Submitted:
24 February 2023
Posted:
27 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of titanate nanostructured coating
2.2. Loading of metronidazole in nanostructures
2.3. Polymeric coating of nanostructured samples
2.4. Crosslinking of polymeric coating
2.5. Microstructural characterization
2.6. Apatite deposition – “In vitro” Bioactivity Assay
2.7. “In vitro” drug release
2.8. Statistical analysis
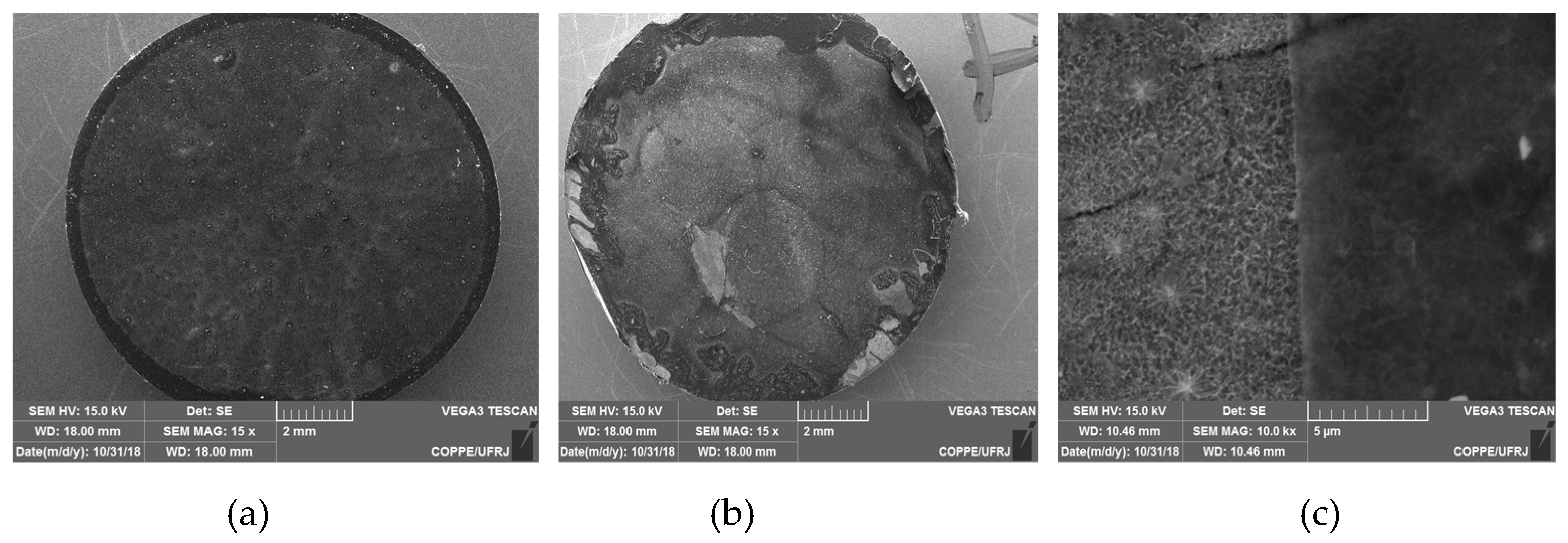
3. Results and Discussion
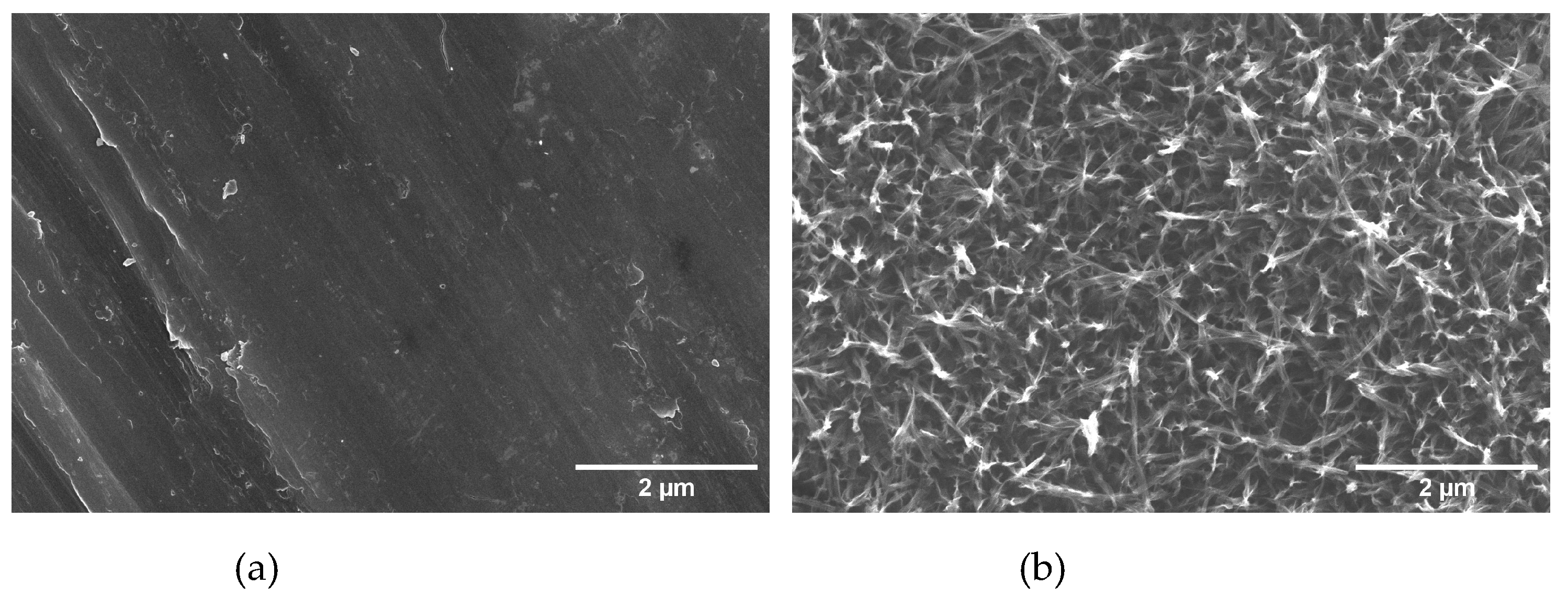
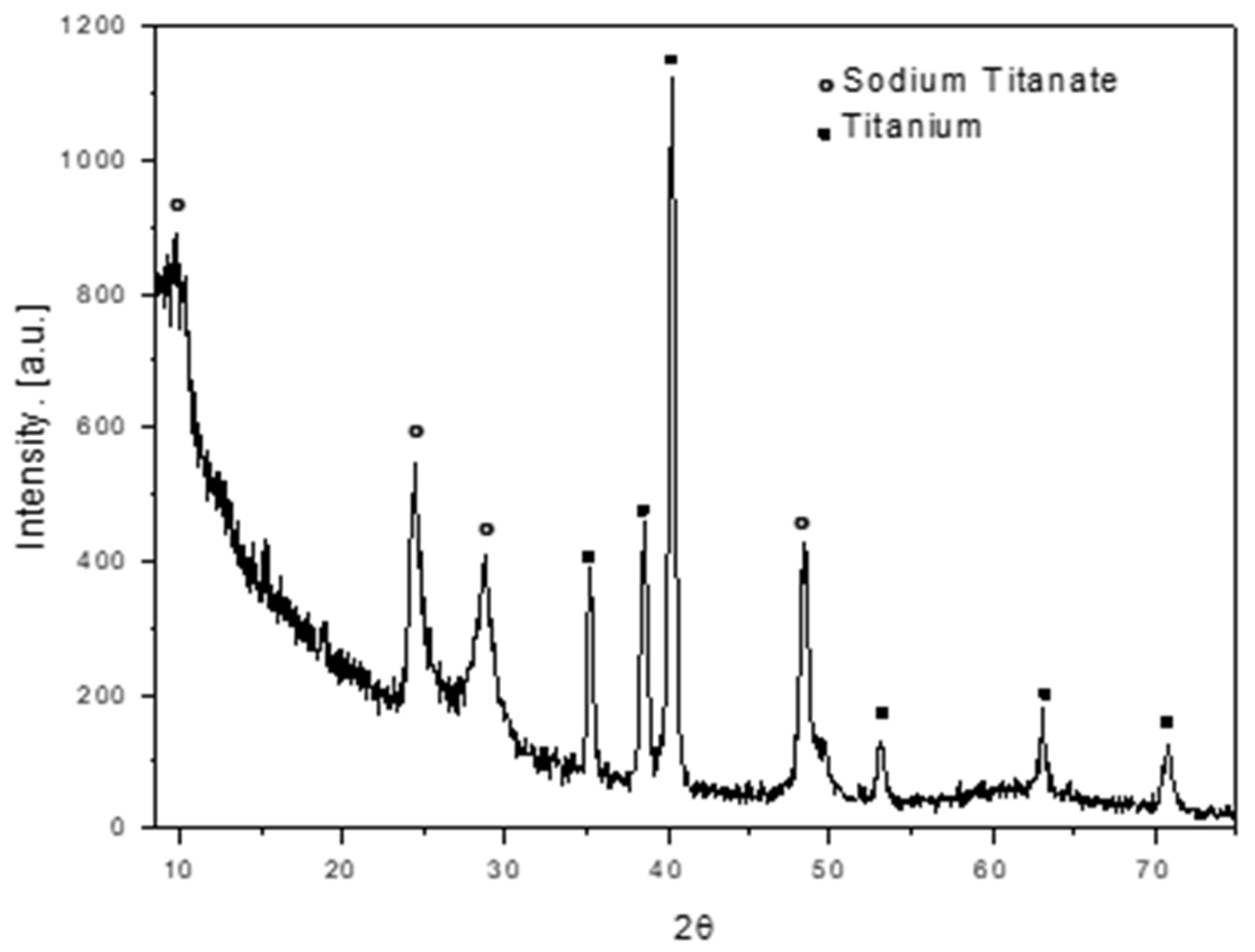
3.1. Titanate nanostructured coating
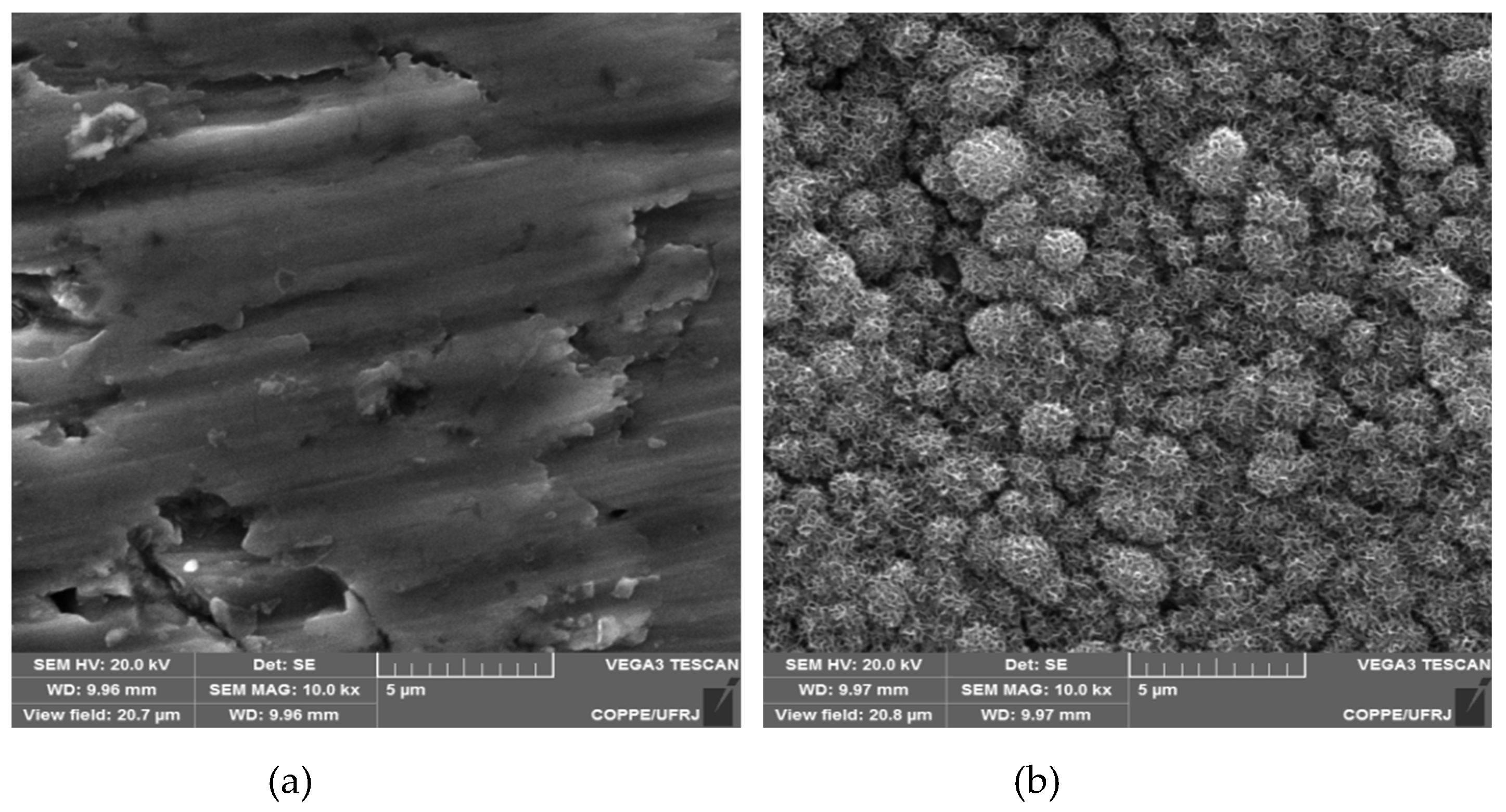
3.2. “In vitro” bioactivity assay
3.3. Surface wettability
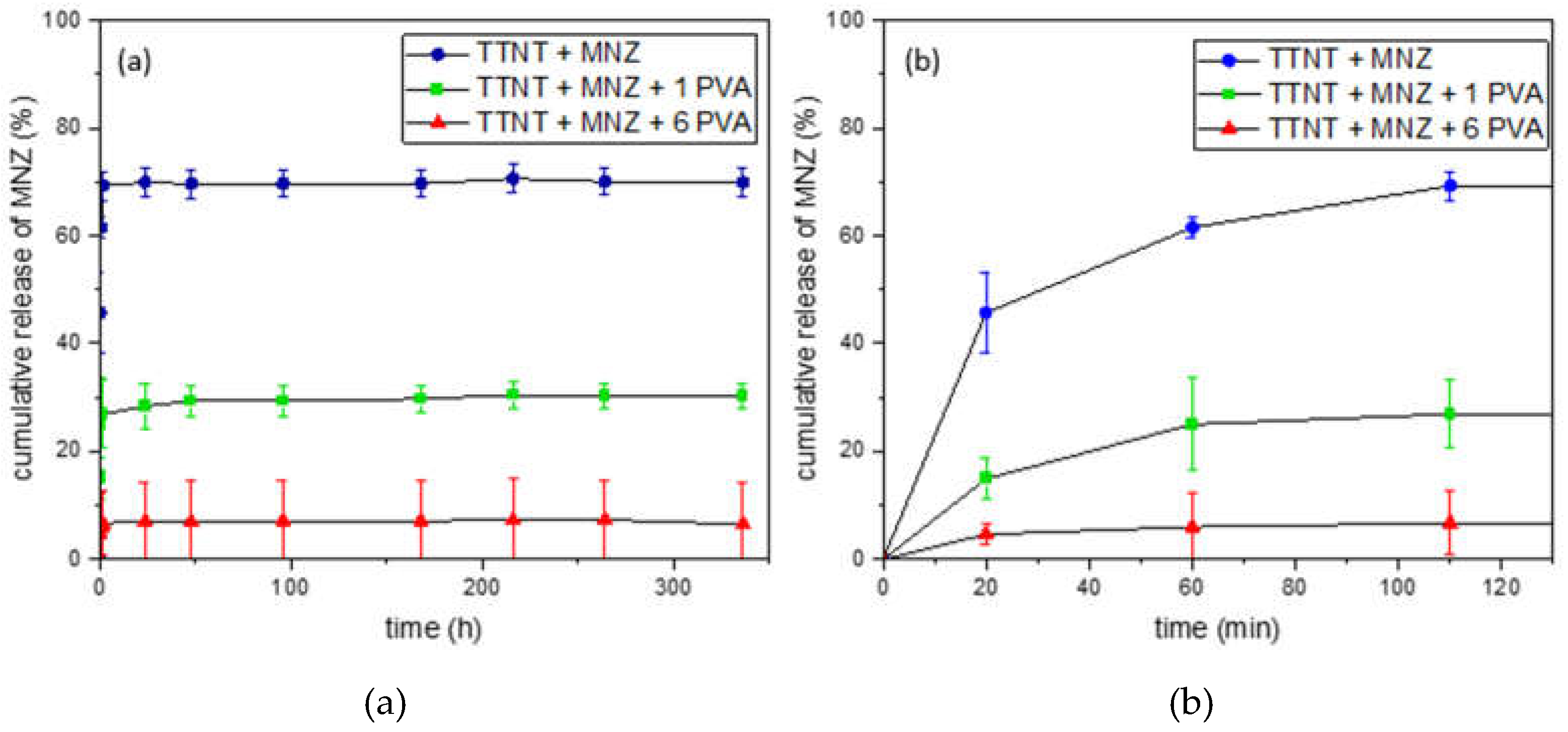
3.4. “In vitro” metronidazole release evaluation
3.5. Chemical composition evaluation after “in vitro” metronidazole release analysis
4. Conclusions
- (1)
- It did not change the surface treatment and consequently the properties of the material;
- (2)
- All groups reached the minimum inhibitory concentration to help fight bacteria and reduce the body's inflammatory response;
- (3)
- All groups allowed an immediate delivery of metronidazole, which could reduce implant complications during the early wound healing processes;
- (4)
- Although TTNT+MNZ showed higher percentage of antibiotic release within the studied groups, the PVA coating may absorb body fluids and water that provide distribution of MNZ throughout the wound surgery site, besides contributing to the hydration of the implant site, facilitating the wound healing;
- (5)
- The design with 1 layer of PVA (TTNT + MNZ + 1PVA) showed to be the best option, since it can combine the water absorption capacity of the PVA-coated regions with the higher bioactivity of titanate nanostructure exposed in the degraded regions of the coating. Nevertheless, the effect of the crosslinking degree of PVA on the kinetic release of metronidazole should be better investigated.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, H.; Ni, M.; Rui, Y.; Cheng, T.; Cheng, C.; Pan, X.; Li, G.; Lin, C. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J. Orthop. Transl. 2014, 2, 35–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.P.; Palmieri, A.; Carinci, F.; Bolfarini, C. Osteoblasts behavior on chemically treated commercially pure titanium surfaces. J. Biomed. Mater. Res. 2014, 102, 1816–1822. [Google Scholar] [CrossRef]
- Kang, B.; Lan, D.; Liu, L.; Dang, R.; Yao, C.; Liu, P.; Ma, F.; Qi, S.; Chen, X. Antibacterial Activity and Bioactivity of Zn-Doped TiO2 Coating for Implants. Coatings 2022, 12, 1264. [Google Scholar] [CrossRef]
- Eggert, F.; Levin, L. Biology of teeth and implants: The external environment, biology of structures, and clinical aspects. Quintessence Int. 2018, 49, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Leal, B.F.; Hubler, R.; de Oliveira, S.D.; Teixeira, E.R. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express 2019, 9. [Google Scholar] [CrossRef]
- Pecoraro, C.; Carbon, D.; Deng, D.; Cascioferro, S.M.; Diana, P.; Giovannetti, E. Biofilm Formation as Valuable Target to Fight against Severe Chronic Infections. Curr. Med. Chem. 2022, 29, 4307–4310. [Google Scholar] [CrossRef]
- Li, Z.; Xu, W.; Wang, X.; Jiang, W.; Ma, X.; Wang, F.; et al. Fabrication of PVA/PAAm IPN hydrogel with high adhesion and enhanced mechanical properties for body sensors and antibacterial activity. Eur. Polym. J. 2021, 146, 110253. [Google Scholar] [CrossRef]
- Ebhodaghe, O.S. Hydrogel – based biopolymers for regenerative medicine applications: a critical review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 155–172. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Muthuraj, M.S.A. Antimicrobial Susceptibility of Periodontopathogens. Clin. Dent. 2021, 9, 0974–3979. [Google Scholar]
- Dilley, M.; Geng, B. Immediate and delayed hypersensitivity reactions to antibiotics: aminoglycosides, clindamycin, linezolid, and metronidazole. Clin. Rev. Allergy Immunol. 2022, 62, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.N.; Rouze, R.; Quilty, B.; Alves, G.G.; Soares, G.D. A.; Thire, R.M.S.M.; McGuinness, G.B. Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interface Focus 2013, 4, 20130049–20130049. [Google Scholar] [CrossRef]
- Da Silva, M.A.C. ; Membranas bioreabsorvíveis de Poli(3- Hidroxibutirato) carregadas com metronidazol para regeneração tecidual guiada. Doctoral Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Anitha, V.C.; Banerjee, A.N.; Joo, S.W.; Min, B.K. Morphology-dependent low macroscopic field emission properties of titania/titanate nanorods synthesized by alkali-controlled hydrothermal treatment of a metallic Ti surface. Nanotechnology 2015, 26, 355705. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, V.K.; Popat, K.C. Hydrothermally treated titanium surfaces for enhanced osteogenic differentiation of adipose derived stem cells. Mater. Sci. Eng. C 2021, 128, 112315. [Google Scholar] [CrossRef]
- Kokubo, T.; Matsushita, T.; Takadama, H.; Kizuki, T. Development of bioactive materials based on surface chemistry. J. Eur. Ceram. Soc. 2009, 29, 1267–1274. [Google Scholar] [CrossRef]
- Huang, Y.Z.; He, S.K.; Guo, Z.J.; Pi, J.K.; Deng, L.; Dong, L.; et al. Nanostructured titanium surfaces fabricated by hydrothermal method: influence of alkali conditions on the osteogenic performance of implants. Mater. Sci. Eng. C 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Bright, R.; Hayles, A.; Wood, J.; Ninan, N.; Palms, D.; Visalakshan, R.M.; Burzava, A.; Brown, T.; Barker, D.; Vasilev, K. Bio-Inspired Nanostructured Ti-6Al-4V Alloy: The Role of Two Alkaline Etchants and the Hydrothermal Processing Duration on Antibacterial Activity. Nanomaterials 2022, 12, 1140. [Google Scholar] [CrossRef]
- Morgado, E.; De Abreu, M.A.S.; Pravia, O.R.C.; et al. A study on the structure and thermal stability of titanate nanotubes as a function of sodium content. Solid State Sci. 2006, 8, 888–900. [Google Scholar] [CrossRef]
- Scarano, A.; Rexhep, S.T.; Lorusso, F.; Leo, L. Wettability of implant surfaces: Blood vs autologous platelet liquid (APL). J. Mech. Behav. Biomed. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Kazemian, M.; Ghorbanzadeh, S. A brief overview of cellular and molecular mechanisms of osseointegration. Int. J. Contemp. Dent. Med. Rev. 2015, 010415. [Google Scholar]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration – communication of cells. Clin. Oral. Implants Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gao, Y.; Ma, Q.; Zhang, X.; Zhang, Y.; Song, W. Nanotopographical cues for regulation of macrophages and osteoclasts: emerging opportunities for osseointegration. J. Nanobiotechnology 2022, 20, 1–22. [Google Scholar] [CrossRef]
- Ferencík, M.; Rovensky, J.; Matha, V.; Herold, M. Kompendium der Immunologie: Grundlagen und Klinik; Springer-Verlag, 2006. [Google Scholar]
- Poulet, P.P.; Duffaut, D.; Lodter, J.P. Metronidazole susceptibility testing of anaerobic bacteria associated with periodontal disease. J. Clin. Periodontol. 1999, 26, 261–263. [Google Scholar] [CrossRef]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2(B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatments. J. Solid State Chem. 2005, 178, 2179–2185. [Google Scholar] [CrossRef]
- Xie, J.; Wang, X.; Zhou, Y. Understanding Formation Mechanism of Titanate Nanowires through Hydrothermal Treatment of Various Ti-Containing Precursors in Basic Solutions. J. Mater. Sci. Technol. 2012, 28, 488–494. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Ni, M.; Rui, Y.; Cheng, T.-Y.; Cheng, C.-K.; Lin, C. Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J. Orthop. Transl. 2014, 2, 35–42. [Google Scholar] [CrossRef]
- Zainuddin Hill, D.J.T.; Le, T.T. An ESR study on γ-irradiated poly(vinyl alcohol). Radiat. Phys. Chem. 2001, 62, 283–291. [Google Scholar] [CrossRef]
- Herculano, R.D.; Guimarães, S.A.C.; Belmonte, G.C.; Duarte, M.A.H.; Oliveira Júnior, O.N.; de Kinoshita, A.; de Graeff, C.F.O. Metronidazole release using natural rubber latex as matrix. Mater. Res. 2010, 13, 57–61. [Google Scholar] [CrossRef]
- Brako, F.; Luo, C.; Matharu, R.K.; Ciric, L.; Harker, A.; Edirisinghe, M.; Craig, D.Q.M. A Portable Device for the Generation of Drug-Loaded Three-Compartmental Fibers Containing Metronidazole and Iodine for Topical Application. Pharmaceutics 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to spectroscopy. Cengage Learn. 2014.


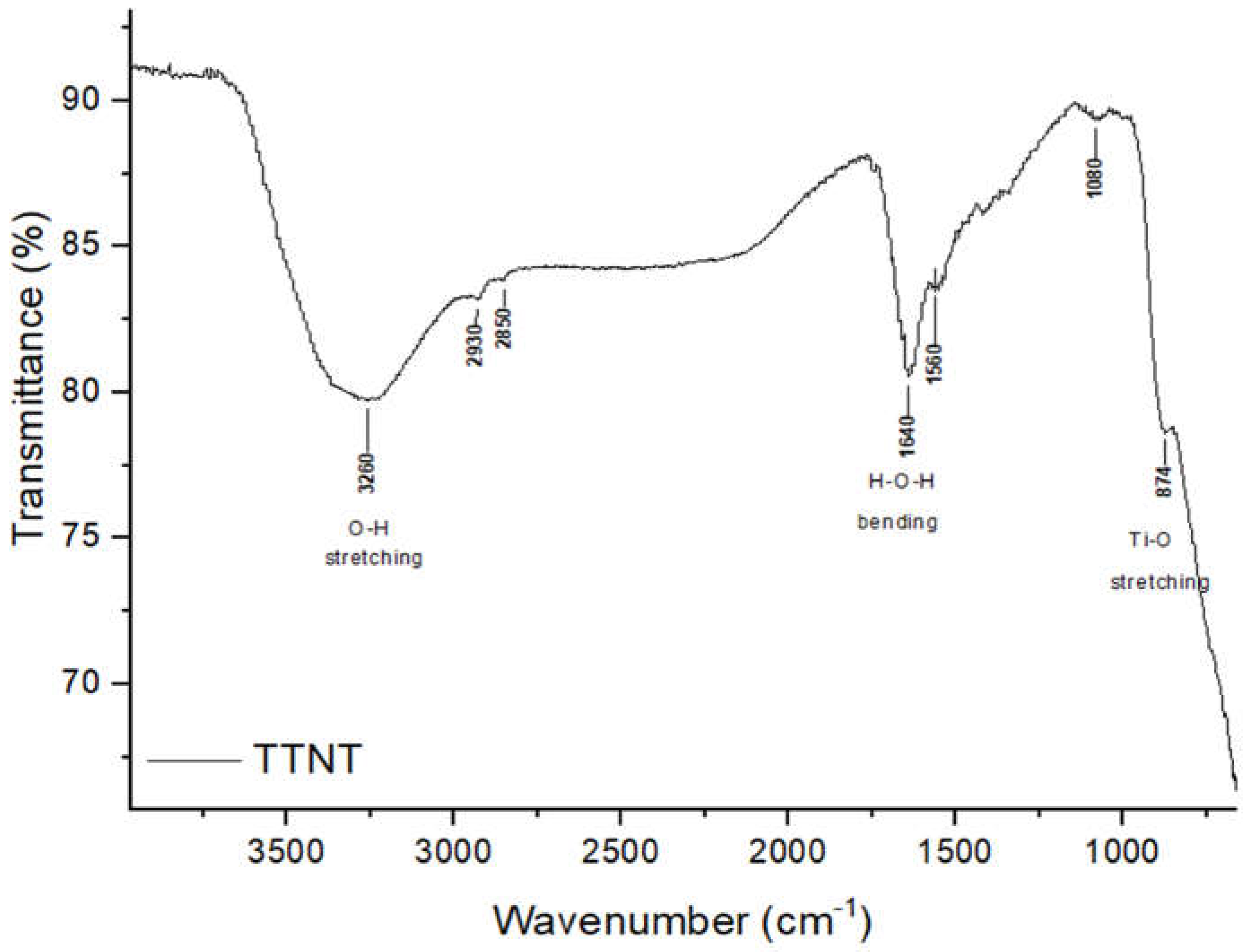
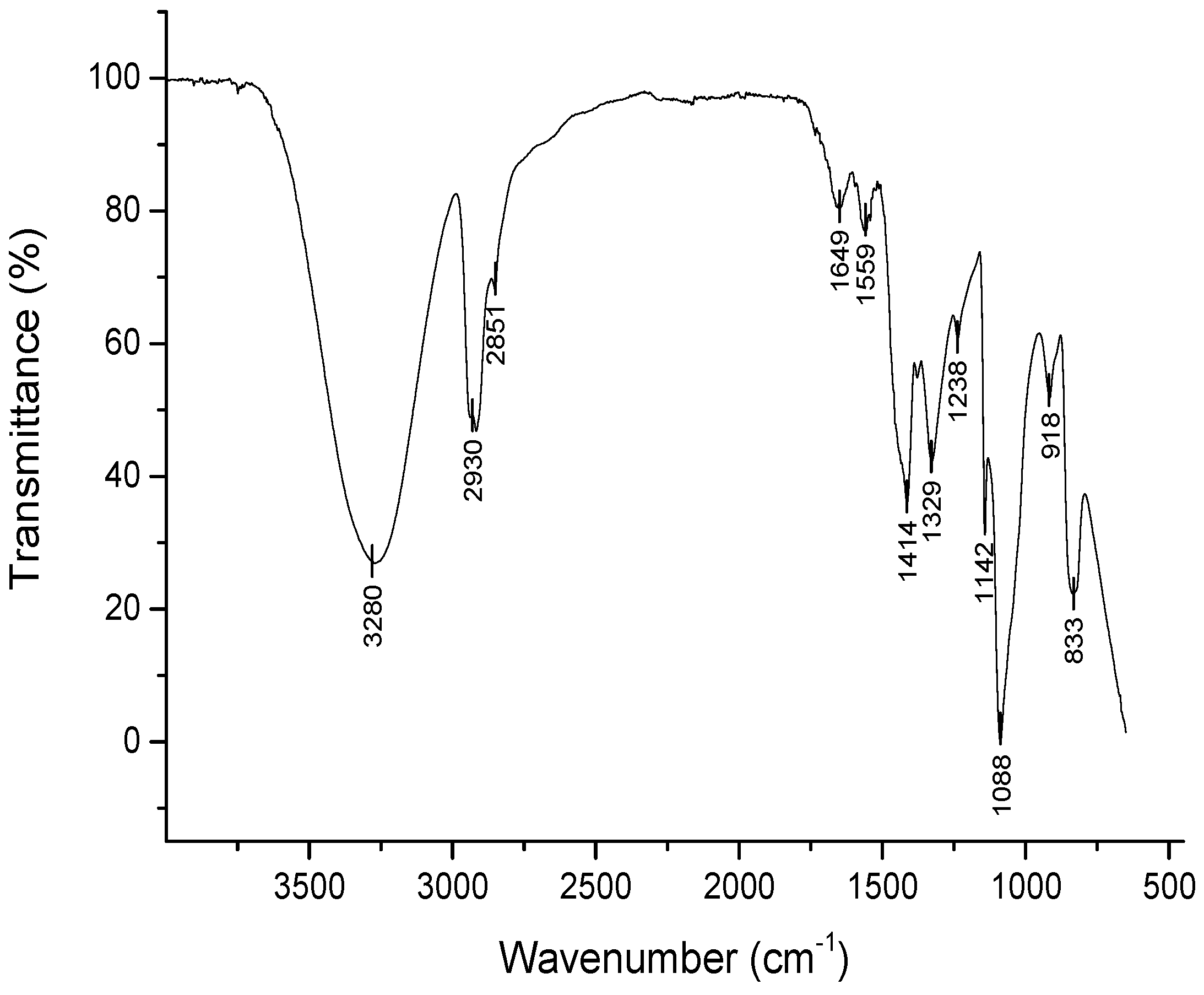
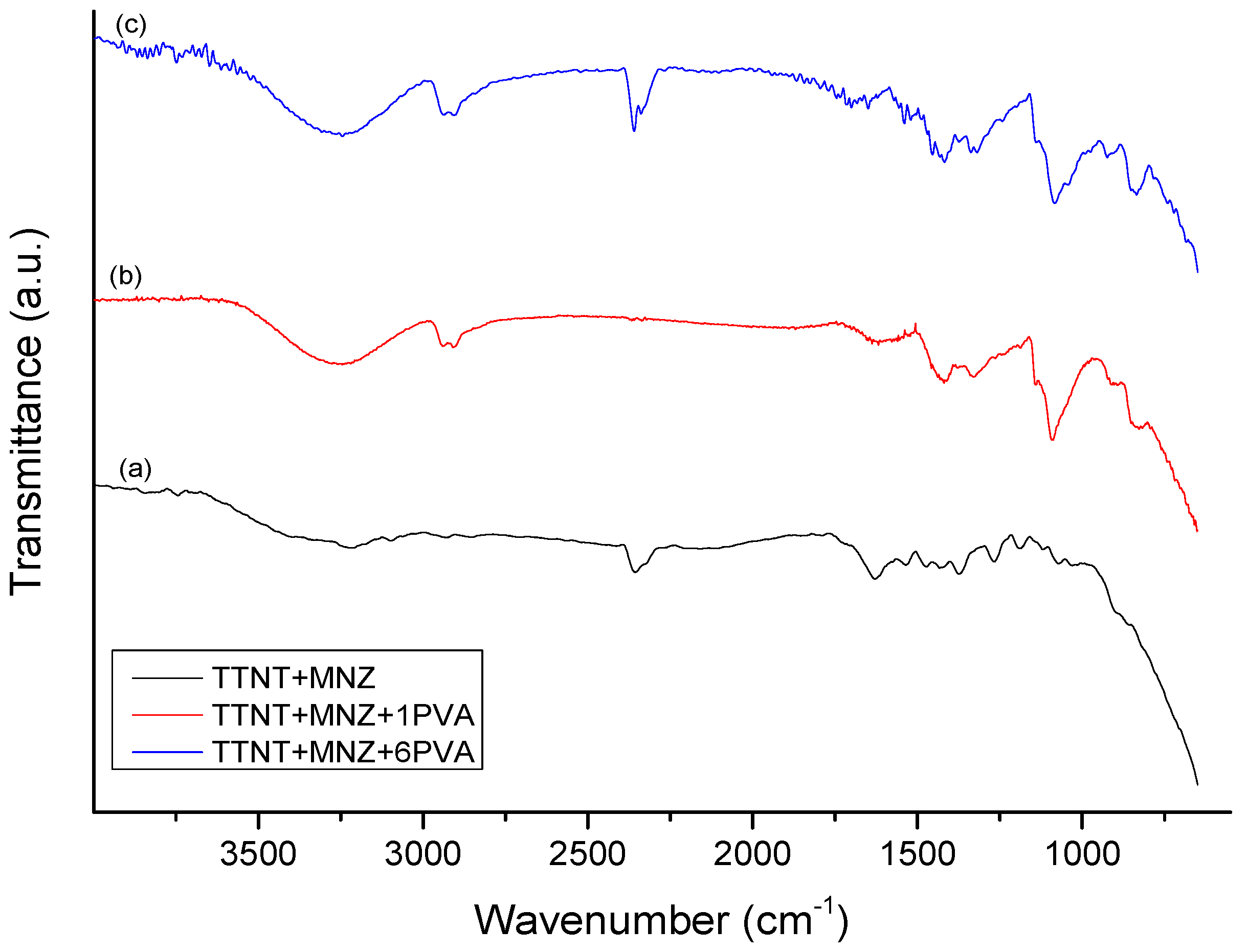
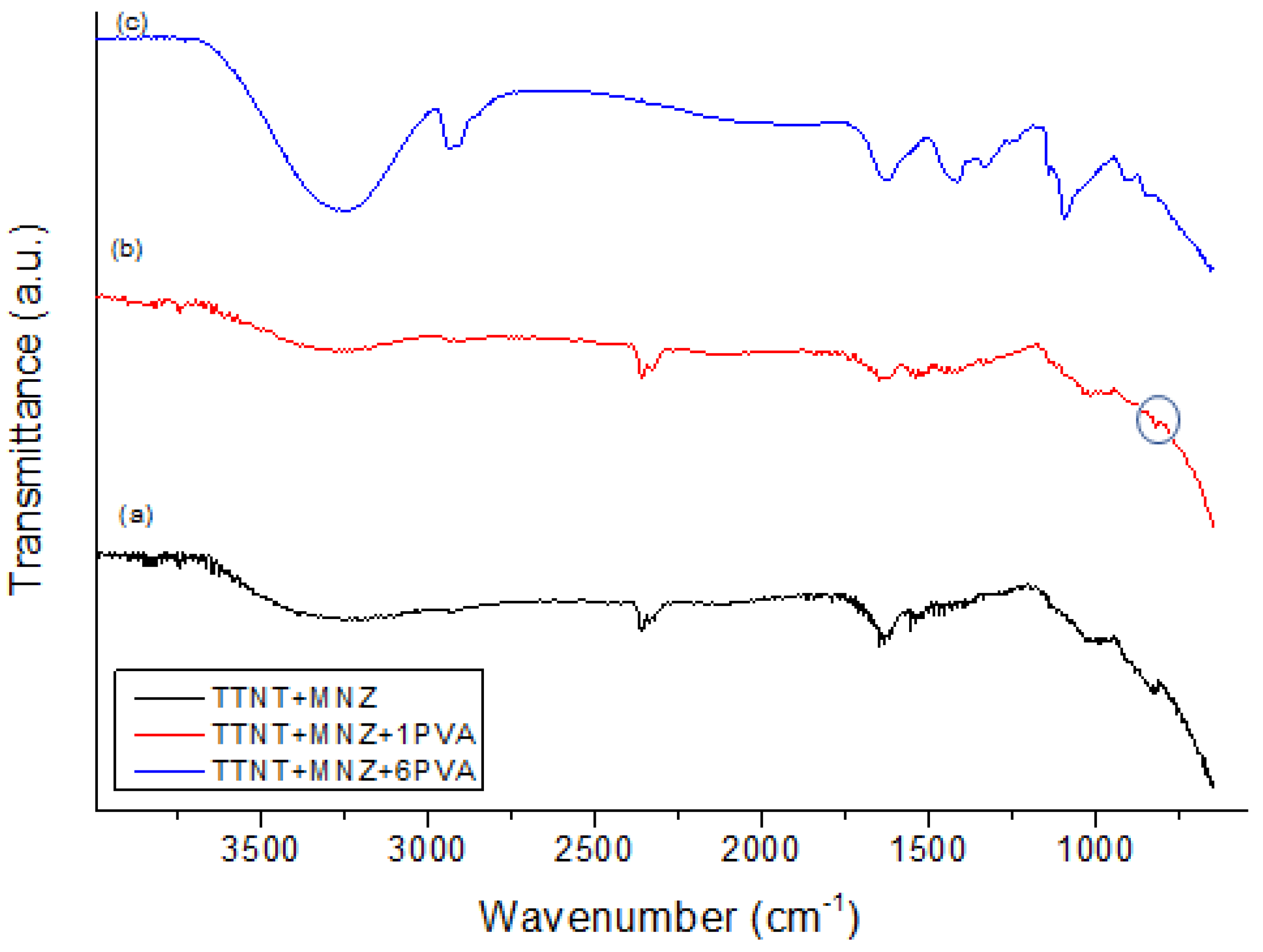
| Groups | Description |
|---|---|
| TTNT | Titanate nanostructures on Ti disk surface via hydrothermal synthesis |
| TTNT+MNZ | Titanate nanostructures on Ti disk surface via hydrothermal synthesis + Metronidazole |
| TTNT+MNZ+1PVA | Titanate nanostructures on Ti disk surface via hydrothermal synthesis + Metronidazole + 1 layer of irradiated PVA film |
| TTNT+MNZ+6PVA | Titanate nanostructures on Ti disk surface via hydrothermal synthesis + Metronidazole + 6 layers of irradiated PVA film |
| % of MNZ released | 20 min | 60 min | 110 min | 24 h | 48 h |
|---|---|---|---|---|---|
| TTNT+MNZ | 45.7cA | 61.5bA | 69.3aA | 69.9aA | 69.6aA |
| TTNT+MNZ+1PVA | 14.9bB | 25.0aB | 26.9aB | 28.4aB | 29.3aB |
| TTNT+MNZ+6PVA | 4.66aC | 6.01aC | 6.72aC | 7.01aC | 7.02aC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





