Submitted:
13 February 2023
Posted:
16 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatment
2.2. Measurement of Cardiac Function
2.3. Haematoxylin and Eosin (HE) Staining and Wheat Germ Agglutinin (WGA) Staining
2.4. Transcriptome Sequencing
2.5. Western Blot
2.6. Statistical Analysis
3. Results
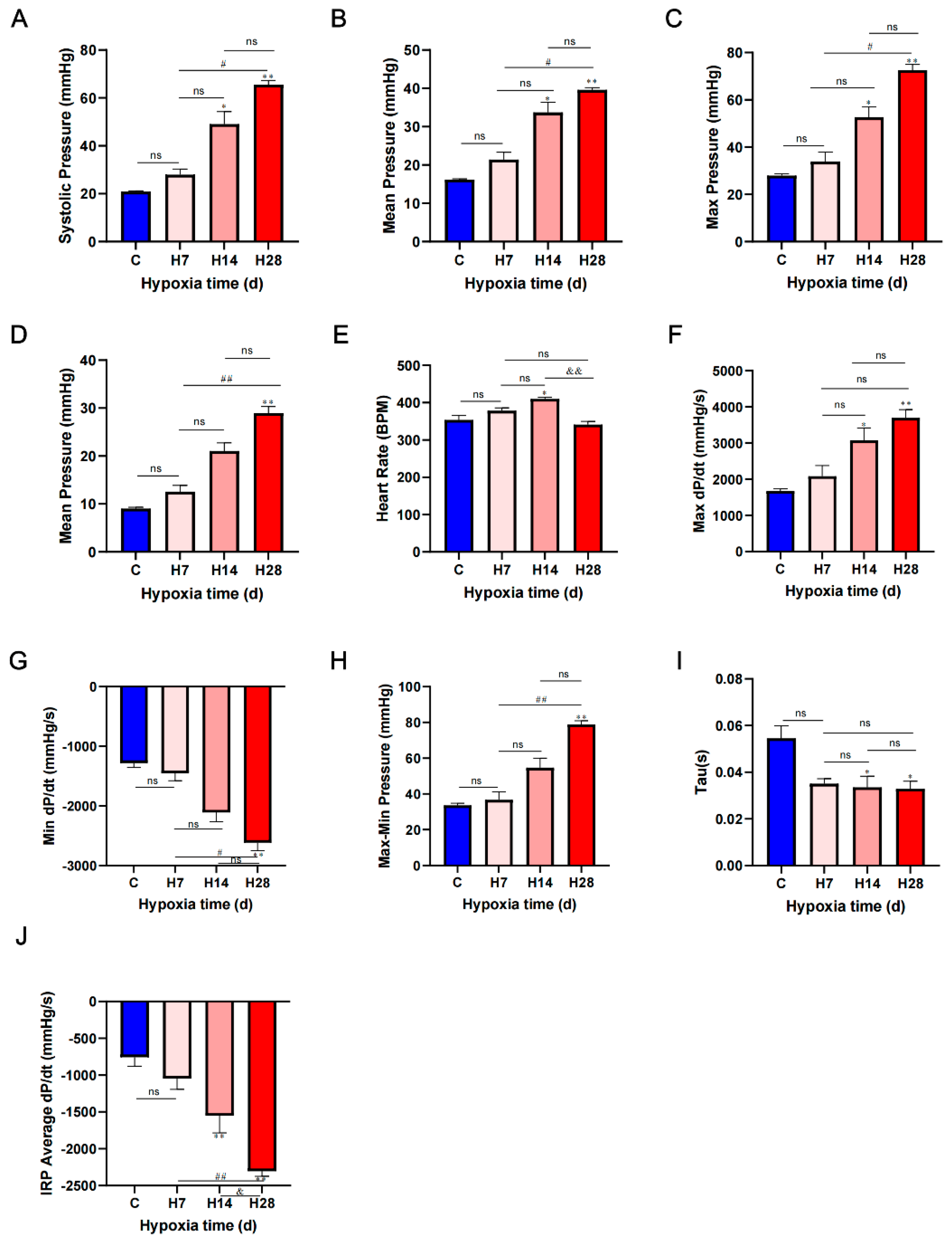
3.1. Hypoxia Developed Pulmonary Hypertension, Right Ventricular Diastolic and Systolic Functions Were Enhanced in Rats
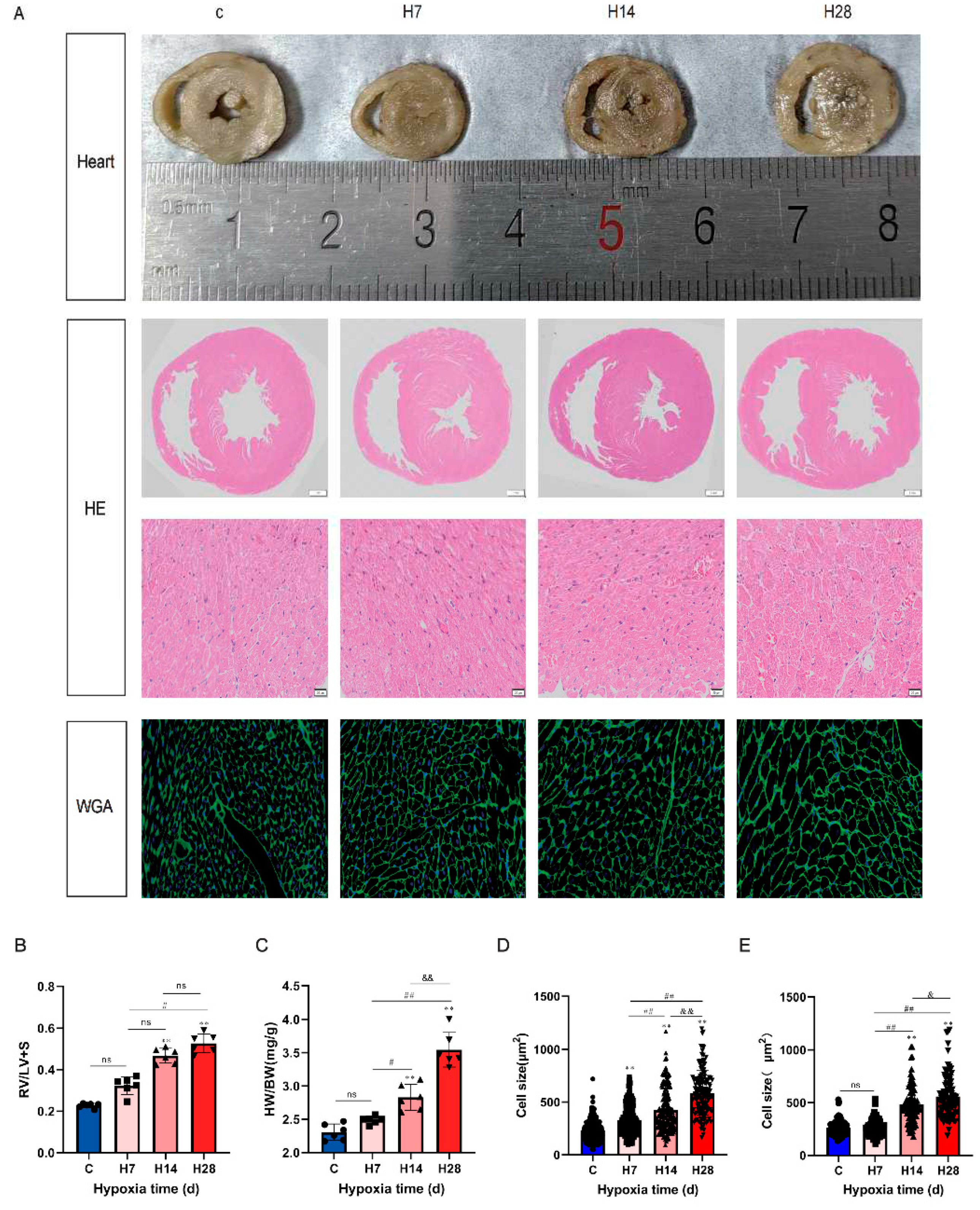
3.2. Hypoxia Caused Cardiomyocyte Hypertrophy
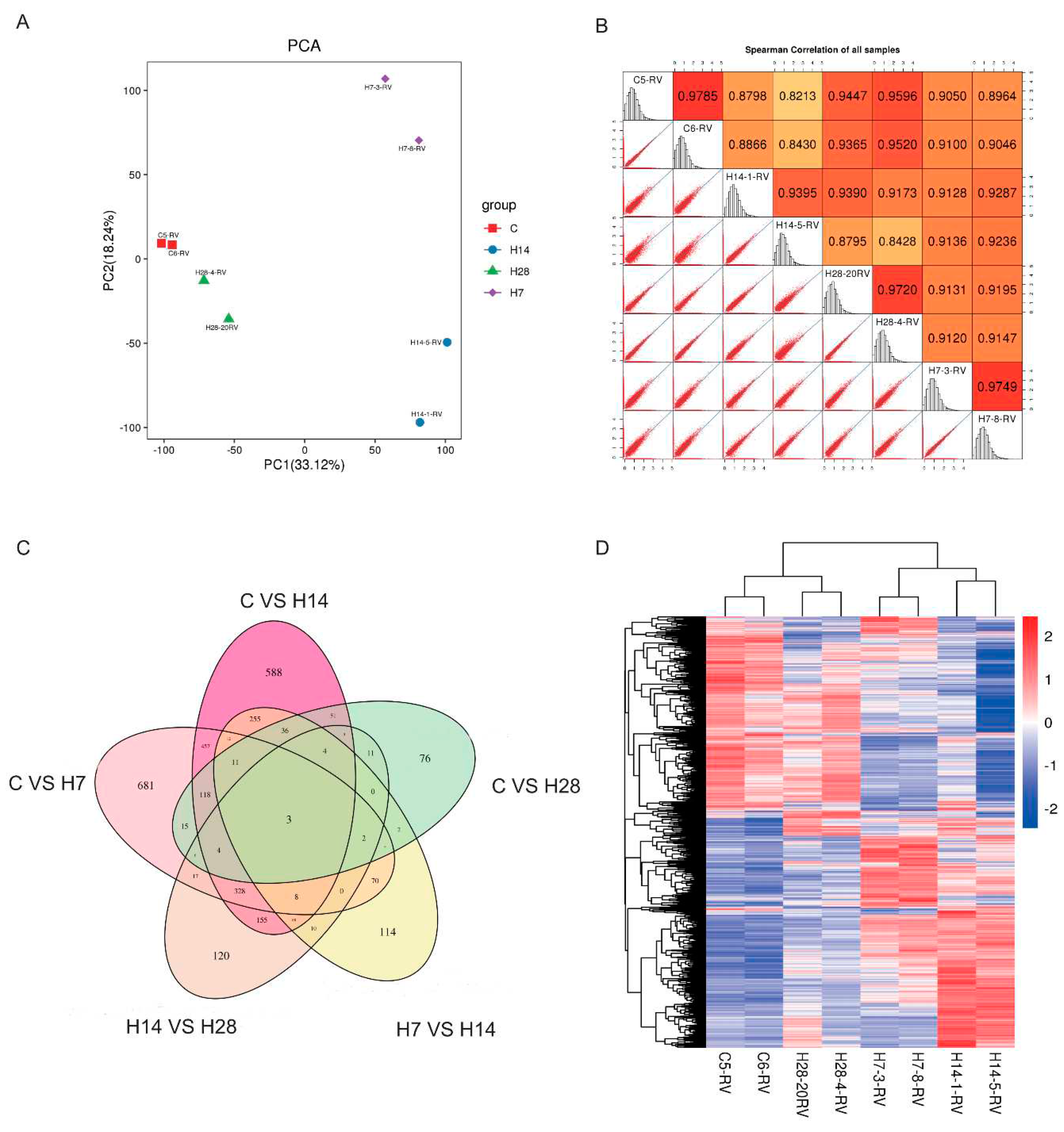
3.3. Transcriptomic Screening of Right Ventricular
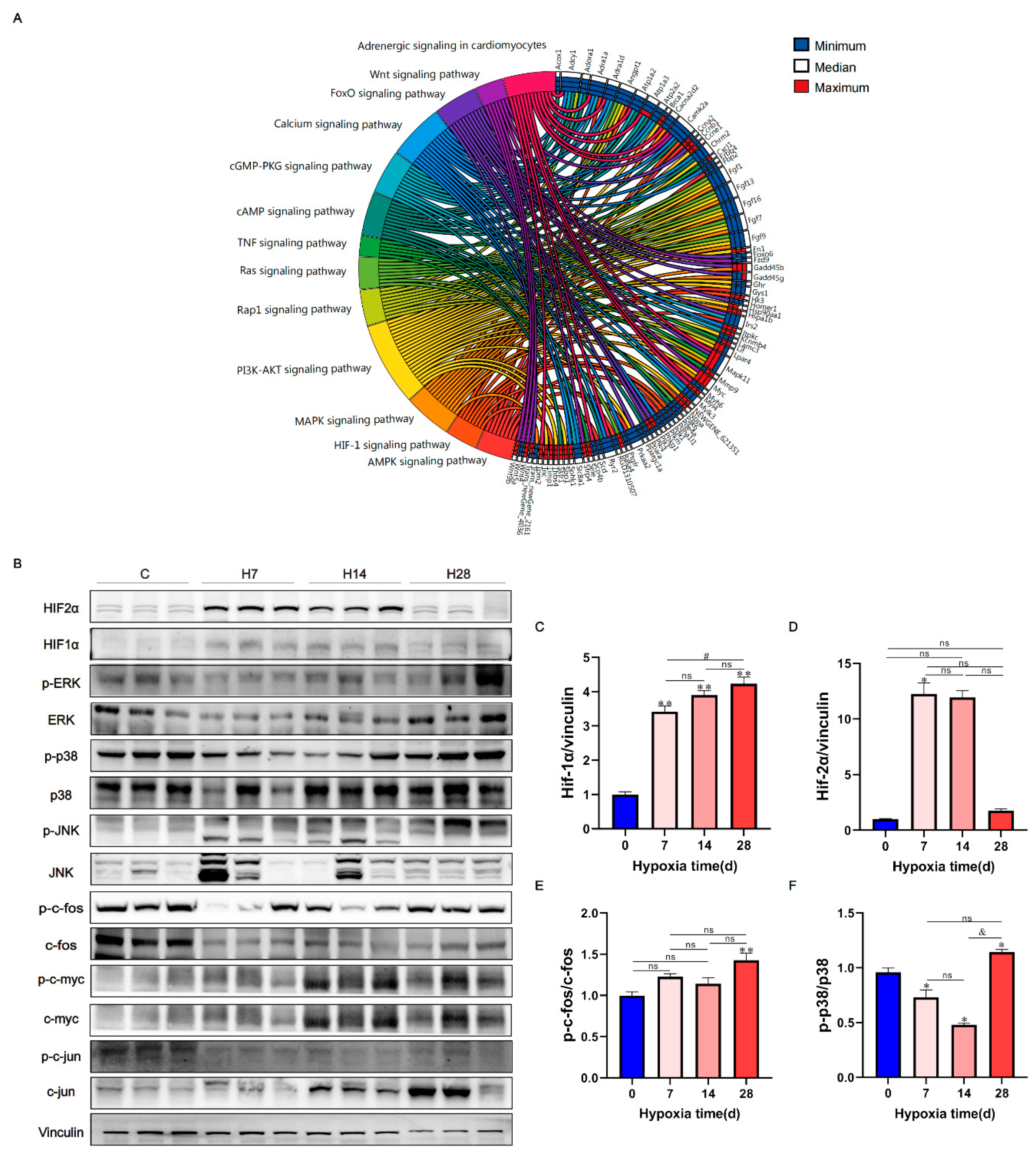
3.4. The HIF-1 Signaling Pathway and MAPK Signaling Pathway Were Activated by Hypoxia
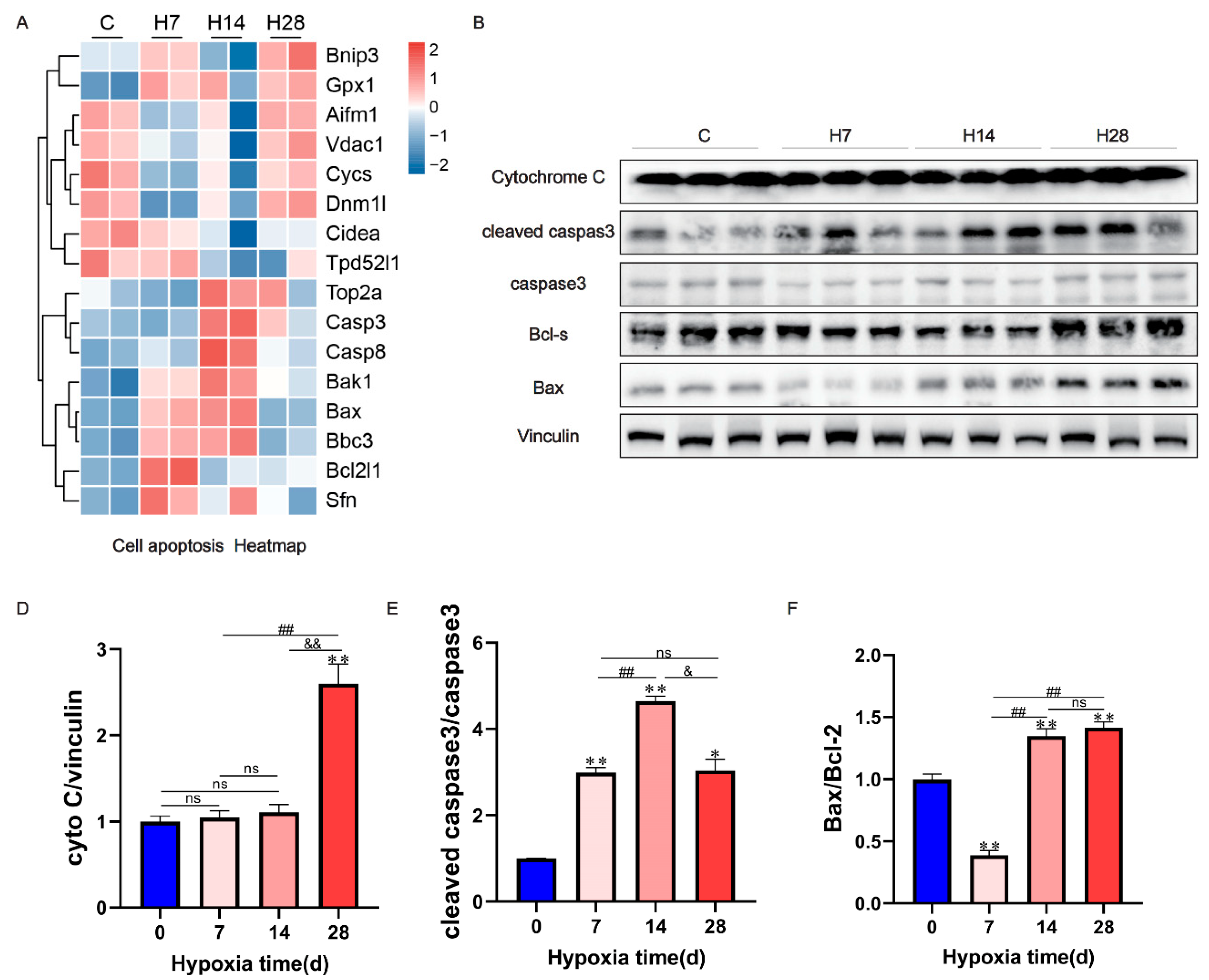
3.5. Apoptosis Occurred in Hypoxic Right Heart Tissue
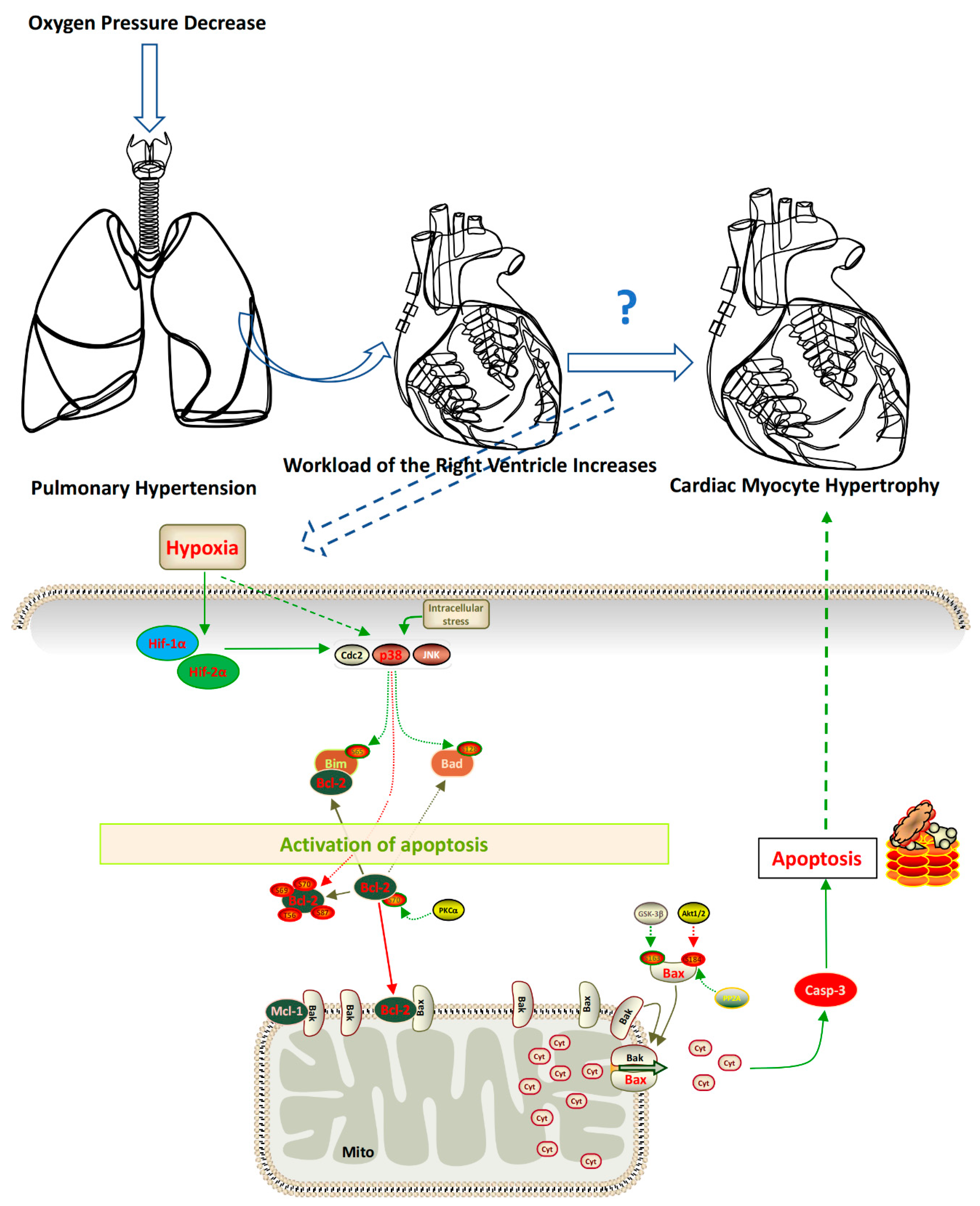
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aaronson, P.I. Pulmonary hypertension associated with chronic hypoxia: just ASIC-ness? J Physiol 2021, 599, 4731–4732. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; et al. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Song, P.; Zou, M.H. AMPK and Pulmonary Hypertension: Crossroads Between Vasoconstriction and Vascular Remodeling. Front Cell Dev Biol 2021, 9, 691585. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; et al. Novel Targets in a High-Altitude Pulmonary Hypertension Rat Model Based on RNA-seq and Proteomics. Front Med (Lausanne) 2021, 8, 742436. [Google Scholar] [CrossRef] [PubMed]
- Yasukochi, Y.; et al. Transcriptomic Changes in Young Japanese Males After Exposure to Acute Hypobaric Hypoxia. Front Genet 2020, 11, 559074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; et al. Drag-reducing polymers attenuates pulmonary vascular remodeling and right ventricular dysfunction in a rat model of chronic hypoxia-induced pulmonary hypertension. Clin Hemorheol Microcirc 2020, 74, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; et al. Tsantan Sumtang Restored Right Ventricular Function in Chronic Hypoxia-Induced Pulmonary Hypertension Rats. Front Pharmacol 2020, 11, 607384. [Google Scholar] [CrossRef]
- Gao, A.R.; et al. Xinyang Tablet attenuates chronic hypoxia-induced right ventricular remodeling via inhibiting cardiomyocytes apoptosis. Chin Med 2022, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The physiological basis of pulmonary arterial hypertension. Eur Respir J 2022, 59. [Google Scholar] [CrossRef]
- Zhang, L.; et al. Clinical and translational values of spatial transcriptomics. Signal Transduct Target Ther 2022, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, G.; et al. Understanding longitudinal biventricular structural and functional changes in a pulmonary hypertension Sugen-hypoxia rat model by cardiac magnetic resonance imaging. Pulm Circ 2020, 10, 2045894019897513. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; et al. Isosteviol improves cardiac function and promotes angiogenesis after myocardial infarction in rats. Cell Tissue Res 2022, 387, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Baudouy, D.; et al. Echocardiographic and Histological Examination of Cardiac Morphology in the Mouse. J Vis Exp 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; et al. A simplified herbal formula for the treatment of heart failure: Efficacy, bioactive ingredients, and mechanisms. Pharmacol Res 2019, 147, 104251. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; et al. Dapagliflozin Mediates Plin5/PPARalpha Signaling Axis to Attenuate Cardiac Hypertrophy. Front Pharmacol 2021, 12, 730623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; et al. Nobiletin Attenuates Pathological Cardiac Remodeling after Myocardial Infarction via Activating PPARgamma and PGC1alpha. PPAR Res 2021, 2021, 9947656. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; et al. NH(4)Cl treatment prevents doxorubicin-induced myocardial dysfunction in vivo. Life Sci 2019, 227, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; et al. LuQi Formula Regulates NLRP3 Inflammasome to Relieve Myocardial-Infarction-Induced Cardiac Remodeling in Mice. Evid Based Complement Alternat Med 2021, 2021, 5518083. [Google Scholar] [CrossRef] [PubMed]
- Sydykov, A.; et al. Pulmonary Hypertension in Acute and Chronic High Altitude Maladaptation Disorders. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Luks, A.M.; Swenson, E.R.; Bartsch, P. Acute high-altitude sickness. Eur Respir Rev 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazazi, R.; et al. Macrophage-NLRP3 Activation Promotes Right Ventricle Failure in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2022, 206, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Legchenko, E.; et al. PPARgamma agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med 2018, 10. [Google Scholar]
- Kang, Y.; et al. Sulforaphane prevents right ventricular injury and reduces pulmonary vascular remodeling in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2020, 318, H853–H866. [Google Scholar] [CrossRef]
- Wu, W.; Du, Z.; Wu, L. Dexmedetomidine attenuates hypoxia-induced cardiomyocyte injury by promoting telomere/telomerase activity: Possible involvement of ERK1/2-Nrf2 signaling pathway. Cell Biol Int 2022, 46, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.H.; et al. CD38 promotes angiotensin II-induced cardiac hypertrophy. J Cell Mol Med 2017, 21, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, P.; et al. Novel and robust treatment of pulmonary hypertension with netrin-1 and netrin-1-derived small peptides. Redox Biol 2022, 55, 102348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; et al. Targeted inhibition of calpain in mitochondria alleviates oxidative stress-induced myocardial injury. Acta Pharmacol Sin 2021, 42, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; et al. Hypoxia exacerbates cardiomyocyte injury via upregulation of Wnt3a and inhibition of Sirt3. Cytokine 2020, 136, 155237. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; et al. Neutrophil Gelatinase-Associated Lipocalin2 Exaggerates Cardiomyocyte Hypoxia Injury by Inhibiting Integrin beta3 Signaling. Med Sci Monit 2019, 25, 5426–5434. [Google Scholar] [CrossRef]
- Feng, H.; et al. Resveratrol Inhibits Ischemia-Induced Myocardial Senescence Signals and NLRP3 Inflammasome Activation. Oxid Med Cell Longev 2020, 2020, 2647807. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; et al. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cai, D. Midazolam suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis by inhibiting the JNK/p38 MAPK signaling pathway. Can J Physiol Pharmacol 2022, 100, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z. Interleukin 32 participates in cardiomyocyte-induced oxidative stress, inflammation and apoptosis during hypoxia/reoxygenation via the NOD2/NOX2/MAPK signaling pathway. Exp Ther Med 2022, 24, 567. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; et al. Kruppel-Like Factor 15 Reduces Ischemia-Induced Apoptosis Involving Regulation of p38/MAPK Signaling. Hum Gene Ther 2021, 32, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; et al. Multiple pathways are responsible to the inhibitory effect of butorphanol on OGD/R-induced apoptosis in AC16 cardiomyocytes. J Appl Toxicol 2022, 42, 830–840. [Google Scholar] [CrossRef]
- Wei, W.; Peng, J.; Li, J. Curcumin attenuates hypoxia/reoxygenation-induced myocardial injury. Mol Med Rep 2019, 20, 4821–4830. [Google Scholar] [CrossRef]
- Sun, C.; et al. Astragaloside IV Ameliorates Myocardial Infarction Induced Apoptosis and Restores Cardiac Function. Front Cell Dev Biol 2021, 9, 671255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




