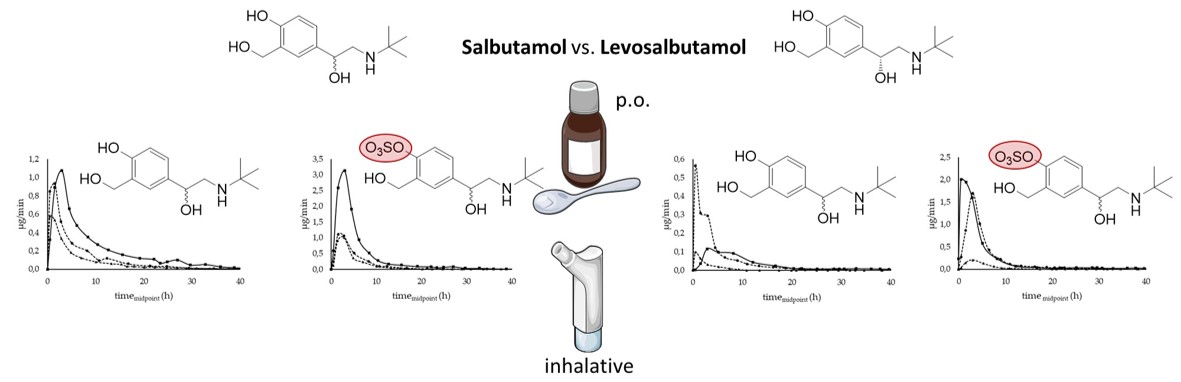
1. Introduction
Salbutamol is a widely known β
2-sympathomimetic drug, commonly prescribed for the treatment of asthmatic patients. It is available as a racemic preparation and recently also approved as the pure enantiomer levosalbutamol ((
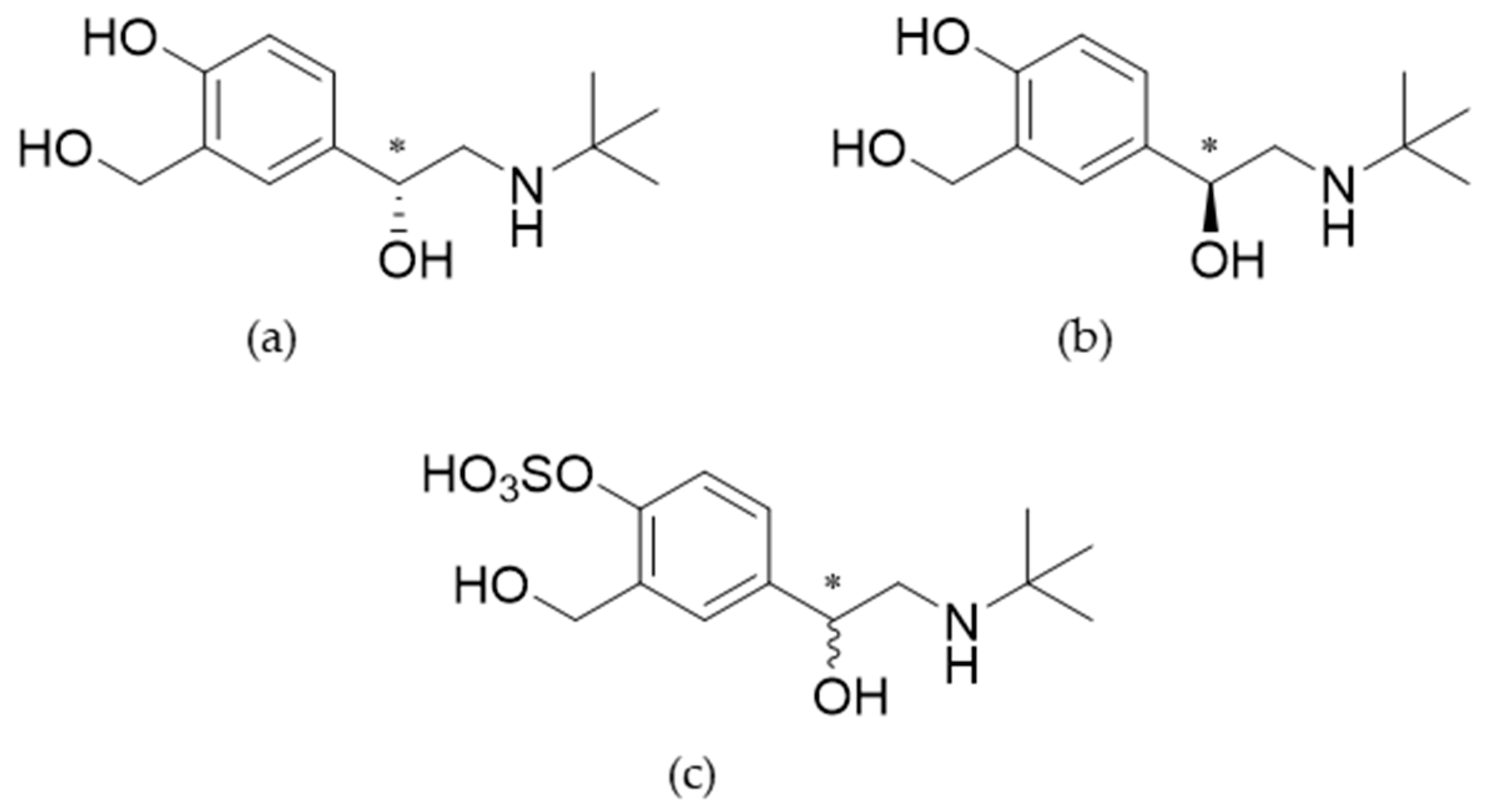
R)-salbutamol), which is the pharmacologically active enantiomer (chemical structures in
Figure 1). However, a clinically relevant advantage of levosalbutamol opposed to racemic formulations has not been shown [
1].
The main metabolic pathway of salbutamol is sulfonation by sulfotransferases (SULTs), more precisely by the phenol-sulfotransferase SULT1A3 while SULT1A1, SULT1B1 and SULT1C4 do not show activity towards this compound [
1,
2,
3,
4]
. Sulfotransferases catalyze the transfer of a sulfonate group from the cofactor 3’-phosphoadenosine-5’phosphosulfate (PAPS) to the substrate, generally leading to inactive metabolites [
2]. These enzymes can be found in liver, small intestine, kidneys, and the lungs. Most abundant in the small intestine are SULT1B1 and SULT1A3 whereas in the liver SULT1A1 is the major enzyme isoform and SULT1A3 is not present. The lungs and kidneys contain only low levels of SULTs [
2,
5,
6,
7]. The biotransformation of salbutamol by SULT1A3 is a stereoselective process. (
R)-Salbutamol is favored by the enzyme and metabolized up to twelve times faster than the
S-enantiomer which leads to higher plasma concentrations of (
S)-salbutamol as parent compound after administration of the racemate [
1,
8]. Also, it has been reported, that (
S)-salbutamol acts as a competitive inhibitor of the phenol-sulfotransferase which leads to reduced sulfonation and thus to higher plasma concentrations of (
R)-salbutamol when applied as racemate [
9]. Glucuronidation of salbutamol only occurs for up to 3 % after oral administration and glucuronidated metabolites were undetectable after inhalative administration [
10].
Renal excretion is the major pathway to clear salbutamol and its metabolites from the body. Active renal secretion is probable as a higher clearance has been calculated for salbutamol compared to the creatinine clearance [
11,
12]. It has been shown that concentrations of salbutamol in urine and plasma are approximately equal during the period immediately after pulmonary administration, indicating minimal metabolic clearance from the lungs [
1].
The main route of administration for salbutamol is inhalation but oral formulations are also available. Salbutamol is absorbed at 80-90% when administered orally or pulmonarily; however, only 10-20% of an inhaled dose is delivered to the lungs, while the rest of the dose is swallowed [
12,
13]. After oral application the bioavailability of salbutamol reached 44% despite of the first pass effect through sulfonation in the intestinal wall [
14]. Plasma concentrations of the sulfoconjugated metabolite after oral administration were therefore higher than those of salbutamol. Since respiratory cells did not show significant metabolic activity for salbutamol
in vitro, little to no first pass effect was expected for pulmonary application. However,
in vivo occurrence of first pass metabolism was reported [
14] which was explained by an inadvertently swallowed part of the dosage. When administered intravenously, the majority of the dose was excreted unchanged [
15].
In this study, differences in the urinary excretion pattern of salbutamol and salbutamol-4’-O-sulfate were investigated. Moreover, application forms, routes of administration, and enantiopure versus racemic formulations were compared for their influence on the renal excretion.
2. Materials and Methods
2.1. Chemicals and reagents
Salbutamol hemisulfate (> 98.0 %) was obtained from TCI Europe (Zwijndrecht, Belgium). Hydrochloric acid (35 %, analytical grade), citric acid, ATP and salbutamol-(tert-butyl-d9)-acetate were obtained from Sigma Aldrich (Taufkirchen, Germany). Methanol (MeOH, LC-MS grade) and potassium hydrogen phthalate were purchased from Thermo Fisher Scientific (Hennigsdorf, Germany). Ammonium formate (HCOONH4, LC-MS grade) and ammonium chloride were from VWR Chemicals (Darmstadt, Germany). D(+)-glucose, ammonium hydrogen carbonate, di-sodium hydrogen phosphate, ferric chloride hexahydrate, potassium chloride, magnesium chloride hexahydrate, D(+)-biotin, agar, Triton-X100 and Tris were purchased from Carl Roth GmbH (Karlsruhe, Germany). Ammonium sulfate, sodium sulfate, nicotinic acid, boric acid, copper sulfate pentahydrate, manganese sulfate and potassium iodide were obtained from Merck (Darmstadt, Germany). Molybdic acid was purchased from Alfa Aesar (Kandel, Germany) and inositol was from Th. Geyer (Berlin, Germany). DMSO-d6 (> 99.8 %) was purchased from Deutero (Kastellaun, Germany). Ultrapure water was prepared with a Milli-Q water purification system LaboStar 2-DI/UV from SG Wasseraufbereitung und Regenerierstation GmbH (Barsbüttel, Germany) equipped with LC-Pak Polisher and a 0.22-μm membrane point-of-use cartridge (Millipak®, Th Geyer, Berlin, Germany).
2.2. Synthesis of salbutamol-4’-O-sulfate as reference
A suitable reference for quantitation of salbutamol-4’-
O-sulfate was not commercially available. Therefore, it was synthesized following a biochemical approach developed by Sun et al. [
4,
16] using genetically modified fission yeast cells. The method used herein was slightly modified from Sun et al. [
4,
16], who described the successful generation of salbutamol-4’-
O-sulfate using the recombinant fission yeast strain YN20 which provides recombinant human SULT1A3. Briefly, the fission yeast strain expressing SULT1A3 was cultured in 10 ml liquid Edinburgh Minimal Medium (EMM) at 30 °C, 230 rpm and then transferred to a flask with 400 ml liquid EMM to grow a large culture. Subsequently, a certain number of cells was transferred to a centrifuge tube and centrifuged at 4 °C, 4500 rcf for 5 minutes. The supernatant was then discarded, and the cells were incubated in 0.3 % Triton-X100 in Tris-KCl buffer (200 mM KCl, 100 mM Tris, pH 7.8) at 30 °C with agitation for one hour to permeabilize the cells. The cells were then washed thrice with NH
4HCO
3 buffer (50 mM, pH 7.8) and resuspended to a concentration of 2.5 × 10
8 cells per ml in a reaction mixture containing ATP (11 mM), (NH
4)
2SO
4 (5.5 mM), MgCl
2 (20 mM) in NH
4HCO
3 buffer (50 mM, pH 7.8). The reaction was started by adding the substrate (1 mM) to the mixture which was then incubated with agitation for 17 h at 37 °C to allow biotransformation. Finally, the reaction mixture was centrifuged at 4 °C, 3320 rcf for 5 min and the supernatant containing the product was collected. The cells were washed twice with water and the merged supernatants were purified on a silica gravity column and by semi-preparative HPLC separation.
2.3. Characterization of salbutamol-4’-O-sulfate
2.3.1. UHPLC-QTOF-MS
High resolution accurate mass analysis of the biosynthesized salbutamol-4’-O-sulfate was performed in targeted MS/MS mode (2 Hz MS1; 3 Hz MS2) on an Agilent 6550 iFunnel QTOF-MS (G6550A; Agilent Technologies, Santa Clara, USA) coupled to an Agilent 1290 Infinity Ⅱ UHPLC system (Agilent Technologies, Waldbronn, Germany). Ionization was achieved utilizing an ESI source (Dual Agilent Jetstream) in positive mode. Source parameters were 3500 V capillary voltage, 500 V nozzle voltage, drying gas temperature 170 °C, drying gas flow 17 l/min, nebulizer 10 psi, sheath gas temperature 375 °C, and sheath gas flow 12 l/min. The UHPLC was equipped with a Poroshell 120 phenyl-hexyl column (3 x 100 mm; 1.9 µm), gradient elution was performed at a flow rate of 0.400 ml/min at 35 °C column temperature and started with 5 % B (20 mM ammonium formate in MeOH) and 95 % A (20 mM ammonium formate in water) for 1 min. The gradient evolved in 4 min to 40 % B, then in 2 min to 95 % B and was then kept at 95 % B for 1.9 min before re-equilibration.
2.3.2. Nuclear Magnetic Resonance
1H (400 MHz) and 13C NMR (100 MHz) were recorded at 298 K on a Bruker Avance III 400 instrument (Bruker, Rheinstetten, Germany). ERETIC-analysis was performed with 30° angle, 16 scans, and an interscan delay of 40 s. Frequency range was +/- 10 ppm and 64k data points were generated. NMR integrals were referenced to NMR integrals of 10.05 mg 1,3,5-trimethoxybenzene (TraceCERT Lot#BCBO5470) in 0.605 ml DMSO-d6 counting in the content given by its batch analysis certificate. Analytes were dissolved in DMSO-d6 (99.8 %) and measured in Wilmad economy grade NMR sample tubes.
2.4. Study design
Different formulations of racemic salbutamol and the pure enantiomer were administered by a healthy volunteer. A dose of 600 µg of racemic salbutamol was applied by inhalation as aerosol (SADA, 6 x 100 µg puffs; Salbuhexal) and as powder inhalation (SAPI, 3 x 200 µg; Cyclocaps) to evaluate equivalence in excretion of the parent drug and its sulfoconjugate subject to the use of a metered dose inhaler (MDI) and a dry powder inhaler (DPI). Levosalbutamol was administered pulmonary as well, at a low dose of 90 µg (LeSaTD, 2 x 45 µg puffs) and a high dose of 630 µg (LeSa, 14 x 45 µg puffs; Xopenex). Additionally, oral administrations of 2 mg of racemic salbutamol as a liquid (SAPO, 2 ml as drops; SalbuBronch Elixir 1 mg/ml) and 1 mg of levosalbutamol hydrochloride (LSAP, 2 ml of a 0.5 mg/ml levosalbutamol solution) were performed. Administrations were carried out at least one week apart. Urine was collected pre- and for up to 141 hours (6 days) post-administration. All urine samples were collected as they accrued throughout at least the first 48 hours after administration. Afterwards, morning urines were collected. Excreted volumes measured and corresponding collection periods were recorded. Aliquots of the urine samples were stored at -20 °C until analysis.
2.5. Sample preparation
For sample preparation, urine aliquots were thawed at room temperature. To 200 µl of the urine sample 700 µl methanol and 100 µl internal standard containing d9-labeled salbutamol were added (final concentration of internal standard 500 ng/ml). Samples were then cooled for 10 minutes at -20 °C, centrifuged at 14100 rcf for 5 minutes and the supernatant was transferred to glass vials for analysis. Matrix matched calibration was performed in the range of 0.83 ng/ml - 1665 ng/ml for salbutamol and 0.93 ng/ml - 186 ng/ml for salbutamol-4’-O-sulfate. Urine samples with higher concentrations were diluted with blank urine prior to sample preparation.
2.6. Instruments and chromatographic conditions
All quantitative urine analyses were carried out by ultrahigh performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) using an Agilent Infinity 1290 II UHPLC-System (Agilent Technologies, Waldbronn, Germany) coupled to an Agilent 6495 iFunnel QQQ-MS (G6495B; Agilent Technologies, Santa Clara, USA). Chromatography was performed utilizing an InfinityLab Poroshell 120 phenyl-hexyl column (3 x 100 mm, 1.9 µm) at a temperature of 35 °C. Gradient elution was performed using 20 mM ammonium formate in water (A) and 20 mM ammonium formate in methanol (B) at a flow rate of 0.400 ml/min. Details of the gradient are shown in Table 1. The tandem mass spectrometer was operated in multiple reaction monitoring (MRM) using positive/negative electrospray ionization mode. Salbutamol-4’-O-sulfate in ESI negative mode and salbutamol glucuronide were not considered in this study, their mass transitions did not add value to the discussed results. Detailed parameters for all analytes are available from Table 2.
2.7. Data analysis
Proportions of salbutamol-4’-O-sulfate and salbutamol were calculated in relation to the total amount of salbutamol excreted as those compounds. Amounts of salbutamol-4’-O-sulfate were always calculated as the mass of salbutamol metabolized.
A Sigma minus plot (y-axis: (
U∞ -
Ut), logarithmic; x-axis: time) was used for evaluation of urine data. The elimination constant was calculated with the following equation:
The elimination half-life was then calculated by:
Table 1.
Gradient for mobile phase used during chromatography.
Table 1.
Gradient for mobile phase used during chromatography.
| Time (min) |
A1 (%) |
B2 (%) |
| 0.00 |
95 |
5 |
| 1.00 |
95 |
5 |
| 5.00 |
60 |
40 |
| 6.00 |
5 |
95 |
| 7.90 |
5 |
95 |
| 8.00 |
95 |
5 |
Table 2.
Parameters used for ion source and MRM.
Table 2.
Parameters used for ion source and MRM.
| Ion source parameters |
| |
| Gas temperature |
170 °C |
| Gas flow |
17 l/min |
| Nebulizer |
10 psi |
| Sheath gas temperature |
400 °C |
| Sheath gas flow |
12 l/min |
| Capillary voltage |
4000 V |
| Nozzle voltage |
500 V |
| |
|
| MRM parameters |
| |
Precursor ion [m/z] |
Product ion [m/z] |
Collision energy |
| |
|
|
|
| Salbutamol |
[M+H]+ = 240.0
|
222.1
166.1
148.1
121.1
91.0
77.1 |
8
12
16
25
48
56 |
| |
|
|
|
| Salbutamol-4’-O-sulfate |
[M+H]+ = 320.0
|
240.0
222.0
166.0
148.0
77.0 |
4
16
16
32
80 |
| |
|
|
|
| |
[M-H]- = 318.0
|
238.0
|
25
|
| Salbutamol-d9
|
[M+H]+ = 249.2 |
231.1
166.1
148.1
121.1 |
8
12
16
25 |
| |
|
|
|
| Salbutamol glucuronide |
[M+H]+ = 416.0
|
298.0
240.0
224.0
222.0
148.0 |
12
18
29
20
20 |
| |
|
|
|
| |
[M-H]- = 414.0
|
396.0
220.0
146.0 |
18
25
25 |
3. Results
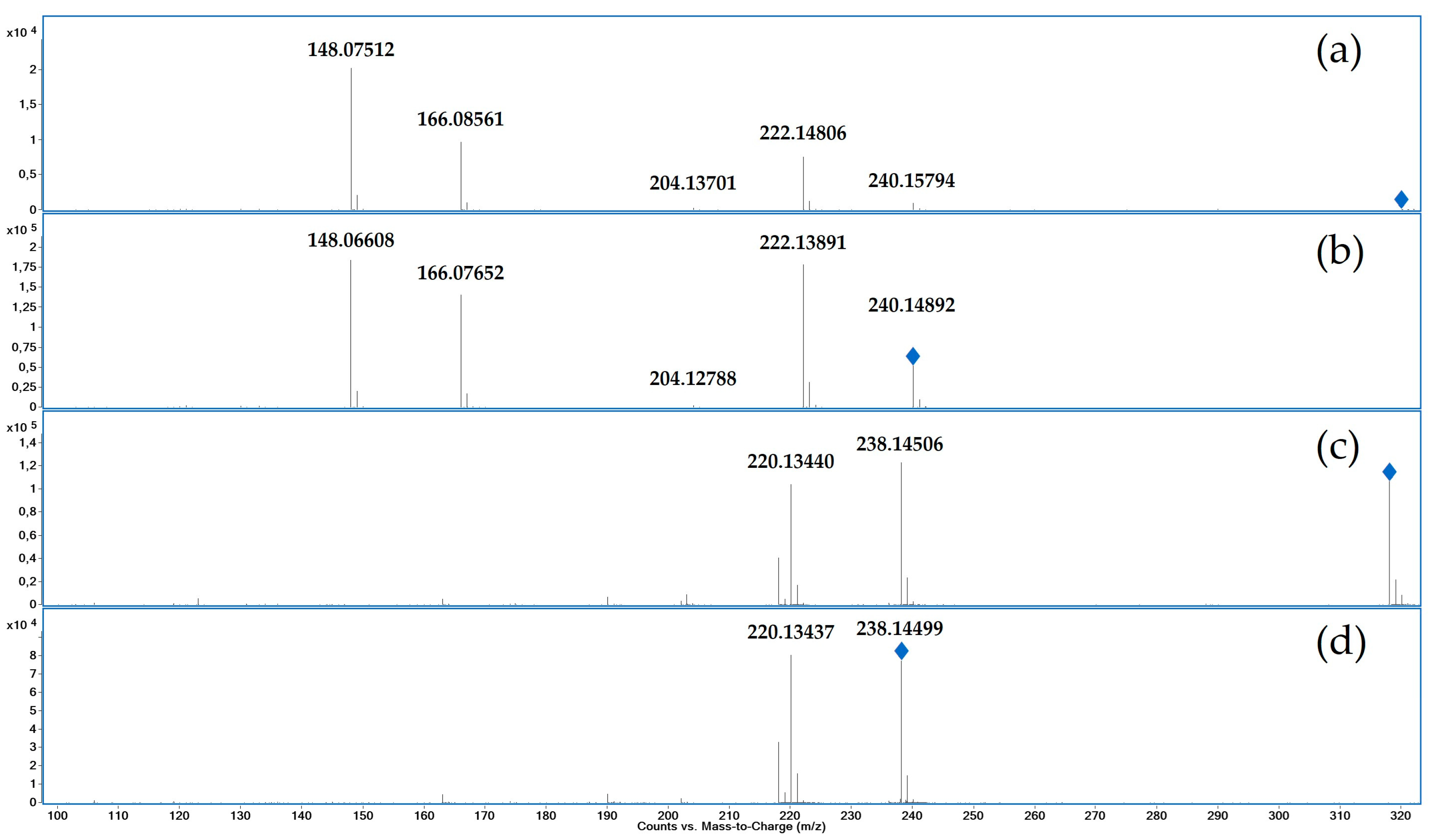
3.1. Characterization of reference by UHPLC-QTOF-MS and NMR
The accurate mass found for salbutamol-4’-
O-sulfate [M+H]
+ at 3.69 min was
m/z 320.11599 (exact mass
m/z 320.11623, mass error Δm/z = -0.75 ppm). The product ion spectra of salbutamol-4’-
O-sulfate (a, c) and salbutamol (b, d) are displayed in
Figure 2 (targeted MS
2). After the loss of SO
3 ([M+H-SO
3]
+ m/z 240.1579) salbutamol-4’-
O-sulfate shows a similar fragmentation as salbutamol. Consecutive water losses lead to the product ions
m/z 222.14806 ([M+H-SO
3-H
2O]
+) and
m/z 204.13701 ([M+H-SO
3-2H
2O]
+). α-Cleavage between position 2 and 3 leads to
m/z 166.08561 ([C
9H
10O
3]
+), a subsequent loss of water leads to
m/z 148.07512 ([C
9H
8O
2]
+).
The fragmentation in negative mode behaves similarly, although only the loss of SO3 ([M-H-SO3]- m/z 238.14506) and a subsequent loss of water ([M+H-SO3-H2O]- m/z 220.13440) can be observed.
MS1 data in positive mode showed the loss of SO3 as in-source fragmentation for salbutamol-4’-O-sulfate. This phenomenon can also be observed in negative mode, but less dominant.
1H and
13C NMR shift data were collected for salbutamol hemisulfate salt and the biosynthesized sulfoconjugated salbutamol. The assignment of all signals was achieved unambiguously using additionally 2D techniques like
1H,
1H COSY,
1H,
13C HSQC and
1H,
13C HMBC for the aliphatic ABX spin system and the AMX system of the aromatic protons. The chemical shifts and couplings are summarized in
Table 3. The carbon attached to the phenol group of the sulfonated hydroxy group (the
ipso position) is shifted upfield by 3.84 ppm, the chemical shifts of the carbon atoms
ortho and
para to the sulfonation site are in contrast shifted downfield in a range of 4.53 to 7.30 ppm. Chemical shifts near other potential sulfonation sites like the amine function or the benzylic or aliphatic hydroxy group are only marginally changed. Diagnostic shift differences are marked bold in
Table 3.
Figure 2.
Product ion spectra (UHPLC-QTOF-MS) of salbutamol-4’-O-sulfate with 20 eV collision energy (a) and salbutamol with 10 eV collision energy (b) in positive mode and salbutamol-4’-O-sulfate with 20 eV collision energy (c) and salbutamol with 10 eV collision energy (d) in negative mode; blue rhombs indicate the respective precursor ion.
Figure 2.
Product ion spectra (UHPLC-QTOF-MS) of salbutamol-4’-O-sulfate with 20 eV collision energy (a) and salbutamol with 10 eV collision energy (b) in positive mode and salbutamol-4’-O-sulfate with 20 eV collision energy (c) and salbutamol with 10 eV collision energy (d) in negative mode; blue rhombs indicate the respective precursor ion.
3.2. Administration of salbutamol through dry powder inhaler and metered dose inhaler
An equal dose (600 µg) of salbutamol was applied using a DPI and a MDI. Administration of racemic salbutamol with an MDI was performed in duplicate. The results for the total quantity excreted and the shares excreted as unchanged drug and sulfonated metabolite are shown in
Table 4. The amount of the dose recovered in the urine was 80 % after administration as powder and 83 - 115 % after using an MDI. The proportion of cumulative excreted salbutamol and salbutamol-4’-
O-sulfate related to the total amount excreted was similar for both administration types. Excretion of salbutamol and the sulfoconjugate appears to be equivalent for administration by MDI and DPI. Hence, only results from the MDI trial were representatively used for comparison of inhalative racemic salbutamol and levosalbutamol.
3.3. Pharmacokinetic evaluation of urine data
The following pharmacokinetic parameters were calculated with the generated urine data: time of maximum excretion rate (tmax (urine)), elimination constant (ke), elimination half-life (t ½ (urine)), cumulative amount excreted as parent compound and sulfate-metabolite as percentage of the administered dose, salbutamol-4’-O-sulfate proportions for every collection period.
3.3.1. Cumulative excretion of salbutamol and salbutamol-4’-O-sulfate
Amounts of 74 – 115 % of the applied dose were recovered in the urine as either parent compound or sulfonated metabolite (
Figure 3). The lowest amount was recovered following oral administration of racemic salbutamol, whereas the highest amount appears to be excreted after inhalative application of the racemic drug and oral administration of levosalbutamol. When inhaled as levosalbutamol, 76 – 84 % of the dose was excreted renally.
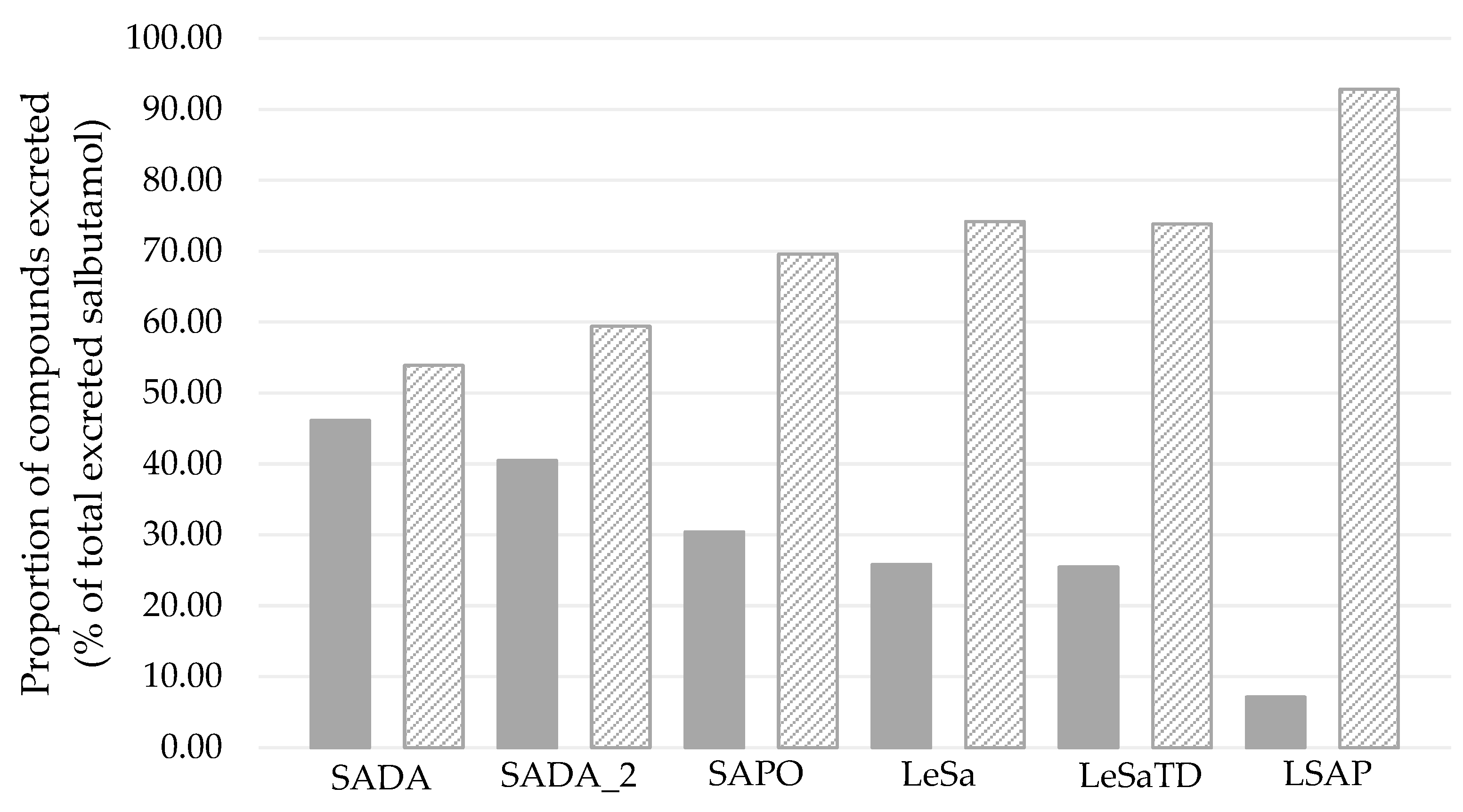
Figure 4 shows the proportion of excreted compounds in percent related to the total excreted amount. For all administrations, the majority was excreted as the sulfoconjugated metabolite, while 7 – 45 % of the excreted amount was recovered as unchanged parent compound.
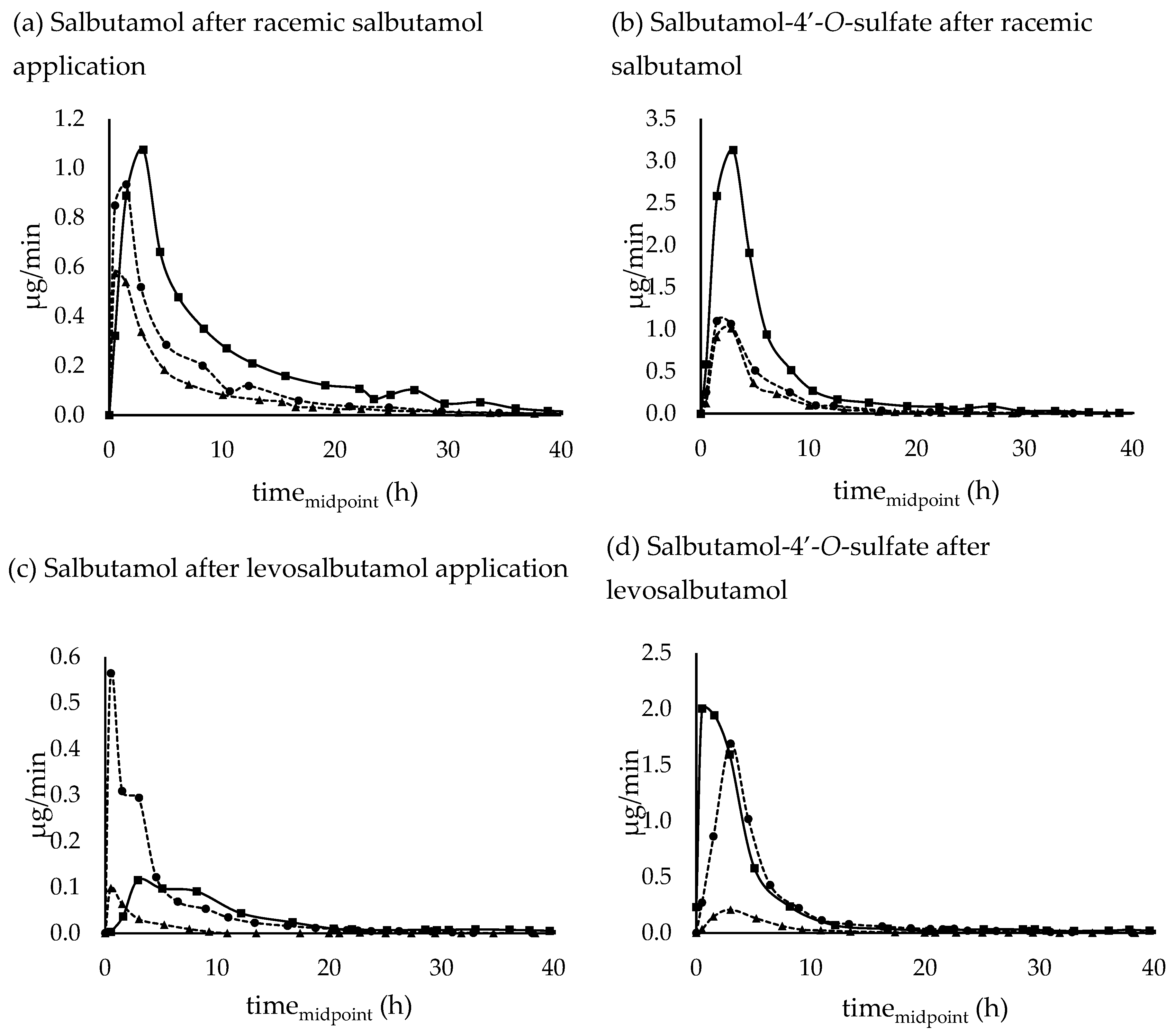
3.3.2. Urinary excretion rates
Renal excretion rates of racemic salbutamol, levosalbutamol and their sulfoconjugated metabolites after pulmonary and oral administration are shown in Figure 5.
Following oral application of the racemic and enantiopure preparation, the highest excretion rate of salbutamol occurred in the collection period between 2-4 hours and for inhalative administration after 1-2 hours. When applied pulmonarily as levosalbutamol, the maximum excretion rate tended to appear earlier, more precisely within the first hour after administration. Excretion rate maxima of salbutamol-4’-O-sulfate on the other hand appeared at a similar time for oral racemic and pulmonary enantiomeric application with a maximum excretion rate after 2-4 hours whereas pulmonary application of racemic salbutamol led to the highest excretion rate within 1-2 hours post administration. In contrast to oral administration of racemic salbutamol, when applied as oral levosalbutamol, the maximum excretion rate for the sulfoconjugate occurred within the first hour post administration.
Excreted parent compound and sulfonated metabolite could be quantified for up to 70 hours post administration for orally applied racemic salbutamol (SAPO), 60 hours for oral levosalbutamol (LSAP), and 46 hours for inhaled racemic drug (SADA). After pulmonary administration of 630 µg levosalbutamol (LeSa) quantitative measurements for unchanged salbutamol were possible for 32 hours and for 90 µg levosalbutamol (LeSaTD) 24 hours post administration. Salbutamol-4’-
O-sulfate on the other hand could also be determined in later samples than the parent compound when pure levosalbutamol was applied with a quantitation window of 48 hours for high dose 630 µg and 46 hours for a therapeutic dosage of 90 µg levosalbutamol. Concentrations measured in later samples were below the calibrated range (0.83 ng/ml to 1664 ng/ml for salbutamol and 0.93 ng/ml to 186 ng/ml for sulfoconjugate). The total renally excreted amount and the time of the highest excretion rate
tmax (urine) are shown in
Table 5.
Figure 5.
Urinary excretion rates of (a) salbutamol and (b) salbutamol-4’-O-sulfate after 600 µg inhalative (SADA - circles with dashed lines, SADA_2 – triangles with dashed lines) and 2 mg oral (SAPO - squares with full lines) administration of racemic salbutamol, (c) salbutamol and (d) salbutamol-4’-O-sulfate after 630 µg inhalative (LeSa - circles with dashed lines), 90 µg inhalative (LeSaTD – triangle with dashed lines), and 1 mg oral (squares with full lines) administration of enantiopure levosalbutamol. Salbutamol-4’-O-sulfate was calculated as amount of salbutamol that was sulfonated.
Figure 5.
Urinary excretion rates of (a) salbutamol and (b) salbutamol-4’-O-sulfate after 600 µg inhalative (SADA - circles with dashed lines, SADA_2 – triangles with dashed lines) and 2 mg oral (SAPO - squares with full lines) administration of racemic salbutamol, (c) salbutamol and (d) salbutamol-4’-O-sulfate after 630 µg inhalative (LeSa - circles with dashed lines), 90 µg inhalative (LeSaTD – triangle with dashed lines), and 1 mg oral (squares with full lines) administration of enantiopure levosalbutamol. Salbutamol-4’-O-sulfate was calculated as amount of salbutamol that was sulfonated.
3.3.2. Salbutamol-4’-O-sulfate in relation to unchanged salbutamol
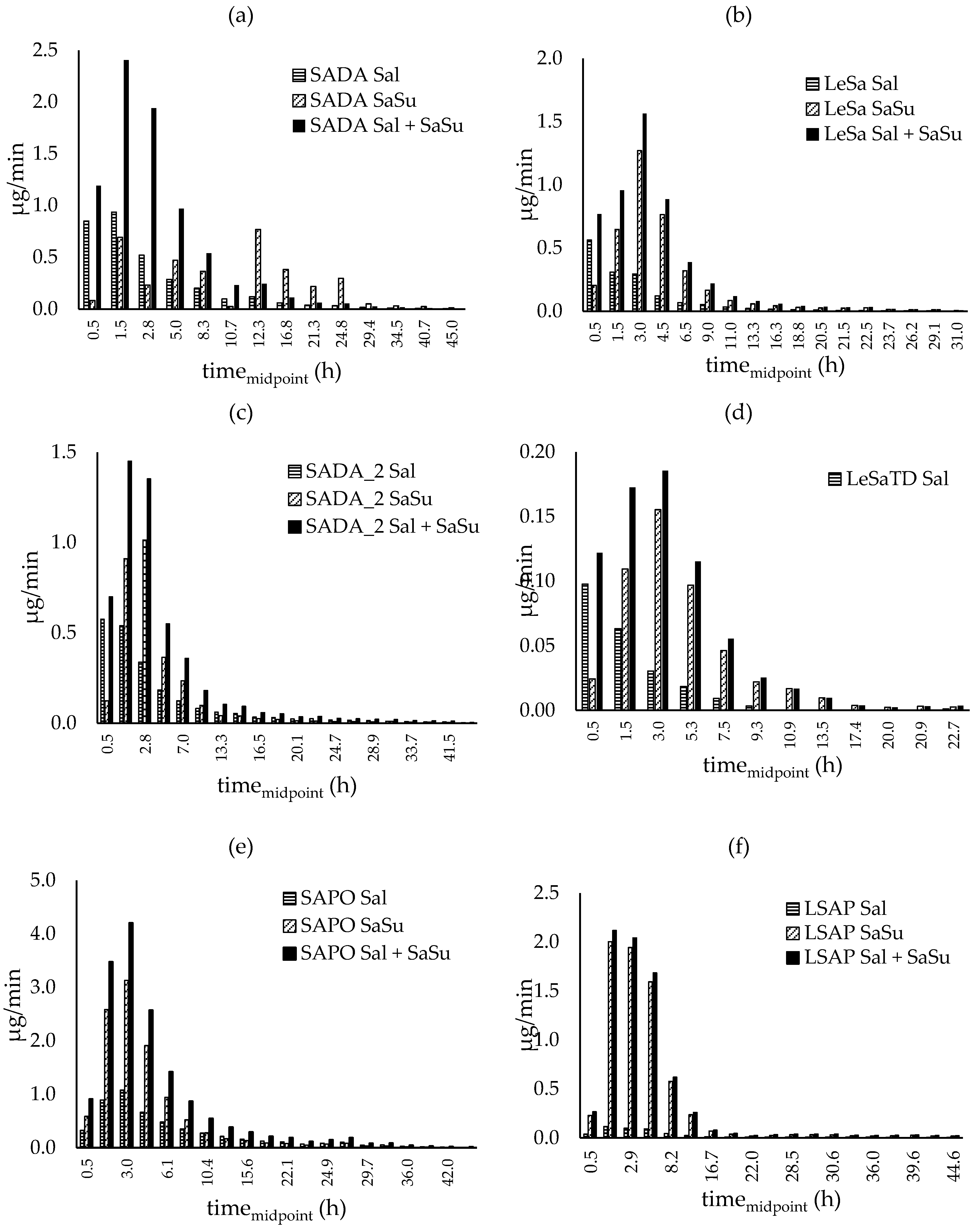
Excretion rate proportions of salbutamol, salbutamol-4’-O-sulfate as well as the excretion rates for both compounds together are shown in Figure 6.
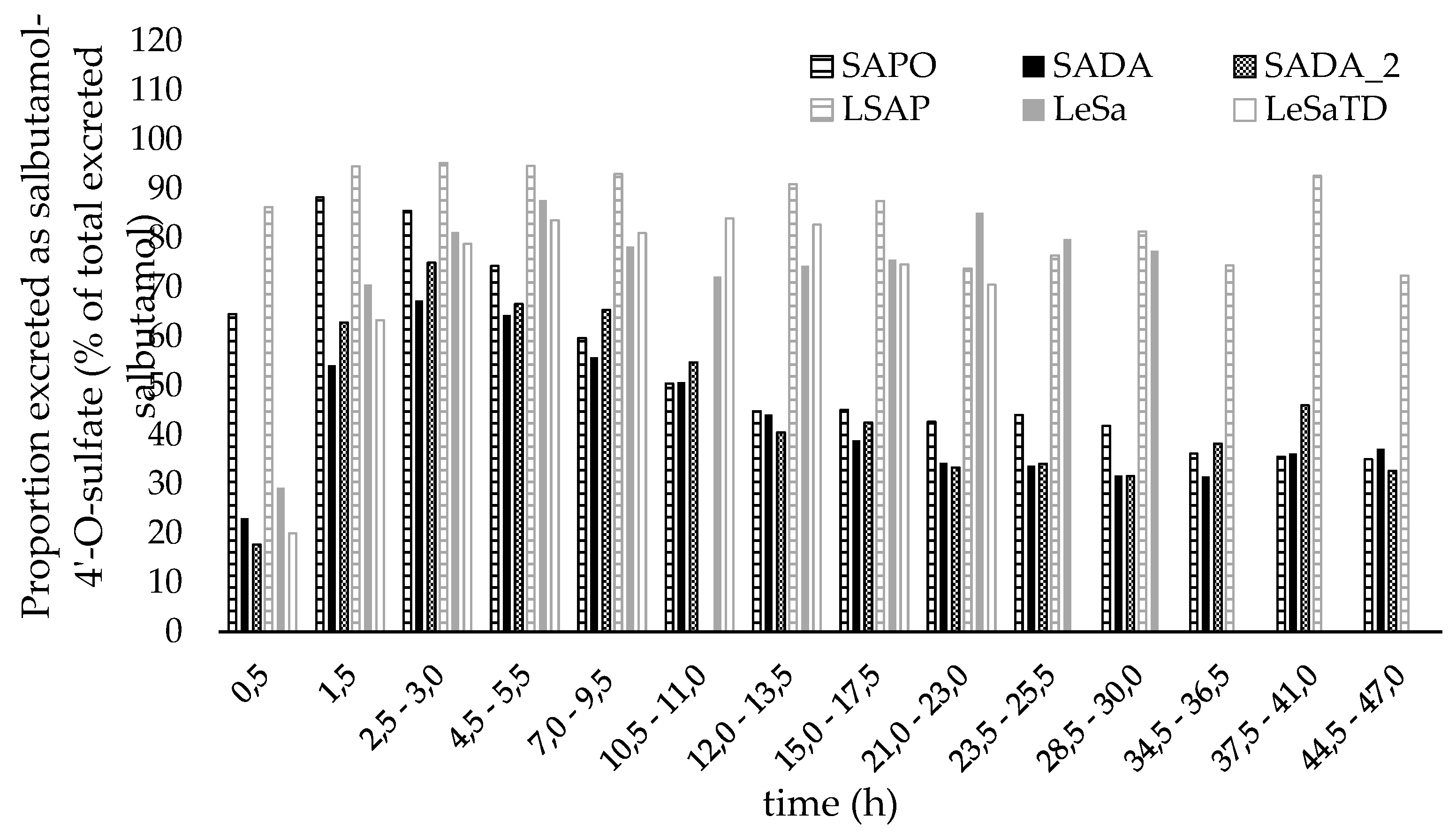
After pulmonary application (Figure 6 (a) - (d)) over the first hour, 2-3 times more unconjugated salbutamol was recovered in urine than sulfate metabolite. During the following collection periods correlation was reversed and salbutamol-4’-O-sulfate predominated in the amount excreted. Though, differences in the sulfate metabolite proportion for racemic formulation and the pure enantiomer were observed. For the entire time of renal elimination, the proportion of salbutamol-4’-O-sulfate (shown in Figure 7) did not exceed 75 % and shifted towards salbutamol again after twelve hours when racemic salbutamol was applied inhalative whereas for pure levosalbutamol the metabolite proportion climbed up to 88 % for high (630 µg) dose and 84 % for low dose (90 µg) levosalbutamol. When administered orally as racemate, the sulfonated metabolite predominates the excreted amount throughout twelve hours post administration followed by a slight shift towards an equal amount of both compounds and a slight tendency towards unchanged salbutamol respectively. In contrast to that, after oral administration of levosalbutamol the sulfate proportion was higher than 85 % from the very beginning of urinary excretion and did not fall below 72 %, showing that most of the compound renally excreted was the sulfoconjugate at all collection times.
Figure 6.
Excretion rates of parent compound (Sal) and metabolite (SaSu) excreted after inhalative administration of (a) and (c) 600 µg racemic drug (SADA, SADA_2) (b) 630 µg levosalbutamol (LeSa) (d) 90 µg levosalbutamol (LeSaTD) and after oral administration of (e) 2 mg racemic salbutamol (SAPO) and (f) 1 mg levosalbutamol (LSAP). Salbutamol-4’-O-sulfate was calculated as amount of salbutamol that is sulfonated.
Figure 6.
Excretion rates of parent compound (Sal) and metabolite (SaSu) excreted after inhalative administration of (a) and (c) 600 µg racemic drug (SADA, SADA_2) (b) 630 µg levosalbutamol (LeSa) (d) 90 µg levosalbutamol (LeSaTD) and after oral administration of (e) 2 mg racemic salbutamol (SAPO) and (f) 1 mg levosalbutamol (LSAP). Salbutamol-4’-O-sulfate was calculated as amount of salbutamol that is sulfonated.
Figure 7.
Proportion of salbutamol-4’-O-sulfate excreted in urine in relation to total salbutamol excreted. Salbutamol-4’-O-sulfate was calculated as amount of salbutamol excreted as sulfate metabolite. SADA – 600 µg inhalative racemic salbutamol, SAPO – 2 mg oral racemic salbutamol, LeSa – 630 µg inhalative levosalbutamol and LeSaTD – 90 µg inhalative levosalbutamol, LSAP – 1 mg oral levosalbutamol.
Figure 7.
Proportion of salbutamol-4’-O-sulfate excreted in urine in relation to total salbutamol excreted. Salbutamol-4’-O-sulfate was calculated as amount of salbutamol excreted as sulfate metabolite. SADA – 600 µg inhalative racemic salbutamol, SAPO – 2 mg oral racemic salbutamol, LeSa – 630 µg inhalative levosalbutamol and LeSaTD – 90 µg inhalative levosalbutamol, LSAP – 1 mg oral levosalbutamol.
3.3.4. Elimination constant and elimination half-life
The renal elimination half-life varied for salbutamol and salbutamol-4’-
O-sulfate for different administration types between 3.3 h – 8.9 h for salbutamol and 3.7 – 6.9 h for salbutamol-4’-
O-sulfate (
Table 6). When inhaled levosalbutamol renal elimination half-life tended to be shorter than for levosalbutamol and its sulfonated metabolite than for racemic salbutamol and its sulfonated metabolite. In contrast to that, the elimination half-life of both, salbutamol and sulfoconjugate, appeared to be longer for levosalbutamol than for racemic salbutamol when administered orally.
4. Discussion
4.1. Biosynthesis and characterization of salbutamol-4’-O-sulfate
To directly quantify salbutamol-4’-
O-sulfate, reference material was biosynthesized and characterized by UHPLC-QTOF-MS and NMR. Due to the three hydroxy groups in salbutamol chemical synthesis proved challenging due to lack of selectivity. Similar to analogous sympathomimetic drugs protection group strategies failed [
17], while utilizing recombinant human SULT1A3 provided in genetically modified fission yeasts resulted in a one-time sulfonated product.
To prove the sulfonation site of salbutamol
1H and
13C NMR shift data were collected for salbutamol hemisulfate salt and the biosynthesized sulfoconjugate of salbutamol. The problem of determining the sulfonation site in substituted phenols has been clearly described by Purchartová
et al. [
18], as a direct proof is not possible. The position of sulfonation is identified indirectly by the effects on chemical shifts on neighboring atoms, most prominently on the carbon atoms. In general, for simple phenols and also more complex structures and natural products with higher substituted aromatic rings the following effects upon sulfonation are observed in deuterated water, methanol or dimethyl sulfoxide [
18,
19,
20]: The carbon attached to the phenol group of the sulfonated hydroxy group (the
ipso position) is shifted upfield by 4 to 6 ppm, the chemical shifts of the carbon atoms
ortho and
para to the sulfonation site in contrast are shifted downfield in a range of 3 to 7 ppm, the
meta positions are not influenced to such a large extent. Similarly, the chemical shifts of the protons in
ortho position of the sulfonation site are downfield shifted by 0.4 to 0.6 ppm, other protons in the ring system exhibit only a small low field shift in the range of 0.1 ppm. These effects can be observed without exception in the case of sulfonated salbutamol and the chemical shifts near other potential sulfonation sites like the amine function or the benzylic or aliphatic hydroxy group are only marginally changed. So, the 4’-hydroxy group is clearly identified as sulfonation site. Due to fast exchange processes the protons of the amine and hydroxy functions cannot be observed as separated signals, which could be taken as an independent proof of the sulfonation of the phenolic hydroxy group as well. After purification the amount of salbutamol-4’-
O-sulfate was determined by qNMR prior to its use as reference for metabolite determination in the urine samples.
4.2. Administration route and pharmakokinetic of salbutamol and its sulfo metabolite
Inhalation by the two different application forms, i.e. DPI and MDI, was compared and it was found to lead to the same proportion of parent compound and salbutamol-4’-O-sulfate for the total excreted amount. Administration of MDI was repeated due to an amount of more than 100 % of the dose recovered in the urine. Possible reasons for this high amount may be a higher dose that is released with the first puffs of a new inhaler. The repetition of the administration of 600 µg racemic salbutamol from a MDI led to a recovery of 83 % of the dosage in the urine. The same MDI was used, supporting the assumption of the first doses released from the inhaler being higher than 100 µg per puff. However, as another source for a recovery over 100 % might serve the relatively high uncertainty in urine volume determination. The volumetric device used was not qualified for highly precision volume determination of very low amounts of urines. This uncertainty may contribute to a inaccuracy for the total amount of recovered salbutamol in the first trial of inhalative applyication of 600 µg racemic salbutamol (SADA). In the repetion of the trial (SADA_2) the urine collection was performed utilizing more accurate equipment for low volums, if necessary.
Considering the different routes of administration (i.e. oral (aqueous solution)
versus inhalative (MDI)) it became apparent, that longer detection times were achieved in case of oral administration. In particular, longer detection windows were observed after oral administration of the racemic drug in by contrast with oral administration of levosalbutamol. As the amount of administered racemic salbutamol was higher than the amount of levosalbutamol in oral application, the observed longer occurrence in urine result was not surprising. By comparing the inhalation of similar doses (~600 µg) of racemic and enantiopure salbutamol a shorter detection window was observed after enantiopure administration. Also, pulmonary applicated levosalbutamol led to the shortest found renal elimination half-life for salbutamol and the sulfonated metabolite. This is in line with reports from literature [
1,
8], which found a higher affinity towards levosalbutamol for SULT1A3. By contrast, in oral administration a higher excretion half-life was observed for levosalbutamol; this finding needs to be corroborated in further studies for confirmation. Similar observations were made for the sulfoconjugated metabolite.
When administered as levosalbutamol, the parent compound was sulfoconjugated to a higher extent than racemic salbutamol. The highest amounts of sulfoconjugate were found when levosalbutamol was administered orally which is in accordance with literature, that SULT1A3 is mainly localized in the jejunum [
2,
5]. According to Ward
et al. and Melchor
et al. [
12,
13] only 10-20 % [
21] of an inhaled dose was delivered to the lungs whereas the rest of the dose was swallowed leading to relatively high amounts of salbutamol-4’-
O-sulfate recovered in urine after inhalative administration. In line with these findings in the current study the highest amounts of sulfoconjugate were also found after oral administration of racemic salbutamol. Whereas the proportion of sulfate metabolite in the total excreted amount was smaller after racemic administration than after enantiopure administration. Considering the same applied amount of levosalbutamol in racemic or enatiopure administration (LSAP 1 mg vs. SAPO 2 mg) the results reflect and support the above mentioned higher affinity of SULT1A3 towards (
R)-salbutamol which was already reported by Boulton
et al. and Walle
et al. [
1,
8]. Opposed to oral administration, after inhalative administration, less sulfoconjugate was formed from racemic salbutamol as well as from levosalbutamol. Due to the missing first pass effect in the lungs [
14] the truly pulmonary applied part of the dosage contibutes less to the genereration of salbutamol-4’-
O-sulfate. Further metabolic and kinetic investigations may profit from the additional availability of serum samples and an enhanced number of participants.
Author Contributions
Conceptualization, ALJ, LCH, FMB, XdT, MKP; methodology, ALJ, LCH, SY, MB, UG; validation, ALJ, LCH; formal analysis, ALJ, UG; investigation, ALJ, LCH, MKP; resources, MKP, MB, FMB; data curation, ALJ; writing—original draft preparation, ALJ; writing—review and editing, LCH, FB, MB, UG, FMB, XdT, MKP; visualization, ALJ, FB; supervision, MKP, MB, FMB; project administration, MKP; funding acquisition, MKP, MB, FMB. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the World Anti-Doping Agency (WADA), grant number WADA 19A10MP. The Article processing charges (APC) are covered by the Open Access Publication Initiative of Freie Universität Berlin.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The volunteer provided an informed consent before participation.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
We would like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. The authors thank the OpenAccess Publication Fund of Freie Universität Berlin for support of the APC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boulton, D.W.; Fawcett, J.P. The pharmacokinetics of levosalbutamol: what are the clinical implications? Clinical pharmacokinetics 2001, 40, 23-40. [CrossRef]
- Ko, K.; Kurogi, K.; Davidson, G.; Liu, M.Y.; Sakakibara, Y.; Suiko, M.; Liu, M.C. Sulfation of ractopamine and salbutamol by the human cytosolic sulfotransferases. J Biochem 2012, 152, 275-283. [CrossRef]
- Jacobson, G.A.; Raidal, S.; Robson, K.; Narkowicz, C.K.; Nichols, D.S.; Haydn Walters, E. Bronchopulmonary pharmacokinetics of (R)-salbutamol and (S)-salbutamol enantiomers in pulmonary epithelial lining fluid and lung tissue of horses. British journal of clinical pharmacology 2017, 83, 1436-1445. [CrossRef]
- Sun, Y.; Harps, L.C.; Bureik, M.; Parr, M.K. Human Sulfotransferase Assays With PAPS Production in situ. Frontiers in Molecular Biosciences 2022, 9. [CrossRef]
- Teubner, W. Charakterisierung von Sulfotransferasen im Gastrointestinaltrakt von Mensch und Ratte und Aktivierung von Promutagenen in V79- Zellen, die eine intestinale Form (1B1) des Menschen und der Ratte exprimieren. 2001.
- Gamage, N.; Barnett, A.; Hempel, N.; Duggleby, R.G.; Windmill, K.F.; Martin, J.L.; McManus, M.E. Human sulfotransferases and their role in chemical metabolism. Toxicol Sci 2006, 90, 5-22. [CrossRef]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W.H. Quantitative Evaluation of the Expression and Activity of Five Major Sulfotransferases (SULTs) in Human Tissues: The SULT “Pie”. Drug Metabolism and Disposition 2009, 37, 2255. [CrossRef]
- Walle, T.; Eaton, E.A.; Walle, U.K.; Pesola, G.R. Stereoselective metabolism ofRS-albuterol in humans. Clinical Reviews in Allergy & Immunology 1996, 14, 101-113. [CrossRef]
- Boulton, D.W.; Fawcett, J.P. Pharmacokinetics and pharmacodynamics of single oral doses of albuterol and its enantiomers in humans. Clin Pharmacol Ther 1997, 62, 138-144. [CrossRef]
- Mareck, U.; Guddat, S.; Schwenke, A.; Beuck, S.; Geyer, H.; Flenker, U.; Elers, J.; Backer, V.; Thevis, M.; Schänzer, W. Determination of salbutamol and salbutamol glucuronide in human urine by means of liquid chromatography-tandem mass spectrometry. Drug Test Anal 2011, 3, 820-827. [CrossRef]
- Boulton, D.W.; Fawcett, J.P. Enantioselective disposition of salbutamol in man following oral and intravenous administration. British journal of clinical pharmacology 1996, 41, 35-40. [CrossRef]
- Ward, J.K.; Dow, J.; Dallow, N.; Eynott, P.; Milleri, S.; Ventresca, G.P. Enantiomeric disposition of inhaled, intravenous and oral racemic-salbutamol in man--no evidence of enantioselective lung metabolism. British journal of clinical pharmacology 2000, 49, 15-22. [CrossRef]
- Melchor, R.; Biddiscombe, M.F.; Mak, V.H.; Short, M.D.; Spiro, S.G. Lung deposition patterns of directly labelled salbutamol in normal subjects and in patients with reversible airflow obstruction. Thorax 1993, 48, 506-511. [CrossRef]
- Nakpheng, T.; Songkarak, S.; Suwandecha, T.; Sritharadol, R.; Chunhachaichana, C.; Srichana, T. Evidences for salbutamol metabolism by respiratory and liver cell lines. Drug metabolism and pharmacokinetics 2017, 32, 127-134. [CrossRef]
- Morgan, D.J.; Paull, J.D.; Richmond, B.H.; Wilson-Evered, E.; Ziccone, S.P. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol 1986, 22, 587-593. [CrossRef]
- Sun, Y.; Machalz, D.; Wolber, G.; Parr, M.K.; Bureik, M. Functional Expression of All Human Sulfotransferases in Fission Yeast, Assay Development, and Structural Models for Isoforms SULT4A1 and SULT6B1. Biomolecules 2020, 10, 1517. [CrossRef]
- Orlovius, A.-K.L. Sulfokonjugierte Sypathomimetika in der Dopinganalytik: Synthese, Charakterisierung und Analyse.; Rheinische Friedrich-Wilhelms-Universität Bonn: https://hdl.handle.net/20.500.11811/6086, 2014.
- Purchartová, K.; Valentová, K.; Pelantová, H.; Marhol, P.; Cvačka, J.; Havlíček, L.; Křenková, A.; Vavříková, E.; Biedermann, D.; Chambers, C.S.; et al. Prokaryotic and Eukaryotic Aryl Sulfotransferases: Sulfation of Quercetin and Its Derivatives. ChemCatChem 2015, 7, 3152-3162. [CrossRef]
- Horst, M.; Hartog, A.; Morabet, R.; Marais, A.; Kircz, M.; Wever, R. Enzymatic Sulfation of Phenolic Hydroxy Groups of Various Plant Metabolites by an Arylsulfotransferase. European Journal of Organic Chemistry 2015, 2015. [CrossRef]
- Ragan, M.A. Phenol sulfate esters: ultraviolet, infrared, 1H and 13C nuclear magnetic resonance spectroscopic investigation. Canadian Journal of Chemistry 1978, 56, 2681-2685. [CrossRef]
- Nishikawa, M.; Masuyama, Y.; Nunome, M.; Yasuda, K.; Sakaki, T.; Ikushiro, S. Whole-cell-dependent biosynthesis of sulfo-conjugate using human sulfotransferase expressing budding yeast. Applied Microbiology and Biotechnology 2018, 102, 723-732. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).