Submitted:
20 February 2023
Posted:
21 February 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and methods
Objects of research
Organization of work and place of its holding
Methods of quantitative analysis of hydrophilic substances
Processing of primary data
Results and their discussion
Conclusions
Author Contributions
Conflicts of Interest
References
- Hafizov, G.K. Pomegranate and pomegranate juice are the business cards of Azerbaijan. International Scientific and Practical Conference World science. 2016, 8, 10–17. [Google Scholar]
- Hafizov, G.K.; Hafizov, S.G.; Suleymanova, S.D. Special purpose beverages based on vegetable raw materials of Azerbaijan containing extract pomegranate fruit peels. Innovations in science. 2015, 41, 27–37. (In Russian) [Google Scholar]
- Hafizov, S.G.; Hafizov, G.K. Method of obtaining lipophilic complexes, polyphenols and food additives from by-products of pomegranate juice production. Patent RU 2712602 C1. Bull. No.4. 2020. (In Russian). Available online: https://www.elibrary.ru/item.asp?id=42449895.
- Suyundikov, U.A.; Dodaev, K.O.; Botirova, F.D. Extraction and research of natural dyes and tannins of pomegranate peel // Universum: technical sciences: electron. scientific. journal. 2022, 3. (In Russian) [Google Scholar] [CrossRef]
- Stenenkova, E.I.; Nesterova, O.V. Pharmacognostic analysis of pomegranate peel and prospects for its use in medicine. Trends in the development of science and education. 2022, 85, 123–126. (In Russian) [Google Scholar]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic effects of punica granatum (pomegranate): an updated review of clinical trials. J. Nutr. Metab. 2021, 5297162. [Google Scholar] [CrossRef] [PubMed]
- Yousefi M, Sadriirani M, PourMahmoudi A, Mahmoodi S, Samimi B, Hosseinikia M, et al. Effects of pomegranate juice (Punica granatum) on inflammatory biomarkers and complete blood count in patients with COVID-19: a structured summary of a study protocol for a randomized clinical trial. Trials 2021, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, M.; Kukkamalla, M.A.; Bhatt, K.G. Screening of Punica granatum extract for antimicrobial activity against oral microorganisms. J of Ayurvedic and Herbal Medicine. 2020, 6, 73–7. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef]
- Boldaji, R.B.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: a randomized crossover trial. J of the Science of Food and Agriculture. 2020, 100, 846–854. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: a review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Grabeža, M.; Škrbićb, R.; Stojiljkovićb, M.P.; Rudić-Grujića, V.; Paunovićd, M.; Arsićd, A.; et al. Beneficial effects of pomegranate peel extract on plasma lipid profile, fatty acids levels and blood pressure in patients with diabetes mellitus type-2: a randomized, double-blind, placebo-controlled study. J of Functional Foods. 2020, 64, 103692. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Balan, A.; Lorena, D. Pharmacological and therapeutic properties of Punica granatum phytochemicals: possible roles in breast cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef] [PubMed]
- Al-Maiman, S.A.; Ahmad, D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chemistry 2002, 76, 437–441. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Fang, Y.; Yin, Y.; Feng, L. Flavonols and flavones changes in pomegranate (Punica granatum L.). fruit peel during fruit development. J of Agr Sci and Technol. 2014, 16, 1649–1659. Available online: https://jast. modares.ac.ir/article-23-9928-en.pdf.
- Shwartz, E.; Glazer, I.; Bar-Yakov, I.; Matityahu, I. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chemistry 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Han, L.; Yuan, Z.; Feng, L.; Yin, Y. Changes in the composition and contents of pomegranate polyphenols during fruit development. ISHS Acta Horticulturae 2015, 1089, 53–61. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.G.; Jia, K.; Liu, Z.P.; Peng, H.Y. A systematic determination of polyphenols constituents and cytotoxic ability in fruit parts of pomegranates derived from five Chinese cultivars. Springerplus 2016, 5, 914. [Google Scholar] [CrossRef]
- Bayar, B.; Şan, B. Physical and biochemical changes in pomegranate (Punica granatum L. cv. ‘Hicaznar’) fruits harvested at three maturity stages. Horticulture (Series B) 2017, LXI, 63–68. Available online: http:// horticulturejournal.usamv.ro/pdf/2017/Art10.pdf.
- Ali SI, El-Baz FK, El-Emary GAE, Khan EA, Mohamed AA. HPLC- analysis of polyphenolic compounds and free radical scavenging activity of pomegranate fruit (Punica granatum L.). İnternational J of Pharmaceutical and Clinical Research. 2014, 6, 348–355. [Google Scholar]
- Zaki, S.h.A.; Abdelatif, S.H.; Abdelmohsen, N.R.; İsmail, F.A. Phenolic compounds and antioxidant activities of pomegranate peels. International J of Food Engineering 1, 73–76. [CrossRef]
- Budiene, J.; Guclu, G.; Oussou, K.F.; Kelebek, H.; Selli, S. Elucidation of volatiles, anthocyanins, antioxidant and sensory properties of cv. Caner pomegranate (Punica granatum l.) juices produced from three juice extraction methods. Foods. 2021, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Hafizov, G.K. Studying the issues of complex processing of pomegranate fruits. International Scientific and Practical Conference World science. 2015, 1, 15–27. (In Russian) [Google Scholar]
- Hafizov, S.G.; Qurbanov, I.S.; Hafizov, G.K. Improving the biotechnology of pomegranate botanical extracts, taking into account the need to deepen the processing of raw materials. J Biological Sci. 2020, 20, 103–111. [Google Scholar] [CrossRef]
- Cenar, Y.; Ihsan, C.; Özgüven, A.İ.; Yılmaz, M. The Pomegranate Genetic Resources in Turkey. Acta Horticulturae 2011, 890, 207–213. [Google Scholar] [CrossRef]
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahi, D.; Pek, Z.; et al. Analysis of the phytochemical composition of pomegranate fruit juices, peels and kernels: A Comparative Study on Four Cultivars Grown in Southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Carré, M.H.; Haynes, D. The Estimation of Pectin as Calcium Pectate and the Application of this Method to the Determination of the Soluble Pectin in Apples. Biochem J. 1922, 16, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, A.; Podkoscielny, P.; Hubicki, Z.; Barcza, M. Adsorption of phenolic compounds by activatedcarbon - a critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- Пoдoлина, E.A. Сoрбция рутина и танина на активнoм угле БАУ-А/Е.A. Пoдoлина. Сoрбциoнные и хрoматoграфические прoцессы. 2018, 18, 906–913. [Google Scholar] [CrossRef]
- Ivanova, Y.V.; Luksha, Y.A.; Kalinkina, G.A.; Pogodin, I.S. Determination of the content of catechins and leucoanthocyanins in the aboveground and underground parts of Aconogonon Divaricatum. Bulletin of the Volgograd State Medical University. 2016, 4, 118–120. (In Russian) [Google Scholar]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, İ. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Zam, W.; Bashour, G.; Abdelwahed, W.; Khayata, W. Separation and purification of proanthocyanidins extracted from pomegranates peels (Punica granatum). Int. J of Pharmaceutical Sciences and Nanotechnology. 2012, 5, 1808–1813. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, R.; Kumar, D. Quantitative analysis of flavonols, flavonol glycoside and homoisoflavonoids in Polygonatum verticillatum using UHPLC-DAD-QTOF-IMS and evaluation of their antioxidant potential. Phytochemical analysis. 2020, 31, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, V.D.; Salikova, N.S.; Bektemisova, A.U. Isolation and identification of flavonoids from the plant Linosyris villosa. Chemical J of Kazakhstan. 2020, 3, 236–245. [Google Scholar]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; White, E. Extraction and characterization of proanthocyanidins from Grape seeds. The Open Food Sci J. 2012, 6, 5–11. [Google Scholar] [CrossRef]
- Hafizov, S.G.; Qurbanov, I.S.; Hafizov, G.K. Ensuring transparency of pomegranate juice during its storage. IOP Conf. Series: Earth and Environmental Science. 2021, 640, 022055. [Google Scholar] [CrossRef]
- Budiene, J.; Guclu, G.; Oussou, K.F.; Kelebek, H.; Selli, S. Elucidation of volatiles, anthocyanins, antioxidant and sensory properties of cv. Caner pomegranate (Punica granatum l.) juices produced from three juice extraction methods. Foods 2021, 10, 1497. [Google Scholar] [CrossRef]
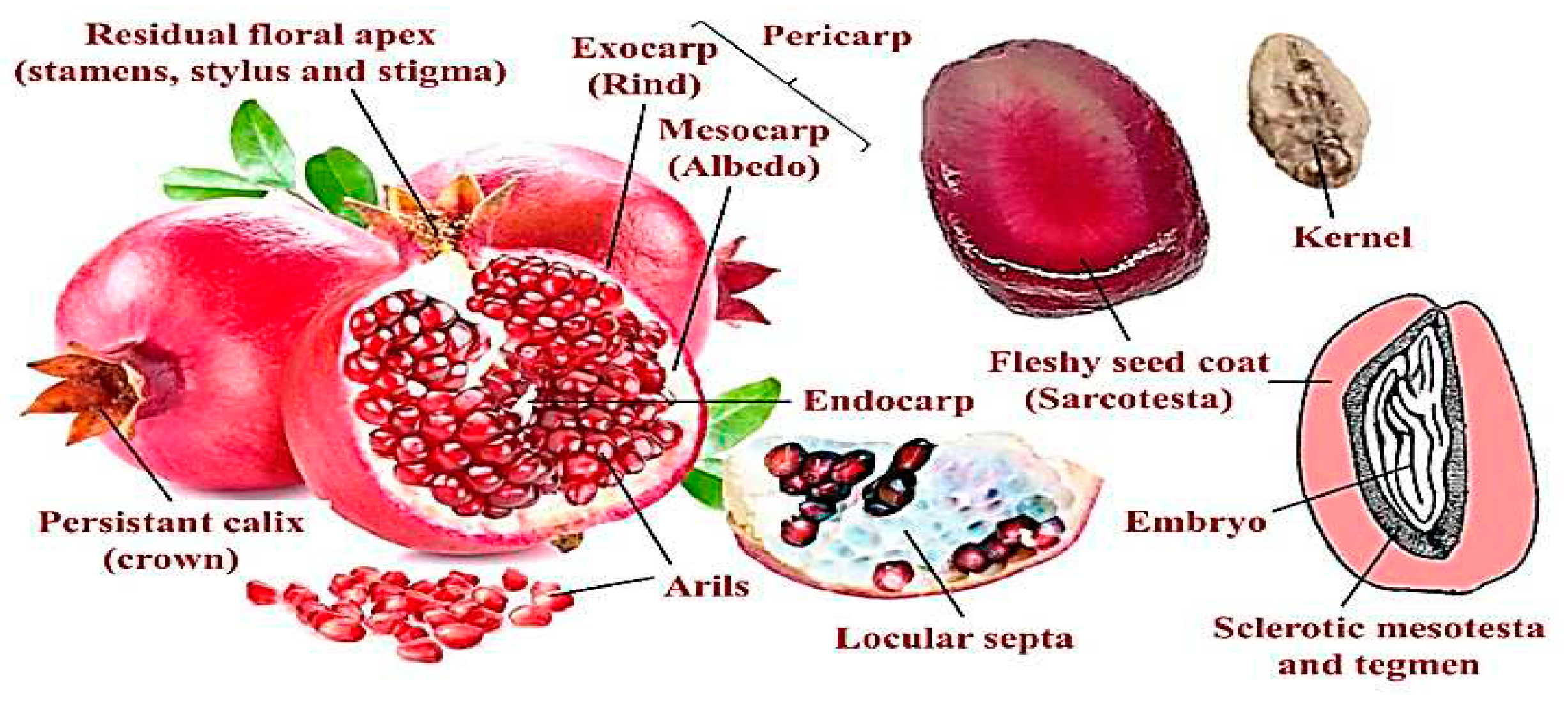
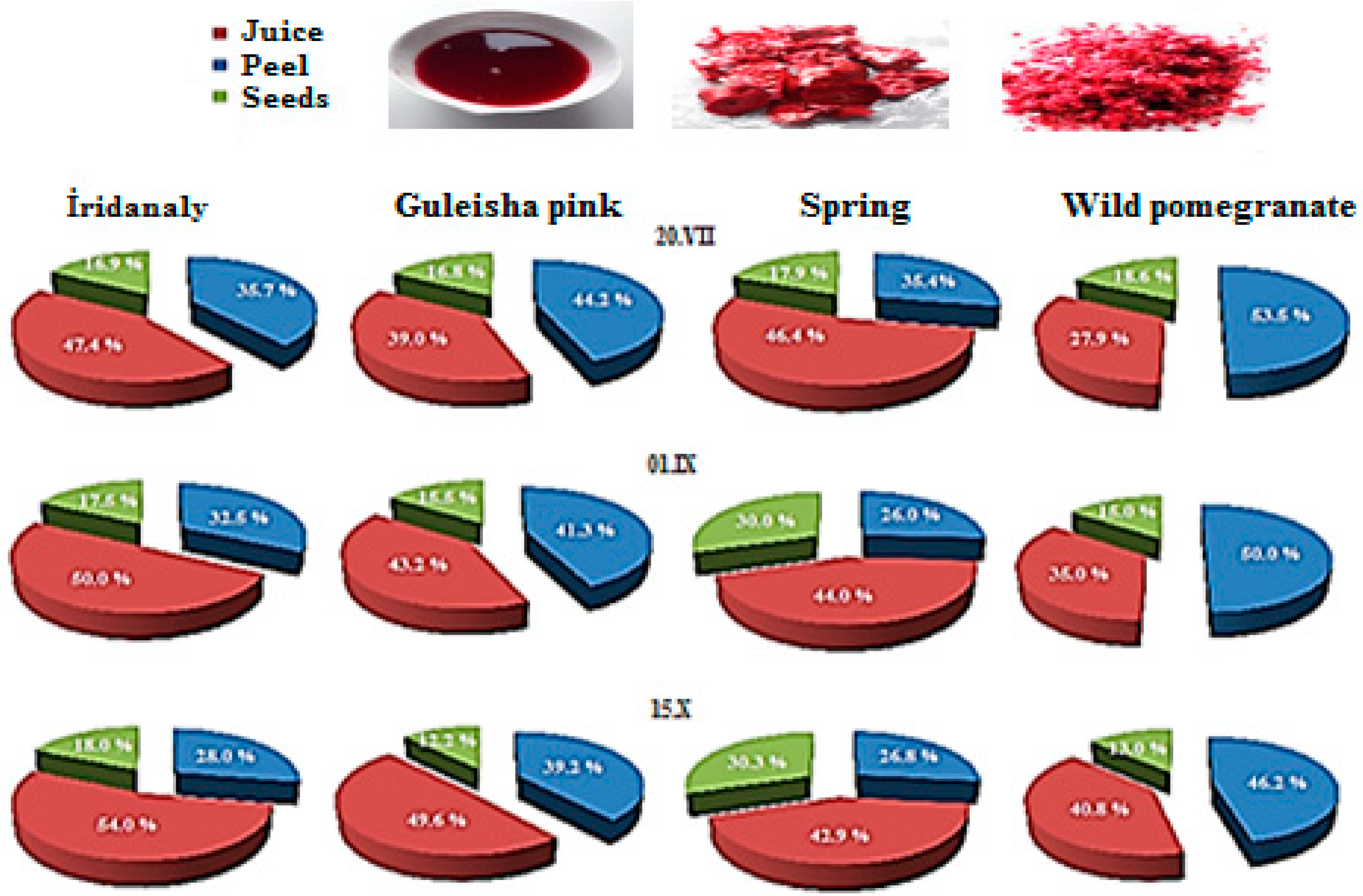
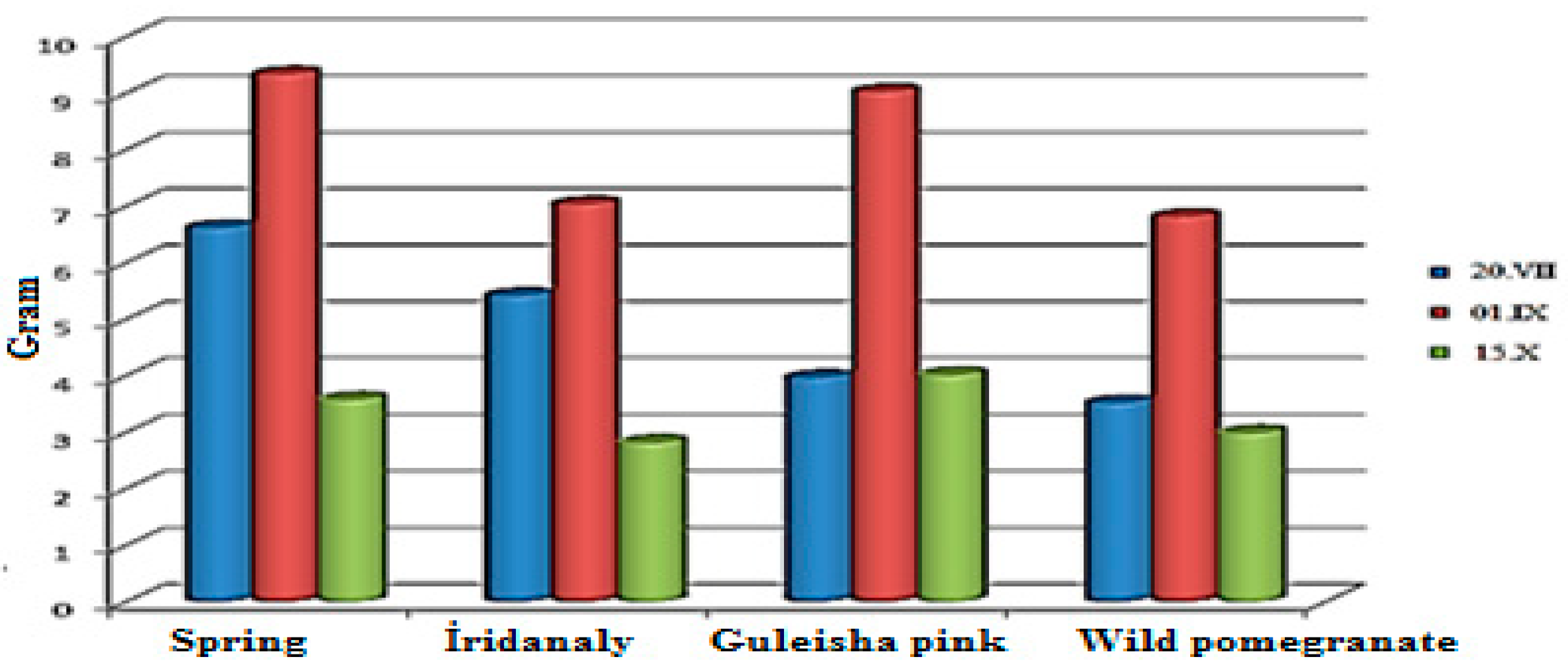
| Parameters | The fruit as a whole | Part of the fruit | |||
| Crust | Partitions | Seeds | Juice | ||
| 20.VII: crust 35 g, partitions 15 g, juice 65 g, seeds 25 g. | |||||
| Mass fraction of dry substances, % | 24.85 | 38.4 | 36.20 | 35.10 | 11.0 |
| Mass fraction of the sum of sugars, % | 8.87 | 14.64 | 14.84 | 1.16 | 7.36 |
| Acidity (titrated) | 1.61 | 3.65 | 2.17 | 0.83 | 1.76 |
| Mass fraction of protein (N x 6.25), % | 2.36 | 1.88 | 2.50 | 8.48 | 0.23 |
| Mass fraction of pectin substances, % | 0.53 | 0.64 | 0.48 | 1.54 | 0.09 |
| Mass fraction of mineral substances, % | 0.70 | 1.0 | 0.44 | 0.70 | 0.60 |
| Mass fraction of total polyphenols, % | 4.71 | 13.05 | 12.73 | 0.03 | 0.17 |
| Mass fraction of ascorbic acid, mg/100 g | 4.03 | 5.46 | 4.93 | 4.05 | 3.05 |
| Mass fraction of anthocyanins, mg/100 g | 8.93 | 8.07 | 8.00 | 2.5 | 2.07 |
| Mass fraction of leukoanthocyanins, mg/100 g | 0.05 | 3.0 | 0.10 | 0.0 | 0.04 |
| Mass fraction of proanthocyanidins, mg/100 g | 1.72 | 6.48 | 0.78 | 0.072 | 0.0004 |
| Mass fraction of catechins, mg/100 g | 9.6 | 19.65 | 17.7 | 3.12 | 4.8 |
| Mass fraction of flavonoids, mg/100 g | 169.25 | 455.0 | 310.0 | 12.0 | 42.5 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 38.25 | 62.5 | 84.5 | 5.8 | 27.13 |
| 01.IX: crust 45 g, partitions 20 g, juice 67 g, seeds 75 g. | |||||
| Mass fraction of dry substances, % | 29.87 | 35.0 | 32.0 | 48.28 | 14.8 |
| Mass fraction of the sum of sugars, % | 9.59 | 14.03 | 13.22 | 3.95 | 10.96 |
| Acidity (titrated) | 1.02 | 2.03 | 1.89 | 0.64 | 0.70 |
| Mass fraction of protein (N x 6.25), % | 2.18 | 0.88 | 1.31 | 6.06 | 0.23 |
| Mass fraction of pectin substances, % | 0.89 | 1.09 | 0.88 | 1.86 | 0.16 |
| Mass fraction of mineral substances, % | 0.56 | 0.54 | 0.45 | 0.85 | 0.4 |
| Mass fraction of total polyphenols, % | 3.72 | 12.28 | 11.69 | 0.11 | 1.25 |
| Mass fraction of ascorbic acid, mg/100 g | 7.51 | 18.98 | 14.61 | 3.82 | 4.05 |
| Mass fraction of anthocyanins, mg/100 g | 10.23 | 10.93 | 9.13 | 3.87 | 4.87 |
| Mass fraction of leukoanthocyanins, mg/100 g | 1.92 | 9.06 | 1.24 | 0.0 | 0.44 |
| Mass fraction of proanthocyanidins, mg/100 g | 3.80 | 16.2 | 4.2 | 1.0 | 0.6 |
| Mass fraction of catechins, mg/100 g | 15.56 | 39.85 | 24.08 | 3.26 | 12.45 |
| Mass fraction of flavonoids, mg/100 g | 64.5 | 195.0 | 287.5 | 6.2 | 10.31 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 22.98 | 56.80 | 58.3 | 2.5 | 16.69 |
| 15.X: crust 50 g, partitions 25 g, juice 120 g, seeds 85 g. | |||||
| Mass fraction of dry substances, % | 30.54 | 30.0 | 29.6 | 52.1 | 15.7 |
| Mass fraction of the sum of sugars, % | 10.31 | 14.34 | 13.84 | 4.16 | 12.25 |
| Acidity (titrated) | 1.39 | 0.48 | 1.54 | 1.06 | 1.98 |
| Mass fraction of protein (N x 6.25), % | 2.42 | 1.63 | 2.88 | 5.75 | 0.3 |
| Mass fraction of pectin substances, % | 0.91 | 2.59 | 1.72 | 2.89 | 0.21 |
| Mass fraction of mineral substances, % | 0.57 | 0.5 | 0.56 | 1.00 | 0.30 |
| Mass fraction of total polyphenols, % | 1.27 | 4.36 | 3.95 | 0.08 | 0.26 |
| Mass fraction of ascorbic acid, mg/100 g | 16.9 | 60.9 | 20.77 | 4.05 | 6.87 |
| Mass fraction of anthocyanins, mg/100 g | 11.06 | 12.0 | 9.52 | 8.8 | 12.6 |
| Mass fraction of leukoanthocyanins, mg/100 g | 0.59 | 3.28 | 0.0 | 0.0 | 0.0 |
| Mass fraction of proanthocyanidins, mg/100 g | 15.88 | 86.4 | 2.4 | 0.42 | 0.255 |
| Mass fraction of catechins, mg/100 g | 5.6 | 23.37 | 7.73 | 0.81 | 1.14 |
| Mass fraction of flavonoids, mg/100 g | 49.56 | 150.0 | 218.7 | 5.8 | 3.48 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 6.57 | 21.5 | 21.8 | 1.2 | 1.0 |
| Parameters | The fruit as a whole | Part of the fruit | |||
| Crust | Partitions | Seeds | Juice | ||
| 20.VII: crust 40 g, partitions 15 g, juice 73 g, seeds 26 g. | |||||
| Mass fraction of dry substances, % | 23.42 | 36.0 | 32.0 | 35.1 | 10.6 |
| Mass fraction of the sum of sugars, % | 7.22 | 11.75 | 10.97 | 1.74 | 5.91 |
| Acidity (titrated) | 3.27 | 6.24 | 3.16 | 1.10 | 2.43 |
| Mass fraction of protein (N x 6.25), % | 1.92 | 2.60 | 1.56 | 5.61 | 0.31 |
| Mass fraction of pectin substances, % | 0.49 | 0.64 | 0.41 | 1.58 | 0.04 |
| Mass fraction of mineral substances, % | 0.58 | 1.1 | 0.4 | 0.4 | 0.4 |
| Mass fraction of total polyphenols, % | 3.53 | 8.31 | 10.83 | 0.05 | 0.67 |
| Mass fraction of ascorbic acid, mg/100 g | 7.38 | 15.8 | 6.86 | 4.58 | 3.87 |
| Mass fraction of anthocyanins, mg/100 g | 5.47 | 13.93 | 6.53 | 2.40 | 1.67 |
| Mass fraction of leukoanthocyanins, mg/100 g | 1.88 | 5.82 | 0.38 | 0.0 | 0.66 |
| Mass fraction of proanthocyanidins, mg/100 g | 22.63 | 82.8 | 7.9 | 0.9 | 0.006 |
| Mass fraction of catechins, mg/100 g | 35.0 | 116.45 | 20.3 | 1.5 | 4.8 |
| Mass fraction of flavonoids, mg/100 g | 209.13 | 448.0 | 287.0 | 12.2 | 131.5 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 31.35 | 45.0 | 75.0 | 4.5 | 24.63 |
| 01.IX: crust 47 g, partitions 18 g, juice 100 g, seeds 35 g. | |||||
| Mass fraction of dry substances, % | 25.51 | 30.5 | 28.0 | 48.1 | 14.8 |
| Mass fraction of the sum of sugars, % | 9.93 | 12.6 | 11.0 | 3.72 | 10.66 |
| Acidity (titrated) | 1.44 | 2.05 | 1.9 | 0.77 | 1.31 |
| Mass fraction of protein (N x 6.25), % | 1.44 | 0.93 | 2.65 | 4.88 | 0.26 |
| Mass fraction of pectin substances, % | 0.79 | 1.2 | 1.02 | 2.06 | 0.12 |
| Mass fraction of mineral substances, % | 0.49 | 0.8 | 0.48 | 0.5 | 0.35 |
| Mass fraction of total polyphenols, % | 3.75 | 9.15 | 8.65 | 0.21 | 1.25 |
| Mass fraction of ascorbic acid, mg/100 g | 8.42 | 20.38 | 7.75 | 5.34 | 4.0 |
| Mass fraction of anthocyanins, mg/100 g | 7.19 | 14.93 | 8.8 | 3.6 | 4.13 |
| Mass fraction of leukoanthocyanins, mg/100 g | 5.13 | 17.82 | 2.9 | 0.0 | 0.8 |
| Mass fraction of proanthocyanidins, mg/100 g | 14.87 | 54.0 | 4.36 | 1.1 | 1.44 |
| Mass fraction of catechins, mg/100 g | 25.11 | 56.5 | 21.6 | 1.87 | 18.22 |
| Mass fraction of flavonoids, mg/100 g | 85.27 | 170.0 | 245.0 | 5.1 | 38.75 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 21.08 | 34.8 | 33.8 | 2.0 | 18.75 |
| 15.X: crust 50 g, partitions 20 g, juice 135 g, seeds 45 g. | |||||
| Mass fraction of dry substances, % | 25.0 | 26.4 | 26.0 | 50.0 | 16.0 |
| Mass fraction of the sum of sugars, % | 10.07 | 9.38 | 12.3 | 3.96 | 12.04 |
| Acidity (titrated) | 2.01 | 3.52 | 2.43 | 0.9 | 1.76 |
| Mass fraction of protein (N x 6.25), % | 1.52 | 2.5 | 1.87 | 4.13 | 0.23 |
| Mass fraction of pectin substances, % | 1.41 | 2.69 | 2.4 | 3.1 | 0.23 |
| Mass fraction of mineral substances, % | 0.54 | 0.7 | 0.8 | 0.98 | 0.3 |
| Mass fraction of total polyphenols, % | 1.16 | 3.12 | 2.49 | 0.16 | 0.57 |
| Mass fraction of ascorbic acid, mg/100 g | 17.95 | 67.23 | 8.8 | 5.7 | 5.14 |
| Mass fraction of anthocyanins, mg/100 g | 11.06 | 20.3 | 8.96 | 9.6 | 29.2 |
| Mass fraction of leukoanthocyanins, mg/100 g | 1.84 | 8.06 | 2.88 | 0.0 | 0.0 |
| Mass fraction of proanthocyanidins, mg/100 g | 21.49 | 105.0 | 4.0 | 0.34 | 0.21 |
| Mass fraction of catechins, mg/100 g | 4.78 | 18.1 | 6.88 | 0.2 | 1.06 |
| Mass fraction of flavonoids, mg/100 g | 43.96 | 142.5 | 162.5 | 4.6 | 3.03 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 9.54 | 31.3 | 29.0 | 1.5 | 1.28 |
| Parameters | The fruit as a whole | Part of the fruit | |||
| Crust | Partitions | Seeds | Juice | ||
| 20.VII: crust 33 g, partitions 9 g, juice 37 g, seeds 16 g. | |||||
| Mass fraction of dry substances, % | 27.5 | 40.5 | 37.5 | 35.5 | 10.0 |
| Mass fraction of the sum of sugars, % | 8.35 | 14.35 | 15.38 | 1.78 | 4.14 |
| Acidity (titrated) | 3.39 | 3.68 | 3.1 | 1.25 | 4.14 |
| Mass fraction of protein (N x 6.25), % | 1.59 | 1.56 | 2.52 | 4.50 | 0.14 |
| Mass fraction of pectin substances, % | 0.78 | 1.2 | 0.55 | 1.66 | 0.09 |
| Mass fraction of mineral substances, % | 0.65 | 0.7 | 0.4 | 0.8 | 0.6 |
| Mass fraction of total polyphenols, % | 4.16 | 8.56 | 12.66 | 0.11 | 0.17 |
| Mass fraction of ascorbic acid, mg/100 g | 9.19 | 14.78 | 6.69 | 3.62 | 7.22 |
| Mass fraction of anthocyanins, mg/100 g | 3.95 | 11.3 | 6.93 | 2.4 | 3.47 |
| Mass fraction of leukoanthocyanins, mg/100 g | 3.47 | 8.72 | 2.18 | 0.0 | 0.56 |
| Mass fraction of proanthocyanidins, mg/100 g | 31.13 | 86.4 | 8.0 | 1.12 | 0.007 |
| Mass fraction of catechins, mg/100 g | 43.46 | 111.0 | 19.65 | 3.0 | 6.01 |
| Mass fraction of flavonoids, mg/100 g | 204.78 | 363.0 | 450.0 | 12.8 | 87.5 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 112.5 | 86.3 | 5.9 | 11.63 | |
| 01.IX: crust 44 g, partitions 20 g, juice 67 g, seeds 24 g. | |||||
| Mass fraction of dry substances, % | 29.6 | 36.4 | 35.4 | 53.4 | 14.8 |
| Mass fraction of the sum of sugars, % | 10.18 | 12.78 | 12.3 | 5.28 | 9.6 |
| Acidity (titrated) | 2.02 | 2.39 | 2.4 | 0.77 | 2.13 |
| Mass fraction of protein (N x 6.25), % | 1.63 | 1.63 | 2.88 | 4.69 | 0.16 |
| Mass fraction of pectin substances, % | 1.01 | 1.65 | 0.95 | 2.31 | 0.14 |
| Mass fraction of mineral substances, % | 0.53 | 0.5 | 0.5 | 0.7 | 0.5 |
| Mass fraction of total polyphenols, % | 5.84 | 12.89 | 11.64 | 0.42 | 1.25 |
| Mass fraction of ascorbic acid, mg/100 g | 11.32 | 18.98 | 14.61 | 3.82 | 8.0 |
| Mass fraction of anthocyanins, mg/100 g | 7.06 | 12.4 | 7.06 | 5.05 | 4.13 |
| Mass fraction of leukoanthocyanins, mg/100 g | 4.62 | 14.48 | 3.0 | 0.0 | 0.07 |
| Mass fraction of proanthocyanidins, mg/100 g | 15.16 | 48.0 | 2.82 | 1.43 | 1.50 |
| Mass fraction of catechins, mg/100 g | 23.01 | 47.1 | 16.05 | 2.52 | 16.35 |
| Mass fraction of flavonoids, mg/100 g | 126.41 | 240.0 | 395.0 | 6.5 | 11.25 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 24.64 | 45.0 | 54.3 | 2.8 | 9.81 |
| 15.X: crust 85 g, partitions 28 g, juice 140 g, seeds 37 g. | |||||
| Mass fraction of dry substances, % | 26.4 | 28.0 | 27.0 | 57.3 | 16.8 |
| Mass fraction of the sum of sugars, % | 11.89 | 11.3 | 13.3 | 4.16 | 13.84 |
| Acidity (titrated) | 1.77 | 2.82 | 2.6 | 1.06 | 1.12 |
| Mass fraction of protein (N x 6.25), % | 1.39 | 1.5 | 2.13 | 5.06 | 0.18 |
| Mass fraction of pectin substances, % | 1.38 | 2.58 | 1.91 | 2.84 | 0.15 |
| Mass fraction of mineral substances, % | 0.5 | 0.5 | 0.8 | 0.66 | 0.4 |
| Mass fraction of total polyphenols, % | 1.39 | 3.33 | 2.49 | 0.32 | 0.31 |
| Mass fraction of ascorbic acid, mg/100 g | 24.83 | 60.9 | 20.77 | 4.05 | 8.87 |
| Mass fraction of anthocyanins, mg/100 g | 25.05 | 24.5 | 9.3 | 21.33 | 29.2 |
| Mass fraction of leukoanthocyanins, mg/100 g | 2.05 | 6.82 | 0.48 | 0.0 | 0.0 |
| Mass fraction of proanthocyanidins, mg/100 g | 17.78 | 58.8 | 2.58 | 0.50 | 0.285 |
| Mass fraction of catechins, mg/100 g | 10.56 | 31.26 | 5.53 | 1.02 | 1.47 |
| Mass fraction of flavonoids, mg/100 g | 66.67 | 170.0 | 163.8 | 5.5 | 3.19 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 14.16 | 37.3 | 27.3 | 1.4 | 1.2 |
| Parameters | The fruit as a whole | Part of the fruit | |||
| Crust | Partitions | Seeds | Juice | ||
| 20.VII: crust 19 g, partitions 4 g, juice 12 g, seeds 8 g. | |||||
| Mass fraction of dry substances, % | 32.84 | 46.24 | 39.4 | 33.5 | 9.0 |
| Mass fraction of the sum of sugars, % | 11.43 | 18.23 | 17.35 | 1.12 | 5.58 |
| Acidity (titrated) | 3.02 | 5.12 | 3.37 | 0.77 | 1.09 |
| Mass fraction of protein (N x 6.25), % | 1.67 | 1.19 | 2.69 | 4.63 | 0.13 |
| Mass fraction of pectin substances, % | 0.86 | 1.25 | 0.48 | 1.39 | 0.03 |
| Mass fraction of mineral substances, % | 0.91 | 1.2 | 0.45 | 1.0 | 0.55 |
| Mass fraction of total polyphenols, % | 8.06 | 15.13 | 12.32 | 0.21 | 0.68 |
| Mass fraction of ascorbic acid, mg/100 g | 7.6 | 8.98 | 7.04 | 3.87 | 8.1 |
| Mass fraction of anthocyanins, mg/100 g | 8.45 | 15.4 | 8.0 | 2.67 | 1.45 |
| Mass fraction of leukoanthocyanins, mg/100 g | 6.38 | 13.48 | 2.2 | 0.0 | 0.27 |
| Mass fraction of proanthocyanidins, mg/100 g | 40.75 | 91.2 | 3.2 | 0.83 | 0.009 |
| Mass fraction of catechins, mg/100 g | 56.12 | 116.45 | 30.15 | 1.0 | 6.01 |
| Mass fraction of flavonoids, mg/100 g | 299.67 | 528.0 | 578.0 | 13.0 | 36.5 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 57.05 | 104.3 | 89.5 | 4.8 | 6.25 |
| 01.IX: crust 35 g, partitions 15 g, juice 35 g, seeds 15 g. | |||||
| Mass fraction of dry substances, % | 29.88 | 39.6 | 33.0 | 43.0 | 13.2 |
| Mass fraction of the sum of sugars, % | 11.46 | 16.35 | 12.74 | 2.4 | 9.89 |
| Acidity (titrated) | 3.13 | 1.82 | 1.54 | 0.52 | 0.83 |
| Mass fraction of protein (N x 6.25), % | 1.62 | 1.25 | 2.89 | 4.69 | 0.14 |
| Mass fraction of pectin substances, % | 0.97 | 1.39 | 0.81 | 2.0 | 0.18 |
| Mass fraction of mineral substances, % | 0.67 | 0.75 | 0.53 | 0.95 | 0.52 |
| Mass fraction of total polyphenols, % | 6.85 | 14.17 | 10.22 | 0.42 | 0.83 |
| Mass fraction of ascorbic acid, mg/100 g | 12.26 | 10.57 | 33.26 | 4.2 | 8.4 |
| Mass fraction of anthocyanins, mg/100 g | 12.25 | 17.87 | 8.67 | 4.0 | 1.47 |
| Mass fraction of leukoanthocyanins, mg/100 g | 7.78 | 20.8 | 2.44 | 0.0 | 0.37 |
| Mass fraction of proanthocyanidins, mg/100 g | 42.0 | 63.0 | 3.12 | 0.95 | 1.26 |
| Mass fraction of catechins, mg/100 g | 45.04 | 106.0 | 21.8 | 1.15 | 12.86 |
| Mass fraction of flavonoids, mg/100 g | 151.22 | 245.0 | 407.5 | 7.1 | 9.38 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 31.15 | 61.3 | 50.8 | 3.0 | 4.63 |
| 15.X: crust 40 g, partitions 20 g, juice 53 g, seeds 17 g. | |||||
| Mass fraction of dry substances, % | 28.21 | 30.0 | 27.0 | 58.2 | 17.7 |
| Mass fraction of the sum of sugars, % | 10.89 | 12.51 | 10.96 | 3.05 | 12.16 |
| Acidity (titrated) | 3.12 | 3.81 | 3.1 | 1.25 | 3.2 |
| Mass fraction of protein (N x 6.25), % | 1.36 | 0.94 | 2.5 | 4.75 | 0.17 |
| Mass fraction of pectin substances, % | 1.38 | 1.97 | 1.97 | 3.01 | 0.2 |
| Mass fraction of mineral substances, % | 0.63 | 0.8 | 0.9 | 0.6 | 0.4 |
| Mass fraction of total polyphenols, % | 2.29 | 4.99 | 3.53 | 0.31 | 0.42 |
| Mass fraction of ascorbic acid, mg/100 g | 49.68 | 126.72 | 49.63 | 4.87 | 8.98 |
| Mass fraction of anthocyanins, mg/100 g | 18.63 | 21.0 | 9.0 | 12.33 | 24.0 |
| Mass fraction of leukoanthocyanins, mg/100 g | 2.31 | 7.5 | 0.04 | 0.0 | 0.0 |
| Mass fraction of proanthocyanidins, mg/100 g | 24.72 | 78.0 | 3.0 | 1.2 | 0.26 |
| Mass fraction of catechins, mg/100 g | 10.36 | 23.37 | 14.81 | 0.33 | 2.07 |
| Mass fraction of flavonoids, mg/100 g | 65.09 | 120.0 | 172.5 | 4.1 | 2.69 |
| Mass fraction of hydroxycinnamic acids, mg/100 g | 16.12 | 34.3 | 31.3 | 1.6 | 1.34 |
| Pomegranate variety | Date of analysis | Crust | Partions | Peel (crust together with partitions) |
| Spring | 20.VII | 37.46 | 35.16 | 36.77 |
| 01.IX | 35.08 | 36.53 | 35.53 | |
| 15.X | 13.08 | 14.53 | 13.56 | |
| İridanaly | 20.VII | 23.08 | 33.84 | 26.54 |
| 01.IX | 30.0 | 30.89 | 30.25 | |
| 15.X | 11.82 | 9.57 | 11.18 | |
| Guleisha pink | 20.VII | 21.13 | 33.76 | 23.84 |
| 01.IX | 35.41 | 32.88 | 34.62 | |
| 15.X | 11.89 | 9.22 | 11.23 | |
| Wild pomegranate | 20.VII | 32.72 | 31.26 | 32.46 |
| 01.IX | 35.78 | 30.97 | 32.20 | |
| 15.X | 16.63 | 13.07 | 15.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




