Submitted:
03 February 2023
Posted:
07 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Status Quo of Osteosarcoma Therapies
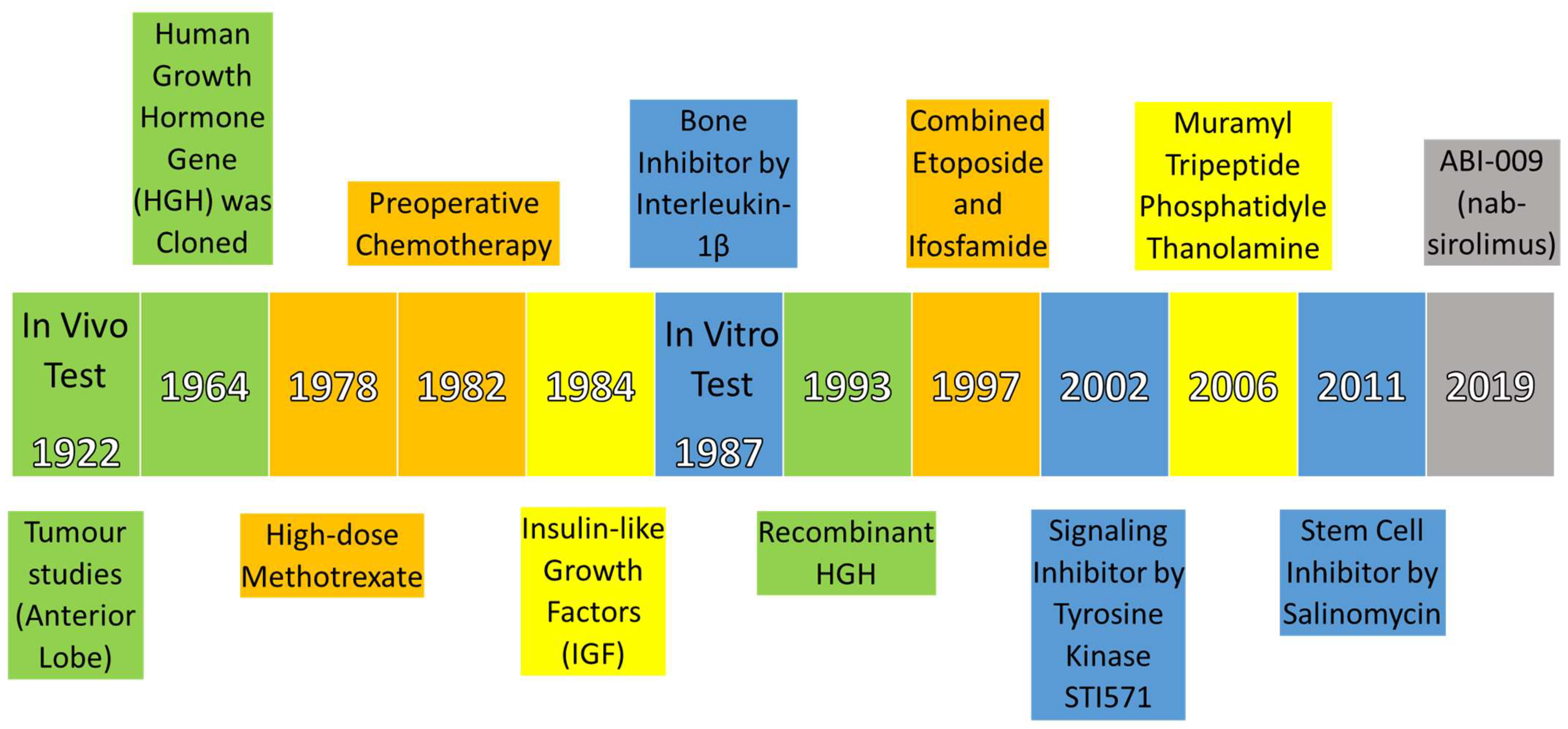
2.1. Historical Development
2.2. Clinical Advancement
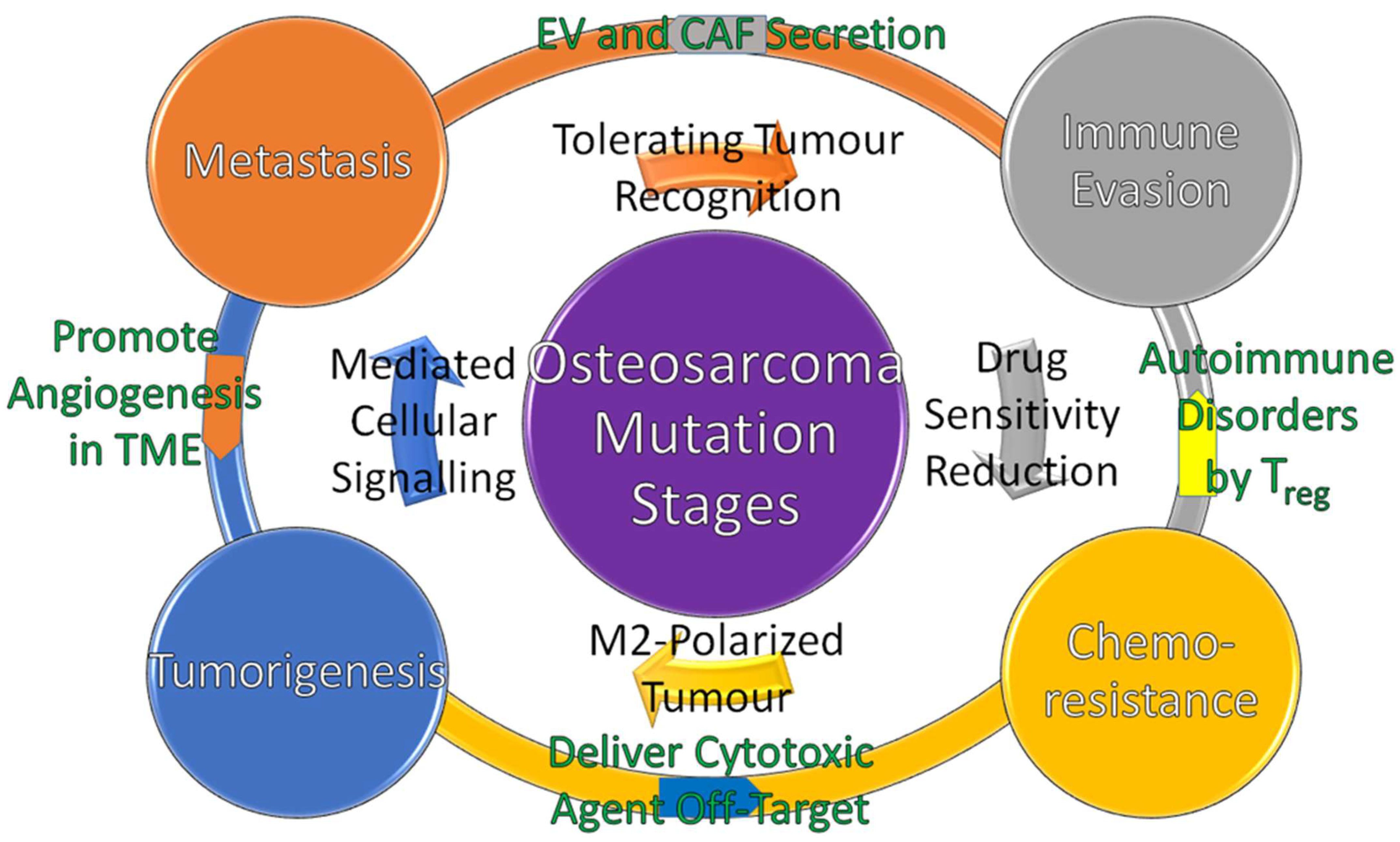
3. Osteosarcoma Bidirectional Mutation Stages
3.1. Tumorigenesis
3.2. Metastasis
3.3. Immune Evasion
3.4. Chemoresistance
4. Recent Osteosarcoma Therapies
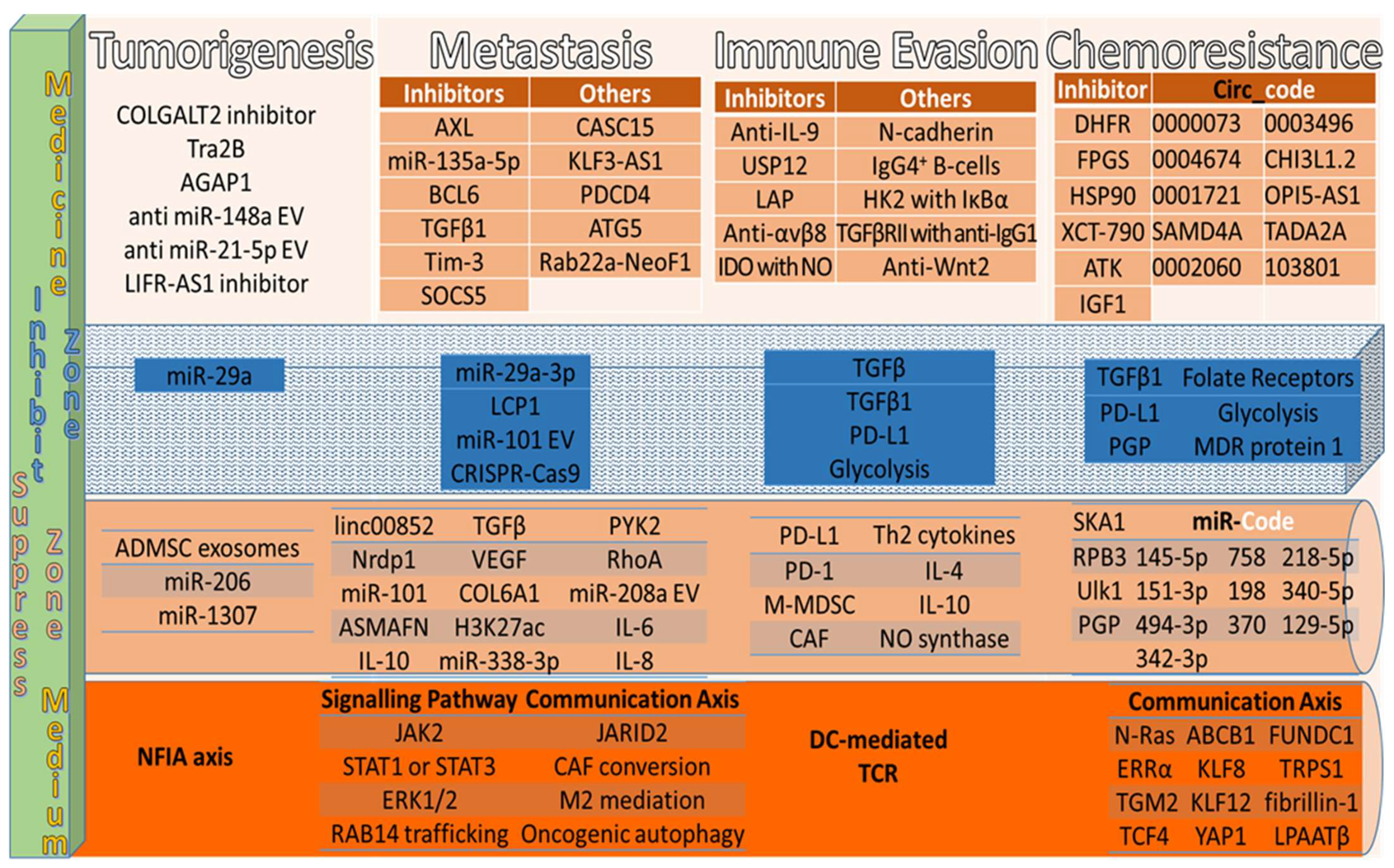
4.1. Tumorigenesis Therapies
4.2. Interfere Communication Mediators’ Therapies
4.3. Immune Evasion Therapies
4.4. Chemoresistance Therapies
5. Conclusions
6. Challenges and Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moukengue, B.; Lallier, M.; Marchandet, L.; Baud’huin, M.; Verrecchia, F.; Ory, B.; Lamoureux, F. Origin and Therapies of Osteosarcoma. Cancers (Basel). 2022, 14, 3503. [CrossRef]
- Stiller, C.A.; Bielack, S.S.; Jundt, G.; Steliarova-Foucher, E. Bone Tumours in European Children and Adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System Project. Eur. J. Cancer 2006, 42, 2124–2135. [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program, SEER*Stat Database: Incidence—SEER Research Data, 9 Registries, Nov 2019 Sub (1975–2017). Available: https://seer.cancer.gov/ (accessed on 24 January 2023).
- Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020, 43. [CrossRef]
- Albuquerque M. Silva1, J.; Marchiori2, E.; Carvalho de Macedo1, F.; Ricardo Garcia da Silva1, P.; Brandão Amorim1, 3, V. Pulmonary Metastasis of Osteosarcoma: Multiple Presentations in a Single Patient. J. Bras. Pneumol. 2022, e20210478. [CrossRef]
- American Cancer Society. Survival Rates for Osteosarcoma. Available: https://www.cancer.org/cancer/osteosarcoma/detection-diagnosis-staging/survival-rates.html (accessed on 28 January 2023).
- Hattinger, C.M.; Salaroglio, I.C.; Fantoni, L.; Godel, M.; Casotti, C.; Kopecka, J.; Scotlandi, K.; Ibrahim, T.; Riganti, C.; Serra, M. Strategies to Overcome Resistance to Immune-Based Therapies in Osteosarcoma. Int. J. Mol. Sci. 2023, 24, 799. [CrossRef]
- Evdokimova, V.; Gassmann, H.; Radvanyi, L.; Burdach, S.E.G. Current State of Immunotherapy and Mechanisms of Immune Evasion in Ewing Sarcoma and Osteosarcoma. Cancers (Basel). 2022, 15, 272. [CrossRef]
- Li, E.; Zhong, S.; Ma, G.; Wang, Q.; Gao, Y. MIR503HG Overexpression Inhibits the Malignant Behaviors of Osteosarcoma Cells by Sponging MiR-103a-3p. Crit. Rev. Eukaryot. Gene Expr. 2022. [CrossRef]
- Clapp, B.; Portela, R.; Sharma, I.; Nakanishi, H.; Marrero, K.; Schauer, P.; Halfdanarson, T.R.; Abu Dayyeh, B.; Kendrick, M.; Ghanem, O.M. Risk of Non-Hormonal Cancer after Bariatric Surgery: Meta-Analysis of Retrospective Observational Studies. Br. J. Surg. 2022, 110, 24–33. [CrossRef]
- Yeo, S.Y.; Bratke, G.; Grüll, H. High Intensity Focused Ultrasound for Treatment of Bone Malignancies—20 Years of History. Cancers (Basel). 2022, 15, 108. [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36–50. [CrossRef]
- Singh, R.; Valluri, A.; Didwania, P.; Lehrer, E.J.; Baliga, S.; Hiniker, S.; Braunstein, S.E.; Murphy, E.S.; Lazarev, S.; Tinkle, C.; et al. Efficacy and Safety of Stereotactic Body Radiation Therapy (SBRT) for Pediatric Malignancies: The LITE-SABR Systematic Review and Meta-Analysis. Adv. Radiat. Oncol. 2023, 101123. [CrossRef]
- Ahmed, G.; Zamzam, M.; Kamel, A.; Ahmed, S.; Salama, A.; Zaki, I.; Kamal, N.; Elshafiey, M. Effect of Timing of Pulmonary Metastasis Occurrence on the Outcome of Metastasectomy in Osteosarcoma Patients. J. Pediatr. Surg. 2019, 54, 775–779. [CrossRef]
- Khanna, C.; Fan, T.M.; Gorlick, R.; Helman, L.J.; Kleinerman, E.S.; Adamson, P.C.; Houghton, P.J.; Tap, W.D.; Welch, D.R.; Steeg, P.S.; et al. Toward a Drug Development Path That Targets Metastatic Progression in Osteosarcoma. Clin. Cancer Res. 2014, 20, 4200–4209. [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [CrossRef]
- Guo, S.; Zhu, X.; Huang, Z.; Wei, C.; Yu, J.; Zhang, L.; Feng, J.; Li, M.; Li, Z. Genomic Instability Drives Tumorigenesis and Metastasis and Its Implications for Cancer Therapy. Biomed. Pharmacother. 2023, 157, 114036. [CrossRef]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-Hallmarks of Aging and Cancer. Cell Metab. 2023, 35, 12–35. [CrossRef]
- Ni, M. [Update and Interpretation of 2021 National Comprehensive Cancer Network (NCCN) “Clinical Practice Guidelines for Bone Tumors”]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2021, 35, 1186–1191. [CrossRef]
- Tan, G.; Xu, J.; Yu, Q.; Yang, Z.; Zhang, H. The Safety and Efficiency of Photodynamic Therapy for the Treatment of Osteosarcoma: A Systematic Review of in Vitro Experiment and Animal Model Reports. Photodiagnosis Photodyn. Ther. 2022, 40, 103093. [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials (Basel). 2022, 15, 1421. [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. Structural Strength Analyses for Low Brass Filler Biomaterial with Anti-Trauma Effects in Articular Cartilage Scaffold Design. Materials (Basel). 2022, 15, 4446. [CrossRef]
- Fierro Pineda, J.C.; Wedekind, M.F.; Glod, J.W. Immunotherapy Approaches for Rare Pediatric Solid Tumors: Advances and Future Directions. Curr. Opin. Pediatr. 2023, 35, 63–74. [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Numerical Simulation Study on Relationship between the Fracture Mechanisms and Residual Membrane Stresses of Metallic Material. J. Funct. Biomater. 2022, 13, 20. [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials (Basel). 2022, 15, 7672. [CrossRef]
- Ke, C.-H.; Chiu, Y.-H.; Huang, K.-C.; Lin, C.-S. Exposure of Immunogenic Tumor Antigens in Surrendered Immunity and the Significance of Autologous Tumor Cell-Based Vaccination in Precision Medicine. Int. J. Mol. Sci. 2022, 24, 147. [CrossRef]
- Fazaeli, H.; Sheikholeslami, A.; Ghasemian, F.; Amini, E.; Sheykhhasan, M. The Emerging Role of LncRNA FENDRR in Multiple Cancer: A Review Study. Curr. Mol. Med. 2022, 22. [CrossRef]
- Shahpouri, M.; Adili-Aghdam, M.A.; Mahmudi, H.; Jaymand, M.; Amoozgar, Z.; Akbari, M.; Hamblin, M.R.; Jahanban-Esfahlan, R. Prospects for Hypoxia-Based Drug Delivery Platforms for the Elimination of Advanced Metastatic Tumors: From 3D Modeling to Clinical Concepts. J. Control. Release 2023, 353, 1002–1022. [CrossRef]
- Zheng, Y.; Chang, X.; Huang, Y.; He, D. The Application of Antidepressant Drugs in Cancer Treatment. Biomed. Pharmacother. 2023, 157, 113985. [CrossRef]
- Khadembaschi, D.; Jafri, M.; Praveen, P.; Parmar, S.; Breik, O. Does Neoadjuvant Chemotherapy Provide a Survival Benefit in Maxillofacial Osteosarcoma: A Systematic Review and Pooled Analysis. Oral Oncol. 2022, 135, 106133. [CrossRef]
- Lim, Y.Y.; Zaidi, A.M.A.; Miskon, A. Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy. Pharmaceuticals 2022, 15, 1330. [CrossRef]
- Evans, H.M.; Long, J.A. Characteristic Effects upon Growth, Oestrus and Ovulation Induced by the Intraperitoneal Administration of Fresh Anterior Hypophyseal Substance. Proc. Natl. Acad. Sci. 1922, 8, 38–39. [CrossRef]
- Li, C.H.; Liu, W.-K. Human Pituitary Growth Hormone. Experientia 1964, 20, 169–178. [CrossRef]
- Jaffe, N.; Frei, E.; Watts, H.; Traggis, D. High-Dose Methotrexate in Osteogenic Sarcoma: A 5-Year Experience. Cancer Treat. Rep. 1978, 62, 259–264.
- Rosen, G.; Caparros, B.; Huvos, A.G.; Kosloff, C.; Nirenberg, A.; Cacavio, A.; Marcove, R.C.; Lane, J.M.; Mehta, B.; Urban, C. Preoperative Chemotherapy for Osteogenic Sarcoma:Selection of Postoperative Adjuvant Chemotherapy Based on the Response of the Primary Tumor to Preoperative Chemotherapy. Cancer 1982, 49, 1221–1230. [CrossRef]
- Zapf, J.; Schmid, C.H.; Froesch, E.R. 1 Biological and Immunological Properties of Insulin-like Growth Factors (IGF) I and II. Clin. Endocrinol. Metab. 1984, 13, 3–30. [CrossRef]
- Stashenko, P.; Dewhirst, F.E.; Rooney, M.L.; Desjardins, L.A.; Heeley, J.D. Interleukin-1β Is a Potent Inhibitor of Bone Formation in Vitro. J. Bone Miner. Res. 2009, 2, 559–565. [CrossRef]
- Bengtsson, B.A.; Edén, S.; Lönn, L.; Kvist, H.; Stokland, A.; Lindstedt, G.; Bosaeus, I.; Tölli, J.; Sjöström, L.; Isaksson, O.G. Treatment of Adults with Growth Hormone (GH) Deficiency with Recombinant Human GH. J. Clin. Endocrinol. Metab. 1993, 76, 309–317. [CrossRef]
- Gentet, J.-C.; Brunat-Mentigny, M.; Demaille, M.C.; Pein, F.; Avet-Loiseau, H.; Berger, C.; De Lumley, L.; Pacquement, H.; Schmitt, C.; Sariban, E.; et al. Ifosfamide and Etoposide in Childhood Osteosarcoma. A Phase II Study of the French Society of Paediatric Oncology. Eur. J. Cancer 1997, 33, 232–237. [CrossRef]
- McGary, E.C.; Weber, K.; Mills, L.; Doucet, M.; Lewis, V.; Lev, D.C.; Fidler, I.J.; Bar-Eli, M. Inhibition of Platelet-Derived Growth Factor-Mediated Proliferation of Osteosarcoma Cells by the Novel Tyrosine Kinase Inhibitor STI571. Clin. Cancer Res. 2002, 8, 3584–3591.
- Nardin, A.; Lefebvre, M.; Labroquere, K.; Faure, O.; Abastado, J. Liposomal Muramyl Tripeptide Phosphatidylethanolamine: Targeting and Activating Macrophages for Adjuvant Treatment of Osteosarcoma. Curr. Cancer Drug Targets 2006, 6, 123–133. [CrossRef]
- Tang, Q.-L.; Zhao, Z.-Q.; Li, J.; Liang, Y.; Yin, J.-Q.; Zou, C.-Y.; Xie, X.-B.; Zeng, Y.-X.; Shen, J.-N.; Kang, T.; et al. Salinomycin Inhibits Osteosarcoma by Targeting Its Tumor Stem Cells. Cancer Lett. 2011, 311, 113–121. [CrossRef]
- Gordon, E.M.; Chua-Alcala, V.S.; Kim, K.; Baby, R.; Angel, N.; Quon, D.; Wong, S.; Chawla, S.P. A Phase I/II Investigation of Nivolumab and ABI-009 ( Nab -Sirolimus) in Advanced Undifferentiated Pleomorphic Sarcoma (UPS), Liposarcoma (LPS), Chondrosarcoma (CS), Osteosarcoma (OS), and Ewing Sarcoma: Preliminary Efficacy and Safety Results. J. Clin. Oncol. 2019, 37, 11057–11057. [CrossRef]
- Manji, A.; Samson, Y.; Deyell, R.J.; Johnston, D.L.; Lewis, V.A.; Zorzi, A.P.; Berman, J.N.; Brodeur-Robb, K.; Morrison, E.; Kee, L.; et al. Low-Dose Metronomic Topotecan and Pazopanib (TOPAZ) in Children with Relapsed or Refractory Solid Tumors: A C17 Canadian Phase I Clinical Trial. Cancers (Basel). 2022, 14, 2985. [CrossRef]
- Gartrell, J.; Panetta, J.C.; Baker, S.D.; Chen, Y.L.; Hawkins, D.S.; Ostrenga, A.; Scharschmidt, T.J.; Spunt, S.L.; Wang, D.; Weiss, A.R. The Effects of Pazopanib on Doxorubicin Pharmacokinetics in Children and Adults with Non-Rhabdomyosarcoma Soft Tissue Sarcoma: A Report from Children’s Oncology Group and NRG Oncology Study ARST1321. Cancer Chemother. Pharmacol. 2022, 89, 551–557. [CrossRef]
- Szkandera, J. Keeping up the ‘Race Pace’ in a Patient with Nonuterine Leiomyosarcoma. Futur. Oncol. 2022, 18, 12–16. [CrossRef]
- Liu, Z.; Wang, X.; Wang, J.; Zhang, P.; Li, C.; Wang, B.; Liu, G.; Yao, W. Gemcitabine Plus Anlotinib Is Effective and Safe Compared to Gemcitabine Plus Docetaxel in Advanced Soft Tissue Sarcoma. Front. Oncol. 2022, 12. [CrossRef]
- Villaruz, L.C.; Kelly, K.; Waqar, S.N.; Davis, E.J.; Shapiro, G.; LoRusso, P.; Dees, E.C.; Normolle, D.P.; Rhee, J.C.; Chu, E.; et al. NCI 9938: Phase I Clinical Trial of ATR Inhibitor Berzosertib (M6620, VX-970) in Combination with Irinotecan in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2022, 40, 3012–3012. [CrossRef]
- Plummer, R.; Dean, E.; Arkenau, H.-T.; Redfern, C.; Spira, A.I.; Melear, J.M.; Chung, K.Y.; Ferrer-Playan, J.; Goddemeier, T.; Locatelli, G.; et al. A Phase 1b Study Evaluating the Safety and Preliminary Efficacy of Berzosertib in Combination with Gemcitabine in Patients with Advanced Non-Small Cell Lung Cancer. Lung Cancer 2022, 163, 19–26. [CrossRef]
- Brahmi, M.; Gautier, J.; Dufresne, A.; Marec-Berard, P.; Cropet, C.; Vizoso, S.; Bissuel, L.; Valentin, T.; Entz-Werle, N.; Bompas, E.; et al. REGOMAIN: A Randomized, Placebo-Controlled, Double-Blinded, Multicenter, Comparative Phase II Study of the Efficacy of Regorafenib as Maintenance Treatment in Patients (Pts) with High-Grade Bone Sarcomas (HGBS) at Diagnosis or Relapse and without Complete. J. Clin. Oncol. 2022, 40, TPS11585–TPS11585. [CrossRef]
- Vienot, A.; Vernerey, D.; Bouard, A.; Klajer, E.; Asgarov, K.; Kim, S.; Tournigand, C.; Louvet, C.; André, T.; Rousseau, B.; et al. SO-20 Stanniocalcin 1 (STC1) in Patients with Refractory Colorectal Cancer (CRC) Treated with Regorafenib: An Exploratory Analysis of the CORRECT Trial. Ann. Oncol. 2022, 33, S365. [CrossRef]
- Lee, S.; Cavaliere, A.; Gallezot, J.-D.; Keler, T.; Michelhaugh, S.K.; Belitzky, E.; Liu, M.; Mulnix, T.; Maher, S.E.; Bothwell, A.L.M.; et al. [89Zr]ZrDFO-CR011 PET Correlates with Response to Glycoprotein Nonmetastatic Melanoma B–Targeted Therapy in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2022, 21, 440–447. [CrossRef]
- Lazaratos, A.-M.; Annis, M.G.; Siegel, P.M. GPNMB: A Potent Inducer of Immunosuppression in Cancer. Oncogene 2022, 41, 4573–4590. [CrossRef]
- Albarrán, V.; Villamayor, M.L.; Chamorro, J.; Rosero, D.I.; Pozas, J.; San Román, M.; Calvo, J.C.; Pérez de Aguado, P.; Moreno, J.; Guerrero, P.; et al. Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 13784. [CrossRef]
- Pearson, A.D.; Gaspar, N.; Janeway, K.; Campbell-Hewson, Q.; Lawlor, E.R.; Copland, C.; Karres, D.; Norga, K.; Benzaghou, F.; Weiner, S.; et al. Paediatric Strategy Forum for Medicinal Product Development of Multi-Targeted Kinase Inhibitors in Bone Sarcomas. Eur. J. Cancer 2022, 173, 71–90. [CrossRef]
- Teo, M.Y.M.; Fong, J.Y.; Lim, W.M.; In, L.L.A. Current Advances and Trends in KRAS Targeted Therapies for Colorectal Cancer. Mol. Cancer Res. 2022, 20, 30–44. [CrossRef]
- Al Shihabi, A.; Davarifar, A.; Nguyen, H.T.L.; Tavanaie, N.; Nelson, S.D.; Yanagawa, J.; Federman, N.; Bernthal, N.; Hornicek, F.; Soragni, A. Personalized Chordoma Organoids for Drug Discovery Studies. Sci. Adv. 2022, 8. [CrossRef]
- Cash, T.; Jonus, H.C.; Tsvetkova, M.; Beumer, J.H.; Lee, J.Y.; Henry, C.; Aguilera, D.; Harvey, R.D.; Goldsmith, K.C. A Phase I Study of Simvastatin in Combination with Topotecan and Cyclophosphamide in Pediatric Patients with Relapsed and/or Refractory Solid and CNS Tumors. J. Clin. Oncol. 2020, 38, 10541–10541. [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The Potential Use of Simvastatin for Cancer Treatment: A Review. Biomed. Pharmacother. 2021, 141, 111858. [CrossRef]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Prim. 2022, 8, 77. [CrossRef]
- Pascual-Pasto, G.; Castillo-Ecija, H.; Unceta, N.; Aschero, R.; Resa-Pares, C.; Gómez-Caballero, A.; Vila-Ubach, M.; Muñoz-Aznar, O.; Suñol, M.; Burgueño, V.; et al. SPARC-Mediated Long-Term Retention of Nab-Paclitaxel in Pediatric Sarcomas. J. Control. Release 2022, 342, 81–92. [CrossRef]
- Digklia, A.; Kollar, A.; Kronig, M.-N.; Britschgi, C.; Rordorf, T.; Joerger, M.; Krasniqi, F.; Metaxas, Y.; Colombo, I.; Dietrich, D.; et al. 1495P SAKK 57/16 Nab-Paclitaxel and Gemcitabine in Soft Tissue Sarcoma (NAPAGE): Final Results from the Phase Ib/II Trial with >2y Median Follow Up. Ann. Oncol. 2022, 33, S1230. [CrossRef]
- Omar, S.; Albritton, K.; Heym, K.; Wang, J.; Ray, A. Multimodal Treatment of Sarcomas Linked to BCOR-CCNB3 Fusion in Pediatrics: A 3-Patient Case Series. Clin. Pediatr. Hematol. 2022, 29, 60–64. [CrossRef]
- Smida, M.; Ammar, A.; Fedhila, F.; Douira, W.; Sassi, S. Periosteal Preservation: A New Technique in Resection of Bone High-Grade Malignant Tumors in Children—about Eleven Cases. World J. Surg. Oncol. 2022, 20, 312. [CrossRef]
- Puppo, M.; Jaafar, M.; Diaz, J.-J.; Marcel, V.; Clézardin, P. MiRNAs and SnoRNAs in Bone Metastasis: Functional Roles and Clinical Potential. Cancers (Basel). 2022, 15, 242. [CrossRef]
- Sadeh, M.; Toledano, H.; Cohen, I.J. A Comprehensive Review of Neuropsychologic Studies Supports the Concept That Adequate Folinic Acid Rescue Prevents Post Methotrexate Neurotoxicity. J. Pediatr. Hematol. Oncol. 2023, 45, 1–11. [CrossRef]
- Li, S.; Wang, X. The Potential Roles of Exosomal Noncoding RNAs in Osteosarcoma. J. Cell. Physiol. 2021, 236, 3354–3365. [CrossRef]
- Abhange, K.; Makler, A.; Wen, Y.; Ramnauth, N.; Mao, W.; Asghar, W.; Wan, Y. Small Extracellular Vesicles in Cancer. Bioact. Mater. 2021, 6, 3705–3743. [CrossRef]
- Kluszczynska, K.; Czyz, M. Extracellular Vesicles-Based Cell-Cell Communication in Melanoma: New Perspectives in Diagnostics and Therapy. Int. J. Mol. Sci. 2023, 24, 965. [CrossRef]
- Song, H.; Zhao, J.; Cheng, J.; Feng, Z.; Wang, J.; Momtazi-Borojeni, A.A.; Liang, Y. Extracellular Vesicles in Chondrogenesis and Cartilage Regeneration. J. Cell. Mol. Med. 2021, 25, 4883–4892. [CrossRef]
- Jothimani, G.; Pathak, S.; Dutta, S.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Cancer-Associated MicroRNA Expression Profiling and Proteomic Analysis of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes. Tissue Eng. Regen. Med. 2022, 19, 1013–1031. [CrossRef]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [CrossRef]
- Ross, J.; McIver, Z.; Lambert, T.; Piergentili, C.; Bird, J.E.; Gallagher, K.J.; Cruickshank, F.L.; James, P.; Zarazúa-Arvizu, E.; Horsfall, L.E.; et al. Pore Dynamics and Asymmetric Cargo Loading in an Encapsulin Nanocompartment. Sci. Adv. 2022, 8. [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [CrossRef]
- Xiang, Y.; Hu, C.; Wu, G.; Xu, S.; Li, Y. Nanomaterial-Based Microfluidic Systems for Cancer Biomarker Detection: Recent Applications and Future Perspectives. TrAC Trends Anal. Chem. 2023, 158, 116835. [CrossRef]
- Liang, Y.; Fang, D.; Gao, X.; Deng, X.; Chen, N.; Wu, J.; Zeng, M.; Luo, M. Circulating MicroRNAs as Emerging Regulators of COVID-19. Theranostics 2023, 13, 125–147. [CrossRef]
- Laviron, M.; Boissonnas, A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019, 10. [CrossRef]
- Wei, Z.; Zheng, D.; Pi, W.; Qiu, Y.; Xia, K.; Guo, W. Isoquercitrin Restrains the Proliferation and Promotes Apoptosis of Human Osteosarcoma Cells by Inhibiting the Wnt/β-Catenin Pathway. J. Bone Oncol. 2023, 100468. [CrossRef]
- Lu, D.; Liu, R.; Zhou, Y.; Zhang, Z.; Jiang, X.; Xu, J.; Su, A.; Hu, Z. FOXO3a-Dependent up-Regulation of HSP90 Alleviates Cisplatin-Induced Apoptosis by Activating FUNDC1-Mediated Mitophagy in Hypoxic Osteosarcoma Cells. Cell. Signal. 2023, 101, 110500. [CrossRef]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma Cell-Derived Exosomes Affect Tumor Microenvironment by Specific Packaging of MicroRNAs. Carcinogenesis 2020, 41, 666–677. [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [CrossRef]
- Wolf-Dennen, K.; Gordon, N.; Kleinerman, E.S. Exosomal Communication by Metastatic Osteosarcoma Cells Modulates Alveolar Macrophages to an M2 Tumor-Promoting Phenotype and Inhibits Tumoricidal Functions. Oncoimmunology 2020, 9. [CrossRef]
- Lopes, N.; Vivier, E.; Narni-Mancinelli, E. Natural Killer Cells and Type 1 Innate Lymphoid Cells in Cancer. Semin. Immunol. 2023, 66, 101709. [CrossRef]
- Wu, C.-C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.-L.; Ju, Z.; et al. Immuno-Genomic Landscape of Osteosarcoma. Nat. Commun. 2020, 11, 1008. [CrossRef]
- Verma, A.; Mathur, R.; Farooque, A.; Kaul, V.; Gupta, S.; Dwarakanath, B.S. T-Regulatory Cells In Tumor Progression And Therapy. Cancer Manag. Res. 2019, Volume 11, 10731–10747. [CrossRef]
- Ray, S.K.; Mukherjee, S. Altering Landscape of Cancer Vaccines: Unique Platforms, Research on Therapeutic Applications and Recent Patents. Recent Pat. Anticancer. Drug Discov. 2023, 18, 133–146. [CrossRef]
- Bayatipoor, H.; Mehdizadeh, S.; Jafarpour, R.; Shojaei, Z.; Pashangzadeh, S.; Motallebnezhad, M. Role of NKT Cells in Cancer Immunotherapy—from Bench to Bed. Med. Oncol. 2022, 40, 29. [CrossRef]
- Metelli, A.; Wu, B.X.; Riesenberg, B.; Guglietta, S.; Huck, J.D.; Mills, C.; Li, A.; Rachidi, S.; Krieg, C.; Rubinstein, M.P.; et al. Thrombin Contributes to Cancer Immune Evasion via Proteolysis of Platelet-Bound GARP to Activate LTGF-β. Sci. Transl. Med. 2020, 12. [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature 2018, 554, 538–543. [CrossRef]
- Shima, T.; Shimoda, M.; Shigenobu, T.; Ohtsuka, T.; Nishimura, T.; Emoto, K.; Hayashi, Y.; Iwasaki, T.; Abe, T.; Asamura, H.; et al. Infiltration of Tumor-associated Macrophages Is Involved in Tumor Programmed Death-ligand 1 Expression in Early Lung Adenocarcinoma. Cancer Sci. 2020, 111, 727–738. [CrossRef]
- Wang, X.; Chen, Z.; Li, B.; Fan, J.; Xu, W.; Xiao, J. Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review. Cancers (Basel). 2022, 15, 264. [CrossRef]
- Huang, T.; Tan, X.; Huang, H.; et, al Targeting Cancer-Associated Fibroblast-Secreted WNT2 Restores Dendritic Cell-Mediated Antitumour Immunity. Gut 2022, 71, 333–344.
- Yue, P.; Han, B.; Zhao, Y. Focus on the Molecular Mechanisms of Cisplatin Resistance Based on Multi-Omics Approaches. Mol. Omi. 2023. [CrossRef]
- Fan, L.; Qu, Y.; Tong, W.; Lin, H.; Xiao, B. Application Value of a Selenium-Hydroxyapatite Nanodelivery System as Osteosarcoma Treatment. Mater. Express 2022, 12, 1033–1041. [CrossRef]
- Garcia-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sánchez, H.S.; Cruz-Ramos, M. An Overview of Resistance to Chemotherapy in Osteosarcoma and Future Perspectives. Cancer Drug Resist. 2022, 5, 762–793. [CrossRef]
- Inui, T.; Nomoto, R.; Yokota, J.; Yamashita, T.; Okada, K.; Kishimoto, W.; Nakase, H.; Mizuguchi, H. Establishment of MDR1-Knockout Human Enteroids for Pharmaceutical Application. Drug Metab. Pharmacokinet. 2023, 48, 100476. [CrossRef]
- Cai, J.-X.; Liu, J.-H.; Wu, J.-Y.; Li, Y.-J.; Qiu, X.-H.; Xu, W.-J.; Xu, P.; Xiang, D.-X. Hybrid Cell Membrane-Functionalized Biomimetic Nanoparticles for Targeted Therapy of Osteosarcoma. Int. J. Nanomedicine 2022, Volume 17, 837–854. [CrossRef]
- Zhang, X.-B.; Zhang, R.-H.; Su, X.; Qi, J.; Hu, Y.-C.; Shi, J.-T.; Zhang, K.; Wang, K.-P.; Zhou, H.-Y. Exosomes in Osteosarcoma Research and Preclinical Practice. Am. J. Transl. Res. 2021, 13, 882–897.
- Weinman, M.A.; Ramsey, S.A.; Leeper, H.J.; Brady, J. V.; Schlueter, A.; Stanisheuski, S.; Maier, C.S.; Miller, T.; Ruby, C.E.; Bracha, S. Exosomal Proteomic Signatures Correlate with Drug Resistance and Carboplatin Treatment Outcome in a Spontaneous Model of Canine Osteosarcoma. Cancer Cell Int. 2021, 21, 245. [CrossRef]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential Anticancer Properties and Mechanisms of Thymoquinone in Osteosarcoma and Bone Metastasis. Cell. Mol. Biol. Lett. 2022, 27, 21. [CrossRef]
- Cheng, B.; Yu, Q.; Wang, W. Intimate Communications within the Tumor Microenvironment: Stromal Factors Function as an Orchestra. J. Biomed. Sci. 2023, 30, 1. [CrossRef]
- Chaudhary, A.; Raza, S.S.; Haque, R. Transcriptional Factors Targeting in Cancer Stem Cells for Tumor Modulation. Semin. Cancer Biol. 2023, 88, 123–137. [CrossRef]
- Wu, T.; Yang, W.; Sun, A.; Wei, Z.; Lin, Q. The Role of CXC Chemokines in Cancer Progression. Cancers (Basel). 2022, 15, 167. [CrossRef]
- Wang, Y.; Chu, Y.; Li, K.; Zhang, G.; Guo, Z.; Wu, X.; Qiu, C.; Li, Y.; Wan, X.; Sui, J.; et al. Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front. Cell Dev. Biol. 2020, 8. [CrossRef]
- Kehayova, Y.S.; Wilkinson, J.M.; Rice, S.J.; Loughlin, J. Independent Osteoarthritis Risk-conferring Alleles Mediate the Same Epigenetic and Transcriptional Effect on a Shared Target Gene, COLGALT2. Arthritis Rheumatol. 2022. [CrossRef]
- Zhang, H.; Wang, J.; Ren, T.; Huang, Y.; Liang, X.; Yu, Y.; Wang, W.; Niu, J.; Guo, W. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MiR-206 Inhibits Osteosarcoma Progression by Targeting TRA2B. Cancer Lett. 2020, 490, 54–65. [CrossRef]
- Tian, B.; Du, X.; Zheng, S.; Zhang, Y. The Role of Tumor Microenvironment in Regulating the Plasticity of Osteosarcoma Cells. Int. J. Mol. Sci. 2022, 23, 16155. [CrossRef]
- Han, F.; Pu, P.; Wang, C.; Ding, X.; Zhu, Z.; Xiang, W.; Wang, W. Osteosarcoma Cell-Derived Exosomal MiR-1307 Promotes Tumorgenesis via Targeting AGAP1. Biomed Res. Int. 2021, 2021, 1–17. [CrossRef]
- Chen, P.-W.; Gasilina, A.; Yadav, M.P.; Randazzo, P.A. Control of Cell Signaling by Arf GTPases and Their Regulators: Focus on Links to Cancer and Other GTPase Families. Biochim. Biophys. Acta - Mol. Cell Res. 2022, 1869, 119171. [CrossRef]
- Vail, D.J.; Somoza, R.A.; Caplan, A.I. MicroRNA Regulation of Bone Marrow Mesenchymal Stem Cell Chondrogenesis: Toward Articular Cartilage. Tissue Eng. Part A 2022, 28, 254–269. [CrossRef]
- Zhang, H.; Yu, Y.; Wang, J.; Han, Y.; Ren, T.; Huang, Y.; Chen, C.; Huang, Q.; Wang, W.; Niu, J.; et al. Macrophages-Derived Exosomal LncRNA LIFR-AS1 Promotes Osteosarcoma Cell Progression via MiR-29a/NFIA Axis. Cancer Cell Int. 2021, 21, 192. [CrossRef]
- Bai, Z.; Wang, X.; Zhang, Z. Long Noncoding RNA LIFR-AS1: A New Player in Human Cancers. Biomed Res. Int. 2022, 2022, 1–11. [CrossRef]
- Alfieri, M.; Meo, L.; Ragno, P. Posttranscriptional Regulation of the Plasminogen Activation System by Non-Coding RNA in Cancer. Int. J. Mol. Sci. 2023, 24, 962. [CrossRef]
- Luo, X.; Li, Y.; Hua, Z.; Xue, X.; Wang, X.; Pang, M.; Xiao, C.; Zhao, H.; Lyu, A.; Liu, Y. Exosomes-Mediated Tumor Metastasis through Reshaping Tumor Microenvironment and Distant Niche. J. Control. Release 2023, 353, 327–336. [CrossRef]
- Zhang, W.; Yan, Y.; Peng, J.; Thakur, A.; Bai, N.; Yang, K.; Xu, Z. Decoding Roles of Exosomal LncRNAs in Tumor-Immune Regulation and Therapeutic Potential. Cancers (Basel). 2022, 15, 286. [CrossRef]
- Li, Q.; Wang, X.; Jiang, N.; Xie, X.; Liu, N.; Liu, J.; Shen, J.; Peng, T. Exosome-transmitted Linc00852 Associated with Receptor Tyrosine Kinase AXL Dysregulates the Proliferation and Invasion of Osteosarcoma. Cancer Med. 2020, 9, 6354–6366. [CrossRef]
- Zhang, H.; Du, Y.; Xin, P.; Man, X. The LINC00852/MiR-29a-3p/JARID2 Axis Regulates the Proliferation and Invasion of Prostate Cancer Cell. BMC Cancer 2022, 22, 1269. [CrossRef]
- Ge, X.; Liu, W.; Zhao, W.; Feng, S.; Duan, A.; Ji, C.; Shen, K.; Liu, W.; Zhou, J.; Jiang, D.; et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol. Ther. - Nucleic Acids 2020, 21, 900–915. [CrossRef]
- Zhang, H.; Song, J.; Zhou, X. Long Noncoding RNA P53 Upregulated Regulator of P53 Levels Promotes Osteogenic Differentiation in Osteoporosis Progression Through Sponging MiR-135a-5p. J. Biomater. Tissue Eng. 2022, 12, 2085–2091. [CrossRef]
- Zhang, K.; Dong, C.; Chen, M.; Yang, T.; Wang, X.; Gao, Y.; Wang, L.; Wen, Y.; Chen, G.; Wang, X.; et al. Extracellular Vesicle-Mediated Delivery of MiR-101 Inhibits Lung Metastasis in Osteosarcoma. Theranostics 2020, 10, 411–425. [CrossRef]
- Mei, X.; Zhang, B.; Zhao, M.; Lu, Q. An Update on Epigenetic Regulation in Autoimmune Diseases. J. Transl. Autoimmun. 2022, 5, 100176. [CrossRef]
- Mazumdar, A.; Urdinez, J.; Boro, A.; Migliavacca, J.; Arlt, M.J.E.; Muff, R.; Fuchs, B.; Snedeker, J.G.; Gvozdenovic, A. Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int. J. Mol. Sci. 2020, 21, 5451. [CrossRef]
- Tripathi, R.; Sinha, N.R.; Kempuraj, D.; Balne, P.K.; Landreneau, J.R.; Juneja, A.; Webel, A.D.; Mohan, R.R. Evaluation of CRISPR/Cas9 Mediated TGIF Gene Editing to Inhibit Corneal Fibrosis in Vitro. Exp. Eye Res. 2022, 220, 109113. [CrossRef]
- Cheng, Z.; Wang, L.; Wu, C.; Huang, L.; Ruan, Y.; Xue, W. Tumor-Derived Exosomes Induced M2 Macrophage Polarization and Promoted the Metastasis of Osteosarcoma Cells Through Tim-3. Arch. Med. Res. 2021, 52, 200–210. [CrossRef]
- Gomes de Morais, A.L.; Cerdá, S.; de Miguel, M. New Checkpoint Inhibitors on the Road: Targeting TIM-3 in Solid Tumors. Curr. Oncol. Rep. 2022, 24, 651–658. [CrossRef]
- Zhang, Y.; Liu, Z.; Yang, X.; Lu, W.; Chen, Y.; Lin, Y.; Wang, J.; Lin, S.; Yun, J.-P. H3K27 Acetylation Activated-COL6A1 Promotes Osteosarcoma Lung Metastasis by Repressing STAT1 and Activating Pulmonary Cancer-Associated Fibroblasts. Theranostics 2021, 11, 1473–1492. [CrossRef]
- Wang, Y.; Han, Y.; Wang, L.; Zou, M.; Sun, Y.; Sun, H.; Guo, Q.; Peng, X. Mycoplasma Gallisepticum Escapes the Host Immune Response via Gga-MiR-365-3p/SOCS5/STATs Axis. Vet. Res. 2022, 53, 103. [CrossRef]
- Zhang, H.; Wang, J.; Ren, T.; Huang, Y.; Yu, Y.; Chen, C.; Huang, Q.; Guo, W. LncRNA CASC15 Is Upregulated in Osteosarcoma Plasma Exosomes and CASC15 Knockdown Inhibits Osteosarcoma Progression by Regulating MiR-338-3p/RAB14 Axis. Onco. Targets. Ther. 2020, Volume 13, 12055–12066. [CrossRef]
- Chen, C.; Liu, L. Silencing of LncRNA KLF3-AS1 Represses Cell Growth in Osteosarcoma via MiR-338-3p/MEF2C Axis. J. Clin. Lab. Anal. 2022, 36. [CrossRef]
- Qin, F.; Tang, H.; Zhang, Y.; Zhang, Z.; Huang, P.; Zhu, J. Bone Marrow-derived Mesenchymal Stem Cell-derived Exosomal MicroRNA-208a Promotes Osteosarcoma Cell Proliferation, Migration, and Invasion. J. Cell. Physiol. 2020, 235, 4734–4745. [CrossRef]
- Wang, Q.; Zhou, H.; Zhu, X.; Jiang, F.; Yu, Q.; Zhang, J.; Ji, Y. MiR-208 Inhibits Myocardial Tissues Apoptosis in Mice with Acute Myocardial Infarction by Targeting Inhibition of PDCD4. J. Biochem. Mol. Toxicol. 2022, 36. [CrossRef]
- Huang, Y.; Liu, W.; He, B.; Wang, L.; Zhang, F.; Shu, H.; Sun, L. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Osteosarcoma Development by Activating Oncogenic Autophagy. J. Bone Oncol. 2020, 21, 100280. [CrossRef]
- Yu, Z.; Tang, H.; Chen, S.; Xie, Y.; Shi, L.; Xia, S.; Jiang, M.; Li, J.; Chen, D. Exosomal LOC85009 Inhibits Docetaxel Resistance in Lung Adenocarcinoma through Regulating ATG5-Induced Autophagy. Drug Resist. Updat. 2022, 100915. [CrossRef]
- Zhong, L.; Liao, D.; Li, J.; Liu, W.; Wang, J.; Zeng, C.; Wang, X.; Cao, Z.; Zhang, R.; Li, M.; et al. Rab22a-NeoF1 Fusion Protein Promotes Osteosarcoma Lung Metastasis through Its Secretion into Exosomes. Signal Transduct. Target. Ther. 2021, 6, 59. [CrossRef]
- Zeng, C.; Zhong, L.; Liu, W.; Zhang, Y.; Yu, X.; Wang, X.; Zhang, R.; Kang, T.; Liao, D. Targeting the Lysosomal Degradation of Rab22a-NeoF1 Fusion Protein for Osteosarcoma Lung Metastasis. Adv. Sci. 2022, 2205483. [CrossRef]
- Ye, H.; Hu, X.; Wen, Y.; Tu, C.; Hornicek, F.; Duan, Z.; Min, L. Exosomes in the Tumor Microenvironment of Sarcoma: From Biological Functions to Clinical Applications. J. Nanobiotechnology 2022, 20, 403. [CrossRef]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13. [CrossRef]
- Liu, Z.; Zhou, Z.; Dang, Q.; Xu, H.; Lv, J.; Li, H.; Han, X. Immunosuppression in Tumor Immune Microenvironment and Its Optimization from CAR-T Cell Therapy. Theranostics 2022, 12, 6273–6290. [CrossRef]
- Wang, J.; Zhang, H.; Sun, X.; Wang, X.; Ren, T.; Huang, Y.; Zhang, R.; Zheng, B.; Guo, W. Exosomal PD-L1 and N-Cadherin Predict Pulmonary Metastasis Progression for Osteosarcoma Patients. J. Nanobiotechnology 2020, 18, 151. [CrossRef]
- Tomassetti, A. Editorial to the Special Issue “Activations of Cadherin Signaling in Cancer.” Int. J. Mol. Sci. 2022, 23, 7022. [CrossRef]
- Heim, L.; Yang, Z.; Tausche, P.; Hohenberger, K.; Chiriac, M.T.; Koelle, J.; Geppert, C.-I.; Kachler, K.; Miksch, S.; Graser, A.; et al. IL-9 Producing Tumor-Infiltrating Lymphocytes and Treg Subsets Drive Immune Escape of Tumor Cells in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13. [CrossRef]
- Feng, Y.; Yan, S.; Lam, S.K.; Ko, F.C.F.; Chen, C.; Khan, M.; Ho, J.C.-M. IL-9 Stimulates an Anti-Tumor Immune Response and Facilitates Immune Checkpoint Blockade in the CMT167 Mouse Model. Lung Cancer 2022, 174, 14–26. [CrossRef]
- Zhan, X.; He, Q.; Sheng, J.; Jiang, X.; Lin, L.; Huang, Y.; He, S.; Chen, Y.; Li, L.; Zeng, Z.; et al. USP12 Positively Regulates M-MDSC Function to Inhibit Antitumour Immunity through Deubiquitinating and Stabilizing P65. Immunology 2022, 167, 544–557. [CrossRef]
- Guo, J.; Zhao, J.; Sun, L.; Yang, C. Role of Ubiquitin Specific Proteases in the Immune Microenvironment of Prostate Cancer: A New Direction. Front. Oncol. 2022, 12. [CrossRef]
- Toney, N.J.; Opdenaker, L.M.; Cicek, K.; Frerichs, L.; Kennington, C.R.; Oberly, S.; Archinal, H.; Somasundaram, R.; Sims-Mourtada, J. Tumor-B-Cell Interactions Promote Isotype Switching to an Immunosuppressive IgG4 Antibody Response through Upregulation of IL-10 in Triple Negative Breast Cancers. J. Transl. Med. 2022, 20, 112. [CrossRef]
- Zhang, X.; Jin, X.; Guan, L.; Lin, X.; Li, X.; Li, Y. IgG4-Related Disease With Gastrointestinal Involvement: Case Reports and Literature Review. Front. Immunol. 2022, 13. [CrossRef]
- Cao, Y.; He, H.; Li, R.; Liu, X.; Chen, Y.; Qi, Y.; Yu, K.; Wang, J.; Lin, C.; Liu, H.; et al. Latency-Associated Peptide Identifies Immunoevasive Subtype Gastric Cancer With Poor Prognosis and Inferior Chemotherapeutic Responsiveness. Ann. Surg. 2022, 275, e163–e173. [CrossRef]
- Chen, S.-Y.; Mamai, O.; Akhurst, R.J. TGFβ: Signaling Blockade for Cancer Immunotherapy. Annu. Rev. Cancer Biol. 2022, 6, 123–146. [CrossRef]
- Mohapatra, P.; Chandrasekaran, N. Wnt/β-Catenin Targeting in Liver Carcinoma through Nanotechnology-Based Drug Repurposing: A Review. Biomed. Pharmacother. 2022, 155, 113713. [CrossRef]
- Duan, Z.; Lin, X.; Wang, L.; Zhen, Q.; Jiang, Y.; Chen, C.; Yang, J.; Lee, C.-H.; Qin, Y.; Li, Y.; et al. Specificity of TGF-Β1 Signal Designated by LRRC33 and Integrin AVβ8. Nat. Commun. 2022, 13, 4988. [CrossRef]
- Dodagatta-Marri, E.; Ma, H.-Y.; Liang, B.; Li, J.; Meyer, D.S.; Chen, S.-Y.; Sun, K.-H.; Ren, X.; Zivak, B.; Rosenblum, M.D.; et al. Integrin Avβ8 on T Cells Suppresses Anti-Tumor Immunity in Multiple Models and Is a Promising Target for Tumor Immunotherapy. Cell Rep. 2021, 36, 109309. [CrossRef]
- Guo, D.; Tong, Y.; Jiang, X.; Meng, Y.; Jiang, H.; Du, L.; Wu, Q.; Li, S.; Luo, S.; Li, M.; et al. Aerobic Glycolysis Promotes Tumor Immune Evasion by Hexokinase2-Mediated Phosphorylation of IκBα. Cell Metab. 2022, 34, 1312-1324.e6. [CrossRef]
- Zhang, Y.; Fu, J.; Liu, S.; Wang, L.; Qiu, J.; van Schaik, E.J.; Samuel, J.E.; Song, L.; Luo, Z.-Q. Coxiella Burnetii Inhibits Host Immunity by a Protein Phosphatase Adapted from Glycolysis. Proc. Natl. Acad. Sci. 2022, 119. [CrossRef]
- Du, L.; He, H.; Xiao, Z.; Xiao, H.; An, Y.; Zhong, H.; Lin, M.; Meng, X.; Han, S.; Shuai, X. GSH-Responsive Metal–Organic Framework for Intratumoral Release of NO and IDO Inhibitor to Enhance Antitumor Immunotherapy. Small 2022, 18, 2107732. [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-Dioxygenase (IDO) Inhibitors and Cancer Immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [CrossRef]
- Gulley, J.L.; Schlom, J.; Barcellos-Hoff, M.H.; Wang, X.; Seoane, J.; Audhuy, F.; Lan, Y.; Dussault, I.; Moustakas, A. Dual Inhibition of TGF-β and PD-L1: A Novel Approach to Cancer Treatment. Mol. Oncol. 2022, 16, 2117–2134. [CrossRef]
- Metropulos, A.E.; Munshi, H.G.; Principe, D.R. The Difficulty in Translating the Preclinical Success of Combined TGFβ and Immune Checkpoint Inhibition to Clinical Trial. eBioMedicine 2022, 86, 104380. [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [CrossRef]
- Martin, C.J.; Datta, A.; Littlefield, C.; Kalra, A.; Chapron, C.; Wawersik, S.; Dagbay, K.B.; Brueckner, C.T.; Nikiforov, A.; Danehy, F.T.; et al. Selective Inhibition of TGFβ1 Activation Overcomes Primary Resistance to Checkpoint Blockade Therapy by Altering Tumor Immune Landscape. Sci. Transl. Med. 2020, 12. [CrossRef]
- Chen, C.; Shi, Q.; Xu, J.; Ren, T.; Huang, Y.; Guo, W. Current Progress and Open Challenges for Applying Tyrosine Kinase Inhibitors in Osteosarcoma. Cell Death Discov. 2022, 8, 488. [CrossRef]
- Northcote-Smith, J.; Suntharalingam, K. Targeting Chemotherapy-Resistant Tumour Sub-Populations Using Inorganic Chemistry: Anti-Cancer Stem Cell Metal Complexes. Curr. Opin. Chem. Biol. 2023, 72, 102237. [CrossRef]
- Costa, A.R.; Duarte, A.C.; Costa-Brito, A.R.; Gonçalves, I.; Santos, C.R.A. Bitter Taste Signaling in Cancer. Life Sci. 2023, 121363. [CrossRef]
- Aprile, M.; Costa, V.; Cimmino, A.; Calin, G.A. Emerging Role of Oncogenic Long Noncoding RNA as Cancer Biomarkers. Int. J. Cancer 2023, 152, 822–834. [CrossRef]
- Zhang, Q.; Liu, N.; Wang, J.; Liu, Y.; Wang, K.; Zhang, J.; Pan, X. The Recent Advance of Cell-Penetrating and Tumor-Targeting Peptides as Drug Delivery Systems Based on Tumor Microenvironment. Mol. Pharm. 2023. [CrossRef]
- de Castro, K.C.; Coco, J.C.; dos Santos, É.M.; Ataide, J.A.; Martinez, R.M.; do Nascimento, M.H.M.; Prata, J.; da Fonte, P.R.M.L.; Severino, P.; Mazzola, P.G.; et al. Pluronic® Triblock Copolymer-Based Nanoformulations for Cancer Therapy: A 10-Year Overview. J. Control. Release 2023, 353, 802–822. [CrossRef]
- Wang, H.; Zhou, X.; Li, C.; Yan, S.; Feng, C.; He, J.; Li, Z.; Tu, C. The Emerging Role of Pyroptosis in Pediatric Cancers: From Mechanism to Therapy. J. Hematol. Oncol. 2022, 15, 140. [CrossRef]
- Marques da Costa, M.E.; Marchais, A.; Gomez-Brouchet, A.; Job, B.; Assoun, N.; Daudigeos-Dubus, E.; Fromigué, O.; Santos, C.; Geoerger, B.; Gaspar, N. In-Vitro and In-Vivo Establishment and Characterization of Bioluminescent Orthotopic Chemotherapy-Resistant Human Osteosarcoma Models in NSG Mice. Cancers (Basel). 2019, 11, 997. [CrossRef]
- Salem, I.M.; Mostafa, S.M.; Salama, I.; El-Sabbagh, O.I.; Hegazy, W.A.H.; Ibrahim, T.S. Design, Synthesis and Antitumor Evaluation of Novel Pyrazolo[3,4- d ]Pyrimidines Incorporating Different Amino Acid Conjugates as Potential DHFR Inhibitors. J. Enzyme Inhib. Med. Chem. 2023, 38, 203–215. [CrossRef]
- Yu, W.; Min, D.; Lin, F.; Zheng, S.; Tang, L.; He, A.; Hu, H.; Shen, Z. SKA1 Induces de Novo MTX-resistance in Osteosarcoma through Inhibiting FPGS Transcription. FEBS J. 2019, 286, 2399–2414. [CrossRef]
- Zhang, Y.; Zhang, C.; Man, X.; Men, Y.; Ren, X.; Li, X.; Han, L.; Sun, Z.; Yang, Y.; Hou, S.; et al. Functional Characterization of the SiFPGS2 Gene of Foxtail Millet in Folate Accumulation and Root Development. Plant Growth Regul. 2022. [CrossRef]
- Xiao, X.; Wang, W.; Li, Y.; Yang, D.; Li, X.; Shen, C.; Liu, Y.; Ke, X.; Guo, S.; Guo, Z. HSP90AA1-Mediated Autophagy Promotes Drug Resistance in Osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 201. [CrossRef]
- Chen, Y.; Zhang, K.; Li, Y.; Guo, R.; Zhang, K.; Zhong, G.; He, Q. Oestrogen-Related Receptor Alpha Mediates Chemotherapy Resistance of Osteosarcoma Cells via Regulation of ABCB1. J. Cell. Mol. Med. 2019, 23, 2115–2124. [CrossRef]
- Wang, G.; Cao, L.; Jiang, Y.; Zhang, T.; Wang, H.; Wang, Z.; Xu, J.; Mao, M.; Hua, Y.; Cai, Z.; et al. Anlotinib Reverses Multidrug Resistance (MDR) in Osteosarcoma by Inhibiting P-Glycoprotein (PGP1) Function In Vitro and In Vivo. Front. Pharmacol. 2022, 12. [CrossRef]
- He, Q.; Hao, P.; He, G.; Mai, H.; Liu, W.; Zhang, W.; Zhang, K.; Zhong, G.; Guo, R.; Yu, C.; et al. IGF2BP1-Regulated Expression of ERRα Is Involved in Metabolic Reprogramming of Chemotherapy Resistant Osteosarcoma Cells. J. Transl. Med. 2022, 20, 348. [CrossRef]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Kipanyula, M.J.; Vecchio, L.; Tagne Simo, R.; Njamnshi, A.K.; Lukong, K.E.; Mimche, P.N. Insulin-like Growth Factor-1 Signaling in the Tumor Microenvironment: Carcinogenesis, Cancer Drug Resistance, and Therapeutic Potential. Front. Endocrinol. (Lausanne). 2022, 13. [CrossRef]
- Celik, B.; Cicek, K.; Leal, A.F.; Tomatsu, S. Regulation of Molecular Targets in Osteosarcoma Treatment. Int. J. Mol. Sci. 2022, 23, 12583. [CrossRef]
- Li, X.; Liu, Y.; Zhang, X.; Shen, J.; Xu, R.; Liu, Y.; Yu, X. Circular RNA Hsa_circ_0000073 Contributes to Osteosarcoma Cell Proliferation, Migration, Invasion and Methotrexate Resistance by Sponging MiR-145-5p and MiR-151-3p and Upregulating NRAS. Aging (Albany. NY). 2020, 12, 14157–14173. [CrossRef]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [CrossRef]
- Wei, W.; Ji, L.; Duan, W.; Zhu, J. Circular RNA Circ_0081001 Knockdown Enhances Methotrexate Sensitivity in Osteosarcoma Cells by Regulating MiR-494-3p/TGM2 Axis. J. Orthop. Surg. Res. 2021, 16, 50. [CrossRef]
- Liu, S.; Duan, K.; Zhang, X.; Cao, X.; Wang, X.; Meng, F.; Liu, H.; Xu, B.; Wang, X. Circ_0081001 Down-Regulates MiR-494-3p to Enhance BACH1 Expression and Promotes Osteosarcoma Progression. Aging (Albany. NY). 2021, 13, 17274–17284. [CrossRef]
- Bai, Y.; Li, Y.; Bai, J.; Zhang, Y. Hsa_circ_0004674 Promotes Osteosarcoma Doxorubicin Resistance by Regulating the MiR-342-3p/FBN1 Axis. J. Orthop. Surg. Res. 2021, 16, 510. [CrossRef]
- Ma, X.-L.; Zhan, T.-C.; Hu, J.-P.; Zhang, C.-L.; Zhu, K.-P. Doxorubicin-Induced Novel CircRNA_0004674 Facilitates Osteosarcoma Progression and Chemoresistance by Upregulating MCL1 through MiR-142-5p. Cell Death Discov. 2021, 7, 309. [CrossRef]
- Guan, H.; Xu, H.; Chen, J.; Wu, W.; Chen, D.; Chen, Y.; Sun, J. Circ_0001721 Enhances Doxorubicin Resistance and Promotes Tumorigenesis in Osteosarcoma through MiR-758/TCF4 Axis. Cancer Cell Int. 2021, 21, 336. [CrossRef]
- Gao, Y.; Ma, H.; Gao, Y.; Tao, K.; Fu, L.; Ren, R.; Hu, X.; Kou, M.; Chen, B.; Shi, J.; et al. CircRNA Circ_0001721 Promotes the Progression of Osteosarcoma Through MiR-372-3p/MAPK7 Axis. Cancer Manag. Res. 2020, Volume 12, 8287–8302. [CrossRef]
- Wei, W.; Ji, L.; Duan, W.; Zhu, J. CircSAMD4A Contributes to Cell Doxorubicin Resistance in Osteosarcoma by Regulating the MiR-218-5p/KLF8 Axis. Open Life Sci. 2020, 15, 848–859. [CrossRef]
- Yanbin, Z.; Jing, Z. CircSAMD4A Accelerates Cell Proliferation of Osteosarcoma by Sponging MiR-1244 and Regulating MDM2 MRNA Expression. Biochem. Biophys. Res. Commun. 2019, 516, 102–111. [CrossRef]
- Ji, Y.; Liu, J.; Zhu, W.; Ji, J. Circ_0002060 Enhances Doxorubicin Resistance in Osteosarcoma by Regulating the MiR-198/ABCB1 Axis. Cancer Biother. Radiopharm. 2020, cbr.2020.4240. [CrossRef]
- Huang, Y.; Xie, J.; Li, E. Comprehensive Circular RNA Profiling Reveals Circ_0002060 as a Potential Diagnostic Biomarkers for Osteoporosis. J. Cell. Biochem. 2019, 120, 15688–15694. [CrossRef]
- Xie, C.; Liang, G.; Xu, Y.; Lin, E. Circular RNA Hsa_circ_0003496 Contributes to Tumorigenesis and Chemoresistance in Osteosarcoma Through Targeting (MicroRNA) MiR-370/Krüppel-Like Factor 12 Axis. Cancer Manag. Res. 2020, Volume 12, 8229–8240. [CrossRef]
- Lin, Z.; Xie, X.; Lu, S.; Liu, T. Noncoding RNAs in Osteosarcoma: Implications for Drug Resistance. Cancer Lett. 2021, 504, 91–103. [CrossRef]
- Zhang, Z.; Zhou, Q.; Luo, F.; Zhou, R.; Xu, J.; Xiao, J.; Dai, F.; Song, L. Circular RNA Circ-CHI3L1.2 Modulates Cisplatin Resistance of Osteosarcoma Cells via the MiR-340-5p/LPAATβ Axis. Hum. Cell 2021, 34, 1558–1568. [CrossRef]
- Song, L.; Zhou, Z.; Gan, Y.; Li, P.; Xu, Y.; Zhang, Z.; Luo, F.; Xu, J.; Zhou, Q.; Dai, F. Long Noncoding RNA OIP5-AS1 Causes Cisplatin Resistance in Osteosarcoma through Inducing the LPAATβ/PI3K/AKT/MTOR Signaling Pathway by Sponging the MiR-340-5p. J. Cell. Biochem. 2019, 120, 9656–9666. [CrossRef]
- Zhang, J.; Ma, X.; Zhou, R.; Zhou, Y. TRPS1 and YAP1 Regulate Cell Proliferation and Drug Resistance of Osteosarcoma via Competitively Binding to the Target of CircTADA2A – MiR-129-5p. Onco. Targets. Ther. 2020, Volume 13, 12397–12407. [CrossRef]
- Wu, Y.; Xie, Z.; Chen, J.; Chen, J.; Ni, W.; Ma, Y.; Huang, K.; Wang, G.; Wang, J.; Ma, J.; et al. Circular RNA CircTADA2A Promotes Osteosarcoma Progression and Metastasis by Sponging MiR-203a-3p and Regulating CREB3 Expression. Mol. Cancer 2019, 18, 73. [CrossRef]
- Pan, Y.; Lin, Y.; Mi, C. Cisplatin-resistant Osteosarcoma Cell-derived Exosomes Confer Cisplatin Resistance to Recipient Cells in an Exosomal Circ_103801-dependent Manner. Cell Biol. Int. 2021, 45, 858–868. [CrossRef]
- Soghli, N.; Qujeq, D.; Yousefi, T.; Soghli, N. The Regulatory Functions of Circular RNAs in Osteosarcoma. Genomics 2020, 112, 2845–2856. [CrossRef]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68. [CrossRef]
- Prater S, M.B. Osteosarcoma. [Updated 2022 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available: https://www.ncbi.nlm.nih.gov/books/NBK549868/ (accessed on 28 January 2023).
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers (Basel). 2020, 12, 2130. [CrossRef]
- Synoradzki, K.J.; Bartnik, E.; Czarnecka, A.M.; Fiedorowicz, M.; Firlej, W.; Brodziak, A.; Stasinska, A.; Rutkowski, P.; Grieb, P. TP53 in Biology and Treatment of Osteosarcoma. Cancers (Basel). 2021, 13, 4284. [CrossRef]
- Brady, S.W.; Ma, X.; Bahrami, A.; Satas, G.; Wu, G.; Newman, S.; Rusch, M.; Putnam, D.K.; Mulder, H.L.; Yergeau, D.A.; et al. The Clonal Evolution of Metastatic Osteosarcoma as Shaped by Cisplatin Treatment. Mol. Cancer Res. 2019, 17, 895–906. [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-Derived Suppressor Cells as Immunosuppressive Regulators and Therapeutic Targets in Cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [CrossRef]
- Bleve, A.; Consonni, F.M.; Porta, C.; Garlatti, V.; Sica, A. Evolution and Targeting of Myeloid Suppressor Cells in Cancer: A Translational Perspective. Cancers (Basel). 2022, 14, 510. [CrossRef]
- Deng, C.; Xu, Y.; Fu, J.; Zhu, X.; Chen, H.; Xu, H.; Wang, G.; Song, Y.; Song, G.; Lu, J.; et al. Reprograming the Tumor Immunologic Microenvironment Using Neoadjuvant Chemotherapy in Osteosarcoma. Cancer Sci. 2020, 111, 1899–1909. [CrossRef]
- Uehara, T.; Eikawa, S.; Nishida, M.; Kunisada, Y.; Yoshida, A.; Fujiwara, T.; Kunisada, T.; Ozaki, T.; Udono, H. Metformin Induces CD11b+-Cell-Mediated Growth Inhibition of an Osteosarcoma: Implications for Metabolic Reprogramming of Myeloid Cells and Anti-Tumor Effects. Int. Immunol. 2019, 31, 187–198. [CrossRef]
- Wright, E.M. SGLT2 and Cancer. Pflügers Arch. - Eur. J. Physiol. 2020, 472, 1407–1414. [CrossRef]
- Barbosa, A.M.; Martel, F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers (Basel). 2020, 12, 154. [CrossRef]
- Wu, W.; Zhang, Z.; Jing, D.; Huang, X.; Ren, D.; Shao, Z.; Zhang, Z. SGLT2 Inhibitor Activates the STING/IRF3/IFN-β Pathway and Induces Immune Infiltration in Osteosarcoma. Cell Death Dis. 2022, 13, 523. [CrossRef]
| No | Completed Year | GCTI | Sarcoma Type | API | Primary Test | Refs. |
|---|---|---|---|---|---|---|
| 1 | 2022 | NCT02357810 | SSM | Tpt and Pzp | Laboratory Biomarker Analysis | [45,46] |
| 2 | 2021 | NCT01532687 | Refractory Soft | Pzp, Plb, and Gct | Laboratory Biomarker Analysis | [47,48] |
| 3 | 2022 | NCT03718091 | Advanced Solid | M6620 (VX-970) | Laboratory Biomarker Analysis | [49,50] |
| 4 | 2022 | NCT02048371 | Selected Subtypes | Plb and Rgf | Laboratory Biomarker Analysis | [51,52] |
| 5 | 2022 | NCT02487979 | RRS | GV in GPNMB carrier | Laboratory Biomarker Analysis | [53,54] |
| 6 | 2022 | NCT02432274 | RRS Malignancies | Lenvatinib, EnI | Dose escalation study | [55,56] |
| 7 | 2021 | NCT03190174 | Advanced | Nab-Rpm in Nvl carrier | Dose escalation study | [57,58] |
| 8 | 2020 | NCT02390843 | RRS | Cfa, Sim, Tpt, and MGF | Dose escalation study | [59,60] |
| 9 | 2019 | NCT01962103 | RRS | Nab-paclitaxel | Dose escalation study | [62,63] |
| 10 | 2005 | NCT00180908 | Solid | EnI, Mtx, and Dox | Laboratory Biomarker Analysis | [64,65] |
| Medicine | Tumorigenesis | Treatment Mechanisms | Ref. |
|---|---|---|---|
| COLGALT2 inhibitor | Proliferation, migration, and invasion | Suppress ADMSC exosome-mediated | [105,106] |
| Tra2B | Suppress BMSC-derived exosomal miR-206 | [107,108] | |
| AGAP1 | Suppress OS cell-derived exosomal miR-1307 | [109,110] | |
| miR-148a and miR-21-5p EVs | Chondrogenesis | Increase genes to mimic UVEC formation in TME | [81,111] |
| LIFR-AS1 inhibitor | Progression | Inhibit miR-29a in the NFIA axis | [112,113] |
| Medicine | Interfere Communication Mediator in Signalling Pathway | Ref. |
|---|---|---|
| AXL inhibitor | Interfere miR-29a-3p by suppressing linc00852 in JARID2 axis | [117,118] |
| miR-135a-5p inhibitor | Interfere BMSC-derived exosomal LCP1 by suppressing Nrdp1 in JAK2/STAT3 signalling pathway | [119,120] |
| BCL6 inhibitor | Interfere miR-101 EV by suppressing ADMSC-derived miR-101 | [121,122] |
| TGFβ1 inhibitor | Interfere CRISPR-Cas9 by suppressing CAF and ASMAFN differentiation | [123,124] |
| Tim-3 inhibitor | Interfere the M2 mediation by suppressing IL-10, TGFβ, and VEGF secretions | [125,126] |
| SOCS5 inhibitor | Interfere STAT1 mediation by suppressing COL6A1 from H3K27ac activated in CAF conversion with IL-6 and IL-8 secretions | [127,128] |
| CASC15 or KLF3-AS1 | Interfere RAB14 trafficking by suppressing miR-338-3p | [129,130] |
| PDCD4 | Interfere ERK1/2 signalling pathway by suppressing miR-208a EV | [131,132] |
| ATG5 | Interfere oncogenic autophagy by suppressing BMSC-derived EV | [133,134] |
| Rab22a-NeoF1 | Interfere M2 with RGD peptide internalisation in STAT3 by suppressing PYK2 and RhoA | [135,136] |
| Medicine | Prevention Mechanisms | Ref. |
|---|---|---|
| mRNA N-cadherin | Suppress PD-L1 to reduce immunosuppression and tumorigenesis | [140,141] |
| Anti-IL-9 | Suppress IL-10 expression and tumour infiltrating T cells | [142,143] |
| USP12 inhibitor | Suppress M-MDSC, NO synthase, and PD-L1 to activate CD8+ T cells to stabilise p65 | [144,145] |
| IgG4+ B-cells | Suppress Th2 cytokines IL-4 and IL-10 | [146,147] |
| LAP inhibitor | Suppress PD-1 to activate CD8+ T cells with effector molecule phenotypes | [148,149] |
| Anti-Wnt2 mAb | Suppress CAF and PD-1 to activate DC-mediated anti-tumour TCR | [93,150] |
| Anti-αvβ8 integrin | Inhibit TGFβ or TGFβ1 immunosuppression to activate TCR or Treg cells | [151,152] |
| HK2 with IκBα | Inhibit PD-L1 expression and activate CD8+ T-cell | [153,154] |
| IDO inhibitor with NO | Inhibit glycolysis to increase the functions of CD8+ T-cells and Treg cells | [155,156] |
| TGFβRII with anti-IgG1 | Inhibit TGFβ and PD-L1 | [157,158] |
| Inhibitor | Resistance | Chemoresistance Prevention | Ref. |
|---|---|---|---|
| DHFR | Mtx and Dox | Reduce folate receptors to induce apoptosis in cancer cells | [168,169] |
| FPGS | Mtx | Reduce folate receptors by inhibiting the interaction of SKA1 and RPB3 | [170,171] |
| HSP90 | CDDP | Inhibit Ulk1 in FUNDC1 mediation for mitophagy activation | [172,80] |
| XCT-790 or ATK | Dox | Inhibit PGP for ABCB1 in the ERRα axis | [173,174] |
| IGF1 | Dox | Inhibit ABCB1 in the ERRα axis to reverse metabolic disorder | [175,176] |
| Gene Knockdown | Resistance | Chemoresistance Prevention | Ref. |
|---|---|---|---|
| circ_0000073 | Mtx | Inhibit N-Ras pathway by sponging miR-145-5p and miR-151-3p | [178,179] |
| circ_0081001 | Mtx | Inhibit TGM2 axis by sponging miR-494-3p | [180,181] |
| circ_0004674 | Dox | Inhibit fibrillin-1 axis by sponging miR-342-3p | [182,183] |
| circ_0001721 | Dox | Inhibit TCF4 axis by sponging miR-758 | [184,185] |
| circ_SAMD4A | Dox | Inhibit KLF8 axis by sponging miR-218-5p | [186,187] |
| circ_0002060 | Dox | Inhibit ABCB1 axis by sponging miR-198 | [188,189] |
| circ_0003496 | Dox | Inhibit KLF12 axis by sponging miR-370 | [190,191] |
| circ_CHI3L1.2 or OPI5-AS1 | CDDP | Inhibit LPAATβ axis by sponging miR-340-5p | [192,193] |
| circ_TADA2A | CDDP | Inhibit TRPS1 and YAP1 axis by sponging miR-129-5p | [194,195] |
| circ_103801 | CDDP | Inhibit MDR-associated protein 1 and PGP | [196,197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





