1. Introduction
Infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for millions of confirmed coronavirus disease 2019 (COVID-19) cases worldwide. Several studies have shown that a significant number of people affected by COVID-19 have persistent symptomatology, even 4 weeks after the onset of acute symptoms. This scenario is commonly referred to as long COVID, in which most patients experience a wide range of symptoms, indicating multi-organ involvement [
1,
2,
3,
4].
Among the various clinical and laboratory manifestations of the disease described throughout the COVID-19 pandemic, investigations of the haematological profile have proven important in the risk assessment of severe cases of COVID-19. Hypercoagulable state and changes in platelet, leucocyte, and erythrocyte counts are found in patients who develop an unfavourable evolution of the disease, indicating a poor prognosis and that these should be observed carefully [
3,
5,
6].
Studies indicate that the haematological profile can remain altered in the long COVID phase [
5,
6]; however, the literature is still scarce in longer-lasting investigations, such as in long COVID for up to 1 year, and even more so in the Amazon region. Therefore, this study aimed to evaluate haematological laboratory markers, linking them to clinical findings and long-term outcomes in patients with long COVID. Our results suggest a potential compensatory mechanism for erythrogram-related markers within up to 985 days of long COVID. Increased levels of leukogram-related markers and increased coagulation activity were also observed in the worse long COVID groups.
2. Materials and Methods
This was an observational, cross-sectional, prospective study approved by the Ethics Committee for Research Involving Human Beings of the State University of Pará (opinion 4.252.664). This study followed the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology guidelines supporting the construction of this article. Written consent was obtained from all participants.
Adults (aged ≥18 years) of both sexes enrolled in a clinical care programme for patients with long COVID, conducted in the Amazon region, Brazil, after an open call. Between March 2020 and June 2022, a total of 260 patients voluntarily enrolled in the programme. Diagnosis of long COVID was carried out using the following criteria: (a) confirmation of acute symptomatic infection by SARS-CoV-2 by real-time polymerase chain reaction amplification – acute symptoms needed to be consistent with COVID-19 and not attributable to any other cause – and (b) presence of at least one prolonged symptom of COVID-19 (post-acute COVID-19) that cannot be assigned to another cause, such as fatigue, dyspnoea, cough, chest pain, muscle pain or weakness, headache, insomnia, visual disturbances, tremor, loss of balance, lower limb oedema, arthralgia, palate, and/or olfactory disorders, for at least 4 weeks (28 days) past the acute onset of symptoms.
None of the included patients had any clinical infectious conditions that could confuse the interpretation of the variables studied here. In vaccinated patients, the time interval from the time of the last vaccination to the collection of blood samples ranged from 49 to 377 days. Thus, the 260 patients were divided into the following groups: ‘hospitalisation in the acute phase’; ‘long COVID period’; and ‘number of long COVID symptoms’, which allowed comparisons, correlations, and associations to be carried out in the studied population (
Figure 1).
The first stage of the collection consisted of venepuncture for blood collection, with the patient fasting for at least 8 h, being collected 3 mL of blood in two Vacuette® tubes (Greiner Bio-One, Kremsmünster, Austria): (a) tube with ethylenediaminetetraacetic acid (EDTA) anticoagulant, for whole-blood analysis, which consisted of the quantification of red blood cells (RBCs), haemoglobin, haematocrit, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red cell distribution width (RDW), erythrocyte sedimentation rate (ESR), white blood cells (WBCs), neutrophils, eosinophils, basophils, monocytes, lymphocytes, platelets, mean platelet volume, and plateletcrit and platelet distribution width (PDW); and (b) tube with sodium citrate for coagulation analysis, including prothrombin time (PT), PT activity, and activated partial thromboplastin time. The normal reference ranges for the aforementioned examinations were adopted from the respective reagent manufacturers (
Supplementary Table S1). The second collection stage, which occurred within a maximum of 24 hours after the first stage, consisted of face-to-face interviews to collect clinical and baseline demographic data of the participants, such as sex, age, presence of comorbidities or concomitant infections, date of acute onset of symptoms, self-reported long COVID symptomatology, medications used, hospital admission in the COVID-19 acute phase, and length of stay.
Whole blood samples in tubes with EDTA were analysed using the Zybio Z3 haematological analyser (Zybio Inc., Chongqing, China), using the reagent line for haematological tests from Labtest (Lagoa Santa, Brazil). After 1 h of rest, the ESR was also determined in whole blood samples. The sodium citrate tube was centrifuged at 3,000 rpm for 5 min (Daiki 80-2B centrifuge, Ionlab, Araucária, Brazil), followed by the determination of clotting parameters in the HumaClot Junior coagulation analyser, with its own reagent kit line for haemostasis tests (HUMAN, Wiesbaden, Germany).
GraphPad Prism™ software version 8.4.3 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. The D’Agostino–Pearson test was used to assess data normality. The mean and standard deviation (SD) were the dispersion measures used to describe continuous variables. Analysis of variance was used to compare variables with a normal distribution, and the Mann–Whitney U test was performed to compare variables with non-normal distribution. The chi-square test was used to compare the categorical variables. Associations between risk variables and long COVID outcomes were assessed using multiple logistic regression analysis. In addition, linear correlation using Pearson’s coefficient was used to evaluate correlations between the acute hospitalisation period and leucocyte series count in long COVID. Statistical significance was defined as a two-tailed P-value of <0.05.
3. Results
Of the 260 selected patients, the majority were female (n=166) and not elderly (n=198), with 34.2% being hospitalised in COVID-19 acute phase (n=89). Fatigue (n=181), dyspnoea (n=176), and muscle weakness (n=159) were the most self-reported long COVID symptoms. The mean long COVID period was 308.1 days (SD, 171.5) with a mean of approximately six concomitant symptoms (mean ± SD, 6.1 ± 3.3). The longest reported long COVID period was 985 days. Most of the mean laboratory levels were within the reference ranges; however, the mean ESR levels increased, and the mean PDW levels were below the minimum adopted threshold (
Table 1 and
Supplementary Table S1). Only four patients (1.5%) presented with thrombocytopenia (platelet count, <150,000 thousand/mm3).
Values are expressed as the mean ± standard deviation (SD). (a) Heart, kidney, liver, or neurological diseases; (b) n=89; (c) shown for ≥4 weeks from symptom onset. RBCs, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; RDW, red cell distribution width; ESR, erythrocyte sedimentation rate; WBCs, white blood cells; MPV, mean platelet volume; PDW, platelet distribution width; PT, prothrombin time; aPTT, activated partial thromboplastin time.
Hospitalised patients in the acute COVID-19 phase presented with increased levels of RBCs, WBCs, eosinophils, lymphocytes, and platelets and increased percentages of RDW and plateletcrit compared with non-hospitalised patients (n=171). In patients with up to 90 days of long COVID (n=31), the MCV, MCH, and MCHC levels were lower than those in patients with long COVID for >90 days (n=229), which can also be observed when comparing patients with up to 180 days (n=60) and those with >180 days of long COVID (n=200). In contrast, patients with up to 365 days of long COVID (n=172) presented with increased levels of MCV, MCH, and MCHC. The RBC count and RDW percentages increased in patients with up to 90 and 180 days of long COVID. Patients with more than six concomitant symptoms (n=119) presented with increased levels of WBCs, neutrophils, and lymphocytes, shorter PT, and higher PT activity (
Table 2).
Shorter periods of long COVID, such as up to 90 or 365 days, were associated with female sex, acute hospitalisation, and an increased platelet count. On the other hand, having long COVID for >1 year was associated with a higher lymphocyte count and low haemoglobin levels. Female sex and acute hospitalisation were associated with more than six concomitant symptoms (
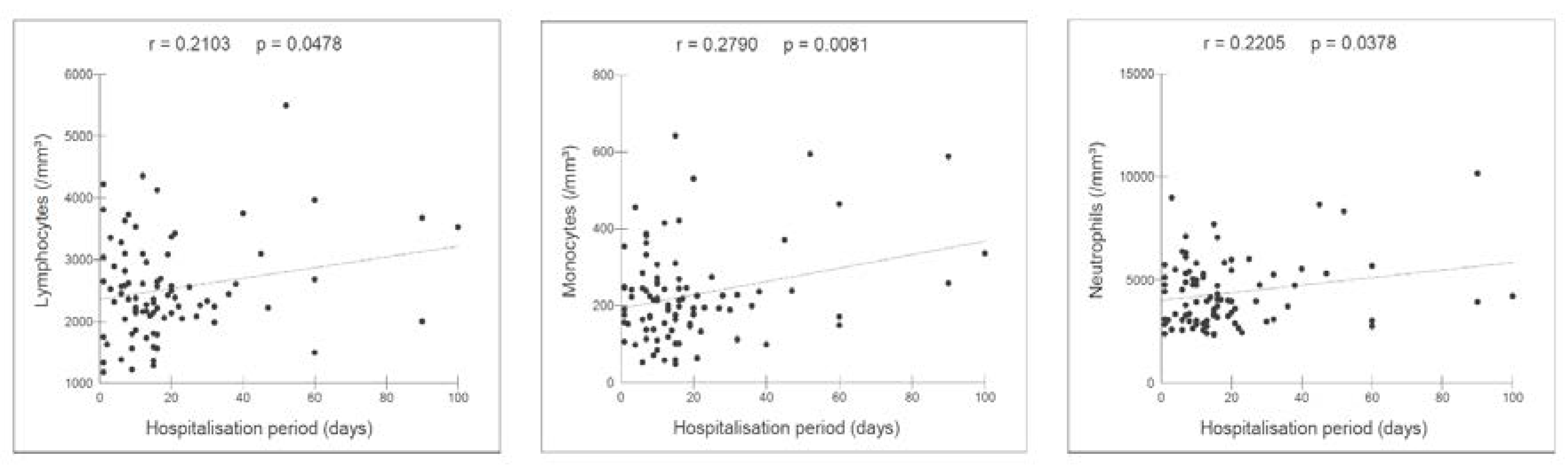
Table 3). In hospitalised patients (n=89), the neutrophil, monocyte, and lymphocyte levels were correlated with the hospitalisation period (
Figure 2).
4. Discussion
Our main findings suggest that the study population had, on average, a positive recovery in the haematological profile, even if symptoms of long COVID were reported for up to 985 days. Among the 89 hospitalised patients, there were higher mean levels of red/white cells and platelets and percentages of plateletcrit and RDW. In addition, haematimetric parameters, such as MCV, MCH, and MCHC, were higher in shorter periods of long COVID. Patients presenting with more than six concomitant long COVID symptoms had a higher WBC count, lower PT, and increased PT activity. More than 1 year of long COVID was associated with low haemoglobin levels and increased lymphocyte counts.
Table 2.
Comparison of the haematological levels and clotting parameters in the long COVID outcome groups.
Table 2.
Comparison of the haematological levels and clotting parameters in the long COVID outcome groups.
| Variable |
Hospitalised in acute phase |
Long COVID period |
Number of long COVID symptoms |
| Yes |
No |
p* |
≤90 days |
>90 days |
p* |
≤180 days |
>180 days |
p* |
≤365 days |
>365 days |
p* |
≤6 |
>6 |
p* |
| RBCs, mean ± SD, millions/mm³ |
4.8 ± 0.6 |
4.6 ± 0.6 |
0.0179 |
4.9 ± 0.5 |
4.6 ± 0.6 |
0.0104 |
4.8 ± 0.6 |
4.6 ± 0.6 |
0.0175 |
4.6 ± 0.6 |
4.7 ± 0.6 |
0.0943 |
4.6 ± 0.6 |
4.7 ± 0.6 |
0.7387 |
| Haemoglobin, mean ± SD, g/dL |
13.3 ± 1.2 |
13.1 ± 1.3 |
0.1644 |
13.3 ± 1.5 |
13.1 ± 1.3 |
0.6262 |
13.2 ± 1.3 |
13.1 ± 1.3 |
0.4144 |
13.2 ± 1.2 |
13 ± 1.4 |
0.2598 |
13.2 ± 1.4 |
13.1 ± 1.2 |
0.5828 |
| Haematocrit, mean ± SD, % |
39.5 ± 4 |
39 ± 4.1 |
0.6307 |
40.2 ± 3.6 |
39.1 ± 4.2 |
0.1340 |
39.8 ± 4.1 |
39 ± 4.1 |
0.2023 |
39.3 ± 4.1 |
39.1 ± 4.1 |
0.7379 |
39.3 ± 4.3 |
39 ± 3.8 |
0.5742 |
| MCV, mean ± SD, fL |
83.3 ± 8.5 |
84.3 ± 9.2 |
0.0502 |
81.8 ± 5.7 |
84.3 ± 9.3 |
0.0160 |
82.6 ± 6.5 |
84.4 ± 9.5 |
0.0317 |
85.2 ± 7.5 |
81.5 ± 10.9 |
0.0018 |
84.9 ± 8.3 |
82.8 ± 9.5 |
0.0849 |
| MCH, mean ± SD, pg |
28.1 ± 3.8 |
28.4 ± 3.1 |
0.0601 |
27.2 ± 2.2 |
28.5 ± 3.4 |
0.0053 |
27.6 ± 2.8 |
28.5 ± 3.5 |
0.0123 |
28.8 ± 3.2 |
27.4 ± 3.4 |
0.0019 |
28.6 ± 3.4 |
28 ± 3.2 |
0.0548 |
| MCHC, mean ± SD, % |
33.6 ± 1.3 |
33.5 ± 1.2 |
0.4145 |
33 ± 0.8 |
33.6 ± 1.2 |
0.0023 |
33.3 ± 1.1 |
33.6 ± 1.2 |
0.0150 |
33.7 ± 1.3 |
33.3 ± 1.1 |
0.0270 |
33.6 ± 1.3 |
33.5 ± 1.1 |
0.5651 |
| RDW, mean ± SD, % |
14.1 ± 1.1 |
13.4 ± 1.4 |
<0.0001 |
14.2 ± 0.8 |
13.6 ± 1.4 |
<0.0001 |
14.1 ± 1 |
13.5 ± 1.4 |
<0.0001 |
13.6 ± 1.4 |
13.7 ± 1.2 |
0.8452 |
13.7 ± 1.6 |
13.6 ± 1 |
0.2541 |
| ESR, mean ± SD, mm |
41.6 ± 28.4 |
39.8 ± 25.3 |
0.8307 |
44.9 ± 22.9 |
39.8 ± 26.8 |
0.1164 |
44.9 ± 28.8 |
39 ± 25.5 |
0.1614 |
40 ± 24.8 |
41.2 ± 29.3 |
0.8974 |
41.4 ± 27.5 |
39.2 ± 25 |
0.6760 |
| WBCs, mean ± SD, thousands/mm³ |
7.2 ± 2.2 |
6.6 ± 2.1 |
0.0179 |
6.6 ± 1.6 |
6.9 ± 2.2 |
0.7055 |
6.9 ± 2.2 |
6.8 ± 2.1 |
0.8166 |
6.8 ± 1.9 |
6.9 ± 2.5 |
0.7137 |
6.5 ± 2.1 |
7.2 ± 2.2 |
0.0083 |
| Neutrophils, mean ± SD, thousands/mm³ |
4.3 ± 1.6 |
4 ± 1.4 |
0.2369 |
3.9 ± 1.1 |
4.1 ± 1.5 |
0.6755 |
4.2 ± 1.5 |
4.1 ± 1.4 |
0.6646 |
4.1 ± 1.3 |
4 ± 1.7 |
0.3108 |
3.9 ± 1.3 |
4.3 ± 1.6 |
0.0356 |
| Eosinophils, mean ± SD, /mm³ |
232.7 ± 105.7 |
214 ± 125.7 |
0.0366 |
261.4 ± 85.6 |
214.9 ± 122.3 |
0.0017 |
232.6 ± 108.2 |
216.8 ± 122.5 |
0.1303 |
228.3 ± 124.1 |
205.1 ± 108.6 |
0.1653 |
216.5 ± 116.7 |
225.1 ± 122.8 |
0.6985 |
| Basophils, mean ± SD, /mm³ |
28.4 ± 34.4 |
31.8 ± 38.4 |
0.4869 |
37 ± 36.3 |
29.7 ± 37.1 |
0.2224 |
29.3 ± 33.6 |
31 ± 38.1 |
0.9104 |
33.6 ± 36.3 |
24.8 ± 38 |
0.0268 |
29.3 ± 37 |
32.1 ± 37.2 |
0.4484 |
| Monocytes, mean ± SD, /mm³ |
225.7 ± 121.3 |
200.9 ± 104.7 |
0.0938 |
164.4± 76.3 |
215.5 ± 113.7 |
0.0130 |
213.8 ± 142.8 |
208.1 ± 100 |
0.5884 |
204.6 ± 116.2 |
218.7 ± 100.3 |
0.0729 |
193.7 ± 89.2 |
228 ± 130.3 |
0.0703 |
| Lymphocytes, mean ± SD, thousands/mm³ |
2.5 ± 0.7 |
2.2 ± 1 |
<0.0001 |
2.2 ± 0.6 |
2.3 ± 1 |
0.4582 |
2.2 ± 0.8 |
2.3 ± 1 |
0.6933 |
2.2 ± 0.8 |
2.4 ± 1.2 |
0.1387 |
2.2 ± 1.1 |
2.4 ± 0.8 |
0.0281 |
| Platelets, mean ± SD, thousands/mm³ |
320 ± 101.1 |
293.6 ± 78.8 |
0.0486 |
339 ± 98.8 |
297.7 ± 85.2 |
0.0225 |
313.3 ± 98.5 |
299.4 ± 84.3 |
0.4683 |
295.7 ± 82.4 |
316.2 ± 96.4 |
0.1260 |
297.6 ± 87.4 |
308.6 ± 88.2 |
0.1854 |
| MPV, mean ± SD, fL |
8.5 ± 0.8 |
8.6 ± 1.1 |
0.7327 |
8.5 ± 0.9 |
8.5 ± 1 |
0.6287 |
8.5 ± 0.8 |
8.5 ± 1.1 |
0.7758 |
8.5 ± 1 |
8.5 ± 1 |
0.6898 |
8.6 ± 1 |
8.5 ± 1 |
0.3808 |
| Plateletcrit, mean ± SD, % |
0.270 ± 0.079 |
0.249 ± 0.068 |
0.0244 |
0.290 ± 0.091 |
0.251 ± 0.069 |
0.0151 |
0.267 ± 0.086 |
0.252 ± 0.068 |
0.3336 |
0.251 ± 0.073 |
0.266 ± 0.071 |
0.1437 |
0.254 ± 0.072 |
0.259 ± 0.073 |
0.4371 |
| PDW, mean ± SD, % |
16.1 ± 0.7 |
15.9 ± 7.9 |
0.0819 |
16.1 ± 0.3 |
16 ± 6.8 |
0.3911 |
15.8 ± 1.3 |
16 ± 7.3 |
0.4211 |
15.4 ± 1.8 |
17 ± 10.7 |
0.8994 |
16.4 ± 8.6 |
15.5 ± 1.6 |
0.1396 |
| PT, mean ± SD, s |
12.3 ± 1 |
12.4 ± 1.2 |
0.2488 |
12.6 ± 1.1 |
12.3 ± 1.1 |
0.1864 |
12.4 ± 1.1 |
12.3 ± 1.1 |
0.7004 |
12.3 ± 1.2 |
12.4 ± 0.9 |
0.7998 |
12.4 ± 1.1 |
12.2 ± 1.1 |
0.0380 |
| PT activity, mean ± SD, % |
96.3 ± 19.2 |
95.9 ± 24.7 |
0.2439 |
91.5 ± 18.5 |
96.7 ± 23.5 |
0.3222 |
95.1 ± 19.5 |
96.4 ± 23.9 |
0.8425 |
97.2 ± 24.7 |
93.9 ± 19 |
0.7544 |
94.6 ± 23.9 |
97.8 ± 21.8 |
0.0443 |
| aPTT, mean ± SD, s |
29.5 ± 4.9 |
30.6 ± 6.1 |
0.1334 |
29.6 ± 6.4 |
30.3 ± 5.6 |
0.5338 |
29.6 ± 5.4 |
30.4 ± 5.8 |
0.3875 |
30.3 ± 5.4 |
30.1 ± 6.2 |
0.9229 |
30.3 ± 4.5 |
30.1 ± 6.9 |
0.4766 |
| Total, n (%) |
89 (34.2) |
171 (65.7) |
- |
31 (11.9) |
229 (88) |
- |
60 (23) |
200 (76) |
- |
172 (66.1) |
88 (33.8) |
- |
141 (54.2) |
119 (45.7) |
- |
Table 3.
Association between the haematological and clotting abnormalities and long COVID outcomes.
Table 3.
Association between the haematological and clotting abnormalities and long COVID outcomes.
| Variables |
Long COVID outcomes |
| Long COVID period >90 days (n=229) |
Long COVID period >365 days (n=88) |
Number of long COVID symptoms >6 (n=119) |
| Coefficient |
p-value |
Odds ratio |
Coefficient |
p-value |
Odds ratio |
Coefficient |
p-value |
Odds ratio |
| Female gender |
0.9822 |
0.0337 |
2.6703 |
-0.4808 |
0.1180 |
0.6183 |
0.7387 |
0.0179 |
2.0931 |
| Age ≥60 years |
-0.3446 |
0.4360 |
0.7085 |
-0.3289 |
0.3320 |
0.7197 |
-0.6762 |
0.0384 |
0.5086 |
| Hospitalisation in acute phase |
-0.9319 |
0.0395 |
0.3938 |
-0.6692 |
0.0399 |
0.5121 |
1.2739 |
< 0.0001 |
3.5748 |
| Long COVID period, ≤90 days |
- |
- |
- |
- |
- |
- |
-0.1852 |
0.6724 |
0.8310 |
| RBCs <4 million/mm3
|
1.8704 |
0.0906 |
6.4909 |
-0.7894 |
0.1112 |
0.4541 |
-0.1674 |
0.6939 |
0.8459 |
| Haemoglobin <12 g/dL |
-0.2671 |
0.6735 |
0.7656 |
1.0673 |
0.0057 |
2.9076 |
-0.2053 |
0.5918 |
0.8144 |
| Neutrophils >5 thousands/mm3
|
-0.0267 |
0.9585 |
0.9737 |
-0.6306 |
0.0718 |
0.5322 |
0.4955 |
0.1261 |
1.6414 |
| Lymphocytes >2,5 thousands/mm3
|
1.1104 |
0.0508 |
3.0355 |
0.6254 |
0.0495 |
1.8690 |
0.0559 |
0.8567 |
1.0575 |
| Platelets >450 thousands/mm3
|
-2.1664 |
0.0012 |
0.1146 |
0.3219 |
0.5889 |
1.3797 |
-0.3558 |
0.5662 |
0.7006 |
Evidence indicates that thrombocytopenia and anaemia influence the severity of acute COVID-19 [
7,
8,
9], increasing the risk of fatal outcomes. Furthermore, immune thrombocytopenia was reported 4 weeks after the onset of COVID-19 symptoms, even if limited to 1-week maximum [
5]. In contrast, in the present study, patients who were hospitalised during the acute phase of COVID-19, which can characterise a more severe acute involvement, presented with higher levels of haematological markers in the long COVID phase than those who were not hospitalised. This suggests homeostatic compensation in the convalescence phase after hospital discharge, which is further corroborated by the haematimetric parameters that tend to increase over the months, at least until the first year of long COVID, and with a higher RBC count and RDW percentages in lower periods of long COVID.
In acute COVID-19, leucocytosis and lymphopenia are frequent findings [
10,
11], making the WBC count indicative of severity, whereas the normalisation of lymphocyte levels suggests convalescence [
12,
13,
14]. Here, in both worse outcome groups, patients with more than six simultaneous long COVID symptoms, and patients hospitalised in the acute phase, an increase in WBC count was observed, with higher levels mainly of lymphocytes, in addition to increased coagulation activity, when compared with its counter groups. In the same way that the red series behaved in this study, this increase suggests a response that exacerbates the effects of the acute disturbance emerging in the long COVID phase [
6]; however, further investigations are needed.
This study has several limitations. Having additional study groups, such as the control group of patients without long-term symptoms, would allow interesting comparisons regarding the haematological status of patients with long COVID. Furthermore, assessing the patient’s haematological profile at the time of the COVID-19 acute phase could indicate the course of the haematological implications of long COVID, which was not performed in this study. However, to our knowledge, this is the first study to provide clues about the haematological condition of patients with prolonged symptoms of COVID-19 long after the acute phase, demonstrating profiles of important markers in determining the severity of the involvement of SARS-CoV-2 within up to 985 days prior to the onset of symptoms.
Analysing recognisably impactful severity-predictor markers of COVID-19 in post-acute scenarios is extremely important to identify possible long-term changes in the haematological system. Therefore, observing abnormalities in screening markers, such as those investigated here, is an important strategy to identify more severe haematological diseases in the future, long after the acute SARS-CoV-2 infection. Moreover, relating the profile of these markers to clinical outcomes in patients with long COVID provides a general overview for future studies that aim to build a risk stratification for patients with several months of long COVID.
5. Conclusions
We evaluated how the laboratory haematological levels behave in patients presenting with long-term COVID-19 symptoms for up to 985 days. Hospitalised patients presented with increased levels of haematological parameters, such as higher mean levels of RBCs and platelets, which suggests a benign compensation after the acute COVID-19 phase. In the groups with worse long COVID outcomes, such as the hospitalisation group itself, or the group with more simultaneous symptoms, increased levels of WBCs and lymphocytes and a shorter PT were observed, which can also indicate an exacerbated response to acute involvement; however, it is uncertain. These findings provide an interesting insight into the haematological profile of long COVID while promoting reflection for future studies. It is suggested that future investigations address the issue of increased leucocyte and lymphocyte levels, as well as increased coagulation activity, even 4 weeks after the onset of symptoms. In addition, follow-up studies of long COVID are important, as they would help to better visualise long-term haematological changes.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Adopted reference ranges for laboratory exams.
Author Contributions
Conceptualization, V.G, D.M and L.F.; methodology, V.G, D.M, L.F and P.L; software, D.M; validation, L.F and P.L.; formal analysis and investigation, V.G and D.M.; resources, L.F.; data curation, V.G, D.M and L.F.; writing—original draft preparation, V.G, D.M. and L.F.; writing—review and editing, V.G, D.M., P.L., V.P., P.V., J.Q and L.F.; visualization, supervision and project administration, V.P., P.V., J.Q and L.F.; funding acquisition, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the AMAZON FOUNDATION FOR RESEARCH SUPPORT (FAPESPA), grant number #006/2020, the SECRETARY OF SCIENCE, TECHNOLOGY, AND HIGHER, PROFESSIONAL AND TECHNOLOGICAL EDUCATION (SECTET), grant number #09/2021, the COORDENAÇÃO DE APERFEIÇOAMENTO DE PESSOAL DE NÍVEL SUPERIOR – Brazil (CAPES), grant number “Notice n° 13/2020” and the CONSELHO NACIONAL DE DESENVOLVIMENTO CIENTÍFICO E TECNOLÓGICO – Brazil, grant number “INCT: 406360/2022-7”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the STATE UNIVERSITY OF PARÁ (protocol code No. 4.252.664 / September 1, 2020) for studies involving humans.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, L.F. The data are not publicly available due to containing information that could compromise the privacy of research participants.
Acknowledgments
The authors would like to thank the support of Federal University of Pará for this publication through the PROPESP/UFPA (PAPQ - Programa de Apoio a Publicação Qualificada).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Á Steig, B.; Gaini, S.; et al. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin Infect Dis 2021, 73, e4058–e4063. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr 2021, 15, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Yang, B.; Wang, P.; Zhou, Q.; Zhang, Z.; et al. Delayed-phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19). Br J Haematol 2020, 190, 179–184. [Google Scholar] [CrossRef]
- Mohiuddin Chowdhury, A.T.M.; Karim, M.R.; Ali, M.A.; Islam, J.; Li, Y.; He, S. Clinical Characteristics and the Long-Term Post-Recovery Manifestations of the COVID-19 Patients-A Prospective Multicenter Cross-Sectional Study. Front Med (Lausanne) 2021, 8, 663670. [Google Scholar] [CrossRef] [PubMed]
- Karimi Shahri, M.; Niazkar, H.R.; Rad, F. COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int J Lab Hematol 2021, 43, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; et al. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, A.K.; Rath, D.; Geisler, T.; Gawaz, M. Platelets and COVID-19. Hamostaseologie 2021, 41, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; et al. Hematological findings and complications of COVID-19. Am J Hematol 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).