Submitted:
09 February 2023
Posted:
13 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Alphavirus Genus
2.1. Mayaro Virus (MAYV)
2.2. Eastern Equine Encephalitis Virus (EEEV)
2.3. Western Equine Encephalitis Virus (WEEV)
2.4. Chikungunya Virus (CHIKV)
2.5. Pixuna Virus (PIXV), Mucambo Virus (MUCV), Una Virus (UNAV), Aura Virus (AURAV) and Triniti Virus (TNTV)
3. Flavivirus Genus
3.1. Dengue Virus (DENV)
3.2. Saint Louis Encephalitis Virus (SLEV)
3.3. West Nile Virus (WNV)
3.4. Cacipacoré Virus (CACV), Ilhéus Virus (ILHV) and Bussuquara Virus (BUSV)
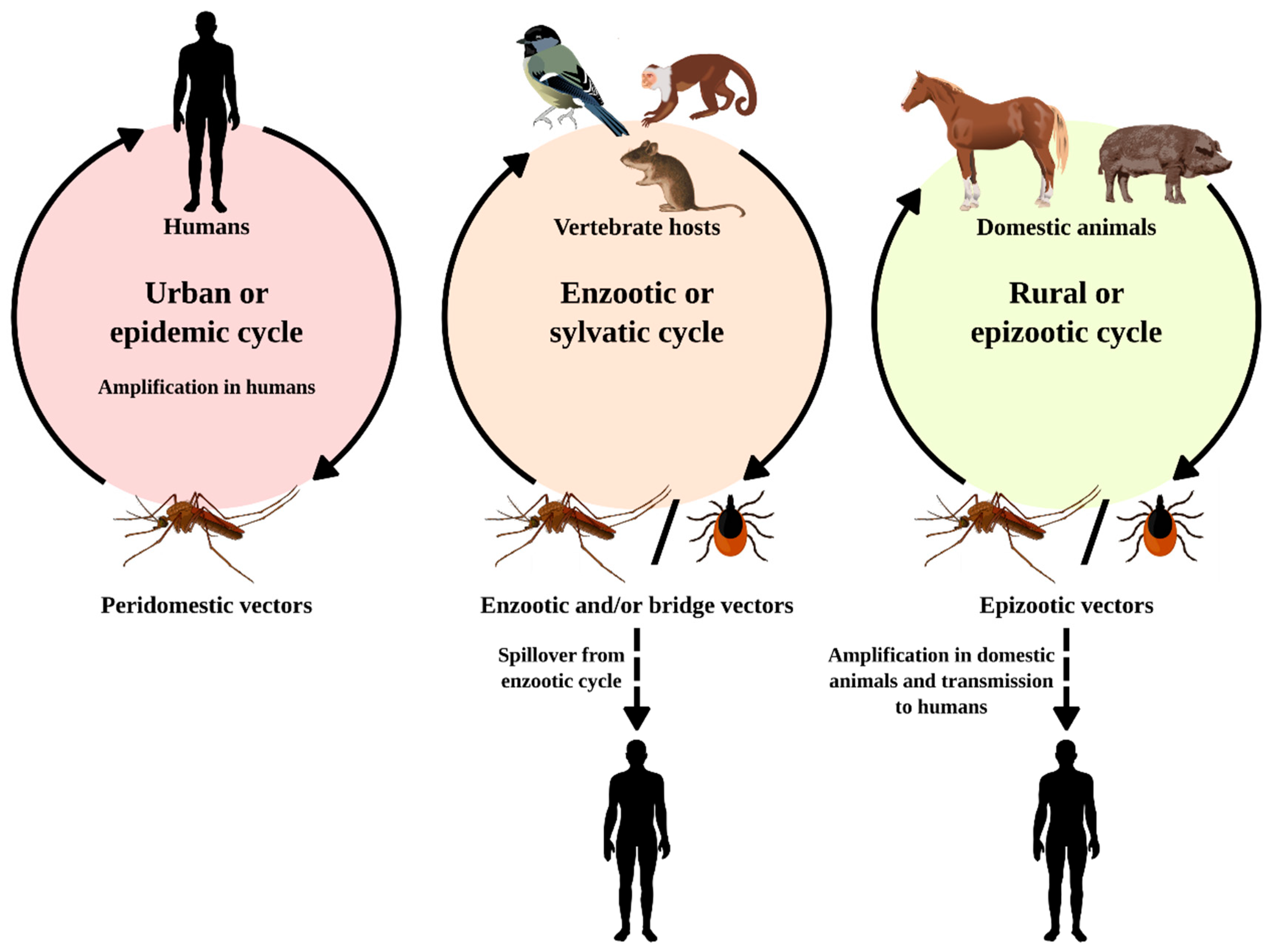
4. Viral Isolation
4.1. Isolation in Mice
4.2. Cell Culture
4.3. Origin of Viral Isolates
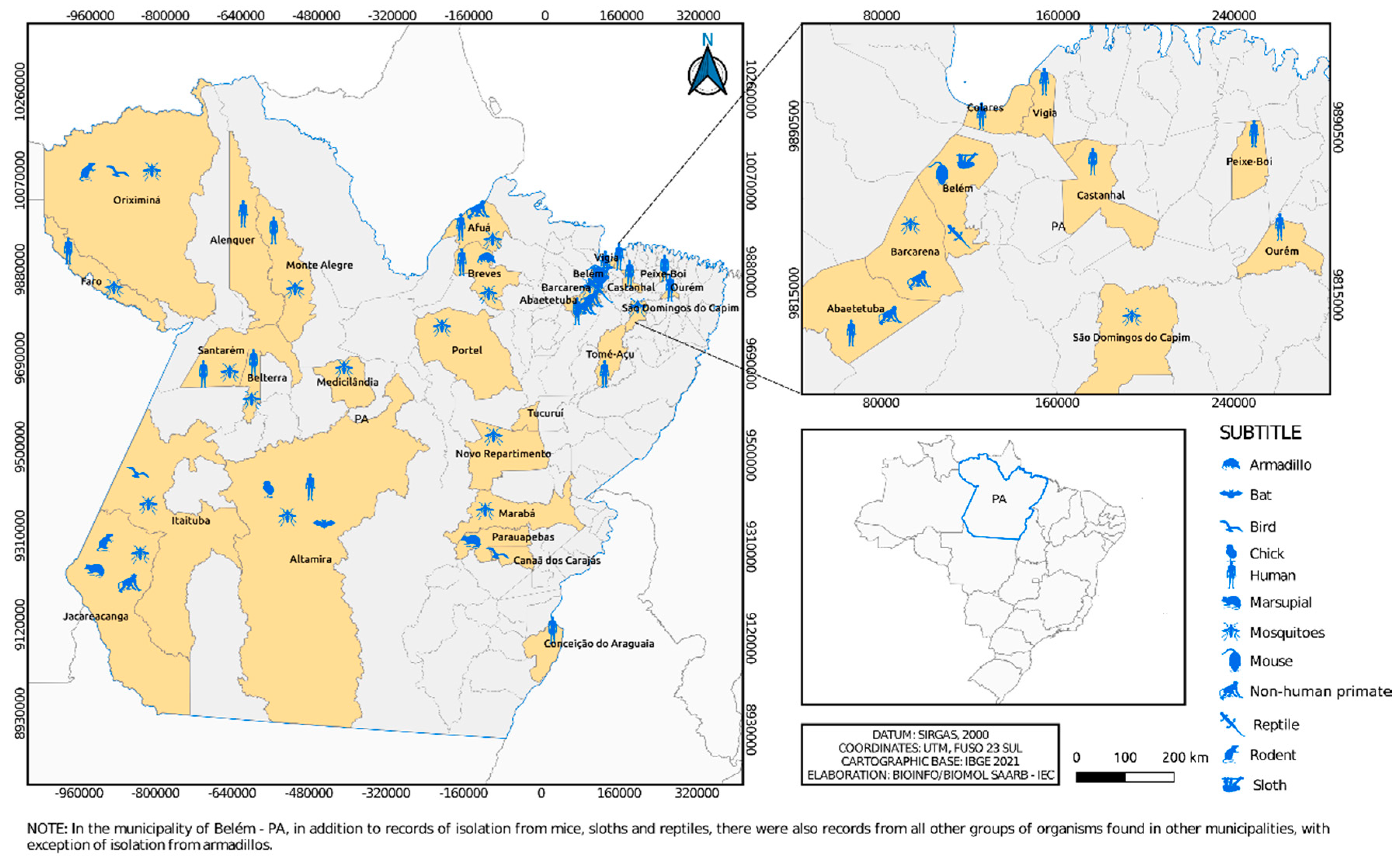
5. Alphavirus and Flavivirus Collection
5.1. Isolated Samples from Mice
5.2. Isolated Samples in Cell Culture
6. Legacy and Future Concerns
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). 1985. Arthropod-borne and rodent-borne viral diseases. Technical Report Series, n. 719. Geneva: World Health Organization.
- Brazil. Ministry of Health. Manual de Vigilância Sentinela de Doenças Neuroinvasivas por Arbovírus; Ministry of Health: Brasília, Brazil, 2017. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Arboviral diseases, neuroinvasive and non-neuroinvasive. Available online: https://ndc.services.cdc.gov/case-definitions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive-2015/ (accessed on 17 March 2022).
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- WHO (World Health Organization). 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization.
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. New Eng J Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Lopes, N.; Nozawa, C.; Linhares, R.E.C. Características gerais e epidemiologia dos arbovírus emergentes no Brasil. Rev Pan-Amaz Saude 2014, 5, 55–64. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Freitas, R.R.; Von Zuben, A.P.B. Arboviruses emerging in Brazil: challenges for clinic and implications for public health. Rev. Saude Publica 2017, 51, 1–6. [Google Scholar] [CrossRef]
- Pimentel, V.; Afonso, R.; Nunes, M.; Vieira, M.L.; Bravo-Barriga, D.; Frontera, E.; Martinez, M.; Pereira, A.; Maia, C.; Paiva-Cardoso, M.N.; et al. Geographic dispersal and genetic diversity of tick-borne phleboviruses (Phenuiviridae, Phlebovirus) as revealed by the analysis of L segment sequences. Ticks Tick Borne Dis. 2019, 10, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Tesh, R.B.; Popov, V.L.; Widen, S.G.; Wood, T.G.; Forrester, N.L.; Gonzalez, J.P.; Saluzzo, J.F.; Alkhovsky, S.; Lam, S.K.; et al. Exploiting the Legacy of the Arbovirus Hunters. Viruses 2019, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Travassos da Rosa, J.F.S.; Travassos da Rosa, A.P.A.; Vasconcelos, P.F.C.; Pinheiro, F.P.; Rodrigues, S.G.; Travassos da Rosa, E.S.; Dias, L.B.; Cruz, A.C.R. Arboviruses isolated in the Evandro Chagas Institute, including some described for the first time in the Brazilian Amazon region, their know hosts, and their pathology for man. In An Overview of Arbovirology in Brazil and Neighbouring Countries; Travassos da Rosa, A.P., Vasconcelos, P.F.C., Travassos da Rosa, J.F.S., Eds.; Instituto Evandro Chagas: Belém, Brazil, 1998; pp. 19–31. [Google Scholar]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J Gen Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Lutomiah, J.; Obanda, V.; Gakuya, F.; Mutisya, J.; Mulwa, F.; Michuki, G.; Chepkorir, E.; Fischer, A.; Venter, M.; et al. Isolation of tick and mosquito-borne arboviruses from ticks sampled from livestock and wild animal hosts in Ijara District, Kenya. Vector Borne Zoonotic Dis. 2013, 13, 637–642. [Google Scholar] [CrossRef]
- Hayes, R.O.; Francy, D.B.; Lazuick, J.S.; Smith, G.C.; Gibbs, E.P.J. Role of the Cliff Swallow Bug (Oeciacus vicarius) in the Natural Cycle of a Western Equine Encephalitis-Related Alphavirus. J Med Entomol. 1977, 14, 257–262. [Google Scholar] [CrossRef]
- Kuhn, R.J. Togaviridae. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, USA, 2013; pp. 629–650. [Google Scholar]
- Simmond, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J Gen Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- ICTV (International Committee on Taxonomy of Viruses). Currently ICTV Taxonomy Release. Available on: https://talk.ictvonline.org/ taxonomy/ (accessed on 04 April 2022).
- Pinheiro, F.P.; Freitas, R.B.; Travassos da Rosa, J.F.; Gabbay, Y.B.; Mello, W.A.; LeDuc, J.W. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am J Trop Med Hyg. 1981, 30, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C.; Travassos da Rosa, A.P.A.; Rodrigues, S.G.; Rosa, E.S.T.; Dégallier, N.; Rosa, J.F.S.T.; et al. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad. Saude Publica 2001, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, T.L.M.; Santos, C.L.S.; Suzuki, A.; Petrella, S.M.C.; Bisordi, I.; Nagamori, A.H.; Marti, A.T.; Santos, R.N.; Fialho, D.M.; Lavigne, S.; et al. Mayaro virus: imported cases of human infection in São Paulo State, Brazil. Rev Int Med Trop. 2007, 4, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.; Silva, E.V.; Carvalho, V.L.; Rodrigues, S.G.; Nunes-Neto, J.P.; Monteiro, H.; Peixoto, V.S.; Chiang, J.O.; Nunes, M.R.; Vasconcelos, P.F. Mayaro Fever Virus, Brazilian Amazon. Emerg Infect Dis. 2009, 15, 1830–1832. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, N.; Heinen, L.B.; Santos, M.A.; Pereira, F.C.; Slhessarenko, R.D. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz 2014, 109, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.S.; Chiang, J.O.; Ferreira, M.S.; Henriques, D.F.; Oliveira, C.F.; Carvalho, V.L.; Silva, E.V.P.; Azevedo, R.S.S.; Martins, L.C. Research on the circulation of arboviruses in human populations living in the Municipalities of Parauapebas and Canaã de Carajás, located in the southeastern mesoregion of the state of Pará. Research, Society and Development 2022, 11, e6211326043. [Google Scholar] [CrossRef]
- Pereira, N.T.; Rocha, M.N.; Sucupira, P.H.F.; Carvalho, F.D.; Moreira, L.A. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci Rep 2018, 10, 6889. [Google Scholar] [CrossRef] [PubMed]
- Theilacker, C.; Held, J.; Allering, L.; Emmerich, P.; Schmidt-Chanasit, J.; Kern, W.V.; Panning, M. Prolonged polyarthralgia in a German traveller with Mayaro virus infection without inflammatory correlates. BMC Infect Dis. 2013, 13, 369. [Google Scholar] [CrossRef]
- Neufeld, P.M. About Mayaro Fever: an emergin arbovirus. Rev Bras Anal Clin. 2017, 49, 118–119. [Google Scholar] [CrossRef]
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef]
- Barba, M.; Fairbanks, E.L.; Daly, J.M. Equine viral encephalitis: prevalence, impact, and management strategies. Vet Med (Auckl). 2019, 10, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Beer, J. Doenças infecciosas em animais domésticos. Roca: São Paulo, Brazil, 1999; ISBN 978-8572412483.
- Gil, L.H.V.G.; Magalhaes, T.; Santos, B.S.A.S.; Oliveira, L.V.; Oliveira-Filho, E.F.; Cunha, J.L.R.; Fraiha, A.L.S.; Rocha, B.M.M.; Longo, B.C.; Ecco, R.; et al. Active Circulation of Madariaga Virus, a Member of the Eastern Equine Encephalitis Virus Complex, in Northeast Brazil. Pathogens 2021, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Alice, F.J. Encefalomielite equina na Bahia, estudo de três amostras isoladas. Rev Bras Biol. 1951, 11, 125–144. [Google Scholar]
- Cunha, R. Estudos sobre uma amostra de vírus da encefalomielite equina isolada de material proveniente de Recife. Bol Soc Bras Med Vet. 1954, 14, 201–215. [Google Scholar]
- Causey, O.R.; Shope, R.E.; Laemmert, H.W. Report of an epizootic of encephalomyelitis virus in Pará, Brazil. Rev Serv Esp Saude Publica. 1962, 12, 47–50. [Google Scholar]
- Correa, W.M.; Correa, C.N.M. Encefalomielite equina. In Enfermidades infecciosas dos mamíferos domésticos, 2nd ed. MEDSI: Rio de Janeiro, Brazil, 1992; pp. 635–642.
- Iversson, L.B.; Silva, R.A.M.S.; Travassos da Rosa, A.P.A.; Barros, V.L.R.S. Circulation of Eastern Equine Encephalitis, Western equine encephalitis, Ilhéus, Maguari and Tacaiuma viruses in equines of the Brazilian Pantanal, South America. Rev Inst Med Trop. 1993, 35, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.R.; Sugay, W. Ocorrência da encefalomielite equina em Itaporanga, Estado de São Paulo. Arq Inst Biol. 1962, 29, 63–68. [Google Scholar]
- Campos, K.F.; de Oliveira, C.H.S.; Reis, A.B.; Vamasaki, E.M.; Brito, M.F.; Andrade, S.T.J.; Duarte, M.D.; Barbosa, J.D. Surto de encefalomielite equina Leste na Ilha de Marajó, Pará. Pesq Vet Bras. 2013, 33, 443–448. [Google Scholar] [CrossRef]
- Casseb, A.R.; Casseb, L.M.N.; Silva, S.P.; Vasconcelos, P.F.C. Abovírus: Importante zoonose na Amazônia Brasileira. Vet e Zootec. 2013, 20, 391–403. [Google Scholar]
- Fernández, Z.; Richartz, R.; Travassos da Rosa, A.P.A.; Soccol, V.T. Identification of the encephalitis equine virus, Brazil. Rev Saude Pública. 2000, 34, 232–235. [Google Scholar] [CrossRef]
- Wigg, M.D. Isolamento de uma amostra de vírus WEE em Haemagogous janthinomys. Master’s dissertation, Rio de Janeiro Federal University, Rio de Janeiro, Brazil, 1977.
- Heinemann, M.B.; Souza, M.C.C.; Cortez, A.; Ferreira, F.; Homem, V.S.F.; Ferreira-Neto, J.S.; Soares, R.M.; Cunha, E.M.S.; Richtzenhain, L.J. Soroprevalência da encefalomielite equina do leste e do oeste no Município de Uruará, PA, Brasil. Brazilian Journal of Veterinary Research and Animal Science 2006, 43, 137–139. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.C.; Travassos da Rosa, J.F.S.; Travassos da Rosa, A.P.A.; Dégallier, N.; Pinheiro, F.D.P. Epidemiologia das encefalites por arbovírus na Amazônia brasileira. Rev Inst Med Trop S Paulo. 1991, 33, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Dexter, E.P.; Dexter, D.D.; Lindsay, C.W.; Ross, R.R.; Lutwick, L. Case of fatal eastern equine encephalitis. ID Cases 2021, 26, e01288. [Google Scholar] [CrossRef]
- Hervé, J.P.; Dégallier, N.; Travassos da Rosa, A.P.A.; Pinheiro, F.P.; Sá Filho, G.C. Arboviroses: aspectos ecológicos. In Instituto Evandro Chagas: 50 Anos de Contribuição às Ciências Biológicas e à Medicina Tropical. Fundação SESP: Belém Brazil, 1986; pp. 409–437.
- Rust, R.S. Human arboviral encephalitis. Semin Pediatr Neurol. 2012, 19, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Acha, P.N.; Szyfres, B. Zoonosis y enfermedades transmisibles comunes al hombre y a los animales. Volumen 1: Bacteriosis y Micosis, 3rd ed. Pan American Health Organization: Washington, USA, 2003.
- Bruno-Lobo, G.; Bruno-Lobo, M.; Travassos, J.; Pinheiro, F.; Pazin, I.P. Estudos sobre arbovírus. III. Isolamento de vírus sorologicamente relacionado ao sub-grupo Western - Sindbis de um caso de encefalomielite equina no Rio de Janeiro. An Microbiol. 1961, 9, 183–195. [Google Scholar]
- Jonkers, A.H.; Downs, W.G.; Spence, L.; Aitken, T.H.G. Arthropod borne encephalitis viruses in Northern South America. II. A serological survey of northeastern Venezuela. Am J Trop Med Hyg. 1965, 14, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.L.; Kenney, J.L.; Deardoff, E.; Wang, E.; Weaver, S.C. Western Equine Encephalitis submergence: lack of evidence for a decline in virus virulence. Virology 2008, 380, 170–172. [Google Scholar] [CrossRef] [PubMed]
- BVS (Biblioteca Virtual em Saúde). Febre Chikungunya. Available online: https://bvsms.saude.gov.br/febre-de-chikungunya/ (accessed on 17 May 2022).
- Montalbano, C.A.; Bezerra, W.S.P.; Ribeiro, K.M.; Rosa, S.B.A. Doenças infecciosas de relevância no Brasil. Atena Editora: Ponta Grossa, Brazil, 2021, 340 pp. ISBN 978-655-983-610-9.
- Brazilian Ministry of Health. Boletim epidemiológico v. 54, n. 01, 2023. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2023/boletim-epidemiologico-volume-54-no-01/ (accessed on 07 February 2022).
- Cruz, A.C.R.; Vasconcelos, P.F.C. Arbovírus no Brasil. Biológico, 2008, 70, 45–46. [Google Scholar]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the Flavivirus life cycle. Nat Rev Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Coimbra, T.L.; Nassar, E.S.; Nagamori, A.H.; Ferreira, I.B.; Pereira, L.E.; Rocco, I.M.; Ueda-Ito, M.; Romano, N.S. Iguape: a newly recognized flavivirus from São Paulo State, Brazil. Intervirology. 1993, 36, 144–152. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Catão, R.C. Dengue no Brasil: abordagem geográfica na escala nacional. Cultura acadêmica: São Paulo, Brazil, 2012, 176 pp. ISBN 978-857-983-332-8.
- Bezerra, J.M.T.; Sousa, S.C.; Tauil, P.L.; Carneiro, M.; Barbosa, D.S. Entry of dengue virus serotypes and their geographic distribution in Brazilian federative units: a systematic review. Rev Bras Epidemiol. 2021, 24, E210020. [Google Scholar] [CrossRef] [PubMed]
- Oneda, R.M.; Basso, S.R.; Frasson, L.R.; Mottecy, N.M.; Saraiva, L.; Bassani, C. Epidemiological profile of dengue in Brazil between the years 2014 and 2019. Rev Assoc Med Bras. 2021, 67, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.L.; Amarilla, A.A.; Poloni, T.R.; Covas, D.T.; Aquino, V.H.; Figueiredo, L.T.M. Detection of dengue virus in sera of Brazilian blood donors. Transfusion 2012, 52, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Santos, E.V.; Hespanhol, M.R.; Marta, R.; Rodrigues, E.S.; Haddad, R.; Ubiali, E.M.A.; Covas, D.T.; Kashima, S. Dengue RNA detection and seroprevalence in blood donors during an outbreak in the São Paulo State, Brazil, 2016. J Med Virol. 2021, 93, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Kubizeski, J.R. Arboviroses emergentes no município de Sinop-MT: pesquisa de vetores. Bachelor’s dissertation, Mato Grosso Federal University, Sinop, Brazil, 2017.
- Lopes, S.F. Identificação de Flavivirus em aves silvestres da Amazônia Central. Master’s dissertation, Amazonas Federal University, Manaus, Brazil, 2011.
- Rocco, I.M.; Santos, C.L.; Bisordi, I.; Petrella, S.M.; Pereira, L.E.; Souza, R.P.; Suzuki, A. St. Louis encephalitis vírus: first isolation from a human in São Paulo state, Brasil. Rev Inst Med Trop S Paulo. 2005, 47, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.M.; Kubiszeski, J.R.; Vieira, C.J.S.; Gusmão, A.F.; Pratis, T.S.; Colombo, T.E.; Thies, S.F.; Araujo, F.C.; Zanelli, C.F.; Milhim, B.H.G.A.; et al. Detection of Saint Louis encephalitis virus in two Brazilian states. J Med Virol. 2021, 94, 776–781. [Google Scholar] [CrossRef]
- Lavezzo, L.C. Estudo de arboviruses em dodores de sangue na região Amazônica e em uma cidade do interior de São Paulo. Master’s dissertation, São Paulo State University, São Paulo, Brazil, 2010.
- Barbosa, B.C.M. Arbovírus emergentes no Brasil e seu risco de transmissão por transfusão sanguínea: uma revisão da literatura. Master’s dissertation, Minas Gerais Federal University, Belo Horizonte, Brazil, 2017.
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am J Trop Med Hyg. 1940, 20, 471–472. [Google Scholar] [CrossRef]
- Murgue, B.; Zeller, H.; Deubel, V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol. 2002, 267, 195–221. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile. Lancet Infect Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Melandri, V.; Guimarães, A.E.; Komar, N.; Nogueira, M.L.; Mondini, A.; Fernandez-Sesma, A.; Alencar, J.; Bosch, I. Serological detection of West Nile virus in horses and chicken from Pantanal, Brazil. Mem Inst Oswaldo Cruz. 2012, 107, 1073–1075. [Google Scholar] [CrossRef]
- Ometto, T.; Durigon, E.L.; de Araujo, J.; Aprelon, R.; de Aguiar, D.M.; Cavalcante, G.T.; Melo, R.M.; Levi, J.E.; de Azevedo Júnior, S.M.; Petry, M.V.; et al. West Nile virus surveillance, Brazil, 2008-2010. Trans R Soc Trop Med Hyg. 2013, 107, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez, J.; Nogueira, R.M.R.; Komar, N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Neg Trop Dis. 2014, 8, e2706. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.C.; Silva, E.V.P.; Casseb, L.M.N.; Silva, S.P.; Cruz, A.C.R.; Pantoja, J.A.S.; Medeiros, D.B.A.; Filho, A.J.M.; Cruz, E.R.M.; Araújo, M.T.F.; et al. First isolation of West Nile virus in Brazil. Mem Inst Oswaldo Cruz. 2019, 114, e180332. [Google Scholar] [CrossRef]
- Silva, A.S.G.; Rehfeld, I.S.; Santos, B.S.A.S.; Franklin, L.F.S.; Teixeira, R.B.C.; Lobato, Z.I.P.; Guedes, M.I.M.C.; Costa, E.A. Febre do Nilo Ocidental no Brasil: o novo desafio aos medicos-veterinários. Rev Educ Cont Med Vet Zootec. 2021, 19, e38082. [Google Scholar] [CrossRef]
- Costa, E.A.; Bayeux, J.J.M.; Silva, A.S.G.; de Queiroz, G.A.; Santos, B.S.A.S.; Rocha, M.N.; Rehfeld, I.S.; Franklin, L.F.S.; Valle, L.B. Epidemiological surveillance of West Nile virus in the world and Brazil: relevance of equine surveillance in the context of “One Health”. Brazilian Journal of Veterinary Research and Animal Science 2019, 56, e164335. [Google Scholar] [CrossRef]
- Meireles, A.R.; Fernandes, L.F.; Fernande, P.M.G.; da Cruz, G.H.S. Primeiro diagnóstico de febre do Nilo Ocidental em humano em Minas Gerais: relato de caso. Braz J Infect Dis. 2022, 26, 102294. [Google Scholar] [CrossRef]
- Vieira, M.A.C.S.; Romano, A.P.M.; Borba, A.S.; Silva, E.V.P.; Chiang, J.O.; Eulálio, K.D.; Azevedo, R.S.S.; Rodrigues, S.G.; Almeida-Neto, W.S.; Vasconcelos, P.F.C. West Nile virus encephalitis: The first human case recorded in Brazil. Am J Trop Med Hyg. 2015, 93, 377–379. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Davis, L.E.; Debiasi, R.; Goade, D.E.; Haaland, K.T.; Harrington, J.A.; Harnar, J.B.; Pergam, S.A.; King, M.K.; Demasters, B.K.; Tyler, K.L. West Nile virus neuroinvasive disease. Ann Neurol. 2006, 60, 286–300. [Google Scholar] [CrossRef]
- Chapman, G.E.; Baylis, M.; Archer, D.; Daly, J.M. The challenges posed by equine arboviruses. Equine Vet J. 2018, 50, 436–445. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Research International. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J. Transfusion-Transmitted Diseases. In Transfus Medicine, 5th ed.; McCullough, J; Wiley-Blackwell: Hoboken, NJ, 2021, pp. 422-452.
- Pealer, L.N.; Marfin, A.A.; Petersen, L.R.; Lanciotti, R.S.; Page, P.L.; Stramer, S.L.; Stobierski, M.G.; Signs, K.; Newman, B.; Kapoor, H.; et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003, 349, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Sylvester, D.; Chancey, C.; Hewlett, I.K.; Stramer, S.L. West Nile Virus Adheres to Human Red Blood Cells in Whole Blood. Clin Infect Dis. 2007, 45, 181–186. [Google Scholar] [CrossRef]
- Harrington, T.; Kuehnert, M.J.; Kamel, H.; Lanciotti, R.S.; Hand, S.; Currier, M.; Chamberland, M.E.; Petersen, L.R.; Marfin, A.A. West Nile virus infection transmitted by blood transfusion. Transfusion 2003, 43, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.L.G.; Amarilla, A.A.; Figueiredo, G.G.; Alfonso, H.L.; Lippi, V.; Maia, F.G.M.; Morais, F.A.; Costa, C.A.D.; Henriques, D.A.; Durigon, E.L.; et al. Cacipacore virus as an emergent mosquito-borne Flavivirus. Rev Soc Bras Med Trop. 2017 50, 539–542. [CrossRef]
- Batista, W.C.; Tavares, G.S.B.; Vieira, D.S.; Honda, E.R.; Pereira, S.S.; Tada, M.S. Notification of the first isolation of Cacipacore virus in a human in the State of Rondônia, Brazil. Rev Soc Bras Med Trop. 2011, 44, 528–530. [Google Scholar] [CrossRef]
- Laemmert, H.W.; Hughes, T.P. The virus of Ilhéus encephalitis: isolation, serological specificity and transmission. J Immunol. 1947, 55, 61–67. [Google Scholar] [CrossRef]
- Bernal, M.K.M.; Chiang, J.O.; Mendes, F.F.; Andrade, S.L.S.; da Silva, S.K.S.M.; Pereira, W.L.A. Study of Arboviruses in Philander opossum, Didelphis marsupialis and Nectomys rattus captured from forest fragments in the municipality of Belém, Pará, Brazil. Cienc Rural. 2021, 51, n 4, e20200515. [Google Scholar] [CrossRef]
- Pereira, L.E.; Suzuki, A.; Coimbra, T.L.M.; Souza, R.P.; Chamelet, E.L.B. Arbovírus Ilheus em aves silvestres (Sporophila caerulescens e Molothrus bonariensis). Rev. Saude Publica. 2001, 35, 119–123. [Google Scholar] [CrossRef]
- Gomes, G.; Causey, O.R. Bussuquara, a new arthropod-borne virus. Proc Soc Exp Biol Med. 1959, 101, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Srihongse, S.; Johnson, C.M. The first isolation of Bussuquara virus from man. Trans R Soc Trop Med Hyg. 1971, 65, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Gibrail, M.M. Detecção de anticorpos para arbovírus em primatas não humanos no município de Goiânia, Goiás. Master’s dissertation, Goiás Federal University, Goiânia, Brazil, 2015.
- Casals, J. The arthropod-borne group of animal viruses. Trans R Soc Trop Med Hyg. 1957, 19, 219–235. [Google Scholar]
- Gubler, D.J.; Kuno, G.; Sather, G.E.; Velez, M.; Olivier, A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am J Trop Med Hyg. 1984, 33, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Meslin, F.X.; Stohr, K.; Heymann, D. Public health implications of emerging zoonoses. Rev Sci Tech. 2001, 19, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.H.N.G. Estudos epidemiológicos sobre arbovírus em populações rurais e urbanas do Estado do Amazonas. Master’s dissertation, Amazonas Federal University, Manaus, Brazil, 2009.
- IEC (Evandro Chagas Institute). O estudo da Arbovirologia em defesa da Amazônia. Available online: https://antigo.iec.gov.br/especial-85-anos-arbovirologia/ (accessed on 04 April 2022).
- Vasconcelos, H.B.; Nunes, M.R.; Casseb, L.M.; Carvalho, V.L.; Pinto da Silva, E.V.; Silva, M.; Casseb, S.M.; Vasconcelos, P.F. Molecular epidemiology of Oropouche virus, Brazil. Emerg Infect Dis. 2011, 17, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Chastel, C. [Asymptomatic infections in man: a Trojan horse for the introduction and spread of mosquito-borne arboviruses in non-endemic areas? ] Bull Soc Pathol Exot. 2011, 104, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo, R.S.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Marbán-Castro, E.; Goncé, A.; Fumadó, V.; Romero-Acevedo, L.; Bardají, A. Zika virus infection in pregnant women and their children: a review. Eur J Obstet Gynecol Reprod Biol. 2021, 265, 162–168. [Google Scholar] [CrossRef]
- Lowe, R.; Lee, S.; Martins, L.R.; Torres, C.C.; Castro, M.C.; Pascual, M. Emerging arboviruses in the urbanized Amazon rainforest. BMJ. 2020, 371, m4385. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; McCarthy, K.; Bedford, P.; Johnson, C.T.; Nichols, R.; Yoksan, S.; Marchesani, R.; Knauber, M.; Wells, K.H.; Arroyo, J.; et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002, 20, 1004–1018. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




