Introduction
In both primary and secondary cardiovascular (CV) prevention, the effectiveness of the programs depends on the adherence of professionals to clinical guidelines, access to indicated drugs by patients and patient adherence to the guidelines prescribed in the medium and long term. Clinical practice shows that the indications are often not followed by an important part of the actors, decreasing the effectiveness of cardiovascular prevention programs. An initiative proposes using polypills to improve adherence among healthcare providers and patients to these programs.[1]
The three most prevalent chronic conditions – diabetes mellitus (DM), hypertension (HTN) and dyslipidemia (PLD) – stand out as the diseases or cardiovascular risk factors (CVR) with the highest avoidable costs, for which each additional dollar spent on medications for patients who do adhere can generate between 3 and 13 dollars of savings alone. in preventable emergencies, visits to health care providers and hospitalizations.[2]
In primary CV prevention there are data demonstrating the effectiveness of prevention programs even when applied to the general population or people with low CVR; however, therapeutic strategies aimed at simultaneously controlling several CVR factors in patients without declared cardiovascular disease (primary prevention) are expensive and difficult to implement.[3,4]
In secondary CV prevention , patients with various CVR factors or with a history of ischemic heart disease (IC) have an elevated risk of recurrence of new coronary events. Combined pharmacological therapy is a common practice in secondary cardiovascular prevention even in geriatric patients and its benefits in morbidity and mortality are widely documented; However, the complexity of the therapeutic regimen often means that professionals tend not to implement a complete preventive regimen with lack of adherence to clinical guidelines, do not question the patient about their adherence to treatment and, in turn, that [5,6,7]patients present a low adherence to the therapeutic regimen with multiple medications; in these cases adherence to the therapeutic regimen is usually low after 6 months [4]even after acute myocardial infarction (AMI), while the use of a polypill after this period reduces the rate of major cardiovascular events.[8,9,10]
The consequences of this lack of therapeutic adherence are: increase in the rate of major CV episodes (and,[3] consequently, morbidity and mortality in both primary and secondary prevention), non-adherence to treatments for other related diseases or their delayed diagnosis (such as DM) due to less medical consultation; All this leads to an increase in the care burden and an increase in healthcare costs. Therapeutic adherence is a key factor in ensuring the sustainability of the healthcare system, as non-adherence is linked to poorer health outcomes and higher costs for the system.[11,12,13]
The polymer containing in different doses Acetylsalicylic Acid (ASA) plus Ramipril and Atorvastatin has shown its clinical effectiveness and high tolerability. According to its technical sheet, the indication focuses on [14,15,16] the secondary prevention of cardiovascular accidents as substitution treatment in adult patients adequately controlled with the three substances taken at the same time at equivalent doses to reduce the risk of suffering a CV accident when the patient has already suffered a previous event, not including the primary prevention of accidents CV, despite the evidence in the sense of providing benefits.[12,17]
The strategies using polypills for secondary CV prevention have shown greater comfort for the patient and an increase in adherence to treatment up to 20%, improving not only the CVR factors [8,13,17,18,19,20] but also reducing CV events and the health expenditure derived from them, being considered a strategy of great cost-effectiveness. [20,21,22]
The change to a polypill with this composition has as advantages the increase in the use [13,18] of Antiplatelet Agents (AAP) and [11] more favorably modify the levels of total cholesterol, LDL-cabbage, HDL-cabbage and[23] blood pressure, [11] than in patients who follow treatment with [14]three separate drugs, especially in people with a history of non-adherence or who have some [24] Predictor of lack of pharmacological adherence, in patients who are not well controlled with equipotent doses and with adherence problems, in patients who are controlled with individual drugs, and in patients with comorbidities and polymedicated.
The benefits of the association of ASA + Ramipril + statins are essentially due[18] to the increase in therapeutic compliance (more accentuated in antihypertensive drugs and in ASA, above statins). [24] The simplification of the therapeutic regimen and the increase in adherence in the short, medium and long term[11] show that for every 10% increase in adherence, cardiovascular complications decrease by 6.7%; assuming that the polypill increases adherence by up to 20%, the reduction in complications could be around 12.6% (up to 11 fatal and 46 non-fatal episodes per 1 [24]. 000 patients treated), pointing out its enormous cost-effectiveness, especially in a [13,21,22]country like Spain where the polypill has a price identical to the sum of its components in a separate generic version; in polymedicated patients, the simplification of the therapeutic regimen also results in better compliance with treatment guidelines for other conditions and diseases by indirectly increasing adherence to treatment treatments the others because they have a simpler regimen of administration of all medications. The use of fixed combination treatments is associated with a greater than expected reduction in blood pressure and lipid levels, due to increased therapeutic adherence. In phase IV studies, the reduction in blood pressure and LDL-cabbage was kept after one year of treatment, reducing cardiovascular risk factors.[13,32,35]
The indications of a polypill may include patients with high or very high CVR ([1]subclinical CV disease) to control their risk factors and as organic protection, provided that they do not present a high risk of bleeding in three types of patients: hypertensive patients with high CVR, primary CV prevention in patients with all three CVR factors present and in secondary CV prevention in patients controlled with all three components. [13,15,18,25] First, hypertensive patients with high CVR, defined by one or more of the following criteria: age ≥70 years, risk ≥10% at 10 years in the SCORE2 table adapted to the risk of their European region, risk ≥5% at 10 years in the SCORE table calibrated for Spain, risk ≥10% for the REGICOR or Framingham tables, left ventricular hypertrophy, microalbuminuria[16,26,27,28,29,30] or proteinuria, renal failure, increased pulse wave velocity, increased carotid intimomedial thickness, presence of atheromatous plaques and pathological ankle-brachial index. [31] Secondly, as primary prevention of cardiovascular events in patients with indications for treatment with all three components, patients with subclinical CV disease (patients with elevated or very high CVR and low risk of bleeding),[31,32] diabetics over 50 years of age with at least one associated CVR factor or with chronic kidney disease and microalbuminuria or macroalbuminuria, hypertensive patients with high CVR , patients with high CVR with clinical or subclinical ventricular dysfunction. Thirdly, it could also be indicated as secondary prevention of cardiovascular events in adult patients adequately controlled with monocomponents administered concomitantly at equivalent therapeutic doses, coronary complications, ischemic cerebrovascular or symptomatic peripheral arterial disease and patients with coronary stent.[5,16,25,31,32,33,34]
The use of a polypill could increase the adherence of patients to medication by reducing their CVR in the medium and long term, resulting in greater effectiveness of cardiovascular prevention programs without increasing costs or pressure on the health system.
Objectives
Study #Trigeria: to specify the combined prevalence of hypertension, dyslipidemia and atrial fibrillation in nursing homes, including the rate of prescription of drugs for their control or for the control of cardiovascular risk and to analyze the possible advantages of the prescription of a polypill over the polymedication guidelines.
Methods
Study #Trigeria: observational analytical study conducted from the analysis of medical records and pharmaceutical prescriptions of people institutionalized in nursing homes.
Data collection was during the third quarter of 2022 in the four nursing homes taking part in the study. Data were collected anonymously from all people who at the time of the study were institutionalized in the centers by the staff in charge of each of the patients, following current ethical standards. The variables analyzed were age, gender, personal history of hypertension (HTN), dyslipidemia (PLD), atrial fibrillation (AF), cognitive impairment, heart disease, diabetes, stroke, chronic kidney disease (CKD), hypothyroidism, chronic obstructive pulmonary disease (COPMP) or other antecedents of interest. We collected pharmacological prescription data on antiplatelets, statins and antihypertensives from the angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor 2 (ARB) inhibitor (ARB) families and the total number of oral drugs prescribed. Data were collected in a database and analyzed using the IBM SPSS® v.2 7 software.
Results
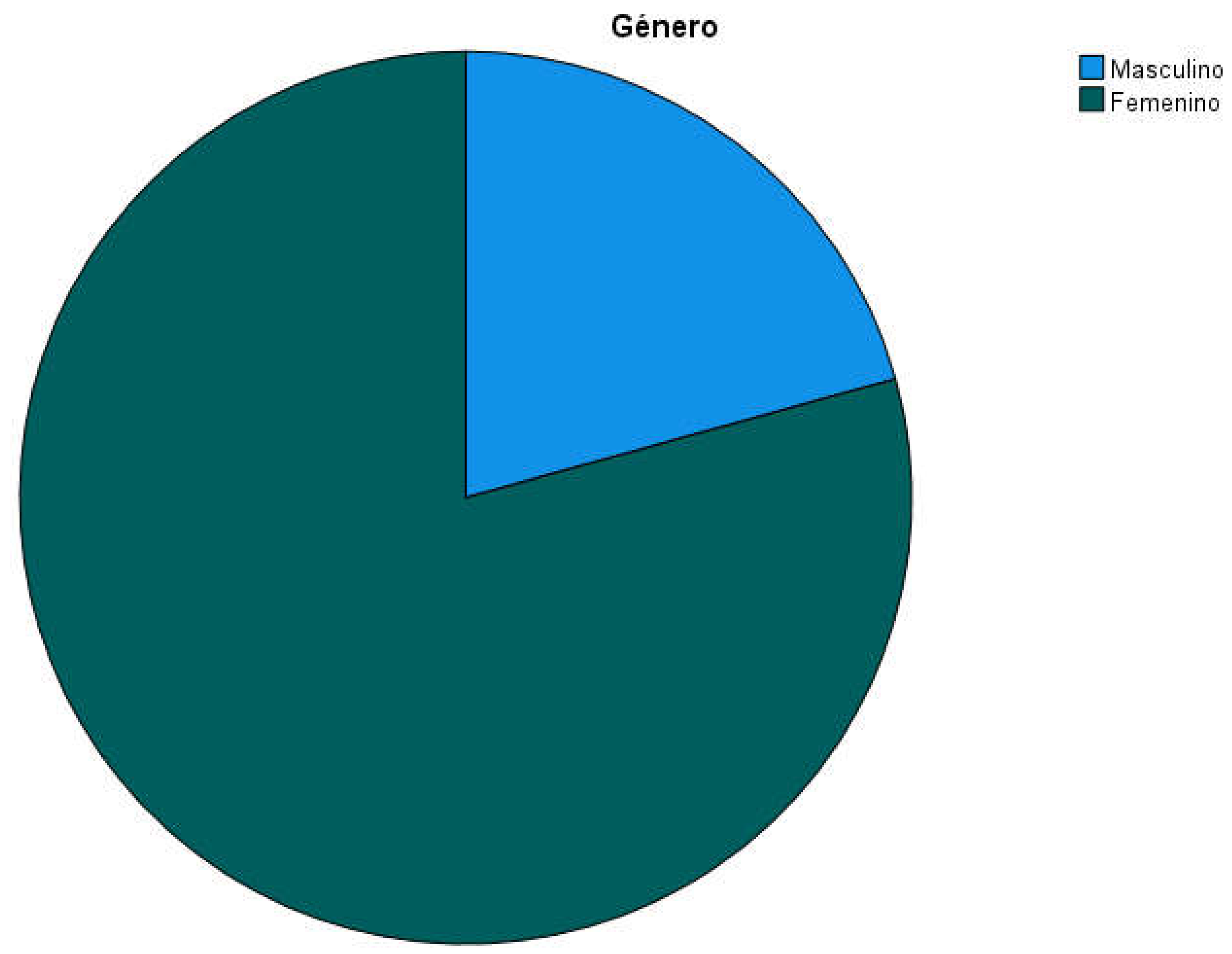
The final sample consisted of 169 people institutionalized in 4 residential centers (two of 25, one of 32 and another of 87 places, see
Table 1). 79.3% of participants (n=134) were women while 20.7% were men (n=35; see
Table 2 and
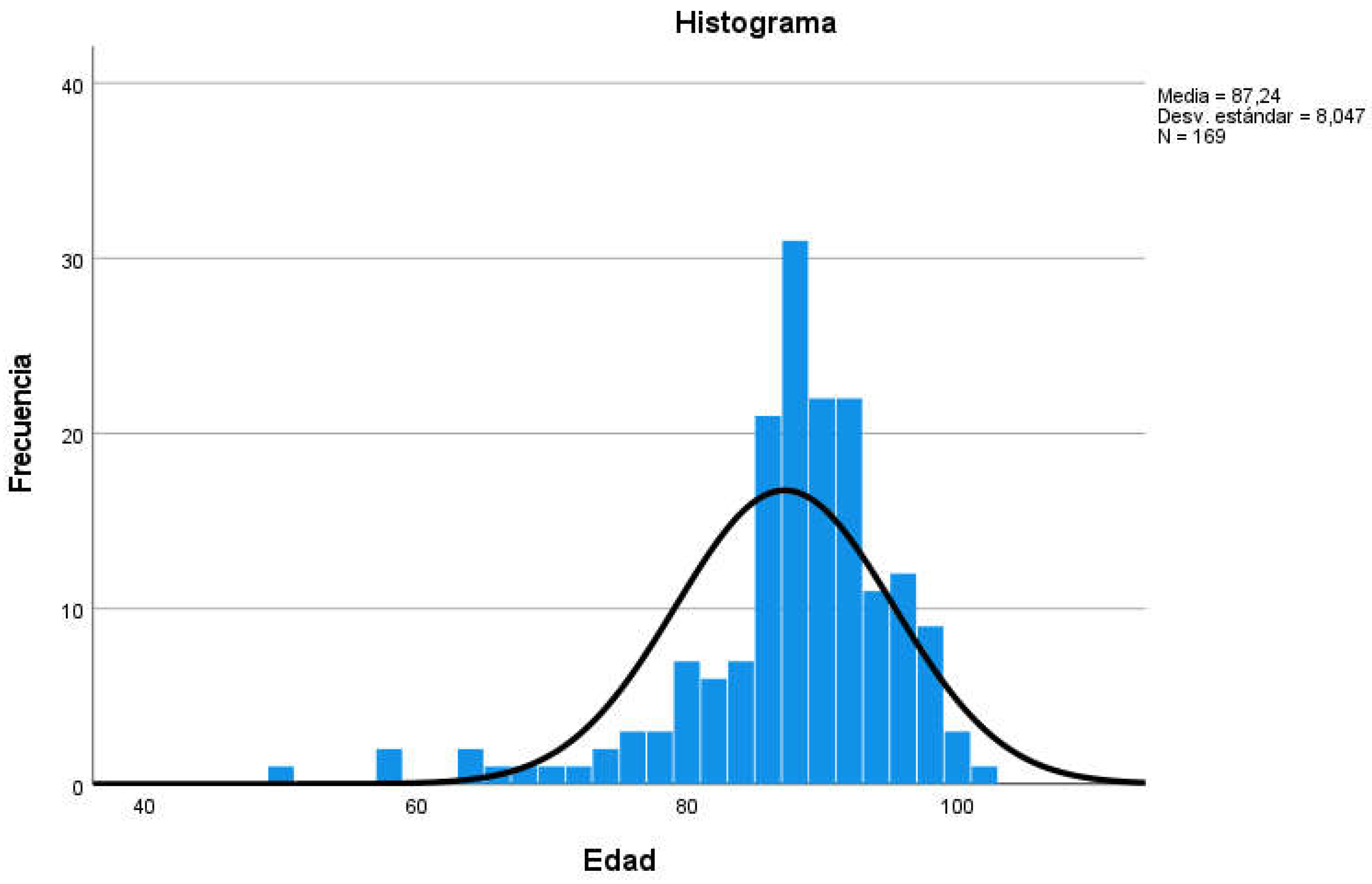
Figure 1); the mean age of participants was 87.24 years (Standard Deviation SD=8,047, min=50, max=101, range=51; see
Table 3 and
Figure 2).
68.6% of participants had a history of hypertension (n=116, see
Table 4) and 20.1% of AF (
Table 5 and
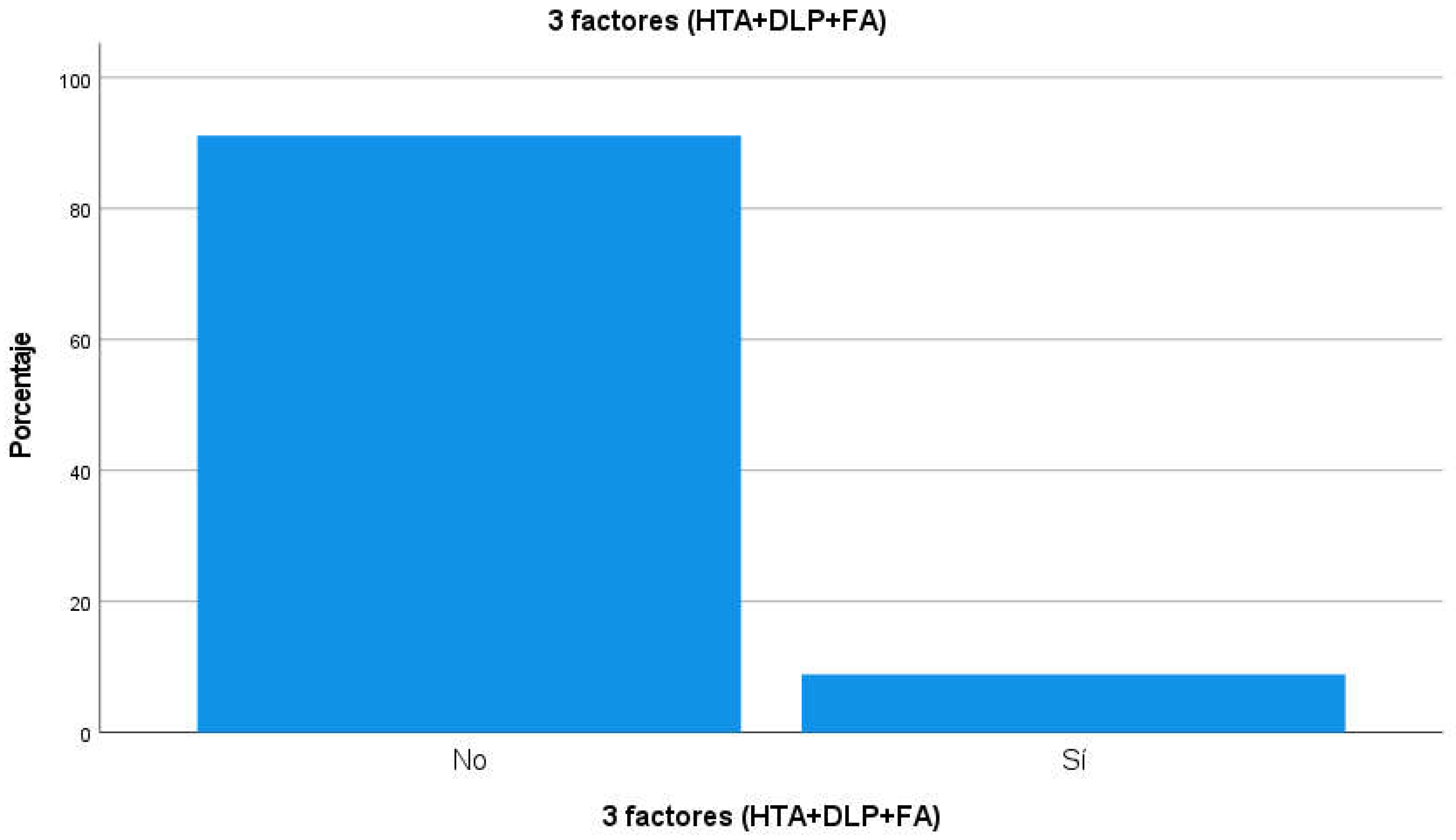
Table 6). The prevalence of participants presenting with hypertension, PLD and AF at the same time was 15 people (8.9% of the total participants, see
Table 7 and
Figure 3). Among other pathological history of the participants, 58% had cognitive impairment or dementia (n=98), 18.3% some type of heart disease (n=31), 26.6% had diabetes (n=45), 17.2% had suffered a stroke (stroke, n=29), 21.3% had moderate or advanced chronic kidney disease (CKD, n=36), 13.6% hypothyroidism (n=23) and 7.7% chronic obstructive pulmonary disease (n=13);
Table 8 shows the distribution of medical pathological history of interest other than AF, PLD and hypertension. Analysis of medication regimens shows that the mean number of oral drugs prescribed in the 165 patients from whom data were obtained was 6.94 (min=0, max=15, SD=2,982; p=0.000,
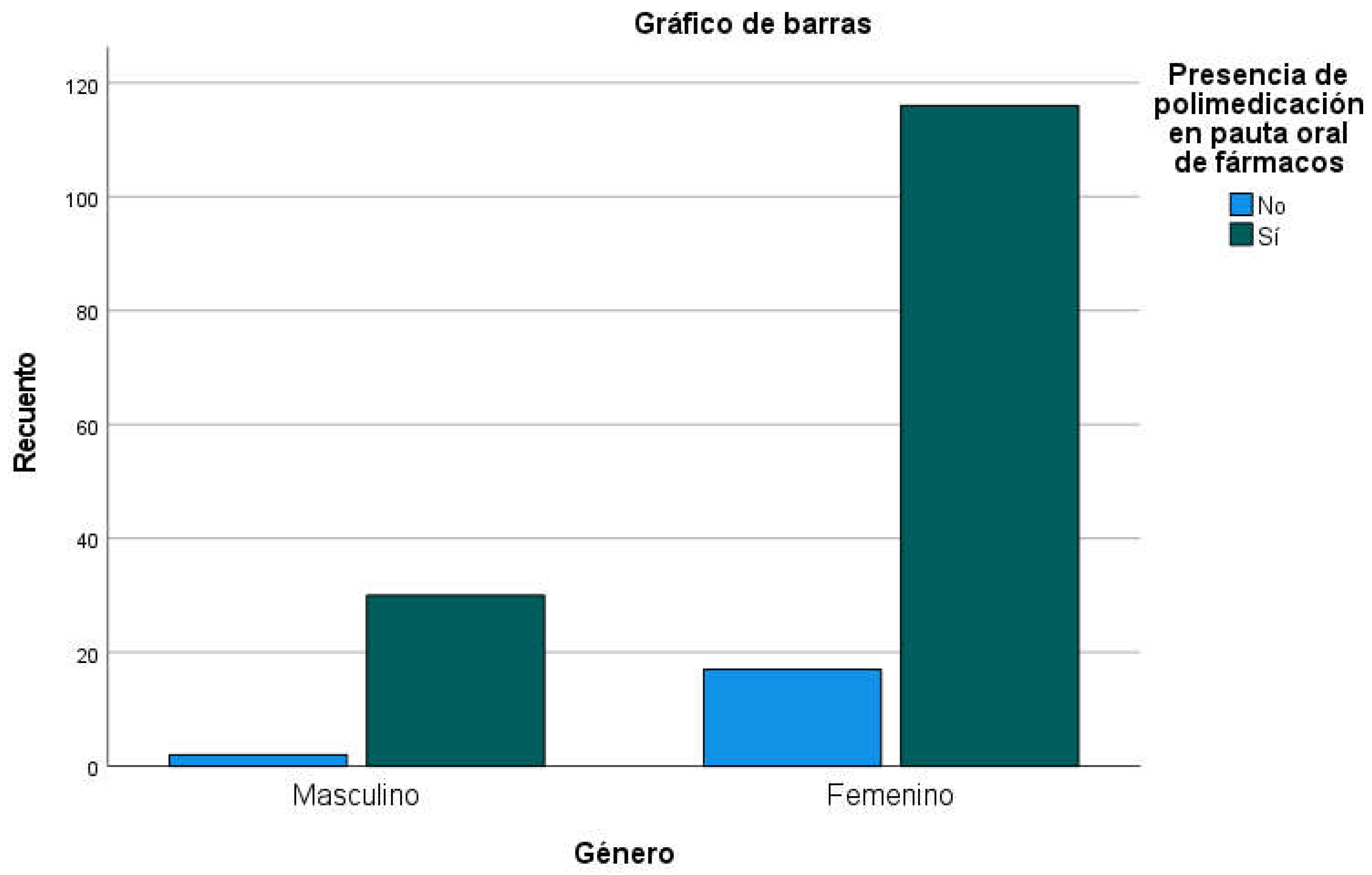
Table 9), with the presence of polymedication (defined as 4 or more orally prescribed drugs) in 88.5% of residents (men: 93.8%; women: 87.2%) without significant differences between both genders (
Table 10,
Figure 4). By sex, women had a mean of 6.65 oral drugs prescribed (SD=2,847) and men 8.09 (SD=3,273), with significant differences between genders (p=0.013;
Table 11).
Analyzing both genders separately, it can be seen that women had a mean age of 88.81 years (SD=5.953) while the mean age in men was 81.23 years (SD=11.596), showing a statistically significant difference between the groups (p<0.0001). 62.9% of men (n=22) and 70.1% of women (n=94) had a history of hypertension; 40% of men (n=14) and 48.5% of women (n=65) had a history of PLD; 14.3% of men (n=5) and 21.6% of women (n=29) had AF, with no statistically significant differences between the sexes in the prevalence of the three circumstances studied.
17.1% of men (n=6) and 14.9% of women (n=20) were under treatment with oral anticoagulants (OAC), while 31.4% of men (n=11) and 25.4% of women (n=34) were on AA P treatment. There were also no statistically significant differences on the prescription of OAC or AAP. Thus, in total 42.0% (n=71) was following treatment with OAC or PAA: 48.6% of men (n=17) and 40.3% of women (n=54), with no statistically significant differences between the sexes.
Prescription of Antiplatelets, Statins and Antihypertensives
Among the 34 people with AF, only 26.5% were on PAA treatment (n=9), while the remaining 73.5 % were not prescribed (n=25); in contrast, 26.7% of people without AF were on antiplatelet therapy, mainly for other cardiac causes. According to the history of AF, in twenty-five cases (73.5% of the total) the prescription of antiplatelet agents could be written down and were not prescribed.
A total of statins were prescribed in 17.2% of participants (n=29): in 25.7% of men (n=9) and in 14.9% of women (n=20), with no statistically significant differences between the sexes. Among people with a history of PLD, only 24.1% were following lipid-lowering therapy (n=19, for good control with diet and physical exercise in the remaining 75.9%), while 11.1% of people without PLD were following statin treatment (n=10, possibly for another cardiac cause; p<0.021).
In total, antihypertensives of the ACE inhibitor or ARB-II families were prescribed in 33.7% of participants (n=57): in 42.9% of men (n=15) and in 31.3% of women (n=42), with no statistically significant differences between the sexes. Among those with a history of hypertension, only 44% were following antihypertensive treatment with one of these two families of drugs (n=51) while 56% did not have them scheduled (because they were prescribed another type of drug or because they had good control of blood pressure through non-pharmacological methods), while 11.3 % of people without hypertension were following antihypertensive treatment with ACE inhibitors or ARB-II (n=6, possibly for another cardiac causes; p<0.001).
Number of People With All 3 Factors (HTA+DLP+FA)
Of the total of 169 people included, 8.9% had the three risk factors combined (HTN+PLD+AF, n=15), 14 women and one man (10.4% of women and 2.9% of men, with no statistically significant differences between the sexes, see
Table 12); the mean age of people with all three factors was 88.20 years (SD=
Table 12), without statistically significant differences with the rest of the participants. There were also no significant differences between people with or without the three factors studied in relation to most of the other pathologies analyzed (cognitive impairment, diabetes, chronic kidney disease, hypothyroidism or chronic obstructive pulmonary disease).
Significant differences were observed between people with the three factors studied and the presence of heart disease (p=0.025): 42.9% of the 14 women with all three factors had heart disease (n=6), while only 15.8% of the 120who did not have all three factors had some heart disease (n=19). In the case of men, it was not possible to study because only one man was represented, but when both sexes were analyzed together, significant differences were observed in the association between heart disease and the presence of the three factors analyzed together (p=0.035): 40% of the 15 people who presented all three factors had a history of heart disease (n=6, all of them women).
Significant differences were also observed between people with the three factors studied and a history of cerebral vascular accident (CVA). Women with all three factors had a longer history of stroke: 35.7% of the 14 women with all three factors had had a stroke (n=5), while only 12.5% of the 120 women who did not have all three factors (n=15; p=0.037) had suffered a stroke. In the case of men, it was not possible to study because only one man was represented, but when both sexes were analyzed together, significant differences were observed in the association between stroke and the presence of the three factors analyzed together (p = 0.025): 40% of the 15 people who presented the three factors had a history of stroke (n = 6, five women and one man).
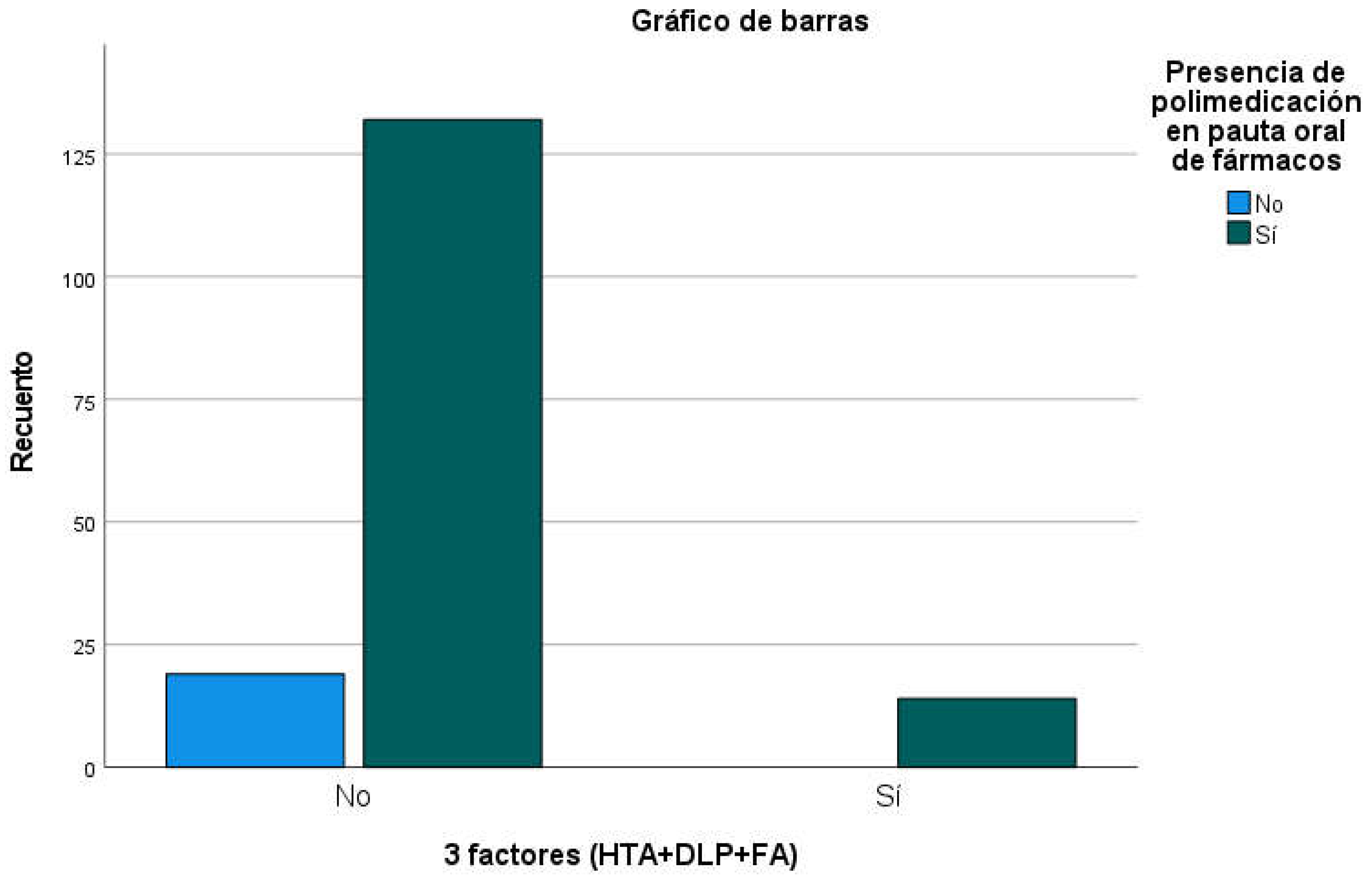
There were also no significant differences in the percentage of presence of polymedication between people with all three factors and the rest of the participants (p=0.167;
Table 13); it should be noted that 100% of the 15 people with all three factors had polymedication while 87.4% of people without all three factors had 4 or more oral drugs prescribed (
Figure 5). Among the participants who had prescribed drugs from the 3 groups (AAP + Statins + ACE inhibitors or ARBs), the mean number of oral drugs prescribed was 8.60 (SD=3,209), while in the rest of the participants it was 6.89 oral drugs (SD=2,971;
Table 14); although the differences were not significant (p=0.207), a large effect size was observed between the two groups (Cohen's d=0.575).
Among the 15 people who presented the three factors studied (HTN, PLD and AF), only 4 (26.66%, all of them women, see
Table 15 three factors (26.7%, p<0.001) while the rest did not (n=11, 73.34%) . When adding to this figure the people with indication of the three groups of drugs to which an OAC had been indicated in substitution of a AAG, only one person was in this condition, with which finally there were 5 people (33.3% of the total, all of them women) with possible indication and prescription of AAP or OAC, statin and antihypertensive of the ACE inhibitor or ARB-II families (p<0.001, see
Table 16).
If in the 5 people who had a prescription of the 3 drugs studied (AAP + Statin + ACE INHIBITOR-ARA-II) a polypill containing the three components in a single capsule or tablet was prescribed, the average number of drugs prescribed would go from 8.60 (SD = 3.209) to 6.60 (SD = 3.209), showing a reduction of l 23.25% (see
Table 17 ); although the differences are not significant by the number of participants in these conditions, the effect size can be considered strong (d of Cohen=0.575).
Discussion
The 169 people included in the final sample, comprising all residents of the participating centers, followed the usual distribution of approximately 80% women and 20% men, with an age measure of 87 years, women being on average 7.6 years older than men, significant differences that follow the usual proportion of life expectancy in our country. No significant differences were observed between genders in terms of the prevalence of the most important pathologies for our study, with 68.6% of hypertension, 46.7% of PLD and 20.1% of AF; A significantly higher incidence of heart disease and stroke was observed among those women with all three factors (42.9% and 35.7%, respectively) than in the rest of the participants (15.8% and 12.5%, respectively); Possibly in men the proportion was similar, but it cannot be estimated because only one had all three factors. Or after pathologies of high prevalence were cognitive impairment (58%), diabetes (26.6%), CKD (21.3%), heart disease (18.3%) or stroke (17.2%) among other antecedents, showing a distribution of frequent polypathologies in geriatric residential centers. Considering only oral drugs, the rate of polymedication was also high (88.5%), with an overall mean of 6.94 drugs prescribed per person (SD=2,982,
Table 9). No significant differences were found between the sexes with respect to the prescription of AAP or OAC.
A low rate of prescription of antiplatelet agents is seen among people with an indication for AF, with just over a quarter of people with indication and prescription of this group of drugs. A low rate of statin prescription is also observed among people with a history of PLD (only 17.2%), but this may be due to lack of indication by age or by good lipemic control through diet and physical exercise. In the case of ACE inhibitors or ARBs of the ACE inhibitor or ARB families, these were prescribed in 44% of patients with a history of hypertension, also due to good blood pressure control through non-pharmacological methods or by the prescription of other types of antihypertensive drugs (probably diuretics, calcium channel blockers or beta-blockers). The most recent evidence[36] shows that the indication of ASA as primary prevention does not increase disability-free life expectancy in people over 70 years of age, that the use of statins as primary prevention [37] in people with limited life expectancy or frailty may not bring r benefits or that[38] s prescription of an antihypertensive drug (in people without associated heart disease) does not reduce the blood pressure control figures at 12 weeks in people over 80 years of age with prescription of various antihypertensive drugs, all of them reasons that could justify a process of deprescription in [39]elderly patients in primary prevention.
8.9% of the people analyzed presented the three main factors analyzed (HTN, DLP and AF), making a total of 15, without statistically significant differences between both sexes; The mean age in these patients was 88.2 years, with no statistically significant differences with the rest of the population studied. Significant differences were observed in the presence of heart disease between women with all three risk factors present (42.9%) and those who did not have all three factors at the same time (15.8%); significant differences were also observed regarding stroke history among women with or without all three factors (35.7%) (12.5%). As there was only one man in the group with all three factors present, the analysis could not be performed in the male gender. No significant differences were detected with the presence of other pathological antecedents (other than heart disease or stroke) or polymedication.
Only 26.7% of the participants with the three factors present had prescribed drugs against all of them, either for reasons of undertreatment or for lack of indication or for sufficient control with non-pharmacological measures. However, the control of hypertension or PLD in this type of patients can hardly explain this low rate of joint treatment, and may indicate a certain rate of undertreatment in this group of patients.
The rate of polymedication in these patients is very high, and any measure to simplify prescribing and administration guidelines should be welcomed, especially if the objectives are optimization, safety, adherence and containment of pharmaceutical expenditure. Polypharmacy, as a result of multimorbidity, has been shown to be considerably high in geriatrics and may contribute to medication non-adherence due to the significant burden on the patient's life. In this environment, the appearance and use of [40] drugs with fixed associations can be a tool of great value; in this case, the use of a polypill containing the active ingredients for the control of hypertension, PLD and AF is a tool capable of simplifying drug administration guidelines, to increase adherence to prescriptions and can also be a tool to maximize the optimization of prescriptions: surely some of the drugs for the control of the factors analyzed are not prescribed by the doctors in charge due to the presence of polymedication in these multipathological patients; doctors Responsible may not prescribe all the drugs indicated by a concern about increased polymedication. It is possible that the low prescription rates detected in patients with a history of AF, PLD or hypertension may be due to a concern on the part of prescribing physicians not to excessively increase polymedication, especially for pathologies with long latency time regarding their consequences on health. The polypill in our sample could reduce from 8.60 to 6.60 the number of medications in people who already had the three prescribed drugs, enabling a reduction of 23.25% of polymedication in these patients, simplifying the regimen of administration of the medication and allowing to increase adherence and the prescription rates to all those patients who have it indicated, without increasing the pharmaceutical expenditure by the equivalence in the price of the three drugs separately with respect to the polypill. The use of a polypill to increase patient adherence, especially in diseases such as AF, PLD or hypertension could not only be cost-effective, but also reduce the number of hospitalizations, medical visits and premature deaths.[2]
Conclusions
In the sample studied, a significantly higher incidence of heart disease and cerebral vascular accidents is seen in those people with the three factors studied (Atrial Fibrillation, Dyslipidemia and Arterial Hypertension). A low rate of prescription of Antiplatelet medications is also detected among people with Atrial Fibrillation. Low rates of prescription of statins and antihypertensive ACE inhibitors or ARBs are also observed, although these may be influenced by good control with non-pharmacological measures or by the use of other groups of drugs.
The use of drugs in association such as the polypill for the three pathologies studied (Atrial Fibrillation, Dyslipidemia and Arterial Hypertension) can increase the suitability of prescriptions in people with polypathology simplifying administration, increasing adherence to pharmacological treatments without increasing pharmaceutical expenditure and reducing the number of visits to health providers, hospital admissions and avoidable emergencies.
Conflicts of Interest
No conflict of interest is declared.
References
- Esquirol Caussa J, Herrero Vila E, Sánchez Aldeguer J. Use of a Polypill to reduce Cardiovascular Risk Factors: Review and use Guide. 2022; Available from: https://www.preprints.org/manuscript/202203.0404/v2.
- Khan R, Socha-Dietrich K. Investing in medication adherence improves health outcomes and health system efficiency: Adherence to medicines for diabetes, hypertension, and hyperlipidaemia. OECD Health Working Papers [Internet]. 2018;(105). Available from:. [CrossRef]
- Rao S, Jamal Siddiqi T, Khan MS, Michos ED, Navar AM, Wang TJ, et al. Association of polypill therapy with cardiovascular outcomes, mortality, and adherence: A systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis. 2022 Jul;73:48–55. [CrossRef]
- Fuster V, Sanz G. Fixed-dose compounds in the secondary prevention of ischemic heart disease. Rev Esp Cardiol. 2011;64(SUPPL. 2):3–9. [CrossRef]
- Liuzzo G, Patrono C. A SECURE polypill as a strategy at the heart of secondary prevention. Eur Heart J [Internet]. 2022 Sep 15 [cited 2022 Oct 12];1–2. Available from: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehac518/6711731. [CrossRef]
- Sanz G, Fuster V. Maximizing Therapeutic Envelope for Prevention of Cardiovascular Disease: Role of Polypill. Mount Sinai Journal of Medicine. 2012;79:683–8. [CrossRef]
- Armstrong PW, McAlister FA. Searching for Adherence: Can We Fulfill the Promise of Evidence-Based Medicines? J Am Coll Cardiol. 2016;68(8):802–4. [CrossRef]
- Castellano JM, Sanz G, Peñalvo JL, Bansilal S, Fernández-Ortiz A, Alvarez L, et al. A polypill strategy to improve adherence. J Am Coll Cardiol. 2014;64(20):2071–82. [CrossRef]
- Huynh K. Polypills for the secondary prevention of MACE. Nat Rev Cardiol. 2022 Sep 20; [CrossRef]
- Castellano JM, Pocock SJ, Bhatt DL, Quesada AJ, Owen R, Fernandez-Ortiz A, et al. Polypill Strategy in Secondary Cardiovascular Prevention. New England Journal of Medicine. 2022 Sep 15;387(11):967–77. [CrossRef]
- González-Juanatey JR, Cordero A, Castellano JM, Masana L, Dalmau R, Ruiz E, et al. Reduction of cardiovascular events in patients with cardiovascular disease with the CV-polypill: a retrospective and propensity score matching study. In: European Society of Cardiology, editor. ESC Congress 2021 - The Digital Experience [Internet]. 2021. Available from: https://esc365.escardio.org/presentation/237242.
- Brainin M, Teuschl Y, Martins S. Polypill: Benefits Seen for Stroke and Other Outcomes. Stroke. 2022 Aug;53(8):2695–701. [CrossRef]
- González-Juanatey JR, Mostaza JM, Lobos JM, Abarca B, Llisterri JL, Baron-Esquivias G, et al. Consensus document for the use of the Polypill in the secondary prevention of cardiovascular disease. Med Clin (Barc) [Internet]. 2017;148(3):139.e1-139.e15. Available from:. [CrossRef]
- González-Juanatey JR, Tamargo J, Torres F, Weisser B, Oudovenko N. Pharmacodynamic study of the cardiovascular polypill. Is there any interaction among the monocomponents? Revista Española de Cardiología (English Edition). 2021;74(1):51–8. [CrossRef]
- Coca A, Kreutz R, Manolis AJ, Mancia G. A practical approach to switch from a multiple pill therapeutic strategy to a polypill-based strategy for cardiovascular prevention in patients with hypertension. J Hypertens. 2020;38(10):1890–8. [CrossRef]
- AEMPS. Technical Data Sheet - Trinomy [Internet]. UP. Madrid. Available from: https://cima.aemps.es/cima/pdfs/es/ft/78575/78575_ft.pdf.
- Abushouk AI, Sayed A, Munir M, Ghanem E, Abdelfattah O, Michos ED, et al. Fixed-Dose Combination (Polypill) for Cardiovascular Disease Prevention: A Meta-Analysis. Am J Prev Med. 2022 Sep;63(3):440–9. [CrossRef]
- González-Juanatey JR, Mostaza JM, Lobos JM, Abarca B, Llisterri JL. A Step Ahead in Secondary Prevention of Cardiovascular Risk. Consensus Document on Clinical Use of the Polypill. Rev Esp Cardiol. 2016;69(6):547–50. [CrossRef]
- Mohamed MMG, Osman M, Kheiri B, Saleem M, Lacasse A, Alkhouli M. Polypill for cardiovascular disease prevention: Systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2022 Aug;360:91–8. [CrossRef]
- Castellano JM, Bueno H, Fuster V. The cardiovascular polypill: Clinical data and ongoing studies. Int J Cardiol. 2015;201:S8–14. [CrossRef]
- Jahangiri R, Rezapour A, Malekzadeh R, Olyaeemanesh A, Roshandel G, Motevalian SA. Cost-effectiveness of fixed-dose combination pill (Polypill) in primary and secondary prevention of cardiovascular disease: A systematic literature review. Vol. 17, PLoS ONE. Public Library of Science; 2022. [CrossRef]
- Barrios V, Kaskens L, Castellano JM, Cosin-Sales J, Ruiz JE, Zsolt I, et al. Usefulness of a cardiovascular polytablet in the treatment of patients in secondary prevention in Spain: a cost-effectiveness study. Rev Esp Cardiol. 2017;70(1):42–9. [CrossRef]
- Gómez-Álvarez E, Verdejo J, Ocampo S, Ponte-Negretti CI, Ruíz E, Ríos MM. The CNIC-polypill improves atherogenic dyslipidemia markers in patients at high risk or with cardiovascular disease: Results from a real-world setting in Mexico. IJC Heart and Vasculature. 2020;29. [CrossRef]
- Lafeber M, Spiering W, Visseren FLJ, Grobbee DE, Bots ML, Stanton A, et al. Impact of switching from different treatment regimens to a fixed-dose combination pill (polypill) in patients with cardiovascular disease or similarly high risk. Eur J Prev Cardiol. 2017;24(9):951–61. [CrossRef]
- Marzal D, Rodríguez Padial L, Arnáiz JA, Castro A, Cosín J, Lekuona I, et al. Use of the cardiovascular polypill 40 mg in secondary cardiovascular prevention. Clinica e Investigacion en Arteriosclerosis. 2018;30(5):240–7. [CrossRef]
- Mazón-Ramos P. Cardiovascular risk in the 21st century: Identifying risk in primary prevention. Controlling risk in secondary prevention. Rev Esp Cardiol. 2012;65(SUPPL.2):3–9. [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J [Internet]. 2021;42(25):2439–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34120177. [CrossRef]
- AEMPS. Data sheet - Ramipril [Internet]. Madrid; 2019. Available from: https://cima.aemps.es/cima/pdfs/es/ft/67932/67932_ft.pdf.
- Sans S, Fitzgerald AP, Royo D, Conroy R, Graham I. Calibration of the cardiovascular irrigation SCORE table for Spain. Rev Esp Cardiol. 2007;60(5):476–85. [CrossRef]
- Buitrago Ramírez F, Cañón-Barroso L, Díaz-Herrera N, Cruces-Muro E, Escobar-Fernández M, Serrano-Arias JM. Comparison of the REGICOR and SCORE tables for the classification of cardiovascular risk and the identification of patients who are candidates for lipid-lowering or antihypertensive treatment. Rev Esp Cardiol. 2007;60(2):139–47. [CrossRef]
- González-Juanatey JR. Update of the consensus document on the clinical use of polytablet: new dose with atorvastatin 40 mg. Journal E [Internet]. 2018;71(7):595–7. Available from: https://www.revespcardiol.org/es-pdf-S030089321830071X. [CrossRef]
- Castellano JM, Verdejo J, Ocampo S, Rios MM, Gómez-Álvarez E, Borrayo G, et al. Clinical Effectiveness of the Cardiovascular Polypill in a Real-Life Setting in Patients With Cardiovascular Risk in Mexico: The SORS Study. Arch Med Res. 2019;50(1):31–40. [CrossRef]
- Masjuan J, Gállego J, Aguilera JM, Arenillas JF, Castellanos M, Díaz F, et al. Use of cardiovascular polypills for the secondary prevention of cerebrovascular disease. Neurologia. 2021;36(1):1–8. [CrossRef]
- Ros-Castelló V, Natera-Villalba E, Gómez-López A, Sánchez-Sánchez A, Chico-García JL, García-Madrona S, et al. Use of the Cardiovascular Polypill in Secondary Prevention of Cerebrovascular Disease: A Real-Life Tertiary Hospital Cohort Study of 104 Patients. Cerebrovasc Dis Extra. 2020;10(3):166–73. [CrossRef]
- Gómez-Álvarez E, Verdejo J, Ocampo S, Ruiz E, Martinez-Rios MA. Reaching blood pressure guideline targets with the CNIC polypill in patients with a previous cardiovascular event in Mexico: A post hoc analysis of the SORS study. Future Cardiol. 2019;16(1):53–60. [CrossRef]
- Morgan-Gouveia M, Drago K, Harris P, Sheffrin M. Geriatric update 2022: Preventing Alzheimer disease and more. Vol. 89, Cleveland Clinic journal of medicine. NLM (Medline); 2022. p. 617–24. [CrossRef]
- Ryan J, Storey E, Murray AM, Woods RL, Wolfe R, Reid CM, et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020 Jul 21;95(3):E320–31. [CrossRef]
- Yourman LC, Cenzer IS, Boscardin WJ, Nguyen BT, Smith AK, Schonberg MA, et al. Evaluation of Time to Benefit of Statins for the Primary Prevention of Cardiovascular Events in Adults Aged 50 to 75 Years: A Meta-Analysis. JAMA Intern Med. 2021 Feb 1;181(2):179–85. [CrossRef]
- 26 May; Sheppard JP, Burt J, Lown M, Temple E, Lowe R, Fraser R, et al. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients with Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial. JAMA - Journal of the American Medical Association. 2020 May 26;323(20):2039–51. [CrossRef]
- Carvalho M, Almeida IF. The Role of Pharmaceutical Compounding in Promoting Medication Adherence. Vol. 15, Pharmaceuticals. MDPI; 2022. [CrossRef]
Figure 1.
Gender of participants.
Figure 1.
Gender of participants.
Figure 2.
Age distribution of participants.
Figure 2.
Age distribution of participants.
Figure 3.
Prevalence of participants with a history of hypertension, PLD and AF.
Figure 3.
Prevalence of participants with a history of hypertension, PLD and AF.
Figure 4.
Oral polymedication, by gender (absolute numbers).
Figure 4.
Oral polymedication, by gender (absolute numbers).
Figure 5.
Presence of polymedication in people with or without all three factors.
Figure 5.
Presence of polymedication in people with or without all three factors.
Table 1.
Center of origin of the participants.
Table 1.
Center of origin of the participants.
| |
Frequency |
Percentage |
| Valid |
1 |
25 |
14,8 |
| 2 |
87 |
51,5 |
| 3 |
32 |
18,9 |
| 4 |
25 |
14,8 |
| Total |
169 |
100,0 |
Table 2.
Gender of participants.
Table 2.
Gender of participants.
| |
|
Frequency |
Percentage |
| Valid |
Male |
35 |
20,7 |
| Female |
134 |
79,3 |
| Total |
169 |
100,0 |
Table 3.
Age distribution of participants.
Table 3.
Age distribution of participants.
| Media |
87,24 |
| St. Deviation |
8,047 |
| Rank |
51 |
| Minimal |
50 |
| Maximum |
101 |
Table 4.
Prevalence of hypertension.
Table 4.
Prevalence of hypertension.
| |
Frequency |
Percentage |
| Valid |
No |
53 |
31,4 |
| Yes |
116 |
68,6 |
| Total |
169 |
100,0 |
Table 5.
Prevalence of dyslipidemia.
Table 5.
Prevalence of dyslipidemia.
| |
Frequency |
Percentage |
| Valid |
No |
90 |
53,3 |
| Yes |
79 |
46,7 |
| Total |
169 |
100,0 |
Table 6.
Prevalence of atrial fibrillation.
Table 6.
Prevalence of atrial fibrillation.
| |
Frequency |
Percentage |
| Valid |
No |
135 |
79,9 |
| Yes |
34 |
20,1 |
| Total |
169 |
100,0 |
Table 7.
Prevalence of participants with a history of hypertension, PLD and AF.
Table 7.
Prevalence of participants with a history of hypertension, PLD and AF.
| |
Frequency |
Percentage |
| Valid |
No |
154 |
91,1 |
| Yes |
15 |
8,9 |
| Total |
169 |
100,0 |
Table 8.
Prevalence of other important pathological antecedents.
Table 8.
Prevalence of other important pathological antecedents.
| |
Frequency |
Percentage |
| Osteoporosis |
4 |
2,4 |
| TEP |
3 |
1,8 |
| HDA |
2 |
1,2 |
| HCV |
2 |
1,2 |
| Monoclonal gammopathy |
1 |
,6 |
| Hyperuricemia |
1 |
,6 |
| Intolerance to AAS |
1 |
,6 |
| Myasthenia Gravis |
1 |
,6 |
| MM |
1 |
,6 |
| MPOC |
1 |
,6 |
| Park |
1 |
,6 |
| Polycythemia Vera |
1 |
,6 |
| Psychosis |
1 |
,6 |
| TCE |
1 |
,6 |
| TVP |
1 |
,6 |
| Valvopathy |
1 |
,6 |
Table 9.
distribution of the number of oral medications prescribed.
Table 9.
distribution of the number of oral medications prescribed.
| |
N |
Minimal |
Maximum |
Average |
Standard deviation |
| Number of oral drugs prescribed |
166 |
0 |
15 |
6,94 |
2,982 |
| Valid N (per list) |
166 |
|
|
|
|
Table 10.
Table of prescribed polymedication, total and by gender.
Table 10.
Table of prescribed polymedication, total and by gender.
| |
Presence of polymedication in oral drug regimen |
Total |
| No |
Yes |
| Gender |
Male |
Recount |
2 |
30 |
32 |
| % within Gender |
6,3% |
93,8% |
100,0% |
| Female |
Recount |
17 |
116 |
133 |
| % within Gender |
12,8% |
87,2% |
100,0% |
| Total |
Recount |
19 |
146 |
165 |
| % within Gender |
11,5% |
88,5% |
100,0% |
Table 11.
Differences in the number of oral medications prescribed, by gender.
Table 11.
Differences in the number of oral medications prescribed, by gender.
| |
Gender |
N |
Average |
Standard deviation |
Standard average error |
| Number of oral drugs prescribed |
Male |
33 |
8,09 |
3,273 |
,570 |
| Female |
133 |
6,65 |
2,847 |
,247 |
Table 12.
Cross table Gender*3 factors (HTA+DLP+FA).
Table 12.
Cross table Gender*3 factors (HTA+DLP+FA).
| |
3 factors (HTA+DLP+FA) |
Total |
| No |
Yes |
| Gender |
Male |
Recount |
34 |
1 |
35 |
| % within Gender |
97,1% |
2,9% |
100,0% |
| Female |
Recount |
120 |
14 |
134 |
| % within Gender |
89,6% |
10,4% |
100,0% |
| Total |
Recount |
154 |
15 |
169 |
| % within Gender |
91,1% |
8,9% |
100,0% |
Table 13.
Cross-table of 3 factors (HTN + DLP + FA) and presence of polymedication in oral drug regimen.
Table 13.
Cross-table of 3 factors (HTN + DLP + FA) and presence of polymedication in oral drug regimen.
| |
Presence of polymedication in oral drug regimen |
Total |
| No |
Yes |
| 3 factors (HTA+DLP+FA) |
No |
Recount |
19 |
132 |
151 |
| % within 3 factors (HTA+DLP+FA) |
12,6% |
87,4% |
100,0% |
| Yes |
Recount |
0 |
14 |
14 |
| % within 3 factors (HTA+DLP+FA) |
0,0% |
100,0% |
100,0% |
| Total |
Recount |
19 |
146 |
165 |
| % within 3 factors (HTA+DLP+FA) |
11,5% |
88,5% |
100,0% |
Table 14.
Comparison of the number of medications prescribed in participants prescribed for the three drug families or without them.
Table 14.
Comparison of the number of medications prescribed in participants prescribed for the three drug families or without them.
| |
Prescription of 3 drugs (Anplatelet+Statin+ACE Inhibitor/ARA-II) |
N |
Average |
Standard deviation |
Standard average error |
| Number of oral drugs prescribed |
Yes |
5 |
8,60 |
3,209 |
1,435 |
| No |
161 |
6,89 |
2,971 |
,234 |
Table 15.
Cross-table 3 factors (HTN+DLP+FA) and Prescription of the 3 drugs (Antiagr+Statin+ACE/ARA-II), by gender.
Table 15.
Cross-table 3 factors (HTN+DLP+FA) and Prescription of the 3 drugs (Antiagr+Statin+ACE/ARA-II), by gender.
| Gender |
Prescription of 3 drugs (Antiagr+Statin+ACE INHIBITOR/ARA-II) |
Total |
| No |
Yes |
| Male |
3 factors (HTA+DLP+FA) |
No |
Recount |
34 |
|
34 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
|
100,0% |
| Yes |
Recount |
1 |
|
1 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
|
100,0% |
| Total |
Recount |
35 |
|
35 |
| % within 3 factors (HTA+DLP+FA) |
100,0% |
|
100,0% |
Female
p=0.000 |
3 factors (HTA+DLP+FA) |
No |
Recount |
120 |
0 |
120 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
0,0% |
100,0% |
| Yes |
Recount |
9 |
5 |
14 |
| % Within 3 factors (HTA+DLP+FA) |
64,3% |
35,7% |
100,0% |
| Total |
Recount |
129 |
5 |
134 |
| % Within 3 factors (HTA+DLP+FA) |
96,3% |
3,7% |
100,0% |
Total
p=0.000 |
3 factors (HTA+DLP+FA) |
No |
Recount |
154 |
0 |
154 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
0,0% |
100,0% |
| Yes |
Recount |
10 |
5 |
15 |
| % Within 3 factors (HTA+DLP+FA) |
66,7% |
33,3% |
100,0% |
| Total |
Recount |
164 |
5 |
169 |
| % within 3 factors (HTA+DLP+FA) |
97,0% |
3,0% |
100,0% |
Table 16.
Cross-table of 3 factors (HTN+DLP+FA) and Prescription of 3 drugs (Antiplatelet/ACO+Statin+ACE/ARA-II) by Gender.
Table 16.
Cross-table of 3 factors (HTN+DLP+FA) and Prescription of 3 drugs (Antiplatelet/ACO+Statin+ACE/ARA-II) by Gender.
| Gender |
Prescription of 3 drugs (Anplatelet+Statin+ACE Inhibitor/ARA-II) |
Total |
| No |
Yes |
| Male |
3 factors (HTA+DLP+FA) |
No |
Recount |
32 |
2 |
34 |
| % Within 3 factors (HTA+DLP+FA) |
94,1% |
5,9% |
100,0% |
| Yes |
Recount |
1 |
0 |
1 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
0,0% |
100,0% |
| Total |
Recount |
33 |
2 |
35 |
| % Within 3 factors (HTA+DLP+FA) |
94,3% |
5,7% |
100,0% |
Female
p=0.000 |
3 factors (HTA+DLP+FA) |
No |
Recount |
120 |
0 |
120 |
| % Within 3 factors (HTA+DLP+FA) |
100,0% |
0,0% |
100,0% |
| Yes |
Recount |
9 |
5 |
14 |
| % within 3 factors (HTA+DLP+FA) |
64,3% |
35,7% |
100,0% |
| Total |
Recount |
129 |
5 |
134 |
| % Within 3 factors (HTA+DLP+FA) |
96,3% |
3,7% |
100,0% |
Total
p=0.000 |
3 factors (HTA+DLP+FA) |
No |
Recount |
152 |
2 |
154 |
| % Within 3 factors (HTA+DLP+FA) |
98,7% |
1,3% |
100,0% |
| Yes |
Recount |
10 |
5 |
15 |
| % within 3 factors (HTA+DLP+FA) |
66,7% |
33,3% |
100,0% |
| Total |
Recount |
162 |
7 |
169 |
| % Within 3 factors (HTA+DLP+FA) |
95,9% |
4,1% |
100,0% |
Table 17.
rescribed in case of prescription of polypill to people with sign of the three drugs.
Table 17.
rescribed in case of prescription of polypill to people with sign of the three drugs.
| |
Prescription of 3 drugs (Antiagr+Statin+ACE INHIBITOR/ARA-II) |
N |
Average |
Standard deviation |
Standard average error |
| Number of oral drugs prescribed |
Yes |
5 |
8,60 |
3,209 |
1,435 |
| Number of oral drugs if prescribed polypill |
Yes |
5 |
6,60 |
3,209 |
1,435 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).