Submitted:
14 February 2023
Posted:
15 February 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
2.1. Sample collection
2.2. CTLs sampling
2.3. Amplicon sequencing
2.4. Profiling of volatiles by HS-SPME-GC-MS during stacking fermentation
2.5. Sensory quality analysis
2.6. Data analysis
3. Results
3.1. Microbial community changes during stacking fermentation
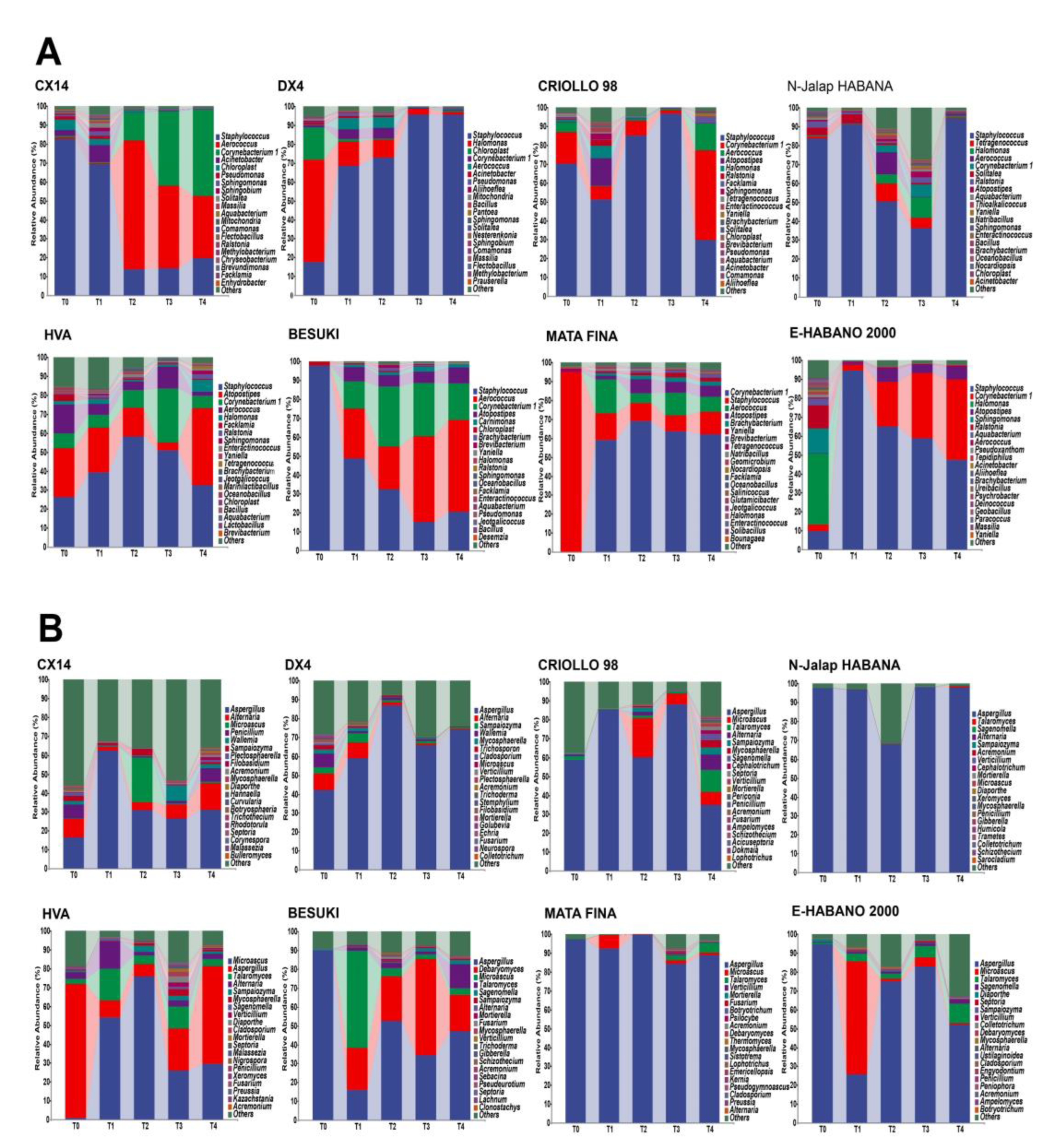
3.2. Characteristic microorganisms from different varieties of CTLs
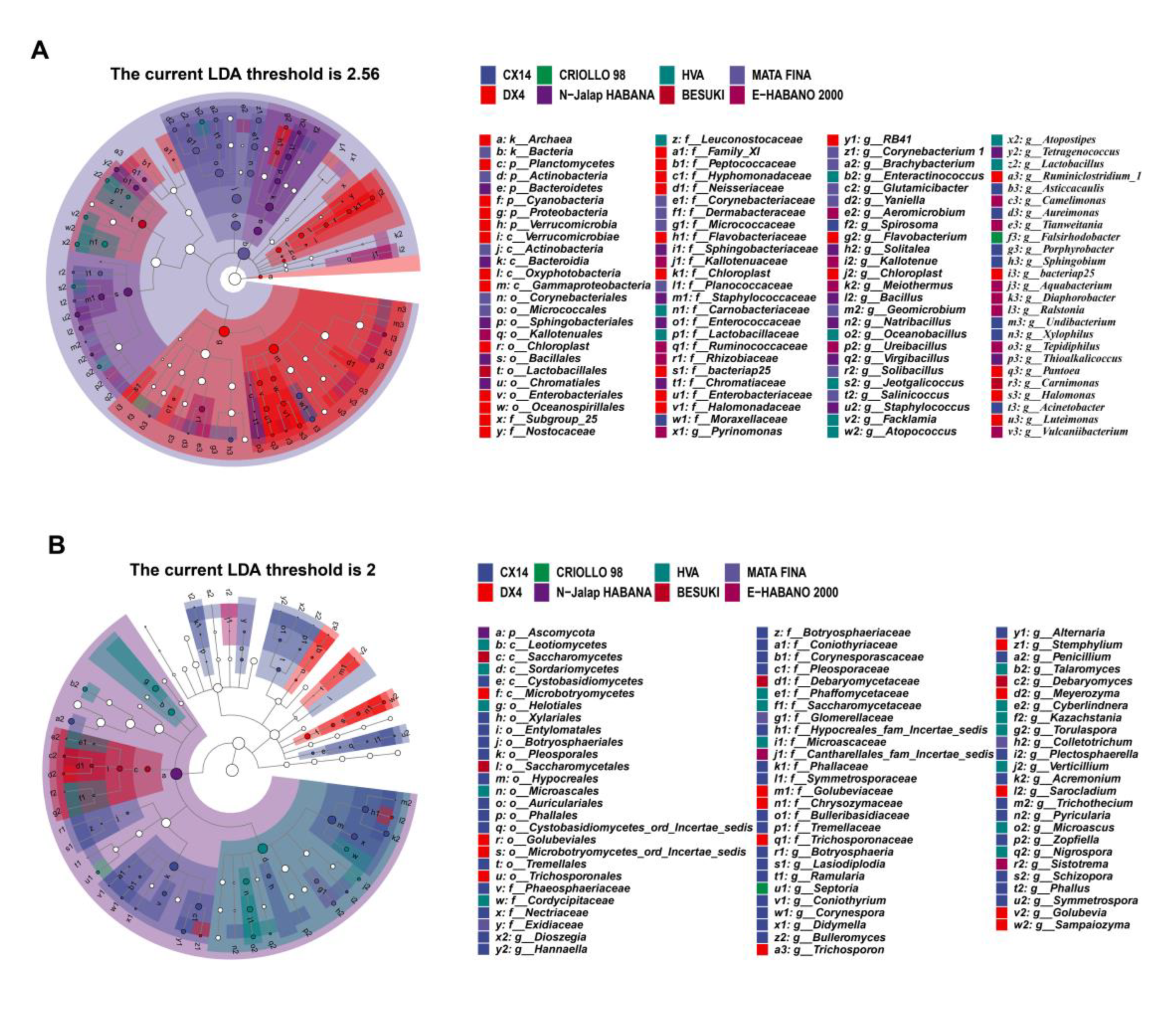
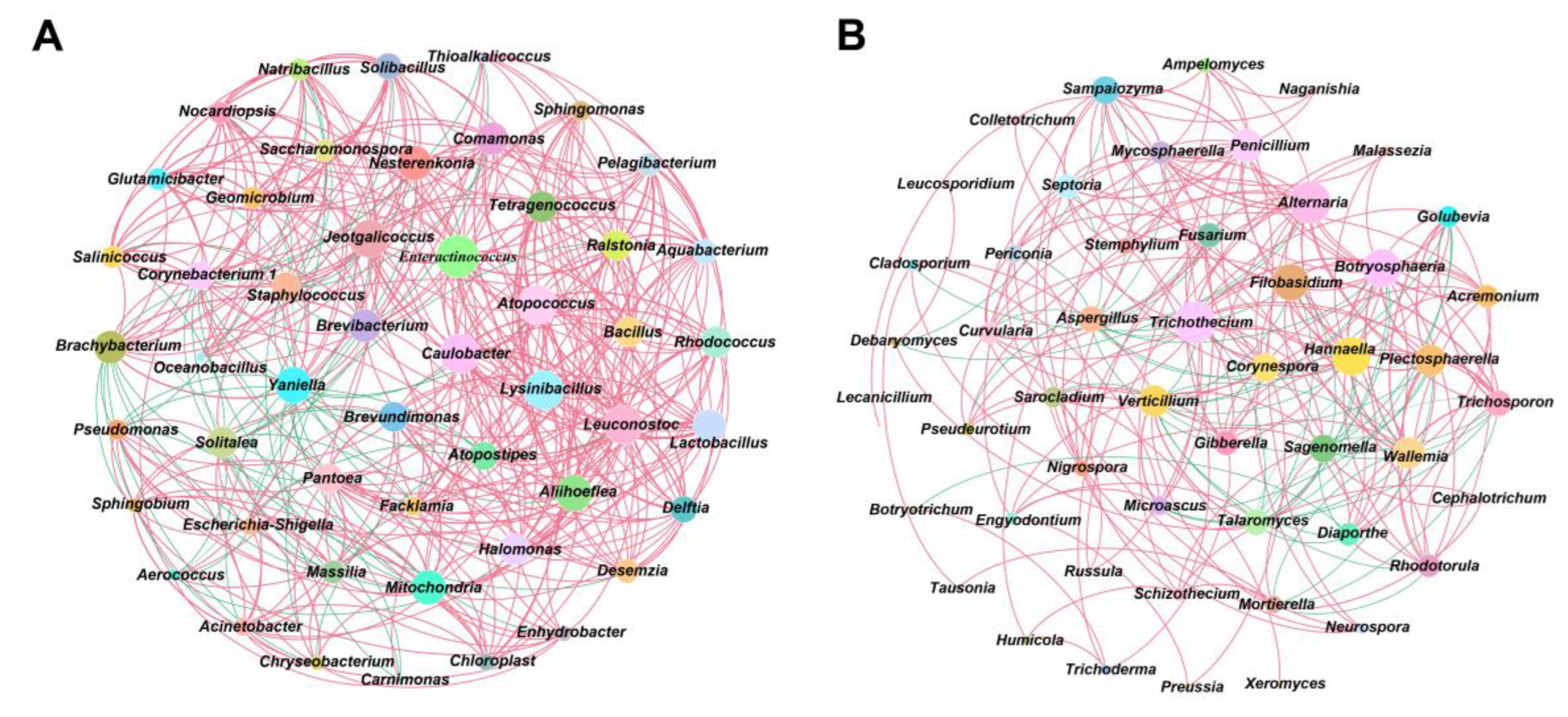
3.3. Interaction relationship between microbial community
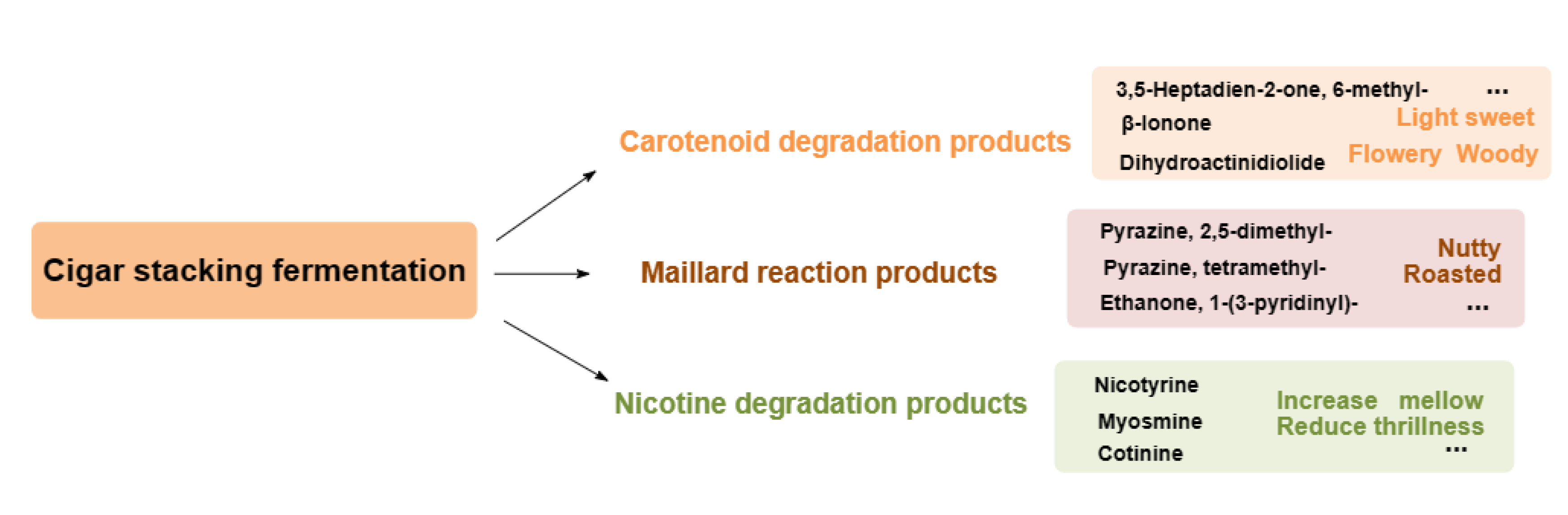
3.4. Changes of volatiles in the stacking fermentation
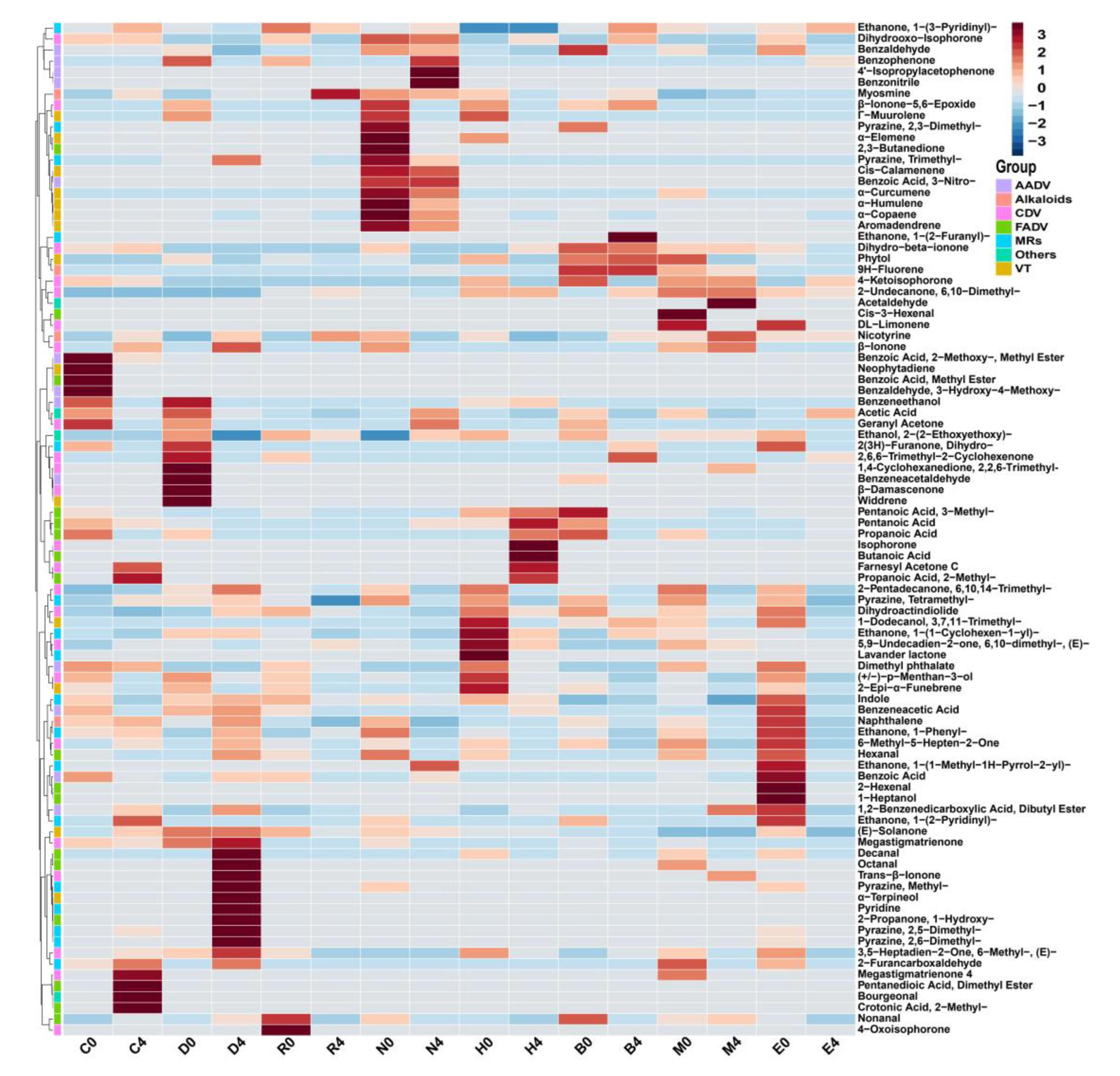
3.5. Characteristic volatiles from different CTL varieties
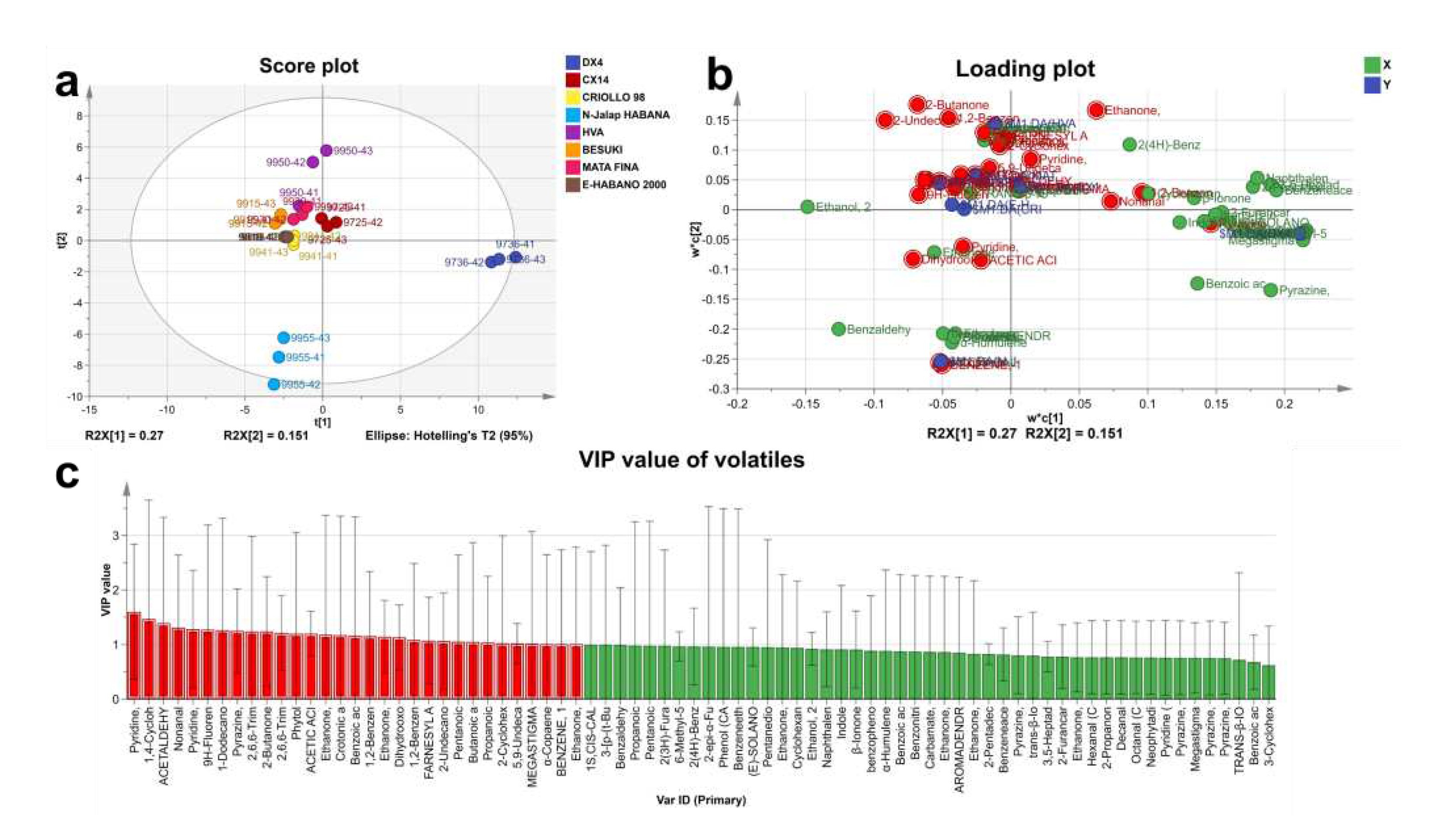
| Varieties | Characteristic volatiles |
| CX14 | Ethanone, 1-(2-pyridinyl)-、Tiglic acid、Benzoic acid, 2-methoxy-, methyl ester、Dimethyl phthalate、Megastigmatrienone 4、Bourgeonal |
| DX4 | Nonanal、Pyrazine, tetramethyl-、Ethanone, 1-(1-Cyclohexen-1-yl)- |
| CRIOLLO 98 | Myosmine |
| N-Jalap HABANA | Acetic Acid、Geranyl Acetone、α-Curcumene |
| HVA | Farnesyl acetone C、2-Undecanone, 6,10-dimethyl-、Pentanoic acid, 3-methyl-、Butanoic acid、Propanoic acid, 2-methyl-、Isophorone、α-Copaene |
| BESUKI | 9H-Fluorene、1-Dodecanol, 3,7,11-Trimethyl-、2,6,6-Trimethyl-2-Cyclohexenone、Dihydro-beta-ionone、Phytol、Dihydrooxo-isophorone、Ethanone, 1-(2-furanyl)- |
| MATA FINA | 1,4-Cyclohexanedione, 2,2,6-Trimethyl-、Acetaldehyde、Nicotyrine、1,2-Benzenedicarboxylic acid, dibutyl ester、4-Oxoisophorone |
3.6. Correlation analysis between microorganisms-volatiles and volatiles-aroma
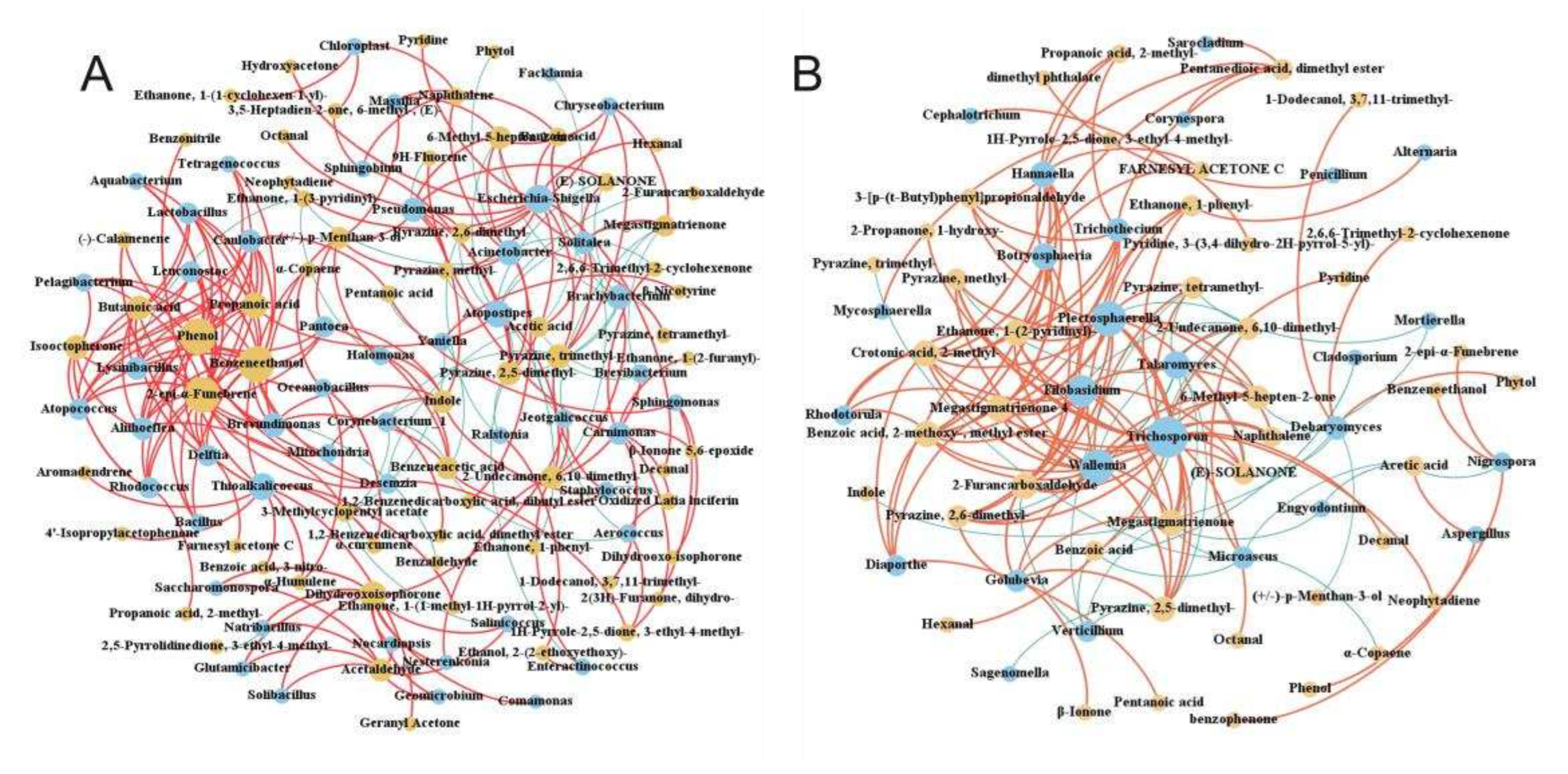
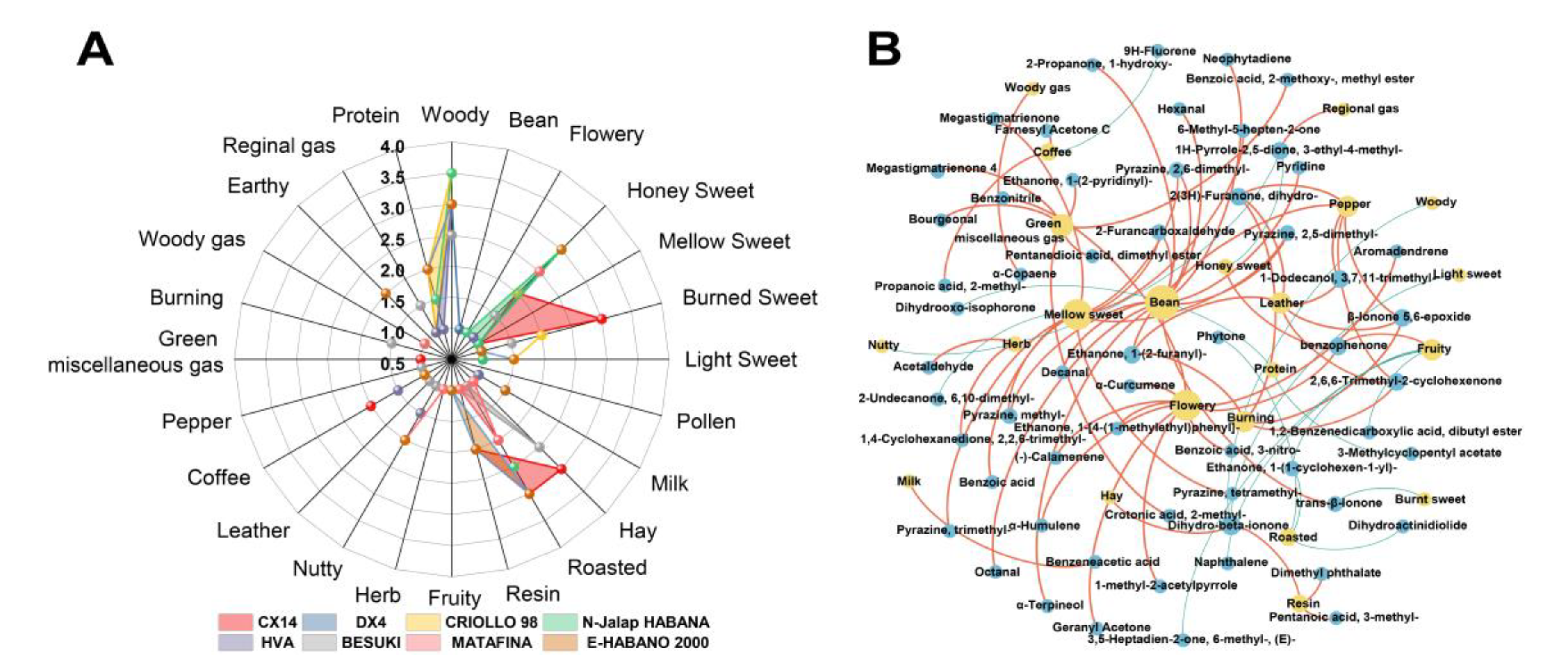
4. Discussion
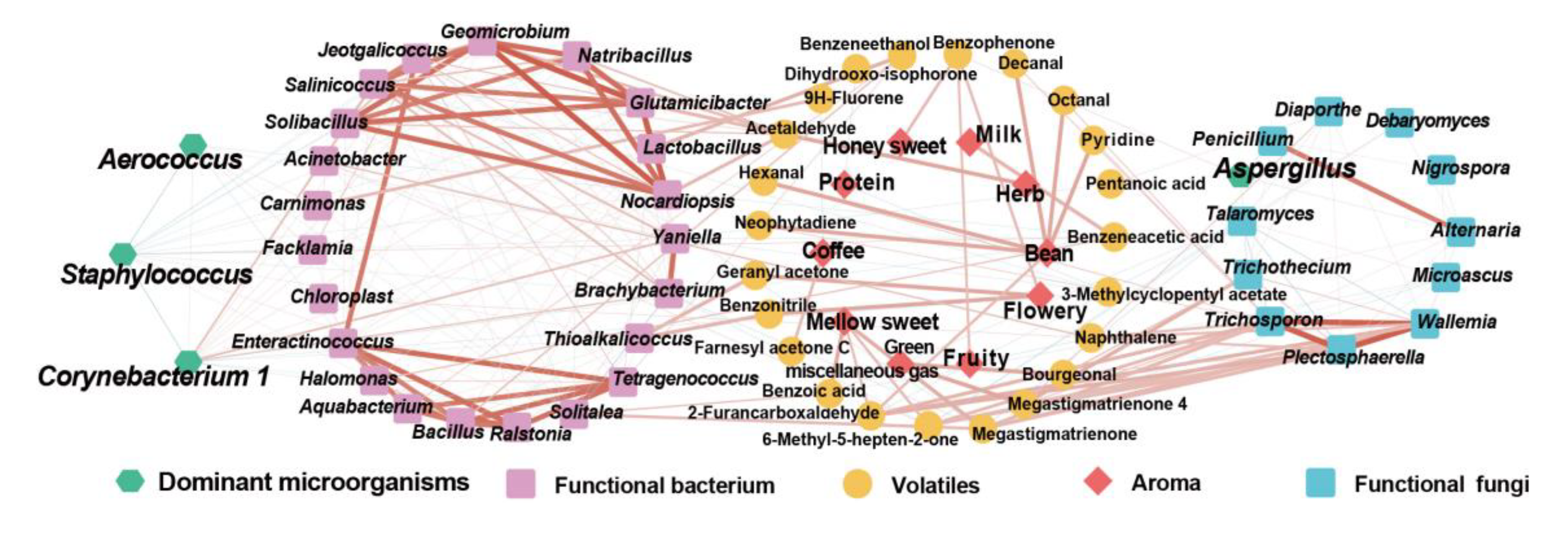
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, G.; Muthumalage, T.; Rahman, I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018, 288, 143–155. [Google Scholar] [CrossRef]
- Spatarella, A.; Folan, P.; Farber, H.J.; American Thoracic Society Tobacco Action, C. Cigars. Am. J. Respir. Crit. Care Med. 2018, 197, P7–P8. [Google Scholar] [CrossRef]
- Gupta, C. A Biotechnological Approach to Microbial Based Perfumes and Flavours. J. Microbiol. Exp. 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Krusemann, E.J.Z.; Pennings, J.L.A.; Cremers, J.; Bakker, F.; Boesveldt, S.; Talhout, R. GC-MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef]
- Banozic, M.; Jokic, S.; Ackar, D.; Blazic, M.; Subaric, D. Carbohydrates-Key Players in Tobacco Aroma Formation and Quality Determination. Molecules 2020, 25. [Google Scholar] [CrossRef]
- ZHANG, L.; LUO, Z.-H.; YANG, M.-C. Diversity of Fermentation Microbes and Changes of Hydrolytic Enzyme Activities of Cigar Leaf Raw Materials. J. Agric. Sci. Technol. 2021, 23, 171–180. (In Chinese) [Google Scholar] [CrossRef]
- LIU, F.-F.; FANG, X.; LI, L.-L.; TAN, Z.-Y.; YE, M.-Q.; ZHU, T.-T.; HUANG, Y.-Y.; PAN, Y. . Changs of Volatiles Components in Cigar Wrapper During Deep Fermentation. Guangdong Agric. Sci. 2022, 49, 158–164. (In Chinese) [Google Scholar] [CrossRef]
- Liu, T.; Guo, S.; Wu, C.; Zhang, R.; Zhong, Q.; Shi, H.; Zhou, R.; Qin, Y.; Jin, Y. Phyllosphere microbial community of cigar tobacco and its corresponding metabolites. Front. Microbiol. 2022, 13, 1025881. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Q.; Li, P.; Wu, X.; Liu, Y.; Yang, Z.; Li, D.; Zhang, J.; Du, G. Analysis of Microbial Community, Volatile Flavor Compounds, and Flavor of Cigar Tobacco Leaves From Different Regions. Front. Microbiol. 2022, 13, 907270. [Google Scholar] [CrossRef]
- ZHANG, Q.-Y.; LUO, C.; LI, D.-L.; CAI, W. . Research Progress in Curing and Fermentation Technology for Cigar Tobacco Leaf Production. Acta Tabacaria Sin. 2020, 26, 1–6. (In Chinese) [Google Scholar] [CrossRef]
- ZHOU, T.; CAI, J.-Y.; XING, L.; ZHOU, W.; JIA, Y.-H.; GAO, J. . Exploration and Refletions on the Application of the Great Wall Cigar to Dometic Cigar Tobacco. Mod. Ind. Econ. Informationization 2021, 11, 140–142. (In Chinese) [Google Scholar] [CrossRef]
- Wu, X.; Zhu, P.; Li, D.; Zheng, T.; Cai, W.; Li, J.; Zhang, B.; Zhu, B.; Zhang, J.; Du, G. Bioaugmentation of Bacillus amyloliquefaciens-Bacillus kochii co-cultivation to improve sensory quality of flue-cured tobacco. Arch. Microbiol. 2021, 203, 5723–5733. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Q.; Wu, Q.; Li, D.; Wu, X.; Li, P.; Zhou, Q.; Cai, W.; Zhang, J.; Du, G. Effects of Inoculation With Acinetobacter on Fermentation of Cigar Tobacco Leaves. Front. Microbiol. 2022, 13, 911791. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Wu, X.; Cai, W.; Zhu, P.; Peng, Z.; Zheng, T.; Li, D.; Li, J.; Zhou, G.; Du, G.; Zhang, J. Profiling the role of microorganisms in quality improvement of the aged flue-cured tobacco. BMC Microbiol. 2022, 22, 197. [Google Scholar] [CrossRef]
- Yu, S.; Drton, M.; Promislow, D.E.L.; Shojaie, A. CorDiffViz: an R package for visualizing multi-omics differential correlation networks. BMC Bioinform. 2021, 22, 486. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar] [CrossRef]
- LIU, X.-Y.; ZHU, Q.; YANG, F.; ZHANG, J.; ZHANG, Q.-L.; LI, J.-H.; WANG, L. Muti-omics Reveal the Formation of Flavor Compounds in Sauce-flavor Daqu. Food Ferment. Ind. 2021, 47, 35–41. (In Chinese) [Google Scholar]
- ZHENG, T.-F.; ZHANG, Q.-Y.; LI, D.-L.; ZHANG, J.; DU, G.-C. . Analysis of Flavor Characteristics and Microbial Community of Cigar Tobacco Leaves from Different Regions. J. Henan Agric. Sci. 2022, 51, 45–54. (In Chinese) [Google Scholar] [CrossRef]
- YAN, T.-J.; ZHENG, L.-L.; LI, X.-N.; LIU, L.-P.; PAN, Y.; WANG, J.; LONG, F.; ZHOU, P.; SHI, X.-D. Diversity Analysis and Function Prediction of Bacterial Communities in Cigar Leaves Based on High-throughput Sequencing Technology. Acta Tabacaria Sin. 2022, 28, 8. (In Chinese) [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Prokopov, T.; Nikolova, M.; Stoyanova, A.; Zheljazkov, V.D. Carotenoid-Related Volatile Compounds of Tobacco (Nicotiana tabacum L.) Essential Oils. Molecules 2019, 24, 3446. [Google Scholar] [CrossRef]
- Liang, M.H.; He, Y.J.; Liu, D.M.; Jiang, J.G. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit. Rev. Biotechnol. 2021, 41, 513–534. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Song, S.; Hayat, K.; Eric, K.; Majeed, H. Tobacco alkaloids reduction by casings added/enzymatic hydrolysis treatments assessed through PLSR analysis. Regul. Toxicol. Pharmacol. 2016, 75, 27–34. [Google Scholar] [CrossRef]
- ZHENG, L.-L.; ZHAO, L.; CAI, X.-H.; CHEN, Z.; CHAI, Z.-S.; SHI, X.-D. . Analysis of Bacterial and Fungal Community Diversity During Indusrial Secondary Fermentation of Cigar Core Tobacco Leaves. Acta Tabacaria Sin. 2022, 1–9. (In Chinese) [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, A.; Patil, U. Optimal production and characterization of alkaline protease from newly isolated halotolerant Jeotgalicoccus sp. Biocatal. Agric. Biotechnol. 2015, 4, 235–243. [Google Scholar] [CrossRef]
- Xiong, Y.-W.; Gong, Y.; Li, X.-W.; Chen, P.; Ju, X.-Y.; Zhang, C.-M.; Yuan, B.; Lv, Z.-P.; Xing, K.; Qin, S. Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium Glutamicibacter halophytocola KLBMP 5180 isolated from a coastal halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, T.; Jia, H.; Guo, C.; Wang, Z.; Yue, T.; Yuan, Y. Comparative evaluation of the effects of natural and artificial inoculation on soybean paste fermentation. Lwt 2022, 155. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Zhang, J.; Liu, J.; Zou, X. Dynamic characteristics and co-occurrence patterns of microbial community in tobacco leaves during the 24-month aging process. Ann. Microbiol. 2021, 71, 1–13. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.Y.; Yang, F.W.; Xie, Y.F.; Guo, Y.H.; Yao, W.R.; Zhou, W.B.A. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Tech. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Tong, J.; Xu, Y. An Alkylpyrazine Synthesis Mechanism Involving l-Threonine-3-Dehydrogenase Describes the Production of 2,5-Dimethylpyrazine and 2,3,5-Trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019, 85, e01807–e01819. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





