Introduction
Acute subdural hematoma (ASH) represents a major clinical entity in traumatic brain injury (TBI), diagnosed on computed tomography (CT) as an extra axial, hyperdense, crescent lesion between the dura, and brain parenchyma [
1]. The prognosis for traumatic ASH is poor [
2], presenting the worst outcome of any category of severe TBI, given that 60% (21-90%) will die or remain severely disabled [
3,
4,
5,
6]. Several variables can help to predict prognosis in these cases, as age, trauma mechanism, presence of intracranial or systemic associated lesions, elapsed time between the traumatic event and the onset of treatment, hypoxia, hypotension and midline shift (MLS) [
7,
8,
9], per example. ASH represents a major clinical entity in severe TBI. Approximately 60% of patients with a severe TBI also have an ASH to some extent [
3]. Severe TBI has been reported to cause circulatory disturbances, specially at an acute stage. Several clinical TBI studies have linked low CBF to poor outcome during the acute stage [
10,
11,
12]. The CBF in patients with ASH decreases markedly immediately after injury [
4,
13].
The ASH pathophysiology, besides the hematoma mass expansion itself, involves hemodynamic and metabolic events that can generate brain swelling [
14,
15,
16,
17]. Moreover, it is known that when the pressure within the cranium begins to rise, venous blood is displaced through the jugular foramen and eventually the right atrium. This displacement is able to compensate the increased pressure for a time until mechanical compensation is overcome [
18,
19]. All this cascade of events cooperates to intracranial pressure (ICP) elevation [
20,
21] and the persistence of decreased brain blood volume and hypoxia [
1,
22,
23].
Recently, perfusion tomography (PCT) became an important diagnostic tool in the emergency settings. The use of PCT allows the evaluation of at least three hemodynamic parameters: cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT). PCT has already demonstrated that decompressive craniectomy surgery improves cerebral hemodynamics in patients sustaining cerebrovascular ischemic stroke [
20,
24,
25], nevertheless, the usefulness of PCT for assessment of ASH has been less explored. PCT may be helpful in a neurosurgical perspective to choose the best treatment strategy: craniotomy with hematoma evacuation or decompressive craniectomy [
1,
20,
26]. Therefore, the present study aimed to evaluate the brain hemodynamics before and after surgery in a series of patients with ASH. A secondary endpoint was to evaluate the cerebral hemodynamics findings correlation with the outcome of this population.
Methods
This was a single-center, prospective cohort study. The patients were consecutively enrolled at the emergency room of a tertiary hospital and the patients had a 6-month follow-up after the surgical procedure. The clinical and surgical treatments were performed in accordance with the hospital guidelines. This study was approved by the local institutional ethics committee, (IRB 38212714.2.0000.0068), informed consent was obtained with the next of kin for each patient. The study hypothesis was that the hemodynamics parameters would improve after surgery. It was evaluated the MTT, CBF, and CBV before and after surgery. The primary dependent variable was the change in PCT parameters after surgery. Other secondary endpoint was to correlate the same PCT parameters with the extended Glasgow outcome scale in 6 months.
Subjects and procedures
Patients with clinical and radiological signs of ASH and possible indication of surgery were eligible for the study. The decision to perform the procedure was made via the mutual agreement of the neurology and neurosurgery teams. The researchers involved in this study did not participate in the decision to define the appropriate treatment. After defining the operative management, the patients were recruited. All patients were hospitalized in an intensive care unit during the postoperative period. After 6 months, a structured interview was performed by a single evaluator, who was blinded to the information from PCT studies.
The inclusion criteria were as follows: patients >18 years of age, ASH that required surgical decompression, and informed consent obtained from the patient or a lawful representative. The exclusion criteria were as follows: intracerebral hematoma presented on nonenhanced CT on admission; presence of contraindications for PCT, such as renal failure or contrast allergy; hemodynamic instability; fever; pregnancy; hemoglobin <8 mg/dL; patients who qualified for thrombolytic treatment; and poor technical quality of pre- or postoperative PCT. The patients included in this study underwent nonenhanced head CT and PCT before and 24 hours after the surgical procedure.
PCT Protocol
PCT tests were performed using a 64-channel multidetector CT scanner (Philips Medical Systems World Headquarters, Best, the Netherlands). Dynamic conventional scans were obtained in a plane parallel to the orbitomeatal orientation that included the basal ganglia, the thalamus, and parts of the anterior, middle, and posterior cerebral arterial. The acquisition lasted 50 seconds and began 5 seconds after an intravenous infusion of 50 mL iodinated contrast (concentration 300 mg/mL) via an injection pump, which was administered at a rate of 4 mL/s. The imaging parameters were 80 kV and 200 mA.
The images were acquired in a helicoidal cine mode at a rate of 1 image per second and were analyzed manually using the General Electric CT Perfusion Software. The arterial and venous input regions of interest (ROIs) were selected. To produce a proper protocol to evaluate the PCT, it was defined a limited number of ROIs to be studied when analyzing the PCT images. ROIs were positioned at the brain cortical regions and basal ganglia, also at the lesion’s core and around the area of hypoattenuation ipsilateral to the lesion. Mirrored ROIs contralateral to the lesion were also evaluated. The postoperative PCT was performed using the same technique and utilizing the same pattern as the preoperative ROI inputs. The absolute values of MTT, CBF, and CBV were obtained on the workstation. All patient data were pooled and analyzed in terms of their means and confidence intervals. All PCT exams were evaluated by a single experienced radiologist, who was blinded to the clinical data.
Statistical Analysis
The variables were described by the mean value, presented as standard deviation (SD). To compare the PCT parameters values, it was used the Wilcoxon test, which was considered statistically significant for p values < 0.05. Spearman’s correlation test was used to correlate postoperative and preoperative CBF ratio with outcome.
Results
Four male and one female were evaluated,
Table 1 shows epidemiological characteristics of each patient analyzed. Primary mechanism of trauma was fall (four patients), and one patient was a victim of a car crash and evolved to death (three days after surgery). The mean age was 46 years (SD: 8.1). All patients were submitted to surgery due to ASH, the MLS, rCBF, rCBV and rMTT before and after surgery are described at
Table 2.
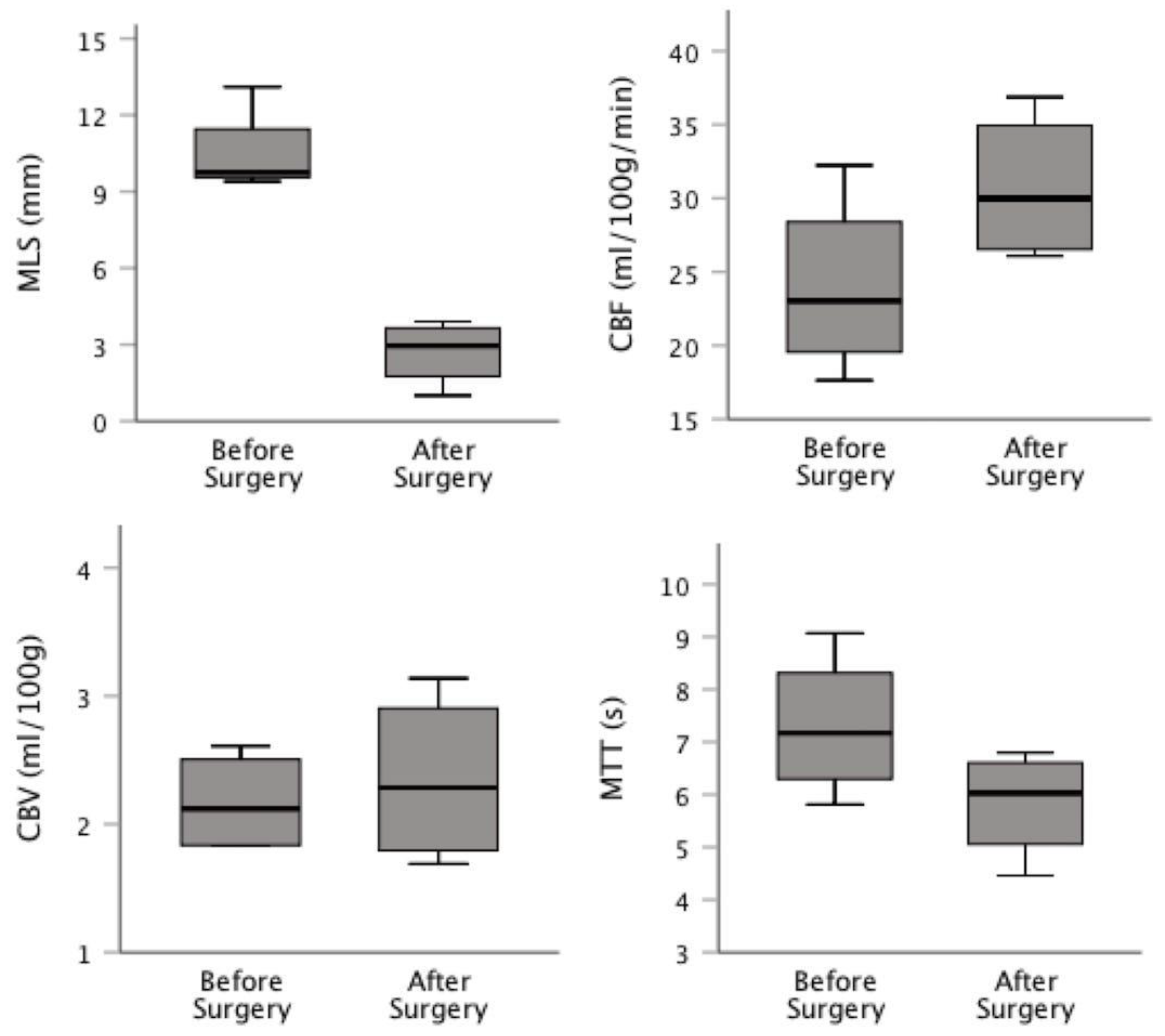
The preoperative mean of the MLS was 10.06 mm (SD: 1.80), which was reduced, after surgery to 2.54 (SD: 1.15; p=0.031). There was a general improvement in CBF after surgery from 23.9 (SD: 6.1) to 30.7 ml/100g/min, (SD: 5.1) and in MTT from 7.3 (SD: 1.3) to 5.8s (SD: 1.0), although it is not statistically significant (p=0.06 and p=0.06, respectively).
There was still a reduction in the CBV media when compared to the value after surgery, from 2.63 (SD: 1.10) to 2.34 ml/100g (SD: 0.67), which is not statistically significant (p=0.31). Post/preoperative CBF ratio correlation with outcome was 0,94 (p=0.054).
Figure 1 presents a box plot comparing the pre- and postoperative values of MLS, CBF, CBV and MTT.
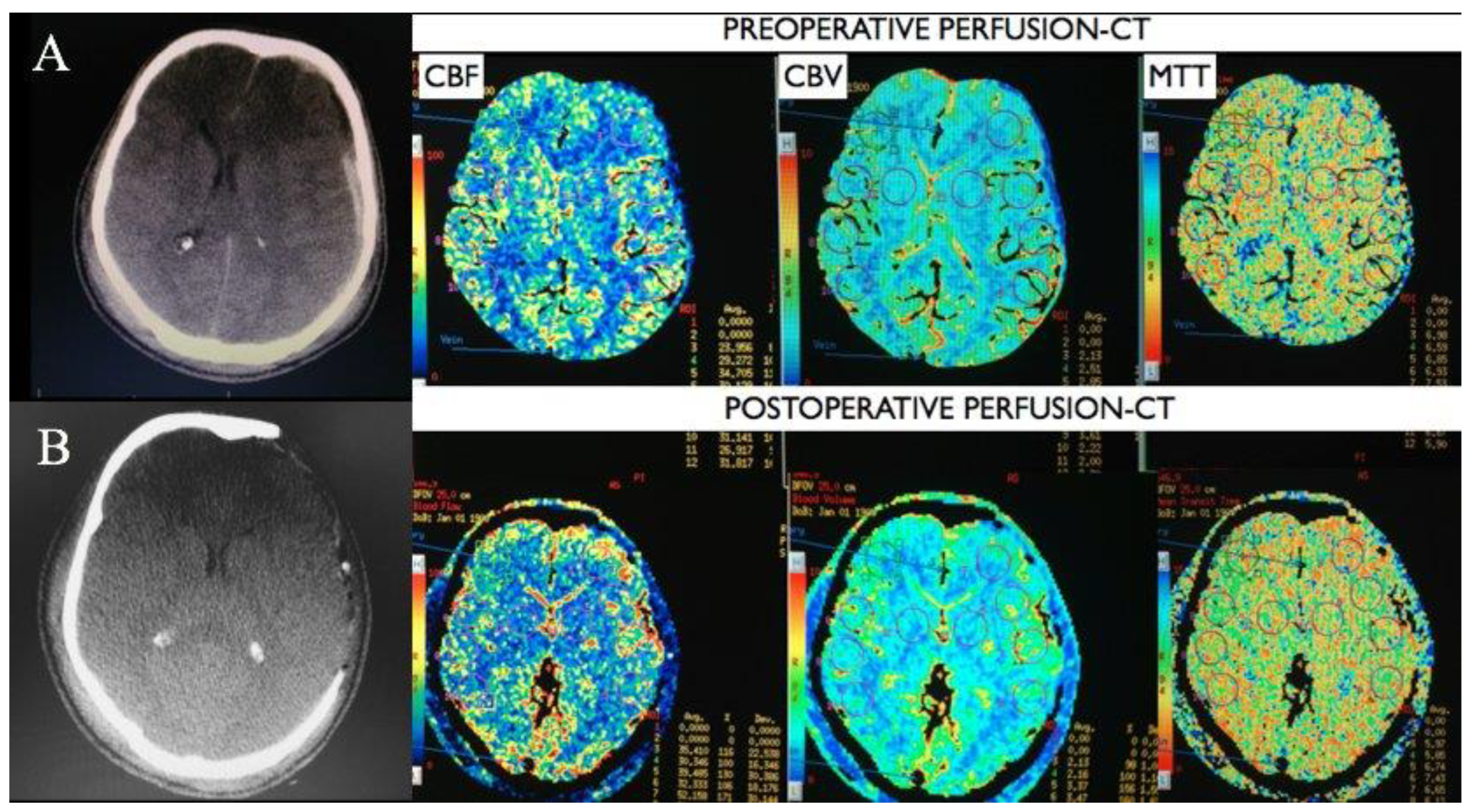
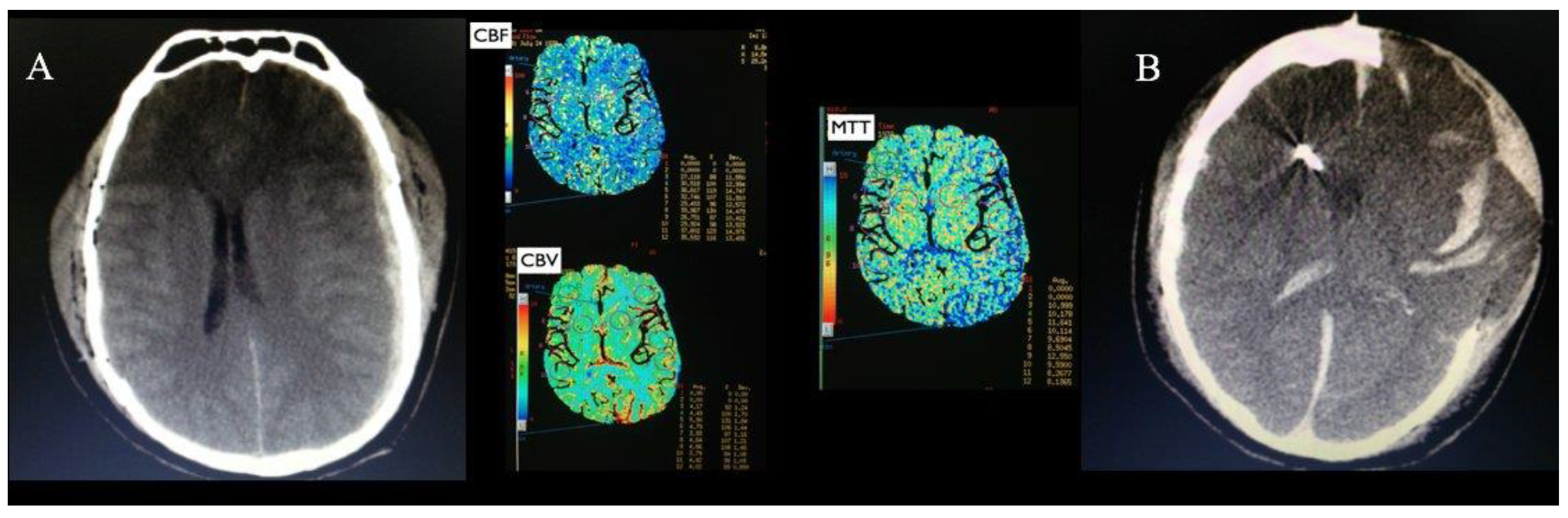
Figure 2 shows the CT scan and PCT pre and postoperatively of a patient, being possible to observe an increase in CBF and a reduction in MTT after surgery. The patient who died had the highest MTT (9.97 seconds) and the highest CBV 4.51 ml/100g. Postoperative PCT was not possible in this patient, however the CT scan post presents multiple hematomas and cerebral herniation, the images of this patient are shown in
Figure 3.
Discussion
The present case series suggest that improvement in PCT parameters from pre to post operative stages, such as reduction in MLS and MTT, as long as elevation in CBF and CBV may have value for the follow-up of ASH patients. To our knowledge, exploring cerebral hemodynamics using PCT on the resection of ASH has not been performed previously.
PCT is a non-invasive hemodynamic assessment method already used in the context of diseases such as cerebral ischemia and spontaneous subarachnoid hemorrhage (SAH). In a prospective study evaluating 50 patients with SAH, the presence of a prolonged MTT was associated with delayed cerebral ischemia and cerebral infarction. Despite not correlating with prognosis [
2,
26]. Dolatowski et al. [
27] proposed the use of PCT as a non-invasive assessment method for early detection of vasospasms. By doing that, PCT assists in the definition of performing angioplasty when treating severe cases, being the MTT the parameter with the highest diagnostic accuracy.
Amorim et al. [
28] have shown that decompressive craniectomy in middle cerebral artery’s stroke is associated with overall brain hemodynamic improvement when evaluated by CT Perfusion. The before-mentioned improvement was represented by the global MTT reduction, increased CBF in the infarction zone, and reduction of MTT in the contralateral hemisphere [
28]. Bendinelli et al. [
15] performed admission PCT in 50 severe TBI patients and showed that CTP variables had great predictive ability to predict poor outcome (AUC for abnormal CTP=0.92), showing the potential of PCT as a tool to evaluate outcome in TBI. However, postoperative PCT in such patients were not performed. Moreover, a detailed analysis of patients sustaining ASH was not available. In our study, the initial analysis of five cases showed a trend of amelioration in brain hemodynamics after surgery and suggested that PCT may be useful as a prognostic tool in patients with ASH.
This study has some limitations. The number of patients included in this study was relatively small. In addition, the hemodynamic study was only performed with PCT. Prospective studies, using transcranial Doppler or CT angiography could allow further evaluation of cerebral collateral circulation and the occurrence of recanalization. Because surgical treatment was performed as needed, at any time of the day, the use of Doppler was not feasible because it could only be performed during the day. Additionally, because CT angiography requires additional exposure to radiation and intravenous iodinated contrast, we preferred not to use it because the patients would have been exposed to 2 PCT studies over a relatively short duration. A study with a greater sample size is necessary to certify if PCT can be used in clinical practice to evaluate intraoperative brain status and long-term patient outcome.
Conclusion
CBF seems to increase after acute subdural hematoma surgery, and it suggests that the improvement in brain hemodynamics correlates to an improved outcome, especially when evaluated in conjugacy with the MTT values, which reduces significantly following decompressive craniectomy.
Funding
No funding was received for this research.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee (Research Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Karibe, H.; Hayashi, T.; Hirano, T.; Kameyama, M.; Nakagawa, A.; Tominaga, T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo) 2014, 54, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, S.; Nakae, R.; Yokota, H.; Spurlock, M.S.; Mondello, S.; Gajavelli, S.; Bullock, R.M. Subdural hematoma decompression model: A model of traumatic brain injury with ischemic-reperfusional pathophysiology: A review of the literature. Behav Brain Res 2018, 340, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.A.; Fitzgerald, S.; Moiel, R.H.; Caram, P. Acute subdural hematomas. Review of 144 cases. J Neurosurg 1975, 42, 37–42. [Google Scholar] [CrossRef]
- Hatashita, S.; Koga, N.; Hosaka, Y.; Takagi, S. Acute subdural hematoma: severity of injury, surgical intervention, and mortality. Neurol Med Chir (Tokyo) 1993, 33, 13–18. [Google Scholar] [CrossRef]
- Sakas, D.E.; Bullock, M.R.; Teasdale, G.M. One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg 1995, 82, 961–965. [Google Scholar] [CrossRef]
- Wilberger, J.E., Jr.; Harris, M.; Diamond, D.L. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 1991, 74, 212–218. [Google Scholar] [CrossRef]
- Braakman, R.; Habbema, J.D.; Gelpke, G.J. Prognosis and prediction of outcome in comatose head injured patients. Acta Neurochir Suppl (Wien) 1986, 36, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Lansberg, M.G. CT perfusion in acute stroke: Practical guidance for implementation in clinical practice. J Cereb Blood Flow Metab 2019, 39, 1664–1668. [Google Scholar] [CrossRef]
- Honda, M.; Ichibayashi, R.; Suzuki, G.; Yokomuro, H.; Seiki, Y.; Sase, S.; Kishi, T. Consideration of the Intracranial Pressure Threshold Value for the Initiation of Traumatic Brain Injury Treatment: A Xenon CT and Perfusion CT Study. Neurocrit Care 2017, 27, 308–315. [Google Scholar] [CrossRef]
- Honda, M.; Ichibayashi, R.; Yokomuro, H.; Yoshihara, K.; Masuda, H.; Haga, D.; Seiki, Y.; Kudoh, C.; Kishi, T. Early Cerebral Circulation Disturbance in Patients Suffering from Severe Traumatic Brain Injury (TBI): A Xenon CT and Perfusion CT Study. Neurol Med Chir (Tokyo) 2016, 56, 501–509. [Google Scholar] [CrossRef]
- Lang, E.W.; Lagopoulos, J.; Griffith, J.; Yip, K.; Mudaliar, Y.; Mehdorn, H.M.; Dorsch, N.W. Noninvasive cerebrovascular autoregulation assessment in traumatic brain injury: validation and utility. J Neurotrauma 2003, 20, 69–75. [Google Scholar] [CrossRef]
- Yokobori, S.; Yamaguchi, M.; Igarashi, Y.; Hironaka, K.; Onda, H.; Kuwamoto, K.; Araki, T.; Fuse, A.; Yokota, H. Outcome and Refractory Factor of Intensive Treatment for Geriatric Traumatic Brain Injury: Analysis of 1165 Cases Registered in the Japan Neurotrauma Data Bank. World Neurosurg 2016, 86, 127–133 e121. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C. Hyperacute measurement of intracranial pressure, cerebral perfusion pressure, jugular venous oxygen saturation, and laser Doppler flowmetry, before and during removal of traumatic acute subdural hematoma. J Neurosurg 2001, 95, 569–572. [Google Scholar] [CrossRef]
- Amorim, R.L.; Bor-Seng-Shu, E.; G, S.G.; Paiva, W.; de Andrade, A.F.; Teixeira, M.J. Decompressive craniectomy and cerebral blood flow regulation in head injured patients: a case studied by perfusion CT. J Neuroradiol 2012, 39, 346–349. [Google Scholar] [CrossRef]
- Bendinelli, C.; Bivard, A.; Nebauer, S.; Parsons, M.W.; Balogh, Z.J. Brain CT perfusion provides additional useful information in severe traumatic brain injury. Injury 2013, 44, 1208–1212. [Google Scholar] [CrossRef]
- Wang, T.D. Brain Perfusion Matters: From Pituitary Function to Blood Pressure Control during Acute Ischemic Stroke. Acta Cardiol Sin 2018, 34, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Hofmeijer, J.; Kappelle, L.J.; Algra, A.; Amelink, G.J.; van Gijn, J.; van der Worp, H.B.; investigators, H. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009, 8, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Brasil, S. Intracranial pressure pulse morphology: the missing link? Intensive Care Med 2022. [Google Scholar] [CrossRef] [PubMed]
- Brasil, S.; Nogueira, R.C.; Salinet, A.S.M.; Yoshikawa, M.H.; Teixeira, M.J.; Paiva, W.; Malbouisson, L.M.S.; Bor-Seng-Shu, E.; Panerai, R.B. The contribution of intracranial pressure to human dynamic cerebral autoregulation after acute brain injury. Am J Physiol Regul Integr Comp Physiol 2022. [Google Scholar] [CrossRef] [PubMed]
- Sawauchi, S.; Marmarou, A.; Beaumont, A.; Signoretti, S.; Fukui, S. Acute subdural hematoma associated with diffuse brain injury and hypoxemia in the rat: effect of surgical evacuation of the hematoma. J Neurotrauma 2004, 21, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lou, H.Y.; Xu, J.; Wang, H.; Huang, X.; Gong, J.B.; Xiong, B.; Yang, X.F. The impact of cranioplasty on cerebral blood perfusion in patients treated with decompressive craniectomy for severe traumatic brain injury. Brain Inj 2015, 29, 1654–1660. [Google Scholar] [CrossRef]
- Hom, J.; Dankbaar, J.W.; Soares, B.P.; Schneider, T.; Cheng, S.C.; Bredno, J.; Lau, B.C.; Smith, W.; Dillon, W.P.; Wintermark, M. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol 2011, 32, 41–48. [Google Scholar] [CrossRef]
- Teasdale, G.; Parker, L.; Murray, G.; Knill-Jones, R.; Jennett, B. Predicting the outcome of individual patients in the first week after severe head injury. Acta Neurochir Suppl (Wien) 1979, 28, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.G.; Ducrocq, S.; Rackelbom, T.; Orliaguet, G.; Renier, D.; Carli, P. Surgical evacuation of acute subdural hematoma improves cerebral hemodynamics in children: a transcranial Doppler evaluation. Childs Nerv Syst 2005, 21, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, S.; Latini, F.; Ceruti, S.; Chieregato, A.; d'Esterre, C.; Lee, T.Y.; Cavallo, M.; Fainardi, E. Temporal changes in CT perfusion values before and after cranioplasty in patients without symptoms related to external decompression: a pilot study. Neuroradiology 2014, 56, 237–243. [Google Scholar] [CrossRef]
- Murphy, A.; Manoel, A.L.; Burgers, K.; Kouzmina, E.; Lee, T.; Macdonald, R.L.; Bharatha, A. Early CT perfusion changes and blood-brain barrier permeability after aneurysmal subarachnoid hemorrhage. Neuroradiology 2015, 57, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Dolatowski, K.; Malinova, V.; Frolich, A.M.; Schramm, R.; Haberland, U.; Klotz, E.; Mielke, D.; Knauth, M.; Schramm, P. Volume perfusion CT (VPCT) for the differential diagnosis of patients with suspected cerebral vasospasm: qualitative and quantitative analysis of 3D parameter maps. Eur J Radiol 2014, 83, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.L.; de Andrade, A.F.; Gattas, G.S.; Paiva, W.S.; Menezes, M.; Teixeira, M.J.; Bor-Seng-Shu, E. Improved hemodynamic parameters in middle cerebral artery infarction after decompressive craniectomy. Stroke 2014, 45, 1375–1380. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).