1. Introduction
Antibiotic resistance can be accelerated by antibiotic overuse [
1], so ensuring appropriate antibiotic prescribing through antibiotic stewardship is crucial. In England, in 2020, >80% of patient antibiotic prescriptions occurred in primary care, notably in the general practice (GP) setting, which accounted for 73% of patient prescriptions [
2]. Hence, primary care is a key target for stewardship interventions. The COVID-19 pandemic has affected antibiotic prescribing and stewardship in primary care. Total antibiotic consumption in the GP setting showed a 9.4% decline between 2019–2020 in England (following a more gradual decline in prior years; 10.4% between 2016–2019). This decline was at least partly due to reduced incidence of bacterial pathogens in 2020, following reduced social mixing [
2]. Meanwhile, there is evidence that optimal primary care antibiotic stewardship behaviour has been disrupted during the pandemic. The pandemic led to a shift towards online consultation, which can increase diagnostic uncertainty, leading to inappropriate prescribing [
3]. For example, general practitioners reported a lower threshold for prescribing for respiratory infections [
4], and increases in antibiotic prescribing in the dental sector were attributed to restricted access to dental care [
2]. Additionally, antibiotic stewardship may have been de-prioritised given pandemic-related pressures [
5,
6]. Therefore, there is a need to re-enforce antibiotic stewardship in the COVID-19 pandemic era.
The UK’s national action plan on antimicrobial resistance outlines ambitions and actions for antibiotic stewardship [
7]. Regarding primary care, a proposed action is to enhance the role of pharmacists in reviewing the dose and duration of prescriptions, especially repeat prescriptions. Through the “Clinical Pharmacists in General Practice” scheme (established in England in 2019), pharmacists work in multidisciplinary primary care teams [
8], and are therefore well placed to support review of repeat antibiotic prescribing. Repeat prescribing encompasses long-term repeat prescribing for chronic conditions (e.g. prophylaxis for immunosuppressed patients), as well as shorter-term repeated prescribing for acute conditions which fail to resolve after a single antibiotic course [
9]. Repeat prescribing is clinically warranted in some cases, but judicious discontinuation of repeat prescriptions is an important aspect of optimal antibiotic stewardship. Bias towards maintaining the status quo, may lead to repeat prescriptions being inappropriately continued [
9]; bias that may have been exacerbated during the COVID-19 pandemic, given time-constraints and de-prioritisation of antibiotic stewardship. Hence, in a pandemic context, antibiotic stewardship in primary care may need to be re-enforced, and repeat antibiotic prescribing is an important focus for stewardship.
Understanding of the nature and scale of antibiotic prescribing in primary care can guide development of stewardship interventions. Previous studies of antibiotic prescribing in England have often used aggregate data published by the national health service (NHS) [
10,
11]. This data does not include patient-level information (demographics, clinical conditions). OpenSAFELY-TPP is a new secure data platform enabling near-real time analysis of primary care electronic health records, covering ~24 million people registered with GP practices in England (~40% of the total English population) [
12] and is broadly representative of the English population. The platform can provide detailed information on patient demographics, clinical conditions, and prescriptions.
Here, we use the OpenSAFELY-TPP platform to investigate patterns of repeat and non-repeat antibiotic prescribing in primary care, in the context of the COVID-19 pandemic. Total repeat/non-repeat prescribing was assessed from January 2020 (pre-pandemic) through to January 2022. More detailed analysis (broken down by patient demographic characteristics, clinical conditions, and prescribed antibiotic class) was conducted on patient cohorts, pre-pandemic (January 2020), and during the pandemic (January 2022). A key aim of this analysis was to highlight patient groups with the highest burden of repeat antibiotic prescribing in the COVID-19 pandemic era. This will inform future development of antibiotic stewardship interventions supporting clinical pharmacists working in primary care to review repeat antibiotic prescriptions.
2. Results
2.1. Frequency of antibiotic prescribing (repeat and non-repeat) during the COVID-19 pandemic
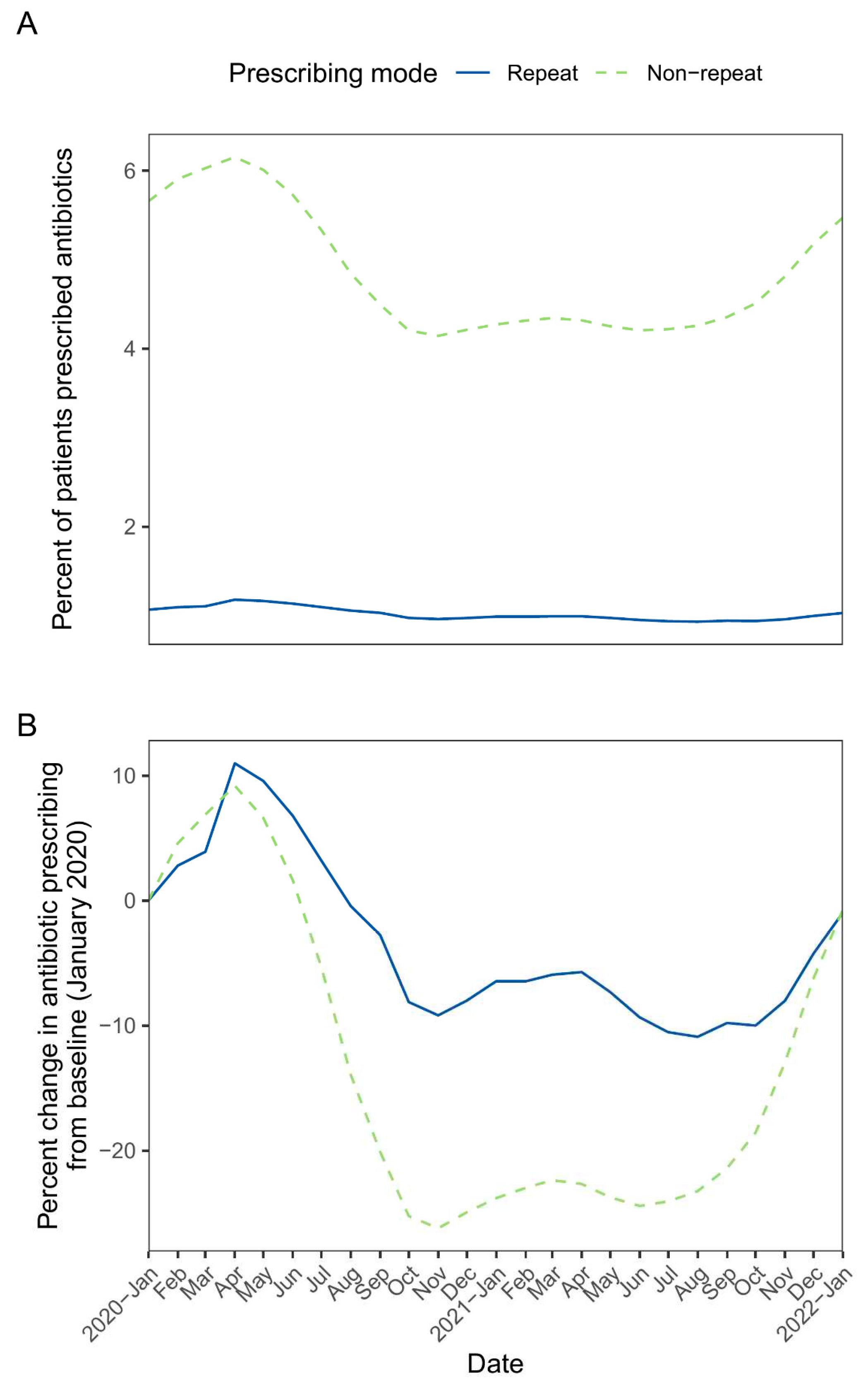
Across monthly rolling patient cohorts from January 2020 to January 2022, the mean study cohort population was 19.58 million. The percentage of monthly cohorts receiving repeat prescriptions ranged from 0.93 to 1.18%, while the percentage receiving non-repeat prescriptions ranged from 4.14 to 6.15% (
Figure 1A). For both prescribing modes, peak prescribing occurred in the April 2020 cohort (patients registered with GP practices as of 1
st April 2020), representing an increase from baseline (January 2020) of ~10%. The percentage of patients prescribed antibiotics declined from May 2020, with non-repeat prescribing showing a more pronounced decline vs repeat prescribing (maximum decline of -26.2% from baseline [non-repeat prescribing, November 2020] vs -10.9% from baseline [repeat prescribing, August 2021]). For both prescribing modes, prescribing increased from ~August 2021, and by January 2022, prescribing was similar to baseline levels (
Figure 1B).
The total study populations in the January 2020 (pre-pandemic) and January 2021 (pandemic) cohorts were 19.38 million and 19.55 million patients, respectively. Of these patient cohorts, 206,865 and 193,517 patients received repeat antibiotic prescriptions (determined based on all antibiotic classes) (
Table 1). This yields overall repeat antibiotic prescribing rates of 10.68 and 9.90 per 1000 patients in pre-pandemic and pandemic cohorts, based on the 6-month lookback period (see Methods). This compares with overall non-repeat prescribing rates of 56.56 and 42.72 per 1000 patients in the pre-pandemic and pandemic cohorts (
Supplementary Table S1). Therefore, in accordance with the monthly percent change analysis (described above), the overall antibiotic prescribing rate was reduced in the pandemic vs pre-pandemic cohort, and non-repeat prescribing showed a steeper overall decline (-24.46%) compared with repeat prescribing (-7.27%).
2.2. Demographics of antibiotic prescribing (repeat and non-repeat) in pre-pandemic and pandemic cohorts
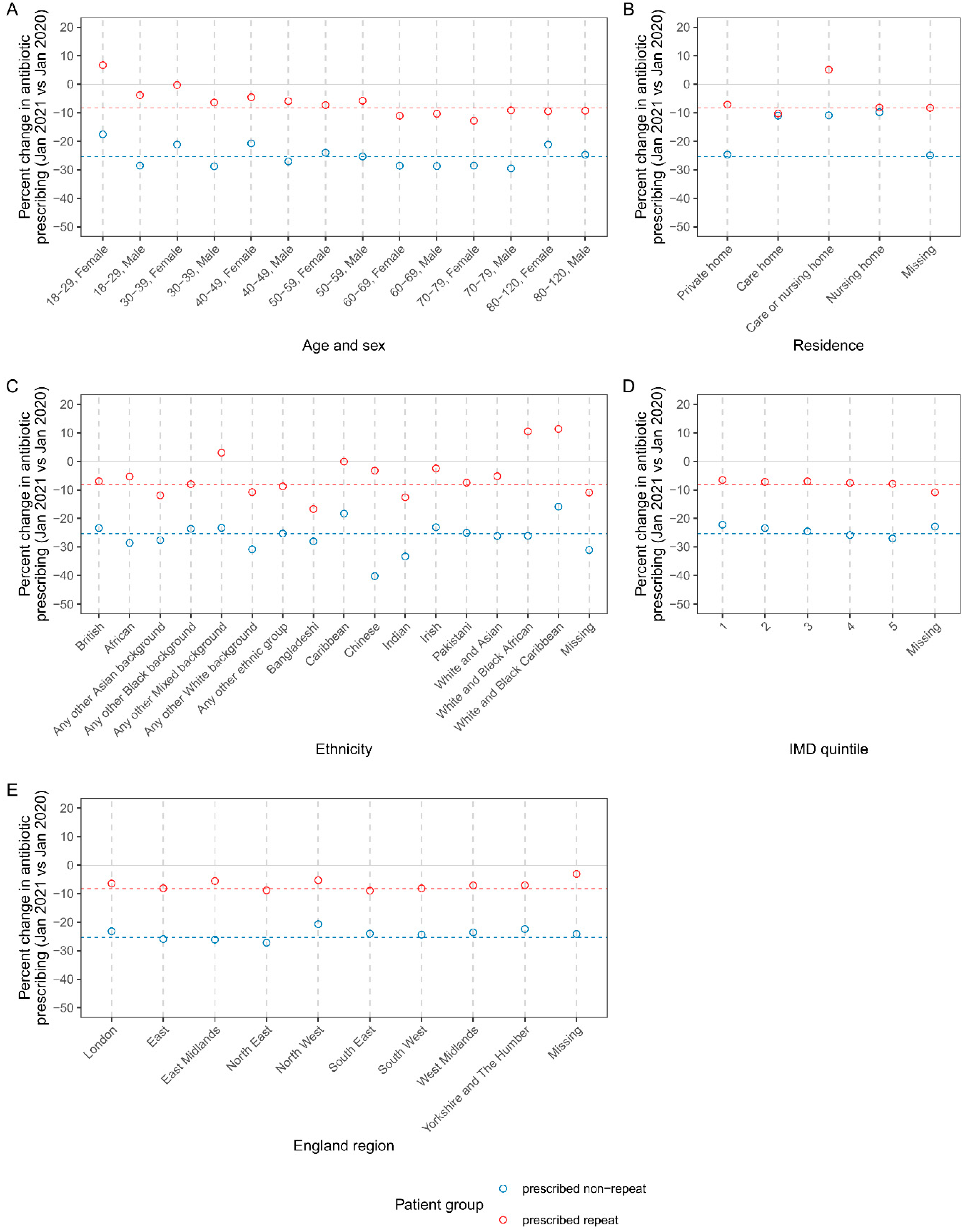
Figure 2 shows the percent change in the rate of repeat and non-repeat antibiotic prescribing in pandemic vs pre-pandemic cohorts, broken down by patient demographics. Across most demographic categories, the percent change in repeat/non-repeat prescribing was negative, and similar to the overall percent change vs pre-pandemic (described above). However, repeat prescribing in young women (aged 18–29) increased 6.72% vs pre-pandemic; also, the decline in non-repeat prescribing was less pronounced in women aged 18–29, compared with all other strata (which more closely aligned to the overall percent change in non-repeat prescribing) (
Figure 2B). Regarding care home residence status, the decline in non-repeat prescribing was smaller in care and nursing home categories. A small number of patients were categorised as being in a care or nursing home (n=2505), rather than explicit categorisation into one or the other. In contrast to the overall trend, this group showed a positive percent change in repeat prescribing. However, this category is small, represents uncertain assignment between care and nursing home, and the result is not reflected in the larger care home and nursing home unambiguous categories. Percent change in antibiotic prescribing showed variation by ethnicity. Notably, compared with the overall trend, there was substantially steeper reduction in non-repeat prescribing among Chinese ethnicity (-40.23% vs pre-pandemic). In contrast to the overall trend for repeat prescribing, there was positive percent change vs pre-pandemic in the following mixed background ethnicities: white and black African, and white and black Caribbean, any other mixed background) (
Figure 2C).
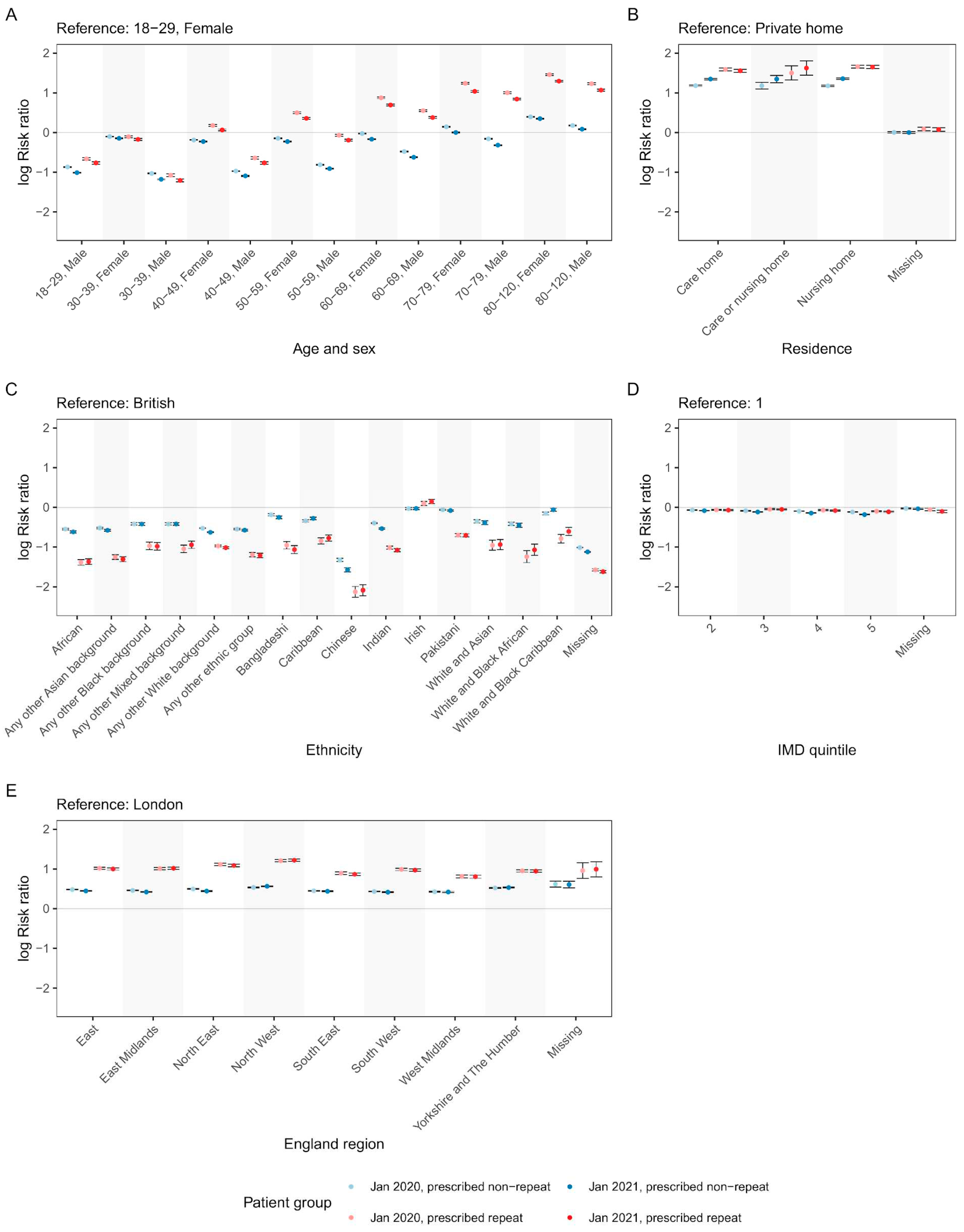
Risk ratios (RRs) were used to explore associations between demographic characteristics and repeat/non-repeat antibiotic prescribing (
Figure 3). Overall trends were similar between pandemic/pre-pandemic cohorts; since changes in prescribing rates are described above, the demographic associations described below are for the pandemic cohort only. The risk of repeat and non-repeat antibiotic prescribing in men was lower than in women across all age brackets. The risk of antibiotic prescribing – especially repeat antibiotic prescribing – increased for patients aged 40–49 and above (
Figure 3A). In under 40s, repeat and non-repeat prescribing rates were similar, whereas for elderly patients, the risk of repeat prescribing was markedly higher. For example, compared with women aged 18–29, women over 79 had a 357% increased risk of repeat prescribing vs 43% increased risk of non-repeat prescribing (RR 3.64 CI=3.57–3.72 vs 1.42 CI=1.40–1.43). There was a strong effect of care home residency status on prescribing; residents of care/nursing homes were at higher risk of antibiotic prescribing, especially repeat prescribing, compared to residents of private homes (
Figure 3B). Compared with white British patients, levels of antibiotic prescribing were relatively similar in Irish patients (16% higher repeat prescribing [RR 1.16 CI=1.10–1.22], and 3% lower non-repeat prescribing [RR 0.97 CI=0.95–0.10]). Compared with white British patients, patients belonging to other ethnicities (besides Irish), were less likely to receive antibiotic prescriptions, especially repeat prescriptions. Chinese ethnicity showed the strongest negative association with antibiotic prescribing (88% lower repeat prescribing [RR 0.12 CI=0.11–0.14], and 79% lower non-repeat prescribing [RR 0.21 CI=0.20–0.22]) (
Figure 3C). Patients from less deprived areas (higher IMD quintile) were less likely to receive antibiotic prescriptions compared with patients from more deprived areas, however, the effect sizes were very modest (
Figure 3D). Compared with London, all other English regions had higher antibiotic prescribing, especially repeat prescribing (
Figure 3E).
2.3. Patterns of prescribing across patient clinical conditions and antibiotic classes in pre-pandemic and pandemic cohorts
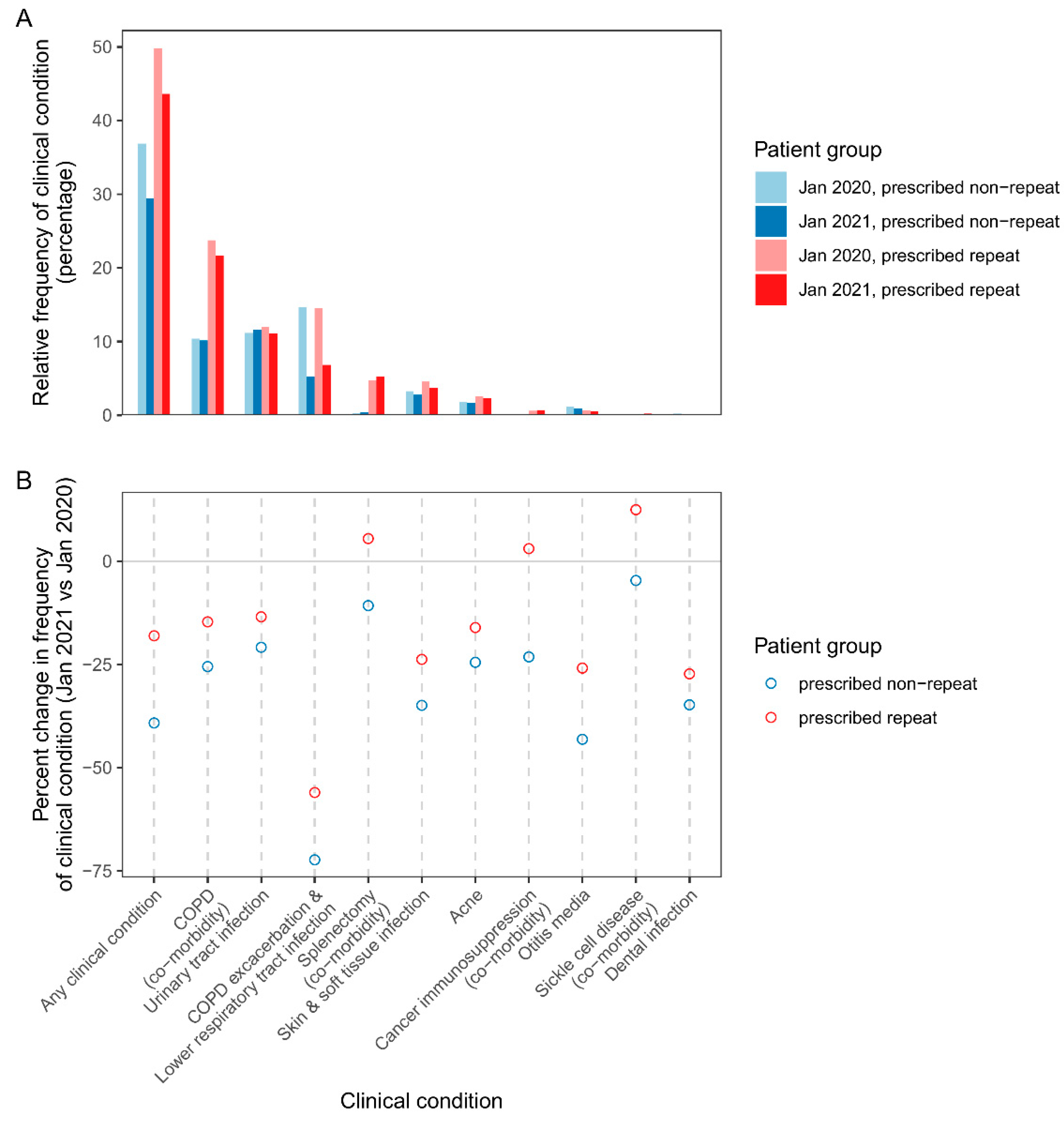
Figure 4A shows the relative frequency of clinical conditions among patients prescribed repeat and non-repeat antibiotics in pre-pandemic and pandemic cohorts. The most frequent clinical conditions linked to repeat prescribing in the pandemic cohort were COPD as a comorbidity, followed by urinary tract infection, COPD exacerbation/lower respiratory tract infection, and splenectomy (comorbidity) (
Figure 4A). Similar patterns were found for repeat and non-repeat prescribing across most clinical conditions except the comorbidities (COPD comorbidity, splenectomy, cancer immunosuppression, sickle cell) which were more common among repeat prescribed patients. For example, in the pandemic cohort, COPD comorbidity was found in 10% of patients receiving non-repeat prescriptions, compared with 22% of patients on repeat prescriptions. Taking all clinical conditions together, in pre-pandemic and pandemic cohorts, respectively, one or more of the clinical conditions was found in 49.8 and 43.6% of patients, compared with 36.8 and 29.4% of patients prescribed non-repeat.
Figure 4B shows percent change in patients prescribed repeat/non-repeat antibiotics for clinical conditions, in pandemic vs pre-pandemic cohorts. There were declines in repeat and non-repeat prescribing for all indications (COPD exacerbation/lower respiratory tract infection, urinary tract infection, skin and soft tissue infection, acne, otitis media, dental infection). Most notable was the 56% and 72% reduction (repeat and non-repeat prescribing, respectively) in the number of patients prescribed for COPD exacerbation/lower respiratory tract. Compared with indications, prescribing for patients with comorbidities generally declined less, and there were moderate increases in the number of patients prescribed repeat antibiotics for splenectomy, cancer immunosuppression, and sickle cell disease.
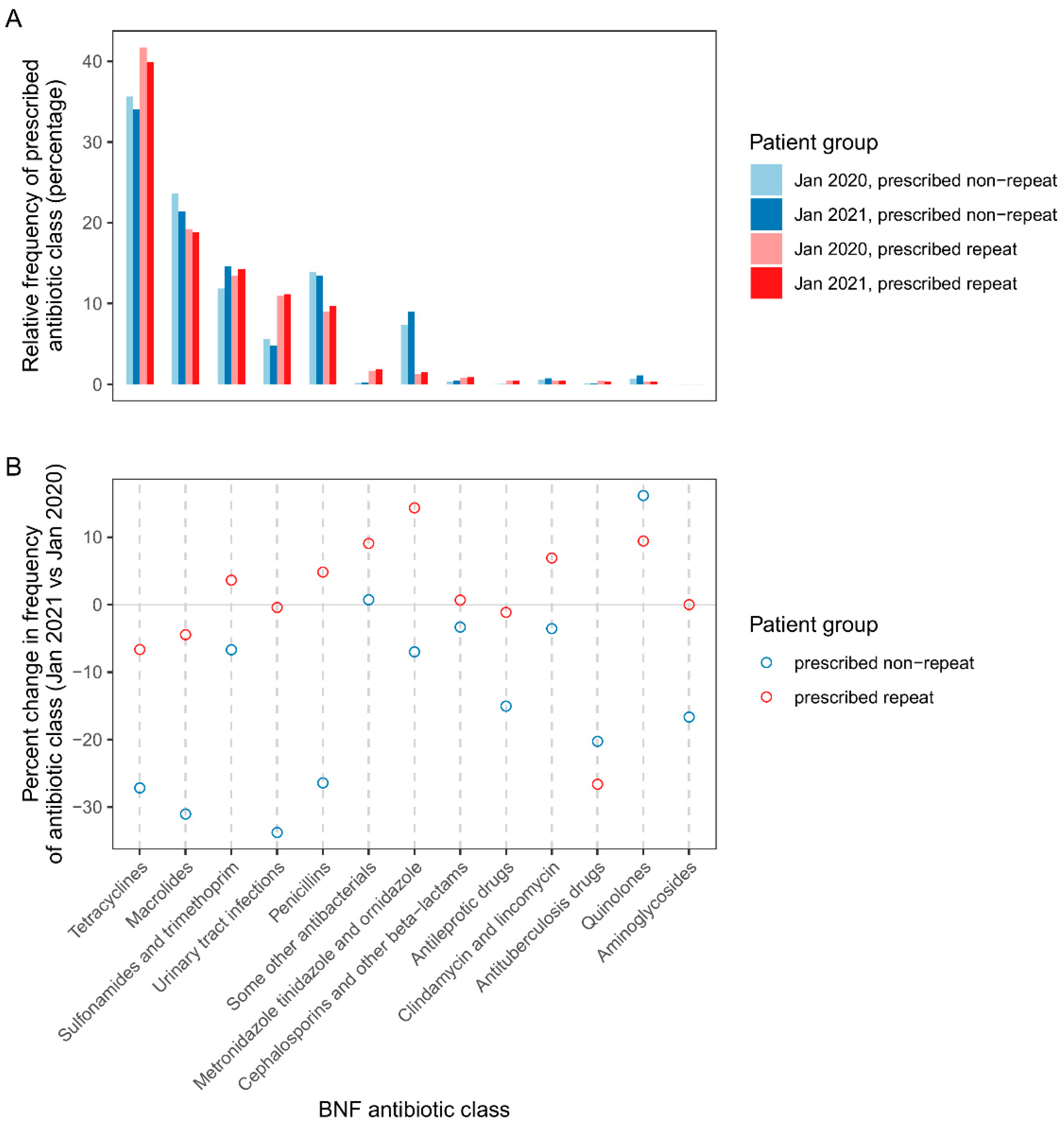
Figure 5A shows the relative frequency of repeat/non-repeat prescribed antibiotic classes in pre-pandemic and pandemic cohorts. The antibiotic classes used most frequently for repeat prescribing were tetracyclines, macrolides, sulfonamides & trimethoprim, urinary tract infection antibiotics (nitrofurantoin and methenamine hippurate), and penicillins. There were some differences in the profile of antibiotic classes for repeat vs non-repeat prescribing, including increased relative frequency of urinary tract infection antibiotics but lower relative frequency of metronidazole, tinidazole & ornidazole. Patterns of prescribing by antibiotic class varied by clinical condition. In contrast to the pattern for all patients, for patients with cancer immunosuppression, sickle cell disease and splenectomy comorbidities, penicillin predominated both repeat and non-repeat prescribing(
Figure S1). Macrolides were the most frequently prescribed class for otitis media; sulfonamides & trimethoprim, followed by urinary tract infection antibiotics were the most frequently prescribed classes linked to urinary tract infections (
Figure S1). Doxycycline was the most frequently prescribed tetracycline sub-class, except for acne where lymecycline prescribing predominated (
Figure S2).
Figure 5B compares the frequency of repeat/non-repeat prescribed antibiotic classes in pandemic vs pre-pandemic cohorts. There were strong declines in non-repeat prescribing of the following antibiotic classes: tetracyclines, macrolides, urinary tract infections, and penicillins, as well as antileprotic drugs, antituberculosis drugs, and aminoglycosides. Conversely, non-repeat quinolone prescribing increased by 16%. Repeat prescribing of antituberculosis drugs showed a strong 27% decline vs pre-pandemic.
3. Discussion
This large population-based study investigated patterns of antibiotic prescribing prior to and during the COVID-19 pandemic in English primary care. Antibiotic prescribing patterns were broken down by patient demographics, clinical conditions, and prescribing modality (non-repeat and repeat prescribing). Repeat prescribing is an important focus for antibiotic stewardship in the COVID-19 pandemic era, because of “status quo bias” in prescribing decisions may have been exacerbated during the pandemic, given time-constraints and de-prioritisation of antibiotic stewardship [
9]. This study reveals the burden of repeat antibiotic prescribing during the COVID-19 pandemic in England, and our findings have guided the development of primary care antibiotic stewardship interventions (discussed below).
Analysis of rolling monthly patient cohorts (patients registered with GP practices as of 1st January 2020, through to 1st January 2022) demonstrated that both repeat and non-repeat antibiotic prescribing increased by ~10% following initial onset of the pandemic, and then reduced following strict COVID-19 lockdown in England in April 2020, corroborating existing reports [
2,
11,
15,
16]. This pattern likely reflects a combination of reduced incidence of bacterial infections (given social distancing measures); and reduced access to/demand for primary care (as people restricted non-essential appointments due to concerns around infection risk, or to “protect the National Health Service”) [
15]. There was a more pronounced reduction in non-repeat prescribing compared with repeat prescribing. This suggests that among patients receiving repeat prescriptions, repeat antibiotic prescribing rates were less influenced by COVID-19-related changes in primary care access/provision, or infection transmission rates. The more in-depth analysis of pandemic (January 2021) and pre-pandemic (January 2020) patient cohorts can inform interpretations. Specifically, analysis of prescribing by demographic characteristics indicated that patients prescribed repeat antibiotics were more likely to be older and/or reside in care/nursing homes – such patients are also more likely to have age-related comorbidities such as cancer immunosuppression, which can be managed through long-term repeat prophylactic prescribing. Maintenance of long-term repeat prescriptions for comorbidities plausibly explains (at least in part) the lower reduction in repeat vs non-repeat prescribing rates during the pandemic. Indeed, analysis of prescribing by clinical conditions demonstrated that levels of repeat prescribing for comorbidities, including cancer immunosuppression, showed a moderate increase in the pandemic vs pre-pandemic cohorts, whereas there were declines in prescribing (especially non-repeat prescribing) for indications (i.e. acute clinical conditions).
Regarding prescribing for indications, the most pronounced reduction observed in pandemic vs pre-pandemic cohorts was for COPD exacerbation/lower respiratory tract infection (56% and 72% reductions for repeat and non-repeat prescribing, respectively). This finding aligns with a previous study of primary care prescribing for respiratory tract infections in English, which found prescriptions roughly halved, when comparing prescribing rates during Winter 2020–2021 with the previous (pre-pandemic) Winter [
10]. Changes in prescribing rates in pandemic vs pre-pandemic cohorts also varied by patient residency and ethnicity. Specifically, there were steeper declines in non-repeat prescribing in private homes (when compared with care/nursing homes) and in patients of Chinese ethnicity (compared with other ethnicities). Overall, these patterns are likely to be driven by (variable) reductions in the incidence of infections, notably respiratory infections. Supporting this, COVID-19 surveillance by the Office for National Statistics (ONS) has demonstrated that COVID-19 infection risk remained higher in care/nursing homes vs private homes, and was lower in Chinese patients vs other ethnic minorities.
Our findings highlight issues around health inequalities in antibiotic prescribing. More deprived areas were moderately more likely to receive repeat and non-repeat antibiotic prescriptions, potentially reflecting poorer health status/increased infection risk. There was lower antibiotic prescribing among non-white ethnic minorities, especially Chinese patients. While this may be partly explained by younger age, the disparity, especially regarding Chinese patients, likely also reflects reduced engagement with primary care services. Comparing pandemic vs pre-pandemic cohorts, there were increases in repeat prescribing among mixed ethnicity black/Caribbean patients.
A key aim of our study was to inform antibiotic stewardship. Findings described above regarding rates of repeat prescribing highlight the importance of regular antibiotic reviews in older patients, care/nursing home residents, and patients with immunosuppressive comorbidities. A stronger evidence base to guide prophylactic prescribing in these patients would be beneficial [
17]. Our analysis of the relative frequency of antibiotic prescribing by class and clinical condition, can help further target stewardship interventions. The most frequent clinical conditions linked to repeat prescribing in the pandemic cohort were COPD as a comorbidity, followed by urinary tract infection, COPD exacerbation/lower respiratory tract infection, and splenectomy (comorbidity). Regarding antibiotic classes, for patients with immunosuppressive comorbidities (identified as at higher risk of repeat prescribing), penicillin antibiotics were the predominantly prescribed antibiotic class. Our analysis of tetracycline sub-classes showed overall predominance of doxycycline, but that lymecycline was the major sub-class prescribed for acne. Based on these findings, primary care antibiotic stewardship intervention toolkits targeting acne, respiratory tract infection, and urinary tract infection are in development. In future, knowledge on health inequalities will be embedded into stewardship tool development.
A strength of this study is the use of OpenSAFELY-TPP data (representing ~40% of general practices) providing a national-level understanding of the burden of antibiotic prescribing, across patient demographic and clinical characteristics. A particularly novel aspect of this study was the distinction between non-repeat and repeat prescribing. This has enabled us to better target stewardship tools towards high burden repeat prescribing. A limitation of the study as with all large database EHR studies is that not all clinical conditions are coded upon infection diagnosis or patient review, or that they may be coded inaccurately. In addition, below 50% of patients prescribed repeat antibiotics were accounted for with the clinical conditions added to codelists, so the burden of repeat prescribing across clinical conditions is incompletely understood. A caveat of comparing prescribing patterns between cohorts is the lack of control for change in case mix over time (changing infection incidence and overall change in study population demographics) Finally, in our observational cohort study design, multiple uncontrolled factors could influence prescribing patterns including background incidence of bacterial infections and primary care prescribing behaviour. However, with clinical interpretation, differences between repeat and non-repeat prescribing patterns can be informative.
4. Materials and Methods
4.1. OpenSAFELY-TPP data source, study design and patient cohorts
We conducted cohort analysis using primary care electronic health record data retrieved from the OpenSAFELY-TPP data analytics platform. Two main patient cohorts were retrieved comprising all patients registered at a GP practice as of 1
st Jan 2020 and as of 1
st Jan 2021; these cohorts represent periods before and during the COVID-19 pandemic, respectively (i.e. “pre-pandemic” and “pandemic” cohorts). The study populations were restricted to patients in England known to be aged 18–120, male or female, and not deceased. In addition to age and sex, other demographic variables were retrieved for each patient: patient geography and deprivation (derived from GP practice address), ethnicity, and care home residency status. Patient geography was categorised into 9 English regions – the Nomenclature of Territorial Units for Statistics 1 (NUTS 1) regions. Deprivation was measured as Index of Multiple Deprivation (IMD) quintile from 1 to 5 (most to least deprived). Ethnicity was divided into the 16 categories of the 2001 UK census [
13], and determined from patient primary care records if present, or otherwise, from hospital Secondary Uses Service (SUS) data. Care home residency status was determined using a previously described address linkage method which matches GP registered addresses to care home addresses [
14]. The method yields the following residency categories: care home, nursing home, either care home or nursing home, private home (neither care home nor nursing home).
For the pre-pandemic and pandemic cohorts, patient-level clinical variables (antibiotic prescribing and clinical conditions) were determined by searching primary care record event data using code lists. Antibiotic prescribing was delineated into 13 antibiotic classes, as defined by the British National Formulary (BNF) (
https://openprescribing.net/bnf/0501/). The number of antibiotic prescriptions within a 6-month window prior to the cohort index dates was determined for each antibiotic class. This allowed us to define repeat and non-repeat patient cohort sub-groups within the pre-pandemic and pandemic cohorts. Repeat antibiotic prescribing was defined as a patient receiving 3 or more prescriptions of a given antibiotic class in the 6-month window; non-repeat prescribing was defined as 1–2 antibiotic prescriptions in the 6 month window. Repeat and non-repeat prescribing was also calculated for all antibiotics combined (in this case, repeat prescribing was defined non-stringently as 3 or more antibiotic prescriptions, irrespective of whether this involved consecutive prescribing of the same antibiotic class). In addition to determining antibiotic prescribing for the pre-pandemic and pandemic cohorts, a monthly rolling measure of the frequency of repeat and non-repeat prescribing was calculated using patient cohorts from 1
st January 2020 to 1
st January 2022. The rationale for distinguishing repeat and non-repeat prescribing was to assist interpretation of results.
For the pre-pandemic and pandemic cohorts, patient clinical conditions were conceptually divided into comorbidities (chronic conditions predisposing for antibiotic use) and indications (infections treated with antibiotics). The following comorbidities were investigated: cancer immunosuppression, chronic obstructive pulmonary disease (COPD), sick cell disease, prior splenectomy. The following indications were investigated: acne, COPD exacerbation/lower-respiratory tract infection, dental infection, otitis media, skin and soft tissue infection, urinary tract infection. A patient was considered to have a comorbidity if the respective clinical condition was recorded at any time on or before the cohort index date. An indication was defined by determining the date of first antibiotic prescription in the 6 month lookback window, then taking a 6 month window beforethis date; if the respective clinical condition was recorded within this window, it was considered an indication for repeat prescribing.
4.2. Statistics and reproducibility
Using data from the OpenSAFELY-TPP platform, the monthly frequency of repeat and non-repeat prescribing from January 2020–January 2022 was visualised (frequency of prescribing, percent change in frequency of prescribing vs January 2020 [baseline]). The percent change in repeat and non-repeat prescribing was also compared in the main study cohorts (January 2021 [pandemic cohort] vs January 2020 [baseline, pre-pandemic cohort]), broken down by demographic characteristics. To explore the association between demographic exposures and antibiotic prescribing, unadjusted risk ratios and 95% confidence intervals were calculated for repeat and non-repeat prescribing for patients in the January 2020 and 2021 cohorts. A Bonferroni-adjusted alpha=0.00024 (52 comparisons, across 4 patient groups [repeat and non-repeat prescribed patients in January 2020 and 2021 cohorts]) was used to guide interpretation of risk ratios. Data visualisation was used to explore patterns of antibiotic prescribing across antibiotic classes and patient clinical conditions. Data management and analysis was carried out using Python.
4.3. Data source
All data were linked, stored and analysed securely within the OpenSAFELY platform:
https://opensafely.org/. Data include pseudonymised data such as coded diagnoses, medications and physiological parameters. No free text data are included. All code is shared openly for review and re-use under MIT open license
https://github.com/opensafely/LT-repeat-antimicrobial-prescribing. Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1. Demographic characteristics of patients prescribed non-repeat antibiotics; Figure S1. The relative frequency of prescribed antibiotic classes is shown as a percentage of total patients within each patient group, broken down by patient clinical condition; Figure S2. The relative frequency of prescribed tetracycline sub-classes is shown as a percentage of total patients within each patient group, broken down by patient clinical condition.
Author Contributions
Conceptualization, Brian MacKenna and Diane Ashiru-Oredope; Data curation, Seb Bacon; Formal analysis, Alex Orlek; Funding acquisition, Ben Goldacre and Diane Ashiru-Oredope; Investigation, Alex Orlek, Eleanor Harvey and Diane Ashiru-Oredope; Methodology, Alex Orlek, Eleanor Harvey, Brian MacKenna and Diane Ashiru-Oredope; Project administration, Alex Orlek, Eleanor Harvey, Louis Fisher and Amir Mehrkar; Resources, Seb Bacon and Diane Ashiru-Oredope; Software, Alex Orlek, Louis Fisher, Seb Bacon and Ben Goldacre; Supervision, Ben Goldacre, Brian MacKenna and Diane Ashiru-Oredope; Validation, Louis Fisher and Amir Mehrkar; Writing – original draft, Alex Orlek and Eleanor Harvey; Writing – review & editing, Alex Orlek, Eleanor Harvey, Louis Fisher, Amir Mehrkar, Seb Bacon, Ben Goldacre, Brian MacKenna and Diane Ashiru-Oredope. All authors have read and agreed to the published version of the manuscript
Funding
This work was funded by UK Health Security Agency and OpenPrescribing. The views expressed are those of the authors and not necessarily those of the NIHR, NHS England, UK Health Security Agency, or the Department of Health and Social Care.
Institutional Review Board Statement
NHS England is the data controller; TPP is the data processor; and the researchers on OpenSAFELY are acting with the approval of NHS England. This implementation of OpenSAFELY is hosted within the TPP environment which is accredited to the ISO 27001 information security standard and is NHS IG Toolkit compliant [18, 19]; patient data has been pseudonymised for analysis and linkage using industry standard cryptographic hashingtechniques; all pseudonymised datasets transmitted for linkage onto OpenSAFELY are encrypted; access to the platform is via a virtual private network (VPN) connection, restricted to a small group of researchers; the researchers hold contracts with NHS England and only access the platform to initiate database queries and statistical models; all database activity is logged; only aggregate statistical outputs leave the platform environment following best practice for anonymisation of results such as statistical disclosure control for low cell counts [
20]. The OpenSAFELY research platform adheres to the obligations of the UK General Data Protection Regulation (GDPR) and the Data Protection Act 2018. In March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 (COPI) to require organisations to process confidential patient information for the purposes of protecting public health, providing healthcare services to the public and monitoring and managing the COVID-19 outbreak and incidents of exposure; this sets aside the requirement for patient consent.22 Taken together, these provide the legal bases to link patient datasets on the OpenSAFELY platform. GP practices, from which the primary care data are obtained, are required to share relevant health information to support the public health response to the pandemic, and have been informed of the OpenSAFELY analytics platform. In accordance with the NHS Health Research Authority guidelines, this study does not require ethical approval as it focuses on service evaluation (and further development of antimicrobial stewardship initiatives) [
21].
Informed Consent Statement
Not applicable: The OpenSAFELY research platform adheres to the obligations of the UK General Data Protection Regulation (GDPR) and the Data Protection Act 2018. In March 2020, the Secretary of State for Health and Social Care used powers under the UK Health Service (Control of Patient Information) Regulations 2002 (COPI) to require organisations to process confidential patient information for the purposes of protecting public health, providing healthcare services to the public and monitoring and managing the COVID-19 outbreak and incidents of exposure; this sets aside the requirement for patient consent.22 Taken together, these provide the legal bases to link patient datasets on the OpenSAFELY platform. GP practices, from which the primary care data are obtained, are required to share relevant health information to support the public health response to the pandemic, and have been informed of the OpenSAFELY analytics platform.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All code for data management and analyses in addition to the prespecified protocol are archived at:
https://github.com/opensafely/LT-repeat-antimicrobial-prescribing All codelists for identifying exposures, covariates and outcomes are openly shared at
https://codelists.opensafely.org/. Access to the platform is via a virtual private network connection, restricted to a small group of researchers. All data relevant to the study are included in the article or uploaded as supplementary information.
Acknowledgments
We are grateful for the generous support received from the TPP Technical Operations team, and from the information governance and database teams at NHS England/NHSX. We would also like to thank Naomi Fleming, Kieran Hand, Helen Kilminster and David Ladenheim for their involvement in broader long term and repeated antibiotics project; Helen Kilminster and David Ladenheim for providing anonymised Primary Care Network data; and Shazia Patel for co-developing the antimicrobial stewardship booklets as a direct result of this study.
Conflicts of Interest
The authors declare no conflict of interest. There were no external funders for the project.
References
- Llor, C. and L. Bjerrum, Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic Advances in Drug Safety, 2014. 5(6): p. 229-241. [CrossRef]
- UKHSA, English surveillance programme for antimicrobial utilisation and resistance (ESPAUR): Report 2020 to 2021. 2021.
- Majeed, A.; Maile, E.J.; Bindman, A.B. The primary care response to COVID-19 in England’s National Health Service. J. R. Soc. Med. 2020, 113, 208–210. [CrossRef]
- Borek, A.J., et al., Impact of the COVID-19 Pandemic on Community Antibiotic Prescribing and Stewardship: A Qualitative Interview Study with General Practitioners in England. Antibiotics, 2021. 10(12): p. 1531. [CrossRef]
- Ashiru-Oredope, D., et al., Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom. Antibiotics, 2021. 10(2): p. 110. [CrossRef]
- Mahida, N., et al., Antimicrobial stewardship in the post COVID-19 pandemic era: an opportunity for renewed focus on controlling the threat of antimicrobial resistance. Journal of Hospital Infection, 2022. 129: p. 121-123. [CrossRef]
- DHSC, UK 5-year action plan for antimicrobial resistance 2019 to 2024. 2019.
- NHS England. Expanding our workforce. 2021 11th October 2021]; Available from: https://www.england.nhs.uk/gp/expanding-our-workforce/.
- Krockow, E.M., E.J. Harvey, and D. Ashiru-Oredope, Addressing long-term and repeat antibiotic prescriptions in primary care: considerations for a behavioural approach. BMJ Quality & Safety, 2022. [CrossRef]
- Andrews, A., et al., Respiratory antibacterial prescribing in primary care and the COVID-19 pandemic in England, winter season 2020–21. Journal of Antimicrobial Chemotherapy, 2022. 77(3): p. 799-802. [CrossRef]
- Hussain, A.Z., V. Paudyal, and M.A. Hadi, Impact of the COVID-19 Pandemic on the Prescribing Patterns of First-Line Antibiotics in English Primary Care: A Longitudinal Analysis of National Prescribing Dataset. Antibiotics, 2021. 10(5): p. 591. [CrossRef]
- Williamson, E.J., et al., Factors associated with COVID-19-related death using OpenSAFELY. Nature, 2020. 584(7821): p. 430-436. [CrossRef]
- Mathur, R., et al., Completeness and usability of ethnicity data in UK-based primary care and hospital databases. Journal of Public Health, 2013. 36(4): p. 684-692. [CrossRef]
- Schultze, A., et al., Identifying Care Home Residents in Electronic Health Records - An OpenSAFELY Short Data Report. Wellcome Open Research, 2021. 6: p. 90. [CrossRef]
- Zhu, N., et al., Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clinical Microbiology and Infection, 2021. 27(5): p. 762-768. [CrossRef]
- Rezel-Potts, E., V. L’Esperance, and M.C. Gulliford, Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. British Journal of General Practice, 2021. 71(706): p. e331-e338. [CrossRef]
- Ahmed, H., et al., Long-term antibiotics for prevention of recurrent urinary tract infection in older adults: systematic review and meta-analysis of randomised trials. BMJ Open, 2017. 7(5): p. e015233. [CrossRef]
- NHS Digital. BETA - Clinical Information Standards. 2022 [cited 2022 24th November]; Available from: https://digital.nhs.uk/about-nhs-digital/our-work/nhs-digital-data-and-technology-standards/clinical-information-standards.
- NHS Digital. Data Security and Protection Toolkit. 2022 [cited 2022 24th November]; Available from: https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit.
- NHS Digital. ISB1523: Anonymisation Standard for Publishing Health and Social Care Data. 2022 [cited 2022 24th November]; Available from: https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/isb1523-anonymisation-standard-for-publishing-health-and-social-care-data.
- NHS Health Research Authority. Is my study research? 2022 [cited 2022 24th November]; Available from: http://www.hra-decisiontools.org.uk/research/index.html.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).