP support. IABP increased the systolic aortic pressure and decreased end-diastolic aortic pressure whereas Impella 2.5 decreased the systolic aortic pressure and increased end-diastolic aortic pressure. Therefore, aortic pulse pressure was higher on IABP support. Impella 2.5 decreased the aortic pulse pressure due to continuous operating speed and unloading of the left ventricle. IABP on the other hand was synchronized with the left ventricle and provided an increased aortic pulse pressure (
Figure 5). Again, the simulation results confirm the clinical findings [
39]. Impella 2.5 support reduced the mean left atrial pressure more than IABP at all operating speeds. Impella 2.5 support is more beneficial in reducing left atrial pressure.
Left ventricular-arterial coupling is ≅1 in healthy conditions [
40] whereas it increases with reduced left ventricular end-systolic elastance during cardiogenic shock [
41]. The baseline left ventricular-arterial coupling was 1.71 in the simulations confirming the clinical data. IABP support decreased left ventricular-arterial coupling whereas Impella 2.5 support increased it (
Figure 6). Coronary, cerebral and renal blood flow rates increased on both IABP and Impella 2.5 support. This was achieved by decreasing vacuum pressure for IABP or increasing operating speed for Impella 2.5. However, IABP support at
Pv=-10 mmHg and
Pv=0 mmHg vacuum pressures increased the blood flow rates in these sections better than Impella 2.5 support (
Figure 6) which may be interpreted as IABP being more beneficial to improve organ perfusion.
Left ventricular external work was reduced on both IABP and Impella 2.5 support. However, Impella 2.5 decreased left ventricular external work in a more remarkable manner (
Figure 7). Therefore, Impella 2.5 seems to reduce myocardial oxygen consumption more effectively compared to IABP. A similar trend in the change of left atrial pressure-volume loop area occurs under the support of both IABP and Impella 2.5 devices. On the other hand, both devices increase right ventricular external work. However, Impella 2.5 support increases right ventricular external work remarkably less than IABP at all simulated operating speeds (
Figure 7). The right atrial pressure-volume loop area also increases following IABP and Impella 2.5 device support.
The outcome of our simulations does confirm the more efficient volume unloading and reduction in myocardial oxygen consumption generated by Impella 2.5. Nevertheless, organ perfusion in terms of coronary, cerebral and renal blood flow is better addressed by IABP assistance. The overall analysis of data suggests that a combined use of IABP and Impella 2.5 may compensate the shortcomings of each device alone and potentially lead to a better level of support. The importance of the context remains to be taken into account considering that IABP availability is higher given its ease of use and patient transfer to a cath lab is not necessarily required: device insertion can be performed at the bedside in intensive care unit or in theatre with or without trans-esophageal echocardiography guidance.
Author Contributions
Conceptualization, C.DeL. and B.DeL.; methodology, B.DeL.; software, B.DeL.; validation, C.DeL., B.DeL. and M.C.; formal analysis, B.DeL. S.B. and R.B.; investigation, C.DeL., R.B. and S.B.; resources, C.DeL and B.DeL.; data curation, B.DeL. R.B., M.C. and S.B.; writing—original draft preparation, B.DeL. and M.C.; writing—review and editing, S.B., R.B., M.C. and C.DeL.; visualization, B.DeL.; supervision, C.DeL. and M.C.; project administration, C.DeL. All authors have read and agreed to the published version of the manuscript.