1. Introduction
Dengue virus (DENV) is a single-stranded RNA virus that belongs to the genus flavivirus, family Flaviviridae. It is the most rapidly spreading arboviral disease in the world and they are mainly found in the tropics, where the mosquito vectors, Aedes aegypti, and Aedes albopictus are found [
1,
2,
3]. According to the WHO, the estimated global population who are at risk for dengue is about 3.9 billion people. There are four serotypes of DENV; DENV serotype 1 (DENV-1), DENV serotype 2 (DENV-2), DENV serotype 3 (DENV-3), and DENV serotype 4 (DENV-4). The genome consists of three structural proteins, capsid (C), premembrane (prM), and envelope (E) proteins, and seven non-structural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. All four DENVs can cause a wide range of illnesses from asymptomatic to flu-like illnesses. Although rare, some people may develop severe dengue which manifests with severe plasma leakage that could result in subsequently several complications including multiple organ dysfunction. Dengue has caused many deaths and the estimated number of dengue deaths is 21,000, annually [
2,
3]. Treatment measures that would prevent the severe manifestations of dengue, hence, are badly needed.
To date, there are two vaccines approved by the WHO, namely CYD-TDV and TAK-003. Despite having these vaccines, the former requires seropositive individuals to be effective while the latter, though it is serostatus-independent, shows an inconsistent efficacy among dengue serotypes. Previous studies have shown that the level of viremia in dengue patients corresponds to the severity of the disease [
4,
5]. Viremia level was shown to be higher in secondary dengue infection which may prolong the disease and increase the chances of manifesting severe dengue symptoms [
4,
5]. Treatments such as with antivirals to reduce the level of viremia, hence, could possibly reduce the severity of dengue. Escalation in research efforts to discover and develop effective antivirals is much needed since the only treatment measure for dengue to date relies mainly on supportive care such as the use of analgesics or antipyretics and fluid management.
Antivirals may act against DENV in several ways including the inhibition of virus replication cycle, direct inhibition, or prophylactic treatment. Understanding DENV replication cycles is therefore important. Replication cycles of DENV consist of adsorption of the virus to the cell, virus entry into the cell, release of the virus genome, assembly, and release of infectious virus particles into the cell. A number of antivirals have been in use for the treatment of virus infection and these include ribavirin and acyclovir. Whereas, antivirals such as virantmycin and alanosine derived from secondary metabolites of soil microorganisms, particularly Streptomyces, have been shown to possess potent antiviral properties [
6,
7,
8]. There are other bioactive compounds derived from Streptomyces have been reported to be effective against other viruses. For example, narasin, an ionophore derived from Streptomyces aureofaciens has been shown to inhibit DENV replication post-entry at a concentration of less than 1 µM against all DENV serotypes [
9].
In a recent study, a methanolic extract of a novel Streptomyces strain KSF 83 recovered from soil sediments of a primary forest in Kuala Sat Forest, Malaysia was described [
10]. The methanolic extract was shown to inhibit the growth of breast and cancer cell lines. Here, the effects of the secondary metabolites of the KSF 103 ME on DENV-2 replication were investigated.
2. Materials and Methods
UHPLC-MS Analysis
Secondary metabolites profiling was performed using the Agilent 1290 Infinity UHPLC system coupled to Agilent 6520 Accurate-Mass Q-TOF mass spectrometer interfaced with a dual ESI source. The column used was Agilent Zorbax Eclipse XDB-C18 (2.1 × 150 mm, 3.5 μm). The temperature of 4°C and 25°C was maintained for auto-sampler and column, respectively. The mobile phase (A) used was 0.1% formic acid solution in water, and acetonitrile while 0.1% formic acid solution was the mobile phase (B). The flow rate of the mobile phase was kept at 0.5 mL/min. The KSF 103 ME (1.0 μL in HPLC grade methanol solvent) was injected and allowed to separate for 25 min, and an additional 5 min was utilized for post-run time. Nitrogen gas with a flow rate of 25 and 600 L/hour was used as a source of the nebulizing and drying gas, respectively, and the temperature was maintained at 350°C. Analysis was performed with a capillary voltage of 3500 V while the fragmentation voltage was optimized to 125 V. Spectral data were analyzed using Agilent MassHunter Qualitative Analysis software and compared with known compounds based on similar molecular features.
Molecular Docking
PyMol, AutoDock Vina 1.5.6, and Discovery Studio Visualizer 2.5 were utilized to prepare the docking files, perform the docking, and analyze the interaction and output. The 2D structures of targeted ligands (compounds) were retrieved from PubChem (
Figure 1), while the 3D structures of targeted receptors (DENV-2 proteins) were retrieved from Protein Data Bank (PDB) and PyMol (
Figure 2). The active sites residue of each DENV-2 protein found to be interacting were shown in
Table 1. In silico study to determine the binding affinity between the ligands and receptors was done as previously described [
13].
Cell Culture and Virus
Vero cells (African green monkey kidney cells) (ATCC, USA) and C6/36 mosquito cells (ATCC, USA) were cultured in Dulbecco’s Minimum Essential Medium (DMEM) (Gibco, Grand Island, NY, USA), and supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA). The cells were incubated at 37°C and 28°C supplemented with 5% and 3% CO2 humidified atmosphere, respectively. The Vero cells were used for antiviral screening and cytotoxicity assay, while C6/36 mosquito cells were used for propagation and titration of the virus. DENV-2 New Guinea C strain (NGC) (ATCC, Manassas, VI, USA) was used in this study.
Cytotoxicity Assay
Briefly, Vero cells were seeded into 96-well cell culture microplates and incubated overnight at 37°C with 5% CO2. After the incubation, the cell monolayers were treated with different concentrations of KSF 103 ME up to 500µg/mL in duplicate. The treated cells were incubated for four days at 37°C with 5% CO2. After the incubation, MTS reagent (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega, Madison, WI, USA) was added to each well and the microplate was incubated at 37°C for 4 h. To determine the cell viability, the optical density (OD) of the treated cells was measured at 490 nm to 700 nm using TECAN 96-well plate reader (TECAN, Switzerland). Dose-response curves were plotted using Graph Pad Prism Version 5 (Graph Pad Software Inc., San Diego, CA, USA), and the values of 50% cytotoxic concentration (CC50) and Maximum Non-Toxic Dose (MNTD) were calculated.
Screening for Inhibitory Activities of KSF 103 ME against DENV
A monolayer of Vero cells cultured in a 24-well cell culture microplate, in DMEM (Gibco, Grand Island, NY, USA) containing 10% FBS (Gibco, Grand Island, NY, USA) was used. The KSF 103 ME, at different concentrations, were added to the wells together with DENV-2 (MOI ≈ 0.1). The plate was then incubated at 37°C with 5% CO2 for 96 hours. The assay was performed in duplicate for each concentration of the extract. After four days, the cell culture supernatant was harvested, aliquoted, and kept at -80°C until needed for virus RNA extraction and evaluation using qRT-PCR.
Identifying the Effects of KSF 103 ME on Different Stages of DENV-2 Replication Cycle
Vero cells were seeded into a 24-well cell culture microplate and incubated overnight or until the cells became confluent, at 37°C supplemented with 5% CO2. For anti-adsorption study, the cell culture microplate was pre-chilled at 4°C for 30 minutes. The cells were treated with different concentrations of KSF 103 ME (50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, and 3.125 µg/mL) and infected with DENV-2 at MOI ≈ 0.1 and incubated at 4°C for 1 hour. Anti-entry study was performed using the confluent Vero cells infected with DENV-2 at MOI ≈ 0.1 and incubated at 4°C for 1 hour for virus adsorption. Then, the cells were treated with different concentrations of KSF 103 ME and incubated at 37°C with 5% CO2 for 1 hour to allow for the inhibitory activity of the extract against DENV-2 entry. Then, the supernatant was removed and 200 µL of citrate buffer (citric acid buffer 40mM, KCl 10mM, NaCl 135 mM, pH 3.0) was added to the cells and incubated for 1 minute to remove unbound DENV-2 particles. For post-adsorption antiviral assay, the confluent cells were infected with DENV-2 at MOI ≈ 0.1 and incubated at 37°C with 5% CO2 for 1 hour. After virus adsorption, the cells were treated with different concentrations of KSF 103 ME. Prophylactic treatment was performed by treating the confluent cells with the KSF 103ME at different concentrations and incubated at 37°C with 5% CO2 for 5 hours. Direct virucidal assay was performed using DENV-2 at MOI ≈ 10. DENV-2 was pre-incubated with the different concentrations of KSF 103 ME at 1:1 ratio for 2 hours at 37°C with 5% CO2. Then, the treated virus inoculum was diluted 100-fold and added to the confluent cells and incubated at 37°C with 5% CO2 for 1 hour to allow adsorption of DENV-2. DMEM with 2% FBS was added and the plates were incubated at 37°C with 5% CO2 for 4 days. After the incubation, the antiviral activity of the extracts was determined by the reduction in RNA copy numbers determined using qRT-PCR.
DENV-2 Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
DENV-2 RNA was extracted from DENV-infected Vero cells using QIAamp® Viral RNA Mini kit (QIAGEN, Hilden, Germany). Reaction mixture was prepared according to the kit protocol (PrecisionPlus OneStep, UK). Known titres of DENV, ranging from 1x102 FFU to 1x106 FFU, were used as reference standards. The qRT-PCR was performed using the DNA Engine Opticon® system (Bio-Rad, Hercules, CA, USA) with the following thermal conditions: i) reverse transcription at 50°C for 30 min, ii) initial denaturation at 95°C for 10 min, iii) 45 cycles of 95°C for 15 sec, 59°C for 30 sec and 72°C for 30 sec. Primer set used for the amplification was as follows; forward primer (5′ CAA TAT GCT GAA ACG CGA GAG AAA 3′) and reverse primer (5′ AAG ACA TTG ATG GCT TTT GA 3′). A standard curve was plotted to determine the absolute quantities of viral RNA in the samples. The percentage of reduction of RNA copy numbers was calculated by comparing the tested concentrations of KSF 103 ME to the untreated virus control.
Statistical Analysis
The value of 50% cytotoxic concentration (CC50) and 50% inhibitory concentration (IC50) of the extract were determined using Graph Pad Prism Version 5 (Graph Pad Software Inc., San Diego, CA, USA). Selectivity Index (SI) value was determined as the ratio of CC50 to IC50 for the extract.
3. Results
UHPLC-MS Analysis
Initially, UHPLC-MS analysis of KSF 103 ME was performed to identify potential compounds that may exhibit antiviral properties against DENV. Negative and positive ion modes were used with the UHPLC-MS. Four different compounds were reported to exhibit potential antiviral properties based on similar molecular features of known compounds (
Table 2).
Binding Affinity of Potential Antiviral Compounds in KSF 103 ME towards DENV-2 Proteins using Molecular Docking
All four identified compounds were analyzed for binding affinity against DENV-2 proteins by molecular docking as shown in
Figure 3,
Figure 4 and
Figure 5. The top-scoring compounds, referred to as “ligands” (1st ligand pose), with respective target DENV-2 proteins were shown in
Table 3. All ligands were shown to possess binding energy (kcal/mol) of -3.9 to -8.8 with the three DENV-2 proteins; namely 2FOM (NS2B/NS3 protease), 2j7u (NS5 polymerase), and 1OKE (envelope protein). Hypoxanthine showed binding energy of -5.1 kcal/mol, -4.9 kcal/mol and -4.6 kcal/mol towards 2FOM, 2j7u and 1OKE, respectively, while purine was shown to possess binding energy of -4.6 kcal/mol, -4.5 kcal/mol, and -4.0 kcal/mol with 2FOM, 2j7u and 1OKE, respectively. Aminocaproic acid exhibited binding energy of -4.7 kcal/mol, -3.9 kcal/mol, and -4.3 kcal/mol towards 2FOM, 2j7u and 1OKE, respectively. Meanwhile, 1α-fluoro-25-hydroxy-16,17,23,23,24,24-hexadehydrovitamin D3 (Vitamin D3) consistently showed the lowest binding energy against 2FOM, 2j7u and 1OKE, at -8.8 kcal/mol, -7.9 kcal/mol, and -6.6kcal/mol, respectively, compared to other ligands. Among the three DENV-2 proteins, vitamin D3 was shown to possess the highest binding affinity towards 2FOM with a binding energy score of -8.8 kcal/mol.
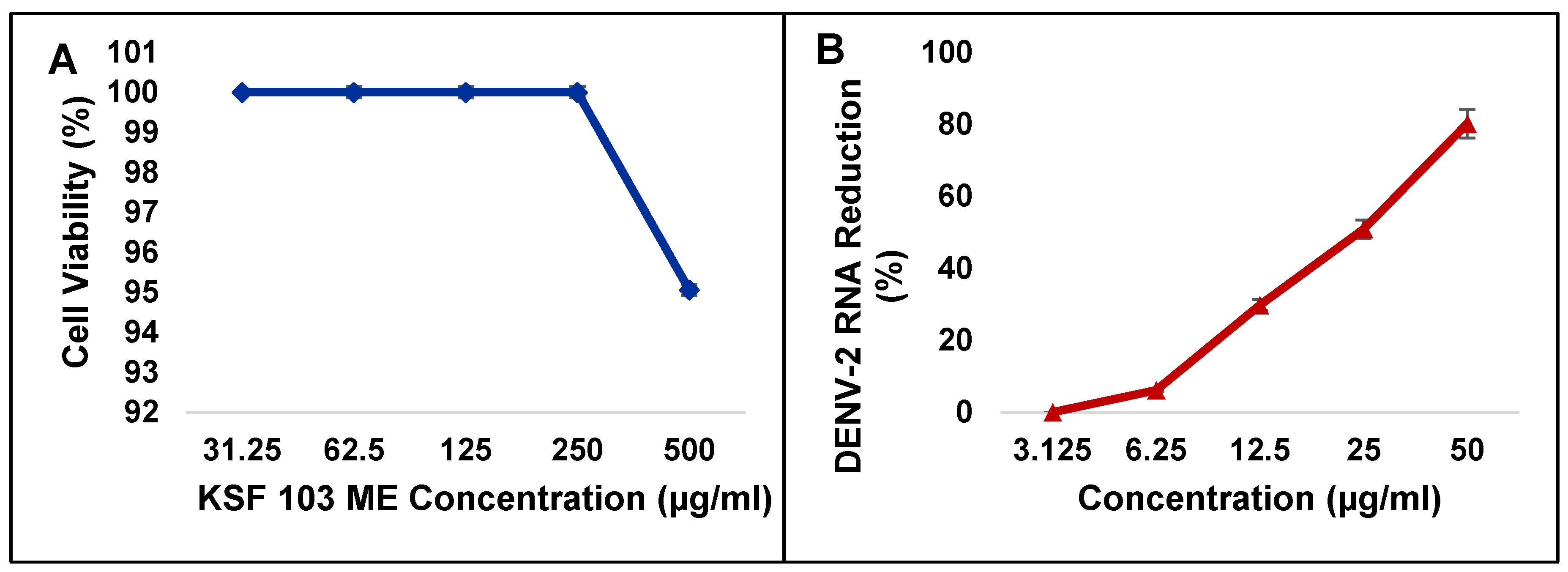
In Vitro Cytotoxicity Assay and Screening for Inhibitory Activities of KSF 103 ME against DENV-2
Cytotoxicity of KSF 103 ME towards Vero cells was first determined. The MNTD and CC
50 were recorded at 512.1 µg/mL and 790.3 µg/mL, respectively (
Table 4). Using these values, the highest concentration of KSF 103 ME (500 µg/mL) used in this study was shown to be non-toxic to the cells (
Figure 6A). The inhibitory activity of KSF 103 ME against DENV-2 was determined by treating the cells with KSF 103 ME at concentrations from 3.125 µg/mL to 50 µg/mL. Results shown in
Table 4 suggested that KSF 103 ME had an IC
50 and SI value of 20.3 µg/mL and 38.9 µg/mL, respectively, with a consistent reduction of DENV-2 RNA from the lowest concentration (3.125 µg/mL) to the highest concentration (50 µg/mL) (
Figure 6B).
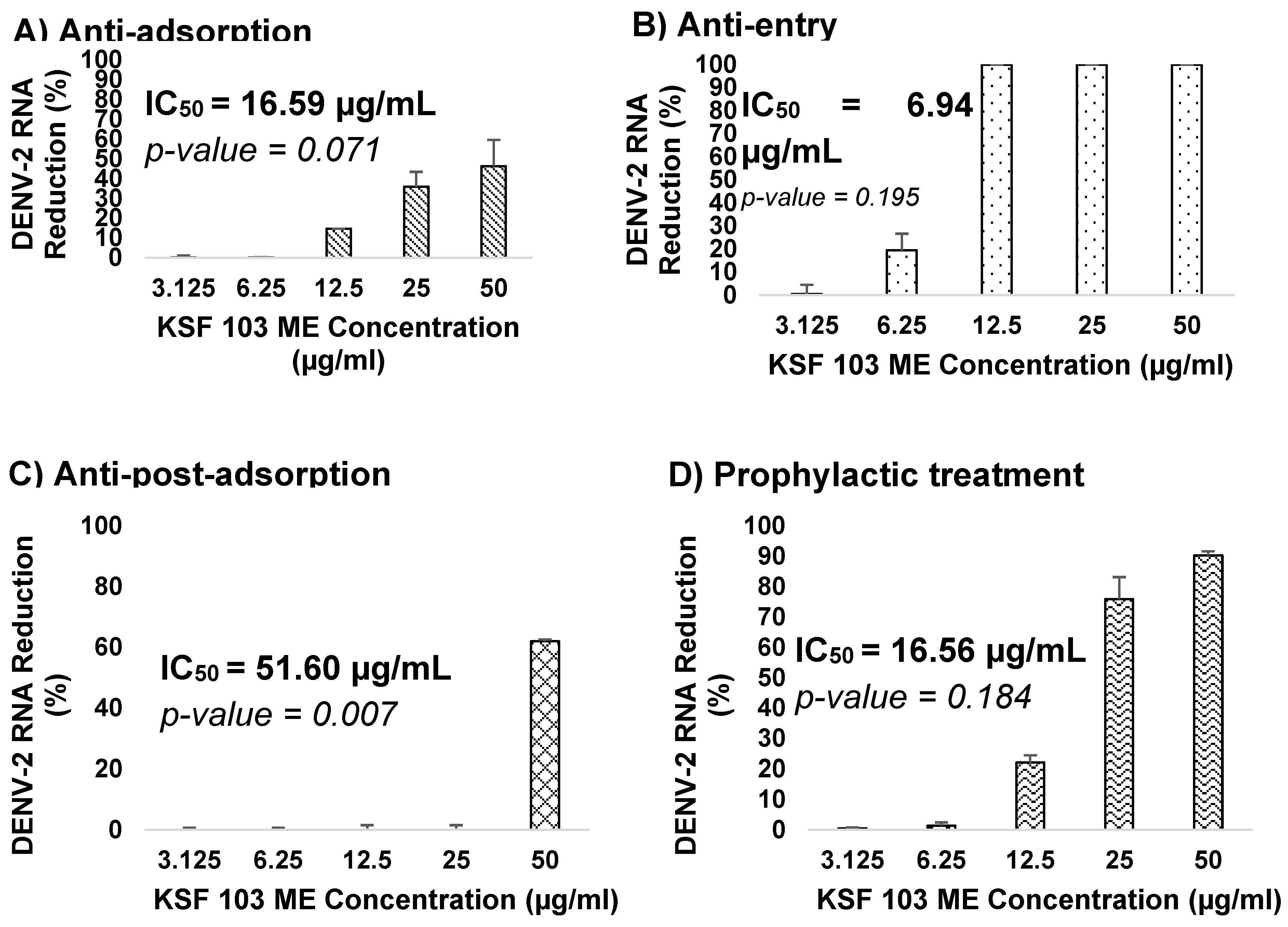
The Effects of KSF 103 ME Treatment at Different Stages of DENV-2 Replication Cycle
KSF 103 ME was evaluated against DENV-2 at different virus replication stages. The antiviral assays include anti-adsorption, anti-entry, anti-post-adsorption, prophylactic, and direct virucidal assays. The graph shows a consistent reduction of virus RNA copy numbers during the virus adsorption to the cells, from 12.5 µg/mL to 50 µg/mL, by 15%, 36%, and 46%, respectively, but exhibited no reduction in the lower concentrations (
Figure 7A). The IC
50 value was 16.59 µg/mL. During the virus internalization into the cells, a reduction of virus RNA copy numbers was consistently observed against the concentration gradient. KSF 103 ME inhibited DENV-2 entry by 1% and 19% at 3.125 µg/mL and 6.25 µg/mL, respectively, and fully inhibited (100%) DENV-2 entry at 12.5 µg/mL, 25 µg/mL, and 50 µg/mL with IC
50 value of 6.94 µg/mL (
Figure 7B). Once the virus entered the cells, the antiviral activity was observed only at the highest concentration (50 µg/mL) by 62% inhibition with IC
50 value of 51.60 µg/mL (
Figure 7C). Antiviral activity of KSF 103 ME was also measured as a prophylactic treatment. There was no inhibition of DENV-2 at 3.125 µg/mL and 6.25 µg/mL but a consistent inhibition was observed at 12.5 µg/mL, 25 µg/mL, and 50 µg/mL by 22%, 76%, and 90%, respectively, with an IC
50 value of 16.56 µg/mL (
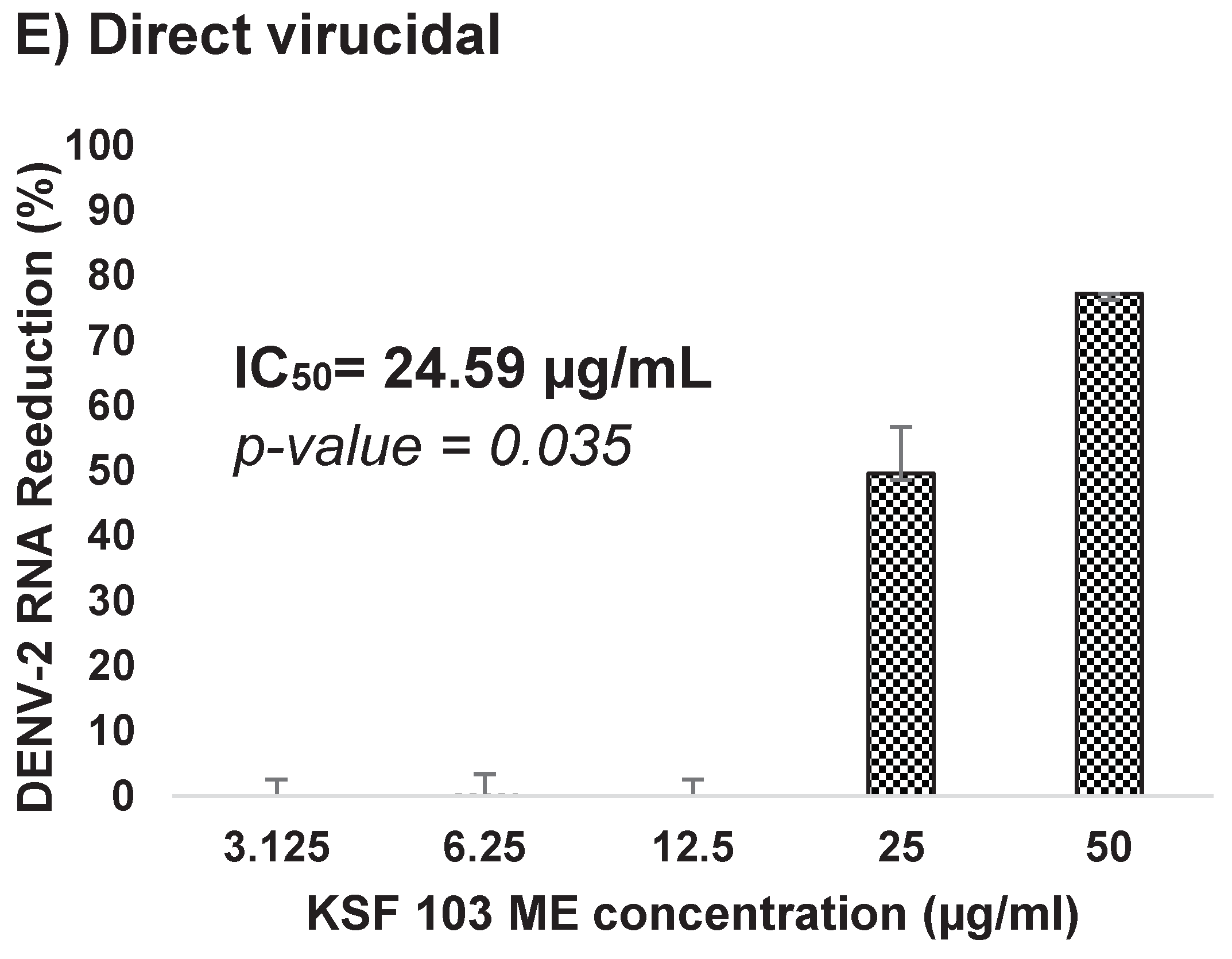
Figure 7D). The extract was also tested as a direct virucidal treatment. No inhibition was recorded at low concentrations (3.125 µg/mL to 12.5 µg/mL), but antiviral activity was observed at 25 µg/mL and 50 µg/mL by 50% and 77%, respectively, with an IC
50 value of 24.59 µg/mL (
Figure 7E).
4. Discussion
No effective treatment for dengue is presently available while efforts to develop vaccines against the infection have resulted in at least two vaccines being approved and to date, no antivirals have successfully been developed. Several efforts to develop dengue antivirals from natural compounds are ongoing. Natural compounds such as plant extracts, marine extracts, soil extracts, or secondary metabolites of microorganisms are widely investigated for potential anti-infectives [
14]. Several studies highlighted the beneficial antiviral properties of Streptomyces bioactive compounds in downregulating some pathogens [15 – 18]. For example, the methanolic fraction of the compound extracted from Streptomyces showed antiviral activity which may interfere with the adsorption and/or penetration of the virus particles into the cells [
19]. The two bioactive compounds from Streptomyces sp., WS-30581 A and WS-30581 B, were investigated and shown to hinder the early stage of EV71 replication (Wei et al., 2014). There are also other studies highlighting the discoveries of Streptomyces bioactive compounds exhibiting antiviral properties against other viruses such as HSV-1, IAV, TMV, and VZV [15 – 17]. In this study, KSF 103 ME was derived from soil sediments of a primary forest in Malaysia. KSF 103 ME was then characterized and tested by antiviral assays for probable antiviral properties against DENV-2 replication.
Based on primary screening through UHPLC-MS, four out of eighty-six compounds were identified from KSF 103 ME that may have potential antiviral properties, namely hypoxanthine, vitamin D3, purine, and aminocaproic acid. Hypoxanthine was previously reported to show inhibitory activity against flaviviruses, alphaviruses, arenaviruses, and rhabdoviruses [
20]. Vitamin D3, among those that existed in KSF 103 ME, was shown to exhibit antiviral activity against hepatitis C, hepatitis B, and herpes simplex virus 1 (HSV-1) [
21,
22,
23,
24]. Purine, a nucleoside analogue, and its derivatives have been widely studied against different viruses such as HIV, HSV-1, HSV-2, varicella zoster virus (VZV), cytomegalovirus (CMV) and hepatitis B [
20,
25,
26,
27,
28]. Finally, aminocaproic acid was also reported in earlier studies to have inhibitory activity against respiratory syncytial virus (RSV) and influenza virus [
29,
30]. The presence of these four compounds may exert antiviral activities against DENV-2 and this was evaluated in the present study.
Molecular docking studies identified the binding affinity of these compounds against three important DENV-2 proteins. All compounds were shown to bind to targeted DENV-2 proteins. Among the three proteins, the highest affinity binding was towards 2FOM (NS2B/NS3 protease). NS2B initially wraps itself around NS3 forming an NS2B/NS3 complex, which plays a vital role in virus maturation and virus RNA replication. KSF 103 ME may inhibit DENV-2 replication by interrupting the activity of NS2B/NS3 protease, suggesting that it is a promising target for anti-DENV-2 drug development [
31]. In addition to 2FOM, these compounds also possessed binding affinity towards 2j7u (NS5 polymerase) and 1OKE (envelope protein). NS5 polymerase is involved in virus replication by the capping of RNA and host cell gene regulation [
32], while envelope protein is the mediator for viral entry and membrane fusion with a receptor on the host cell [
33]. The binding of these identified compounds with NS5 polymerase and envelope protein may hinder viral replication and prevent further spread of dengue infection. Though all compounds were shown to bind to all DENV-2 proteins, vitamin D3 possessed the highest binding affinity towards 2FOM. Little is presently known about KSF 103 ME in inhibiting DENV-2, a strong potential interaction of DENV-2 proteins with the active sites of these compounds identified in silico warrants further investigation [
34].
The CC50 and MNTD of KSF 103 ME were first determined by in vitro cytotoxicity assay. The CC50 described the concentration that resulted in the reduction of cell viability by 50% and any concentrations more than CC50 were considered toxic, while the MNTD was indicative of cell viability at 90% for infection with DENV-2 to occur. Results from this study suggested that KSF 103 ME was non-toxic to the cells as it can be tolerated to as high as 512.1 µg/mL. In the study, the concentrations of KSF 103 ME were fixed at 50 µg/mL since the extract at 50 µg/mL reduced DENV-2 RNA copy numbers by 80% with 100% cell viability. KSF 103 ME showed a consistent inhibition of DENV-2 in a dose-dependent manner. KSF 103 ME was further tested to see the efficacy against DENV-2 at the different replication stages.
Our result suggested KSF 103 ME is most effective in inhibiting DENV-2 during entry. The mechanisms of the inhibition at this stage are still unknown. Inhibition of DENV replication at the entry stage could hinder virus entry into host cells and stop virus replication. This will potentially reduce the level of viremia and prevent the progression of severe dengue. DENV initially infected dendritic cells and Langerhans cells before infecting other surrounding organs such as the liver, spleen, and kidney tissues, and eventually accelerate to severe dengue which manifests severe plasma leakage [
35]. Viremia level was shown to be higher in secondary dengue infection which may prolong the disease and increase the chances of manifesting severe dengue symptoms [
4,
5]. Thus, inhibiting viral entry into the host cells could potentially prevent further viral replication and subsequently curb dengue transmission.
Our findings showed that KSF 103 ME has promising antiviral properties against DENV-2, especially as an inhibitor during virus entry into the host cells. Further studies, however, are required to study the potential mechanisms of the active compounds in KSF 103 ME in inhibiting DENV-2 replication.
5. Conclusions
In summary, the Streptomyces strain KSF 103 methanolic extract with its minimal cytotoxicity and robust antiviral properties at low concentrations, had a potential for further development as an antiviral drug against dengue.
Author Contributions
This work was approved by all the co-authors. Nurfatihah Zulkifli and Jasmine-Elanie Khairat made significant contributions to the concept and study design. Data and logistics for sample acquisition were completed by Adzzie-Shazleen Azman, and Syafiq-Asnawi Zainal Abidin. Nurfatihah Zulkifli, Nur-Faralyza Mohd Baharudin, and Nur-Adila Malek contributed to data analysis and interpretation. Nurfatihah Zulkifli, Sazaly AbuBakar, and Pouya Hassandarvish contributed to writing the draft of the article and critically revising it. Sazaly AbuBakar and Pouya Hassandarcish contributed with grant resources. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We acknowledge the funding from the Ministry of Education, Malaysia for niche area research under the Higher Institution Centre of Excellence (HICoE) program (Project MO002-2019).
Conflicts of Interest
The authors declare no conflicts of interest. The work described in this manuscript is original. It has not been published and is not considered for publication elsewhere, partially, or completely. All authors read and approved the final work.
References
- Gibbons, R. V, & Vaughn, D. W. (2002). Clinical review Dengue : an escalating problem. Bmj, 324, 1563–1566.
- Whitehorn, J., & Simmons, C. P. (2011). The pathogenesis of dengue. Vaccine, 29(42), 7221–7228. [CrossRef] [PubMed]
- Zandi, K., Teoh, B. T., Sam, S. S., Wong, P. F., Mustafa, M. R., & AbuBakar, S. (2012). Novel antiviral activity of baicalein against dengue virus. BMC Complementary and Alternative Medicine, 12(1), 1. [CrossRef]
- De La Cruz-Hernández, S. I., Flores-Aguilar, H., González-Mateos, S., López-Martinez, I., Alpuche-Aranda, C., Ludert, J. E., & Del Angel, R. M. (2013). Determination of viremia and concentration of circulating nonstructural protein 1 in patients infected with dengue virus in Mexico. American Journal of Tropical Medicine and Hygiene, 88(3), 446–454. [CrossRef]
- Vaughn, D. W., Green, S., Kalayanarooj, S., Innis, B. L., Nimmannitya, S., Suntayakorn, S., Endy, T. P., Raengsakulrach, B., Rothman, A. L., Ennis, F. A., & Nisalak, A. (2000). Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. Journal of Infectious Diseases, 181(1), 2–9. [CrossRef]
- Kimura, T., Suga, T., Kameoka, M., Ueno, M., Inahashi, Y., Matsuo, H., Iwatsuki, M., Shigemura, K., Shiomi, K., Takahashi, Y., Ōmura, S., & Nakashima, T. (2019). New tetrahydroquinoline and indoline compounds containing a hydroxy cyclopentenone, virantmycin B and C, produced by Streptomyces sp. AM-2504. Journal of Antibiotics, 72(3), 169–173. [CrossRef]
- Liu, M., Ren, M., Zhang, Y., Wan, Z., Wang, Y., Wu, Z., Wang, K., Fang, W., & Yang, X. (2023). Antiviral Activity of Benzoheterocyclic Compounds from Soil-Derived Streptomyces jiujiangensis NBERC-24992. Molecules, 28(2), 1–12. [CrossRef]
- Murthy, Y. K. S., Thiemann, J. E., Coronelli, C., & Sensi, P. (1966). Alanosine , a New Antiviral and Antitumour Agent isolated from a Streptomyces Trauma and Melanoma Production Transfer of Experimental Nephritis in Rats by means of Lymphatic Duct Lymphocytes. Nature, 211, 1198–1199.
- Low, J. S. Y., Wu, K. X., Chen, K. C., Ng, M. M. L., & Chu, J. J. H. (2011). Narasin, a novel antiviral compound that blocks dengue virus protein expression. Antiviral Therapy, 16(8), 1203–1218. [CrossRef]
- Azman, A. S., Othman, I., Fang, C. M., Chan, K. G., Goh, B. H., & Lee, L. H. (2017). Antibacterial, Anticancer and Neuroprotective Activities of Rare Actinobacteria from Mangrove Forest Soils. Indian Journal of Microbiology, 57(2), 177–187. [CrossRef]
- Lee, L. H., Cheah, Y. K., Sidik, S. M., Mutalib, N. S. A., Tang, Y. L., Lin, H. P., & Hong, K. (2012). Molecular characterization of Antarctic actinobacteria and screening for antimicrobial metabolite production. World Journal of Microbiology and Biotechnology, 28(5), 2125–2137. [CrossRef]
- Ser, H. L., Palanisamy, U. D., Yin, W. F., Chan, K. G., Goh, B. H., & Lee, L. H. (2016). Streptomyces malaysiense sp. nov.: A novel Malaysian mangrove soil actinobacterium with antioxidative activity and cytotoxic potential against human cancer cell lines. Scientific Reports, 6(March), 1–12. [CrossRef]
- Hassandarvish, P., Rothan, H. A., Rezaei, S., Yusof, R., Abubakar, S., & Zandi, K. (2016). In silico study on baicalein and baicalin as inhibitors of dengue virus replication. RSC Advances, 6(37), 31235–31247. [CrossRef]
- Harir, M., Bendif, H., Bellahcene, M., & Fortas and Rebecca Pogni, Z. (2018). Streptomyces Secondary Metabolites. Basic Biology and Applications of Actinobacteria. [CrossRef]
- Fürstner, A., Albert, M., Mlynarski, J., Matheu, M., & DeClercq, E. (2003). Structure Assignment, Total Synthesis, and Antiviral Evaluation of Cycloviracin B1. Journal of the American Chemical Society, 125(43), 13132–13142. [CrossRef]
- Ismet Ara. (2012). Antiviral activities of streptomycetes against tobacco mosaic virus (TMV) in Datura plant: Evaluation of different organic compounds in their metabolites. African Journal of Biotechnology, 11(8), 2130–2138. [CrossRef]
- Li, F., Chen, D., Lu, S., Yang, G., Zhang, X., Chen, Z., Fan, S., Wu, S., & He, J. (2018). Anti-influenza a viral butenolide from streptomyces sp. Smu03 inhabiting the intestine of Elephas maximus. Viruses, 10(7), 1–14. [CrossRef]
- Wei, Y., Fang, W., Wan, Z., Wang, K., Yang, Q., Cai, X., Shi, L., & Yang, Z. (2014). Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virology Journal, 11(1), 1–14. [CrossRef]
- Rodrigues Sacramento, D., Rodrigues Coelho, R. R., Wigg, M. D., Luna Linhares, L. F. D. T., Matos Dos Santos, M. G., Soares Semêdo, L. T. D. A., & Ribeiro Da Silva, A. J. (2004). Antimicrobial and antiviral activities of an actinomycete (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World Journal of Microbiology and Biotechnology, 20(3), 225–229. [CrossRef]
- Nair, V., & Ussery, M. A. (1992). New hypoxanthine nucleosides with RNA antiviral activity. Antiviral Research, 19(2), 173–178. [CrossRef]
- Bitetto, D., Fabris, C., Fornasiere, E., Pipan, C., Fumolo, E., Cussigh, A., Bignulin, S., Cmet, S., Fontanini, E., Falleti, E., Martinella, R., Pirisi, M., & Toniutto, P. (2011). Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transplant International, 24(1), 43–50. [CrossRef]
- Gal-Tanamy, M., Bachmetov, L., Ravid, A., Koren, R., Erman, A., Tur-Kaspa, R., & Zemel, R. (2011). Vitamin D: An innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology, 54(5), 1570–1579. [CrossRef]
- He, L. J., Zhang, H. P., Li, H. J., Wang, J., & Chang, D. D. (2016). Effect of Serum Vitamin D Levels on Cellular Immunity and Antiviral Effects in Chronic Hepatitis B Patients. Clinical Laboratory, 62(10), 1933–1939. [CrossRef]
- Puerta-Guardo, H., De la Cruz Hernández, S. I., Rosales, V. H., Ludert, J. E., & del Angel, R. M. (2012). The 1α,25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antiviral Research, 94(1), 57–61. [CrossRef]
- Clercq, E. De, Sakuma, T., Baba, M., Pauwels, R., Balzarini, J., Rosenberg, I., & Holý, A. (1987). Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Research, 8(5–6), 261–272. [CrossRef]
- Kim, C., Luh, B., Misco, P., Bronson, J., Hitchcock, M., Ghazzouli, I., & Martin, J. (1990). Acyclic Purine Phosphonate Analogues as Antiviral Agents. Synthesis and Structure-Activity Relationships. J Med Chem, 33(4), 1207–1213. [CrossRef]
- Sariri, R., & Khalili, G. (2002). Synthesis of purine antiviral agents, hypoxanthine and 6-mercaptopurine. Russian Journal of Organic Chemistry, 38(7), 1053–1055. [CrossRef]
- Shaw, T., Amor, P., Civitico, G., Boyd, M., & Locarnini, S. (1994). In vitro antiviral activity of penciclovir, a novel purine nucleoside, against duck hepatitis B virus. Antimicrobial Agents and Chemotherapy, 38(4), 719–723. [CrossRef]
- Falynskova, I. N., Ionova, K. S., Dedova, A. V., Leveva, I. A., & Makhmudova, N. R. (2014). Antiviral Activity of Fullerene- ( Tris -Aminocaproic Acid ) Hydrate Against Respiratory Syncytial Virus in HEp-2 Cell Culture. Pharmaceutical Chemistry Journal, May 2014, 85–88. [CrossRef]
- Serkedjieva, J., Nikolova, E., & Kirilov, N. (2010). Synergistic inhibition of Influenza A virus replication by a plant polyphenolrich extract and ε-aminocaproic acid in vitro and in vivo. Acta Virologica, 54(2), 137–145. [CrossRef]
- Rothan, H. A., Mohamed, Z., Paydar, M., Rahman, N. A., & Yusof, R. (2014). Inhibitory effect of doxycycline against dengue virus replication in vitro. Archives of Virology, 159(4), 711–718. [CrossRef]
- Rawlinson, S., Pryor, M., Wright, P., & Jans, D. (2006). Dengue Virus RNA Polymerase NS5: A Potential Therapeutic Target? Current Drug Targets, 7(12), 1623–1638. [CrossRef]
- Huang, Y.-W., Lee, C.-T., Wang, T.-C., Kao, Y.-C., Yang, C.-H., Lin, Y.-M., & Huang, K.-S. (2018). The Development of Peptide-based Antimicrobial Agents against Dengue Virus. Current Protein & Peptide Science, 19(10), 998–1010. [CrossRef]
- Tong, J., Trapido-Rosenthal, H., Wang, J., Wang, Y., Li, Q. X., & Lu, Y. (2012). Antiviral activities and putative identification of compounds in microbial extracts from the Hawaiian coastal waters. Marine Drugs, 10(3), 521–538. [CrossRef]
- Wu, S. J. L., Grouard-Vogel, G., Sun, W., Mascola, J. R., Brachtel, E., Putvatana, R., Louder, M. K., Filgueira, L., Marovich, M. A., Wong, H. K., Blauvelt, A., Murphy, G. S., Robb, M. L., Innes, B. L., Birx, D. L., Hayes, C. G., & Frankel, S. S. (2000). Human skin Langerhans cells are targets of dengue virus infection. Nature Medicine, 6(7), 816–820. [CrossRef]
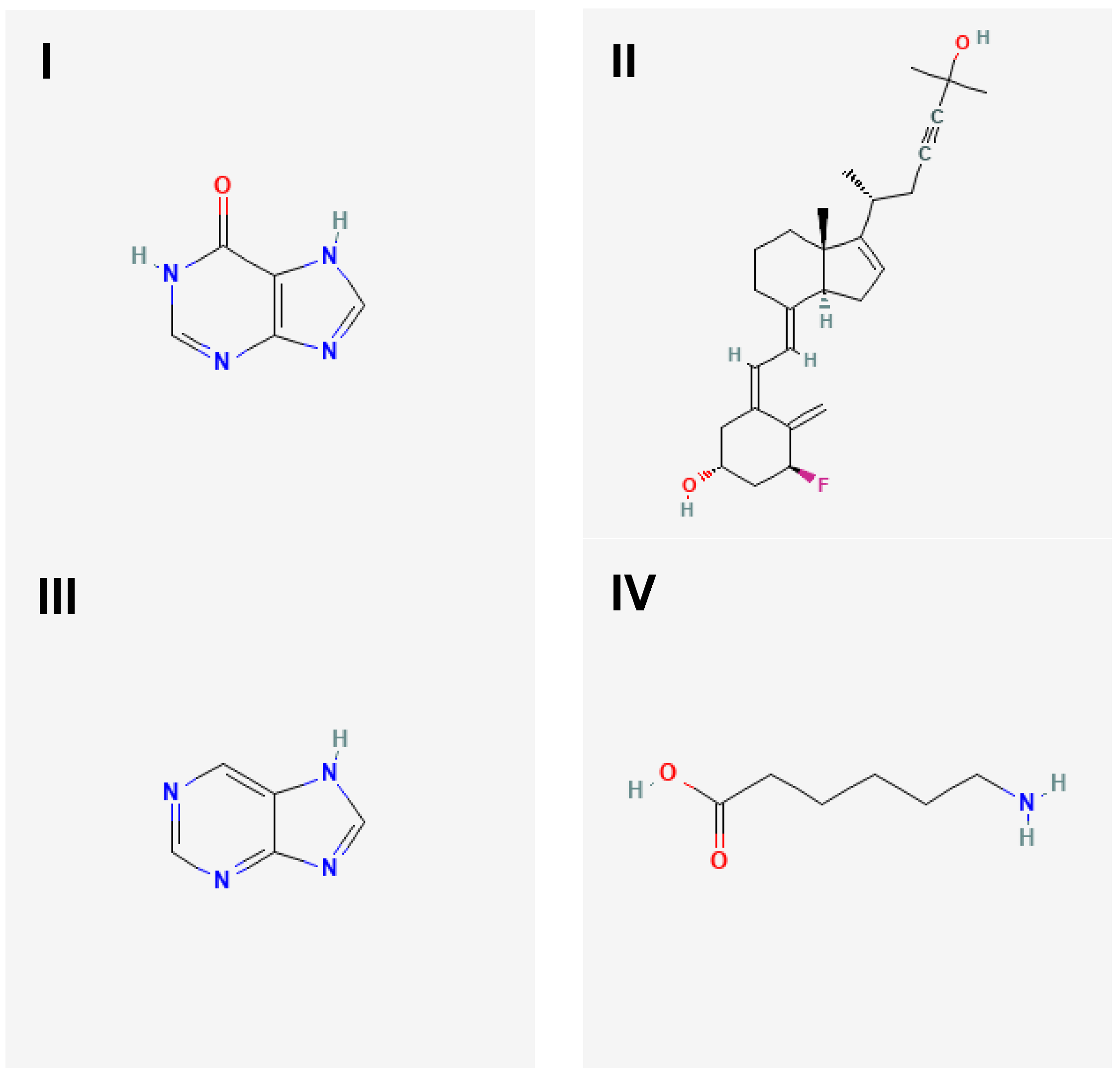
Figure 1.
The 2D molecular structures of targeted ligands. Targeted ligands were hypoxanthine (I), 1α-fluoro-25-hydroxy-16,17,23,23,24,24-hexadehydrovitamin D3 (II), purine (III) and aminocaproic acid (IV).
Figure 1.
The 2D molecular structures of targeted ligands. Targeted ligands were hypoxanthine (I), 1α-fluoro-25-hydroxy-16,17,23,23,24,24-hexadehydrovitamin D3 (II), purine (III) and aminocaproic acid (IV).
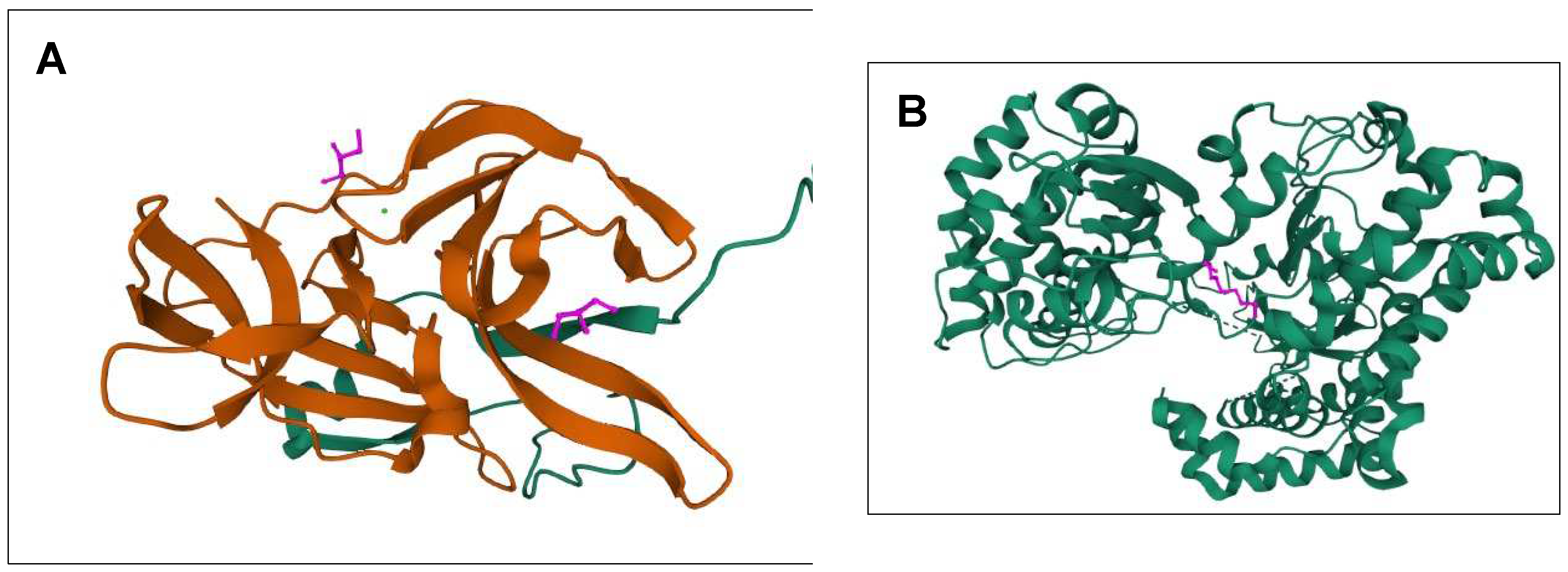
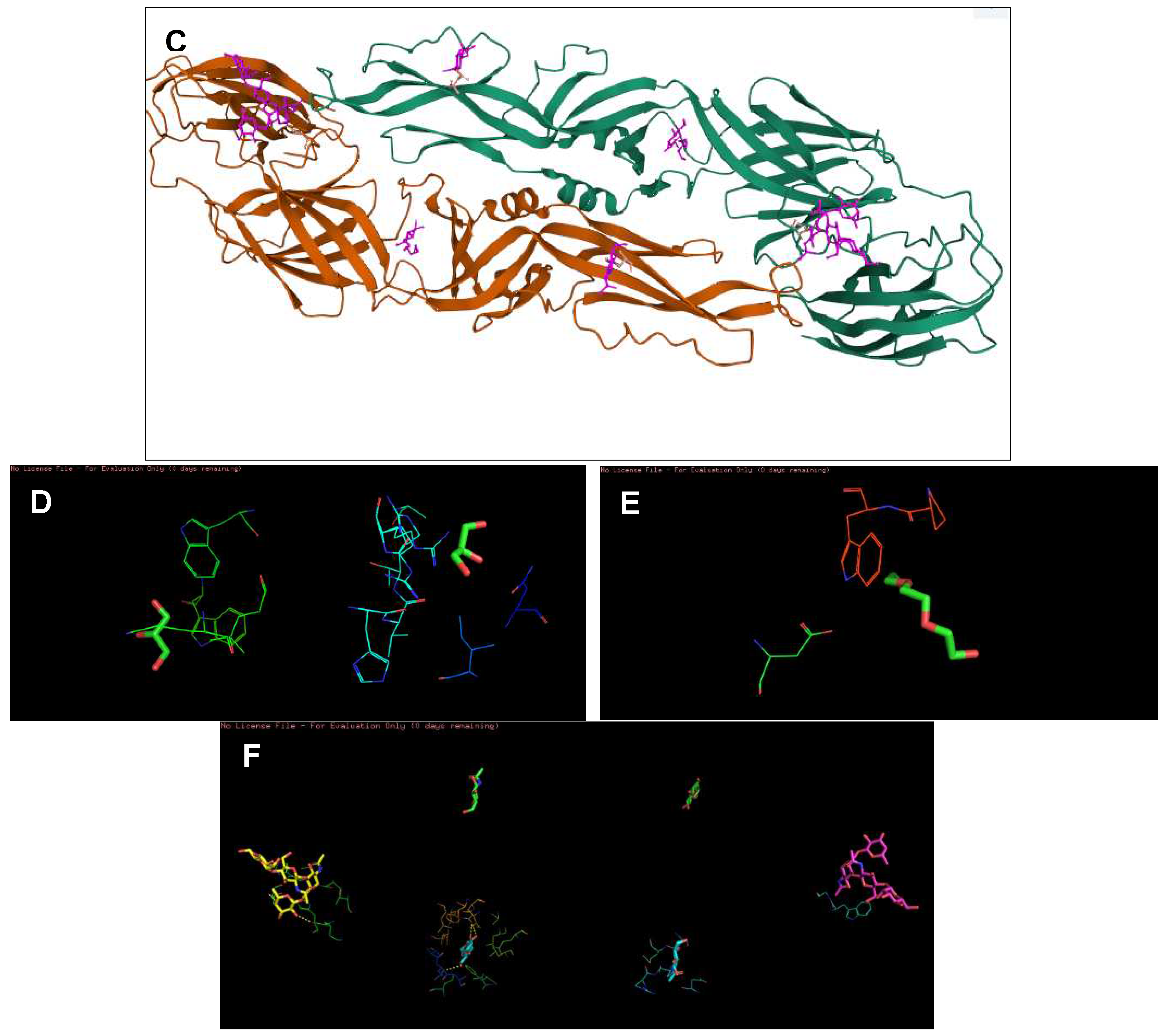
Figure 2.
The 3D structures of targeted DENV-2 proteins derived from PDB server. (A) Structure of NS2B/NS3 protease (ID: 2FOM). Orange: NS3pro, Green: NS2B. Two existing ligands were found interacting in the structure. (B) NS5 polymerase (ID:2j7u). Only one existing ligand found in the structure. (C) E protein (ID: 1OKE). The structure consists of 6 existing ligands. (D–F) Structures of 2FOM, 2j7u and 1OKE visualized in PyMol, respectively.
Figure 2.
The 3D structures of targeted DENV-2 proteins derived from PDB server. (A) Structure of NS2B/NS3 protease (ID: 2FOM). Orange: NS3pro, Green: NS2B. Two existing ligands were found interacting in the structure. (B) NS5 polymerase (ID:2j7u). Only one existing ligand found in the structure. (C) E protein (ID: 1OKE). The structure consists of 6 existing ligands. (D–F) Structures of 2FOM, 2j7u and 1OKE visualized in PyMol, respectively.
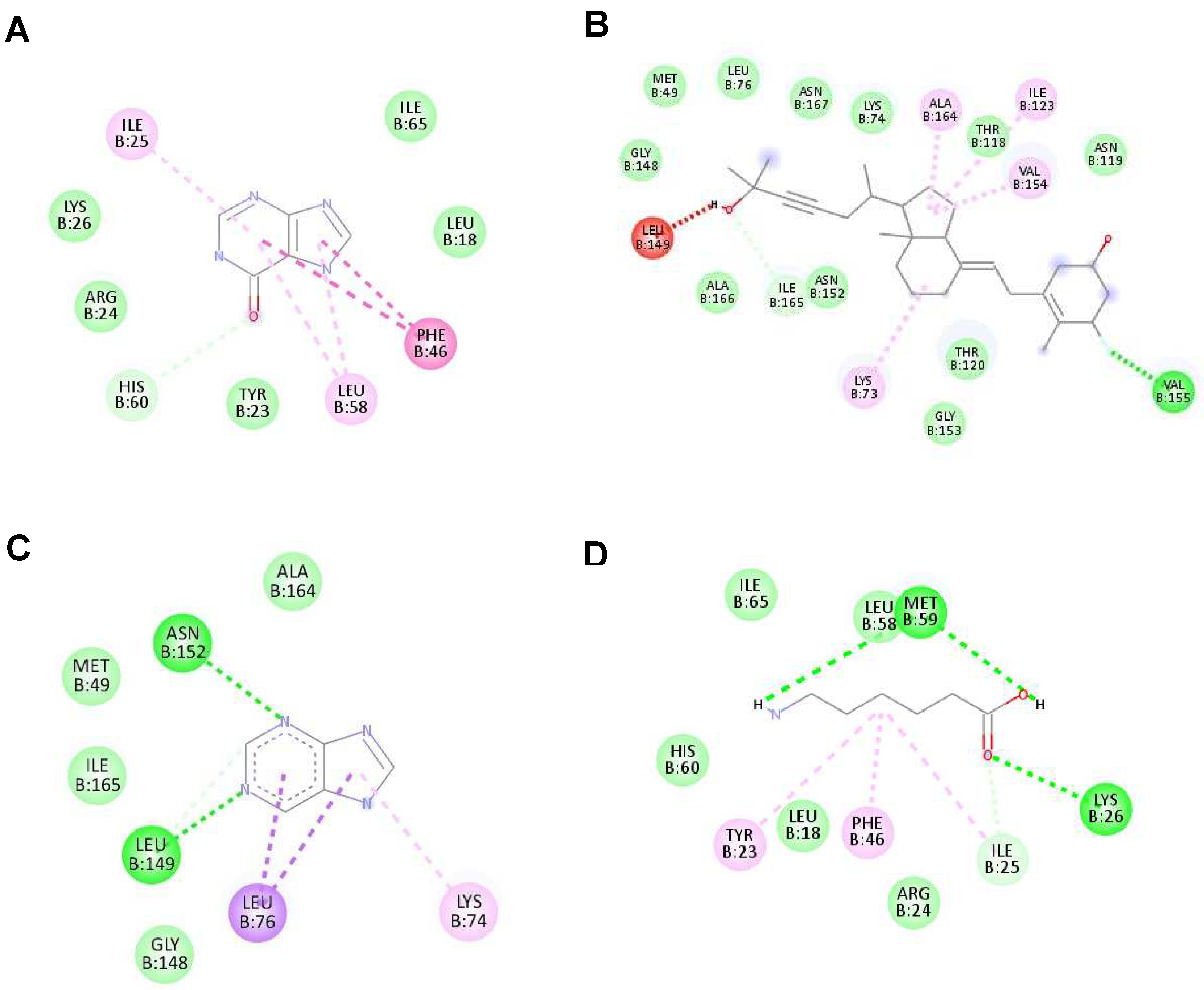
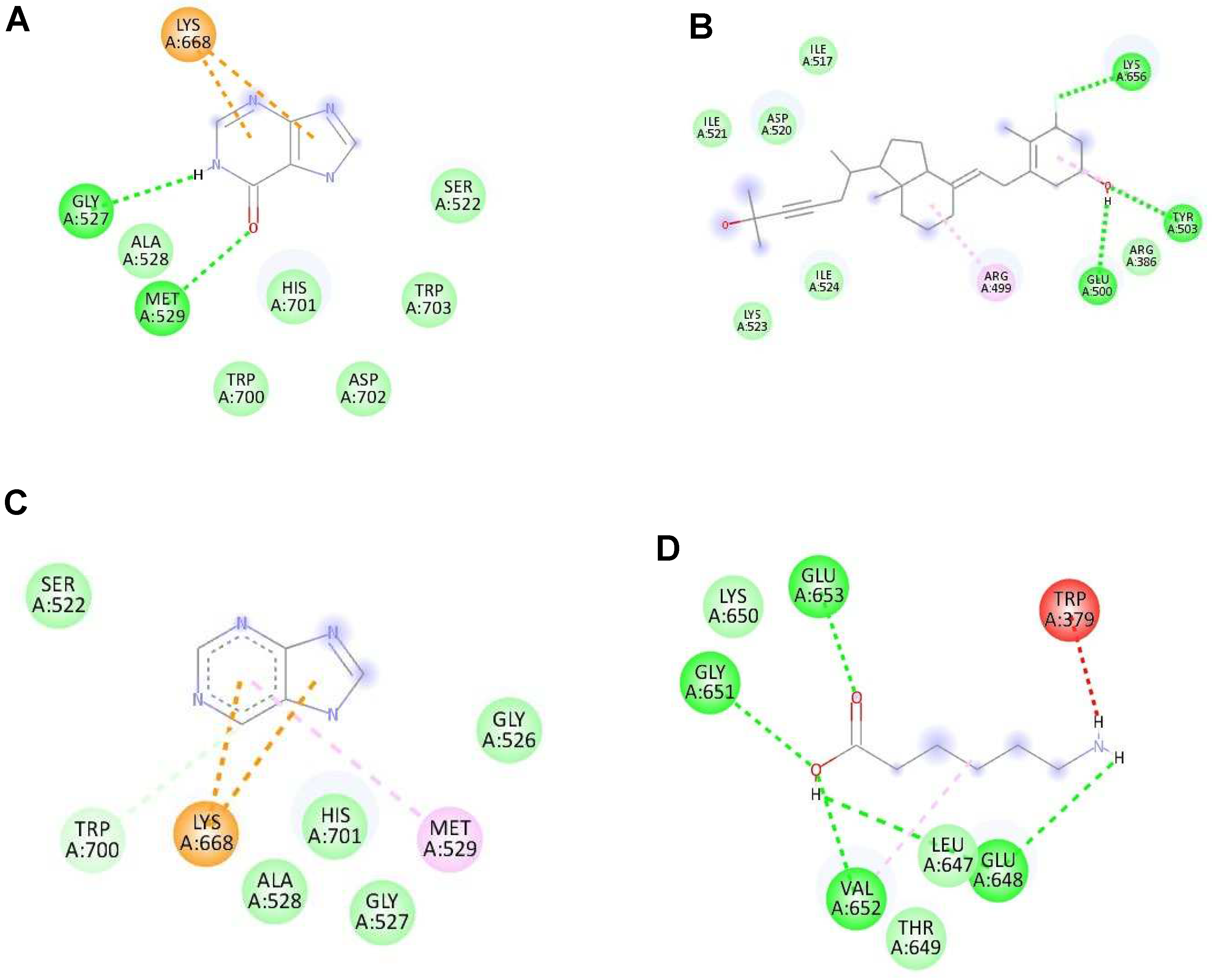
Figure 3.
The highest binding affinity (1st ligand pose) of each ligand with NS2B/NS3 protease (ID:2FOM) generated using Discovery Studio Visualizer. (A–D) The interaction of hypoxanthine, vitamin D3, purine and aminocaproic acid with 2FOM, respectively.
Figure 3.
The highest binding affinity (1st ligand pose) of each ligand with NS2B/NS3 protease (ID:2FOM) generated using Discovery Studio Visualizer. (A–D) The interaction of hypoxanthine, vitamin D3, purine and aminocaproic acid with 2FOM, respectively.
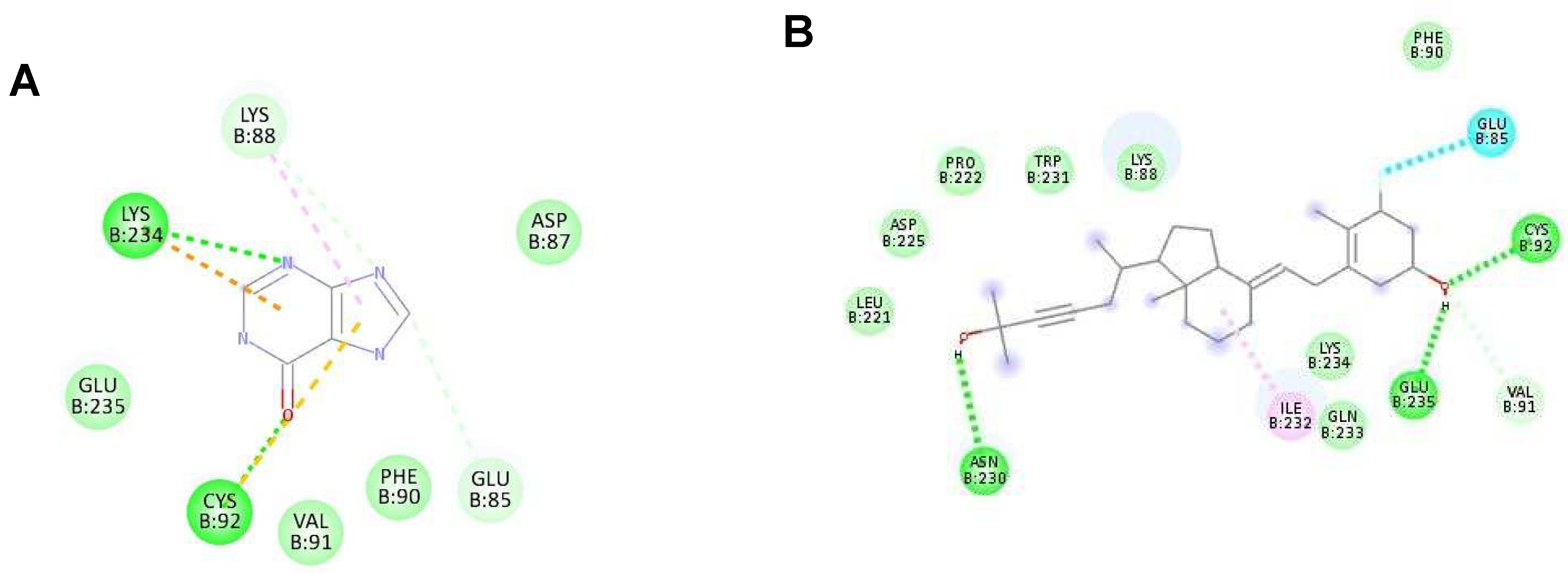
Figure 4.
The interaction of each ligand with NS5 polymerase (ID:2j7u). (A–D) The interaction of hypoxanthine, vitamin D3, purine, and aminocaproic acid with 2j7u, respectively.
Figure 4.
The interaction of each ligand with NS5 polymerase (ID:2j7u). (A–D) The interaction of hypoxanthine, vitamin D3, purine, and aminocaproic acid with 2j7u, respectively.
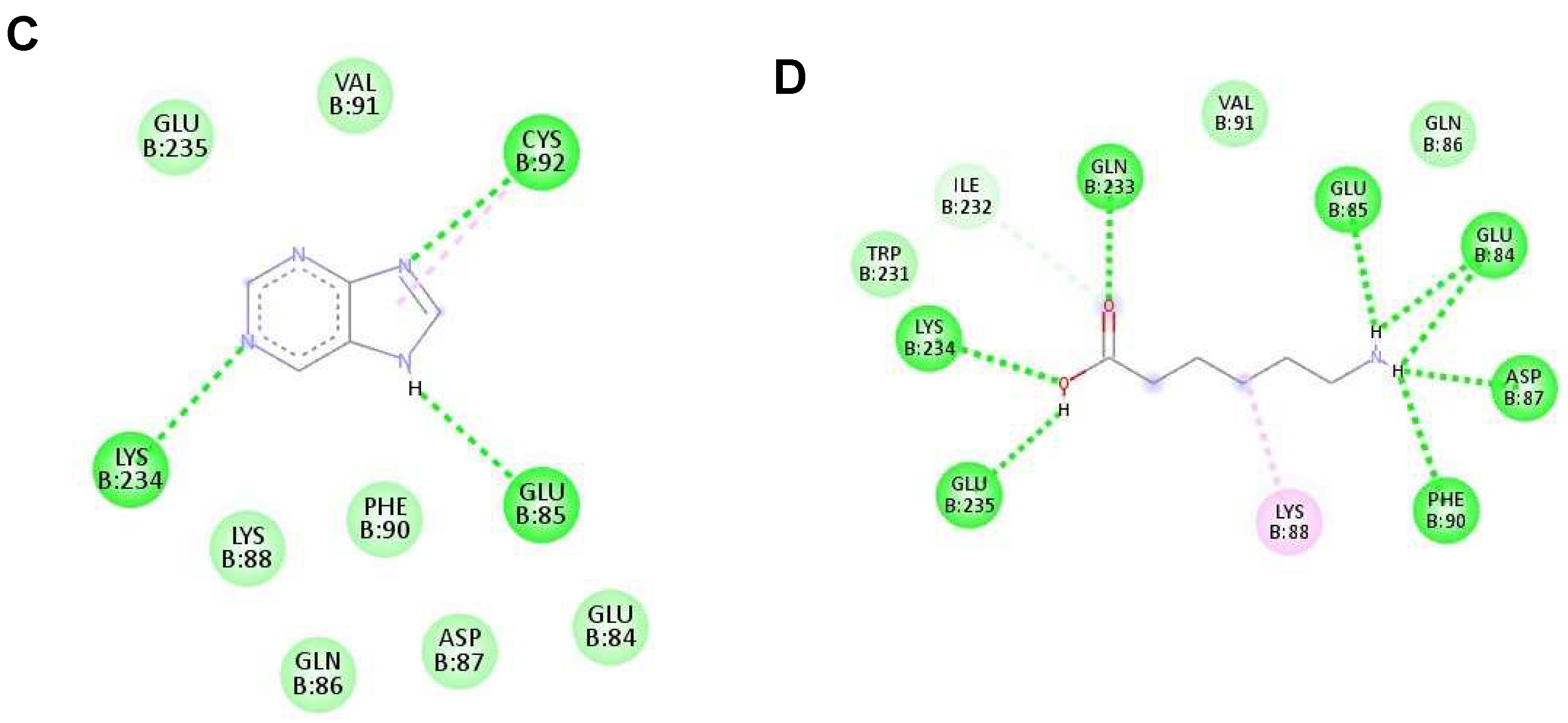
Figure 5.
The interaction of each ligand with envelope protein (ID:1OKE) visualized using Discovery Studio Visualizer. (A–D) The interaction of hypoxanthine, vitamin D, purine, and aminocaproic acid with 1OKE, respectively.
Figure 5.
The interaction of each ligand with envelope protein (ID:1OKE) visualized using Discovery Studio Visualizer. (A–D) The interaction of hypoxanthine, vitamin D, purine, and aminocaproic acid with 1OKE, respectively.
Figure 6.
Cytotoxicity of KSF 103 ME against Vero cells (A) and screening for inhibitory activities of KSF 103 ME against DENV-2 (B). The extract was non-toxic to Vero cells even at the highest concentration and the extract showed a consistent reduction of DENV-2 RNA against the concentration gradient.
Figure 6.
Cytotoxicity of KSF 103 ME against Vero cells (A) and screening for inhibitory activities of KSF 103 ME against DENV-2 (B). The extract was non-toxic to Vero cells even at the highest concentration and the extract showed a consistent reduction of DENV-2 RNA against the concentration gradient.
Figure 7.
(A–E) Dose-dependent antiviral activity of KSF 103 ME at different stages of DENV-2 replication cycle. The DENV-2 infected cells were treated with the shown concentration of KSF 103 ME in duplicate and the graph was presented with error bars representing standard deviation. DENV-2 copy numbers were measured by qRT-PCR and the reduction percentage was calculated as shown.
Figure 7.
(A–E) Dose-dependent antiviral activity of KSF 103 ME at different stages of DENV-2 replication cycle. The DENV-2 infected cells were treated with the shown concentration of KSF 103 ME in duplicate and the graph was presented with error bars representing standard deviation. DENV-2 copy numbers were measured by qRT-PCR and the reduction percentage was calculated as shown.
Table 1.
Predicted active sites residue for each targeted DENV-2 protein obtained using PyMol.
Table 1.
Predicted active sites residue for each targeted DENV-2 protein obtained using PyMol.
| Targeted DENV-2 Protein |
Active Sites Residue |
| NS2B/NS3 protease (ID:2FOM) |
TRP-69, LYS-74, LEU-76, TRP-83 |
| NS5 polymerase (ID: 2j7u) |
ASP-520, PRO-822, TRP-823 |
| Envelope protein (ID: 1OKE) |
ASN-67, GLU-84, ARG-89, PHE-90, MET-118 |
Table 2.
Composition of KSF 103 ME using UHPLC-MS.
Table 2.
Composition of KSF 103 ME using UHPLC-MS.
| NO |
Proposed compounds |
Chemical Formula |
| 1 |
Hypoxanthine [12-14] |
C5H4N4O |
| 2 |
1α-fluoro-25-hydroxy-16,17,23,23,24,24-hexadehydrovitamin D3 / 1α-fluoro-25-hydroxy-16,17,23,23,24,24-hexadehydrocholecalciferol [15-18] |
C27H37FO2
|
| 3 |
Purine [12, 14, 19-21] |
C5H4N4
|
| 4 |
Aminocaproic acid [22, 23] |
C6H13NO2
|
Table 3.
Top binding affinity of each ligand with respective target DENV-2 proteins.
Table 3.
Top binding affinity of each ligand with respective target DENV-2 proteins.
| Protein |
Ligand |
Binding Energy (kcal/mol) |
| 2FOM |
Hypoxanthine |
-5.1 |
| Vitamin D3 |
-8.8 |
| Purine |
-4.6 |
| Aminocaproic acid |
-4.7 |
| 2j7u |
Hypoxanthine |
-4.9 |
| Vitamin D3 |
-7.9 |
| Purine |
-4.5 |
| Aminocaproic acid |
-3.9 |
| 1OKE |
Hypoxanthine |
-4.6 |
| Vitamin D3 |
-6.6 |
| Purine |
-4.0 |
| Aminocaproic acid |
-4.3 |
Table 4.
The CC50, MNTD, IC50, and SI values of KSF 103 ME.
Table 4.
The CC50, MNTD, IC50, and SI values of KSF 103 ME.
| Extract |
CC50 (µg/mL) |
MNTD (µg/mL) |
IC50 (µg/mL) |
SI value |
|
Streptomyces strain KSF 103 methanolic extract |
790.3 |
512.1 |
20.3 |
38.9 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).