Submitted:
25 February 2023
Posted:
27 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Research Questions:
Aims & Objectives of Research:
- To systematically review the diagnostic criteria of THI.
- To identify gaps in the existing literature related to the diagnostic criteria of THI.
2. Methodology
Eligibility Criteria:
| Population | Intervention | Comparison | Outcome | Study Type |
| Infants (Human children aged 4-36 months) | Screening tool or diagnostic criteria used to make a diagnosis. | N/A | Clinical Diagnosis (criteria or clinical assessment to diagnose THI) | All Study Types |
Exclusion Criteria
- Infants diagnosed after age of 36 months (3 years).
- Studies that did not use a screening tool/criterion to make a diagnosis.
- Studies that were abstracts only (did not include full text).
Information Sources
Search Strategy
- Screening tool OR Diagnosis AND Transient Hypogammaglobulinemia AND Infants.
- Transient hypogammaglobulinemia of infancy AND diagnosis.
Selection Process
Data Collection Process
Study Risk of Bias Assessment
Ethical considerations
3. Results
Study Selection:
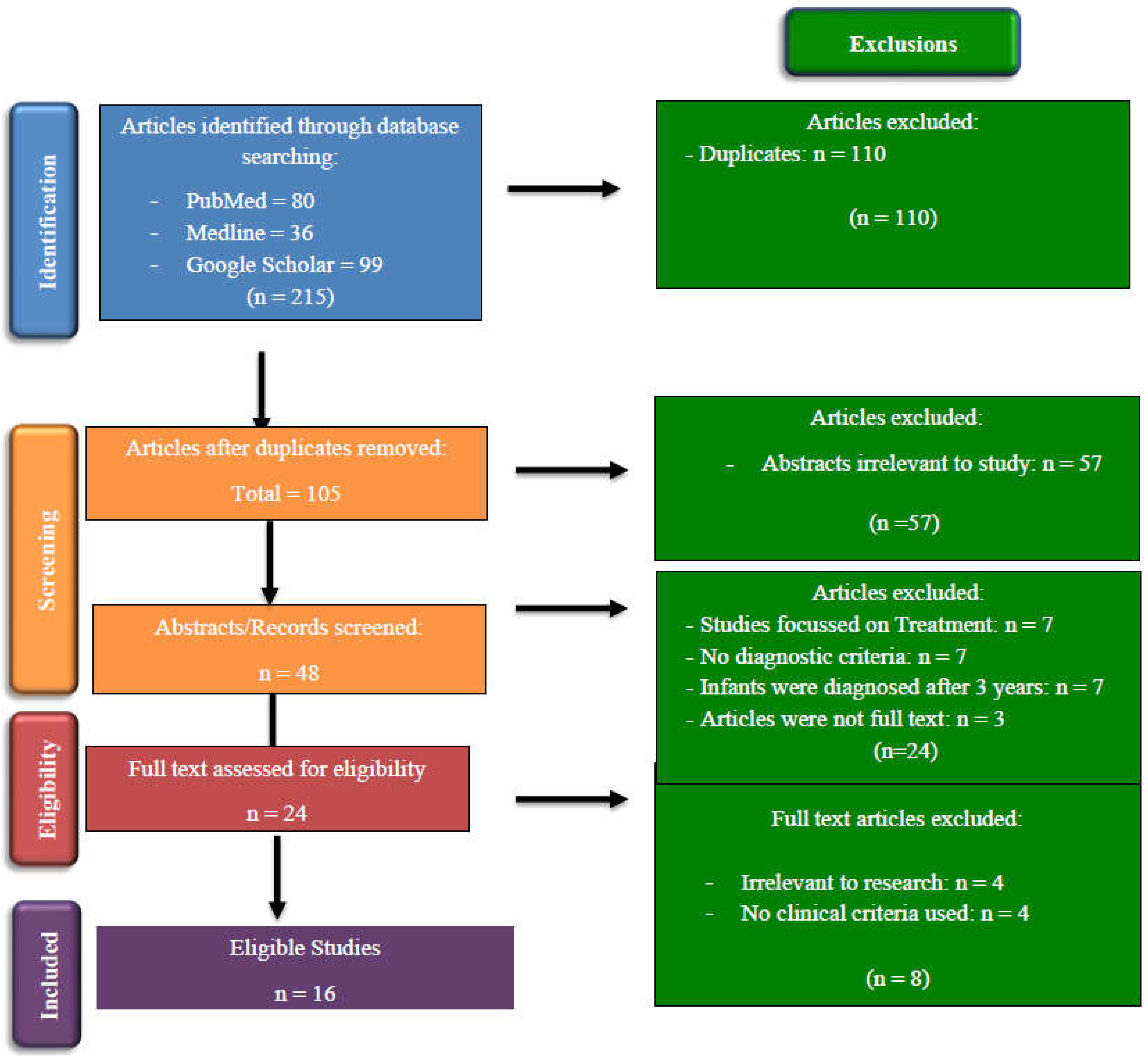
Study Characteristics
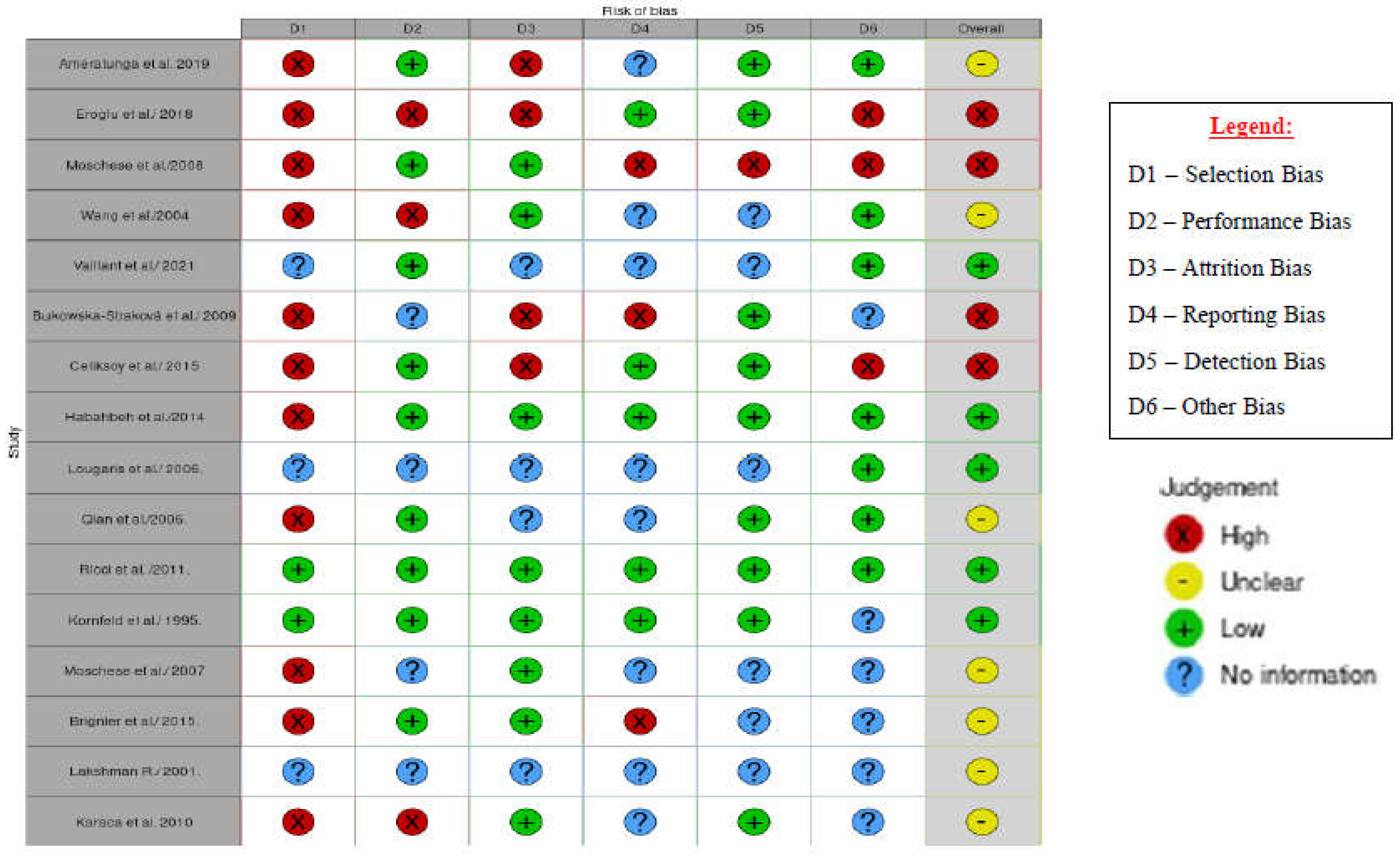
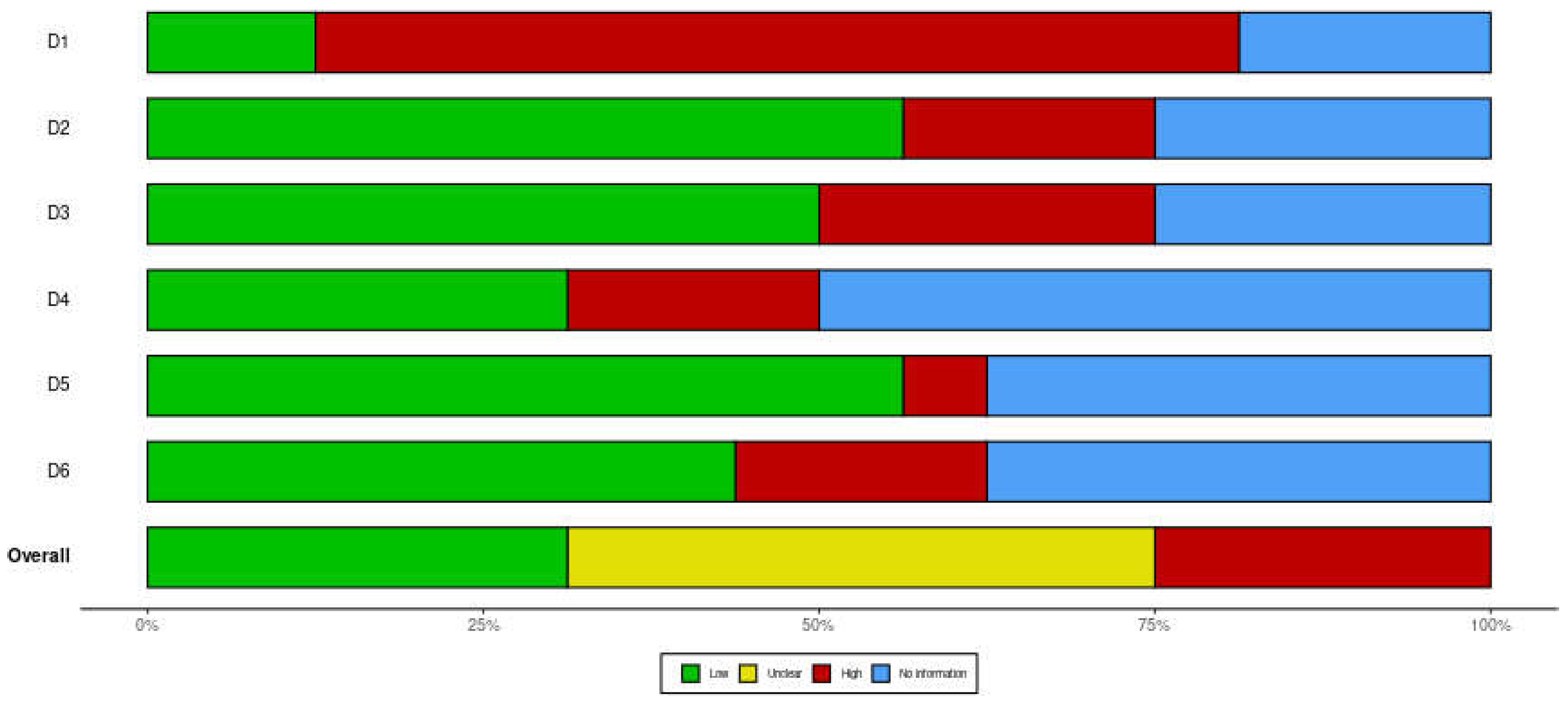
Risk of Bias in Studies
Results of Individual Studies
| Author/Year | Aim | Type of Study/ Design | Participants | Diagnostic Criteria used/Referenced | |
|---|---|---|---|---|---|
| Ameratunga et al./2019 [3] |
To determine clinical features & recovery for patients with THI. |
Retrospective Case Series |
47 patients < 4 years (time of diagnosis), |
History, examination, immunological studies, vaccine response, isohaemagglutinins production. |
|
| Eroglu et al./ 2018 [11] | To analyse B cell subsets of patients with THI diagnosis and compare with healthy age-matched Turkish children | Retrospective Cohort Study | 20 patients, comparison between THI & healthy aged- matched children |
Low levels of IgG (<2 SD) with/without a decrease in IgA or IgM. Lymphocyte subsets, isohaemagglutinins and vaccine responses. Exclusion of defined causes of secondary hypogammaglobulinemia | |
| Moschese et al./2008 [4] | To characterize clinical and immunological features of children with THI and to assess predictive parameters of clinical evolution. | Prospective Cohort Study | 77 THI children at initial diagnosis and of 57 patients at follow-up. |
Exclusion of other causes of hypogammaglobulinemia. Diagnosis made with normalization of IgG levels. Memory B cell subsets and in vitro immunoglobulin production were evaluated |
|
| Wang et al./2004 [7] | To review clinical features, & outcome of children with primary hypogammaglobulinemia |
Retrospective, Case-control Study |
33 patients | Quantifications of serum immunoglobulins (IgA/IgM/IgG) & Lymphocyte subsets were done. | |
| Vaillant & Wilson al./2021 [1] | To review clinical presentation, epidemiology, pathophysiology, treatment, of THI. |
Literature Review | Review paper |
Low serum IgG, Detection of isohemagglutinins (IgM); IgG antibodies (post-exposure) | |
| Bukowska- Straková et al./2009 [17] | To evaluate the B-cell compartment in peripheral blood of children with different types of hypogammaglobulinemia. | Retrospective, longitudinal, observational study. | Children with immunodeficiencies. Included 28 adults with CVID & 12 healthy controls. |
Criteria of the International union of Immunological Society. Patients whose level of Ig normalized before age 4 - THI. | |
| Celiksoy et al./2015 [12] | To analyse the memory B cell subsets of patients with antibody deficiencies. | Retrospective study. | 67 patients; (20 patients with THI (aged 1-3). 28 healthy children of matching ages were included. | Low serum IgG levels, low IgA and/or IgM levels upon admission, Normalisation of low Ig levels during follow- up, Normal production of an antibody specific to isohaemagglutinins, Intact cellular immunity. | |
| Lougaris et al. /2006. [15] | Not stated | Report | Infants, comparison of Ig value with age- matched control. |
Lab analysis of serum IgG, IgM, & IgA. Differential diagnosis between these two conditions (THI & CVID) cannot be made with certainty before 2-3 years of age. |
|
| Qian et al./2006 [19] | To determine clinical signs, immunological changes & outcomes with hypogammaglobulinemia |
Prospective | 91 patients <2 years with warning signs of PID. | Serum immunoglobulins & lymphocyte subsets were analysed. Normalization of Ig levels in follow-up visits to confirm diagnosis. | |
| Ricci et al. /2011 [16] | To assess clinical & immunological evolution of premature & full-term infants with hypogammaglobulinemia |
Prospective, Cross sectional study | 24 children (11 premature and 13 full-term infants) |
Reduction in IgG with/without reduction in IgA and IgM. | |
| Kornfeld et al./1995 [20] | To compare and diagnose a patient with XLA that presented with an initial diagnosis of THI and CVID. |
Retrospective case report | 1 patient, | Low Ig levels, vaccine response (diphtheria and tetanus) evaluated & circulating B cells. | |
| Moschese et al./2007 [14] | To determine if memory B-cell subsets can be used as a predictive marker of outcome |
Retrospective Cohort Study | 36 patients. Compares THI with healthy patients. |
IgG serum levels <2 SDs, circulating B cells >2%, exclusion of known causes of secondary hypogammaglobulinemia. |
|
| Brignier et al./2015 [21] |
To define hypogammaglobulinemia |
Retrospective Cohort |
44 patients with early onset (<6 yrs.) |
Serum immunoglobulin levels, T-cell defects were done. Exclusion of other PADs |
|
| Lakshman R./2001 [22] | To discuss the clinical presentation, laboratory diagnosis and management of hypogammaglobulinemia |
Cross-sectional | Children 6 months to 2 years | Full blood count and peripheral smear examination, Quantitative Ig estimations in serum (IgG, IgA, IgM, IgE), IgG subclass estimation, Lymphocyte subset estimation, & responses to vaccines (diphtheria, tetanus). |
|
| Karaca et al./2010 [13] |
To evaluate clinical, immunological data and outcomes |
Retrospective, Cross-sectional |
101 patients | Serum immunoglobulins (IgG, IgA, IgM), Vaccine responses and lymphocyte subpopulations. |
|
| Habahbeh et al./2014 [18] | To describe the clinical spectrum of primary antibody deficiency, to increase awareness for early referral. |
Retrospective study using medical records |
Medical records of 53 paediatric patients; 19% of which were THI |
Pan-American Group for Immunodeficiency (PAGID) and the European Society for Immunodeficiency (ESID) diagnostic criteria. Molecular diagnosis not available at hospital used in this study. |
|
4. Discussion
5. Conclusion
Recommendations
- We recommend that a policy change be made in both public & private medical centres across Trinidad & Tobago, that mandates routine immunoglobulin level testing & monitoring, especially IgG, be a part of primary level care, for infants around 5-6 months until 2 years.
- We also recommend routine roundtable discussions be held with necessary stakeholders (immunologists, paediatricians, general practitioners, nurses etc.) to prepare RHA’s & other health facilities to discuss current guidelines and its usefulness in the clinical setting.
- In addition, we recommend more research be done specifically focussing on prevalence, treatment & management of THI in Trinidad & Tobago can provide necessary insight, which may also aid the discussions in the point above.
References
- Vaillant AAJ, Wilson AM. Transient Hypogammaglobulinemia of Infancy. StatPearls Internet [Internet]. 2021 Oct 15 [cited 2021 Sep 29]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544356/.
- Knutsen, A. Transient Hypogammaglobulinemia of Infancy: Background, Pathophysiology, Epidemiology [Internet]. Transient Hypogammaglobulinemia of Infancy. 2019 [cited 2022 Mar 20]. Available online: https://emedicine.medscape.com/article/888706-overview#a4.
- Ameratunga, R.; Ahn, Y.; Steele, R.; Woon, S.-T. Transient hypogammaglobulinaemia of infancy: many patients recover in adolescence and adulthood. Clin Exp Immunol. 2019, 198, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Moschese V, S Graziani, S, Avanzini MA, Carsetti R, Marconi, M, La Rocca M, et al. A prospective study on children with initial diagnosis of transient hypogammaglobulinemia of infancy: results from the Italian Primary Immunodeficiency Network - PubMed. [cited 2021 Sep 21]. Available online: https://pubmed.ncbi.nlm.nih.gov/18547478/.
- Immune Deficiency Foundation | [Internet]. [cited 2021 Sep 21]. Available online: https://primaryimmune.org/.
- Duse M, Lacobini M, Leonardi L, Smacchia P, Antonetti L, Giancane G. Transient hypogammaglobulinemia of infancy: intravenous immunoglobulin as first line therapy - PubMed. [cited 2021 Sept 29]. Available online: https://pubmed.ncbi.nlm.nih.gov/20378022/.
- Wang LJ, Yang YH, Lin YT, Chiang BL. Immunological and clinical features of pediatric patients with primary hypogammaglobulinemia in Taiwan - PubMed. [cited 2022 Mar 29]. Available online: https://pubmed.ncbi.nlm.nih.gov/15366655/.
- Data Collection form for Intervention Reviews for RCTs & Non-RCTs- Template [Internet]. Cochrane Developmental, Psychosocial & Learning Problems; [cited 2022 Apr 17]. Available online: https://dplp.cochrane.org/data-extraction-forms.
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cipe, F.E.; Doğu, F.; Güloğlu, D.; Aytekin, C.; Polat, M.; Biyikli, Z.; et al. B-cell subsets in patients with transient hypogammaglobulinemia of infancy, partial IgA deficiency, and selective IgM deficiency. J Investig Allergol Clin Immunol. 2013, 23, 94–100. [Google Scholar] [PubMed]
- Eroglu, F.K.; Aerts Kaya, F.; Cagdas, D.; Özgür, T.T.; Yılmaz, T.; Tezcan, İ.; et al. B lymphocyte subsets and outcomes in patients with an initial diagnosis of transient hypogammaglobulinemia of infancy. Scand J Immunol. 2018, 88, e12709. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy MH, Yildiran A. A comparison of B cell subsets in primary immune deficiencies that progress with antibody deficiency and age-matched healthy children. Available online: https://www.elsevier.es/en-revista-allergologia-et-immunopathologia-105-pdf- S0301054616000094.
- Karaca, N.E.; Aksu, G.; Gulez, N.; Yildiz, B.; Azarsiz, E.; Kutukculer, N. New Laboratory Findings in Turkish Patients with Transient Hypogammaglobulinemia of Infancy. Iran J Allergy Asthma Immunol. 2010, 237–243. [Google Scholar]
- Moschese, V.; Carsetti, R.; Graziani, S.; Chini, L.; Soresina, A.R.; La Rocca, M.; et al. Memory B-cell subsets as a predictive marker of outcome in hypogammaglobulinemia during infancy. J Allergy Clin Immunol. 2007, 120, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Lougaris V, Soresina A, Meini A, Vettore E, Cattaneo G, Plebani A. Diagnostic criteria of hypogammaglobulinemia in infancy. Hematol Meet Rep Former Haematol Rep [Internet]. 2006 [cited 2022 Apr 13];2(10). Available online: https://www.pagepress.org/journals/index.php/hmr/article/view/446.
- Ricci, G.; Piccinno, V.; Giannetti, A.; Miniaci, A.; Specchia, F.; Masi, M. Evolution of Hypogammaglobulinemia in Premature and Full-Term Infants. Int J Immunopathol Pharmacol. 2011, 24, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Bukowska-Straková, K.; Kowalczyk, D.; Baran, J.; Siedlar, M.; Kobylarz, K.; Zembala, M. The B- cell Compartment in the Peripheral Blood of Children With Different Types of Primary Humoral Immunodeficiency. Pediatr Res. 2009, 66, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Habahbeh, Z.M.; Abu-Shukair, M.E.; Almutereen, M.A.; Alzyoud, R.M.; Wahadneh, A.M. Primary Antibody Deficiencies at Queen Rania Children Hospital in Jordan: Single Center Experience. 2014, 10. [Google Scholar]
- Qian J hong;, Chen T xin, Zhu J xing;, Zhu X dong. Clinical features and follow-up of Chinese patients with sym... : Chinese Medical Journal. [cited 2022 Mar 25]. Available online: https://journals.lww.com/cmj/Fulltext/2009/08020/Clinical_features_and_follow_up_of_Chi nese.8.aspx.
- Kornfeld, S.J.; Kratz, J.; Haire, R.N.; Litman, G.W.; Good, R.A. X-linked agammaglobulinemia presenting as transient hypogammaglobulinemia of infancy. J Allergy Clin Immunol. 1995, 95, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Brignier, A.C.; Mahlaoui, N.; Reimann, C.; Picard, C.; Kracker, S.; de Vergnes, N.; et al. Early-onset hypogammaglobulinemia: A survey of 44 patients. J Allergy Clin Immunol. 2015, 136, 1097–1099.e2. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R. Indian Pediatrics - Personal Practice [Internet]. [cited 2022 Mar 25]. Available online: https://www.indianpediatrics.net/aug2001/aug-864-871.htm.
- ESID - European Society for Immunodeficiencies [Internet]. [cited 2022 May 17]. Available online: https://esid.org/Education/Diagnostic-Criteria-PID.
- Conley, M.E.; Notarangelo, L.D.; Etzioni, A. Diagnostic Criteria for Primary Immunodeficiencies. Clin Immunol. 1999, 93, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Bobby Gaspar, H.; Al-Herz, W.; Bousfiha, A.; Casanova, J.L.; Chatila, T.; et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018, 38, 96–128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





