Introduction
Worldwide there are around 30 million stroke survivors, placing stroke second in causes of complex disability (1-4). Symptoms such as spasticity, apraxia, neglect and agnosia are well-known and extensively investigated (5, 6). Besides these symptoms, there are other consequences that recently receives more attention. Mackay-Lyons and Makrides (7) found that the average aerobic capacity of a person with stroke was only 60% of the age and gender related norm. This decreased aerobic capacity is one of the important risk factors in the development of recurrent stroke (8). Considering that recurrent stroke accounts for 20% of all stroke cases (9, 10).
The decreased aerobic capacity after stroke can be explained by a reduction in physical activity, changes in motivation and mood, fatigue, cognitive impairments, and neurological deficits like motor and sensory problems (11, 12). A decreased aerobic capacity may result in a vicious deconditioning cycle in which a reduced maximum oxygen uptake (VO2max) leads to paucity of movements and less weight bearing activities. This can result in altered muscle properties and reduced central command. Both can contribute to a further decrease in VO2max (11, 12). Stimulating people with stroke to be active can break the downward deconditioning spiral. Higher levels of self-efficacy and self-regulation have also been shown to be associated with increased physical activity in individuals (13-16).
Aerobic exercise has proven to be successful in the prevention of prolonged inactivity, avoidance of recurrent stroke and cardiorespiratory events, and improvement of aerobic capacity (17). A minimum of 20 minutes of aerobic exercise, three times a week during minimal 8 weeks, is recommended to achieve a clinically meaningful exercise effect in people with stroke (18).
Since 2011, low to moderate intensity aerobic exercise emerged in the stroke guidelines of the American Stroke Association (19) and in the AEROBICS guidelines (20) are a recommended treatment modality to improve VO2max in people with stroke. In the years following these recommendations, more and more evidence accumulated, including evidence of the benefits of higher intensity training in a supervised, inpatient setting (21-23). Studies in an unsupervised home-based setting are still scarce. Nevertheless, recent guidelines state that aerobic exercise can be delivered in a hospital, in an outpatient clinic but also in an unsupervised home-based setting (18). Home-based training is the most convenient and accessible for most patients but raises concerns about feasibility including safety (19, 24-27).
The delivery of aerobic exercise in a supervised setting has several disadvantages that warrant a more thorough investigation of unsupervised exercise. One of the drawbacks of supervised training is the requirement for transport to the rehabilitation centre which is a frequently reported barrier for physical exercise (21-23, 28-31). It is also difficult to retain the benefits from training in a supervised setting in the long term (18).
In cardiac and obese patients, home-based aerobic exercises have been extensively investigated and have proven to be safe, well tolerated, and effective (32, 33). Here, the training intensities of home-based, unsupervised exercise programmes often vary between 60-80% of the maximal heart rate (HRmax) (1-3). As there is considerable overlap between the physical profile of cardiac and stroke survivors, the question arises whether similar exercise programmes would yield the same benefits in people with stroke.
In the stroke population, home-based physical exercises have shown to be as effective as conventional therapy for improving the activities of daily life (ADL) (34). In existing studies, the absence of supervision during functional exercises does not appear to impact therapy compliance (32). However, research that investigates the effect of unsupervised home-based training on aerobic capacity in people with stroke remains scarce (35, 36).
In one of the limitedly available studies, a 12-week home-based brisk walking programme in people with chronic stroke was researched and showed improved quality of life (37). Mayo, MacKay-Lyons (38), found no significant improvements in the 6-minute walk test after a one-year home-based stationary cycling programme. In 2019, the research group of Krawcyk, Vinther (39) investigated a home-based High Intensity Interval Training (HIIT) programme on a stationary bike in people with stroke. The programme consisted of 15-minute training sessions, five days a week for 12 weeks at an intensity of 77-93% of HRmax. Here HIIT proved to be safe and well-received in home-based people with stroke but did not translate into significant improvements in cardiorespiratory fitness and general well-being (39). Overall, existing home-based interventions either were performed at relatively low intensities, had large variations in intensity, used a short training duration and/or were carried out on device not suitable for participants with more severe impairments. Alternative devices such as recumbent bikes can enlarge the population of suitable participants.
This study therefore aims to investigate the feasibility and preliminary effectiveness of an individually tailored, home-based, 12-weeks moderate intensity training programme on a stationary recumbent bike in people with stroke.
Study Design and Setting
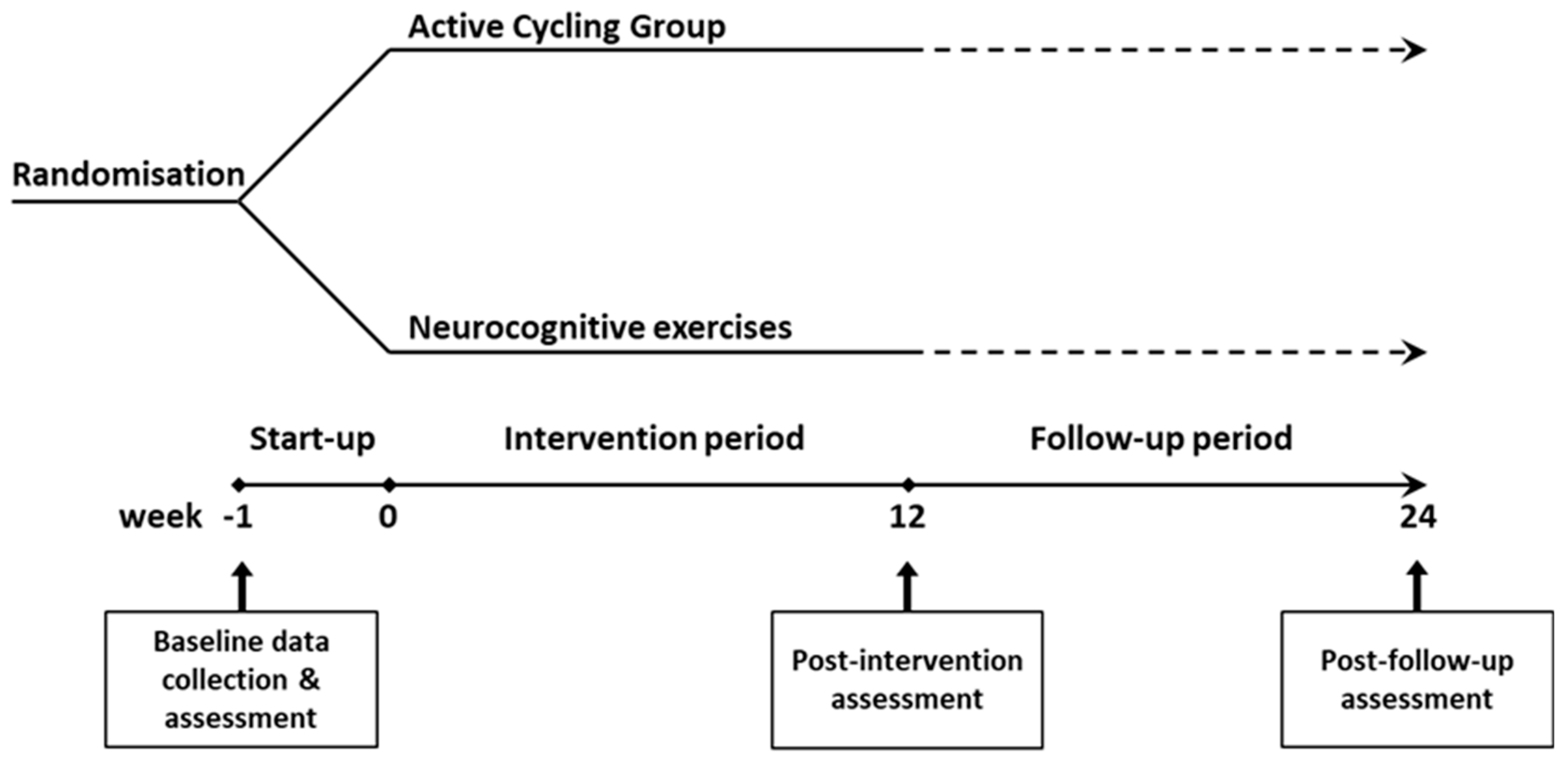
A single-blind, parallel group, randomised controlled design was used. Participants were randomised at a 1:1 ratio to a 12-week active cycling group (ACG) or a control group via sealed envelopes as illustrated in
Figure 1. Group allocation occurred immediately after baseline testing. The intervention starts within one week of baseline testing. Post-intervention assessment is performed immediately after the 12-week programme and after a follow-up period of another 12 weeks. All assessments are carried out at RevArte, a neuro-rehabilitation clinic for outpatients and inpatients in the Antwerp region (Belgium). All training sessions occurred at the participants’ homes. The reporting of this study conforms to the STROBE Guidelines (40).
Participants
Participants will be recruited from the neuro-rehabilitation clinic RevArte by referral from their physical therapist. Participant inclusion criteria are: 1) first-ever stroke except subarachnoid haemorrhage (41), 2) age <80 years, 3) stroke onset between three months and two years, 4) community dwelling, 5) hemodynamically stable, 6) able to follow simple verbal instructions, and 7) able to pedal a Ergoselect 600 recumbent bike (Ergoline) >1 minute. The criteria for exclusion are: 1) history of substance abuse, psychological illnesses or neurological condition prior to stroke, 2) relative and absolute contra-indications for exercise testing (42), and 3) unable to install a recumbent bike at the participant’s home.
This study is approved by the Medical Ethics Committee of the Antwerp University Hospital (UZA; B300201939038). All subjects volunteer to participate and give prior written informed consent.
Treatment Conditions
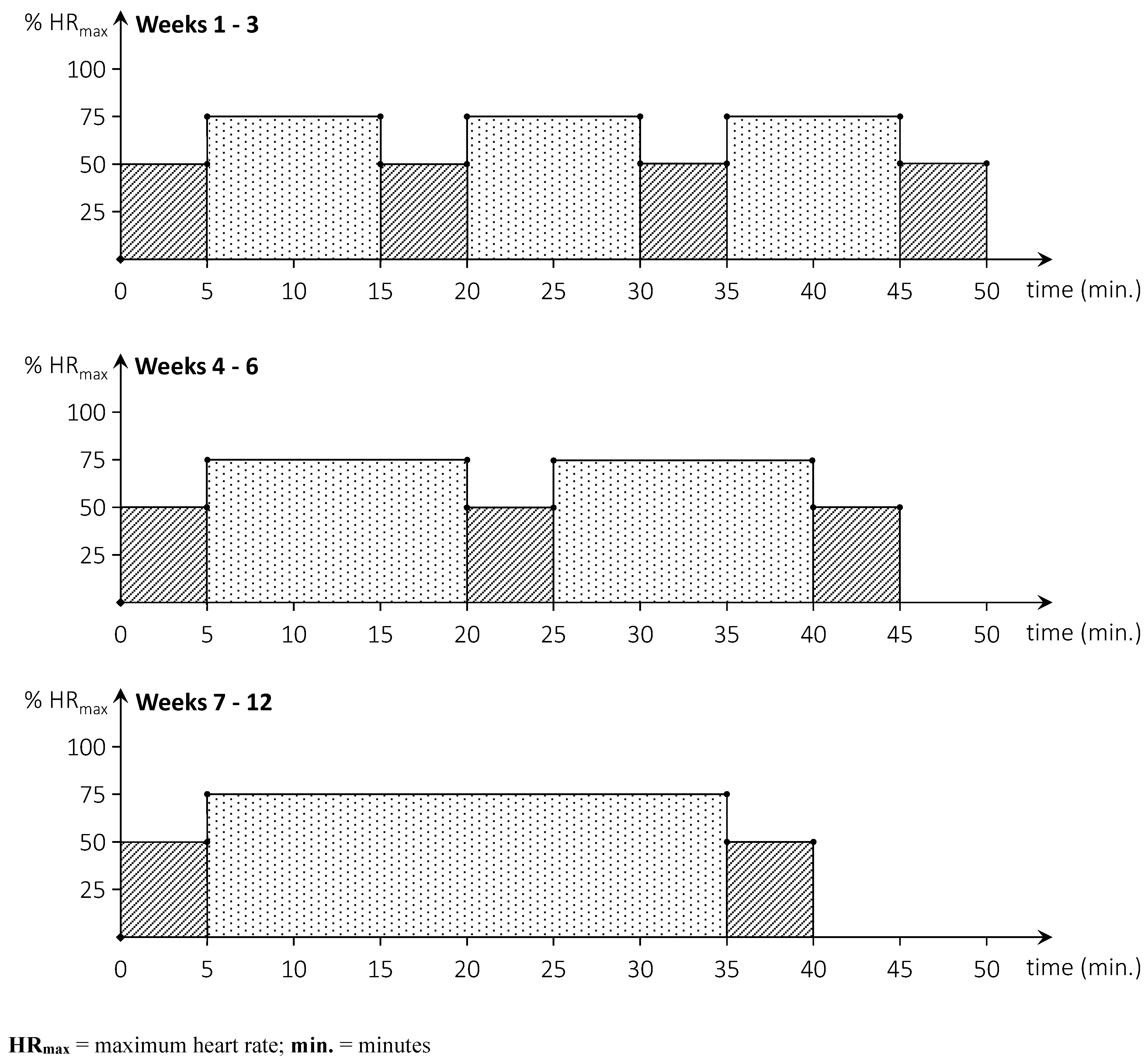
The ACG carries out a home-based cycling programme and the control group executes a home-based neurocognitive exercise programme. Both programmes are performed 3 times per week for a period of 12 weeks in addition to subjects’ usual care. Participants in the ACG executed an aerobic cycling programme and are provided with a recumbent bike (Tunturi E-80R, Tunturi New Fitness B.V., Almere, The Netherlands) and a compatible heart rate chest strap (5.3Khz) at their homes. Each training consists of 30 minutes of cycling in an interval mode (weeks 1-6) or continuously (weeks 7-12) as illustrated in
Figure 2. The training heart rate is set at 75% of the theoretical HR
max, corresponding to the higher end of moderate intensive activity (18). The HR
max is calculated as [220 – age (in years)] (43, 44) or as [168 – 0.51 X age (in years)] for participants taking beta-blocker medication (45). The warm-up, cool-down, and active recovery interval heart rates are set at 50% of the HR
max. Each session started with a warm-up and ended with a cool-down of five minutes. Training consists of three working intervals of 10 minutes (weeks 1-3), two working intervals of 15 minutes (weeks 4-6), or continuous training of 30 minutes (weeks 7-12). An active recovery period of five minutes is programmed between two interval bouts. Participants are instructed to maintain a cycling cadence of 60 – 70 rpm throughout the training sessions. Participants are requested to keep an exercise diary and to export the data of each training session to a USB storage device connected to the bike. A researcher collects training data every four weeks by offloading the training data and monitor compliance, adherence, and adverse events. After the first week of training a researcher collects training data and verifies for malfunctions or problems.
The control group carries out a neurocognitive exercise programme focused on executive functions, visual & spatial abilities, and visual attention. The programme was adapted from an existing visuospatial neglect test battery (46). During each training session, participants were requested to complete a workbook that contained several cancellation, bisection, drawing, visuospatial navigation, and other cognitive tasks. It is estimated that an average participant will take about 30 minutes to complete one workbook. The programme consists of 12 unique workbooks. Every 4 weeks, when 12 workbooks were completed, a researcher visited the participant to collect the workbooks and to leave 12 new workbooks for the next 4 weeks. After the first week of training a researcher collects training data and verifies for problems. Compliance and adverse events are also monitored. Participants are requested to keep an exercise diary to track progress.
Outcome Assessments
All outcome measures are assessed three times (
Figure 1): 1) at baseline (approximately one week before the start of the training), 2) at post-intervention (within one week after the 12 weeks training), and 3) at the primary end-point 12 weeks after the end of the training.
Demographic and stroke information, including medical history are collected (47). The
simplified modified Rankin Scale questionnaire (smRSq) is used as a tool for measurement of baseline stroke severity (48)
Feasibility
Feasibility is split into three categories: compliance with training, adherence to protocol and safety as measured by the occurrence of adverse events. Each category is assessed continuously throughout the intervention in the ACG and control group.
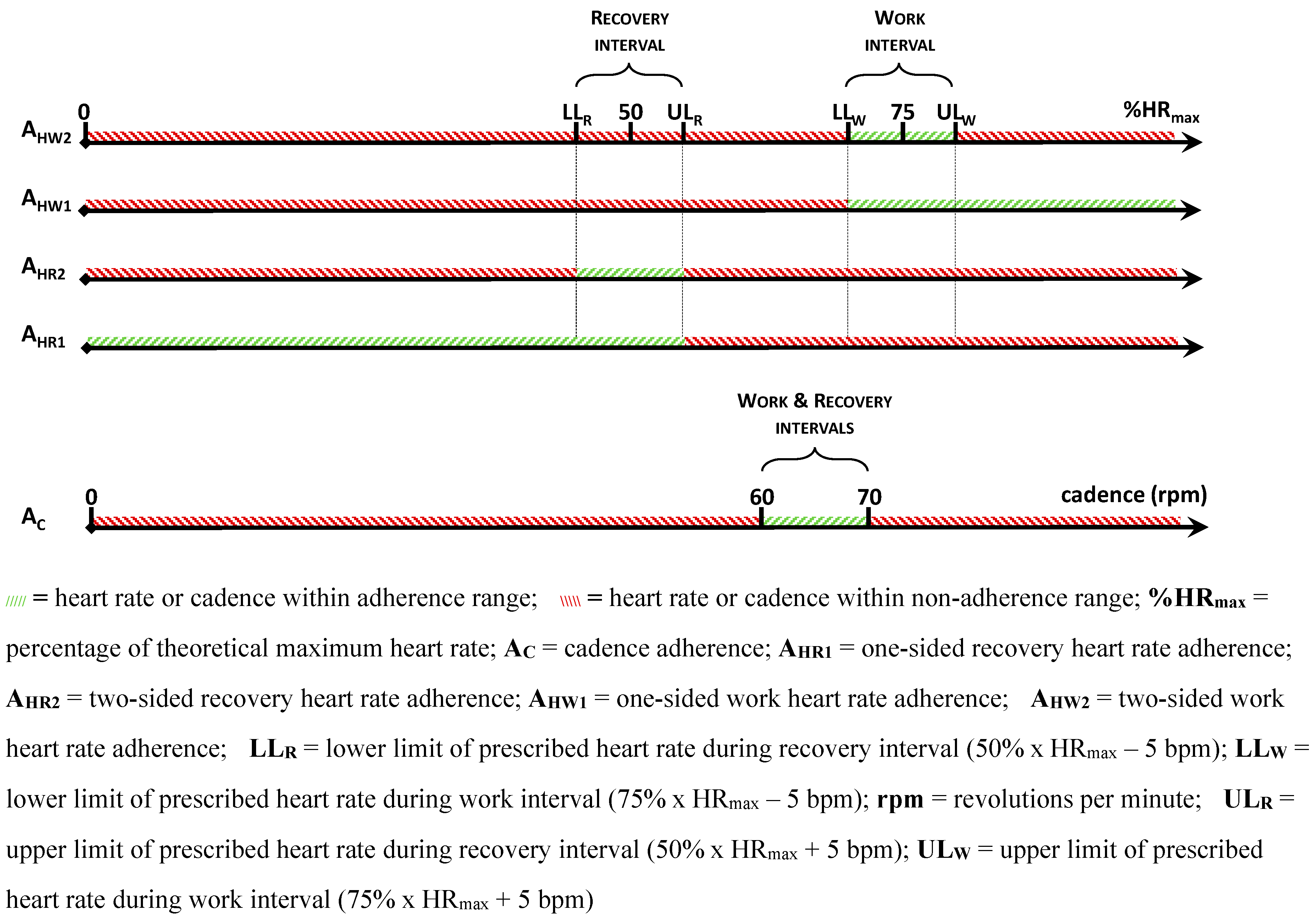
Compliance with training is measured by recording the number of training sessions completed in both groups. In the ACG,
adherence to protocol is recorded by measuring the degree to which participants trained within the requested cycling cadence of 60 – 70 rpm (A
C), and the extent to which they stayed within a 5bpm range of the prescribed heart rate levels (A
H) (
Figure 3). Separate adherence figures are calculated for adherence to the work interval heart rate (A
HW) and recovery interval heart rate (A
HR). For maximum standardisation, both upper (prescribed heart rate + 5bpm) and lower (prescribed heart rate - 5bpm) limits must be respected. In what follows, the latter is referred to as two-sided adherence for work heart rates (A
HW2) and recovery heart rates (A
HR2). To enable in-depth analysis and because of its clinical value, one-sided adherence is also calculated for heart rate levels by only considering the lower limit (work interval) or upper limit (recovery interval). Thus, one-sided adherence is the degree to which participants cycled
at or higher than 75% x HR
max – 5bpm for work heart rates (A
HW1) and
at or lower than 50% x HR
max + 5bpm for recovery heart rates (A
HR1). In the control group, completeness of workbooks was measured for adherence. All participants were requested to write down any adverse events in their exercise diary. During each scheduled visit at a participant’s home, a researcher also verified if any
adverse events had occurred.
Secondary Outcome Measures
The 6-Minute Walk Test (6MWT) assesses walking endurance and was standardized according to the American Thoracic Society guidelines for the 6MWT (49). In addition to distance walked, heart rate throughout the test and step count are measured. Heart rate is measured using a Polar H7 Bluetooth chest strap (Polar Electro, Kempele, Finland). The test is filmed using a mobile phone camera. Step count is measured by post-test video analysis by two researchers. Although stroke-related impairments can strongly influence performance in the 6MWT (49), it is still a reliable (ICC 0.71 -0.99) (50, 51) and valid (ρ = 0.99; p < 0.01) (51) measure for walking ability post-stroke.
The 10-meter Walk Test (10mWT) is performed by monitoring a participant walking 10m on a course of 14m, three times at self-selected (SSP) and three times at maximum (MP) pace (adapted from Cheng, Nelson (52)). Lines are marked at (A) the start (2m) and (B) the end (12m) of the measurement distance. The test execution is filmed in the sagittal plane using a mobile phone camera. The camera is held at a right angle to A until the participant crosses one foot over the line and then immediately held perpendicular to B until the participant again crosses a first foot. The time between these moments is calculated by post-test video analysis. The averages at SSP and at MP are used to calculate the respective walking speeds. The 10mWT is a reliable (53, 54) and valid (52) test to measure gait speed in people with stroke. Mentiplay, Adair (55) found that gait speed has poor correlations with knee extensor (r = 0.18 – 0.55) and ankle plantar flexor strength (r = 0.29 – 0.58). However, moderate correlations exist for the strength of the ankle dorsiflexors of the paretic limb (r = 0.50 – 0.73).
The Rivermead Mobility Index (RMI) is used to measure mobility disability (56). The questionnaire contains 14 closed questions that address fundamental motor skills of increasing difficulty and is administered by a researcher. The Dutch version of the RMI has excellent intra-test reliability (ρ = 0.97) and is strongly correlated with the Barthel Index (ρ = 0.84) (57).
A graded submaximal cycling test measures exercise capacity as registered by power output in Watts (W). The test is performed on a stationary recumbent bike (Ergoline Ergoselect 600, Ergoline, Germany). The start workload of 20W is increased with 15W every minute until 1) 75% of HRmax is reached, 2) a cadence between 55 - 70 rpm can no longer be maintained or 3) the participant wants to stop, whichever occurs first. Followed by five minutes of cooling down at 20W. Heart rate is measured via a Polar H7 Bluetooth chest strap. Blood pressure is measured every minute at the non-paretic side via an arm blood pressure monitor (Panasonic Diagnostec® EW-BU15, Panasonic, Japan).
Physical activity is measured via the Dutch version of the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD), a seven-day recall questionnaire for physical activity based on the Physical Activity Scale for the Elderly (58, 59). The Dutch PASIPD has a test-retest reliability of 0.77 and criterion validity of 0.30, which is in line with other physical activity questionnaires (59, 60).
Health related quality of life (HRQoL) is measured via two self-reported questionnaires. The EuroQol 5 Dimensions 5 Levels (EQ-5D-5L) comprises five dimensions that are rated on a five-level scale (61). The EQ-5D-5L is a recommended core measure to include in every stroke recovery trial (47).
However, as generic HRQoL scales might underestimate the impact of stroke (62, 63), the Stroke Specific Quality of Life Scale (SS-QoL) is additionally included. It consists of 49 items, divided into 12 domains which are scored on a five-point Likert scale (63). Psychometric properties for the instrument have been documented by Lin, Fu (64) who established minimal detectable changes (MDC) in individuals with chronic stroke for the mobility (MDC = 1.5 points), self-care (MDC = 1.2 points) and upper extremity (MDC = 1.2 points) domains.
Cognitive health is measured via the Montreal Cognitive Assessment (MoCA) (65) which has been shown to be the most valid and clinically feasible screening instrument for cognitive impairments post stroke. Scores can vary from 0 to 30, with 26 or higher generally accepted as normal. In people with stroke, a cut-off score of 23-24 has been established for any degree of cognitive impairment within any domain with a sensitivity of 59-92% and a specificity of 67-85% (66).
Perceived self-efficacy is measured via the Dutch General Self-Efficacy Scale (GSES) (15, 67).
The Exercise Self-Regulation Questionnaire (SRQ-E) (16) is used to measure individual differences in the types of motivation or regulation for exercise. Scores on the individual items of the questionnaire are combined to form a Relative Autonomy Index (RAI). Low scores on the RAI suggest a more controlled regulatory style, whereas higher scores are indicative for more autonomous regulation. Psychometric properties of the GSES and SRQ-E have been established in multiple populations, however not in stroke. (16)
Sample Size Calculation
The sample size was calculated using PS Power and Sample Size (68). Oxygen uptake (VO2) was chosen as an outcome variable, given several tests in the test battery are estimations for VO2. Based on data from Gjellesvik, Becker (25) an estimated mean (standard deviation) VO2 of 2.27 (0.45) L·min-1 at inclusion was selected as baseline value. According to the study, after 13 weeks of cardiorespiratory training in stroke patients an improvement of 16.25% can be expected. Based upon these assumptions an estimation of 14 participants was made to reach a power of 80% with a Type I error probability associated with the null hypothesis of 0.05. Taking a drop-out of 10% into account each group must contain at least 21 participants.
Statistical Methods
The statistical program SPSS, version 27, is used for descriptive and non-parametrical statistical analysis (69). The Student t-test is used for comparing the homogeneity between the ACG and control group. Patient characteristics are described. As far as feasibility is concerned, linear mixed-effects models are used for analysing the compliance with training and adherence to protocol within a group. A graphical representation of the data is made. Safety is measured by the number and nature of adverse events that occurred. A descriptive comparison of the differences between pre-post values of secondary outcome measures for the ACG and control group is made. Results are compared to the clinical important minimal difference (CIMD). When no stroke specific normative values were available, a 10% measurement error (ME) is used based on comparison. Differences between changes in the ACG (pre – post-intervention assessment) and control group are also described using the MCID or ME. To determine differences between both groups over the three timepoints for continuous outcomes linear mixed-effects models are used with factors time and group, time-by-group interaction and subject-specific intercept. Time effects and group specific effects of training are compared between groups by means of post hoc testing with Bonferroni-Holm correction. These tests are conducted for all outcome measures. Statistical significance is set at p < 0.05 (two-tailed).
References
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet (London, England). 2014;383(9913):245-54. [CrossRef] [PubMed]
- Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology. 2015;45(3):161-76. Epub 2015/10/28. [CrossRef] [PubMed]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197-223. Epub 2012/12/19. [CrossRef] [PubMed]
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052-90. Epub 2018/10/21. [CrossRef] [PubMed]
- Li S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front Neurol. 2017;8:120. Epub 2017/04/20. [CrossRef] [PubMed]
- Coslett HB. Apraxia, Neglect, and Agnosia. Continuum (Minneap Minn). 2018;24(3, behavioral neurology and psychiatry):768-82. Epub 2018/06/01. [CrossRef] [PubMed]
- Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83(12):1697-702. Epub 2002/12/11. [CrossRef] [PubMed]
- Boehme AK, Esenwa C, Elkind MS. Stroke Risk Factors, Genetics, and Prevention. Circ Res. 2017;120(3):472-95. Epub 2017/02/06. [CrossRef] [PubMed]
- Coull AJ, Lovett JK, Rothwell PM, Oxford Vascular S. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ (Clinical research ed). 2004;328(7435):326-. Epub 2004/01/26. [CrossRef] [PubMed]
- Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30(2):338-49. Epub 1999/02/05. [CrossRef] [PubMed]
- Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. Int J Stroke. 2012;7(6):499-510. Epub 2012/05/10. [CrossRef] [PubMed]
- Bailey RR. Promoting Physical Activity and Nutrition in People With Stroke. Am J Occup Ther. 2017;71(5):7105360010p1-p5. Epub 2017/08/16. [CrossRef] [PubMed]
- McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long-term follow-up of physical activity behavior in older adults. Health Psychol. 2007;26(3):375-80. Epub 2007/05/16. [CrossRef] [PubMed]
- Pekmezi D, Jennings E, Marcus BH. Evaluating and Enhancing Self-Efficacy for Physical Activity. ACSMs Health Fit J. 2009;13(2):16-21. Epub 2009/03/01. [CrossRef] [PubMed]
- Schwarzer R, Jerusalem M. Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Causal and Control Beliefs. 1995;1:35-7.
- Center for Self-Determination Theory. Self-Regulation Questionnaires Florida, USA [11 September 2020]. Available from: https://selfdeterminationtheory.org/self-regulation-questionnaires/.
- Gordon NF, Gulanick M, Costa F, Fletcher G, Franklin BA, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109(16):2031-41. Epub 2004/05/01. [CrossRef] [PubMed]
- MacKay-Lyons M, Billinger SA, Eng JJ, Dromerick A, Giacomantonio N, Hafer-Macko C, et al. Aerobic Exercise Recommendations to Optimize Best Practices in Care After Stroke: AEROBICS 2019 Update. Phys Ther. 2020;100(1):149-56. Epub 2019/10/10. [CrossRef] [PubMed]
- Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532-53. Epub 2014/05/23. [CrossRef] [PubMed]
- MacKay-Lyons, M. Aerobic exercise recommendations to optimize best practices in care after stroke (aerobics)2011. E614-E p.
- Luo L, Meng H, Wang Z, Zhu S, Yuan S, Wang Y, et al. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2020;63(1):59-68. Epub 2019/08/30. [CrossRef] [PubMed]
- Luo L, Zhu S, Shi L, Wang P, Li M, Yuan S. High Intensity Exercise for Walking Competency in Individuals with Stroke: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2019;28(12):104414. Epub 2019/10/02. [CrossRef] [PubMed]
- Wiener J, McIntyre A, Janssen S, Chow JT, Batey C, Teasell R. Effectiveness of High-Intensity Interval Training for Fitness and Mobility Post Stroke: A Systematic Review. Pm r. 2019;11(8):868-78. Epub 2019/03/13. [CrossRef] [PubMed]
- Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis. 2013;35(1):7-22. Epub 2013/02/23. [CrossRef] [PubMed]
- Gjellesvik TI, Becker F, Tjonna AE, Indredavik B, Nilsen H, Brurok B. High-intensity interval training after stroke (HIIT-Stroke study) – effects on peak oxygen uptake – a randomized controlled trial. European stroke journal. 2019;4:57. [CrossRef] [PubMed]
- Pallesen H, Bjerk M, Pedersen AR, Nielsen JF, Evaid L. The Effects of High-Intensity Aerobic Exercise on Cognitive Performance After Stroke: A Pilot Randomised Controlled Trial. Journal of Central Nervous System Disease. 2019;11. [CrossRef] [PubMed]
- Sandberg K, Kleist M, Falk L, Enthoven P. Effects of Twice-Weekly Intense Aerobic Exercise in Early Subacute Stroke: A Randomized Controlled Trial. Archives of Physical Medicine and Rehabilitation. 2016;97(8):1244-53. [CrossRef] [PubMed]
- Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. High-Intensity Interval Training in Stroke Rehabilitation. Topics in Stroke Rehabilitation. 2013;20(4):317-30. [CrossRef] [PubMed]
- Hornby TG, Moore JL, Lovell L, Roth EJ. Influence of skill and exercise training parameters on locomotor recovery during stroke rehabilitation. Current Opinion in Neurology. 2016;29(6):677-83. [CrossRef] [PubMed]
- Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. Within-Session Responses to High-Intensity Interval Training in Chronic Stroke. Medicine & Science in Sports & Exercise. 2015;47(3):476-84. [CrossRef] [PubMed]
- Boyne P, Dunning K, Carl D, Khoury JC, Gerson M, Rockwell B, et al. High Intensity Interval Training May Be Superior to Moderate Intensity Continuous Exercise in Chronic Stroke. Stroke. 2015;46. PubMed PMID: WOS:000349634701417.
- Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomized, controlled trial. Stroke. 2012;43(8):2168-74. Epub 2012/05/26. [CrossRef] [PubMed]
- Chen J, Jin W, Dong WS, Jin Y, Qiao FL, Zhou YF, et al. Effects of Home-based Telesupervising Rehabilitation on Physical Function for Stroke Survivors with Hemiplegia: A Randomized Controlled Trial. Am J Phys Med Rehabil. 2017;96(3):152-60. Epub 2016/07/09. [CrossRef] [PubMed]
- Gelaw AY, Janakiraman B, Gebremeskel BF, Ravichandran H. Effectiveness of Home-based rehabilitation in improving physical function of persons with Stroke and other physical disability: A systematic review of randomized controlled trials. J Stroke Cerebrovasc Dis. 2020;29(6):104800. Epub 2020/04/13. [CrossRef] [PubMed]
- Newitt R, Barnett F, Crowe M. Understanding factors that influence participation in physical activity among people with a neuromusculoskeletal condition: a review of qualitative studies. Disabil Rehabil. 2016;38(1):1-10. Epub 2015/01/15. [CrossRef] [PubMed]
- Nicholson S, Sniehotta FF, van Wijck F, Greig CA, Johnston M, McMurdo ME, et al. A systematic review of perceived barriers and motivators to physical activity after stroke. Int J Stroke. 2013;8(5):357-64. Epub 2012/09/15. [CrossRef] [PubMed]
- Gordon CD, Wilks R, McCaw-Binns A. Effect of aerobic exercise (walking) training on functional status and health-related quality of life in chronic stroke survivors: a randomized controlled trial. Stroke. 2013;44(4):1179-81. Epub 2013/03/09. [CrossRef] [PubMed]
- Mayo NE, MacKay-Lyons MJ, Scott SC, Moriello C, Brophy J. A randomized trial of two home-based exercise programmes to improve functional walking post-stroke. Clin Rehabil. 2013;27(7):659-71. Epub 2013/03/19. [CrossRef] [PubMed]
- Krawcyk RS, Vinther A, Petersen NC, Faber J, Iversen HK, Christensen T, et al. Effect of Home-Based High-Intensity Interval Training in Patients With Lacunar Stroke: A Randomized Controlled Trial. Frontiers in Neurology. 2019;10. [CrossRef] [PubMed]
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31-S4. Epub 2019/04/02. [CrossRef] [PubMed]
- Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113-30. Epub 1980/01/01. PubMed PMID: 6966542; PubMed Central PMCID: PMCPMC2395897.
- Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation. 1997;96(1):345-54. Epub 1997/07/01. [CrossRef] [PubMed]
- Fox SM, Haskell, W. L. The exercise stress test: Needs for standardization. Cardiology: Current Topics and Progress. 1970:149-54.
- Fox SM, 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3(6):404-32. Epub 1971/12/01. [PubMed]
- Godlasky E, Hoffman T, Weber-Peters S, Bradford R, Miller N, Kunselman AR, et al. Effects of β-Blockers on Maximal Heart Rate Prediction Equations in a Cardiac Population. J Cardiopulm Rehabil Prev. 2018;38(2):111-7. Epub 2018/02/22. [CrossRef] [PubMed]
- Vaes N, Lafosse C, Nys G, Schevernels H, Dereymaeker L, Oostra K, et al. Capturing peripersonal spatial neglect: an electronic method to quantify visuospatial processes. Behav Res Methods. 2015;47(1):27-44. Epub 2014/02/26. [CrossRef] [PubMed]
- Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2017;12(5):451-61. Epub 2017/07/13. [CrossRef] [PubMed]
- Bruno A, Shah N, Lin C, Close B, Hess DC, Davis K, et al. Improving modified Rankin Scale assessment with a simplified questionnaire. Stroke. 2010;41(5):1048-50. Epub 2010/03/13. [CrossRef] [PubMed]
- Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167(9):1287. Epub 2003/04/26. [CrossRef] [PubMed]
- Kosak M, Smith T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. 2005;42(1):103-7. Epub 2005/03/03. [CrossRef] [PubMed]
- Lam HS, Lau FW, Chan GK, Sykes K. The validity and reliability of a 6-Metre Timed Walk for the functional assessment of patients with stroke. Physiother Theory Pract. 2010;26(4):251-5. Epub 2010/04/20. [CrossRef] [PubMed]
- Cheng DK, Nelson M, Brooks D, Salbach NM. Validation of stroke-specific protocols for the 10-meter walk test and 6-minute walk test conducted using 15-meter and 30-meter walkways. Top Stroke Rehabil. 2020;27(4):251-61. Epub 2019/11/23. [CrossRef] [PubMed]
- Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. 1990;12(1):6-9. Epub 1990/01/01. [CrossRef] [PubMed]
- Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32(1):8-13. Epub 2008/05/09. [CrossRef] [PubMed]
- Mentiplay BF, Adair B, Bower KJ, Williams G, Tole G, Clark RA. Associations between lower limb strength and gait velocity following stroke: a systematic review. Brain Inj. 2015;29(4):409-22. Epub 2014/12/31. [CrossRef] [PubMed]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud. 1991;13(2):50-4. Epub 1991/04/01. [CrossRef] [PubMed]
- Roorda LD, Green J, De Kluis KR, Molenaar IW, Bagley P, Smith J, et al. Excellent cross-cultural validity, intra-test reliability and construct validity of the Dutch Rivermead Mobility Index in patients after stroke undergoing rehabilitation. J Rehabil Med. 2008;40(9):727-32. Epub 2008/10/10. [CrossRef] [PubMed]
- Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83(2):193-200. Epub 2002/02/08. [CrossRef] [PubMed]
- van der Ploeg HP, Streppel KR, van der Beek AJ, van der Woude LH, Vollenbroek-Hutten M, van Mechelen W. The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health. 2007;4(1):96-100. Epub 2007/05/10. [CrossRef] [PubMed]
- Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14. Epub 2000/08/05. [PubMed]
- EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. Epub 1990/11/05. [CrossRef] [PubMed]
- Owolabi MO. Which is more valid for stroke patients: generic or stroke-specific quality of life measures? Neuroepidemiology. 2010;34(1):8-12. Epub 2009/11/07. [CrossRef] [PubMed]
- Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30(7):1362-9. Epub 1999/07/02. [CrossRef] [PubMed]
- Lin KC, Fu T, Wu CY, Hsieh CJ. Assessing the stroke-specific quality of life for outcome measurement in stroke rehabilitation: minimal detectable change and clinically important difference. Health Qual Life Outcomes. 2011;9:5. Epub 2011/01/21. [CrossRef] [PubMed]
- Nasreddine Z. Montreal Cognitive Assessment23 August 2020. Available from: www.mocatest.org.
- Burton L, Tyson SF. Screening for cognitive impairment after stroke: A systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47(3):193-203. Epub 2015/01/16. [CrossRef] [PubMed]
- Teeuw B, Schwarzer R, Jerusalem M. Dutch Adaptation of the General Self-Efficacy Scale Berlin, Germany: Freie Universität Berlin; 1994 [updated 26 December 199712 April 2021]. Available from: http://userpage.fu-berlin.de/~health/dutch.htm.
- Dupont W, Plummer W. PS power and sample size program available for free on the Internet. Controlled Clin Trials. 18:274 ed1997.
- IBM C. IBM SPSS Statistics for Windows. 27.0 ed2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).