Submitted:
27 February 2023
Posted:
28 February 2023
You are already at the latest version
Abstract
Keywords:
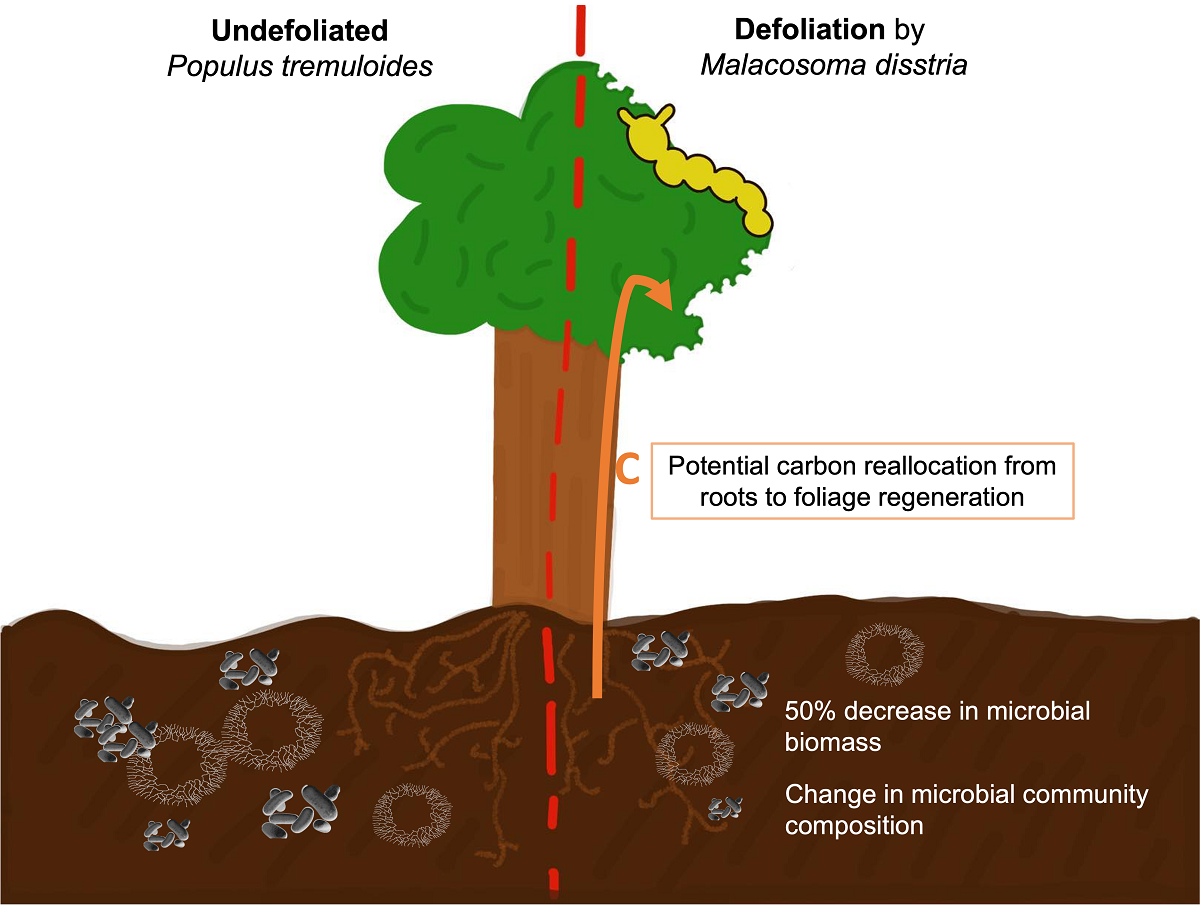
1. Introduction
2. Materials and Methods
3. Results
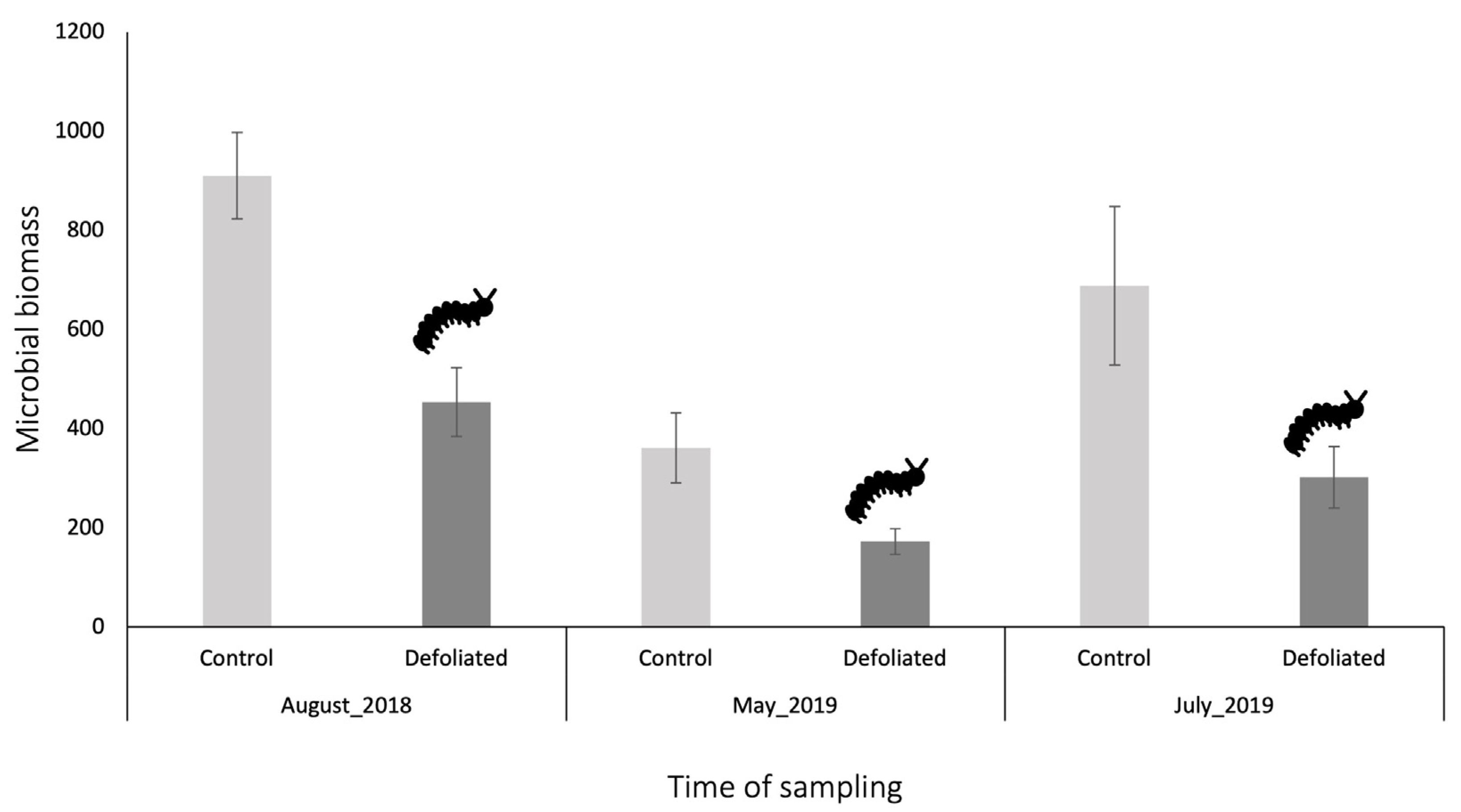
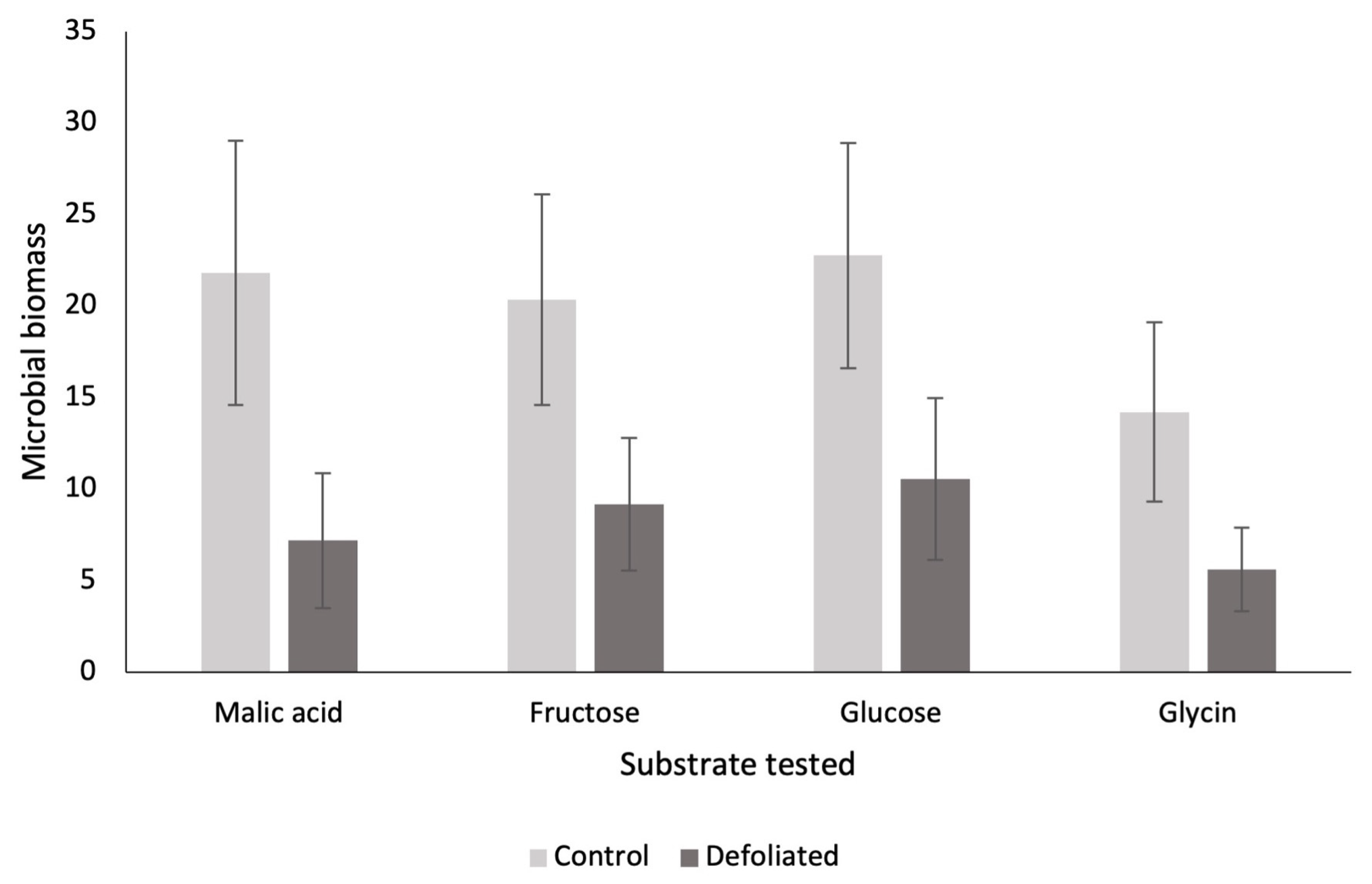
3.1. Soil microbial biomass
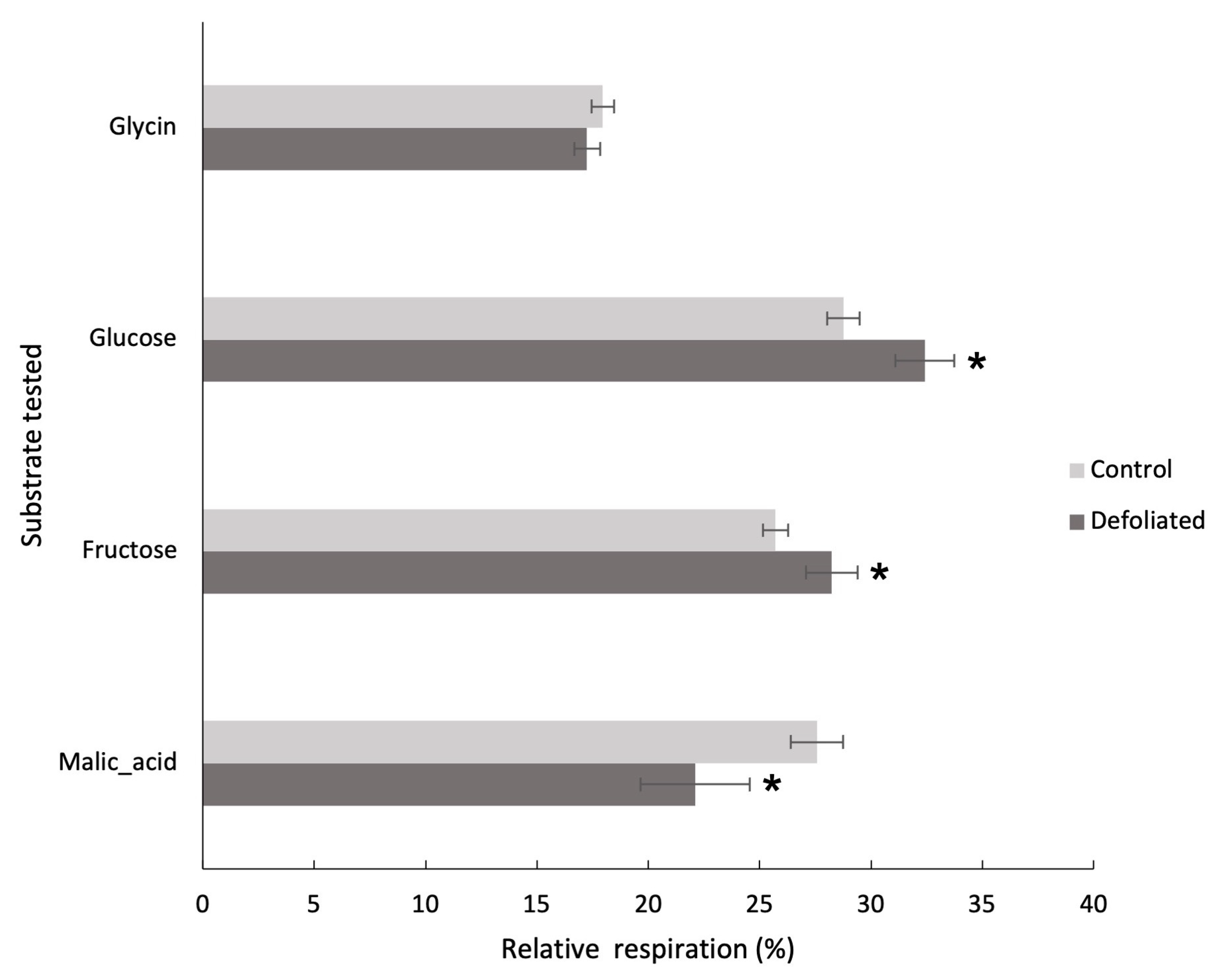
3.2. Soil microbial community
3.3. Fungal ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree Mortality from Drought, Insects, and Their Interactions in a Changing Climate. New Phytologist 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ramsfield, T.D.; Bentz, B.J.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest Health in a Changing World: Effects of Globalization and Climate Change on Forest Insect and Pathogen Impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and Landscape Dynamics in a Changing World. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.A.; Dymond, C.C.; Stinson, G.; Rampley, G.J.; Neilson, E.T.; Carroll, A.L.; Ebata, T.; Safranyik, L. Mountain Pine Beetle and Forest Carbon Feedback to Climate Change. Nature 2008, 452, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Mitton, J.B.; Ferrenberg, S.M. Mountain Pine Beetle Develops an Unprecedented Summer Generation in Response to Climate Warming. The American Naturalist 2012, 179, E163–E171. [Google Scholar] [CrossRef] [PubMed]
- Uelmen, J.A.; Lindroth, R.L.; Tobin, P.C.; Reich, P.B.; Schwartzberg, E.G.; Raffa, K.F. Effects of Winter Temperatures, Spring Degree-Day Accumulation, and Insect Population Source on Phenological Synchrony between Forest Tent Caterpillar and Host Trees. Forest Ecology and Management 2016, 362, 241–250. [Google Scholar] [CrossRef]
- Silfver, T.; Heiskanen, L.; Aurela, M.; Myller, K.; Karhu, K.; Meyer, N.; Tuovinen, J.-P.; Oksanen, E.; Rousi, M.; Mikola, J. Insect Herbivory Dampens Subarctic Birch Forest C Sink Response to Warming. Nature Communications 2020, 11, 2529. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr Forestry Rep 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. HERBIVORE-MEDIATED LINKAGES BETWEEN ABOVEGROUND AND BELOWGROUND COMMUNITIES. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Hunter, M.D. Insect Population Dynamics Meets Ecosystem Ecology: Effects of Herbivory on Soil Nutrient Dynamics: Insect Population Dynamics Meets Ecosystem Ecology. Agricultural and Forest Entomology 2001, 3, 77–84. [Google Scholar] [CrossRef]
- Grüning, M.M.; Beule, L.; Meyer, S.; Karlovsky, P.; I. -M.-Arnold, A. The Abundance of Fungi, Bacteria and Denitrification Genes during Insect Outbreaks in Scots Pine Forests. Forests 2018, 9, 497. [CrossRef]
- Donaldson, J.R.; Lindroth, R.L. GENETICS, ENVIRONMENT, AND THEIR INTERACTION DETERMINE EFFICACY OF CHEMICAL DEFENSE IN TREMBLING ASPEN. Ecology 2007, 88, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.Å.; Rousk, J.; Metcalfe, D.B. Below-ground Responses to Insect Herbivory in Ecosystems with Woody Plant Canopies: A Meta-analysis. J Ecol 2020, 108, 917–930. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Wardle, D.A.; Tate, K.R. Effects of Plant Species Diversity and Composition on Nitrogen Cycling and the Trace Gas Balance of Soils. Plant Soil 2006, 282, 83–98. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect Canopy Herbivory and Frass Deposition Affect Soil Nutrient Dynamics and Export in Oak Mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- le Mellec, A.; Gerold, G.; Michalzik, B. Insect Herbivory, Organic Matter Deposition and Effects on Belowground Organic Matter Fluxes in a Central European Oak Forest. Plant Soil 2011, 342, 393–403. [Google Scholar] [CrossRef]
- Streminska Microbial Abundance and Some of Their Physiological Activities in Soil Organic Horizon of Pine Forest Affected by Insect Herbivory. Pol. J. Environ. Stud. 2006, 15, 905–914.
- Grüning, M.M.; Simon, J.; Rennenberg, H.; l-M-Arnold, A. Defoliating Insect Mass Outbreak Affects Soil N Fluxes and Tree N Nutrition in Scots Pine Forests. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Lovett, G.M.; Ruesink, A.E. Carbon and Nitrogen Mineralization from Decomposing Gypsy Moth Frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef]
- Reynolds, B.C.; Hunter, M.D. Responses of Soil Respiration, Soil Nutrients, and Litter Decomposition to Inputs from Canopy Herbivores. Soil Biology and Biochemistry 2001, 33, 1641–1652. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Perevoznikova, V.D.; Vishnyakova, Z.V. Carbon Emission by Soils in Forests Damaged by the Siberian Moth. 2002, 33, 4.
- le Mellec, A.; Habermann, M.; Michalzik, B. Canopy Herbivory Altering C to N Ratios and Soil Input Patterns of Different Organic Matter Fractions in a Scots Pine Forest. Plant Soil 2009, 325, 255–262. [Google Scholar] [CrossRef]
- Morehouse, K.; Johns, T.; Kaye, J.; Kaye, M. Carbon and Nitrogen Cycling Immediately Following Bark Beetle Outbreaks in Southwestern Ponderosa Pine Forests. Forest Ecology and Management 2008, 255, 2698–2708. [Google Scholar] [CrossRef]
- Baldrian, P. Forest Microbiome: Diversity, Complexity and Dynamics. FEMS Microbiol Rev 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.; Demarco, J.; Hart, S.; Whitham, T.; Cobb, N.; Koch, G. Impacts of Herbivorous Insects on Decomposer Communities during the Early Stages of Primary Succession in a Semi-Arid Woodland. Soil Biology and Biochemistry 2006, 38, 972–982. [Google Scholar] [CrossRef]
- Castaño, C.; Camarero, J.J.; Zas, R.; Sampedro, L.; Bonet, J.A.; Alday, J.G.; Oliva, J. Insect Defoliation Is Linked to a Decrease in Soil Ectomycorrhizal Biomass and Shifts in Needle Endophytic Communities. Tree Physiology 2020, 40, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Oneț, A.; Teușdea, A.; Boja, N.; Domuța, C.; Oneț, C. Effects of Common Oak (Quercus Robur L.) Defolition on the Soil Properties of an Oak Forest in Western Plain of Romania. Ann. For. Res. 2016, 59, 1. [Google Scholar] [CrossRef]
- Mattson, W.J.; Herms, D.A.; Witter, J.A.; Allen, D.C. Woody Plant Grazing Systems: North American Outbreak Folivores and Their Host Plants. General technical report NE - U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station 1991.
- Schowalter, T.D. Biology and Management of the Forest Tent Caterpillar (Lepidoptera: Lasiocampidae). J Integr Pest Manag 2017, 8. [Google Scholar] [CrossRef]
- Cooke, B.J.; Nealis, V.G.; Régnière, J. Insect Defoliators as Periodic Disturbances in Northern Forest Ecosystems. 2009, 39.
- Cooke, B.J.; Lorenzetti, F. The Dynamics of Forest Tent Caterpillar Outbreaks in Québec, Canada. Forest Ecology and Management 2006, 226, 110–121. [Google Scholar] [CrossRef]
- Sutton, A.S.; Tardif, J.C.T.C. Dendrochronological Reconstruction of Forest Tent Caterpillar Outbreaks in Time and Space, Western Manitoba, Canada. Canadian Journal of Forest Research 2007. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Mattson, W.J.; Hain, F.P.; Payne, T.L. Forest Insect Guilds: Patterns of Interaction with Host Trees; 1989 August 13-17; Abakan, Siberia, U.S.S.R; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, 1991; p. NE-GTR-153;
- Moulinier, J.; Lorenzetti, F.; Bergeron, Y. Effects of a Forest Tent Caterpillar Outbreak on the Dynamics of Mixedwood Boreal Forests of Eastern Canada. Écoscience 2013, 20, 182–193. [Google Scholar] [CrossRef]
- Bergeron, Y. SPECIES AND STAND DYNAMICS IN THE MIXED WOODS OF QUEBEC’S SOUTHERN BOREAL FOREST. Ecology 2000, 81, 1500–1516. [Google Scholar] [CrossRef]
- Spencer, K.L.; Harvey, G.L. Understanding System Disturbance and Ecosystem Services in Restored Saltmarshes: Integrating Physical and Biogeochemical Processes. Estuarine, Coastal and Shelf Science 2012, 106, 23–32. [Google Scholar] [CrossRef]
- Vincent, J.-S.; Hardy, L. L’évolution et l’extension Des Lacs Glaciaires Barlow et Ojibway En Territoire Québécois. gpq 2011, 31, 357–372. [Google Scholar] [CrossRef]
- Canada, E. and C.C. Canadian Climate Normals - Climate - Environment and Climate Change Canada. Available online: https://climate.weather.gc.ca/climate_normals/ (accessed on 16 December 2022).
- Ministère de la Faune, des Forêts et des Parc (MFFP) Aires Infestées Par La Livrées Des Forêts Au Québec En 2016; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2016; p. 10;
- Ministère de la Faune, des Forêts et des Parc (MFFP) Aires Infestées Par La Livrée Des Forêts Au Québec En 2017; Gouvernement du Québec, Direction de la protection des forêts: Québec, 2017; p. 14;
- Ministère de la Faune, des Forêts et des Parc (MFFP) Aires infestées par la livrée des forêts au Québec en 2018; 2018;
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method To Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.B.; Dollinger, J.; Renault, P.; Tlili, A.; Bérard, A. The FungiResp Method: An Application of the MicroRespTM Method to Assess Fungi in Microbial Communities as Soil Biological Indicators. Ecological Indicators 2012, 23, 482–490. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A Physiological Method for the Quantitative Measurement of Microbial Biomass in Soils. Soil Biology and Biochemistry 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Campbell, C.D.; Grayston, S.J.; Hirst, D.J. Use of Rhizosphere Carbon Sources in Sole Carbon Source Tests to Discriminate Soil Microbial Communities. Journal of Microbiological Methods 1997, 30, 33–41. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. Journal of Statistical Software 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Mellec, A. le M. le; Michalzik, B.M. Impact of a Pine Lappet (Dendrolimus Pini) Mass Outbreak on C and N Fluxes to the Forest Floor and Soil Microbial Properties in a Scots Pine Forest in Germany. Canadian Journal of Forest Research 2008. [CrossRef]
- Kristensen, J.A. The Biogeochemical Consequences of Litter Transformation by Insect Herbivory in the Subarctic: A Microcosm Simulation Experiment. 2018, 14.
- Donaldson, J.R.; Lindroth, R.L. Effects of Variable Phytochemistry and Budbreak Phenology on Defoliation of Aspen during a Forest Tent Caterpillar Outbreak. Agricultural and Forest Entomology 2008, 10, 399–410. [Google Scholar] [CrossRef]
- Hagerman, A.E. Recent Advances in Polyphenol Research | Wiley Online Books; 2012;
- Coq, S.; Souquet, J.-M.; Meudec, E.; Cheynier, V.; Hättenschwiler, S. Interspecific Variation in Leaf Litter Tannins Drives Decomposition in a Tropical Rain Forest of French Guiana. Ecology 2010, 91, 2080–2091. [Google Scholar] [CrossRef]
- Joanisse, G.D.; Bradley, R.L.; Preston, C.M.; Bending, G.D. Sequestration of Soil Nitrogen as Tannin–Protein Complexes May Improve the Competitive Ability of Sheep Laurel (Kalmia Angustifolia) Relative to Black Spruce (Picea Mariana). New Phytologist 2009, 181, 187–198. [Google Scholar] [CrossRef]
- Adamczyk, B.; Sietiö, O.-M.; Biasi, C.; Heinonsalo, J. Interaction between Tannins and Fungal Necromass Stabilizes Fungal Residues in Boreal Forest Soils. New Phytologist 2019, 223, 16–21. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Sun, T.; Coq, S. The Chitin Connection of Polyphenols and Its Ecosystem Consequences. New Phytologist 2019, 223, 5–7. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Cates, R.G.; Zou, J. In¯uence of Balsam Poplar Tannin Fractions on Carbon and Nitrogen Dynamics in Alaskan Taiga ¯oodplain Soils. Soil Biology 2001, 13. [Google Scholar] [CrossRef]
- Schimel, J.P.; Cleve, K.V.; Cates, R.G.; Clausen, T.P.; Reichardt, P.B. Effects of Balsam Poplar (Populus Balsamifera) Tannins and Low Molecular Weight Phenolics on Microbial Activity in Taiga Floodplain Soil: Implications for Changes in N Cycling during Succession. Can. J. Bot. 1996, 74, 84–90. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen Additions and Microbial Biomass: A Meta-analysis of Ecosystem Studies. 2008, 10.
- Parker, T.C.; Sadowsky, J.; Dunleavy, H.; Subke, J.-A.; Frey, S.D.; Wookey, P.A. Slowed Biogeochemical Cycling in Sub-Arctic Birch Forest Linked to Reduced Mycorrhizal Growth and Community Change after a Defoliation Event. Ecosystems 2017, 20, 316–330. [Google Scholar] [CrossRef]
- Saravesi, K.; Aikio, S.; Wäli, P.R.; Ruotsalainen, A.L.; Kaukonen, M.; Huusko, K.; Suokas, M.; Brown, S.P.; Jumpponen, A.; Tuomi, J.; et al. Moth Outbreaks Alter Root-Associated Fungal Communities in Subarctic Mountain Birch Forests. Microb Ecol 2015, 69, 788–797. [Google Scholar] [CrossRef]
- Frey, B.R.; Lieffers, V.J.; Landhäusser, S.M.; Comeau, P.G.; Greenway, K.J. An Analysis of Sucker Regeneration of Trembling Aspen. Can. J. For. Res. 2003, 33, 1169–1179. [Google Scholar] [CrossRef]
- Légaré, S.; Paré, D.; Bergeron, Y. Influence of Aspen on Forest Floor Properties in Black Spruce-Dominated Stands. Plant Soil 2005, 275, 207–220. [Google Scholar] [CrossRef]
- Chodak, M.; Klimek, B.; Niklińska, M. Composition and Activity of Soil Microbial Communities in Different Types of Temperate Forests. Biol Fertil Soils 2016, 52, 1093–1104. [Google Scholar] [CrossRef]
- Gartzia-Bengoetxea, N.; Kandeler, E.; Martínez de Arano, I.; Arias-González, A. Soil Microbial Functional Activity Is Governed by a Combination of Tree Species Composition and Soil Properties in Temperate Forests. Applied Soil Ecology 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Paré, D.; Bernier, P.; Lafleur, B.; Titus, B.D.; Thiffault, E.; Maynard, D.G.; Guo, X. Estimating Stand-Scale Biomass, Nutrient Contents, and Associated Uncertainties for Tree Species of Canadian Forests. Can. J. For. Res. 2013, 43, 599–608. [Google Scholar] [CrossRef]
- Carter, M.R. Soil Sampling and Methods of Analysis; CRC Press, 2007. ISBN 978-0-429-12622-2.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




