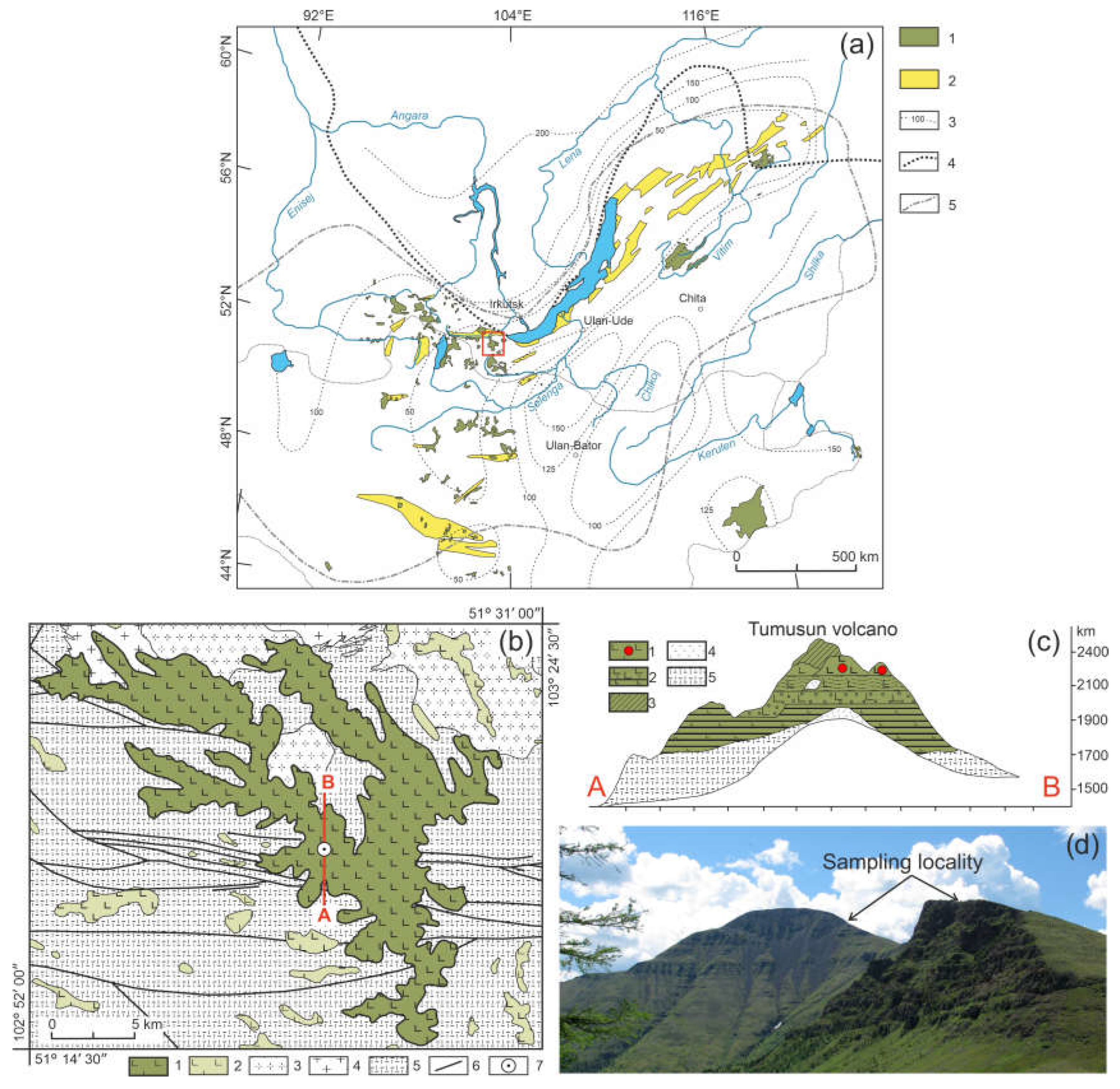
3.1. Petrography
The studied xenoliths are spinel lherzolites. They have medium-grained protogranular textures. Olivine and orthopyroxene both have similar size of ~ 2 mm, but some samples contain rare larger (up to 6 mm) orthopyroxene. Clinopyroxene has smaller size (up to 1 mm). The pyroxenes do not contain exsolution lamellae. Spinel has irregular shape and occurs in an interstitial space.
The modal composition of peridotite xenoliths is: olivine (55.4-61.0 %), orthopyroxene (22.7-28.9 %), clinopyroxene (10.8-18.6 %), spinel (1.8-3.5 %). In all of the studied samples, the minerals exhibit reaction rims, and also thin veinlets in the interstitial space. Serpentine, amphibole, phlogopite, and other hydrous minerals are absent.
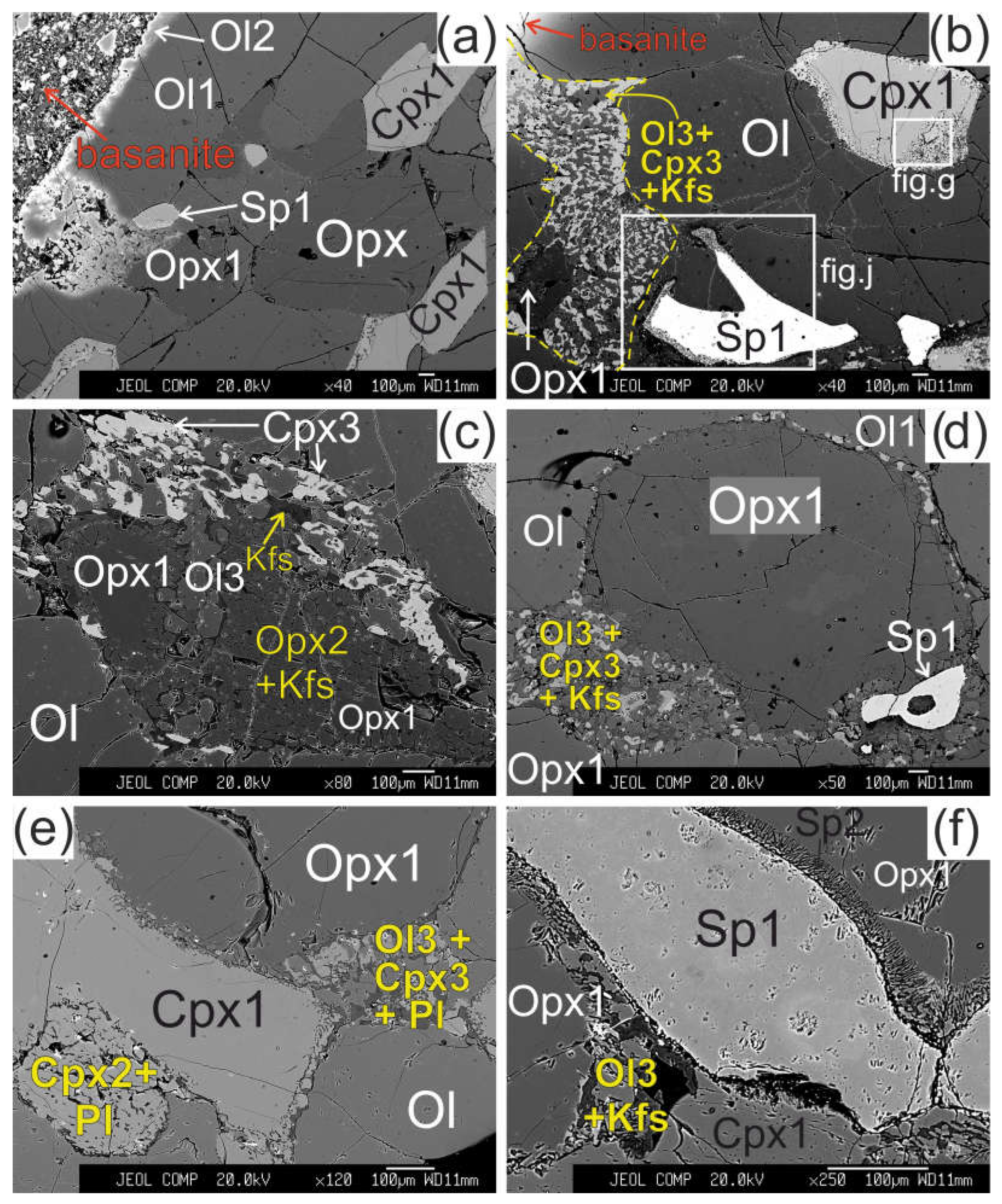
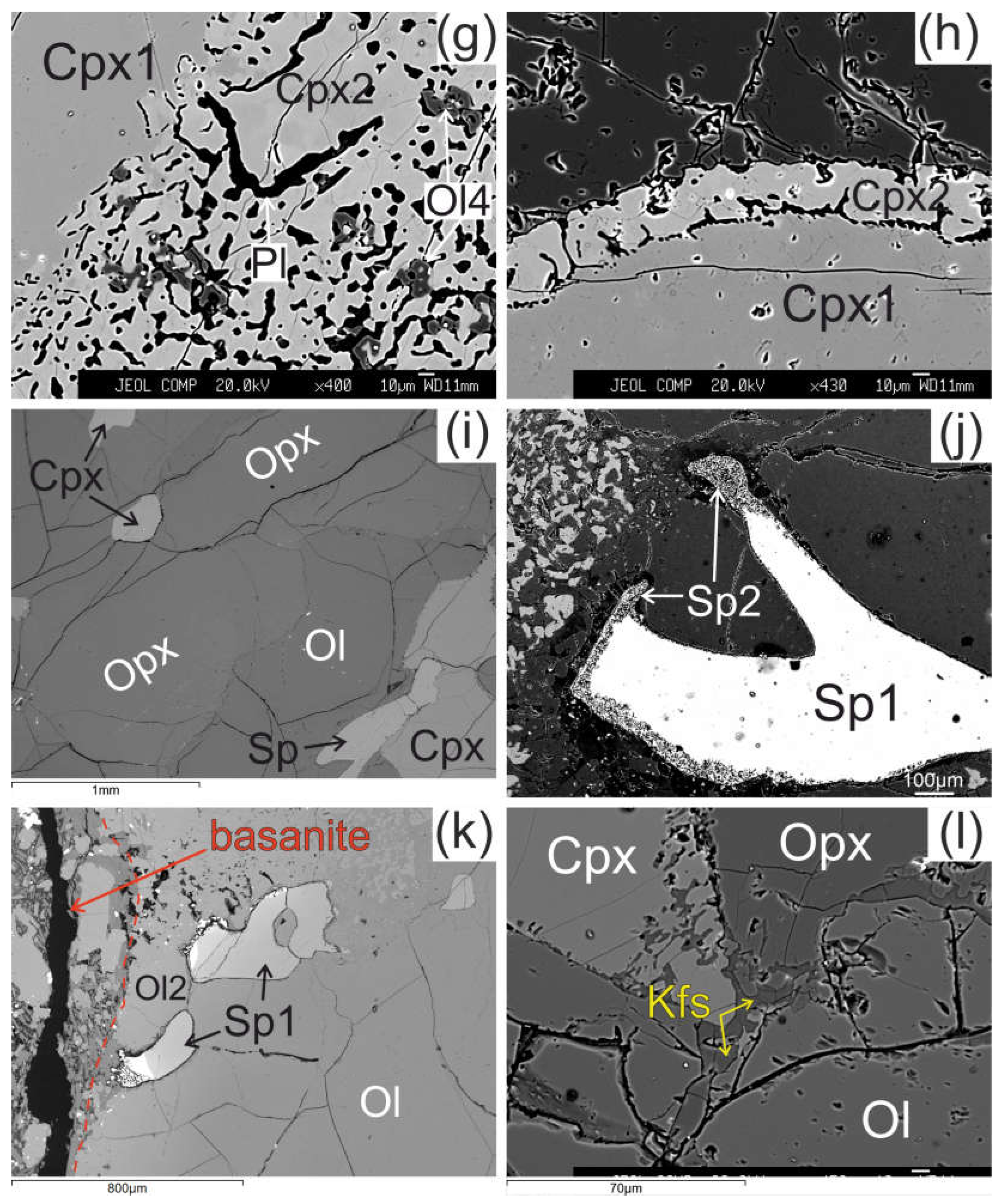
The olivine (Ol1) has a 50-100 μm wide reaction zone at the direct contact of xenolith with basanite. The reaction zone shows undulated border and is represented by olivine of different composition (Ol2) (
Figure 2a).
The orthopyroxene (Opx) in direct contact with basanite has preserved only small areas of Opx1 surrounded by reaction zone with width up to hundreds of μm (see
Figure 2b). The reaction rim of Opx1 is composed of intergrowth of small subhedral grains of olivine (Ol3) and clinopyroxene (Cpx3) and vermicular aggregates of alkali feldspar. The proportions of olivine, clinopyroxene and alkali feldspar in the reaction zone are ca. 50:40:10 %, respectively. Spinel (Sp3) and pentlandite is also found in these reaction zones. The border between reaction zone and orthopyroxene is sharp.
Orthopyroxene at distance of ~2mm from basanite/xenolith contact also has preserved areas of Opx1 surrounded by complex reaction zone, consisting of two parts. A smaller part of the reaction zone is made of Ol3, Cpx3 and alkali feldspar, similar the reaction zones at basanite/xenolith contact. A larger part of the reaction zone is composed of small orthopyroxene grains (Opx2), divided by vermicular aggregates of alkali feldspar (see
Figure 2c). This complex reaction zone is hereafter referred to as orthopyroxene reaction zone of second type.
In all samples, from basanite/xenolith contact toward the central part of xenoliths the orthopyroxene reaction zones become narrower (see
Figure 2d). In central parts of largest xenoliths, the reaction zone of orthopyroxene is absent, or developed not around whole grain and similar to an interstitial aggregate (see
Figure 2e, f, i, l). The reaction zones of orthopyroxene could have “zoning” – those parts of the reaction zone near to orthopyroxene are dominated by clinopyroxene, and those far from orthopyroxene are dominated by olivine (see
Figure 2e). In the central parts of xenoliths, the reaction zone of orthopyroxene is made only of olivine and alkali feldspar (see
Figure 2f).
The reaction zone around clinopyroxene (Cpx1) is formed by the rim of secondary clinopyroxene (Cpx2), which contains vermicular aggregates of plagioclase and less abundant small grains of olivine (Ol4) and spinel (Sp4). The transition from central part of clinopyroxene to the reaction rim is sharp (see
Figure 2g, h). These clinopyroxene microtextures in xenoliths are described as “spongy” or “sieve” [
4,
5,
9,
23]. The width of reaction zone around clinopyroxene varies and is maximal at the basanite/xenolith contact and at contact with orthopyroxene reaction zone (see
Figure 7l, 7i, 8c). In the closely located crystals of clinopyroxene, the width of reaction zone can be different (see
Figure 8c). Toward the center of xenoliths, the width of clinopyroxene reaction zone become narrow (10-30 μm;
Figure 2h) until its location only at certain crystal sides or complete disappearance (see
Figure 2i, 2l, 7c).
The reaction zone of spinel (Sp1) is represented by intergrowth of small subhedral spinel (Sp2) and alkali feldspar. Such spinel microtexture was described as “sieve” texture [
5,
23]. It is developed either at basanite/xenolith contact or at contact with orthopyroxene reaction zone or feldspar veinlets (see
Figure 2j, k). In other cases, the reaction zone of spinel is absent. This is observed at margins or in central parts of xenoliths.
In central parts of xenoliths there are thin (up to 10 μm) veinlets of alkali feldspar, located between grains of minerals which does not have reaction zones (see
Figure 2l).
3.2. Mineral Composition
Hereafter we use the term primary mineral, meaning the mineral without a reaction rim.
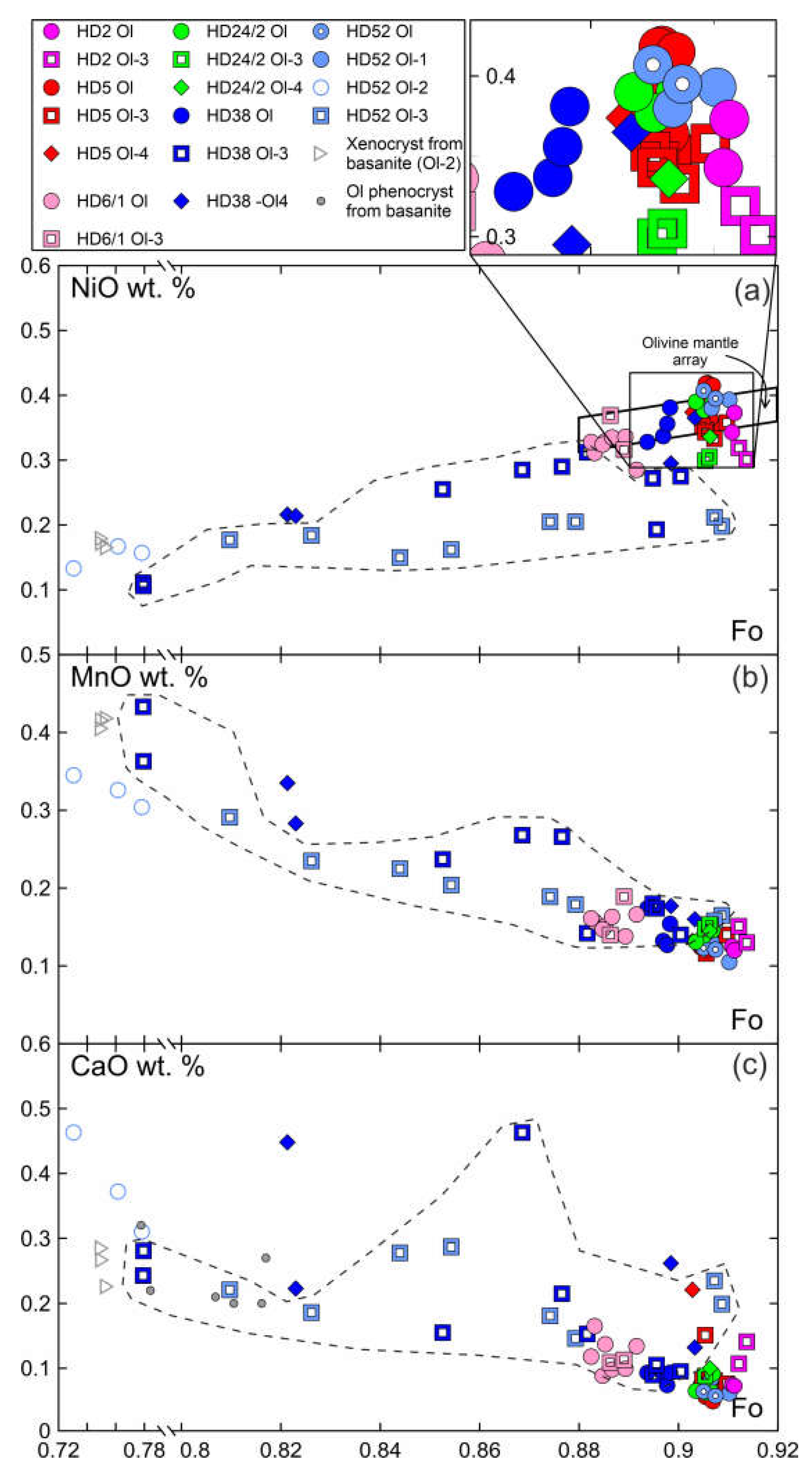
Primary olivine (Ol) is homogenous and have the same composition at margins and in the centers of xenoliths (
Figure 3, Electronic
Supplementary Table S1). The central parts of olivine grain (Ol1) rimned by Ol2 have the same composition as primary olivine. Olivine has the composition common for the fertile mantle lherzolites, and on Mg# – NiO the composition points are located within the mantle array (
Figure 3). Olivine of reaction rims (Ol2) has notably lower Mg# (0.73-0.78) and NiO (0.13-0.16 wt. %) and higher CaO (0.31-0.46 wt.%), compared to grain cores (Mg# 0.91, 0.38 wt. % NiO, 0.08 wt. % CaO) (
Figure 3, sample HD-52, Electronic
Supplementary Table S1). Ol2 is similar to olivine phenocrysts from basanite. Ol3 from orthopyroxene reaction zone near basanite/xenolite contact has low Mg# (0.78-0.81), relatively low NiO (0.1-0.18 wt. %) and relatively high CaO (0.22-0.28 wt. %), i.e., similar in composition to olivine phenocrysts in basanite. Within a single reaction zone, over a distance of ~500 μm from basanite contact inward xenoliths, there is a growth in Mg# in Ol3 until Mg# became similar to that in primary olivine. At the same time, NiO is lower and CaO is higher in Ol3 compared to the composition of primary olivine (
Figure 3, samples HD38, HD52, delineated by dashed line). In central parts of xenoliths, orthopyroxene reaction zones are narrow and their Ol3 has composition virtually similar to that of primary olivine (
Figure 3, Electronic
Supplementary Table S1).
In reaction zones of clinopyroxene at basanite/xenolith contact, Ol4 has low Mg# (0.82) and NiO (0.21 wt. %) and high CaO (0.22-0.45 wt. %) (see
Figure 3, sample HD38; Electronic
Supplementary Table S1). In clinopyroxene reaction zones inside the xenoliths, Ol 4 has Mg# close to that of primary olivine, while NiO is similar to or lower and CaO is close to or higher than that of Ol1 (see
Figure 3; Electronic
Supplementary Table S1).
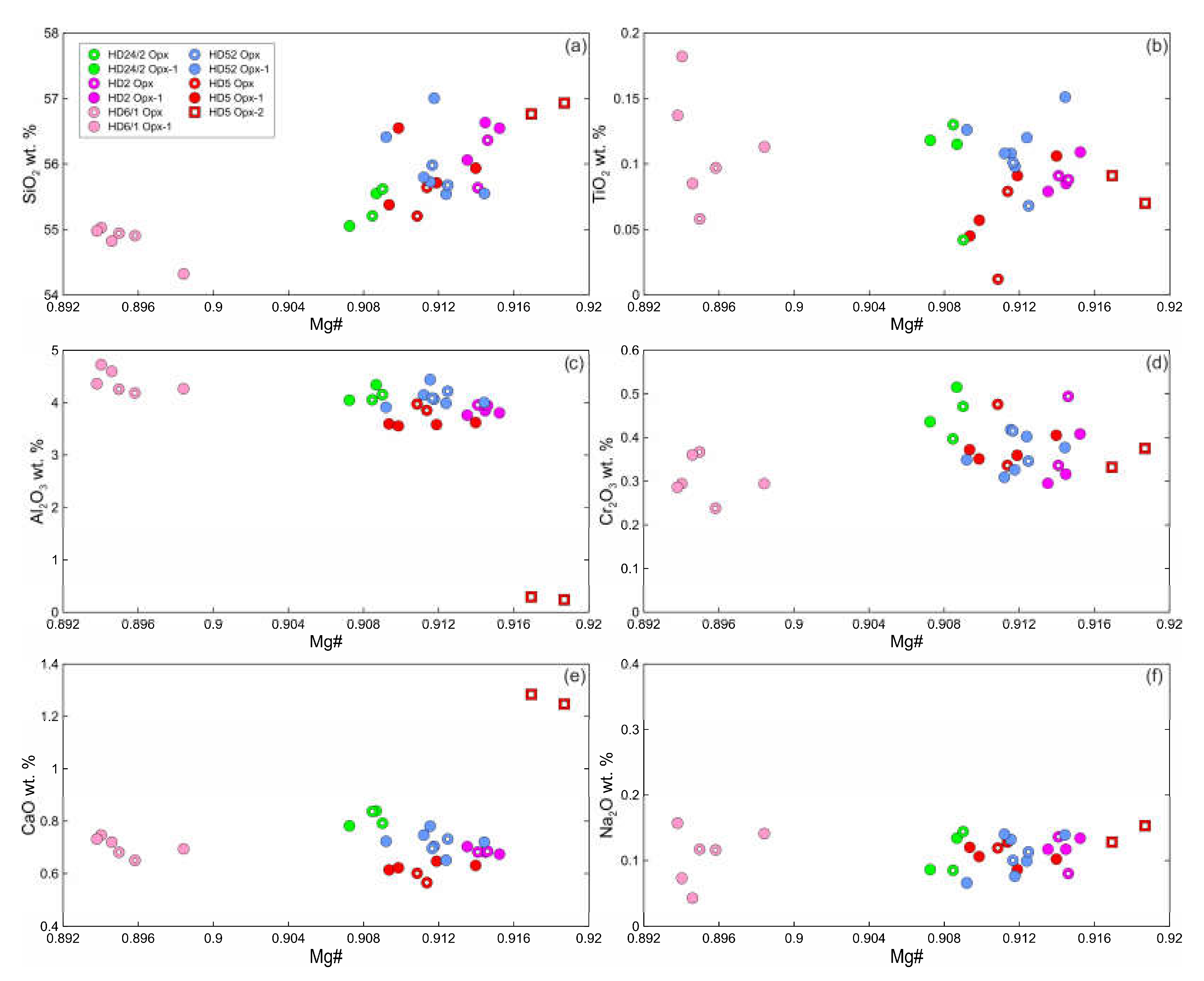
Primary orthopyroxene (Opx) is homogeneous enstatite. At xenolith margins, non-reacted Opx1 is also homogenous and have composition similar to that of primary orthopyroxene from centers of xenoliths. Opx2 from rare reaction zone (Opx2 + Kfs; see
Figure 2c) has lower Al
2O
3, higher Mg# and CaO abundance (
Figure 4; Electronic
Supplementary Table S1).
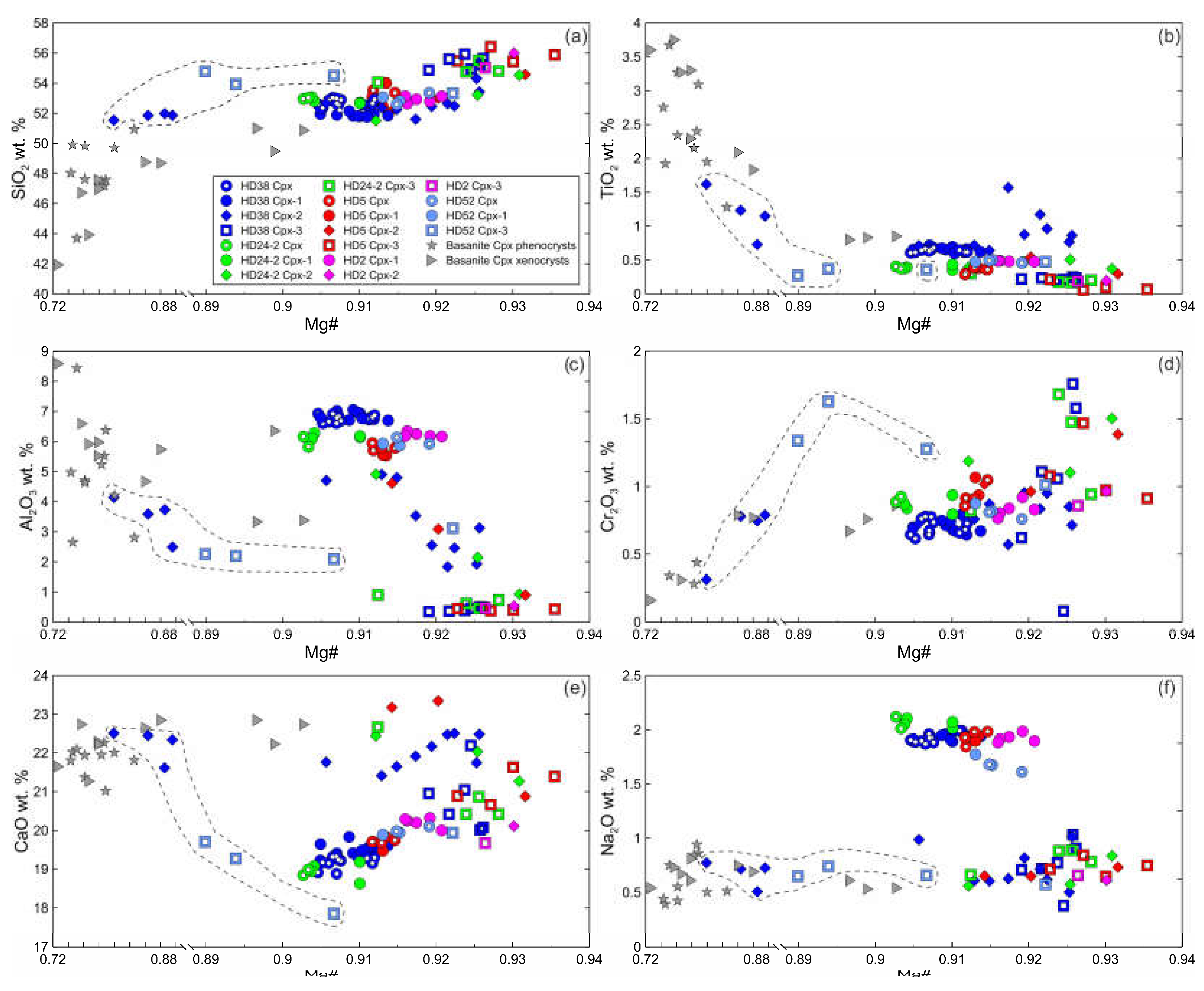
The primary clinopyroxene (Cpx) is diopside. Non-reacted parts of clinopyroxenes (Cpx1) surrounded by reaction rim are homogenous and have the same composition as primary clinopyroxenes without reaction rim (
Figure 5, Electronic
Supplementary Table S1). The secondary clinopyroxene (Cpx2) of reaction rims Cpx1 is diopside (Electronic
Supplementary Table S1). Inside xenoliths, Cpx2 has lower Al
2O
3, Al
VI/Al
IV ratio, and Na
2O and higher SiO
2 and CaO compared to Cpx1. Mg#, TiO
2 and Cr
2O
3 in Cpx2 vary from levels as in Cpx1 to higher values (
Figure 5). In outer rim of a clinopyroxene grain that is in immediate contact with basanite, Cpx2 has low Mg#, Al
2O
3, Na
2O and high TiO
2 and CaO and is similar in composition to clinopyroxene phenocrysts from basanite (
Figure 5, sample HD38, delineated by dashed line). Cpx2 from internal rim of the same grain has composition similar to that of Cpx2 located farther from basanite/xenolith contact (
Figure 5).
Cpx3 from orthopyroxene reaction zones is augite (Electronic
Supplementary Table S1). Composition of Cpx3 varies and depends on the position relative to basanite/xenolith contact. At first 100 μm from the contact, Cpx3 has lower Mg#, Al
2O
3, Na
2O, similar or lower CaO and TiO
2, and higher SiO
2 and Cr
2O
3 compared to primary clinopyroxene (see
Figure 5, sample HD52, delineated by dashed line). At distance more than 100 μm from the contact, Cpx3 has higher Mg#, SiO
2, CaO, and lower Al
2O
3, TiO
2, Na
2O compared to Cpx1 (see
Figure 5). Therefore, all secondary clinopyroxenes have less Al
2O
3 and Na
2O abundances then primary Cpx.
Primary chromium spinel is homogenous within a single sample. Secondary spinel from reaction zones of spinel (Sp2) and orthopyroxene (Sp3) exhibits higher Cr#, TiO
2, MnO, V
2O
5 and lower Mg# and NiO, compared to primary spinel (Electronic
Supplementary Table S1).
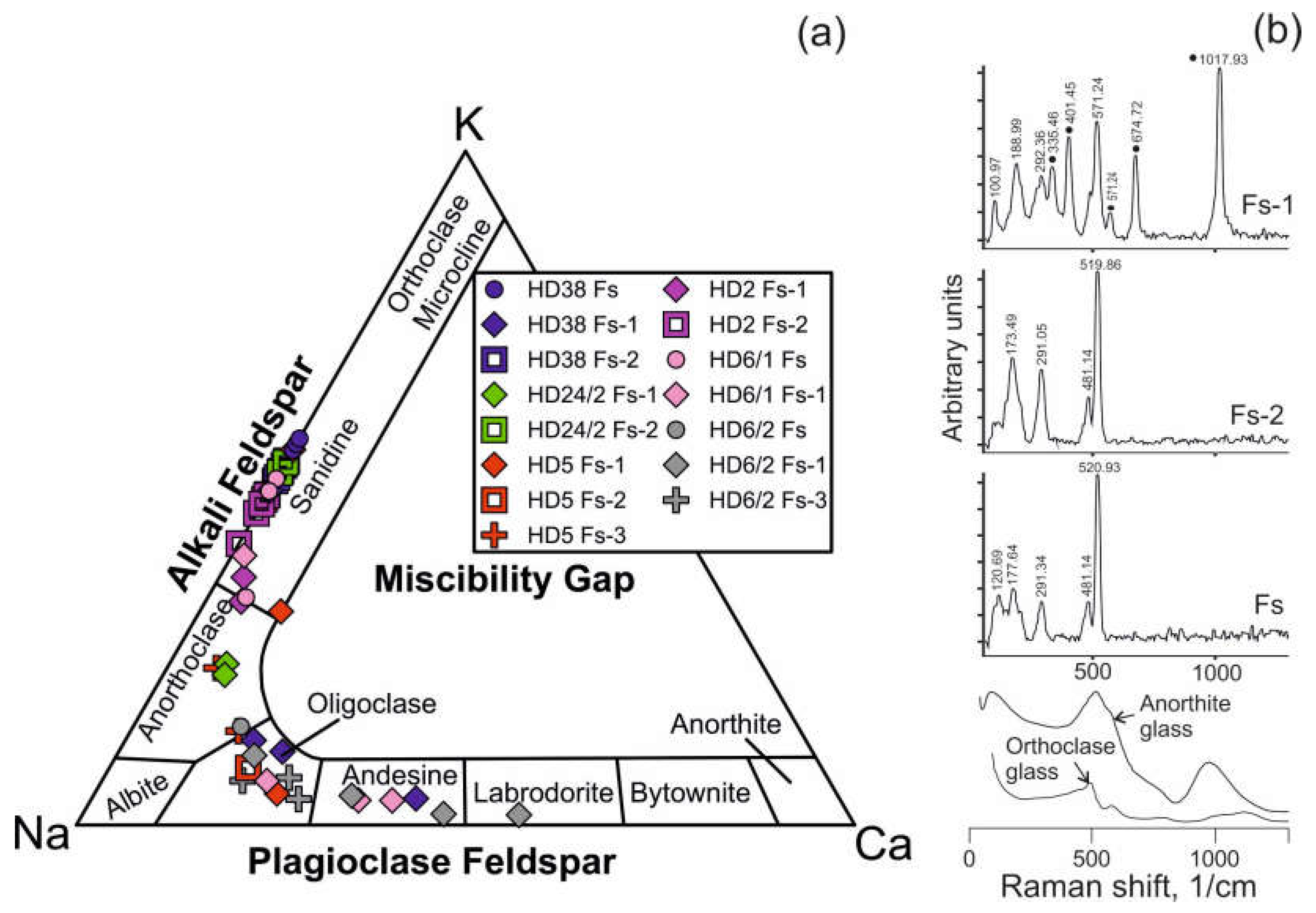
Feldspar in reaction zones of orthopyroxene and spinel, as well as in veinlets between mineral grains is alkali feldspar (
Figure 6a, Electronic
Supplementary Table S1). Feldspar in reaction zones of clinopyroxene is plagioclase. Close to the basanite/xenolith contact, leucite is present in reaction zones of pyroxenes (Electronic
Supplementary Table S1).
Raman spectra of feldspar from orthopyroxene reaction zones as well as from veinlets in interstitial space resemble those of sanidine (
Figure 6b). Raman spectra of feldspar from clinopyroxene reaction zones correspond to that of anorthite (see
Figure 6b).
Figure 6.
(
a) Compositions of feldspar from Tumusun lherzolite xenoliths on ternary classification diagram. Fs – feldspar from veinlets in interstitial space, Fs-1 – feldspar from clinopyroxene reaction zones, Fs-2 – feldspar from orthopyroxene reaction zones, Fs-3 – feldspar from spinel reaction zones. (
b) Raman spectra of feldspars from Tumusun lherzolite xenoliths. The spectrum of Fs-1 is influenced by signal of Cpx2 forming tight intergrowth with feldspar, and clinopyroxene Raman bands are marked by circles. Raman spectra of glasses of anorthite and orthoclase compositions [
24] are shown for comparison.
Figure 6.
(
a) Compositions of feldspar from Tumusun lherzolite xenoliths on ternary classification diagram. Fs – feldspar from veinlets in interstitial space, Fs-1 – feldspar from clinopyroxene reaction zones, Fs-2 – feldspar from orthopyroxene reaction zones, Fs-3 – feldspar from spinel reaction zones. (
b) Raman spectra of feldspars from Tumusun lherzolite xenoliths. The spectrum of Fs-1 is influenced by signal of Cpx2 forming tight intergrowth with feldspar, and clinopyroxene Raman bands are marked by circles. Raman spectra of glasses of anorthite and orthoclase compositions [
24] are shown for comparison.
3.3. Whole-Rock Major and Trace Element Composition
The major and trace element whole-rock composition is listed in
Table 1. In three largest xenoliths, marginal and central parts have been analyzed. The peridotites possess abundances of SiО
2, MgО, Al
2О
3, CaО, TiО
2 similar to that of primitive mantle. The marginal parts of xenoliths have evidently higher concentrations of K
2O and Na
2O compared to central parts, while the difference in other oxides is within analytical uncertainty. Small xenoliths, as well as margins of large xenoliths, have high К
2О and Na
2О, as pointed out earlier [
9].
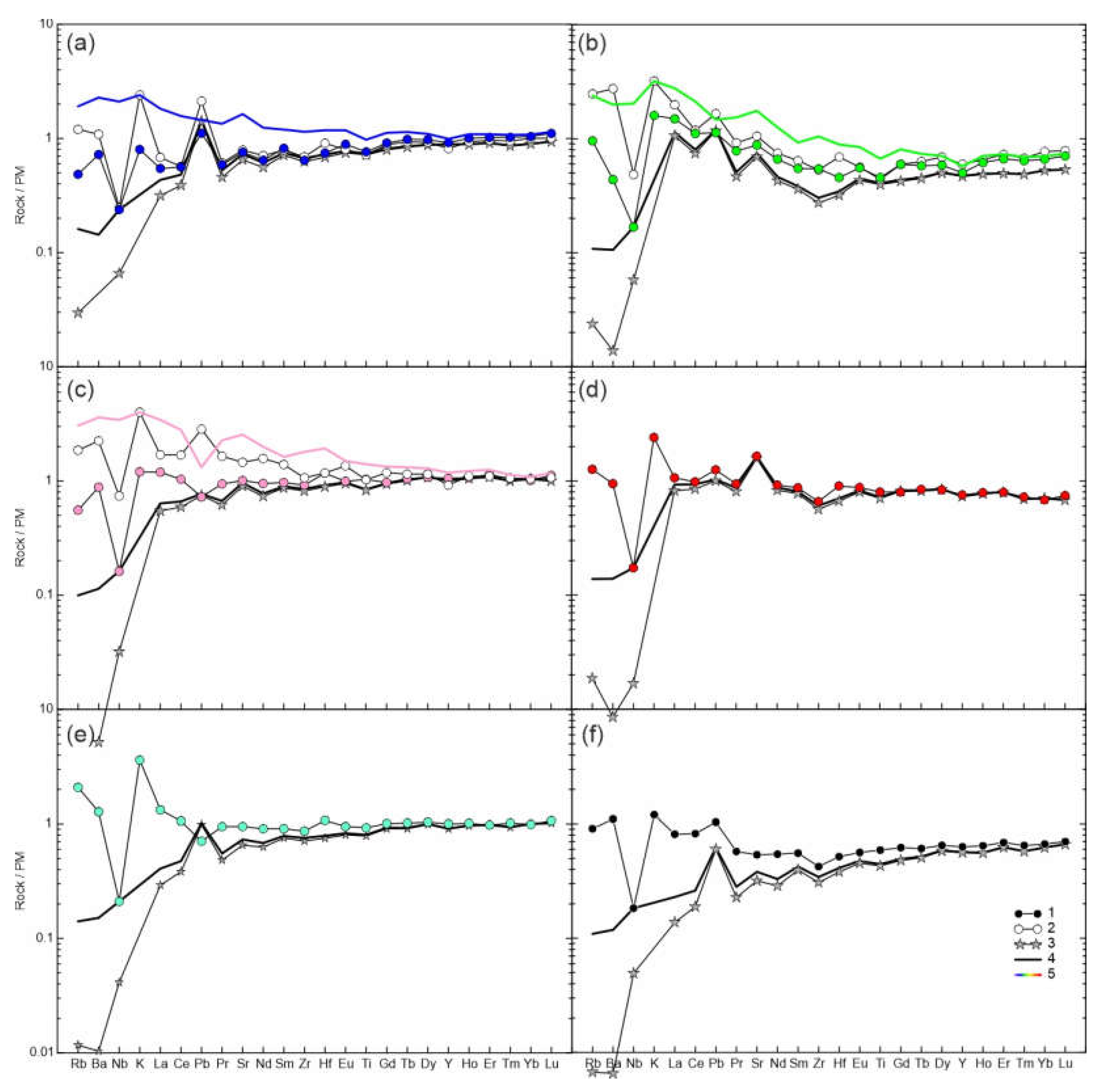
The rocks are characterized by two types of normalized trace element patterns – depleted and enriched. In the depleted type, the trace elements show decrease in normalized abundances toward most incompatible elements (
Figure 7a), but have maxima of Rb, Ba, K, Pb, Sr, and minima of Nb, Zr, Hf, Ti. The enriched rock type exhibits increase in most incompatible elements, and multi-element plots demonstrate maximum of K and minimum Nb, while anomalies of Pb, Sr, Zr, Hf, Ti can be either positive or negative, or lack (
Figure 7b-f). In general, in marginal parts of xenoliths the trace element pattern is similar to that of the central parts, but the marginal parts have higher abundances of LILE. In addition, the marginal part of sample HD6 shows higher LREE-MREE (
Figure 7а-с). All studied small xenoliths demonstrate enriched trace-element patterns.
Figure 7.
Measured and calculated trace element compositions of lherzolite xenoliths from Tumusun volcano. (a) HD38; (b) HD24/2; (c) HD6; (d) HD58; (e) HD34; (f) HD-68. 1 – measured whole-rock composition of central part of xenolith; 2 – measured whole-rock composition of marginal part of xenolith (only for a-c); 3 – calculated whole-rock composition obtained from modal composition and trace element abundances in olivine, ortho- and clinopyroxene; 4 – mix of calculated lherzolite compositions and basanite composition, with basanite proportion (x) defined by difference in Nb abundance between measured and calculated compositions; 5 – Mix of compositions of central part of lherzolite xenolith and basanite proportion (x) defined by difference in measured K abundance between the margin and center of xenolith.
Figure 7.
Measured and calculated trace element compositions of lherzolite xenoliths from Tumusun volcano. (a) HD38; (b) HD24/2; (c) HD6; (d) HD58; (e) HD34; (f) HD-68. 1 – measured whole-rock composition of central part of xenolith; 2 – measured whole-rock composition of marginal part of xenolith (only for a-c); 3 – calculated whole-rock composition obtained from modal composition and trace element abundances in olivine, ortho- and clinopyroxene; 4 – mix of calculated lherzolite compositions and basanite composition, with basanite proportion (x) defined by difference in Nb abundance between measured and calculated compositions; 5 – Mix of compositions of central part of lherzolite xenolith and basanite proportion (x) defined by difference in measured K abundance between the margin and center of xenolith.
3.4. Mineral Trace Element Composition
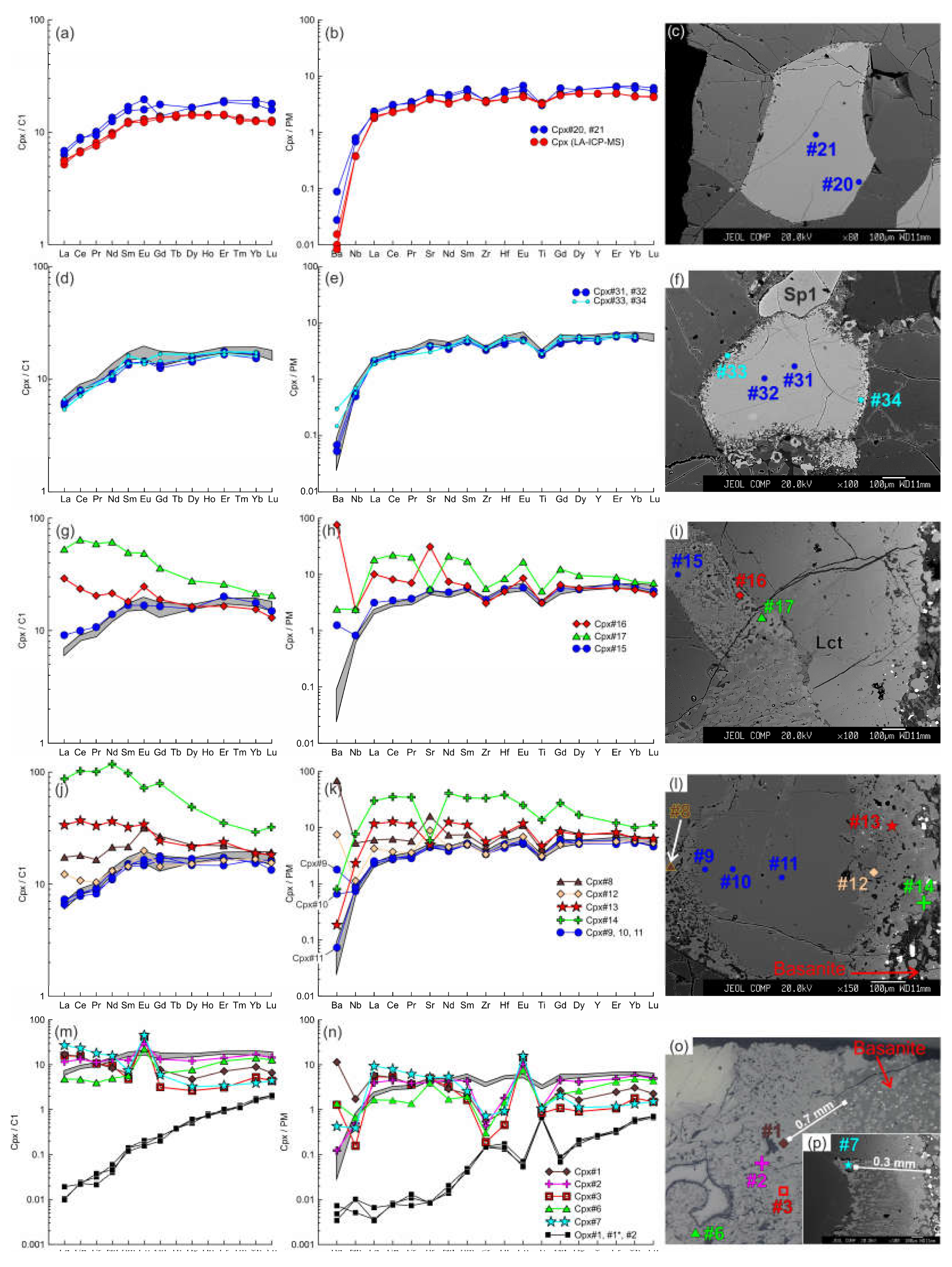
The primary clinopyroxenes without reaction rims have two types of REE patterns. The first type is undifferentiated HREE-MREE and depletion in LREE, i.e., depleted pattern common for residual fertile lherzolites (
Figure 8а). The normalized patterns of trace elements also show depleted shape and depletion in the most incompatible elements (from Ba to Nd) and weak minima of Ti and Zr (
Figure 8b). Inside xenoliths, clinopyroxene with thin reaction zones have the same spectrum type and abundances of trace elements in Cpx1 (core) and Cpx2 (reaction rim), resembling composition of primary clinopyroxene without reaction rim, except for high Ba in Cpx2 (
Figure 8d, e). In clinopyroxene grains located close to basanite/xenolith contact near to orthopyroxene reaction zone (
Figure 8g, h) and at immediate contact with the basanite (
Figure 8j, k), even Cpx1 has higher abundances of Ba and sometimes La and Ce but abundances of other trace element are similar to that of primary clinopyroxene. The composition of Cpx2 varies. Compared with Cpx1, Cpx2 has a gradual increase in trace element abundances toward the edge of reaction zone. In the internal part of reaction rim (Cpx2), the maxima of Ba, Sr and Eu appear, and the minima of Zr, Hf, and Ti remain (
Figure 8g-l; analyses #16, #8, #12). Further in the external part of reaction rim (Cpx2) higher trace element abundances combined with minima of Ba, Sr, and Eu are observed (
Figure 8g-l; analyses #17, #13, #14). It is notable that Cpx1 from the smallest xenolith (1.5 cm in length) has depleted trace element pattern (
Figure 9).
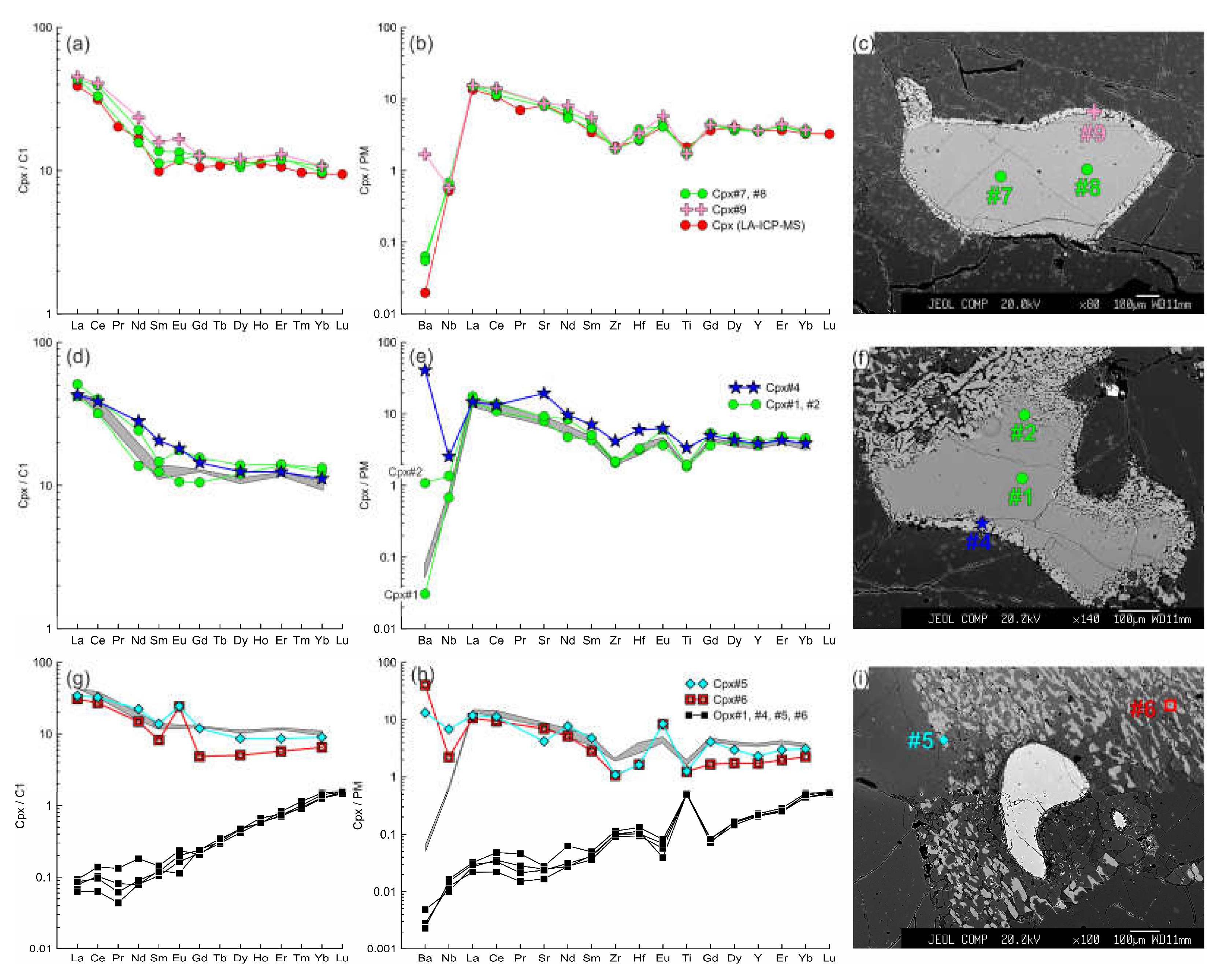
Figure 8.
The trace element composition of the clino- and orthopyroxenes from sample HD38. (a, b, c) Primary clinopyroxene (#20, #21) without reaction rim. Trace element composition of primary clinopyroxene obtained by LA-ICP-MS is shown for comparison. (d, e, f) Cpx1 (#31, #32) with thin reaction rim of Cpx2 (#33, #34). Here and in d, e, g, h, j, k, m, n, the gray field shows the compositions of the primary clinopyroxene (#20, #21). (g, h, i) Cpx1 (Cpx#15) with wide reaction rim of Cpх2 (Cpx#16, Cpx#17) near the basanite/xenolith contact. (j, k, l) Cpx1 (#9 - 11) with wide reaction rim of Cpх2 (#12-14) at basanite/xenolith contact. (m, n, o, p) Cpx3 (#1-7) from orthopyroxene reaction zone at basanite/xenolith contact. Location of primary orthopyroxene (#1, #1*, #2) is beyond the sample areas shown on photo.
Figure 8.
The trace element composition of the clino- and orthopyroxenes from sample HD38. (a, b, c) Primary clinopyroxene (#20, #21) without reaction rim. Trace element composition of primary clinopyroxene obtained by LA-ICP-MS is shown for comparison. (d, e, f) Cpx1 (#31, #32) with thin reaction rim of Cpx2 (#33, #34). Here and in d, e, g, h, j, k, m, n, the gray field shows the compositions of the primary clinopyroxene (#20, #21). (g, h, i) Cpx1 (Cpx#15) with wide reaction rim of Cpх2 (Cpx#16, Cpx#17) near the basanite/xenolith contact. (j, k, l) Cpx1 (#9 - 11) with wide reaction rim of Cpх2 (#12-14) at basanite/xenolith contact. (m, n, o, p) Cpx3 (#1-7) from orthopyroxene reaction zone at basanite/xenolith contact. Location of primary orthopyroxene (#1, #1*, #2) is beyond the sample areas shown on photo.
Cpx1 from some of the samples is enriched in LREE both near the center of xenolith and at basanite/xenolith contact (
Figure 10a, 11a). External parts of Cpx1 show more Ba near to the wider reaction rim (
Figure 10e). Both Cpx1 and Cpx2 have similar REE patterns (
Figure 10a, 10d, 11a). Cpx1 and Cpx2 normalized trace-element patterns demonstrate minima of Zr and Ti; Cpx1 shows minima of Ba and Nb but Cpx2 has maximum of Ba and sometimes Sr (
Figure 10b, 10e, 11b).
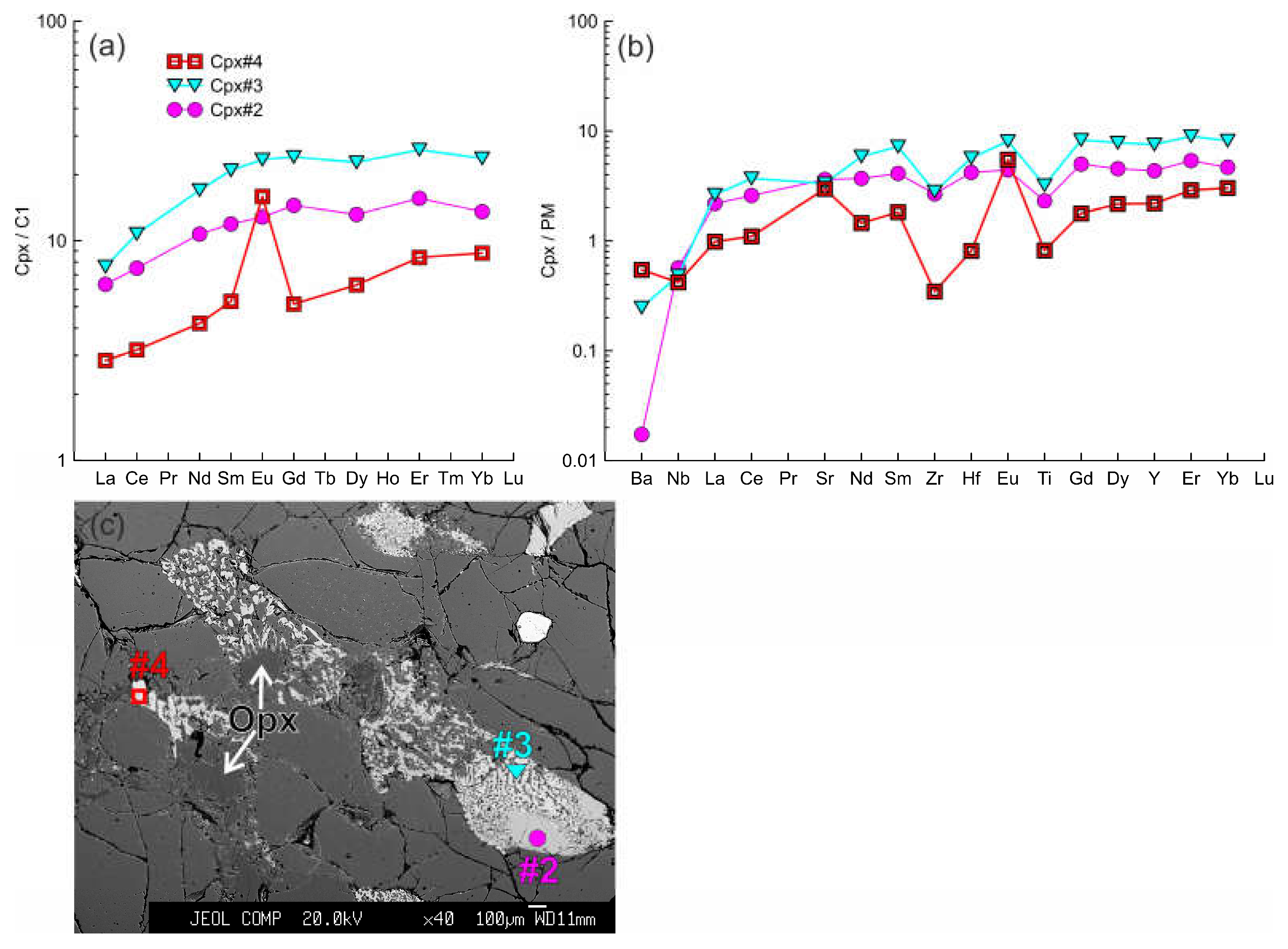
Figure 9.
(a, b) The trace element composition of the clino- and orthopyroxenes from sample HD2 (xenolith of 1.5 cm in size). Cpx 1 (#2) with wide reaction rim of Сpx2 (#3) at the contact with orthopyroxene reaction zone. Сpx3 (#4) from reaction zone of Opx1. (с) Image showing different width of pyroxene reaction zones. Clinopyroxene located in upper right corner contacts with olivine only and shows narrow reaction rim.
Figure 9.
(a, b) The trace element composition of the clino- and orthopyroxenes from sample HD2 (xenolith of 1.5 cm in size). Cpx 1 (#2) with wide reaction rim of Сpx2 (#3) at the contact with orthopyroxene reaction zone. Сpx3 (#4) from reaction zone of Opx1. (с) Image showing different width of pyroxene reaction zones. Clinopyroxene located in upper right corner contacts with olivine only and shows narrow reaction rim.
In samples with LREE-enriched primary clinopyroxenes, Cpx3 is also enriched (
Figure 10g, 11d, 11g). In samples with LREE-depleted primary clinopyroxene, Cpx3 can have both depleted (see
Figure 9a) and enriched (see
Figure 8m-o) trace-element pattern. In last case, the enriched Cpx3 is located at distance up to 1 mm from basanite/xenolith contact. Trace element abundances in Cpx3 vary from the levels close to that of primary clinopyroxene to values transitional between primary orthopyroxene and clinopyroxene (see
Figure 8m- n, 9a-b, 10g-h, 11d-h). The general characteristic of Cpx3 is the presence of positive Eu anomaly and negative anomalies of Nb, Zr, Hf, and Ti. Anomalies of Ba and Sr are either positive or lack. Weak negative Eu anomaly was revealed only in Cpx3 from thin reaction rim of orthopyroxene located far from basanite/xenolith contact (
Figure 11g). Notably, in the same orthopyroxene reaction zone there is Cpx3 with weak positive Eu anomaly. Moreover, Cpx3 from this reaction zone is distinct in higher REE abundances and minimum of Sr, compared to Cpx3 near to basanite/xenolith contact.
Figure 10.
The trace element composition of the clino- and orthopyroxenes from sample HD24-2. (a, b, c) Cpx1 (#7, #8) with thin reaction rim of Cpx2 (#9) near the center of xenolith. Trace element composition of primary clinopyroxene obtained by LA-ICP-MS is shown for comparison. (d, e, f) Cpx1 (#1, #2) with wide reaction zone of Cpx2 (#4) near the basanite/xenolith contact. Here and in d, e, g, h, the gray field shows the compositions of Cpx1 (#7, #8). (g, h, i) Cpx3 (#5, #6) from orthopyroxene reaction zone located near basanite/xenolith contact. Location of primary orthopyroxene (#1, #4-6) is beyond the sample areas shown on photo.
Figure 10.
The trace element composition of the clino- and orthopyroxenes from sample HD24-2. (a, b, c) Cpx1 (#7, #8) with thin reaction rim of Cpx2 (#9) near the center of xenolith. Trace element composition of primary clinopyroxene obtained by LA-ICP-MS is shown for comparison. (d, e, f) Cpx1 (#1, #2) with wide reaction zone of Cpx2 (#4) near the basanite/xenolith contact. Here and in d, e, g, h, the gray field shows the compositions of Cpx1 (#7, #8). (g, h, i) Cpx3 (#5, #6) from orthopyroxene reaction zone located near basanite/xenolith contact. Location of primary orthopyroxene (#1, #4-6) is beyond the sample areas shown on photo.
Orthopyroxenes are LREE-depleted in the samples with depleted-type clinopyroxenes (see
Figure 8 m). Orthopyroxenes in samples with LREE-enriched clinopyroxene show higher abundances of La and Ce compared to pattern common for depleted orthopyroxene (
Figure 10g, 11d). On primitive-mantle-normalized plots, orthopyroxenes show maxima of Ti, Zr, Hf, complementary to corresponding minima in clinopyroxenes (
Figure 8n, 10h, 11e). LREE-enriched orthopyroxenes show minima in Ba, Nb, and Sr (
Figure 10h, 11e). In orthopyroxene with reaction zone of the second type, Opx1 has similar trace-element abundances and pattern as primary orthopyroxene from xenolith center, except for low abundances of Ba, La, Ce, and weak Eu maximum in the former (
Figure 11j-l). At the same time, Opx1 preserves enriched type of REE pattern. Opx2 from this reaction zone has U-shaped normalized trace-element pattern with gradual decrease from HREE to Zr, Sm, Nd, and further growth in more incompatible elements, and is also characterized by maxima of Hf, Eu, and Ti (
Figure 11j-l). Opx2 is enriched in highly incompatible elements, and have positive Eu anomaly and inhomogeneous composition, which is different from Opx1 in the same grain.
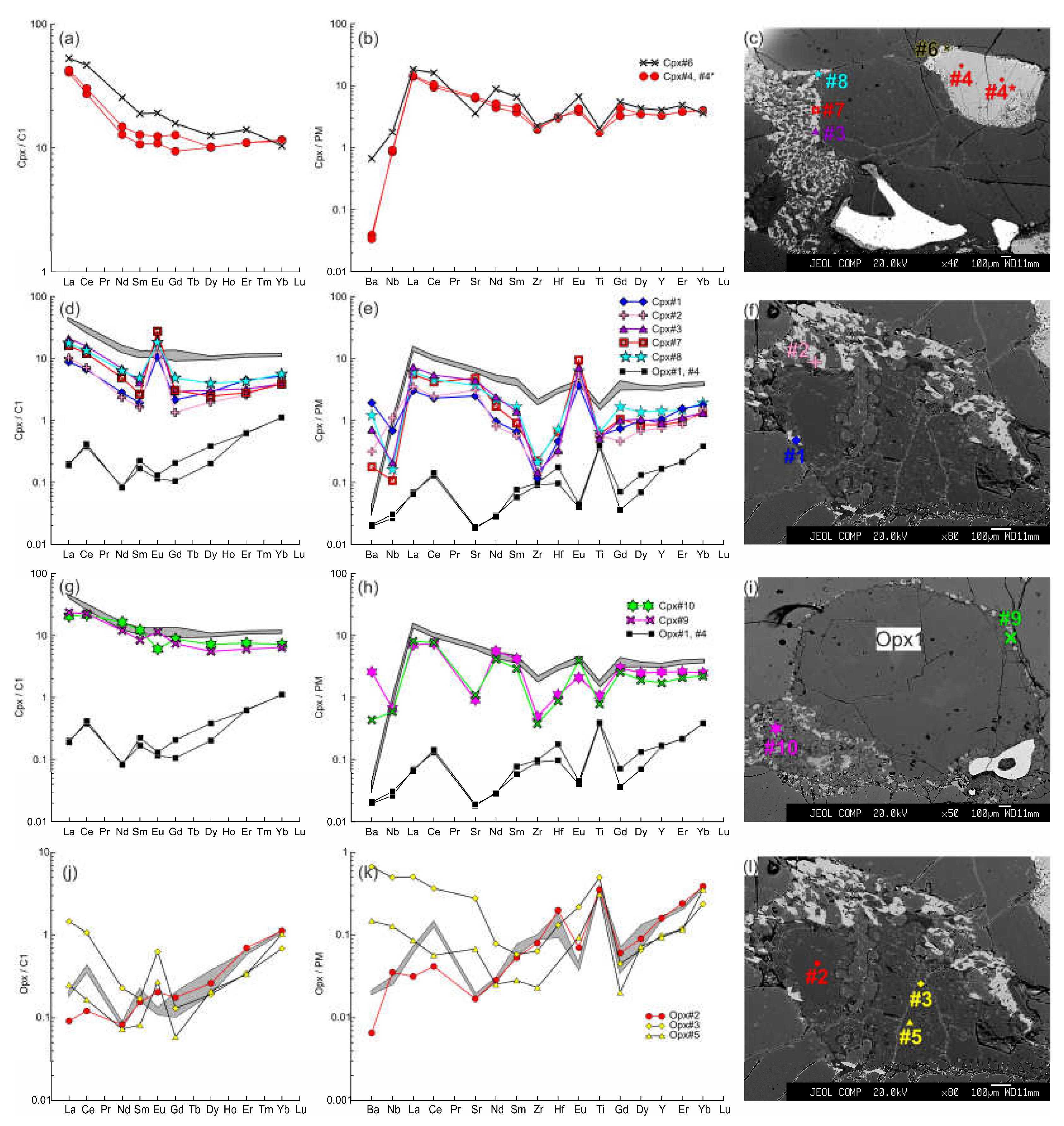
Figure 11.
The trace element composition of the clino- and orthopyroxenes from sample HD5. (a, b, c) Cpx1 (#4, #4*) with wide reaction zone of Cpx2 (#6) near the contact with basanite. (d, e, f) Cpx3 (#1, #2 in f, #3, #7, #8 in c) from orthopyroxene reaction zone, and primary orthopyroxene (Opx#1, #4; the location is beyond the area shown on photo). Here and in g, h, the gray field shows the compositions of the Cpx1 (#4, #4*). (g, h, i) – Cpx3 (#9, #10) from thin reaction zone of Opx1 inside xenolith. (j, k, l) Orthopyroxene reaction zone of second type, with unreacted Opx1 (#2) and secondary Opx2 (#3, #5). The gray field shows the compositions of the primary orthopyroxene (#1, #4).
Figure 11.
The trace element composition of the clino- and orthopyroxenes from sample HD5. (a, b, c) Cpx1 (#4, #4*) with wide reaction zone of Cpx2 (#6) near the contact with basanite. (d, e, f) Cpx3 (#1, #2 in f, #3, #7, #8 in c) from orthopyroxene reaction zone, and primary orthopyroxene (Opx#1, #4; the location is beyond the area shown on photo). Here and in g, h, the gray field shows the compositions of the Cpx1 (#4, #4*). (g, h, i) – Cpx3 (#9, #10) from thin reaction zone of Opx1 inside xenolith. (j, k, l) Orthopyroxene reaction zone of second type, with unreacted Opx1 (#2) and secondary Opx2 (#3, #5). The gray field shows the compositions of the primary orthopyroxene (#1, #4).