Submitted:
23 February 2023
Posted:
24 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Purification of CD19-positive B lymphocytes
2.2. Stimulation of B cells
2.3. Stimulation of cells using Immunocult kit
2.4. Flow cytometry
2.5. Mature B lymphocyte inhibition assay
3. Results
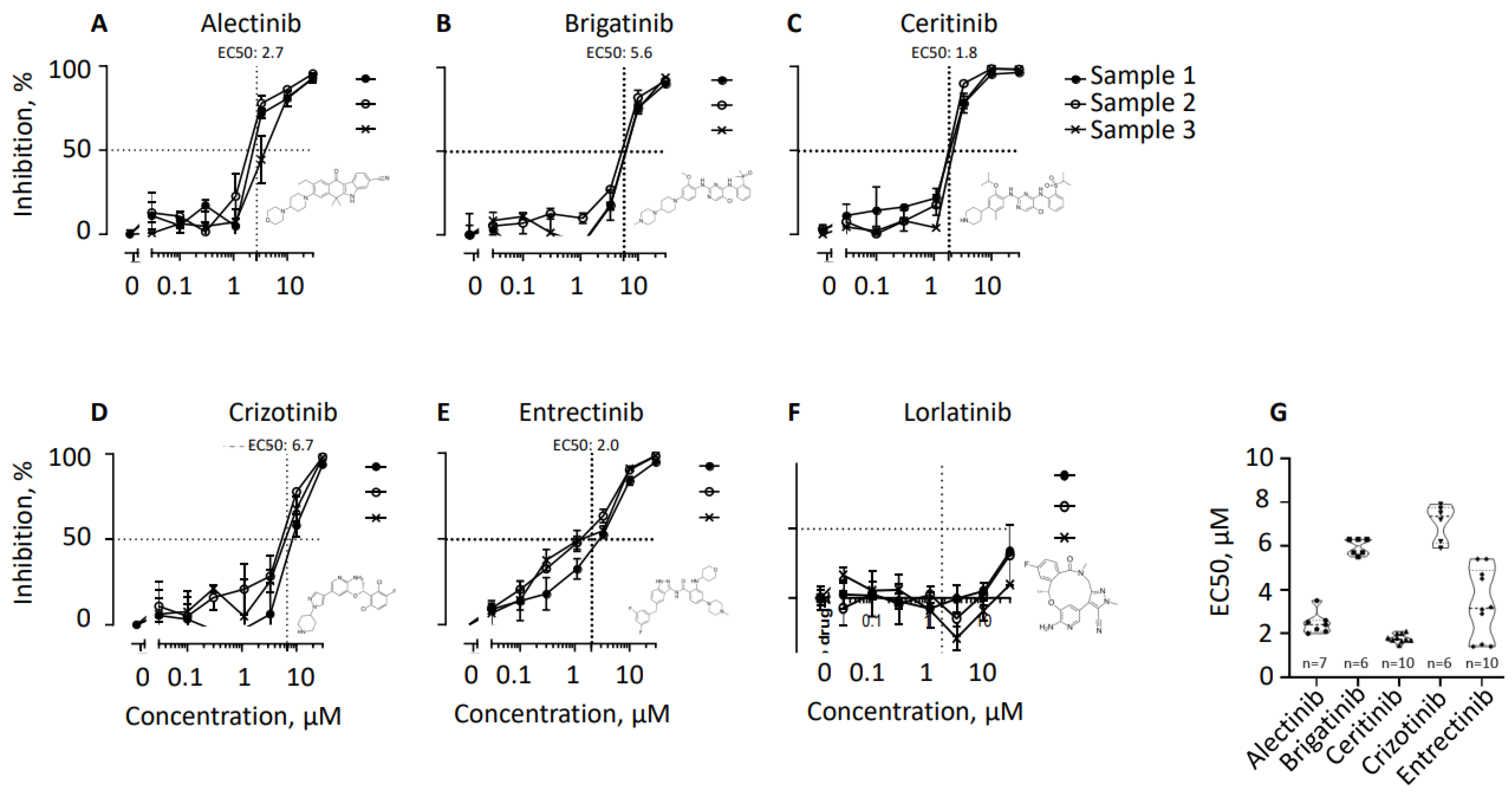
3.1. ALK inhibitors prevent accumulation of thymidine in CD19-positive B cell populations
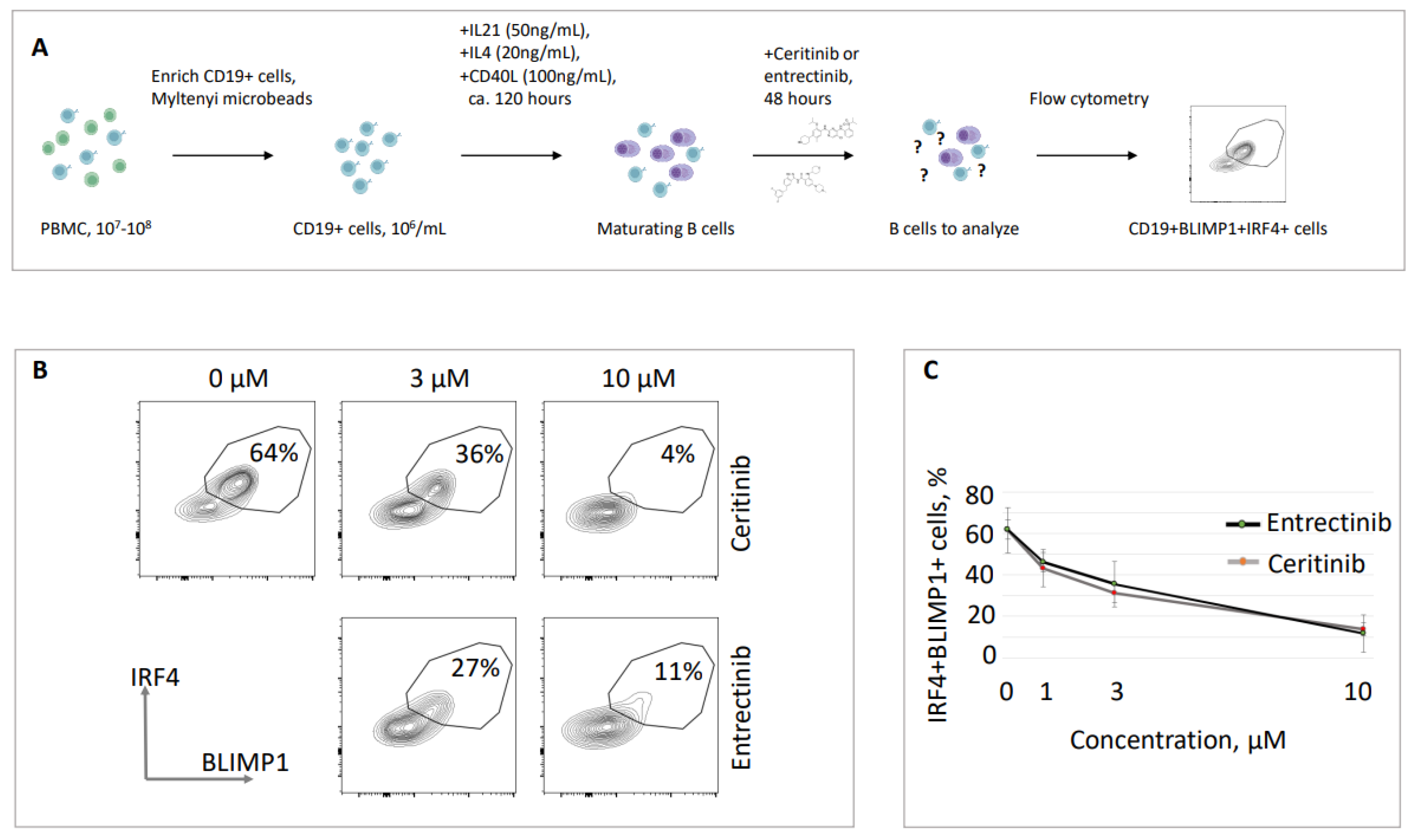
3.2. Ceritinib and entrectinib eliminate BLIMP1/IRF4 double-positive B cell populations
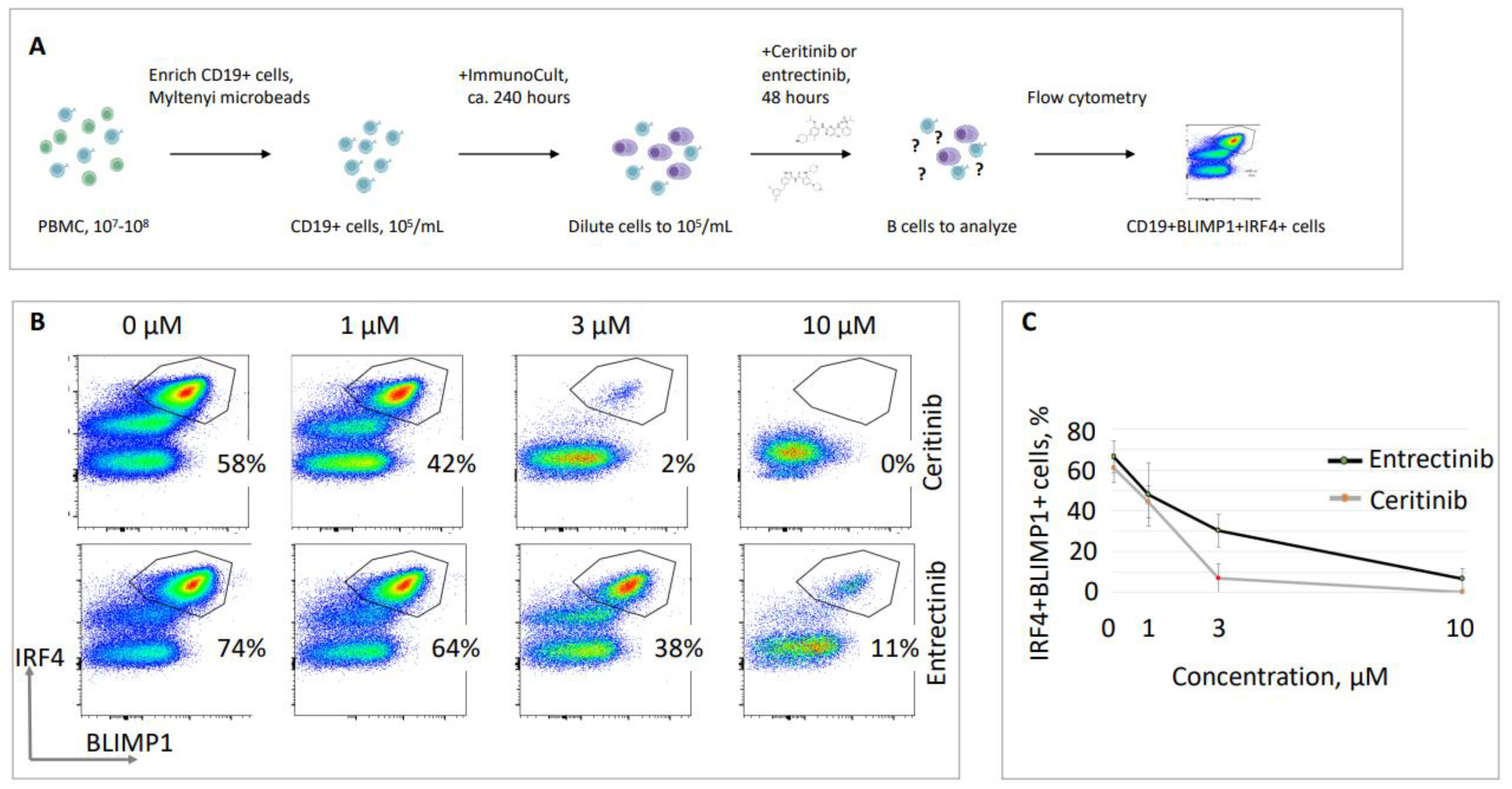
3.3. Validation of ceritinib and entrectinib inhibitory effects on plasma cells
4. Discussion
5. Conclusions
Supplementary Materials
Institutional Review Board Statement
Conflicts of Interest
References
- Alt, F.W.; Zhang, Y.; Meng, F.L.; Guo, C.; Schwer, B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell 2013, 152, 417-429. [CrossRef]
- Rajewsky, K. Clonal selection and learning in the antibody system. Nature 1996, 381, 751-758. [CrossRef]
- Terrell, D.R.; Neunert, C.E.; Cooper, N.; Heitink-Polle, K.M.; Kruse, C.; Imbach, P.; Kuhne, T.; Ghanima, W. Immune Thrombocytopenia (ITP): Current Limitations in Patient Management. Medicina (Kaunas) 2020, 56. [CrossRef]
- Kumar, V.; Alt, F.W.; Oksenych, V. Functional overlaps between XLF and the ATM-dependent DNA double strand break response. DNA Repair (Amst) 2014, 16, 11-22. [CrossRef]
- Castaneda-Zegarra, S.; Fernandez-Berrocal, M.; Tkachov, M.; Yao, R.; Upfold, N.L.E.; Oksenych, V. Genetic interaction between the non-homologous end-joining factors during B and T lymphocyte development: In vivo mouse models. Scand J Immunol 2020, 92, e12936. [CrossRef]
- Wang, X.S.; Lee, B.J.; Zha, S. The recent advances in non-homologous end-joining through the lens of lymphocyte development. DNA Repair (Amst) 2020, 94, 102874. [CrossRef]
- Centonze, F.G.; Reiterer, V.; Nalbach, K.; Saito, K.; Pawlowski, K.; Behrends, C.; Farhan, H. LTK is an ER-resident receptor tyrosine kinase that regulates secretion. J Cell Biol 2019, 218, 2470-2480. [CrossRef]
- Roll, J.D.; Reuther, G.W. ALK-activating homologous mutations in LTK induce cellular transformation. PLoS One 2012, 7, e31733. [CrossRef]
- Farhan, H.O., NO), Munthe, Ludvig (Fjellhamar, NO), Tasken, Kjetil (Rykkinn, NO), Skanland, Sigrid (Oslo, NO), Giliberto, Mariaserena (Oslo, NO), Hellem Schjesvold, Fredrik (Jar, NO), Driessen, Christoph (St. Gallen, CH), Besse, Lenka (St. Gallen, CH), Besse, Andrej (St. Gallen, CH). ALK INHIBITORS FOR TREATMENT OF ALK-NEGATIVE CANCER AND PLASMA CELL-MEDIATED DISEASES. 2022.
- Tao, J.; Zheng, C.; Zhang, C.; Zhou, L.; Liu, Z.; Zhou, Y.; Huang, X.; Lin, L.; Zhai, L. First-line treatments for patients with advanced ALK gene rearrangements in NSCLC: a systematic review and network meta-analysis. J Int Med Res 2022, 50, 3000605221132703. [CrossRef]
- Ianevski, A.; Simonsen, R.M.; Myhre, V.; Tenson, T.; Oksenych, V.; Bjoras, M.; Kainov, D.E. DrugVirus.info 2.0: an integrative data portal for broad-spectrum antivirals (BSA) and BSA-containing drug combinations (BCCs). Nucleic Acids Res 2022. [CrossRef]
- Ianevski, A.; Yao, R.; Simonsen, R.M.; Myhre, V.; Ravlo, E.; Kaynova, G.D.; Zusinaite, E.; White, J.M.; Polyak, S.J.; Oksenych, V.; et al. Mono- and combinational drug therapies for global viral pandemic preparedness. iScience 2022, 25, 104112. [CrossRef]
- Ianevski, A.; Zusinaite, E.; Tenson, T.; Oksenych, V.; Wang, W.; Afset, J.E.; Bjoras, M.; Kainov, D.E. Novel Synergistic Anti-Enteroviral Drug Combinations. Viruses 2022, 14. [CrossRef]
- Ianevski, A.; Yao, R.; Lysvand, H.; Grodeland, G.; Legrand, N.; Oksenych, V.; Zusinaite, E.; Tenson, T.; Bjoras, M.; Kainov, D.E. Nafamostat-Interferon-alpha Combination Suppresses SARS-CoV-2 Infection In Vitro and In Vivo by Cooperatively Targeting Host TMPRSS2. Viruses 2021, 13. [CrossRef]
- Ianevski, A.; Yao, R.; Zusinaite, E.; Lello, L.S.; Wang, S.; Jo, E.; Yang, J.; Ravlo, E.; Wang, W.; Lysvand, H.; et al. Synergistic Interferon-Alpha-Based Combinations for Treatment of SARS-CoV-2 and Other Viral Infections. Viruses 2021, 13. [CrossRef]
- Ianevski, A.; Yao, R.; Zusinaite, E.; Lysvand, H.; Oksenych, V.; Tenson, T.; Bjoras, M.; Kainov, D. Active Components of Commonly Prescribed Medicines Affect Influenza A Virus-Host Cell Interaction: A Pilot Study. Viruses 2021, 13. [CrossRef]
- Oksenych, V.; Kainov, D.E. Broad-Spectrum Antivirals and Antiviral Drug Combinations. Viruses 2022, 14. [CrossRef]
- Kainov, D.; Oksenych, V. Broad-Spectrum Antivirals and Antiviral Combinations: An Editorial Update. Viruses 2022, 14. [CrossRef]
- Ianevski, A.; Kulesskiy, E.; Krpina, K.; Lou, G.; Aman, Y.; Bugai, A.; Aasumets, K.; Akimov, Y.; Bulanova, D.; Gildemann, K.; et al. Chemical, Physical and Biological Triggers of Evolutionary Conserved Bcl-xL-Mediated Apoptosis. Cancers (Basel) 2020, 12. [CrossRef]
- Ianevski, A.; Yao, R.; Biza, S.; Zusinaite, E.; Mannik, A.; Kivi, G.; Planken, A.; Kurg, K.; Tombak, E.M.; Ustav, M., Jr.; et al. Identification and Tracking of Antiviral Drug Combinations. Viruses 2020, 12. [CrossRef]
- Ianevski, A.; Yao, R.; Fenstad, M.H.; Biza, S.; Zusinaite, E.; Reisberg, T.; Lysvand, H.; Loseth, K.; Landsem, V.M.; Malmring, J.F.; et al. Potential Antiviral Options against SARS-CoV-2 Infection. Viruses 2020, 12. [CrossRef]
- Ianevski, A.; Ahmad, S.; Anunnitipat, K.; Oksenych, V.; Zusinaite, E.; Tenson, T.; Bjoras, M.; Kainov, D.E. Seven classes of antiviral agents. Cell Mol Life Sci 2022, 79, 605. [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Johnson, T.W.; Lin, J.J. LTK fusions: A new target emerges in non-small cell lung cancer. Cancer Cell 2022, 40, 23-25. [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535-554. [CrossRef]
- Izumi, H.; Matsumoto, S.; Liu, J.; Tanaka, K.; Mori, S.; Hayashi, K.; Kumagai, S.; Shibata, Y.; Hayashida, T.; Watanabe, K.; et al. The CLIP1-LTK fusion is an oncogenic driver in non-small-cell lung cancer. Nature 2021, 600, 319-323. [CrossRef]
- Szodoray, P.; Andersen, T.K.; Heinzelbecker, J.; Imbery, J.F.; Huszthy, P.C.; Stanford, S.M.; Bogen, B.; Landsverk, O.B.; Bottini, N.; Tveita, A.; et al. Integration of T helper and BCR signals governs enhanced plasma cell differentiation of memory B cells by regulation of CD45 phosphatase activity. Cell Rep 2021, 36, 109525. [CrossRef]
- Dewan, A.; Xing, M.; Lundbaek, M.B.; Gago-Fuentes, R.; Beck, C.; Aas, P.A.; Liabakk, N.B.; Saeterstad, S.; Chau, K.T.P.; Kavli, B.M.; et al. Robust DNA repair in PAXX-deficient mammalian cells. FEBS Open Bio 2018, 8, 442-448. [CrossRef]
- Gago-Fuentes, R.; Xing, M.; Saeterstad, S.; Sarno, A.; Dewan, A.; Beck, C.; Bradamante, S.; Bjoras, M.; Oksenych, V. Normal development of mice lacking PAXX, the paralogue of XRCC4 and XLF. FEBS Open Bio 2018, 8, 426-434. [CrossRef]
- Beck, C.; Castaneda-Zegarra, S.; Huse, C.; Xing, M.; Oksenych, V. Mediator of DNA Damage Checkpoint Protein 1 Facilitates V(D)J Recombination in Cells Lacking DNA Repair Factor XLF. Biomolecules 2019, 10. [CrossRef]
- Castaneda-Zegarra, S.; Huse, C.; Rosand, O.; Sarno, A.; Xing, M.; Gago-Fuentes, R.; Zhang, Q.; Alirezaylavasani, A.; Werner, J.; Ji, P.; et al. Generation of a Mouse Model Lacking the Non-Homologous End-Joining Factor Mri/Cyren. Biomolecules 2019, 9. [CrossRef]
- Castaneda-Zegarra, S.; Xing, M.; Gago-Fuentes, R.; Saeterstad, S.; Oksenych, V. Synthetic lethality between DNA repair factors Xlf and Paxx is rescued by inactivation of Trp53. DNA Repair (Amst) 2019, 73, 164-169. [CrossRef]
- Fauskanger, M.; Haabeth, O.A.W.; Skjeldal, F.M.; Bogen, B.; Tveita, A.A. Tumor Killing by CD4(+) T Cells Is Mediated via Induction of Inducible Nitric Oxide Synthase-Dependent Macrophage Cytotoxicity. Front Immunol 2018, 9, 1684. [CrossRef]
- Khoenkhoen, S.; Adori, M.; Pedersen, G.K.; Karlsson Hedestam, G.B. Flow Cytometry-Based Protocols for the Analysis of Human Plasma Cell Differentiation. Front Immunol 2020, 11, 571321. [CrossRef]
- Hu, V.W.; Black, G.E.; Torres-Duarte, A.; Abramson, F.P. 3H-thymidine is a defective tool with which to measure rates of DNA synthesis. FASEB J 2002, 16, 1456-1457. [CrossRef]
- Jacobsen, J.; Haabeth, O.A.; Tveita, A.A.; Schjetne, K.W.; Munthe, L.A.; Bogen, B. Naive idiotope-specific B and T cells collaborate efficiently in the absence of dendritic cells. J Immunol 2014, 192, 4174-4183. [CrossRef]
- Tveita, A.A.; Schjesvold, F.; Haabeth, O.A.; Fauskanger, M.; Bogen, B. Tumors Escape CD4+ T-cell-Mediated Immunosurveillance by Impairing the Ability of Infiltrating Macrophages to Indirectly Present Tumor Antigens. Cancer Res 2015, 75, 3268-3278. [CrossRef]
- Tveita, A.A.; Schjesvold, F.H.; Sundnes, O.; Haabeth, O.A.; Haraldsen, G.; Bogen, B. Indirect CD4+ T-cell-mediated elimination of MHC II(NEG) tumor cells is spatially restricted and fails to prevent escape of antigen-negative cells. Eur J Immunol 2014, 44, 2625-2637. [CrossRef]
- De Munck, S.; Provost, M.; Kurikawa, M.; Omori, I.; Mukohyama, J.; Felix, J.; Bloch, Y.; Abdel-Wahab, O.; Bazan, J.F.; Yoshimi, A.; et al. Structural basis of cytokine-mediated activation of ALK family receptors. Nature 2021, 600, 143-147. [CrossRef]
- Andersen, P.I.; Ianevski, A.; Lysvand, H.; Vitkauskiene, A.; Oksenych, V.; Bjoras, M.; Telling, K.; Lutsar, I.; Dumpis, U.; Irie, Y.; et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int J Infect Dis 2020, 93, 268-276. [CrossRef]
- Ianevski, A.; Zusinaite, E.; Shtaida, N.; Kallio-Kokko, H.; Valkonen, M.; Kantele, A.; Telling, K.; Lutsar, I.; Letjuka, P.; Metelitsa, N.; et al. Low Temperature and Low UV Indexes Correlated with Peaks of Influenza Virus Activity in Northern Europe during 2010(-)2018. Viruses 2019, 11. [CrossRef]
- Bosl, K.; Ianevski, A.; Than, T.T.; Andersen, P.I.; Kuivanen, S.; Teppor, M.; Zusinaite, E.; Dumpis, U.; Vitkauskiene, A.; Cox, R.J.; et al. Common Nodes of Virus-Host Interaction Revealed Through an Integrated Network Analysis. Front Immunol 2019, 10, 2186. [CrossRef]
- Ianevski, A.; Zusinaite, E.; Kuivanen, S.; Strand, M.; Lysvand, H.; Teppor, M.; Kakkola, L.; Paavilainen, H.; Laajala, M.; Kallio-Kokko, H.; et al. Novel activities of safe-in-human broad-spectrum antiviral agents. Antiviral Res 2018, 154, 174-182. [CrossRef]
- Bulanova, D.; Ianevski, A.; Bugai, A.; Akimov, Y.; Kuivanen, S.; Paavilainen, H.; Kakkola, L.; Nandania, J.; Turunen, L.; Ohman, T.; et al. Antiviral Properties of Chemical Inhibitors of Cellular Anti-Apoptotic Bcl-2 Proteins. Viruses 2017, 9. [CrossRef]
- Helleday, T. PARP inhibitor receives FDA breakthrough therapy designation in castration resistant prostate cancer: beyond germline BRCA mutations. Ann Oncol 2016, 27, 755-757. [CrossRef]
- Zha, S.; Guo, C.; Boboila, C.; Oksenych, V.; Cheng, H.L.; Zhang, Y.; Wesemann, D.R.; Yuen, G.; Patel, H.; Goff, P.H.; et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nature 2011, 469, 250-254. [CrossRef]
- Oksenych, V.; Alt, F.W.; Kumar, V.; Schwer, B.; Wesemann, D.R.; Hansen, E.; Patel, H.; Su, A.; Guo, C. Functional redundancy between repair factor XLF and damage response mediator 53BP1 in V(D)J recombination and DNA repair. Proc Natl Acad Sci U S A 2012, 109, 2455-2460. [CrossRef]
- Castaneda-Zegarra, S.; Zhang, Q.; Alirezaylavasani, A.; Fernandez-Berrocal, M.; Yao, R.; Oksenych, V. Leaky severe combined immunodeficiency in mice lacking non-homologous end joining factors XLF and MRI. Aging (Albany NY) 2020, 12, 23578-23597. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



