1. Introduction
Intramuscular fat has been used for quality assurance systems within the US and Australia to improve the consistency of tenderness and improved eating experience [
1]. Iida et al. [
2] reported that sensory scores for juiciness and tenderness increased as the fat level increased in steaks from Japanese Black steers. Other researchers have reported that increasing levels of marbling results in a higher likelihood of a tender product [
3,
4]. In general, as subcutaneous fat increases, marbling also increases, and producers have used visual observations of fat and live body weight to determine harvest times. As an animal matures, it exhibits significant shifts in physiological responses. For example, as muscle growth approaches its peak, energy utilization for muscle growth is reduced, resulting in increased energy storage [
3,
5]. These changes encourage the deposition of fat, including marbling. However, animal-to-animal variation results in differing levels of marbling and subcutaneous fat. Thus, there is a need to identify what changes occur at the molecular level that initiates fat deposition to help predict final fat deposition.
While advances have been made in genetic selection and objective measures of fat, there are still animals not reaching desired body composition endpoints after similar days on feed. Engle et al. [
6] reported a significant number of differentially expressed genes in longissimus lumborum muscle from Standard and Choice carcasses (1,258 genes, p < 0.01). Functional analysis of these genes revealed differences in the underlying pathways regulating muscle cell growth and proliferation. Biological processes of upregulated genes were associated with signaling pathways associated with inflammation, growth, and metabolism. Furthermore, the upregulation of processes associated with the extracellular matrix, stem cell differentiation, and focal adhesion was observed. Further investigation into the changes occurring during fat deposition to better predict and select animals that consistently achieve the desired level of marbling and fat in a predictable fashion is important. This would allow for more efficient feeding and management of finishing cattle. We hypothesize that gene expression in adipose tissue changes during fattening and that coordinated changes in expression between muscle and adipose tissues can be detected. This work aimed to provide new insight into the differences in muscle and fat tissues when carcasses have reached marbling associated with different USDA quality grades.
2. Materials and Methods
Data collection protocols were approved by the Montana State University Agriculture Animal Care and Use Committee (Protocol No. 2015-AA17).
Fifteen steers were selected at weaning based on weight and date of birth. Steers were moved to the Montana State University Bozeman Area Research and Teaching Farm and placed in a single pen in the feedlot. At the start of the study, steers weighed an average of 313 ± 14 kg. Each steer received a Synovex One Feedlot implant per standard feedlot protocol. They were fed an ad libitum diet of hay for two weeks to acclimate steers to pens and were then started on a 6-week, step-up program to build up to the full ration (
Table 1), which was a diet of 75% shelled corn, 18% hay, and 7% finisher pellet, fed in a bunk daily and had free access to water. Steers were randomly allocated to one of three endpoints based on body weight and visual evaluation, with average endpoint weights of 431 kg, 522 kg, and 612 kg targeting marbling levels for Standard, Select, and Choice quality grades, respectively. These weight-based endpoints achieved the desired marbling endpoints (
Table 2). Animals were transported 60 miles to a small commercial processor and harvested following normal industry standards. Carcass data was collected 24 hours postmortem to calculate yield grade and determine quality grade following USDA guidelines [
7]. Carcasses were ribbed between the 12th and 13th rib, exposing the longissimus dorsi. Marbling determination was done a minimum of 20 minutes after ribbing to give time for the muscle to oxygenate. Carcass characteristics were measured by an individual with 30 years of experience in carcass data collection. Carcass measurements were collected as outlined by Engle et al. [
6].
Striploins were removed at 24 h postmortem and transported to the Montana State University Meat Laboratory. The striploins were cut into 2.54 cm steaks starting at the anterior end. Steaks were individually vacuum packaged. Steaks were randomly assigned to different days of postmortem aging (24 h, 3, 7, 14, or 21 d postmortem). One steak was cut into five equal pieces for myofibrillar fragmentation index (MFI). These pieces were vacuum packaged and aged as described for steaks. After aging, vacuum-packaged meat was placed in a -20 °C freezer until analysis. Steaks were taken from a -20 °C freezer and placed in a 2 °C cooler approximately 24 hours prior to cooking. Steaks were blotted, tagged, weighed, and a single copper constantan thermocouple (OMEGA Engineering, INC, P.O. Box 4047, Stamford, Connecticut) was placed in the center to track endpoint temperature. They were then placed on an aluminum-covered broiler pan and placed under the broiler of a conventional oven 10.16 cm below the heating element. Steaks were then cooked on broil until reaching an internal temperature of 35 °C. At that point, the steaks were turned and cooked on the other side until reaching an internal temperature of 70 °C [
8]. Steaks were cooled in a 2 °C cooler. After cooling for a minimum of 45 minutes, steaks were taken from the cooler, blotted with towels, and weighed. A minimum of five samples were taken parallel to the muscle fiber, resulting in square samples of 1.27 x 1.27 cm. The samples were then sheared using a TMS 30 Food Texturometer fitted with a Warner-Bratzler shear attachment. The average of the samples sheared was used for statistical analysis.
The MFI was determined following the procedures reported by Culler et. al. [
9], as modified by Hopkins et. al. [
10]. Two samples per steer, each duplicated, yielded four measurements per steer. The MFI average for each sample was calculated, and the average was used for statistical analyses. Carcass data was analyzed using the Proc GLM procedure in SAS (v10.2) with quality grade class as an independent variable. MFI and shear force were analyzed using the Proc GLM procedure of SAS with quality grade and day of aging as independent variables, and the interaction was tested but was not significant, so it was dropped from the analysis. The LSMEANS procedure was used to calculate the means and determine significance. Significance was set at a threshold of p < 0.05.
Subcutaneous and intermuscular adipose tissue samples were taken between the 4th and 5th rib at time of harvest and homogenized immediately in Triazol and then transported on dry ice for subsequent RNA extraction. Longissimus thoracis muscle samples from the same area were snap frozen. Frozen muscle samples and homogenized adipose tissue sample RNA was extracted using a Qiagen RNAeasy Plus Universal Midi kit according to manufacturer recommendations (Qiagen LLC, Georgetown MD, USA).
A total of 3 μg of RNA per sample was used to generate sequencing libraries using NEBNext Ultra RNA Library Prep Kit
® from Illumina (NE, USA) following manufacturer instructions and index codes were added to each sample. RNA was enriched for mRNA and cDNA libraries were created using AMPure XP system (Beckman Coulter, Beverly, USA). Libraries were sequenced on an Illumina Hiseq platform, and 125 bp/150 bp paired-end reads were generated. Read quality metrics are shown in
Table 3.
Reads were aligned to reference genome UMD 3.1. The FPKM, the expected number of fragments per kilobase of transcript sequence per millions of base pairs sequenced of each gene, was then calculated based on the length of the gene and the reads count mapped to said gene.
Prior to differential gene expression analysis, for each sequenced library, the read counts were adjusted by edgeR program package through one scaling normalized factor. Differential expression analysis of two conditions was performed using the DEGSeq R package (1.20.0). The p-values were adjusted using the Benjamini-Hochberg method. A corrected p-value of 0.005 and fold change of 1 were set as the threshold for significantly differential expression.
Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by the GOseq R package, in which gene length bias was corrected. GO terms with FDR corrected P-value < 0.05 were considered significantly enriched by differentially expressed genes. KEGG, a database resource for understanding high-level functions and utilities of the biological system, was used, along with KOBAS software, to test the statistical enrichment of differentially expressed genes in KEGG pathways.
3. Results
As expected, carcass weights from animals with Choice quality grade were greater (P = 0.002) than carcass weights from carcasses with less marbling that would result in Select or Standard quality grades (
Table 2). This is not unexpected as the steers resulting in Choice carcasses were on feed longer than the steers of the other quality classifications (150 days versus 73 and 94). Furthermore, the fat thickness was significantly greater (P = 0.007) for carcasses grading Choice and Select than carcasses grading Standard. Other researchers have reported increased fat thickness as the carcass grade and carcass weight increase [
11]. Conversely, the ribeye area was not different between the carcasses of different grades. This is in contrast to other reported results where the ribeye area was observed to be larger when carcass weights were higher [
12,
13,
14]. Fat thickness was greater (P = 0.007) for Choice and Select carcasses than for Standard carcasses (
Table 2). This is expected, as increasing marbling is needed for higher-quality grades and is usually associated with higher fat content in the whole carcass [
3,
6].
Shear force values for steaks from Choice and Select carcasses were significantly lower than values for steaks from Standard carcasses (
Table 2). Mixed results have been reported for differences in tenderness associated with different marbling degrees. Vierck and co-workers [
4] reported differences in tenderness between steaks from Choice and Select carcasses, but no differences were observed between the different levels of Choice. However, other researchers have reported that marbling had little effect on tenderness [
15,
16,
17]. Additional studies evaluating very high levels of intramuscular fat have consistently shown improved tenderness as the marbling increased [
2,
5]. Nishimura and colleagues [
5] suggested changes in connective tissue structure (electron microscopy) as the fat level in the longissimus contributed to the increase in tenderness. This suggests that comparisons at higher levels of marbling may confound the information on how marbling affects tenderness.
Myofibrillar fragmentation index (MFI) was significantly lower for steaks from Choice and Standard carcasses when compared to steaks from Select carcasses (
Table 2). Higher MFI values are associated with a greater breakdown of fibers and have been related to improved tenderness. Kim and Lee [
18], however saw no effect of marbling on MFI when comparing steaks from Hanwoo cattle of varying grades. The differences in reported MFI values do not match the differences in shear force values. Researchers have reported that higher MFI values are correlated to lower Warner-Bratzler shear values. [
18,
19,
20]. The aging of steaks resulted in a decreased shear force for up to 14 days. The greatest change, however, was seen in the first 3 days of aging. Olson et al. [
21] also reported the greatest change in shear force in the first 3 days of aging. Ilian and co-workers [
20] reported similar results. However, Bratcher and co-workers [
22] found improvement in shear force values up to 14 days of aging. MFI values increased up to 7 days of aging but were only significantly different between 1 day of aging and the rest of the aging times. Ilian et al. [
20] reported significant increases occurred in MFI gradually during days 2 and 3 postmortem, but non-significant changes occurred after 7 days. These researchers also found strong correlations between the kinetics of tenderization and MFI. This data suggests that the sarcomeric structure’s dissolution rate is the fastest early postmortem and slows as the time postmortem increases.
Table 3.
Effect of quality classification on the shear force and myofibrillar fragmentation index (MFI) of beef strip steaks.
Table 3.
Effect of quality classification on the shear force and myofibrillar fragmentation index (MFI) of beef strip steaks.
| Shear force (N) 3
|
MFI |
| 84.34 b
|
59.25 b
|
| 79.20 b
|
64.55 a
|
| 105.70 a
|
55.24 b
|
| |
|
| 118.42 a
|
49.78 b
|
| 95.31 b
|
59.51 a
|
| 86.70 bc
|
62.02 a
|
| 73.55 c
|
64.18 a
|
| 74.76 c
|
62.94 a
|
The RNAseq experiment yielded the expected number of transcripts with, on average, over 50 million mapped reads and high-quality data, as shown in
Table 4.
In four cases, the intermuscular fat RNA was of insufficient quality or quantity for sequencing. We replaced these samples with subcutaneous adipose tissue.
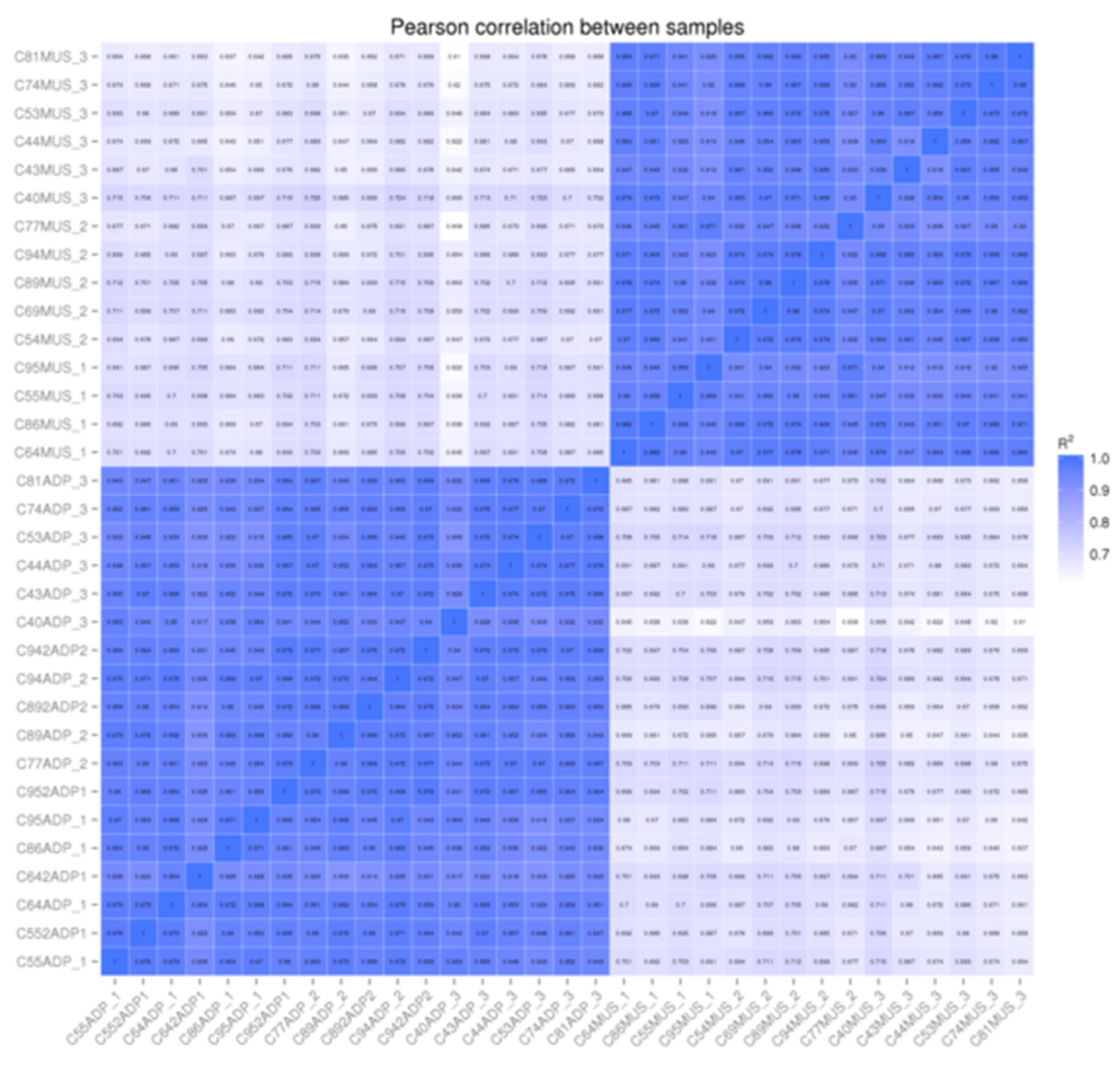
Figure 1 shows the Pearson correlations between each individual sample and the other samples included in the dataset. There are high correlations (>85% on average) between muscle samples, with one sample that was potentially contaminated by connective or vascular tissue, and a high correlation (>90%) between adipose samples, including those subcutaneous samples that were used to replace intermuscular samples. This shows that at these carcass endpoints, there are no detectable differences in gene expression between intermuscular and subcutaneous adipose tissues.
In the comparison between adipose tissue from Standard and Select carcasses, four genes were upregulated, and 29 were downregulated (
Table 5). Notably, two genes associated with WNT signaling were downregulated in adipose tissue from Select carcasses. WNT signaling suppresses adipogenesis by blocking the activation of PPARγ and CEPBα, which are the major regulators of adipogenesis. One upregulated transcript is BOLA-DMB which codes for MHC type 2 and indicates the presence of immune cells such as macrophages [
23]. In comparing adipose tissue from Select to Choice carcasses, eight genes were downregulated, and 15 genes were upregulated (
Table 5). Leptin and ACC1 (Acetyl CoA Carboxylase 1) are two examples of downregulated genes, and this could lead to insulin resistance and potentially inhibit lipogenesis [
24,
25]. There were 49 downregulated genes and 113 upregulated genes in the comparison between adipose tissue from Standard and Choice carcasses (
Table 5). Downregulated genes included: CAB39L, FGF-1, GRIN1, LEP, HK2, YWHAG, ACC1, SCD1, and ELOVL3. These genes are mostly related to fat and energy metabolism and promote adipogenesis and fat deposition (
Table 7,
Table 8 and
Table 9) [
3]. For another example, the downregulation of ACC1, HK2, and the upregulation of EIF43BP1 would inhibit protein synthesis and increase lipogenesis. Furthermore, the down-regulation of leptin can cause insulin resistance [
24,26], and the upregulation of gluconeogenesis, which alters fat metabolism. Additionally, a number of immune markers were upregulated in the adipose tissue from Choice animals compared to Standard, which may be due to inflammation and immune activation in the adipose tissue.
In the comparison between muscle from Standard and Choice carcasses, 15 genes were downregulated, and 20 were upregulated (
Table 6 and
Table 10). The insulin receptor substrate 1 (IRS 1) gene was the only known functionally important gene to be differentially expressed. No transcripts associated with muscular hypertrophy were differentially expressed.
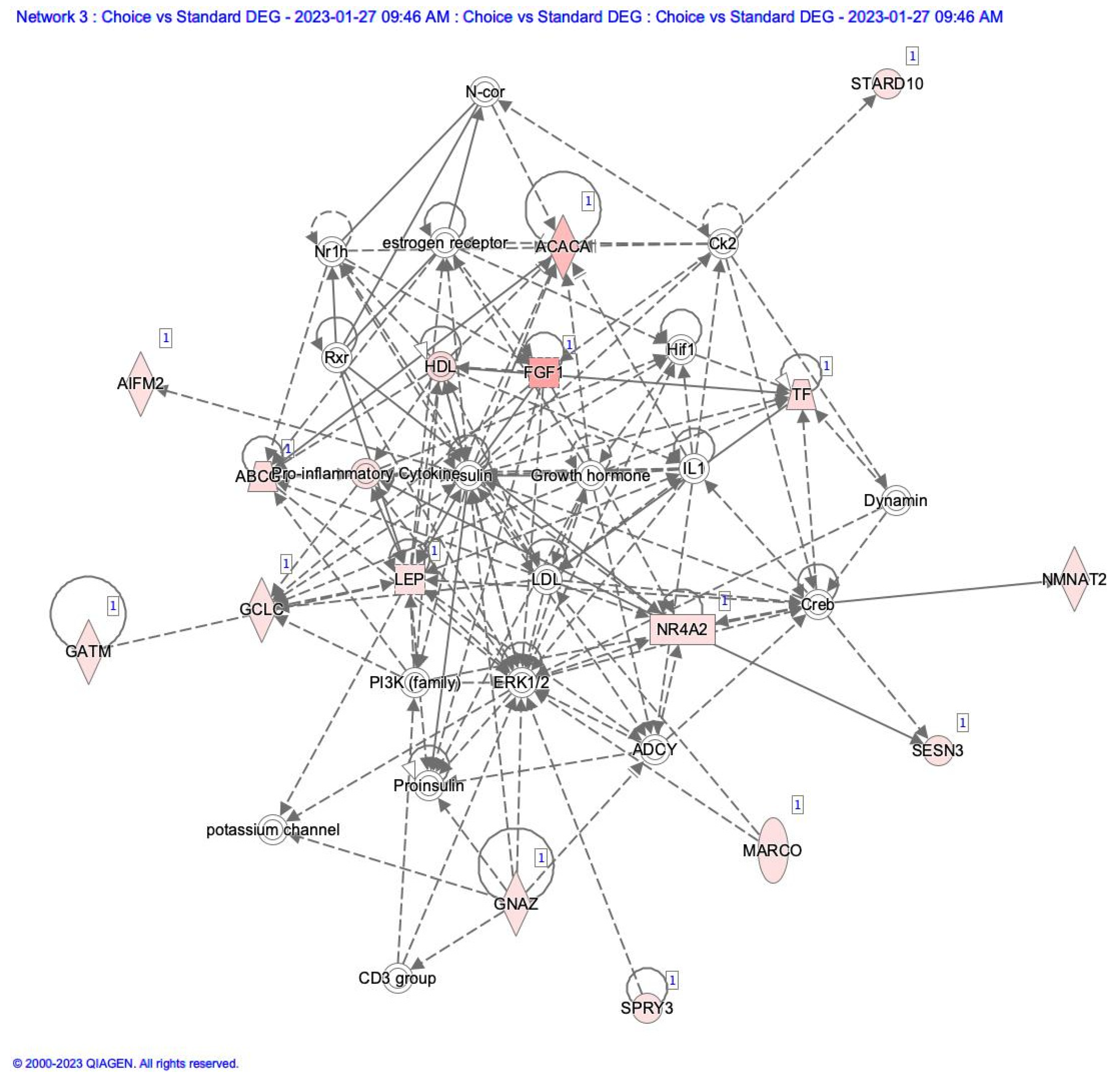
Differentially expressed gene lists were uploaded to Ingenuity Pathway Analysis software (Qiagen Inc.), and network analysis was conducted. In comparing Choice to Standard adipose tissue, a network annotated as Endocrine system disorders, Metabolic Disorders, and Organismal injury was highlighted. This network is centered on insulin and growth hormone, which are not expressed in adipose tissue (
Figure 2). Still, most of the molecules associated with these are upregulated in our dataset. This also indicates that inflammatory processes are being activated in the adipose tissue as fat deposition increases.
Table 5.
Select differentially expressed genes in adipose tissue from beef steers with different USDA Carcass Quality Grades.
Table 5.
Select differentially expressed genes in adipose tissue from beef steers with different USDA Carcass Quality Grades.
| Standard to Select Adipose (1 vs 2) |
|
|
|
| Gene Code |
Fold Change |
Padj |
Gene Abbreviation |
Gene Name |
| Downregulated: |
|
|
|
|
| ENSBTAG00000008063 |
-1.59 |
2.47E-05 |
PPARA |
peroxisome proliferator activated receptor alpha |
| ENSBTAG00000014387 |
-1.85 |
2.19E-05 |
PRKAB2 |
protein kinase AMP-activated non-catalytic subunit beta 2 |
| ENSBTAG00000040128 |
-1.24 |
5.96E-05 |
FZD4 |
frizzled class receptor 4 |
| ENSBTAG00000006037 |
-1.82 |
3.03E-05 |
WISP2 |
WNT1 inducible signaling pathway protein 2 |
| Upregulated: |
|
|
|
|
| ENSBTAG00000021077 |
446 |
3.70E-08 |
BOLA-DMB |
Major histocompatibility complex, class II, DM beta |
| Select to Choice Adipose (2 vs 3) |
|
|
|
|
| Gene Code |
Fold Change |
Padj |
Gene Abbreviation |
Gene Name |
| Downregulated: |
|
|
|
|
| ENSBTAG00000014911 |
3.65 |
2.46E-05 |
LEP |
leptin |
| ENSBTAG00000018777 |
2.64 |
6.08E-07 |
ADCY5 |
adenylate cyclase type 5 |
| Standard to Choice Adipose |
|
|
|
| Gene Code |
Fold Change |
Padj |
Gene Abbreviation |
Gene Name |
| Downregulated: |
|
|
|
|
| ENSBTAG00000034222 |
1.18 |
4.76E-05 |
CAB39L |
calcium binding protein 39 like |
| ENSBTAG00000005198 |
1.80 |
2.62E-07 |
FGF1 |
Fibroblast growth factor 1 |
| ENSBTAG00000047202 |
1.37 |
8.74E-06 |
GRIN1 |
glutamate ionotropic receptor NMDA type subunit 1 |
| ENSBTAG00000014911 |
1.35 |
0.000297 |
LEP |
Leptin |
| ENSBTAG00000013108 |
2.19 |
9.91E-05 |
HK2 |
hexokinase 2 |
| ENSBTAG00000017567 |
1.79 |
1.48E-06 |
ACC1 |
Acetyl-CoA carboxylase alpha |
| ENSBTAG00000045728 |
1.84 |
0.000115 |
SCD1 |
Stearoyl-CoA desaturase |
| ENSBTAG00000008102 |
2.34 |
3.52E-06 |
CRTAC1 |
Cartilage acidic protein 1 isoform 2 precursor |
| ENSBTAG00000008153 |
1.03 |
0.032 |
CAMSAP2 |
Calmodulin regulated spectric associated protein family member 2 |
| ENSBTAG00000011337 |
1.72 |
0.0037 |
ANKRD33B |
Ankyrin repeat domain 33B |
| ENSBTAG00000013107 |
2.12 |
4.76E-06 |
SHANK1 |
SH3 and multiple ankyrin repeat domains 1 |
| ENSBTAG00000018473 |
3.70 |
0.039 |
MARCO |
Macrophage recptor with collagenous structure |
| ENSBTAG00000026156 |
1.63 |
0.025 |
VCL |
Vinculin |
| ENSBTAG00000015690 |
1.03 |
1.88E-06 |
PLIN4 |
Perilipin 4 |
| ENSBTAG00000003359 |
1.44 |
4.39E-06 |
ELOVL5 |
ELOVL fatty acid elongase 5 |
| Upregulated: |
|
|
|
|
| ENSBTAG00000027654 |
-1.43 |
7.97E-05 |
EIF4EBP1 |
eukaryotic translation initiation factor 4E binding protein 1 |
| ENSBTAG00000016071 |
-1.56 |
0.000195 |
HHIP |
hedgehog interacting protein |
| ENSBTAG00000003658 |
-1.23 |
4.50E-05 |
RELN |
reelin precursor |
| ENSBTAG00000007446 |
-1.49 |
8.45E-05 |
NGF |
nerve growth factor |
| ENSBTAG00000007446 |
-1.55 |
0.004 |
SCART1 |
Scavenger receptor family member expressed on T-cells |
| ENSBTAG00000007554 |
-1.26 |
0.022 |
IFI6 |
Interferon alpha inducible protein 6 |
| ENSBTAG00000015182 |
-1.58 |
0.0002 |
STARD10 |
StAR related lipid transfer domain containing 10 |
| ENSBTAG00000039520 |
-2.29 |
0.042 |
SIRPB1 |
Signal Regulatory Protein |
| ENSBTAG00000009656 |
-1.53 |
5.51E-06 |
BOLA-DQA2 |
Major histocompatibility complex, class II, DQ alpha 2 |
| ENSBTAG00000021077 |
-11.68 |
1.40E-08 |
BOLA-DQB |
Major histocompatibility complex, class II, DQ beta |
| ENSBTAG00000038128 |
-2.36 |
1.36E-06 |
BOLA-DQA5 |
Major histocompatibility complex, class II, DQ alpha 5 |
Table 6.
Select differentially expressed genes in muscle tissue from beef steers with different USDA Carcass Quality Grades.
Table 6.
Select differentially expressed genes in muscle tissue from beef steers with different USDA Carcass Quality Grades.
| Standard to Choice Muscle |
|
|
|
| Gene Code |
Fold Change |
padj |
Gene Abbreviation |
Gene Name |
| Downregulated: |
|
|
|
|
| ENSBTAG00000017412 |
-1.23 |
0.0.19 |
SOCS6 |
Suppressor of cytokine signaling 6 |
| ENSBTAG00000021308 |
-1.12 |
0.032 |
IRS1 |
insulin receptor substrate 1 |
| Upregulated: |
|
|
|
|
| ENSBTAG00000002362 |
1.69 |
0.0009 |
APOLD1 |
Apolipoprotein L domain containing 1 |
| ENSBTAG00000032369 |
1.45 |
0.03 |
NMI |
N-myc and STAT interactor |
| ENSBTAG00000009656 |
2.67 |
0.002 |
BOLA-DQA2 |
Major histocompatibility complex, class II, DQ alpha 2 |
| ENSBTAG00000012451 |
1.36 |
0.041 |
BOLA-DMB |
Major histocompatibility complex, class II, DM beta |
Table 7.
GO Enrichment of Select Compared to Standard Adipose Tissue.
Table 7.
GO Enrichment of Select Compared to Standard Adipose Tissue.
| Select compared to Standard Adipose Tissue |
| GO Accession |
Description |
Category |
Padj |
Count |
Up |
Down |
| GO:0006629 |
lipid metabolic process |
Cellular |
0.042 |
956 |
4 |
0 |
| GO:0010887 |
negative regulation of cholesterol storage |
Cellular |
0.005 |
4 |
1 |
0 |
| GO:0046426 |
negative regulation of JAK-STAT cascade |
Cellular |
0.057 |
45 |
1 |
0 |
| GO:0010888 |
negative regulation of lipid storage |
Cellular |
0.017 |
12 |
1 |
0 |
| GO:0010891 |
negative regulation of sequestering of triglyceride |
Cellular |
0.007 |
5 |
1 |
0 |
Table 8.
GO Enrichment of Select Compared to Choice Adipose Tissue.
Table 8.
GO Enrichment of Select Compared to Choice Adipose Tissue.
| Select compared to Choice Adipose Tissue |
| GO Accession |
Description |
Category |
Padj |
Count |
Up |
Down |
| GO:0060612 |
adipose tissue development |
Cellular |
0.031 |
28 |
0 |
1 |
| GO:0046427 |
positive regulation of JAK-STAT cascade |
Cellular |
0.045 |
48 |
0 |
1 |
| GO:00045723 |
positive regulation of fatty acid biosynthetic process |
Cellular |
0.013 |
14 |
0 |
1 |
| GO:0045923 |
postive regulation of fatty acid metabolic process |
Cellular |
0.026 |
26 |
0 |
1 |
| GO:0046889 |
positive regulation of lipid biosynthetic process |
Cellular |
0.043 |
44 |
0 |
1 |
Table 9.
GO Enrichment of Select Compared to Choice Adipose Tissue.
Table 9.
GO Enrichment of Select Compared to Choice Adipose Tissue.
| Choice compared to Standard Adipose Tissue |
| GO Accession |
Description |
Category |
Padj |
Count |
Up |
Down |
| GO:0006633 |
fatty acid biosynthetic process |
Cellular |
0.000 |
104 |
5 |
1 |
| GO:0034625 |
fatty acid elongation, monounsaturated fatty acid |
Cellular |
0.048 |
7 |
2 |
0 |
| GO:0006631 |
fatty acid metabolic process |
Cellular |
0.014 |
254 |
5 |
1 |
| GO:0060612 |
adipose tissue development |
Cellular |
0.024 |
28 |
2 |
0 |
| GO:0045723 |
positive regulation of fatty acid biosynthetic process |
Cellular |
0.004 |
14 |
2 |
0 |
| GO:0045923 |
postive regulation of fatty acid metabolic process |
Cellular |
0.016 |
26 |
2 |
0 |
| GO:0004321 |
fatty-acyl-CoA synthase activity |
Cellular |
0.033 |
4 |
0 |
1 |
Table 10.
GO Enrichment of Standard Compared to Choice Muscle Tissue.
Table 10.
GO Enrichment of Standard Compared to Choice Muscle Tissue.
| Standard compared to Choice Muscle Tissue |
| GO Accession |
Description |
Category |
Padj |
Count |
Up |
Down |
| GO:0001578 |
microtubule bundle formation |
Cellular |
0.006 |
75 |
1 |
1 |
| GO:0046785 |
microtubule polymerization |
Cellular |
0.073 |
51 |
0 |
1 |