1. Introduction
Fragments of asteroidal parent bodies are found on the Earth as meteorites, with unmetamorphosed carbonaceous chondrites (CC) representing some of the most primitive solar system materials analysed to date. Among the extraterrestrial organic matter inventory of CC are amino acids (AA) (1–3). Amino acids are fundamental components within the proteins required by all living organisms on Earth and are thought to be essential for the origin of life (2, 4). Therefore, understanding the types of AA in meteorites and their potential distribution throughout the Solar System in the past is very important for understanding where and when life could have originated.
Amino acids were studied extensively in the Murchison meteorite in the run up to the Apollo missions, in order to develop and validate new analytical techniques and laboratory facilities (5). In one of the first convincing analyses of AA in the Murchison meteorite, it was found that a number AA were present at near racemic abundances, including alanine, valine, sarcosine, glycine, proline and glutaric acid (6). However, approximately 10 g of sample was used and it was quickly realised that more sensitive techniques were required to preserve sample for future analyses. Since the 1970’s many studies have been carried out and it is now known that CC contain many organic compounds including >100 AA (7).
At current, it is still unclear how AA came to be present within meteorite parent bodies, with multiple processes likely combining to provide the entire assemblage present. Interstellar medium (ISM) and outer protosolar nebular (PSN) ice analogue experiments tend to produce simple (low molecular weight) AA, such as glycine, alanine and β-alanine, in higher abundances compared to more complex amino acids (higher molecular weight), such as α-aminobutyric acid, glutaric acid and aspartic acid(8, 9). Meanwhile, more complex AA have been proposed to form during aqueous alteration on meteorite parent bodies, likely via Strecker synthesis involving ammonia, aldehydes and ketones (α-AA) (10), and via Michael addition of ammonia to α,β-unsaturated nitriles, with subsequent hydrolysis (11). Indeed, it was demonstrated that complex mixtures of AA including α, β and γ-AA could have formed from ISM/PSN ice compositions, which were subsequently processed on meteorite parent bodies via formose, condensation and carbonization style reactions (12).
Nevertheless, concerns have been raised over the level of contamination that meteorites have experienced on the Earth’s surface, with suggestions that at least some protein forming AA are derived from terrestrial contamination (5, 13). Accordingly, many studies have investigated the indigeneity of AA within CC and concluded that chirality and isotopic composition can be used to evaluate levels of terrestrial contamination (14–19). Furthermore, an assessment of contamination from the Earth’s biosphere was conducted for Orgueil by searching for DNA and rRNA and found that it was negligible (20).
However, doubts will likely remain over how much contamination has been experienced by meteorites that have fallen to Earth and interacted with its surface, especially when discussing proteogenic AA. As such, while a certain level of contamination is likely inevitable, sample returns offer a very appealing way of obtaining asteroidal material with minimal levels of contamination to assess the indigenous levels of AA in extraterrestrial materials. Nevertheless, one major limitation of sample returns is the limited sample available, with only 5.4 g of material being returned from the asteroid Ryugu by the JAXA Hayabusa2 mission (21). Previous studies that have analysed the AA inventory of CC have typically used >1 g to many 10’s of mg sample sizes (14–19, 22, 23), but these are unlikely to be available to those investigating Ryugu samples, because JAXA has allocated only 10’s of mg to individual curation teams and many analyses must be performed to fully characterise the samples. Furthermore, while a previous study utilised several mg of meteorite to detect the chirality of amino acids, only 5 analytes were targeted and the system used was a highly specialised custom-made instrument that is not commercially available (24). As such, a validated highly sensitive and commercially available technique is required to investigate extraterrestrial materials that comprise small sample sizes, such as those from sample return missions.
Here a highly sensitive ultrahigh performance liquid chromatography-Orbitrap-mass spectrometric (UHPLC-OT-MS) method for analysing AA in meteorite samples is presented and validated. The technique is then applied to detect and quantify amino acids within ~2 mg sample masses of the Murchison (CM2) and Orgueil (CI1) CC.
2. Materials and Methods
2.1. Sample and Standard Preparation
A 2.136 mg sample of Orgueil (CI1), a 2.193 mg sample of Murchison (CM2) and 2 serpentine samples were removed from internal portions of their meteorites and parent rock, respectively, via an ultramicrotome. The Orgueil meteorite was purchased from Meteorites.tv in 2007, while the Murchison sample was purchased from the online store of
www.mars.li which sell meteorites from the private collection of Mr. Eric Haiderer. Both meteorites were kept under nitrogen in a clean room after their purchase. The resulting flakes and powder were then placed within sealable Teflon containers and weighed. Note that the Teflon vials, diamond knife and all tools used to handle the samples were cleaned via ultrasonication with once distilled ion-exchanged (1DIE) water (Puric-ω, Organo Co.) and UHPLC grade MeOH (Kanto Chemical CO., INC, >99.8%) prior to use. The Teflon vials were also soaked in 6M HCl and then 0.5 M HNO
3, prior to cleaning with 1DIE and UHPLC MeOH. Furthermore, all work was undertaken in a class 10 clean room at the Pheasant Memorial Laboratory (PML) for geochemistry and cosmochemistry, Institute for Planetary Materials (IPM), Okayama university at Misasa.
An in-house standard (AA-Mix) containing 26 AA was prepared at ~200 μg g-1 concentration for all the amino acids and included: glycine, sarcosine, alanine, β-alanine, serine, threonine, α-aminobutyric acid, α-aminoisobutyric acid, β-aminobutyric acid, β-aminoisobutyric acid, aspartic acid, valine, norvaline, isovaline, glutamic acid, leucine, isoleucine, alloleucine, α-aminoadipic acid, cycloleucine, pipecolic acid, aminocaproic acid, α-aminopimelic acid, homocycloleucine, phenylalanine and tyrosine. The glycine and alanine standards were obtained from Kanto Chemical CO., INC and the rest of the AA were obtained from Tokyo Chemical Industry CO., LTD and all AA were ≥98% in terms of purity. An aliquot of the standard was then processed to yield AA isopropyl esters (AAIE) (see next section) and diluted to give a range of concentrations from 1 μg g-1 to 0.00075 μg g-1 reducing the concentration by 25% with each dilution, i.e. 1.00, 0.75, 0.50, 0.25, 0.10 μg g-1 and so on.
2.2. Extraction and Sample Processing
After weighing, the Teflon vials containing the samples, including two filled with the serpentine flakes and representing procedural blanks, were filled with 300 µL of 1DIE water, sealed and heated for 20 hours at 110℃. Upon cooling, the 1DIE water supernatant and 3 1DIE water washes of the meteorite residues were transferred to a 10 ml glass tube, sealed and frozen overnight. Then, the frozen water extracts were freeze dried to remove the 1DIE water. The dry extracts were then derivatised with 300 µL 2M HCl/isopropyl alcohol at 110℃, to yield AAIE. After cooling, the derivatised extracts’ volumes were reduced under N2 gas until near dryness. Subsequently, ~1 ml of water was added to the 10 ml tubes, followed by freezing overnight. A second freeze drying step was undertaken to ensure that all water, HCl and isopropyl alcohol were removed from the extracts. Finally, 100 µL of ethyl acetate was added to the 10 ml tubes and ultrasonicated for 10 min to extract the AAIE, before transferring to a 300 µL glass insert vial for UHPLC-OT-MS analysis. Note that the AA standard was also derivatised and processed using the same method, thus allowing for a direct comparison of the concentrations of the standards and the extracted AAIE from the meteorite samples.
2.3. UHPLC-OT-MS Analysis
The UHPLC separation was performed in reversed phase using a ThermoFisher Scientific AccucoreTM 150 Amide HILIC column on a VanquishTM UHPLC unit. The UHPLC unit contained a binary pump system, which allowed for a dynamically changing gradient between two phases. Phase A was 10 mM ammonium formate in water (adjusted to pH 3.5 using formic acid) and phase B was 100% ACN. The gradient was adjusted from 100% B to 79% B over 15 minutes, to 0% B over a further 5 minutes, then held at 0% B for 5 minutes, before increasing to 100% B over 5 minutes and holding at 100% B for an additional 5 minutes. A flow rate of 0.15 mL/minute and a column compartment temperature of 30℃ were used for UHPLC.
The UHPLC unit was coupled to an Orbitrap Fusion Mass Spectrometer (Thermo Scientific) via a heated-electrospray ionisation interface. The following parameters were used for the UHPLC-OT-MS analyses: an ion transfer tube and vaporiser temperature of 300℃, positive ion voltage of 3500 V, sheath gas of 50, auxiliary gas of 15, RF lens of 55%, m/z range of 50–500, OT resolution of 240000 and an AGC target of 2 × 105.
3. Results
3.1. Determination of LOD and LOQ and method validation
The UHPLC-OT-MS method was validated using the results obtained from the in-house AA-Mix standard. The standard was run in triplicate for each concentration (see
Section 2.1) and the 5 concentrations immediately above the concentration at which a given AAIE consistently gave a response were used for limit of detection (LOD) and limit of quantitation (LOQ) determination (
Table 1). A calibration curve was made using the average response value of the triplicate analyses for the 5 concentrations, which was then used to calculate the LOD and LOQ of each AAIE. The LOD and LOQ were defined as 3 and 10 times the standard deviation of the y-intercept divided by the slope of the calibration curve.
The AAIE derivatives of alanine and sarcosine and β-aminobutyric and β-aminoisobutyric acid were found to coelute and as such it is not possible to quantify these amino acids using the method reported here. Threonine displayed two peaks for most standard concentrations (at 13.40 and 15.98 min), with the 15.98 peak being very small at higher concentrations, but becoming larger than the 13.40 peak below 0.25 ppm. Both peaks were found to be suitable for quantitation, with the 13.40 peak being more suited to quantitation at higher concentrations. Meanwhile, the AAIE derivatives of leucine and isoleucine were found to overlap in the extracted ion chromatogram (EIC), but it was possible to observe that two peaks were present. As such, peaks were fitted to either side of the feature observed in the EICs (
Figure S1i–j) and the resulting peak area calibration curves gave good fits, indicated by their R squared (R2) values (>0.980). The R2 values for all amino acids were above 0.980, except for aminocaproic acid (0.940). Accordingly, an LOD and LOQ were not determined for aminocaproic acid. Note that each R2 value reported in table 1 is for the calibration curve fitted to all the data, from the 3 analyses of each of the 5 concentrations for a given AAIE.
In terms of previous studies, the LOD values reported here (8x10-14 to 2x10-15 mol) are comparable to those of ultrahigh performance liquid chromatography-fluorescence detection/time of flight mass spectrometry (22, 25) (~1 × 10-14 to ~1x10-15 mol), but more sensitive than that of two-dimensional gas chromatography-time of flight mass spectrometry (3x10-12 to ~5x10-15 mol) (26).
3.2. Meteorite amino acids
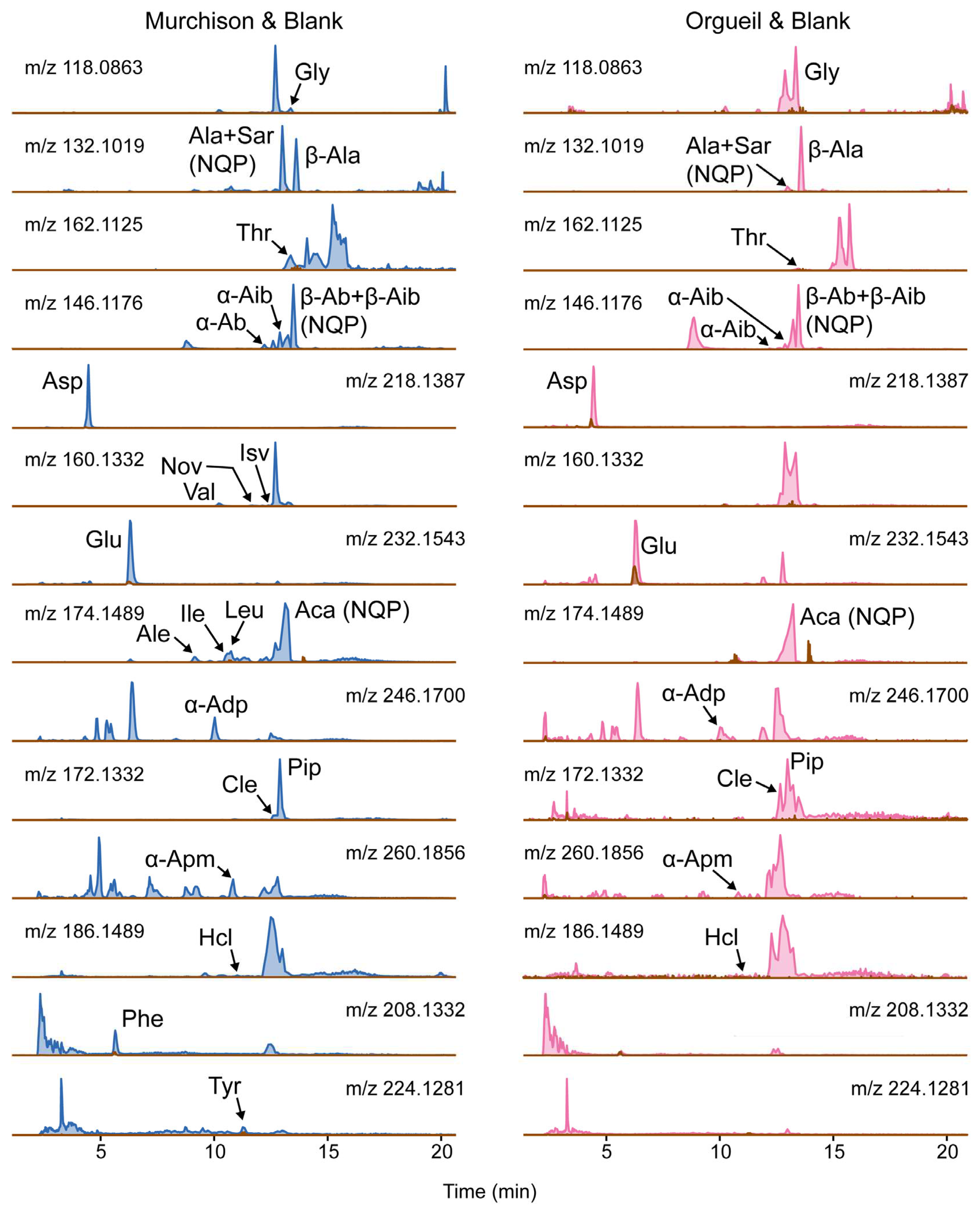
The EICs of Orgueil and Murchison (
Figure 1) for a given mass were visibly different, with the relative heights of peaks within an EIC differing between these samples. On comparison of the peak areas for AAIEs from Murchison and the blank, it was clear that all AAIEs had a blank contribution of <20 %. Whereas, for Orgueil all of the AAIEs had a blank contribution of <20 %, except for glutamic acid (29%). Nevertheless, a blank correction was undertaken for all of the determined AA concentrations.
Meanwhile, in some cases the retention times in the standard and the meteorite were significantly different, defined here as >0.2 min. However, tandem MS (MS
2) data (
Figure S1a-p) for all cases except threonine, isovaline and homocycloleucine, where the responses were too low, indicated that the amino acid was the same as in the standard. Pipecolic acid has the same mass as cycloleucine and the RT value also deviated from the standard by >0.2, but the response in Murchison for pipecolic acid was high enough to yield MS
2 data. Furthermore, both cycloleucine and pipecolic acid elute close together and thus display similar separation characteristics, meaning a matrix affect that causes deviation in the RT, is likely to affect both in the same way. As such, the suspected cycloleucine peak in Murchison was assumed to be cycloleucine.
The AAIEs in Murchison and Orgueil were quantitated (
Table 2) by dividing the average peak area of the unknown AAIE (determined from 3 runs) by the peak area of the AAIE in the standard which gave the closest response and then multiplying by the concentration of the AAIE in the standard. Note that the average standard peak area value was not used, instead the average meteorite AAIE peak area value was divided by each of the standard peak area values from the runs of the triplicate. As a result, 3 concentration values were determined and these were used to generate an error for the meteorite AAIE concentration. Furthermore, a blank correction was applied by subtracting the peak area detected in the blank measurement from that detected in the sample measurement.
In addition, 3 calibration curves were generated across 5 concentrations that intersected the intensity value obtained from the peak area of a given meteorite AAIE, in order to check that the standard data was appropriate for quantitation. The R2 values for all calibration curves were >0.980. However, for α-aminopimelic acid the calibration curve was not linear over the concentration range of interest, but it was possible to fit the curve with a second order polynomial. For α-aminopimelic acid the concentration in Murchison (for Orgueil the AAIE was below the limit of quantitation) was determined by solving the quadratic equations describing the 3 calibration curves of the standards, for more information see (27).
For Murchison, it was possible to determine the concentrations of 19 of the AAIE considered here. In the case of Orgueil, the concentrations of many AAIE were lower, which meant that the concentrations of 10 AAIE were determined. In some cases, the AAIE were above detection limit, but below the limit of quantitation. Furthermore, for alanine and sarcosine (coeluted), β-aminobutyric and β-aminoisobutyric acid (coeluted), and aminocaproic acid (R2 <0.980), it was clear that these AAIE were significantly above the background in both Murchison and Orgueil, but no quantitation was possible. As such, these AAIE are likely present at detectable levels, but the method reported here is not appropriate for quantitating them.
4. Discussion
4.1. Meteorite amino acid concentrations
In terms of previous studies, the LOD values reported here (8x10-14 to 2x10-15 mol) are comparable to those of ultrahigh performance liquid chromatography-fluorescence detection/time of flight mass spectrometry (22, 25) (~1 × 10-14 to ~1 × 10-15 mol), but more sensitive than that of two-dimensional gas chromatography-time of flight mass spectrometry (3 × 10-12 to ~5 × 10-15 mol) (26). Nevertheless, ~100x smaller sample sizes were utilized here, compared to the aforementioned studies.
The concentrations reported here for amino acids from Murchison and Orgueil are different from those reported before via an ultrahigh performance liquid chromatography-fluorescence detection (UHPLC-FD) approach (
17,
22) (
Table 3), with many being at higher concentrations (glycine, α-aminoisobutyric acid, aspartic acid and glutamic acid). Nevertheless, α-aminobutyric acid was found to be much lower for Murchison in the current study compared to that of previous studies. One potential reason for this discrepancy could be heterogeneity between the meteorite samples analysed or heterogeneity introduced by a sampling bias, because of the much smaller amount of sample used by the current study (2.136-2.193 mg) compared to the previous studies discussed here (92-200 mg).
However, it is hard to imagine how heterogeneity or sampling bias could lead to all the observed differences, because the same amino acids that were at higher concentrations in Murchison were also higher in Orgueil. Instead, the difference between the concentrations observed here and those of previous studies may relate to the sample processing procedures. In both of the aforementioned previous studies (17, 22) a desalting step was used after acid hydrolysis, involving a cation exchange column. In order to calculate the recoveries after such a procedure Glavin et al. (2010) used an internal amino acid standard and subsequently corrected all their amino acid concentrations based on the recovery (60-80%) of this one amino acid. The problem with the approach of Glavin et al. (2010) is that it is not clear whether all amino acids would have similar recoveries. Here no desalting step or column chromatography was required. Accordingly, it may be the case that when correcting for the recoveries, some amino acids from the previous studies were overestimated, while others were underestimated. Nonetheless, sample heterogeneity and sampling bias, likely do have some impact on the differences observed and thus cannot be ruled out completely.
Alternatively, the reason for the discrepancy in amino acids observed between the current study and previous studies could relate to potential contamination introduced during curation of the meteorites, because the meteorites utilised here have previous curation histories and were not always housed within the clean room facilities at IPM. As such, additional proteinogenic amino acids could have been added to the meteorites during curation, with glycine, aspartic acid and glutamic acid being protein-forming amino acids.
However, this does not explain why α-aminoisobutyric acid is at a higher or similar concentration for Orgueil compared to previous studies, being that it is a nonproteinogenic amino acid and relatively rare in nature (28). Furthermore, the nonproteinogenic amino acid α-aminobutyric acid is 11× (17) and 2× (22) higher for other studies when compared to the current study. Meanwhile, the nonproteinogenic amino acid α-aminoisobutyric acid is 4× (17) and 7× (22) lower in Murchison from the current study compared to other studies. Such observations clearly indicate that amino acids can differ greatly between studies, likely due to either sample heterogeneity or the analytical protocols employed. For instance, due to the small sample sizes utilised here, some discrepancy between the bulk meteorite amino acid abundances and those detected here are to be expected. Therefore, the differences observed here may in part reflect the probing of a more localised composition in the current study, compared to other studies. Furthermore, the low levels of tyrosine and phenylalanine in Murchison, infers that only small quantities of amino acids have been introduced during past curation of the meteorite. Therefore, contamination seems unlikely to explain the abundance differences between the current study and previous studies.
When calculating the ratios of several amino acids relative to glycine, the ratios show both differences and similarities, indicating that there may be an affect due to heterogeneity or sampling bias between the current study and those of previous studies (
17,
22) (
Table 4). Nevertheless, the difference between the β-alanine/glycine ratio of Orgueil (much higher) and Murchison (much lower) is apparent in the current study and thus supports previous findings that indicate this ratio is sensitive to the degree of aqueous alteration on meteorite parent bodies (
17,
22,
23).
Another potential reason for the differences observed for α-aminoisobutyric acid could be the fact that a seperate hydrolysis step was not included here, and thus it is possible that some AA precursors were not converted to AA in the current study. Nevertheless, during derivatisation the hot water extracts were heated in 2 M HCl and this would have likely converted at least some of the AA precursors to AA. Furthermore, the higher or similar abundances of other amino acids compared to previous studies, likely indicates that most of the AA precursors were converted to AA, similar to studies that utilised a 6 M HCl acid vapour hydrolysis step (17, 22).
4.2. Method suitability for sample return missions
Concerns have been raised in the past over the contamination level of meteorites that have been curated on earth for long periods of time (29–31). Although much work has been done to establish the levels and nature of indigenous amino acids within meteorite samples, doubts still remain (14–19). Sample return missions represent opportunities for the obtainment of extraterrestrial material that has not been affected by passage through the Earth’s atmosphere and interaction with its biologically active surface.
The Hayabusa2 mission, which returned C-Type material from Ryugu in December 2020, acquired only 5.4 g of sample (21). While, the OSIRIS-REx mission is expected to return a larger sample size >60 g, the sample will likely not be available in large quantities for any single destructive analysis. Previously, UHPLC-FD based techniques (17, 22) enabled the detection of many amino acids within relatively small sample sizes (92-200 mg). However, significantly smaller Ryugu sample sizes have been made available to individual facilities (10’s of mg) and multiple analyses are required to be performed. Furthermore, while successful determination of the chirality of amino acids was reported previously for several-mg-sized fraction of meteorites, only 5 analytes were targeted(24). The machine used to undertake the analyses was also a custom-made and highly specialised instrument, which means it is unlikely to be available to other laboratories that may wish to analyse returned samples. As such, commercially available techniques that can quantify organic compounds of particular scientific interest, such as amino acids, in very small sample sizes are required.
The ability of the technique proposed here to detect and quantify multiple amino acids in small sample sizes highlights the appropriateness of this technique for application to extraterrestrial material acquired from sample return missions, such as those from the Hayabusa2 and OSIRIS-REx missions. Nevertheless, in the case of Orgueil, it is likely that a slightly larger sample size (e.g. 3-4 mg) would have enabled the quantitation of more amino acids and as such slightly larger sample masses are recommended for Orgueil-like (CI1 or similar type 1 carbonaceous chondrites) samples.
As mentioned previously, it cannot be ruled out that the meteorite samples analysed here experience some proteinogenic amino acid contamination during their curation before being stored at IPM. However, the fact that many nonproteinogenic amino acids were also detected by the current study at low concentration in such small sample sizes, indicates the method reported here is suitable for returned samples without terrestrial contamination.
Furthermore, the high sensitivity of the technique presented here, makes it suitable for revealing the heterogeneity within individual samples. For instance, several 2-4 mg portions of g-sized meteorite samples, which are separated beyond the mm scale, could be investigated to yield information about amino acid heterogeneity within meteorite samples. Such findings could then be used to better understand the nature of aqueous processes on meteorite parent bodies and answer fundamental questions. For example, how heterogenous are the aqueous alteration conditions that have affected organic matter on meteorite parent bodies? Moreover, are there any mineralogical factors, such as phyllosilicate abundance or composition that correlate with particular amino acid enrichments or depletions? Such questions will be basis of future work utilising the methodology outlined here.
5. Conclusions
While individual amino acid concentrations were found to be different between the current study and previous studies, this may relate to the sample processing procedure used by these previous studies. Here the use of a desalting cation exchange step was avoided and may explain why higher concentrations of amino acids were observed for many of the amino acids reported by the current study. The findings indicate that the methodology is appropriate for the detection and quantitation of amino acids in material acquired by sample return missions. Moreover, the method may be particularly useful in determining the heterogeneity of amino acids within larger extraterrestrial samples through analysis of spatially separated aliquots.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, The tandem mass spectrometry data (MS
2) for the amino acids reported here from the standards, Orgueil and Murchison.
Author Contributions
Conceptualization, Christian Potiszil; Formal analysis, Christian Potiszil; Funding acquisition, Eizo Nakamura; Investigation, Christian Potiszil, Masahiro Yamanaka and Tsutomu Ota; Methodology, Christian Potiszil; Validation, Christian Potiszil; Writing – original draft, Christian Potiszil; Writing – review & editing, Ryoji Tanaka, Katsura Kobayashi and Eizo Nakamura.
Funding
This research was funded by The Ministry of Education, Culture and Sports, Science and Technology (MEXT) of Japan.
Data Availability Statement
The data utilized in the current manuscript is available in text within tables.
Acknowledgments
We are greatly indebted to PML members for their assistance maintaining the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Z. Martins, "Organic Molecules in Meteorites and Their Astrobiological Significance" in Handbook of Astrobiology, V. M. Kolb, Ed. (CRC Press, Boca Raton, Florida : CRC Press, ed. 1st, 2019), pp. 177–194.
- D. P. Glavin, C. M. O. Alexander, J. C. Aponte, J. P. Dworkin, J. E. Elsila, H. Yabuta, "The Origin and Evolution of Organic Matter in Carbonaceous Chondrites and Links to Their Parent Bodies" in Primitive Meteorites and Asteroids. (Elsevier, 2018; https://linkinghub.elsevier.com/retrieve/pii/B9780128133255000033), pp. 205–271.
- J. E. Elsila, J. C. Aponte, D. G. Blackmond, A. S. Burton, J. P. Dworkin, D. P. Glavin, Meteoritic Amino Acids: Diversity in Compositions Reflects Parent Body Histories. ACS Cent Sci. 2, 370–379 (2016). [CrossRef]
- B. Damer, D. Deamer, The Hot Spring Hypothesis for an Origin of Life. Astrobiology. 20, 429–452 (2020). [CrossRef]
- M. A. Sephton, "Organic Geochemistry of Meteorites" in Treatise on Geochemistry: Second Edition, H. D. Holland, K. K. Turekian, Eds. (Elsevier, Oxford, ed. 2nd, 2013; http://linkinghub.elsevier.com/retrieve/pii/C20091284735), vol. 12, pp. 1–31.
- K. KVENVOLDEN, J. LAWLESS, K. PERING, E. PETERSON, J. FLORES, C. PONNAMPERUMA, I. R. KAPLAN, C. MOORE, Evidence for Extraterrestrial Amino-acids and Hydrocarbons in the Murchison Meteorite. Nature. 228, 923–926 (1970). [CrossRef]
- D. P. Glavin, A. S. Burton, J. E. Elsila, J. C. Aponte, J. P. Dworkin, The Search for Chiral Asymmetry as a Potential Biosignature in our Solar System. Chem Rev. 120, 4660–4689 (2020). [CrossRef]
- P. Modica, Z. Martins, C. Meinert, B. Zanda, L. L. S. D’Hendecourt, The Amino Acid Distribution in Laboratory Analogs of Extraterrestrial Organic Matter: A Comparison to CM Chondrites. Astrophys J. 865, 41 (2018). [CrossRef]
- M. Nuevo, G. Auger, D. Blanot, L. d’Hendecourt, A Detailed Study of the Amino Acids Produced from the Vacuum UV Irradiation of Interstellar Ice Analogs. Origins of Life and Evolution of Biospheres. 38, 37–56 (2008). [CrossRef]
- E. T. Peltzer, J. L. Bada, G. Schlesinger, S. L. Miller, The chemical conditions on the parent body of the murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Advances in Space Research. 4, 69–74 (1984). [CrossRef]
- S. L. Miller, The mechanism of synthesis of amino acids by electric discharges. Biochim Biophys Acta. 23, 480–489 (1957). [CrossRef]
- Y. Kebukawa, Q. H. S. Chan, S. Tachibana, K. Kobayashi, M. E. Zolensky, One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci Adv. 3, e1602093 (2017). [CrossRef]
- A. S. Burton, J. C. Stern, J. E. Elsila, D. P. Glavin, J. P. Dworkin, Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev. 41, 5459–5472 (2012). [CrossRef]
- S. Pizzarello, J. R. Cronin, Non-racemic amino acids in the Murray and Murchison meteorites. Geochim Cosmochim Acta. 64, 329–338 (2000). [CrossRef]
- S. Pizzarello, Y. Huang, M. Fuller, The carbon isotopic distribution of Murchison amino acids. Geochim Cosmochim Acta. 68, 4963–4969 (2004). [CrossRef]
- S. Pizzarello, R. v. Krishnamurthy, S. Epstein, J. R. Cronin, Isotopic analyses of amino acids from the Murchison meteorite. Geochim Cosmochim Acta. 55, 905–910 (1991). [CrossRef]
- P. Ehrenfreund, D. P. Glavin, O. Botta, G. Cooper, J. L. Bada, Extraterrestrial amino acids in Orgueil and Ivuna: tracing the parent body of CI type carbonaceous chondrites. Proc Natl Acad Sci U S A. 98, 2138–2141 (2001). [CrossRef]
- M. H. Engel, S. A. Macko, Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature. 389, 265–268 (1997). [CrossRef]
- M. H. Engel, S. A. Macko, J. A. Silfer, Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature. 348 (1990), pp. 47–49. [CrossRef]
- J. W. Aerts, A. Elsaesser, W. F. M. Röling, P. Ehrenfreund, A contamination assessment of the CI carbonaceous meteorite Orgueil using a DNA-directed approach. Meteorit Planet Sci. 51, 920–931 (2016). [CrossRef]
- T. Yada, M. Abe, T. Okada, A. Nakato, K. Yogata, A. Miyazaki, K. Hatakeda, K. Kumagai, M. Nishimura, Y. Hitomi, H. Soejima, M. Yoshitake, A. Iwamae, S. Furuya, M. Uesugi, Y. Karouji, T. Usui, T. Hayashi, D. Yamamoto, R. Fukai, S. Sugita, Y. Cho, K. Yumoto, Y. Yabe, J. P. Bibring, C. Pilorget, V. Hamm, R. Brunetto, L. Riu, L. Lourit, D. Loizeau, G. Lequertier, A. Moussi-Soffys, S. Tachibana, H. Sawada, R. Okazaki, Y. Takano, K. Sakamoto, Y. N. Miura, H. Yano, T. R. Ireland, T. Yamada, M. Fujimoto, K. Kitazato, N. Namiki, M. Arakawa, N. Hirata, H. Yurimoto, T. Nakamura, T. Noguchi, H. Yabuta, H. Naraoka, M. Ito, E. Nakamura, K. Uesugi, K. Kobayashi, T. Michikami, H. Kikuchi, N. Hirata, Y. Ishihara, K. Matsumoto, H. Noda, R. Noguchi, Y. Shimaki, K. Shirai, K. Ogawa, K. Wada, H. Senshu, Y. Yamamoto, T. Morota, R. Honda, C. Honda, Y. Yokota, M. Matsuoka, N. Sakatani, E. Tatsumi, A. Miura, M. Yamada, A. Fujii, C. Hirose, S. Hosoda, H. Ikeda, T. Iwata, S. Kikuchi, Y. Mimasu, O. Mori, N. Ogawa, G. Ono, T. Shimada, S. Soldini, T. Takahashi, Y. Takei, H. Takeuchi, R. Tsukizaki, K. Yoshikawa, F. Terui, S. Nakazawa, S. Tanaka, T. Saiki, M. Yoshikawa, S. ichiro Watanabe, Y. Tsuda, Preliminary analysis of the Hayabusa2 samples returned from C-type asteroid Ryugu. Nat Astron. 6, 214–220 (2022). [CrossRef]
- D. P. Glavin, M. P. Callahan, J. P. Dworkin, J. E. Elsila, The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit Planet Sci. 45, 1948–1972 (2010). [CrossRef]
- Z. Martins, P. Modica, B. Zanda, L. L. S. d’Hendecourt, The amino acid and hydrocarbon contents of the Paris meteorite: Insights into the most primitive CM chondrite. Meteorit Planet Sci. 50, 926–943 (2015). [CrossRef]
- A. Furusho, T. Akita, M. Mita, H. Naraoka, K. Hamase, Three-dimensional high-performance liquid chromatographic analysis of chiral amino acids in carbonaceous chondrites. J Chromatogr A. 1625, 461255 (2020). [CrossRef]
- D. P. Glavin, J. P. Dworkin, A. Aubrey, O. Botta, J. H. Doty, Z. Martins, J. L. Bada, Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit Planet Sci. 41, 889–902 (2006). [CrossRef]
- Myrgorodska, C. Meinert, Z. Martins, L. le Sergeant d’Hendecourt, U. J. Meierhenrich, Quantitative enantioseparation of amino acids by comprehensive two-dimensional gas chromatography applied to non-terrestrial samples. J Chromatogr A. 1433, 131–136 (2016). [CrossRef]
- L. Kirkup, M. Mulholland, Comparison of linear and non-linear equations for univariate calibration. J Chromatogr A. 1029, 1–11 (2004). [CrossRef]
- Schaefer, I. M. Dambuza, S. Dall’Angelo, R. Yuecel, M. Jaspars, L. Trembleau, M. Zanda, G. D. Brown, M. G. Netea, N. A. R. Gow, A Weakened Immune Response to Synthetic Exo-Peptides Predicts a Potential Biosecurity Risk in the Retrieval of Exo-Microorganisms. Microorganisms. 8, 1066 (2020). [CrossRef]
- S. A. Airieau, J. Farquhar, M. H. Thiemens, L. A. Leshin, H. Bao, E. Young, Planetesimal sulfate and aqueous alteration in CM and CI carbonaceous chondrites. Geochim Cosmochim Acta. 69, 4167–4172 (2005). [CrossRef]
- S. Watson, V. K. Pearson, I. Gilmour, M. A. Sephton, Contamination by sesquiterpenoid derivatives in the Orgueil carbonaceous chondrite. Org Geochem. 34, 37–47 (2003). [CrossRef]
- J. Oró, T. Tornabene, Bacterial Contamination of Some Carbonaceous Meteorites. Science (1979). 150, 1046–1048 (1965). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).