Submitted:
28 February 2023
Posted:
28 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Physiological Functions of Copper and Zinc Elements
3. Copper and Zinc for Anti-Chemoresistance in Osteosarcoma Therapies
4. Copper, Zinc, and CuZn Structures in Anti-Chemoresistance
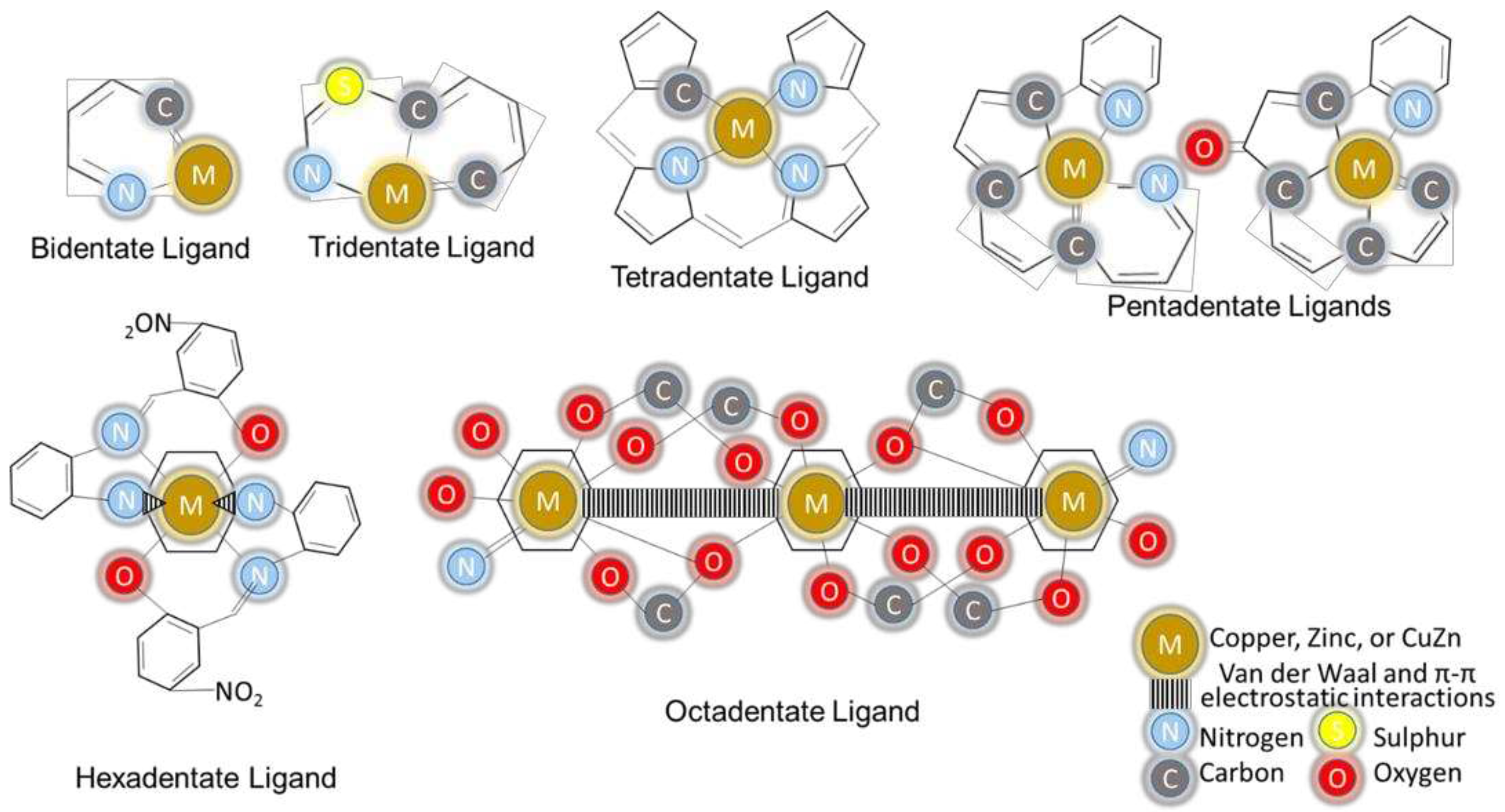
4.1. Copper and Zinc in Chelating Structures
4.2. Copper and Zinc Ions in Metal–Organic Framework Structures
5. Copper, Zinc, and CuZn in Organic Solvent Formation Structures
5.1. CuZn in Planar Aromatic Structures
5.2. CuZn in Schiff-Based and Schiff-Paired Structures
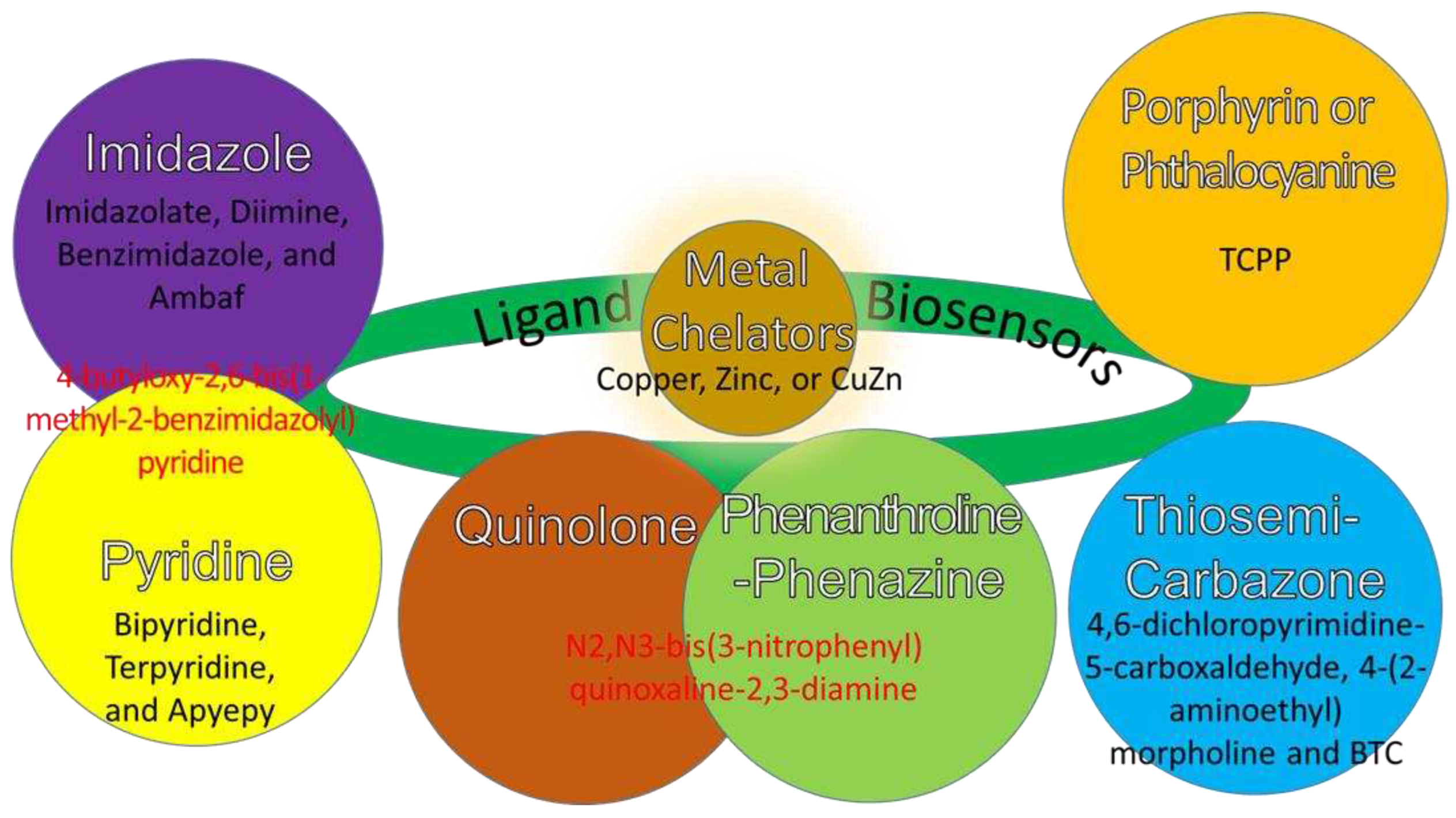
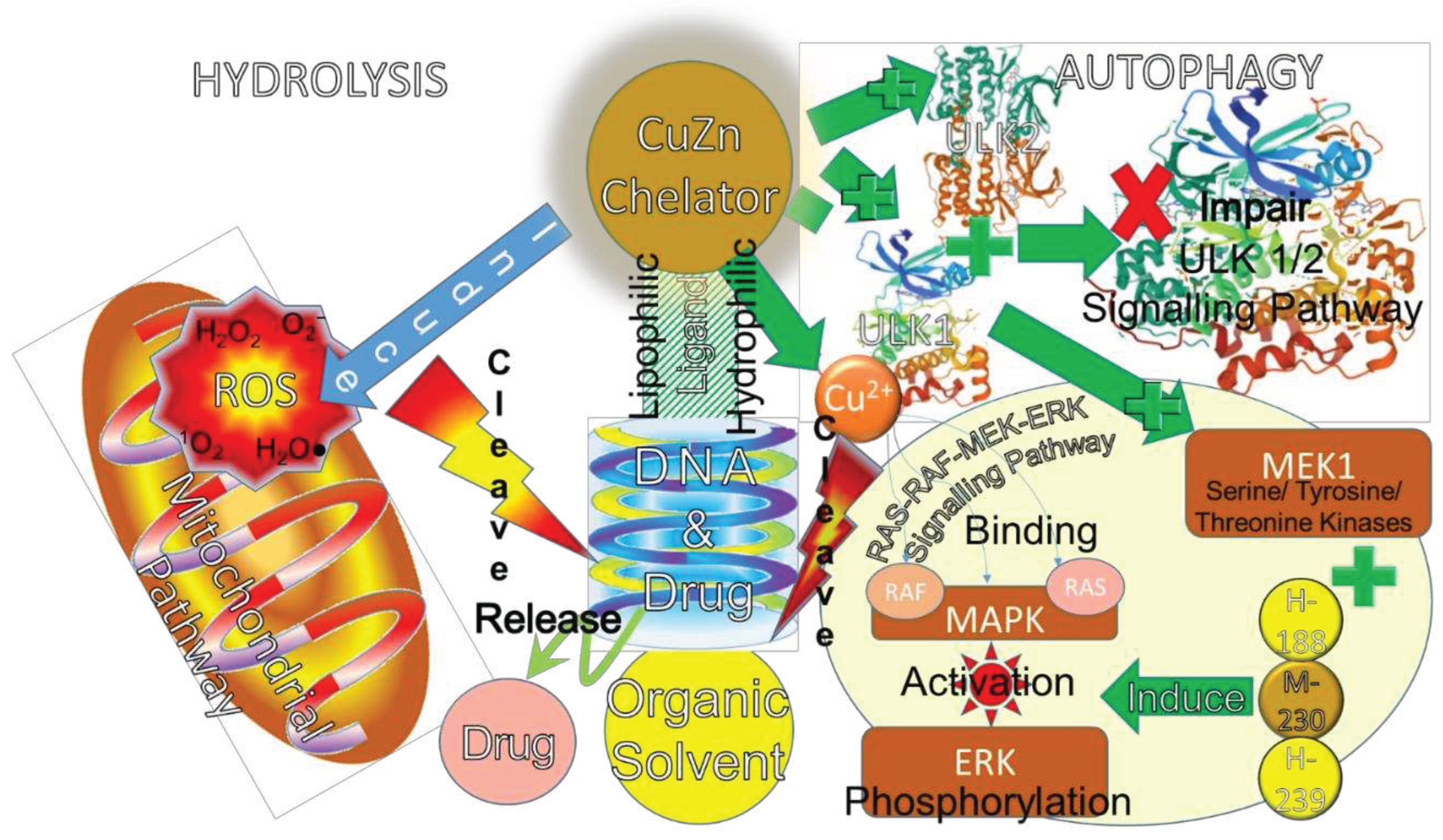
6. Ligand Degradation Properties in Anti-Chemoresistance
7. Ligands’ Functions as Biosensors for Osteosarcoma Therapy
7.1. CuZn Ligands for Redox Biosensor Functions
7.2. CuZn Ligands for Photo-Biosensor Functions
8. Conclusions
9. Challenges and Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todorov, L.; Kostova, I. Recent Trends in the Development of Novel Metal-Based Antineoplastic Drugs. Molecules 2023, 28, 1959. [Google Scholar] [CrossRef] [PubMed]
- Rostán, S.; Mahler, G.; Otero, L. Selenosemicarbazone Metal Complexes as Potential Metal-Based Drugs. Curr. Med. Chem. 2023, 30, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Wang, Y.; Chen, W.-C.; Ju, E.; Mintz, R.L.; Teng, Y.; Zhu, L.; Wang, K.; Lv, S.; Chan, H.F.; et al. Implantable 3D Printed Hydrogel Scaffolds Loading Copper-Doxorubicin Complexes for Postoperative Chemo/Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 4911–4923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kuang, G.; Zhang, L.; Zhu, Y. Nanocarriers for Platinum Drug Delivery. Biomed. Technol. 2023, 2, 77–89. [Google Scholar] [CrossRef]
- Gill, M.R.; Vallis, K.A. Transition Metal Compounds as Cancer Radiosensitizers. Chem. Soc. Rev. 2019, 48, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Olelewe, C.; Awuah, S.G. Mitochondria as a Target of Third Row Transition Metal-Based Anticancer Complexes. Curr. Opin. Chem. Biol. 2023, 72, 102235. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.P.; Patra, M. Platinum Glycoconjugates: “Sweet Bullets” for Targeted Cancer Therapy? Curr. Opin. Chem. Biol. 2023, 72, 102236. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Metal-Based Drugs That Break the Rules. Dalt. Trans. 2016, 45, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Meeran, S.M. Emerging Role of Non-Coding RNAs in Resistance to Platinum-Based Anti-Cancer Agents in Lung Cancer. Front. Pharmacol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, F.; Yasir Khan, H.; Tabassum, S. Progress of Metal-Based Anticancer Chemotherapeutic Agents in Last Two Decades and Their Comprehensive Biological (DNA/RNA Binding, Cleavage and Cytotoxicity Activity) Studies. Chem. Rec. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cun, J.-E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-Based Metal–Organic Frameworks for Biomedical Applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Zehra, S.; Tabassum, S.; Arjmand, F. Biochemical Pathways of Copper Complexes: Progress over the Past 5 Years. Drug Discov. Today 2021, 26, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Azhdari, R. Zinc-Based Metal–Organic Frameworks as Nontoxic and Biodegradable Platforms for Biomedical Applications: Review Study. Drug Metab. Rev. 2019, 51, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Psomas, G. Copper(II) and Zinc(II) Coordination Compounds of Non-Steroidal Anti-Inflammatory Drugs: Structural Features and Antioxidant Activity. Coord. Chem. Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Loubalová, I.; Kopel, P. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules 2023, 28, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper Homeostasis and Copper-Induced Cell Death in the Pathogenesis of Cardiovascular Disease and Therapeutic Strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef]
- Skos, L.; Borutzki, Y.; Gerner, C.; Meier-Menches, S.M. Methods to Identify Protein Targets of Metal-Based Drugs. Curr. Opin. Chem. Biol. 2023, 73, 102257. [Google Scholar] [CrossRef] [PubMed]
- Sumithaa, C.; Ganeshpandian, M. Half-Sandwich Ruthenium Arene Complexes Bearing Clinically Approved Drugs as Ligands: The Importance of Metal–Drug Synergism in Metallodrug Design. Mol. Pharm. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Fang, Y.; Wu, Y.; Zhou, M.; Shen, J.; Fan, X. Metal Ions and Nanometallic Materials in Antitumor Immunity: Function, Application, and Perspective. J. Nanobiotechnology 2023, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, K.; Józkowiak, M.; Angelova Volponi, A.; Shibli, J.A.; Golkar-Narenji, A.; Antosik, P.; Bukowska, D.; Piotrowska-Kempisty, H.; Mozdziak, P.; Dzięgiel, P.; et al. The Role of Exosomes in Human Carcinogenesis and Cancer Therapy—Recent Findings from Molecular and Clinical Research. Cells 2023, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.N.; Kadri, U.; Naha, N. Impact of Heavy Metal-Based Nanomaterials on Environment and Health. In; 2023; pp. 224–277.
- Pham, V.N.; Chang, C.J. Metalloallostery and Transition Metal Signaling: Bioinorganic Copper Chemistry Beyond Active Sites. Angew. Chemie Int. Ed. 2023. [Google Scholar] [CrossRef]
- Huffman, O.G.; Chau, D.B.; Dinicu, A.I.; DeBernardo, R.; Reizes, O. Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Cancers (Basel). 2023, 15, 1402. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Franzè, E.; Maresca, C.; Colella, M.; Pacifico, T.; Stolfi, C. Targeted Therapy of Interleukin-34 as a Promising Approach to Overcome Cancer Therapy Resistance. Cancers (Basel). 2023, 15, 971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Montesdeoca, N.; Karges, J.; Xiao, H. Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy. Angew. Chemie Int. Ed. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Tinazzi, M.; Giacomini, I.; Rosato, B.; Ragazzi, E.; Berretta, M.; Montopoli, M. Clinical Evidence of Interaction Between Nutraceutical Supplementation and Platinum-Based Chemotherapy. Curr. Med. Chem. 2022, 29. [Google Scholar] [CrossRef] [PubMed]
- Thiruchenthooran, V.; Sánchez-López, E.; Gliszczyńska, A. Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers (Basel). 2023, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- Azari, M.; Bahreini, F.; Uversky, V.N.; Rezaei, N. Current Therapeutic Approaches and Promising Perspectives of Using Bioengineered Peptides in Fighting Chemoresistance in Triple-Negative Breast Cancer. Biochem. Pharmacol. 2023, 210, 115459. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Zaidi, A.M.A.; Haque, M.; Miskon, A. Relationship between Osteosarcoma Therapy and Tumorigenesis, Metastasis, Immune Evasion, and Chemoresistance. Cancers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, Q.; Gao, W.; Pu, Y.; He, B. Reversal of Cisplatin Chemotherapy Resistance by Glutathione-Resistant Copper-Based Nanomedicine via Cuproptosis. J. Mater. Chem. B 2022, 10, 6296–6306. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Baião, A.; Ding, T.; Cui, W.; Sarmento, B. Recent Advances in Long-Acting Drug Delivery Systems for Anticancer Drug. Adv. Drug Deliv. Rev. 2023, 194, 114724. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, O. V.; Kolotilina, N.K.; Dolgonosov, A.M.; Khamizov, R.K.; Timerbaev, A.R. A de Novo Nanoplatform for the Delivery of Metal-Based Drugs Studied with High-Resolution ICP-MS. Talanta 2023, 253, 124035. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Zaidi, A.M.A.; Miskon, A. Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy. Pharmaceuticals 2022, 15, 1330. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials (Basel). 2022, 15, 7672. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. Structural Strength Analyses for Low Brass Filler Biomaterial with Anti-Trauma Effects in Articular Cartilage Scaffold Design. Materials (Basel). 2022, 15, 4446. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials (Basel). 2022, 15, 1421. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Numerical Simulation Study on Relationship between the Fracture Mechanisms and Residual Membrane Stresses of Metallic Material. J. Funct. Biomater. 2022, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Y.; Li, H.; Fan, Y. Targeting HER2 Alterations in Non-Small Cell Lung Cancer: Therapeutic Breakthrough and Challenges. Cancer Treat. Rev. 2023, 114, 102520. [Google Scholar] [CrossRef]
- Middya, P.; Roy, D.; Chattopadhyay, S. Synthesis, Structures and Magnetic Properties of End-on Pseudo-Halide Bridged Dinuclear Copper(II) Complexes with N,O-Donor Salicylaldimine Schiff Base Blocking Ligands: A Review. Inorganica Chim. Acta 2023, 548, 121377. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Z.; Kang, Y.; Zhang, W.; Mei, L.; Ji, X. Redox Regulation and Its Emerging Roles in Cancer Treatment. Coord. Chem. Rev. 2023, 475, 214897. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, W.; Ji, X.; Liu, X.; Jin, E. Ionomics-Metabolome Association Analysis as a New Approach to the Impact of Dietary Copper Levels in Suckling Piglets Model. Sci. Rep. 2023, 13, 1164. [Google Scholar] [CrossRef]
- Adusumilli, S.; Haidar, A.; Behbahani-Nejad, N.; Lee, J. 289: MALNUTRITION SHAPING CRITICAL CARE MANAGEMENT: A CASE OF COPPER DEFICIENCY MYELONEUROPATHY. Crit. Care Med. 2023, 51, 130–130. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Lopes, L.; Chéu, M.H.; Soares, E.; Meireles, D.; Machado, J. Updated Mineral Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea Europaea. Plants 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Tatineni, V.; An, J.Y.; Leffew, M.R.; Mahesh, S.A. Anemia from A to Zinc: Hypocupremia in the Setting of Gastric Bypass and Zinc Excess. Clin. Case Reports 2020, 8, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, W.; Tian, S.; Tian, D. Copper Nanoclusters Stabilized by D-Penicillamine for Ultrasensitive and Visual Detection of Oxytetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122286. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, A.; Shiva Priya, K.; Roy Chowdhury, S.; Datta, P.K. Challenges in Anesthesia in Wilson’s Disease: A Systematic Review of the Existing Literature. Cureus 2023. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, J.-L.; Huang, Z.-H.; Ai, G.; Li, G.; Shu, S.-N. Identification of Novel Compound ATP7B Mutations in a Child with Rare Wilson Disease: A Case Report; 2023.
- Daniel, J.-B.; Brugger, D.; van der Drift, S.; van der Merwe, D.; Kendall, N.; Windisch, W.; Doelman, J.; Martín-Tereso, J. Zinc, Copper, and Manganese Homeostasis and Potential Trace Metal Accumulation in Dairy Cows: Longitudinal Study from Late Lactation to Subsequent Mid-Lactation. J. Nutr. 2023. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites 2023, 13, 82. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Izzi, M.; Cioffi, N. Green Synthesis and Analytical Characterization of Core-shell Copper Sub-microparticles. Chem. – A Eur. J. [CrossRef]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta - Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.S.; Ismail, N.S.; Gaber, N.S.; Elzanfaly, E.S. Erdosteine-Based Potentiometric Sensor for Real-Time Surveillance of Copper Traces in Food Supplements and Shredded Canned Tuna. J. Food Compos. Anal. 2023, 115, 105026. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Cheng, Y.; Fu, D.; Chen, Z.; Wang, Y.; Zhang, L.; Yao, C.; Shi, L.; Li, M.; et al. Copper-Based Metal–Organic Framework Overcomes Cancer Chemoresistance through Systemically Disrupting Dynamically Balanced Cellular Redox Homeostasis. J. Am. Chem. Soc. 2022, 144, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- López-Gallego, F.; Salassa, L. Catalysis toward Metal-Based Substrates: A New Prospect for Inorganic Chemistry. Chem Catal. 2023, 3, 100459. [Google Scholar] [CrossRef]
- da Silva, D.A.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.L.; Cerchiaro, G. Copper in Tumors and the Use of Copper-Based Compounds in Cancer Treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhu, S.; Yang, H.; Cui, H.; Guo, H.; Deng, J.; Ren, Z.; Geng, Y.; Ouyang, P.; Xu, Z.; et al. The Dysregulation of Inflammatory Pathways Triggered by Copper Exposure. Biol. Trace Elem. Res. 2023, 201, 539–548. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, S.; Beyens, A.; Callewaert, B. ATP7A-Related Copper Transport Disorders: A Systematic Review and Definition of the Clinical Subtypes. J. Inherit. Metab. Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lubna, S.; Ahmad, R. Clinical and Biochemical Understanding of Zinc Interaction during Liver Diseases: A Paradigm Shift. J. Trace Elem. Med. Biol. 2023, 77, 127130. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc Coordination Complexes as Anticancer Agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J. V. A Review on Current Research Status of the Surface Modification of Zn-Based Biodegradable Metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Pena, E.S.; Lifshits, L.M.; Eckshtain-Levi, M.; Bachelder, E.M.; Ainslie, K.M. Metal–Organic Coordination Polymers for Delivery of Immunomodulatory Agents, and Infectious Disease and Cancer Vaccines. WIREs Nanomedicine and Nanobiotechnology 2023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shao, K.; Zhang, F.; Wang, T.; Han, L.; Kong, X.; Shi, J. “Block and Attack” Strategy for Tumor Therapy through ZnO 2 /SiRNA/NIR-mediating Zn 2+ -overload and Amplified Oxidative Stress. Aggregate 2023. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Zhang, L.; He, F.; Wang, X.; Ye, J. Enhancing Osteoinduction and Bone Regeneration of Biphasic Calcium Phosphate Scaffold Thought Modulating the Balance between Pro-Osteogenesis and Anti-Osteoclastogenesis by Zinc Doping. Mater. Today Chem. 2023, 29, 101410. [Google Scholar] [CrossRef]
- Hussain, M.; Ullah, S.; Raza, M.R.; Abbas, N.; Ali, A. Recent Developments in Zn-Based Biodegradable Materials for Biomedical Applications. J. Funct. Biomater. 2022, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Schio, L.; Bavdek, G.; Grazioli, C.; Gutiérrez Bolaños, C.; Goldoni, A.; Vittadini, A.; Tormen, M.; Floreano, L. Role of Axial Coordination in the Adsorption Configuration of M(II)-Tetraphenylporphyrins (M = Co, Ni, Cu, Zn) on r-TiO <math Altimg="si73.Svg" Display="inline" Id="d1e649"> <msub> <mrow/> <mrow> <mn>2</Mn> </Mrow> </Msub> </Math> (110). Appl. Surf. Sci. 2023, 616, 156548. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Li, L.; Du, Z. Evaluation of Serum Levels of Copper and Zinc in Patients with Celiac Disease Seropositivity: Findings from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2023, 201, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Y.; Huang, C.; Xu, Y.; Xu, Y. Electrogenerated Copper Selenide with Positive Charge to Efficiently Capture and Combat Drug-Resistant Bacteria for Wound Healing. J. Colloid Interface Sci. 2023, 634, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Vančo, J.; Trávníček, Z.; Hošek, J.; Malina, T.; Dvořák, Z. Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-Inflammatory Effects. Int. J. Mol. Sci. 2021, 22, 7626. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Das, D.D.; Chawla, P.A. Exploring the Potential of Trientine Tetrahydrochloride in the Treatment of Wilson Disease. Heal. Sci. Rev. 2023, 6, 100082. [Google Scholar] [CrossRef]
- Chaudhari, V.; Bagwe-Parab, S.; Buttar, H.S.; Gupta, S.; Vora, A.; Kaur, G. Challenges and Opportunities of Metal Chelation Therapy in Trace Metals Overload-Induced Alzheimer’s Disease. Neurotox. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Re-evaluation of the Existing Health-based Guidance Values for Copper and Exposure Assessment from All Sources. EFSA J. 2023, 21. [Google Scholar] [CrossRef]
- Mhaske, A.; Sharma, S.; Shukla, R. Nanotheranostic: The Futuristic Therapy for Copper Mediated Neurological Sequelae. J. Drug Deliv. Sci. Technol. 2023, 80, 104193. [Google Scholar] [CrossRef]
- Wang, X.; Lou, Q.; Fan, T.; Zhang, Q.; Yang, X.; Liu, H.; Fan, R. Copper Transporter Ctr1 Contributes to Enhancement of the Sensitivity of Cisplatin in Esophageal Squamous Cell Carcinoma. Transl. Oncol. 2023, 29, 101626. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, R.; Polishchuk, R.S. Activity and Trafficking of Copper-Transporting ATPases in Tumor Development and Defense against Platinum-Based Drugs. Cells 2019, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cao, W.; Ren, Y.; Zhang, Q.; Liu, J. ATPase Copper Transporter A, Negatively Regulated by MiR-148a-3p, Contributes to Cisplatin Resistance in Breast Cancer Cells. Clin. Transl. Med. 2020, 10, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.; Duarte, D.; Vale, N.; Ricardo, S. The Antineoplastic Effect of Carboplatin Is Potentiated by Combination with Pitavastatin or Metformin in a Chemoresistant High-Grade Serous Carcinoma Cell Line. Int. J. Mol. Sci. 2022, 24, 97. [Google Scholar] [CrossRef]
- Mariniello, M.; Petruzzelli, R.; Wanderlingh, L.G.; La Montagna, R.; Carissimo, A.; Pane, F.; Amoresano, A.; Ilyechova, E.Y.; Galagudza, M.M.; Catalano, F.; et al. Synthetic Lethality Screening Identifies FDA-Approved Drugs That Overcome ATP7B-Mediated Tolerance of Tumor Cells to Cisplatin. Cancers (Basel). 2020, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Huang, Y.-F.; Chou, C.-Y.; Chen, H.H.W. Targeting the Copper Transport System to Improve Treatment Efficacies of Platinum-Containing Drugs in Cancer Chemotherapy. Pharmaceuticals 2021, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, A.; Qiu, Y.; Li, Z.; Wu, X.; Li, Z.; Wu, A.; Yang, F. Navigating Zinc-Involved Nanomedicine in Oncotherapy. Nanoscale 2023. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, A.; Doss, A.; Praveen Pole, R.P.; Pushpa Rani, T.P.K.; Prasad, R.; Satheesh, S. A Mini Review on Plant-Mediated Zinc Oxide Nanoparticles and Their Antibacterial Potency. Biocatal. Agric. Biotechnol. 2023, 48, 102654. [Google Scholar] [CrossRef]
- Liu, H.-M.; Tang, W.; Wang, X.-Y.; Jiang, J.-J.; Zhang, W.; Wang, W. Safe and Effective Antioxidant: The Biological Mechanism and Potential Pathways of Ergothioneine in the Skin. Molecules 2023, 28, 1648. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, R.P.; Nayak, K.H.; Sreelekha, M.K.; Babu, B.P. Progress in Copper-Catalysed/Mediated Intramolecular Dehydrogenative Coupling. Org. Biomol. Chem. 2023, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wu, X.; Kang, X. Integrated the Embedding Delivery System and Targeted Oxygen Scavenger Enhances Free Radical Scavenging Capacity. Food Chem. X 2023, 17, 100558. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 20, 2197. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.-L.; Tong, P.-H.; Meng, Y.-J.; Li, J.-P. Development of a Molecularly Imprinted Electrochemiluminescence Sensor Based on Bifunctional Bilayer Structured ZIF-8-Based Magnetic Particles for Dopamine Sensing. Chinese J. Anal. Chem. 2023, 51, 100226. [Google Scholar] [CrossRef]
- Asensio, G.; Martín-del-Campo, M.; Ramírez, R.A.; Rojo, L.; Vázquez-Lasa, B. New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds. Pharmaceutics 2023, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.D.; Cerchiaro, G.; Morelli Frin, K.P. Rhenium(I) Polypyridine Complexes Coordinated to an Ethyl-Isonicotinate Ligand: Luminescence and in Vitro Anti-Cancer Studies. Inorganica Chim. Acta 2020, 501, 119329. [Google Scholar] [CrossRef]
- Guedes, A.P.M.; Mello-Andrade, F.; Pires, W.C.; de Sousa, M.A.M.; da Silva, P.F.F.; de Camargo, M.S.; Gemeiner, H.; Amauri, M.A.; Gomes Cardoso, C.; de Melo Reis, P.R.; et al. Heterobimetallic Ru( Ii)/Fe( Ii) Complexes as Potent Anticancer Agents against Breast Cancer Cells, Inducing Apoptosis through Multiple Targets. Metallomics 2020, 12, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal Drugs and the Anticancer Immune Response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, X.; Cun, J.-E.; Li, J.; Pu, Y.; He, B. In-Situ Drug Generation and Controllable Loading: Rational Design of Copper-Based Nanosystems for Chemo-Photothermal Cancer Therapy. Chem. Eng. J. 2021, 409, 128222. [Google Scholar] [CrossRef]
- Singh, R.; Singh, G.; George, N.; Singh, G.; Gupta, S.; Singh, H.; Kaur, G.; Singh, J. Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry. Catalysts 2023, 13, 130. [Google Scholar] [CrossRef]
- Mohan, B.; Kamboj, A.; Virender; Singh, K. ; Priyanka; Singh, G.; Pombeiro, A.J.L.; Ren, P. Metal-Organic Frameworks (MOFs) Materials for Pesticides, Heavy Metals, and Drugs Removal: Environmental Safety. Sep. Purif. Technol. 2023, 310, 123175. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Zhang, S.; Shi, S.; Zong, R.; Gao, Y.; Guan, B.; Gamper, N.; Gao, H. Intracellular Zinc Protects Kv7 K+ Channels from Ca2+/Calmodulin-Mediated Inhibition. J. Biol. Chem. 2023, 299, 102819. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, İ.B.; Chłodowska, A.; Olejnik, M. Ionophore Toxicity in Animals: A Review of Clinical and Molecular Aspects. Int. J. Mol. Sci. 2023, 24, 1696. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pang, X.; Nie, L.; Zhu, C.; Zhuo, K.; Zhuo, Q.; Chen, Z.; Liu, G.; Zhang, H.; Lin, Z.; et al. Successive Modification of Polydentate Complexes Gives Access to Planar Carbon- and Nitrogen-Based Ligands. Nat. Commun. 2019, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Tafazzoli, A.; Keypour, H.; Farida, S.H.M.; Ahmadvand, Z.; Gable, R.W. Synthesis, Biological Activities and Theoretical Studies of a New Macroacyclic Schiff Base Ligand and Its Related Co(II), Ni(II), and Cu(II) Complexes: The X-Ray Crystal Structure of the Co(II) Complex. J. Mol. Struct. 2023, 1276, 134770. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Wu, F.-F.; Wang, Z.-X.; He, X.; Xing, F.-F.; Li, M.-X. Syntheses, Crystal Structures, Luminescent and Magnetic Properties of Six 5,5′-(1,2-Phenylenebis(Methoxy))Diisophthalate Coordination Polymers. Inorganica Chim. Acta 2023, 547, 121357. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, X.; Geng, D.; Pan, W.; Li, Z.; Wang, G.; Li, D.; Li, C.; Feng, S.; Zhu, L.; et al. H2O2/NIR-Sensitive “Two-Step” Nano Theranostic System Based Hollow Mesoporous Copper Sulfide/Hyaluronic Acid/JWH133 as an Optimally Designed Delivery System for Multidimensional Treatment of RA. Int. J. Biol. Macromol. 2023, 225, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M. Metallobiology and Therapeutic Chelation of Biometals (Copper, Zinc and Iron) in Alzheimer’s Disease: Limitations, and Current and Future Perspectives. J. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef] [PubMed]
- Pokorný, V.; Štejfa, V.; Havlín, J.; Fulem, M.; Růžička, K. Heat Capacities of L-Cysteine, L-Serine, L-Threonine, L-Lysine, and L-Methionine. Molecules 2023, 28, 451. [Google Scholar] [CrossRef] [PubMed]
- Pooventhiran, T.; Alzahrani, A.Y.A.; Rajimon, K.J.; Thomas, R. Solvent Interaction and Dynamics of Neurotransmitters -aspartic Acid and -glutamic Acid with Water and Ethanol. J. Mol. Struct. 2023, 1273, 134347. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Salem, N.M.H.; Fischer, R.C.; Torvisco, A.; Mautner, F.A.; Vančo, J.; Belza, J.; Dvořák, Z.; Trávníček, Z. Dinuclear Doubly Bridged Phenoxido Copper(II) Complexes as Efficient Anticancer Agents. Eur. J. Med. Chem. 2023, 246, 114992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-L.; Hou, X.-X.; Liu, M.-R.; Huang, F.-P.; Qin, X.-Y. Two Novel Chiral Tetranucleate Copper-Based Complexes: Crystal Structures, Nanoparticles, and Inhibiting Angiogenesis and the Growth of Human Breast Cancer by Regulating the VEGF/VEGFR2 Signal Pathway in Vitro. Dalt. Trans. 2020, 49, 6043–6055. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Johnson, A.; Northcote-Smith, J.; Lu, C.; Suntharalingam, K. Immunogenic Cell Death of Breast Cancer Stem Cells Induced by an Endoplasmic Reticulum-Targeting Copper(II) Complex. ChemBioChem 2020, 21, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi Malekshah, R.; Fahimirad, B.; Khaleghian, A. Synthesis, Characterization, Biomedical Application, Molecular Dynamic Simulation and Molecular Docking of Schiff Base Complex of Cu(II) Supported on Fe3O4/SiO2/APTS. Int. J. Nanomedicine 2020, 15, 2583–2603. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation Mobilizes Copper Metabolism to Promote Colon Tumorigenesis via an IL-17-STEAP4-XIAP Axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, J.; Santos, H.A. Recent Advances in Fenton and Fenton-like Reaction Mediated Nanoparticle in Cancer Therapy. Biomed. Technol. 2023, 3, 40–51. [Google Scholar] [CrossRef]
- Harris, E.D. Cellular Copper Transport and Metabolism. Annu. Rev. Nutr. 2000, 20, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Brady, D.C.; Wittung-Stafshede, P. Evaluation of Copper Chaperone ATOX1 as Prognostic Biomarker in Breast Cancer. Breast Cancer 2020, 27, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chen, C.; Liu, T.; Liu, C.; Liu, S.; Fang, J.; Shangguan, L. Germplasm Resource Evaluation and the Underlying Regulatory Mechanisms of the Differential Copper Stress Tolerance among Vitis Species. Environ. Exp. Bot. 2023, 206, 105198. [Google Scholar] [CrossRef]
- Guffy, S.L.; Pulavarti, S.V.S.R.K.; Harrison, J.; Fleming, D.; Szyperski, T.; Kuhlman, B. Inside-Out Design of Zinc-Binding Proteins with Non-Native Backbones. Biochemistry 2023, 62, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Grant, E.P.; Romain, D.S. St.; Salem, N.M.H.; Louka, F.R.; Massoud, S.S. Copper(II) and Zinc(II) Complexes Bridged by Benzenoid Aromatic Oxocarbon and Dicarboxylate Dianions. Polyhedron 2023, 234, 116327. [Google Scholar] [CrossRef]
- Giacomazzo, G.E.; Paderni, D.; Giorgi, L.; Formica, M.; Mari, L.; Montis, R.; Conti, L.; Macedi, E.; Valtancoli, B.; Giorgi, C.; et al. A New Family of Macrocyclic Polyamino Biphenolic Ligands: Acid-Base Study, Zn(II) Coordination and Glyphosate/AMPA Binding. Molecules 2023, 28, 2031. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhao, L.-R.; Zhang, J.; Wang, X.-Y.; Yu, Y.-M.; Yu, S.-Y. Heteroleptic Copper( i ) Complexes Bearing Functionalized 1 H -Pyrazole-Bipyridine Ligands: Synthesis, Photophysical Properties, Crystal Structures, and Applications in Halogen Sensing. New J. Chem. 2023. [Google Scholar] [CrossRef]
- Pena-Bonhome, C.; Fiaccabrino, D.; Rama, T.; Fernández-Pavón, D.; Southcott, L.; Zhang, Z.; Lin, K.-S.; de Blas, A.; Patrick, B.O.; Schaffer, P.; et al. Toward 68 Ga and 64 Cu Positron Emission Tomography Probes: Is H 2 Dedpa- N, N ′-Pram the Missing Link for Dedpa Conjugation? Inorg. Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, J.-Q.; Liu, Y.-Y.; He, X. Structural Diversity and Luminescence of Zinc Coordination Polymers Constructed by Flexible Ligands. J. Mol. Struct. 2023, 1282, 135183. [Google Scholar] [CrossRef]
- Adhikari, S.; Bhattacharjee, T.; Butcher, R.J.; Porchia, M.; De Franco, M.; Marzano, C.; Gandin, V.; Tisato, F. Synthesis and Characterization of Mixed-Ligand Zn(II) and Cu(II) Complexes Including Polyamines and Dicyano-Dithiolate(2-): In Vitro Cytotoxic Activity of Cu(II) Compounds. Inorganica Chim. Acta 2019, 498, 119098. [Google Scholar] [CrossRef]
- Costa, L.M.O.; Reis, I.S.; Fernandes, C.; Marques, M.M.; Resende, J.A.L.C.; Krenske, E.H.; Schenk, G.; Gahan, L.R.; Horn, A. Synthesis, Characterization and Computational Investigation of the Phosphatase Activity of a Dinuclear Zinc(II) Complex Containing a New Heptadentate Asymmetric Ligand. J. Inorg. Biochem. 2023, 239, 112064. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.E.; Sotorrios, L.; Bisai, M.K.; Yuan, K.; Macgregor, S.A.; Ingleson, M.J. Understanding and Expanding Zinc Cation/Amine Frustrated Lewis Pair Catalyzed C–H Borylation. ACS Catal. 2023, 13, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Ma, Z.-Y.; Qiao, P.-P.; Gao, C.-Y.; Tian, J.-L.; Zhao, J.-Z.; Du, W.-J.; Xu, J.-Y.; Yan, S.-P. Copper Based Metallonucleases as Potential Antitumor Drugs: Synthesis, Structure, in Vitro Cytotoxicity and Apoptosis Inducing Properties. J. Mol. Struct. 2021, 1236, 130278. [Google Scholar] [CrossRef]
- Tsang, C.Y.; Cheung, M.C.Y.; Beyer, S. Assessing the Colloidal Stability of Copper Doped ZIF-8 in Water and Serum. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130452. [Google Scholar] [CrossRef]
- Moharramnejad, M.; Ehsani, A.; Salmani, S.; Shahi, M.; Malekshah, R.E.; Robatjazi, Z.S.; Parsimehr, H. Zinc-Based Metal-Organic Frameworks: Synthesis and Recent Progress in Biomedical Application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3339–3354. [Google Scholar] [CrossRef]
- Lawson, S.; Newport, K.; Schueddig, K.; Rownaghi, A.A.; Rezaei, F. Optimizing Ibuprofen Concentration for Rapid Pharmacokinetics on Biocompatible Zinc-Based MOF-74 and UTSA-74. Mater. Sci. Eng. C 2020, 117, 111336. [Google Scholar] [CrossRef] [PubMed]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. Alginate-Coated ZIF-8 Metal-Organic Framework as a Green and Bioactive Platform for Controlled Drug Release. J. Drug Deliv. Sci. Technol. 2019, 49, 570–576. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Huang, L.; Zhang, W.; Wang, R.; Yue, T.; Sun, J.; Li, G.; Wang, J. The Highly Efficient Elimination of Intracellular Bacteria via a Metal Organic Framework (MOF)-Based Three-in-One Delivery System. Nanoscale 2019, 11, 9468–9477. [Google Scholar] [CrossRef] [PubMed]
- Gharehdaghi, Z.; Rahimi, R.; Naghib, S.M.; Molaabasi, F. Fabrication and Application of Copper Metal–Organic Frameworks as Nanocarriers for PH-Responsive Anticancer Drug Delivery. J. Iran. Chem. Soc. 2022, 19, 2727–2737. [Google Scholar] [CrossRef]
- Liu, X.; Qian, B.; Zhang, D.; Yu, M.; Chang, Z.; Bu, X. Recent Progress in Host–Guest Metal–Organic Frameworks: Construction and Emergent Properties. Coord. Chem. Rev. 2023, 476, 214921. [Google Scholar] [CrossRef]
- Ye, R.; Tan, C.; Chen, B.; Li, R.; Mao, Z. Zinc-Containing Metalloenzymes: Inhibition by Metal-Based Anticancer Agents. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Y.; Liu, Y.; Xiang, Y.; Liu, G.; Zhang, Q.; Yin, Y.; Cai, Y.; Jiang, G. Advances in Bacterial Whole-Cell Biosensors for the Detection of Bioavailable Mercury: A Review. Sci. Total Environ. 2023, 868, 161709. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, F.; He, H.; Du, P.; Liu, Y.; Wang, W.; Wang, S.; Ma, Y.; Chu, X.; Wang, Y.; et al. Predicting the Formation of 2-Amino-3-Methyl-Imidazole[4,5-f]Quinoline (IQ) in the Maillard Reaction Model System under Various Reaction Conditions. LWT 2023, 176, 114551. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Huang, C.-H.; Wibowo, F.S.; Amin, R.; Chen, P.; Li, F. Diethyldithiocarbamate Copper Nanoparticle Overcomes Resistance in Cancer Therapy without Inhibiting P-Glycoprotein. Nanomedicine Nanotechnology, Biol. Med. 2023, 47, 102620. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Kabesova, M.; Benes, J.; Pouckova, P.; Vetvicka, D. Advances in Liposome-Encapsulated Phthalocyanines for Photodynamic Therapy. Life 2023, 13, 305. [Google Scholar] [CrossRef]
- Lin, Y.-D.; Tsai, W.-W.; Lu, C.-W. Exploring the Electroluminescent Applications of Imidazole Derivatives. Chem. – A Eur. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Mavroidi, B.; Zahariou, G.; Pelecanou, M.; Hatzidimitriou, A.G. Structurally Characterized Copper Complexes of Flavonoid Naringenin with Enhanced Radical Scavenging Activity. Inorganica Chim. Acta 2023, 546, 121325. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper(II) Complexes as Potential Anticancer and Nonsteroidal Anti-Inflammatory Agents: In Vitro and in Vivo Studies. Sci. Rep. 2019, 9, 5237. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, T.R.; Deb, J.; Ghosh, I.; Sarkar, S.; Paine, T.K. Combining Anti-Inflammatory and Anti-Proliferative Activities in Ternary Metal-NSAID Complexes of a Polypyridylamine Ligand. Inorganica Chim. Acta 2019, 486, 663–668. [Google Scholar] [CrossRef]
- Yuan, J.; Song, J.-Y.; Yang, H.-H.; Lan, H.-R.; Xing, A.-P.; Li, K.-H.; Zeng, D.; Zhang, Z.-Q.; Feng, S.-Y. Synthesis, Cytotoxicity and DNA Binding of Novel Ni(II), Co(II) and Zn(II) Complexes Bearing Pyrimidinyl Hydrazone Ligand. J. Mol. Struct. 2023, 1276, 134724. [Google Scholar] [CrossRef]
- Christidou, A.; Zavalani, K.; Hatzidimitriou, A.G.; Psomas, G. Copper(II) Complexes with 3,5–Dihalogeno–Salicylaldehydes: Synthesis, Structure and Interaction with DNA and Albumins. J. Inorg. Biochem. 2023, 238, 112049. [Google Scholar] [CrossRef] [PubMed]
- Deb, J.; Lakshman, T.R.; Ghosh, I.; Jana, S.S.; Paine, T.K. Mechanistic Studies of in Vitro Anti-Proliferative and Anti-Inflammatory Activities of the Zn( Ii )–NSAID Complexes of 1,10-Phenanthroline-5,6-Dione in MDA-MB-231 Cells. Dalt. Trans. 2020, 49, 11375–11384. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Akdağ, Ö.; Orman, E.B.; Odabaş, Z.; Özkaya, A.R. Electrochemical and In-Situ Spectroelectrochemical Properties of Novel (5-(Tert-Butyl)-2-((3,4-Dicyanophenoxy)Methyl)Phenyl)Methanolate Substituted Mononuclear Metal Phthalocyanines. J. Mol. Struct. 2023, 1276, 134769. [Google Scholar] [CrossRef]
- Nardi, M.; Cano, N.C.H.; Simeonov, S.; Bence, R.; Kurutos, A.; Scarpelli, R.; Wunderlin, D.; Procopio, A. A Review on the Green Synthesis of Benzimidazole Derivatives and Their Pharmacological Activities. Catalysts 2023, 13, 392. [Google Scholar] [CrossRef]
- Zhi, S.; Li, Y.; Qiang, J.; Hu, J.; Song, W.; Zhao, J. Synthesis and Anticancer Evaluation of Benzo-N-Heterocycles Transition Metal Complexes against Esophageal Cancer Cell Lines. J. Inorg. Biochem. 2019, 201, 110816. [Google Scholar] [CrossRef] [PubMed]
- Baishya, T.; Gomila, R.M.; Barceló-Oliver, M.; Gil, D.M.; Bhattacharyya, M.K.; Frontera, A. Supramolecular Assemblies in Pyridine- and Pyrazole-Based Coordination Compounds of Co(II) and Ni(II): Characterization, Hirshfeld Analysis and Theoretical Studies. Crystals 2023, 13, 203. [Google Scholar] [CrossRef]
- Sankarganesh, M.; Dhaveethu Raja, J.; Adwin Jose, P.R.; Vinoth Kumar, G.G.; Rajesh, J.; Rajasekaran, R. Spectroscopic, Computational, Antimicrobial, DNA Interaction, In Vitro Anticancer and Molecular Docking Properties of Biochemically Active Cu(II) and Zn(II) Complexes of Pyrimidine-Ligand. J. Fluoresc. 2018, 28, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wei, K.; Gao, Y.; Zhou, Z.; Zheng, X.; Li, J.; Qi, J. Comparative Evaluation of the Structure and Antitumor Mechanism of Mononuclear and Trinucleated Thiosemicarbazone Cu(II) Complexes. J. Inorg. Biochem. 2023, 240, 112116. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Rehman, S.; Feroz, I.; Farzia; Khan, R. ; Sinnokrot, M.O.; Subhan, F.; Naeem, M.; Schulzke, C. Synthesis, Spectral, Hirshfeld Surface Analysis and Biological Evaluation of a Schiff Base Copper(II) Complex: Towards a Copper(II) Based Human Anti-Glioblastoma Agent. J. Mol. Struct. 2023, 1278, 134960. [Google Scholar] [CrossRef]
- Alam, M.Z. ; Alimuddin; Khan, S.A. A Review on Schiff Base as a Versatile Fluorescent Chemo-Sensors Tool for Detection of Cu2+ and Fe3+ Metal Ion. J. Fluoresc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, X.; Yang, P.; Zhao, J.; Zhang, W.; Feng, N.; Yang, W.; Tang, J. Defect Self-Assembly of Metal-Organic Framework Triggers Ferroptosis to Overcome Resistance. Bioact. Mater. 2023, 19, 1–11. [Google Scholar] [CrossRef]
- Vieira, A.P.; Wegermann, C.A.; Da Costa Ferreira, A.M. Comparative Studies of Schiff Base-Copper(Ii) and Zinc(Ii) Complexes Regarding Their DNA Binding Ability and Cytotoxicity against Sarcoma Cells. New J. Chem. 2018, 42, 13169–13179. [Google Scholar] [CrossRef]
- Ramírez-Palma, L.G.; Castro-Ramírez, R.; Lozano-Ramos, L.; Galindo-Murillo, R.; Barba-Behrens, N.; Cortés-Guzmán, F. DNA Recognition Site of Anticancer Tinidazole Copper( Ii ) Complexes. Dalt. Trans. 2023, 52, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Jose, J.R.; Krishna, P.M.; Philip, D.; Joy, F.; Vinod, T.P.; Prathapachandra Kurup, M.R.; Nair, Y. In Vitro Analytical Techniques as Screening Tools to Investigate the Metal Chelate-DNA Interactions. ChemistrySelect 2023, 8. [Google Scholar] [CrossRef]
- de Oliveira, J.A.F.; Terra, G.G.; Costa, T.G.; Szpoganicz, B.; Silva-Caldeira, P.P.; de Souza, Í.P.; Pereira-Maia, E.C.; Bortoluzzi, A.J. Synthesis, Characterization and Cytotoxicity of Copper (II) Complex Containing a 2H-Benzo[e][1,3]Oxazin Derivative. J. Inorg. Biochem. 2023, 239, 112087. [Google Scholar] [CrossRef] [PubMed]
- Şahal, H. Zinc(II) Phthalocyanine Substituted by Sulfonamide Derivative: Photophysical and Photochemical Properties. J. Mol. Struct. 2023, 1273, 134275. [Google Scholar] [CrossRef]
- Carroll, G.T.; Dowling, R.C.; Kirschman, D.L.; Masthay, M.B.; Mammana, A. Intrinsic Fluorescence of UV-Irradiated DNA. J. Photochem. Photobiol. A Chem. 2023, 437, 114484. [Google Scholar] [CrossRef]
- AlAjmi, M.; Hussain, A.; Rehman, M.; Khan, A.; Shaikh, P.; Khan, R. Design, Synthesis, and Biological Evaluation of Benzimidazole-Derived Biocompatible Copper(II) and Zinc(II) Complexes as Anticancer Chemotherapeutics. Int. J. Mol. Sci. 2018, 19, 1492. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Han, J.H.; Min, K.S. Ferromagnetic Chloro-Bridged Copper(II) Coordination Polymer: Synthesis, Structure, Magnetism, and DNA Cleavage Effects. J. Mol. Struct. 2023, 1271, 134136. [Google Scholar] [CrossRef]
- Dhanaraj, C.J.; Hassan, I.U.; Johnson, J.; Joseph, J.; Joseyphus, R.S. Synthesis, Spectral Characterization, DNA Interaction, Anticancer and Molecular Docking Studies on Some Transition Metal Complexes with Bidentate Ligand. J. Photochem. Photobiol. B Biol. 2016, 162, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Xie, X.-X.; Huang, Z.-Y.; Kuang, Z.-Y.; Cai, S.-L.; Zhang, W.-G.; Zheng, S.-R. Two Cu(i) Coordination Polymers Based on a New Benzimidazolyl-Tetrazolyl Heterotopic Ligand for Visible-Light-Driven Photocatalytic Dye Degradation. CrystEngComm 2023, 25, 417–424. [Google Scholar] [CrossRef]
- Hendle, J.; Sauder, J.M.; Hickey, M.J.; Rauch, C.T.; Maletic, M.; Schwinn, K.D. ULK1 Unc-51 like Autophagy Activating Kinase in Complex with Inhibitor BTC. Available online: https://www.rcsb.org/structure/6MNH (accessed on 24 February 2023).
- Nicolaou, C.A.; Humblet, C.; Hu, H.; Martin, E.M.; Dorsey, F.C.; Castle, T.M.; Burton, K.I.; Hu, H.; Hendle, J.; Hickey, M.J.; et al. Idea2Data: Toward a New Paradigm for Drug Discovery. ACS Med. Chem. Lett. 2019, 10, 278–286. [Google Scholar] [CrossRef]
- Chaikuad, A.; Ren, H.; Bakas, N.A.; Lambert, L.J.; Cosford, N.D.P.; Knapp, S. Crystal Structure of ULK2 in Complex with SBI-0206965. Available online: https://www.rcsb.org/structure/6YID (accessed on 24 February 2023).
- Ren, H.; Bakas, N.A.; Vamos, M.; Chaikuad, A.; Limpert, A.S.; Wimer, C.D.; Brun, S.N.; Lambert, L.J.; Tautz, L.; Celeridad, M.; et al. Design, Synthesis, and Characterization of an Orally Active Dual-Specific ULK1/2 Autophagy Inhibitor That Synergizes with the PARP Inhibitor Olaparib for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2020, 63, 14609–14625. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, M.; Pan, Y.; Zhang, Y. Ligand-Enhanced Zero-Valent Iron for Organic Contaminants Degradation: A Mini Review. Processes 2023, 11, 620. [Google Scholar] [CrossRef]
- Picariello, G.; Siano, F.; Di Stasio, L.; Mamone, G.; Addeo, F.; Ferranti, P. Structural Properties of Food Proteins Underlying Stability or Susceptibility to Human Gastrointestinal Digestion. Curr. Opin. Food Sci. 2023, 50, 100992. [Google Scholar] [CrossRef]
- Johnson, K.R.; Driscoll, D.M.; Damron, J.T.; Ivanov, A.S.; Jansone-Popova, S. Size Selective Ligand Tug of War Strategy to Separate Rare Earth Elements. JACS Au 2023. [Google Scholar] [CrossRef]
- Rochford, G.; Molphy, Z.; Kavanagh, K.; McCann, M.; Devereux, M.; Kellett, A.; Howe, O. Cu(Ii) Phenanthroline–Phenazine Complexes Dysregulate Mitochondrial Function and Stimulate Apoptosis. Metallomics 2020, 12, 65–78. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.; Karcz, D.; Jenkins, H.; McClean, S.; Devereux, M.; Howe, O.; Pereira, M.D.; May, N. V.; Enyedy, É.A.; Creaven, B.S. Copper(II) Complexes of Coumarin-Derived Schiff Base Ligands: Pro- or Antioxidant Activity in MCF-7 Cells? J. Inorg. Biochem. 2019, 197, 110702. [Google Scholar] [CrossRef] [PubMed]
- Carcelli, M.; Tegoni, M.; Bartoli, J.; Marzano, C.; Pelosi, G.; Salvalaio, M.; Rogolino, D.; Gandin, V. In Vitro and in Vivo Anticancer Activity of Tridentate Thiosemicarbazone Copper Complexes: Unravelling an Unexplored Pharmacological Target. Eur. J. Med. Chem. 2020, 194, 112266. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.-D.; Song, X.-Q.; Kong, Y.; Li, F.-F.; Liao, W.-H.; Zhou, J.; Zhang, J.; Zhao, Q.-H.; Xu, J.-Y.; Chen, C.; et al. A New Schiff Base Copper(II) Complex Induces Cancer Cell Growth Inhibition and Apoptosis by Multiple Mechanisms. J. Inorg. Biochem. 2020, 208, 111103. [Google Scholar] [CrossRef] [PubMed]
- Dankhoff, K.; Gold, M.; Kober, L.; Schmitt, F.; Pfeifer, L.; Dürrmann, A.; Kostrhunova, H.; Rothemund, M.; Brabec, V.; Schobert, R.; et al. Copper(Ii) Complexes with Tridentate Schiff Base-like Ligands: Solid State and Solution Structures and Anticancer Activity. Dalt. Trans. 2019, 48, 15220–15230. [Google Scholar] [CrossRef] [PubMed]
- Naqi Ahamad, M.; Iman, K.; Raza, M.K.; Kumar, M.; Ansari, A.; Ahmad, M.; Shahid, M. Anticancer Properties, Apoptosis and Catecholase Mimic Activities of Dinuclear Cobalt(II) and Copper(II) Schiff Base Complexes. Bioorg. Chem. 2020, 95, 103561. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Zhang, L.; Zhang, J.; Li, C.; Zhang, N.; Xu, H.; Li, Y. A New Schiff Base Coordinated Copper(II) Compound Induces Apoptosis and Inhibits Tumor Growth in Gastric Cancer. Cancer Cell Int. 2019, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Sanz del Olmo, N.; Holota, M.; Michlewska, S.; Gómez, R.; Ortega, P.; Ionov, M.; de la Mata, F.J.; Bryszewska, M. Copper (II) Metallodendrimers Combined with Pro-Apoptotic SiRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics 2020, 12, 727. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Liu, W.; Cahill, P.A.; Redmond, E.M. Caveolin-1 Inhibition Mediates the Opposing Effects of Alcohol on Γ-secretase Activity in Arterial Endothelial and Smooth Muscle Cells. Physiol. Rep. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Sudhahar, V.; Okur, M.N.; O’Bryan, J.P.; Minshall, R.D.; Fulton, D.; Ushio-Fukai, M.; Fukai, T. Caveolin-1 Stabilizes ATP7A, a Copper Transporter for Extracellular SOD, in Vascular Tissue to Maintain Endothelial Function. Am. J. Physiol. Physiol. 2020, 319, C933–C944. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Sun, X.; Xu, L.; Chen, R.A.; Liu, D.X. Coronavirus RNA-Dependent RNA Polymerase Interacts with the P50 Regulatory Subunit of Host DNA Polymerase Delta and Plays a Synergistic Role with RNA Helicase in the Induction of DNA Damage Response and Cell Cycle Arrest in the S Phase. Emerg. Microbes Infect. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potency of Copper(II) Complexes of Thiosemicarbazones. J. Inorg. Biochem. 2020, 210, 111134. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, E.; Gandin, V.; Bertani, R.; Sgarbossa, P.; Natarajan, K.; Bhuvanesh, N.S.P.; Venzo, A.; Zoleo, A.; Mozzon, M.; Dolmella, A.; et al. Synthesis, Characterization and Biological Activity of Novel Cu(II) Complexes of 6-Methyl-2-Oxo-1,2-Dihydroquinoline-3-Carbaldehyde-4n-Substituted Thiosemicarbazones. Molecules 2020, 25, 1868. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ni, H.; Liu, F.; Gu, S.; Yu, P.; Gou, Y. Binuclear Schiff Base Copper(II) Complexes: Syntheses, Crystal Structures, HSA Interaction and Anti-Cancer Properties. Inorganica Chim. Acta 2020, 499, 119186. [Google Scholar] [CrossRef]
- Gu, S.; Yu, P.; Hu, J.; Liu, Y.; Li, Z.; Qian, Y.; Wang, Y.; Gou, Y.; Yang, F. Mitochondria-Localizing N-Heterocyclic Thiosemicarbazone Copper Complexes with Good Cytotoxicity and High Antimetastatic Activity. Eur. J. Med. Chem. 2019, 164, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Y.; Chen, R.; Yu, H.; Li, Y.; Chen, M.; Dou, C.; Yin, P.; Zhang, L.; Tang, P. Ghost Messages: Cell Death Signals Spread. Cell Commun. Signal. 2023, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, U.; Jung, J.-W. Small Molecule Inhibitors for Unc-51-like Autophagy-Activating Kinase Targeting Autophagy in Cancer. Int. J. Mol. Sci. 2023, 24, 953. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, K.; Zhou, Y.-G.; Ren, T. Insight into Autophagy in Platinum Resistance of Cancer. Int. J. Clin. Oncol. 2023. [CrossRef] [PubMed]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper Is an Essential Regulator of the Autophagic Kinases ULK1/2 to Drive Lung Adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Y.; Xu, D.; Xu, K.; Liang, S.-Q.; Yang, Z.; Scherz, A.; Hall, S.R.R.; Forster, S.; Berezowska, S.; et al. MEK1 Drives Oncogenic Signaling and Interacts with PARP1 for Genomic and Metabolic Homeostasis in Malignant Pleural Mesothelioma. Cell Death Discov. 2023, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Lu, B.; Xi, J.; Ocansey, D.K.W.; Mao, F.; Hao, D.; Yan, Y. HucMSC-Ex Alleviates IBD-Associated Intestinal Fibrosis by Inhibiting ERK Phosphorylation in Intestinal Fibroblasts. Stem Cells Int. 2023, 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wattanathamsan, O.; Chantaravisoot, N.; Wongkongkathep, P.; Kungsukool, S.; Chetprayoon, P.; Chanvorachote, P.; Vinayanuwattikun, C.; Pongrakhananon, V. Inhibition of Histone Deacetylase 6 Destabilizes ERK Phosphorylation and Suppresses Cancer Proliferation via Modulation of the Tubulin Acetylation-GRP78 Interaction. J. Biomed. Sci. 2023, 30, 4. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-Dependent Autophagic Degradation of GPX4 Drives Ferroptosis. Autophagy 2023, 1–15. [Google Scholar] [CrossRef]
- Frade, B.B.; Dias, R.B.; Gemini Piperni, S.; Bonfim, D.C. The Role of Macrophages in Fracture Healing: A Narrative Review of the Recent Updates and Therapeutic Perspectives. Stem Cell Investig. 2023, 10, 4–4. [Google Scholar] [CrossRef] [PubMed]
- Fnfoon, D.Y.; Al-Adilee, K.J. Synthesis and Spectral Characterization of Some Metal Complexes with New Heterocyclic Azo Imidazole Dye Ligand and Study Biological Activity as Anticancer. J. Mol. Struct. 2023, 1271, 134089. [Google Scholar] [CrossRef]
- Maret, W. The Redox Biology of Redox-Inert Zinc Ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Wabaidur, S.M.; Alam, M.J.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Alam, M.; Al-Resayes, S.I.; Dwivedi, S.; Khan, M.R.; Islam, M.S.; et al. Synthesis, Structural Investigations and Pharmacological Properties of a New Zinc Complex with a N4-Donor Schiff Base Incorporating 2-Pyridyl Ring. Inorganica Chim. Acta 2019, 487, 97–106. [Google Scholar] [CrossRef]
- Khadem-Ansari, M.-H.; Asoudeh, M.; Gheshlaghi, H.F.K.; Nozari, S.; Zarringol, M.; Maroufi, N.F.; Faridvand, Y. Copper and Zinc in Stage I Multiple Myeloma: Relation with Ceruloplasmin, Lipid Peroxidation, and Superoxide Dismutase Activity. Horm. Mol. Biol. Clin. Investig. 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.P.; Addis, Y.; Nunes, P.; Barroso, S.; Alho, I.; Martins, M.; Matos, A.P.A.; Marques, F.; Cavaco, I.; Costa Pessoa, J.; et al. Exploring the Cytotoxic Activity of New Phenanthroline Salicylaldimine Zn(II) Complexes. J. Inorg. Biochem. 2019, 198, 110727. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Cowan, J.A.; Yu, Z.; Arjmand, F. Enantiomeric Copper Based Anticancer Agents Promoting Sequence-Selective Cleavage of G-Quadruplex Telomeric DNA and Non-Random Cleavage of Plasmid DNA. Metallomics 2020, 12, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Afsan, Z.; Roisnel, T.; Tabassum, S.; Arjmand, F. Structure Elucidation {spectroscopic, Single Crystal X-Ray Diffraction and Computational DFT Studies} of New Tailored Benzenesulfonamide Derived Schiff Base Copper(II) Intercalating Complexes: Comprehensive Biological Profile {DNA Binding, PBR322 DNA Clea. Bioorg. Chem. 2020, 94, 103427. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Y.; Xi, Q.-Y.; Huang, K.-B.; Tang, X.-M.; Chen, Z.-F.; Liu, Y.-C.; Liang, H. Crystal Structure, Cytotoxicity and Action Mechanism of Zn(II)/Mn(II) Complexes with Isoquinoline Ligands. J. Inorg. Biochem. 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fatma, H.; Jameel, M.; Siddique, H.R. An Update on Phytochemicals in Redox Homeostasis: “Virtuous or Evil” in Cancer Chemoprevention? Chemistry (Easton). 2023, 5, 201–222. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Wang, D.; Wang, Y.; Zhou, Z.; Ma, X.; Liu, X.; Dong, Y. Cisplatin-Induced Ototoxicity: From Signaling Network to Therapeutic Targets. Biomed. Pharmacother. 2023, 157, 114045. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Kasamatsu, S.; Ihara, H.; Eaton, P. SOD1 Is an Essential H 2 S Detoxifying Enzyme. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Walke, G.R.; Horvath, I.; Kumar, R.; Blockhuys, S.; Holgersson, S.; Walton, P.H.; Wittung-Stafshede, P. Memo1 Binds Reduced Copper Ions, Interacts with Copper Chaperone Atox1, and Protects against Copper-Mediated Redox Activity in Vitro. Proc. Natl. Acad. Sci. 2022, 119. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-M.; Lee, J.; Song, W.J. Design of Artificial Metalloenzymes with Multiple Inorganic Elements: The More the Merrier. J. Inorg. Biochem. 2021, 223, 111552. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.; Jasmer-McDonald, K.; Zhu, S.; Martin, A.L.; Gudekar, N.; Khan, A.; Ladomersky, E.; Singh, K.; Weisman, G.A.; Petris, M.J. ATP7A Delivers Copper to the Lysyl Oxidase Family of Enzymes and Promotes Tumorigenesis and Metastasis. Proc. Natl. Acad. Sci. 2019, 116, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Larisch, S. Targeting XIAP for Promoting Cancer Cell Death—The Story of ARTS and SMAC. Cells 2020, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, F.-F.; Chen, C.-F.; Li, Y.-L.; Liang, H.; Chen, Z.-F. Antitumor Activity of Synthetic Three Copper(II) Complexes with Terpyridine Ligands. J. Inorg. Biochem. 2023, 240, 112093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Chen, S.; Feng, F.; Bai, J.; Li, J. Ammoniated MOF-74(Zn) Derivatives as Luminescent Sensor for Highly Selective Detection of Tetrabromobisphenol A. Ecotoxicol. Environ. Saf. 2020, 187, 109821. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin–Schiff Base Conjugates Bearing Basic Amino Groups as Antimicrobial Phototherapeutic Agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, R.; Sonoyama, H.; Ide, Y.; Fujimura, T.; Sasai, R.; Nagai, A.; Mori, S.; Kaufman, N.E.M.; Zhou, Z.; Vicente, M.G.H.; et al. Synthesis, Photodynamic Activities, and Cytotoxicity of New Water-Soluble Cationic Gallium(III) and Zinc(II) Phthalocyanines. J. Inorg. Biochem. 2019, 192, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Roguin, L.P.; Chiarante, N.; García Vior, M.C.; Marino, J. Zinc(II) Phthalocyanines as Photosensitizers for Antitumor Photodynamic Therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Z.; Yang, C.; Wang, Z.; Zhong, Z.; Wong, M.-S.; Li, H.-W. Tumor Microenvironment-Responsive Zn/Cu Nanoparticles for Enhanced Chemodynamic Therapy. Smart Mater. Med. 2023, 4, 286–293. [Google Scholar] [CrossRef]
- Zhao, S.; He, L.; Sun, Y.; Xu, T.; Chen, C.; Ouyang, Y.; Chen, Y.; Tan, Y.; Zhou, B.; Liu, H. Acid-Responsive Drug-Loaded Copper Phosphate Nanoparticles for Tumor Cell Therapy through Synergistic Apoptosis and Ferroptosis Strategy. J. Nanoparticle Res. 2023, 25, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Shi, L.; Lu, M.; Lee, K.-J.; Ditty, M.M.; Xing, Y.; He, H.-Z.; Ren, X.; Zheng, S.-Y. Nanoscale Coordination Polymers Enabling Antioxidants Inhibition for Enhanced Chemodynamic Therapy. J. Control. Release 2023, 354, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lei, X.-G. Evidence and Metabolic Implications for a New Non-Canonical Role of Cu-Zn Superoxide Dismutase. Int. J. Mol. Sci. 2023, 24, 3230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, N.; Li, M.; Yao, S.; Sun, X.; Feng, X.; Chen, Y. Light-Related Activities of Metal-Based Nanoparticles and Their Implications on Dermatological Treatment. Drug Deliv. Transl. Res. 2023, 13, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Han, G.; Wan, J.; Zhang, S.; Yang, J.; Zong, W.; Niu, Q.; Liu, R. Catalase and Superoxide Dismutase Response and the Underlying Molecular Mechanism for Naphthalene. Sci. Total Environ. 2020, 736, 139567. [Google Scholar] [CrossRef] [PubMed]
- Surur, A.K.; Momesso, V.M.; Lopes, P.M.; Ferrisse, T.M.; Fontana, C.R. Assessment of Synergism between Enzyme Inhibition of Cu/Zn-SOD and Antimicrobial Photodynamic Therapy in Suspension and E. Coli Biofilm. Photodiagnosis Photodyn. Ther. 2023, 41, 103185. [Google Scholar] [CrossRef] [PubMed]
- Karimov, A.; Orujova, A.; Taslimi, P.; Sadeghian, N.; Mammadov, B.; Karaman, H.S.; Farzaliyev, V.; Sujayev, A.; Tas, R.; Alwasel, S.; et al. Novel Functionally Substituted Esters Based on Sodium Diethyldithiocarbamate Derivatives: Synthesis, Characterization, Biological Activity and Molecular Docking Studies. Bioorg. Chem. 2020, 99, 103762. [Google Scholar] [CrossRef] [PubMed]
- Tosha, T. Visualization of Enzymatic Reaction by Time-Resolved Structural Analysis with Photosensitive Caged Substrate. YAKUGAKU ZASSHI 2022, 142, 21-00203–2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, X.; Huang, Y.; Xu, C.; Zeng, Z.; Shan, T.; Guan, Z.; Xu, X.; Huang, Z.; Zhao, C. Reactive Oxygen Species-Activated Self-Amplifying Prodrug Nanoagent for Tumor-Specific Cu-Chelate Chemotherapy and Cascaded Photodynamic Therapy. Biomaterials 2022, 284, 121513. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Chirila, R.M.; Dronca, R.S. Immune Checkpoint Inhibitor Toxicities. Mayo Clin. Proc. 2019, 94, 1321–1329. [Google Scholar] [CrossRef]
- Forero, J.; Roa, E.; Reyes, J.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (NCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/NHAp) Scaffold. Materials (Basel). 2017, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pal, K. Folic-Acid Adorned Alginate-Polydopamine Modified Paclitaxel/Zn-CuO Nanocomplex for PH Triggered Drug Release and Synergistic Antitumor Efficacy. Int. J. Biol. Macromol. 2023, 234, 123602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Han, W.; Zhao, P.; Wang, Z.; Li, M.; Sui, X.; Liu, Y.; Tian, B.; He, Z.; Fu, Q. Programmed PH-Responsive Core–Shell Nanoparticles for Precisely Targeted Therapy of Ulcerative Colitis. Nanoscale 2023, 15, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, C.; Zhang, Q. Novel Insights into the Involvement of Mitochondrial Fission/Fusion in Heart Failure: From Molecular Mechanisms to Targeted Therapies. Cell Stress Chaperones 2023. [Google Scholar] [CrossRef] [PubMed]
| Drug Carrier | Drug | Efficiency | Ref. |
|---|---|---|---|
| ZIF-74 | Ibuprofen | 80 wt% loading efficiency | [128] |
| ZIF-8/Alg | Metformin | 83.5% loading efficiency, and 6.68 wt.% payload. | [129] |
| ZIF-8/HA | Tetracycline | 98% clearance rate under acidic conditions and pH-responsive. | [130] |
| Cu3-(BTC)2/IONP | Doxorubicin | Adsorbed 40.5% and released 85.5% at pH 5 | [131] |
| Cu-TCPP/GO | Adsorbed 45.7 wt.% and released 98.9% at pH 5. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





