1. Introduction
Breast-conserving surgery with adjuvant radiotherapy (RT) has become one of the standards of care for women with early-stage breast cancer and, as a result, a large number of patients are affected by the cutaneous sequelae of RT; in other cases and stages of the disease chemotherapy (especially epidermal growth factor receptor inhibitors or EGFR) added to surgery, with or without [
1] adjuvant RT, is the protocol of choice in patients with breast cancer, also giving rise to skin alterations as sequelae. In other types of cancer (whether solid organ, hollow organ, or hematology) treatments also produce skin toxicities as sequelae that can affect the quality of life of people during and after cancer treatment, even of sufficient intensity to promote the abandonment or discontinuation of the treatment of the underlying disease itself. These dermatological toxicities, both of[
2]
,[
3] RT and surgery (with localized involvement) and of chemotherapy (affecting a more generalized extension of the skin) can occur during treatment or years after it and can significantly affect the quality of life of patients. Radiation-induced skin toxicities in women with breast cancer include radiation dermatitis, radiation recall, radiation-induced morphea, radiation-induced fibrosis, and skin malignancies on irradiated skin [
1].
Radiation dermatitis is a common sequela of RT that can affect up to 95% of patients, developing moderate to severe skin reactions in up to 30% of them.[
4] Therefore,[
5] women with breast cancer must have timely access to dermatological care in case skin toxicities develop [
1].
The clinical symptoms of acute radiation dermatitis are partially dependent on the cumulative radiation dose. A mild, transient erythema may occur hours after radiation exposure. Classically, acute radiation dermatitis occurs during the second week of RT and presents as a dry, erythematous patch found in the radiation field. After 3 to 4 weeks and high cumulative doses, dry peeling may occur; In severe cases, worsening of edema, pruritus, tenderness, wet peeling, and ulceration may occur.[
6]
It is essential to advise patients on lifestyle changes to minimize the effects of unnecessary friction and trauma within the radiation field. Examples include wearing loose-fitting clothing; avoiding exposure to the sun, extreme heat, or cold and irritating products; using mild emollients; avoiding shaving with disposable or straight-edged razors; and using mild soaps.[
7]
Recent studies have shown that decreasing radiation doses and varying methods of fractionation or administration can reduce the incidence and severity of radiation dermatitis. In particular, the use of hypofractionated RT, intensity-modulated RT, accelerated partial breast irradiation, and prone positioning has resulted in decreased rates of acute radiation dermatitis compared to conventional RT [
5].
The main management strategies include keeping the affected area clean and moist with mild emollients or topical corticosteroids, protecting the area from contamination and infection, and controlling pain. The application of dressings (hydrogel, hydrocolloid, soft silicone, or silver-based dressings) can reduce mechanical injury at the wound site and promote healing. In the presence of superinfection, antimicrobials such as mupirocin ointment or silver sulfadiazine cream may be written down. For grade 3 or 4 reactions, brief interruptions of the RT may be needed along with supportive wound care. Amifostine, zinc, and the combination of oral pentoxifylline and oral vitamin E have been investigated in phase 1 and 2 trials for the treatment of acute radiation dermatitis, with varying success. Other experimental treatments are currently being investigated, including autologous fibroblasts, stem cells, and growth factors (e.g., fibroblast growth factors, platelet-derived growth factor, granulocyte colony-stimulating factors, and granulocytes-macrophages, and transforming growth factor beta-modulators) [
1].
Bioidentical recombinant human epidermal growth factor (rhEGF) is available in concentrations and purity suitable for therapeutic use in stable formulations. The evidence shows beneficial effects in various pathologies and skin lesions (healing of traumatic and surgical wounds, laser-induced wounds, abnormal scars, keloids, dermatitis induced by radiation or chemotherapy, post-inflammatory hyperpigmentation, or for the repair of damage due to skin aging) and having also been considered as a treatment for diseases of the oropharyngeal mucosa and the upper gastroesophageal tract (oral thrush, pharyngeal fistulas, ulcers) and corneal mucosa or conjunctiva. rhEGF has not shown any significant side effects or side effects in humans or laboratory experimental animals, showing the best tolerability and safety with continued use for months. Although EGF is a mitogen for various epithelial and mesenchymal cells, experimental evidence shows that the administration of EGF cannot initiate malignant transformations, as the dysregulation of the receptor is related to mutations of this and not dependent on the activity or concentration of the EGF factor. It has even been suggested that EGF can be cytoprotective for normal cells and even sensitize cancer cells to antitumor therapies, offering a basis for the clinical use of EGF by not initiating any carcinogenetic process in the absence of evidence linking the clinical use of EGF and the appearance of neoplasms. EGF has been used to prevent the side effects of[
8]
,[
9]
,[
10]
,[
11] oncological RT dermatitis[
12]
,[
13]
,[
14]
,[
15] or chemotherapy for EGFR inhibitors [
8].
In general, it is considered that in cancer patients the topical use of preparations with certain preservatives, surfactants, additives, oils, and impurities should be avoided. More specifically, among the preservatives to avoid are: parabens due to their estrogenic activity and because they are absorbed by the skin; formaldehyde donors for possible carcinogenic effects by some of them; benzyl alcohol or benzoate for its irritating effect on the skin; Triclosan for its endocrine disrupting effect, for apparent lack of health benefits and for being a potential inducer of metastasis; para-aminobenzoic acid (PABA) for causing allergic dermatitis and photosensitivity and potentially being able to cause free radical mutations; boric acid (borax) for its possible carcinogenic effect and the possibility of causing skin irritation, among other unwanted effects.[
16]
,[
17]
,[
18]
,[
19]
,[
20]
,[
21]
,[
22]
,[
23]
Among the surfactants for topical products that should be avoided in cancer patients is sodium lauryl sulfate (LSS) for aggressively removing the lipid mantle of the skin.[
24]
Among the additives that should not hold topical creams used in patients undergoing cancer treatment are all kinds of fragrances because they are mixtures of synthesized aromatized molecules, aromatic chemicals of vegetable origin, leaves, or essential oils of which their composition is not usually known exactly for reasons of business secrecy. Pigments such as titanium dioxide (TiO2) are also not recommended for this type of patient because they are possibly carcinogenic to humans.[
25]
,[
26]
Among the oils to avoid are: peanut oil because it can cause allergic reactions and risk of contamination with aflatoxins (carcinogenic); lanolin (obtained from sheep’s wool) because it can contain pesticide residues and can produce allergies; Palmitic acid because it is a saturated fat that could increase the risk of metastasis (in oral ingestion, although its dermal absorption has not been demonstrated).[
27]
,[
28]
,[
29]
,[
30]
Topical creams used in cancer patients should also not hold urea because of the risk of causing itching and consequent scratching injuries, although more evidence is needed.
Among the impurities that should be avoided from holding topical products for cancer patients are phthalates, 1,4-Dioxane[
31]
,[
32] and[
33] nitrosamines because they are considered possibly harmful to health and capable of producing undesirable effects on people in cancer treatment or after they have finished it.[
34]
The primary aim of this study was to evaluate efficacy in the prevention and treatment of radiodermatitis in patients receiving RT therapy. The evaluation of the tolerability and safety of Hydroskin OncologyTM moisturizers in cancer patients treated with RT was defined as a secondary aim.
2. Materials and Methods
A three-arm open-label randomized intervention pilot study was designed. The sample planned for the pilot study was 5 participants per group.
The inclusion criteria were women over 21 years of age and with the capacity to perform the intervention, in treatment for breast cancer using RT including normofractionated and hypofractionated protocols. No exclusion criteria were defined.
The variables were collected before starting treatment, at the beginning of treatment, before session 8, before session 14, at the end of treatment, and one and two weeks after the end of RT treatment.
Study variables: The cutaneous symptomatology was analyzed using the Patient-Observer scale that applies Likert responses between 0 and 3 points to each item (items included: pain, itching, burning, hypersensitivity, involvement of Activities of Daily Living, sleep impairment, and the existence of doubts regarding skin care to be applied); subsequently, the scores of each measurement of the Patient-Observer scale were added to obtain a numerical result. The monitoring of skin condition was analyzed using Mexamer (MultiSkinTestCenter®; analyzing elasticity, hydration, pigmentation, and erythema) as a continuous numerical variable; the results of the Mexamer test were compared with retrospective data from patients who had followed the same treatments and who had applied other commercial preparations for the care of irradiated skin.
The HR cream was composed of the active ingredients Panthenol-B5, 2 types of hyaluronic acid, vitamins C and E; excipients: water, liquid paraffin, microcrystalline hydrogenated wax, glycerin, decyl oleate, glyceryl stearate, peg-100 stearate, acetyl alcohol, carbomer, phenoxyethanol, triethanolamine, polymethyl methacrylate, and caprylyl glycol. The HU cream had the same composition as the HU cream but with a higher fat phase concentration. The RS cream had a composition similar to the HU cream plus the active substance sh-oligopeptide-1 (Epidermal Growth Factor, EGF) and vitamin E; Excipients: water, nucifera coconut oil, Vitis vinifera seed oil, glycerin, peg-6 stearate, peg-8 stearate, dimethicone, dimethicone peg/ppg-4/12, caprylic/capric triglyceride, sodium hyaluronate, carbomer, peg-18 castor oil dioleate, steareth-20, glyceryl stearate, disodium EDTA, ceteth-20, potassium sorbate, BHT, propylene glycol, triisopropanolamine, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate.
Groups: one group (HR) received treatment with the HR moisturizer cream, another group (HU) followed the same pattern with the HU cream, and a third group (RS) with the RS cream.
Intervention: in all groups, applications of the cream corresponding to the intervention group were performed on the irradiated area every 12 hours throughout the RT treatment (application after each RT session and every 12 hours, with the precaution of not applying it within 4 hours before the session).
The results were collected during the first 5 months of 2019 and later analyzed using the statistical program IBM SPSS® v.21.
This study was conducted as a pilot study following the current legislation and the code of good research practice of the Declaration of Helsinki.
3. Limitations
As limitations to the study results, the small number of participants can be determined because it is a pilot test, and the age of the participants could not have been collected.
Nor could we collect Mexamer data on the evolution after the end of the RT regimen in the group that followed treatment with the SR cream, nor the relationship of data on the type of RT regimen at the time of collecting the data of the Patient-Observer scale.
In the retrospective group, data on the fractionation received, nor the characteristics and size of this group were not obtained.
4. Results
The final sample consisted of 15 women over 21 years of age treated with RT for breast cancer, with complementary treatment based on three moisturizers of different compositions (HR, HU, RS) from the beginning of the RT program until 2 weeks after the end of it. As a control group, retrospective data were obtained from patients treated with commercial creams without professional recommendation (among the data of the retrospective control group, no information could be obtained on the fractionation regimen received). There were no undesirable side effects to the application of the creams under study and compliance and tolerance were excellent.
The analysis of the participants was carried out based on the cream received and the fractionation of the RT received: the considered group of patients in hypofractionated treatment was composed of the participants who received 40.05 Gyi in 15 sessions, while the group of patients in normofractionated treatment were those who received 50 Gyi in 25 sessions. The fractionation guideline was decided by optional criteria. In general, the radiodermatitis reaction is different with different dose fractionation: the most important peak of radiodermatitis in hypofractionated regimens occurs a few days after the end of treatment, while in normofractionated regimens it occurs in the last sessions and the days immediately after.
The results of the study are not statistically significant due to the sample size being a pilot study with only 5 cases per group, but it is useful to show the effectiveness of the methods used and observe trends that may recommend a randomized study with a larger number of participants.
Patient-Observer Scale
Due to technical problems, it was not possible to obtain information on the prescription of fractionation of the RT in the patients surveyed in the data collection from the Patient-Observer test; therefore, the data analysis only considers the cream applied to each participant. The Patient-Observer scale analyzes the cutaneous symptomatology and the affectation on the Quality of Life of the patients, being useful to see the evolution of the cutaneous effects of RT.
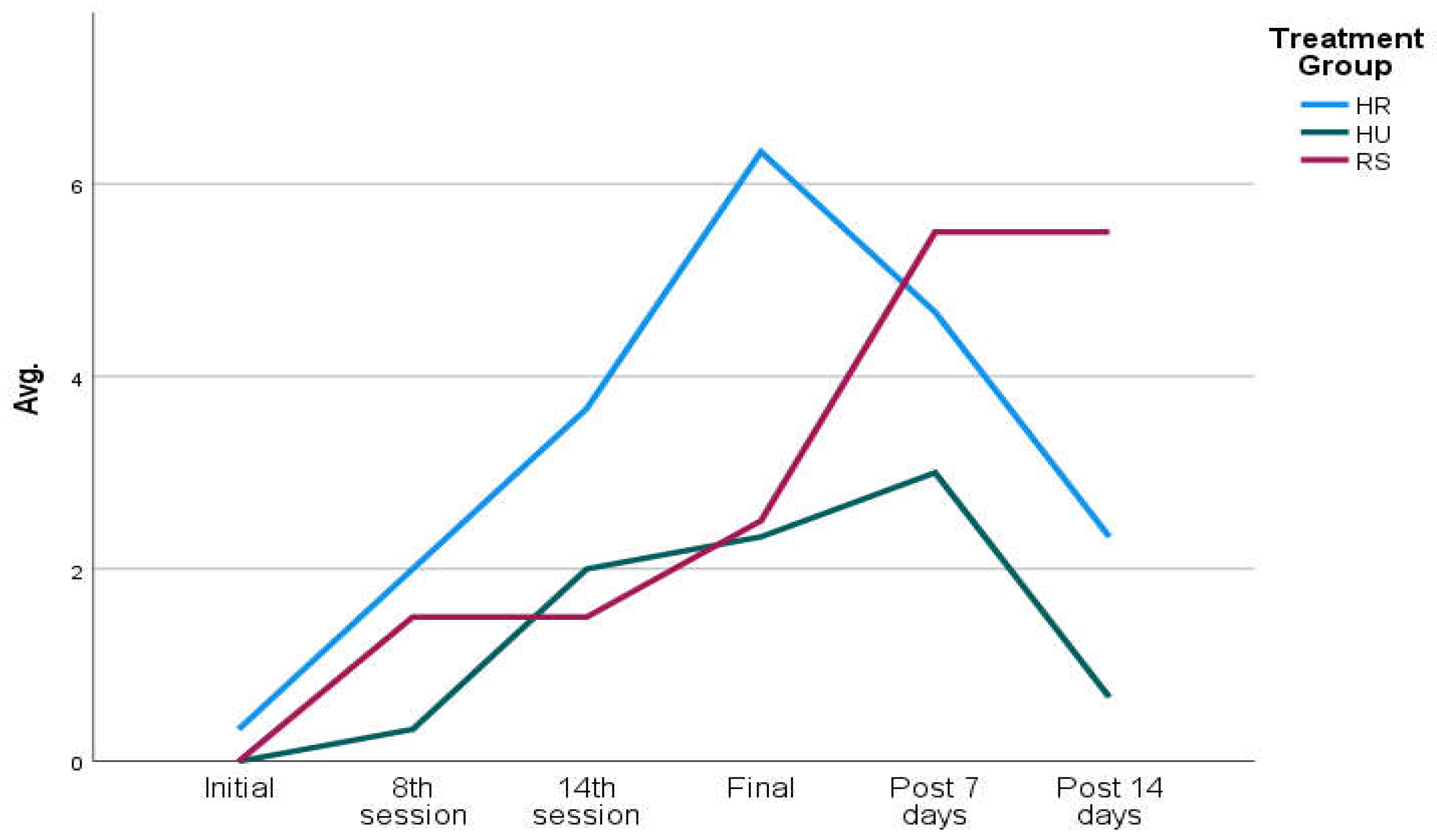
We included 5 participants in each group of application of the creams under study (HR, HU, RS). At baseline, there were no significant differences between study participants on the Patient-Observer scale (HU: 0.40 points; HR: 0.20; RS: 0.00). The mean results of the scale increased as participants received RT sessions until peaking at the end of radiation treatment (HU: 4.80 points; HR:2.40; SR: 2.40), after which the results of the scale reduced their score but without reaching the starting point at 14 days (HU: 2.33; HR: 0.67), except in patients who followed the cream in which they continued to increase the test score during the 14 days after the end of the RT (SR: 5.50 points) (
Table 1). The clinical results of the patients who received RS cream show that on the Patient-Observer scale, the symptoms increase more slightly during RT in this group, comparing the values with the other two creams, but after the completion of the RT regimen, the symptomatology tends to increase when the RS cream continues while in the case of HR and HU creams the symptomatology tends to be reduced (
Figure 1).
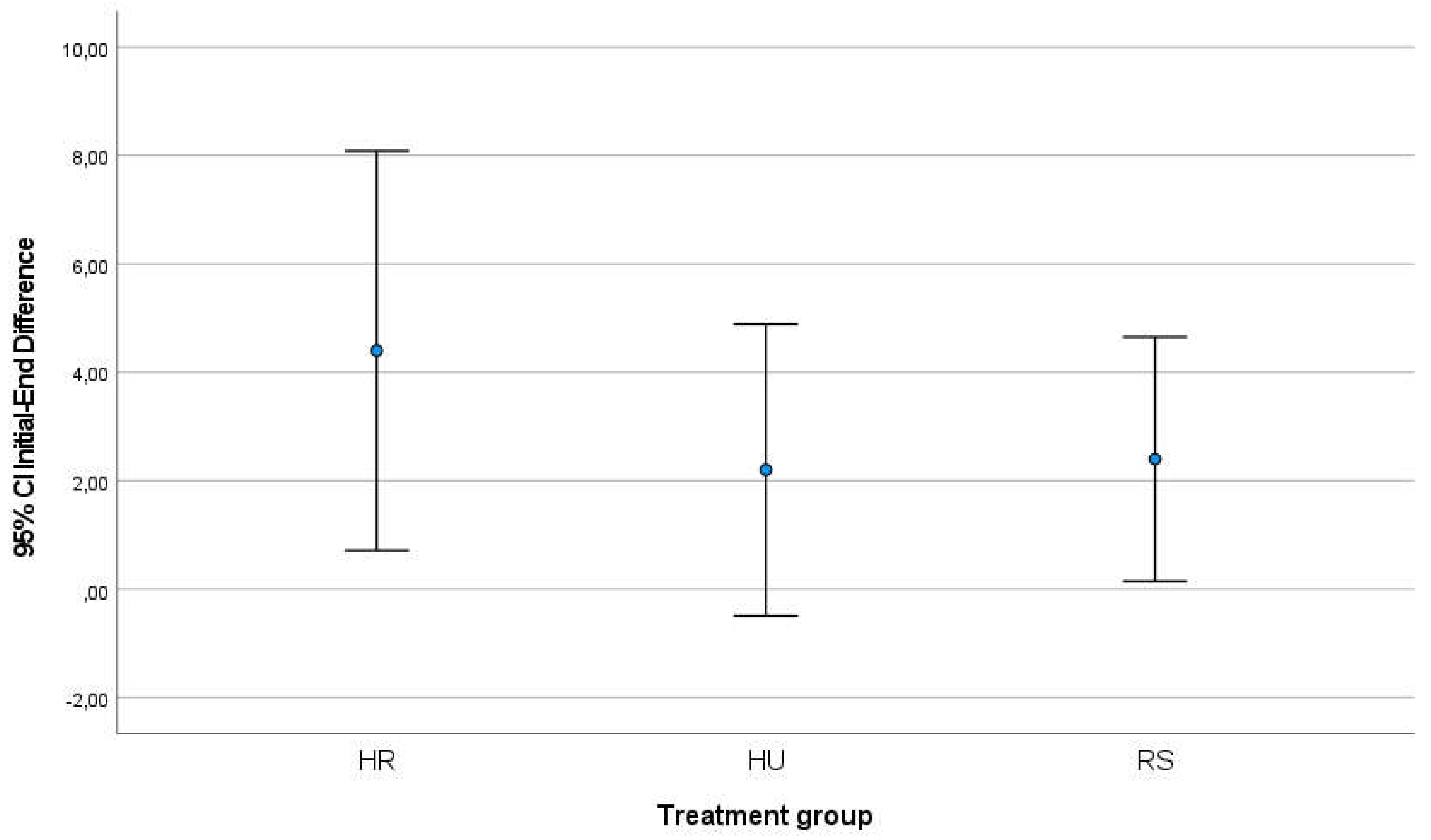
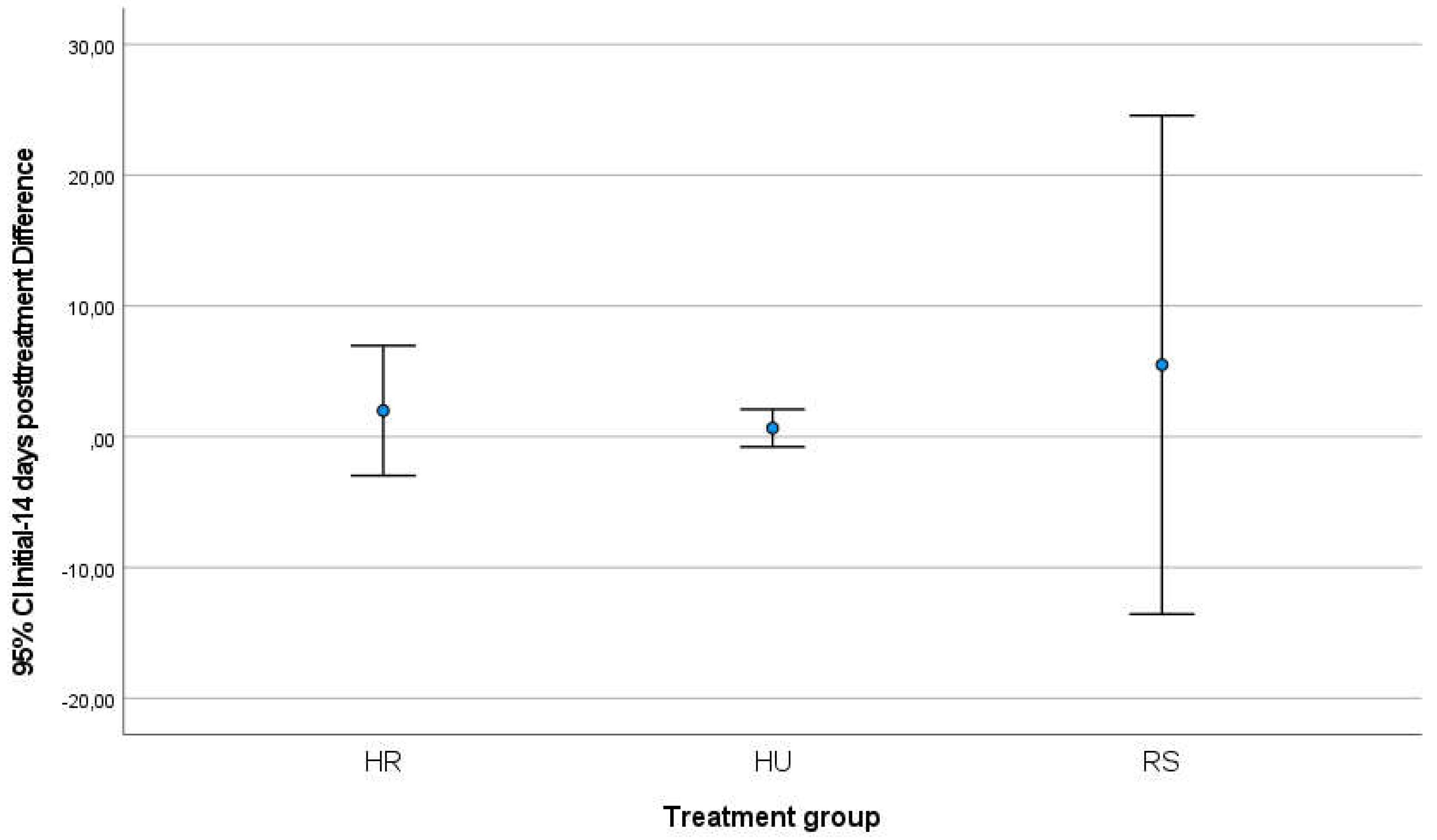
Between the start and end of RT treatment, the HR group increased their score by 4.40 points on the mean (standard deviation 2.96) while the HU group increased the score by 2.20 points (est. v. 2.16) and the RS group increased by 2.40 points (est. 1.81). After 14 days of finishing the RT program and the patients followed the application of the respective creams and comparing the result of the scale with the start of treatment, the HR group had increased by 2.00 points (SD=2.00), the HU group had increased only 0.66 points (Standard Deviation SD=0.577) and the RS group had increased its symptomatology by 5.50 points on average (SD=2.12) (
Table 2). In both comparisons, the analysis of variances using the Levene test did not reveal significant differences, so the groups were considered comparable; ANOVA analysis did not show that differences were significant between groups (initial-final comparison p-value=0.303; initial-post-14day comparison p-value=0.055). In the graphical representation of the comparisons, the differences between the groups can be seen (
Figure 2,
Figure 3).
Mexamer skin analysis
Due to technical problems, data could not be obtained from the Mexamer skin analysis in the RS group after completing the RT treatment, so the post-treatment analysis (7 days and 14 days) could not be performed in this group.
ELASTICITY
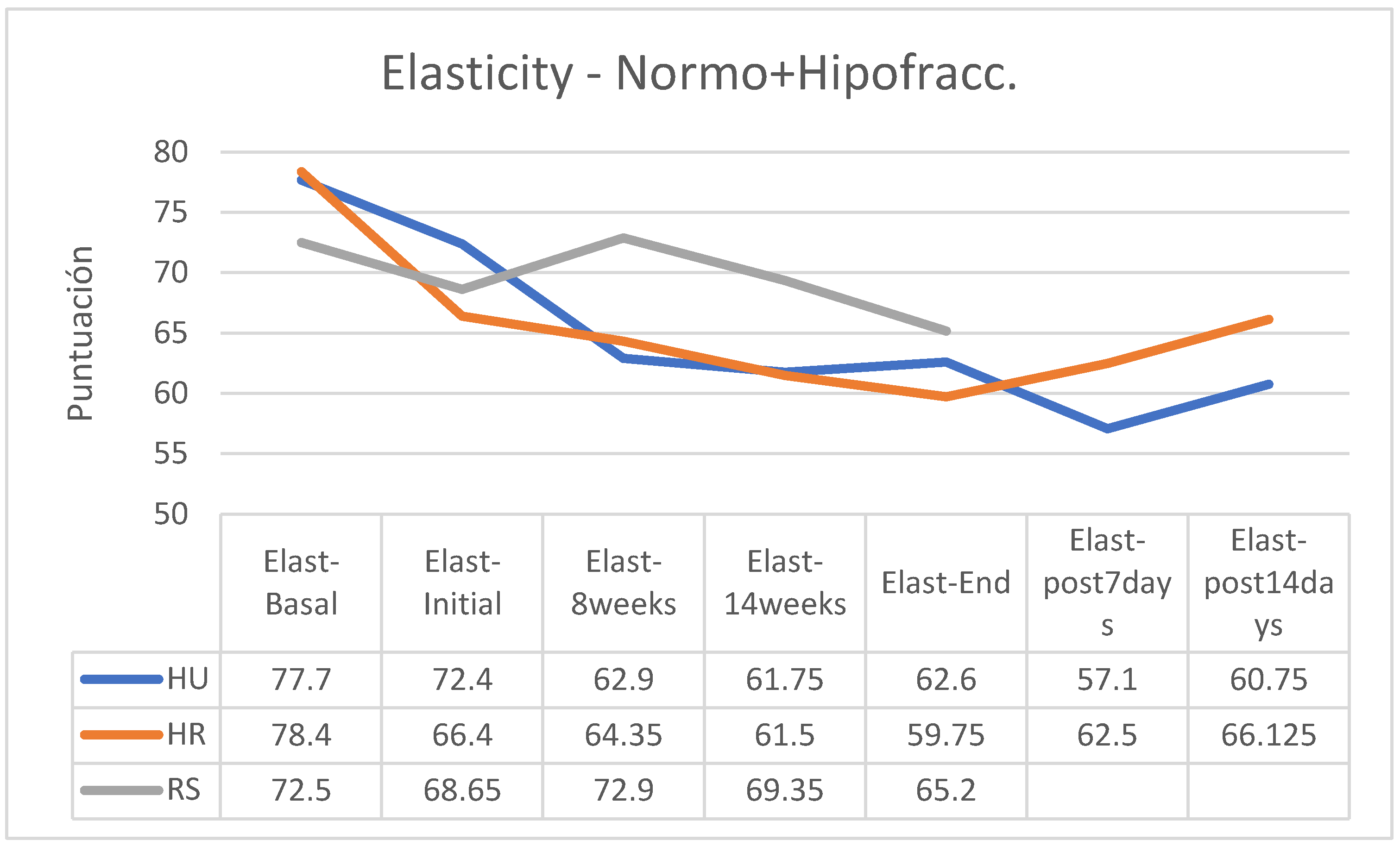
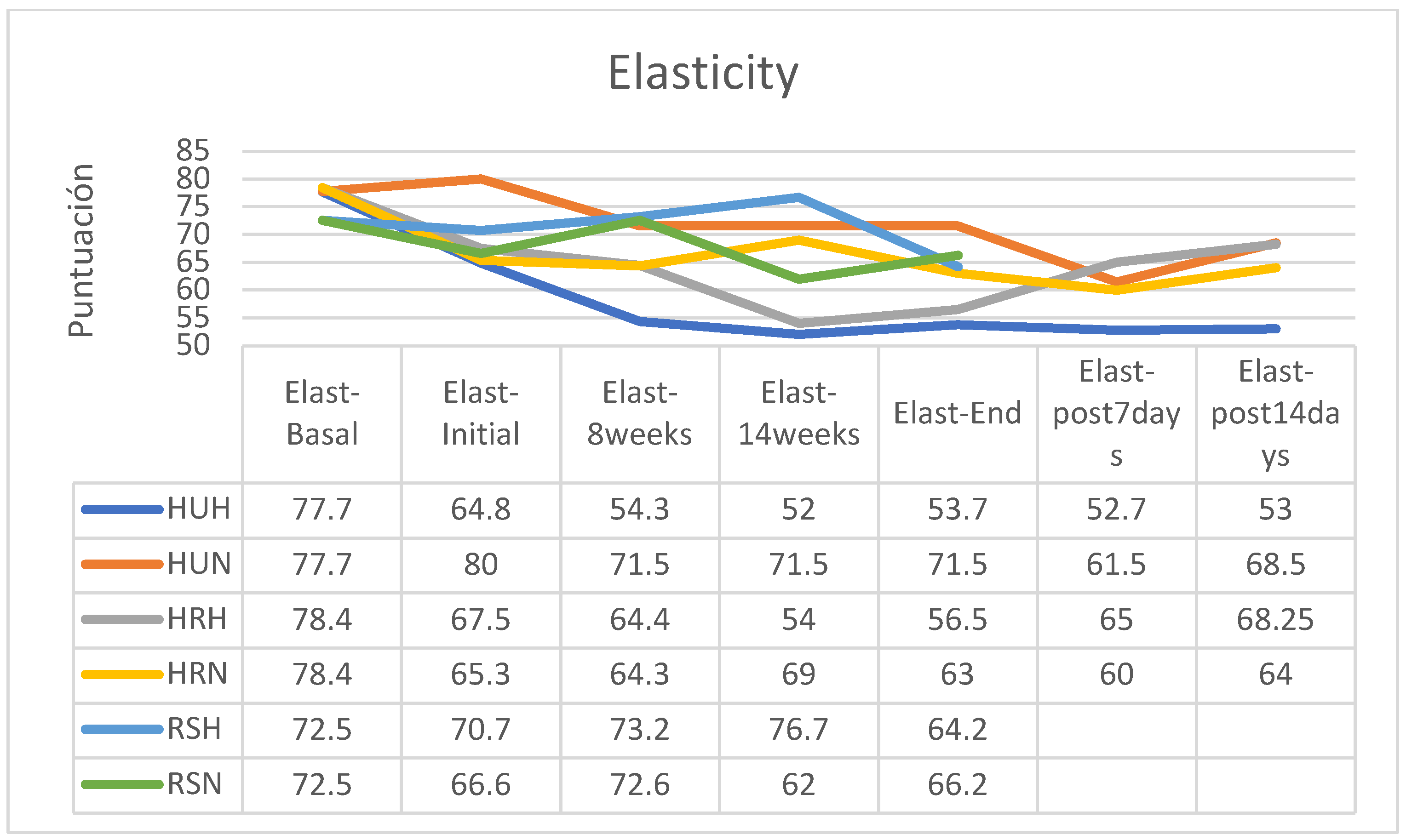
In all patients, skin elasticity was significantly reduced between baseline and during treatment, with an overall trend toward recovery within 14 days after the end of treatment (
Figure 4). However, recovery of elasticity after this period did not achieve pre-treatment normality in any of the groups from which data were obtained (
Figure 5).
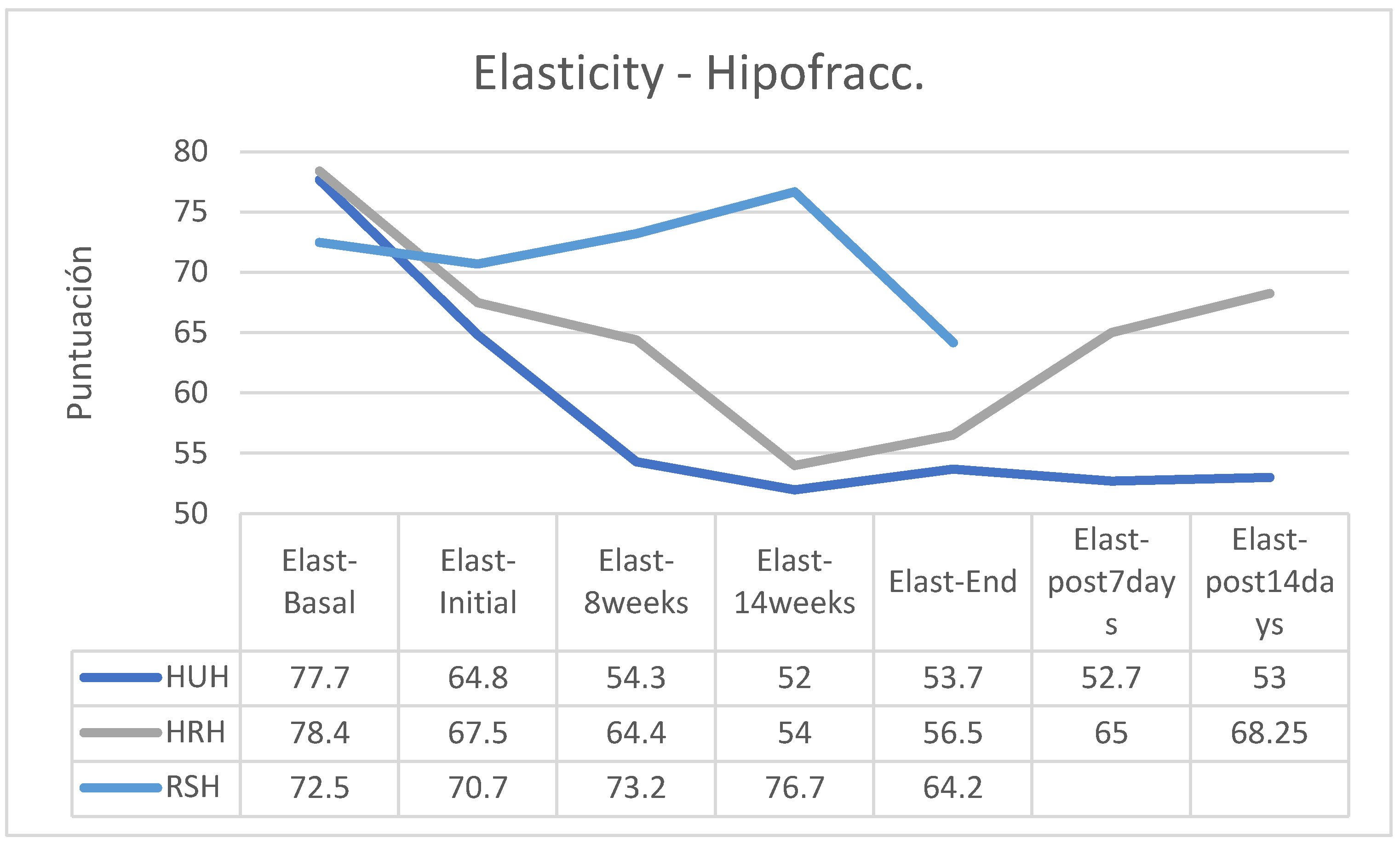
As an exception, the group of patients in the HU group who had followed a hypofractionated treatment regimen (HHUH) did not improve their elasticity after finishing the RT, having the lowest skin elasticity 14 days after the end of treatment. The group that followed treatment with SR cream and hypofractionated regimen increased its skin elasticity between the start of treatment and session 14 and, although the decrease in elasticity was marked between this session and the end of treatment, the loss of final elasticity was less than in the other two groups (
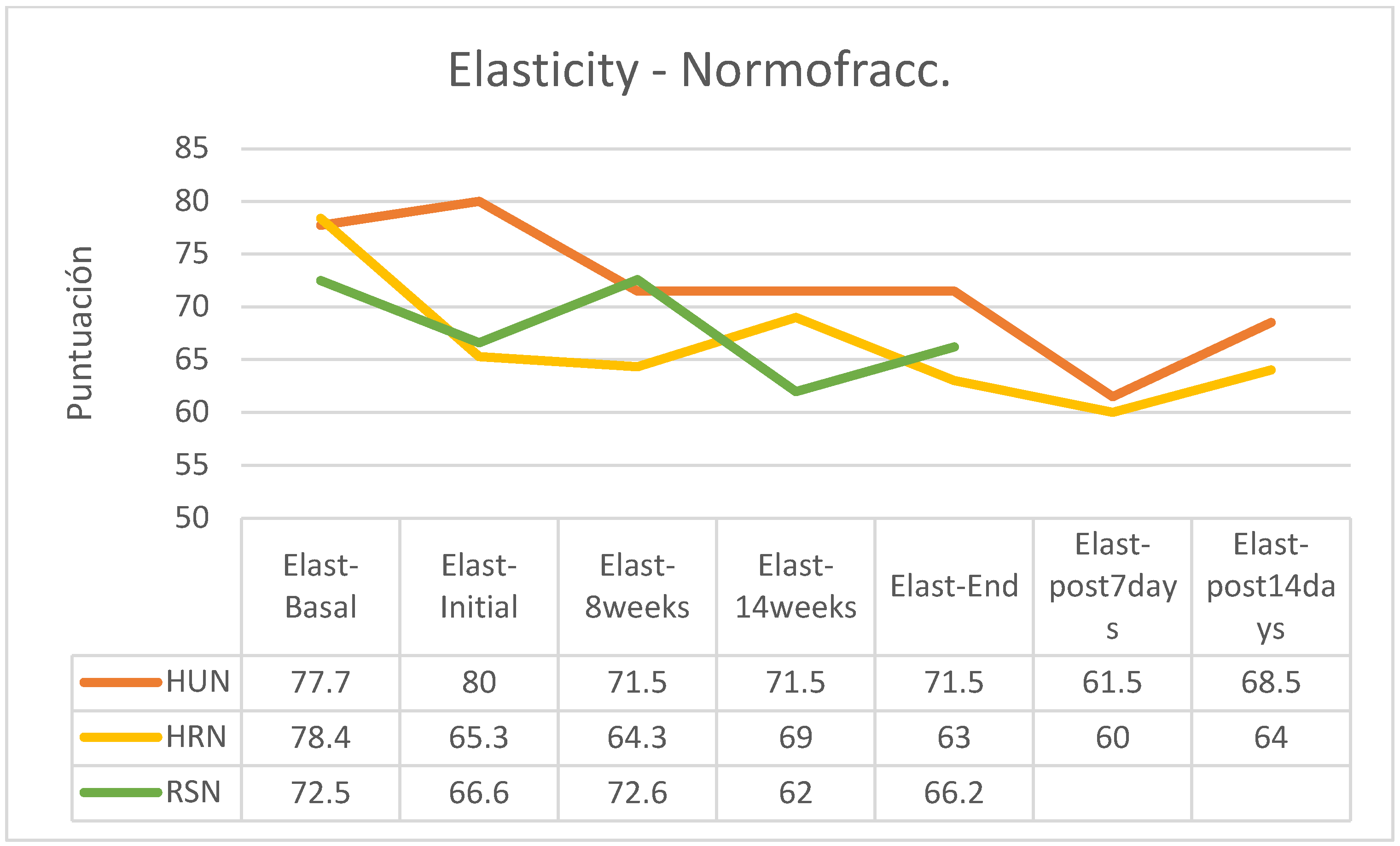
Figure 6); for reasons already explained there are no data on the evolution of this RS group after the end of the treatment regimen. In the group of patients treated using a normofractionated regimen, the behavior was similar in all three groups (
Figure 7).
Between the start and end of treatment, elasticity was reduced by 9.8 points in patients who applied HU cream, by 6.65 points for those who used HR, and only by 3.45 points for those who used SR (
Table 3). Between the start and end of the program, patients who used creams without EGF reduced their elasticity by 8,225 points, while those who used a cream with EGF only reduced their skin elasticity by 3.45 points on average (
Table 4).
Between the beginning and 2 weeks after the end of the RT, the patients who had followed treatment with HU cream had reduced their elasticity by 11.65 points, while for those who had used HR the elasticity was practically the same as at the beginning of treatment (reduction of 0.275 points). As explained, there are no data on the evolution of the RS group after the end of RT (
Table 5).
With the use of any of the 3 creams tested, elasticity was reduced more significantly in hypofractionated treatments (HU -17.3; HR -16.3; RS -9.19) than in normofractionated (HU -10.63; HR -3.52; RS -0.60). In the analysis of the retrospective data of the control group, the mean reduction in elasticity was 12.00%, between -6 and -18% In the retrospective control group, which had followed applications of other commercial preparations, on average the skin elasticity had decreased by 12% (between -6 and -18%, without data on the fractionation applied). (
Table 6).
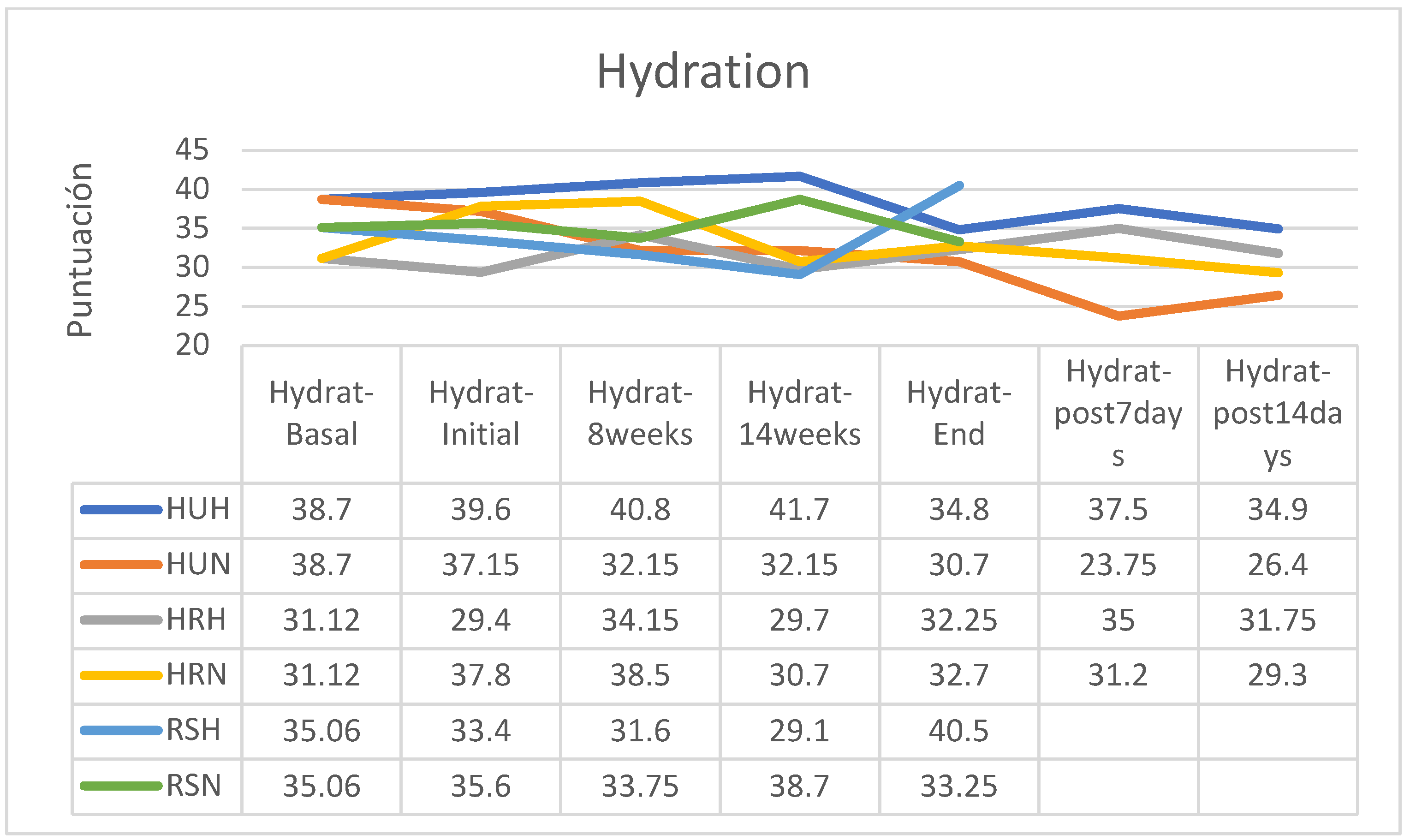
HYDRATION
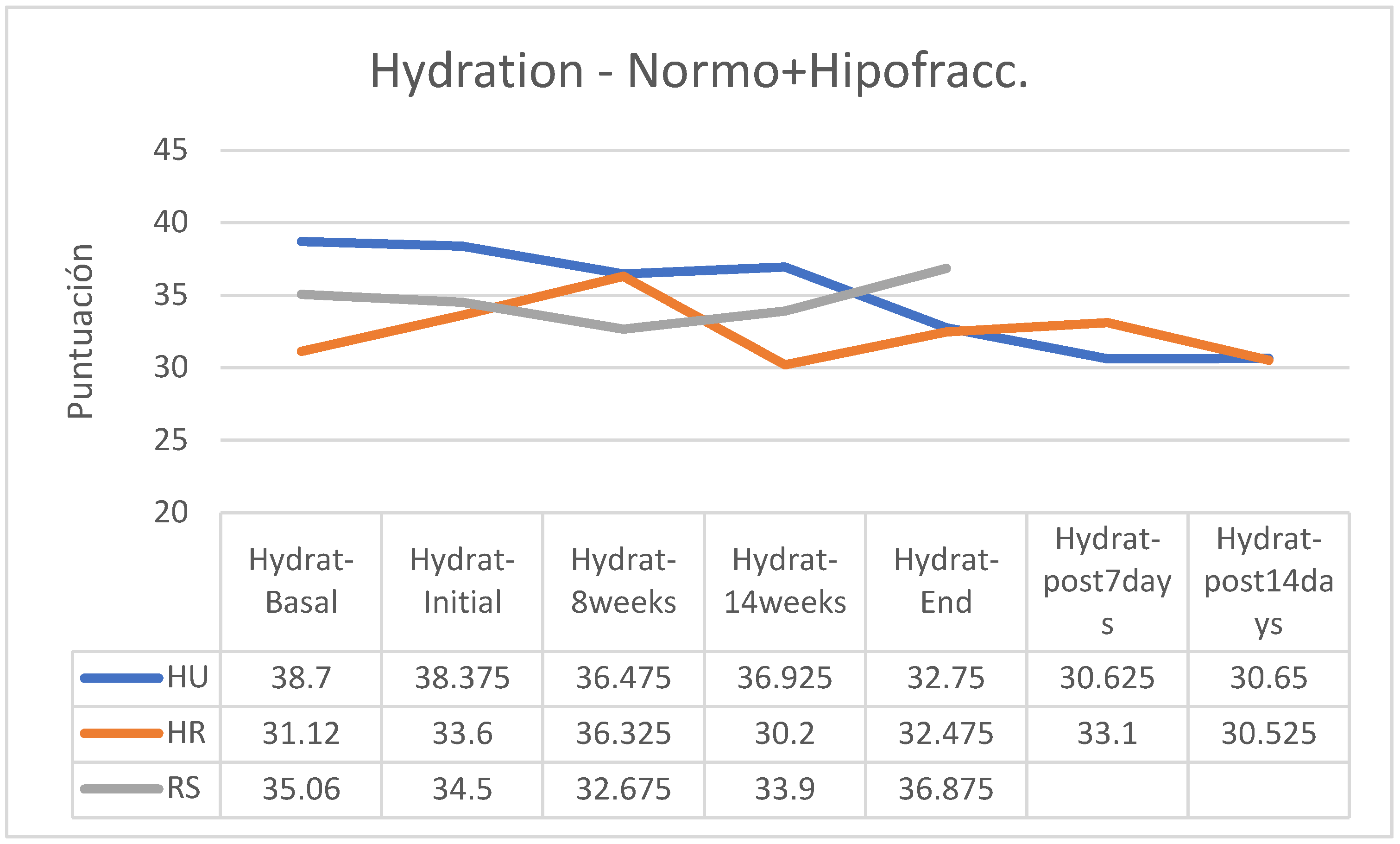
Overall, skin hydration showed fewer changes than elasticity and the tendency to lower hydration was of low intensity in all groups; however, less hydration was observed two weeks after the end of treatment in the group treated with HU in a normofractionated regimen, stability with a tendency to a slight increase in the HR group in hypofractionated regimen and an increase at the end of the treatment of hydration status in patients of the RS group treated with hypofractionated regimen while a slight reduction was observed in those treated with normofractionated regimen (
Figure 8).
Without considering fractionation, in patients who received RS cream, hydration tended to increase at the end of treatment, while the HR and HU groups reduced their hydration during treatment and this reduction was not recovered two weeks after the end of RT (
Figure 9).
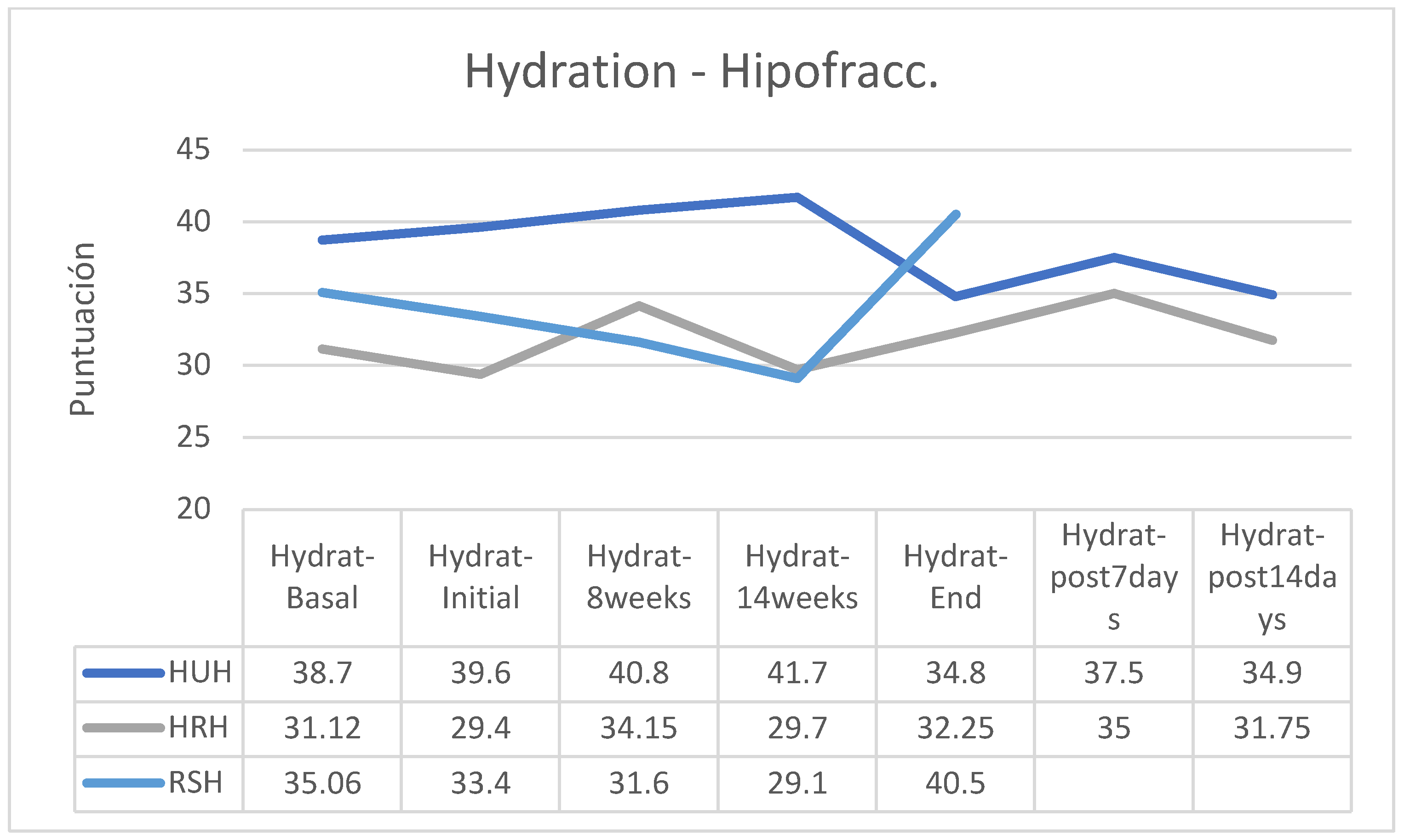
Analyzed in isolation, the group of patients who followed a hypofractionated regimen reduced their skin hydration only in the group using the HU cream, while those who followed the HR cream slightly increased their hydration and the patients who applied RS cream significantly increased their hydration, especially from session 14 of RT treatment (
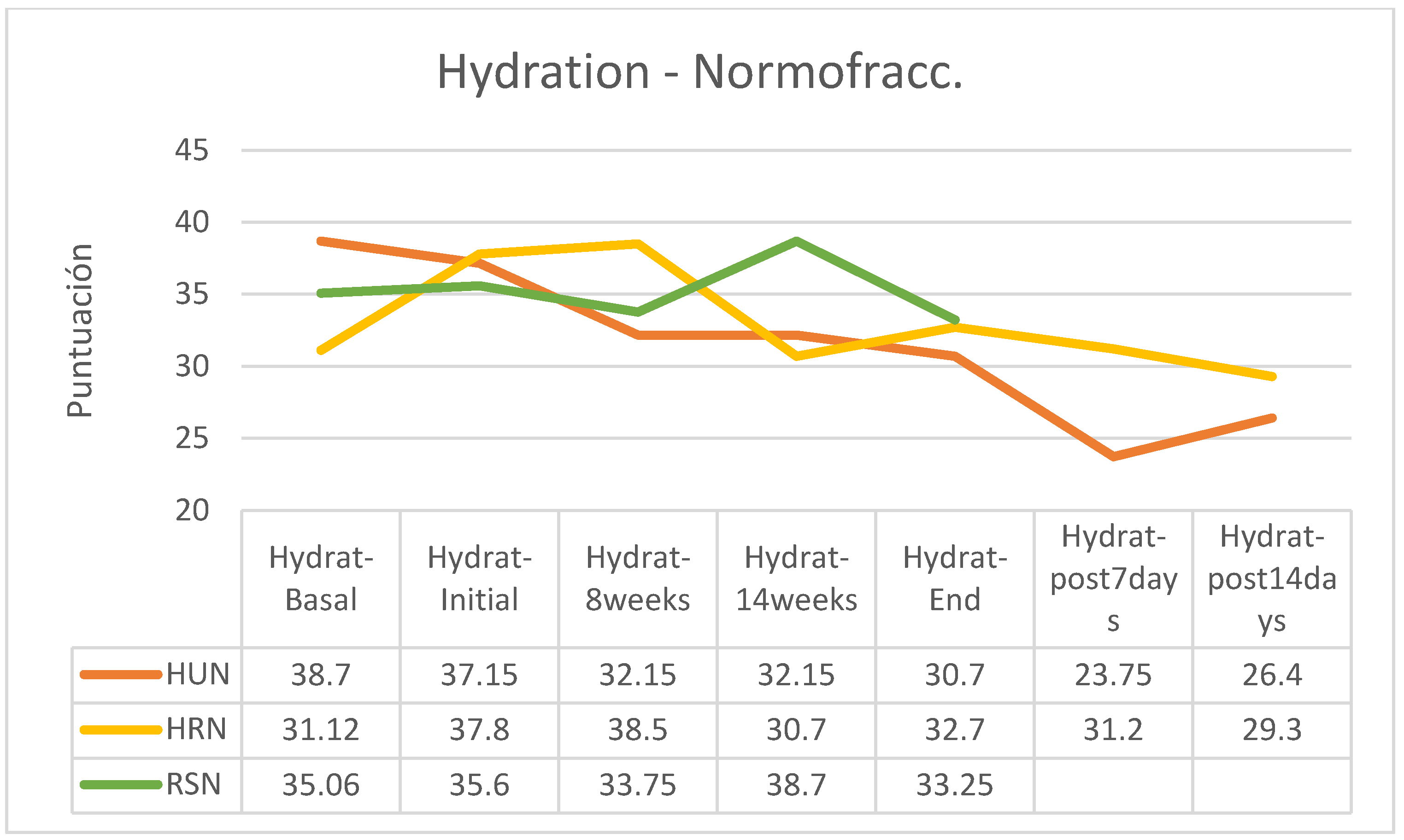
Figure 10). In contrast, all participants who received normofractionated therapy reduced their skin hydration, especially the groups applying HU and HR creams, while in the group that applied RS cream, the reduction was non-existent (
Figure 11).
Between the beginning and the end of the intervention program of application of the creams, there were reductions in hydration in the HU groups (hypofractionated: -12.12%; normofractionated: -17.36%) and in patients who received normofractionated therapy (HR: -13.49%; RS: -6.60%); however, in patients receiving hypofractionated therapy there was increased hydration status in patients receiving HR cream (+9.69) and RS cream (+21.26%). In the retrospective control group, which had followed applications of other commercial preparations, on average pigmentation had increased by 11% (between 7 and 15%). In the retrospective control group, which had followed applications of other commercial preparations, on average the hydration had decreased by 9% (between -3 and -15%, without data on the fractionation applied). (
Table 7).
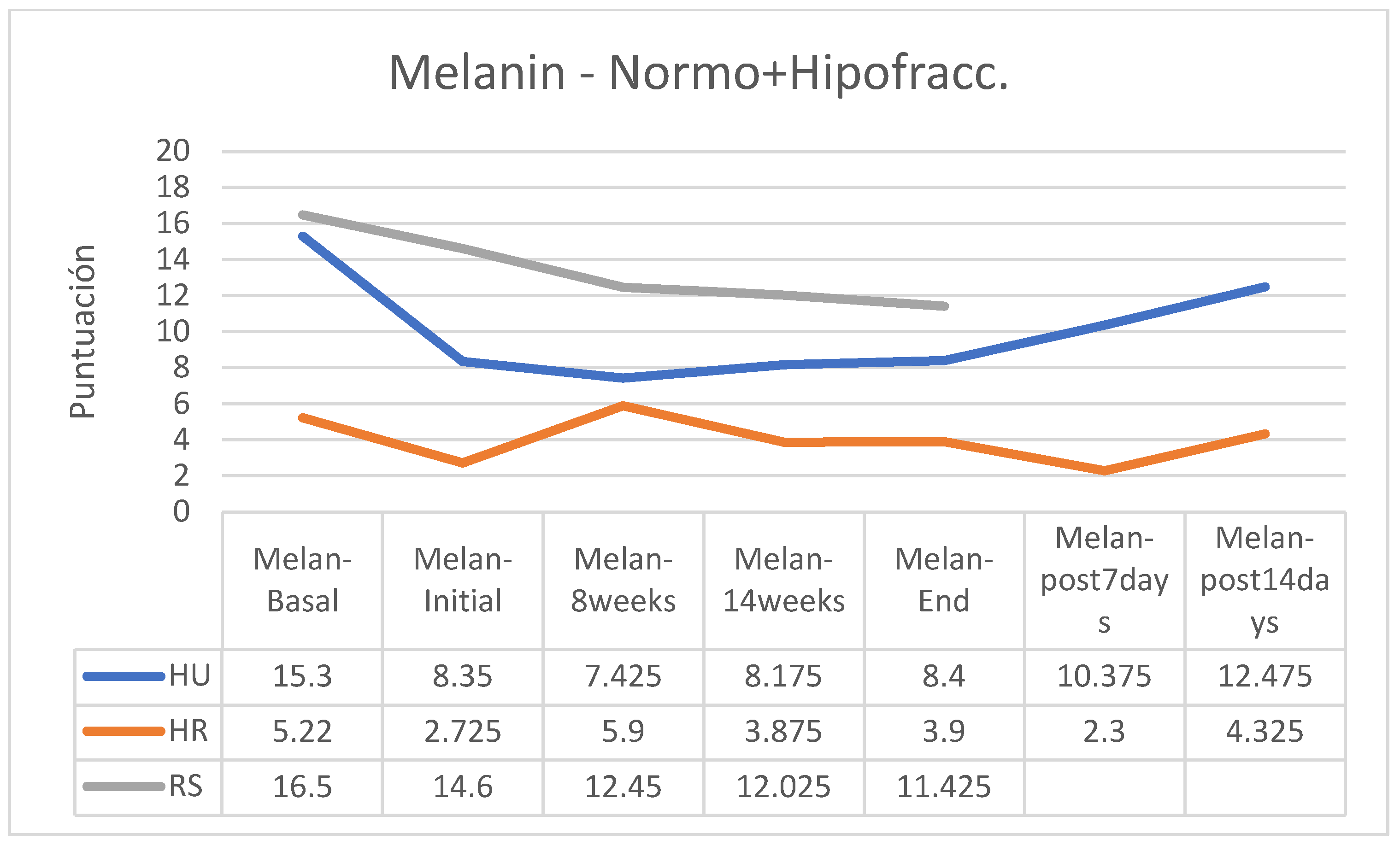
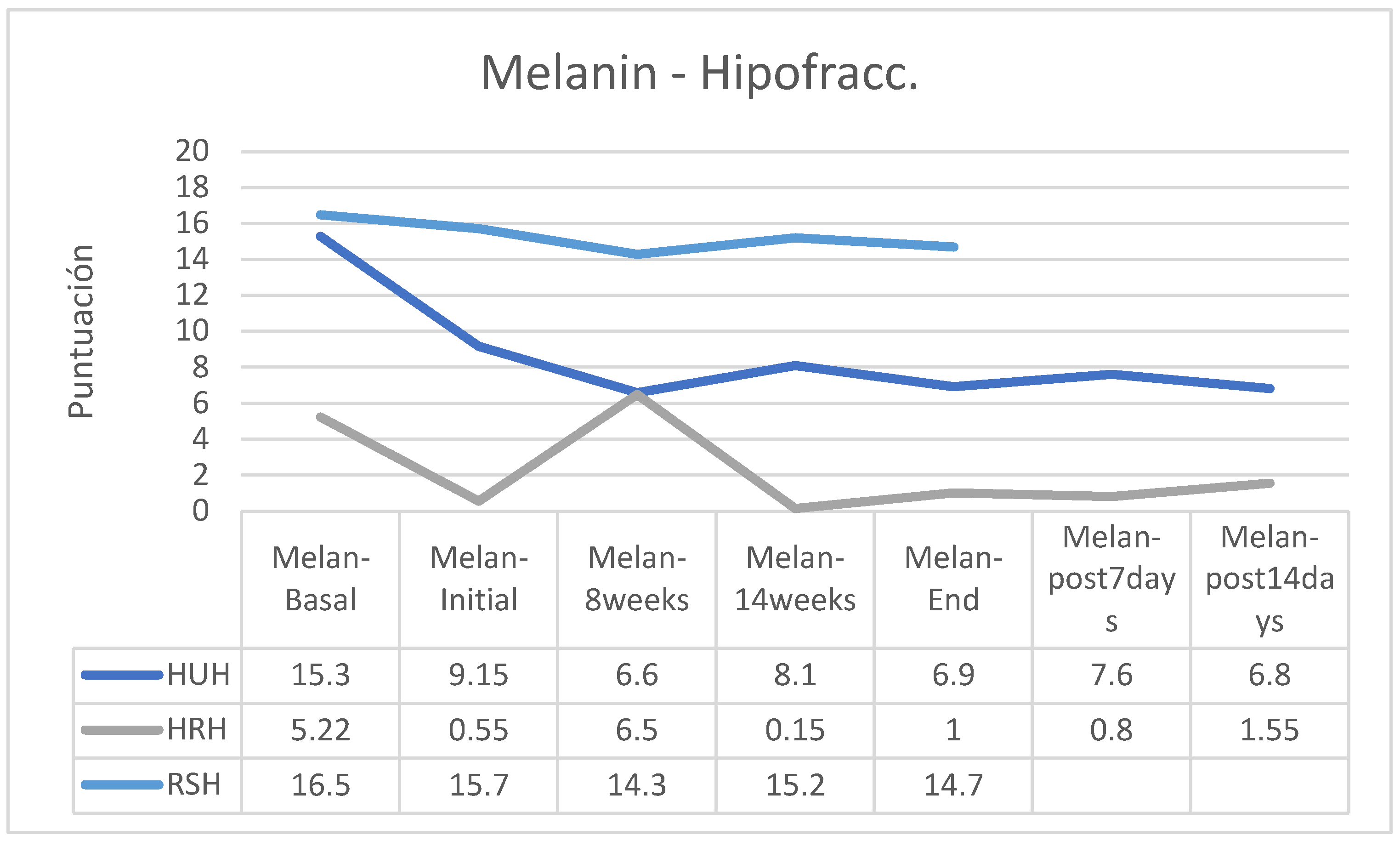
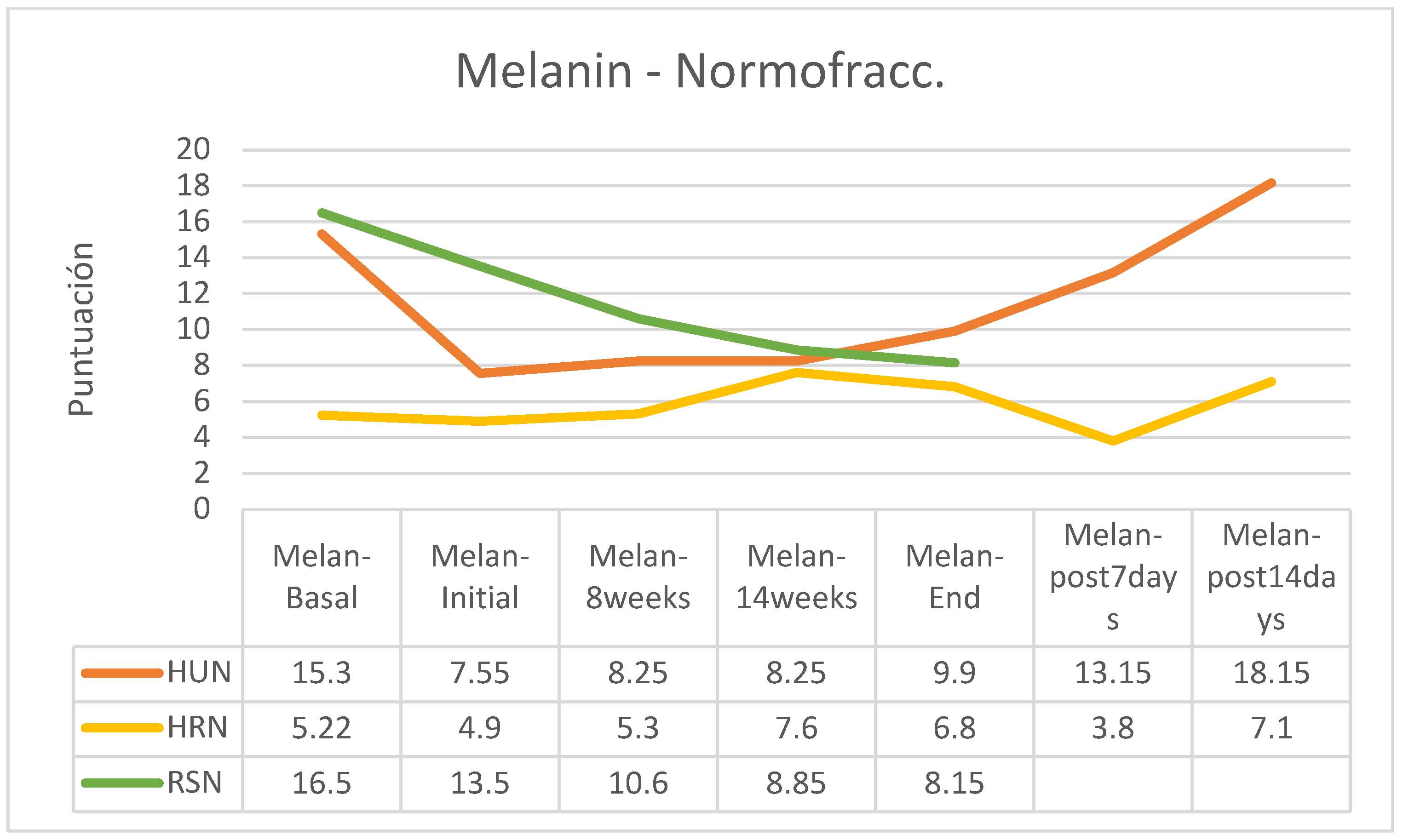
MELANIN
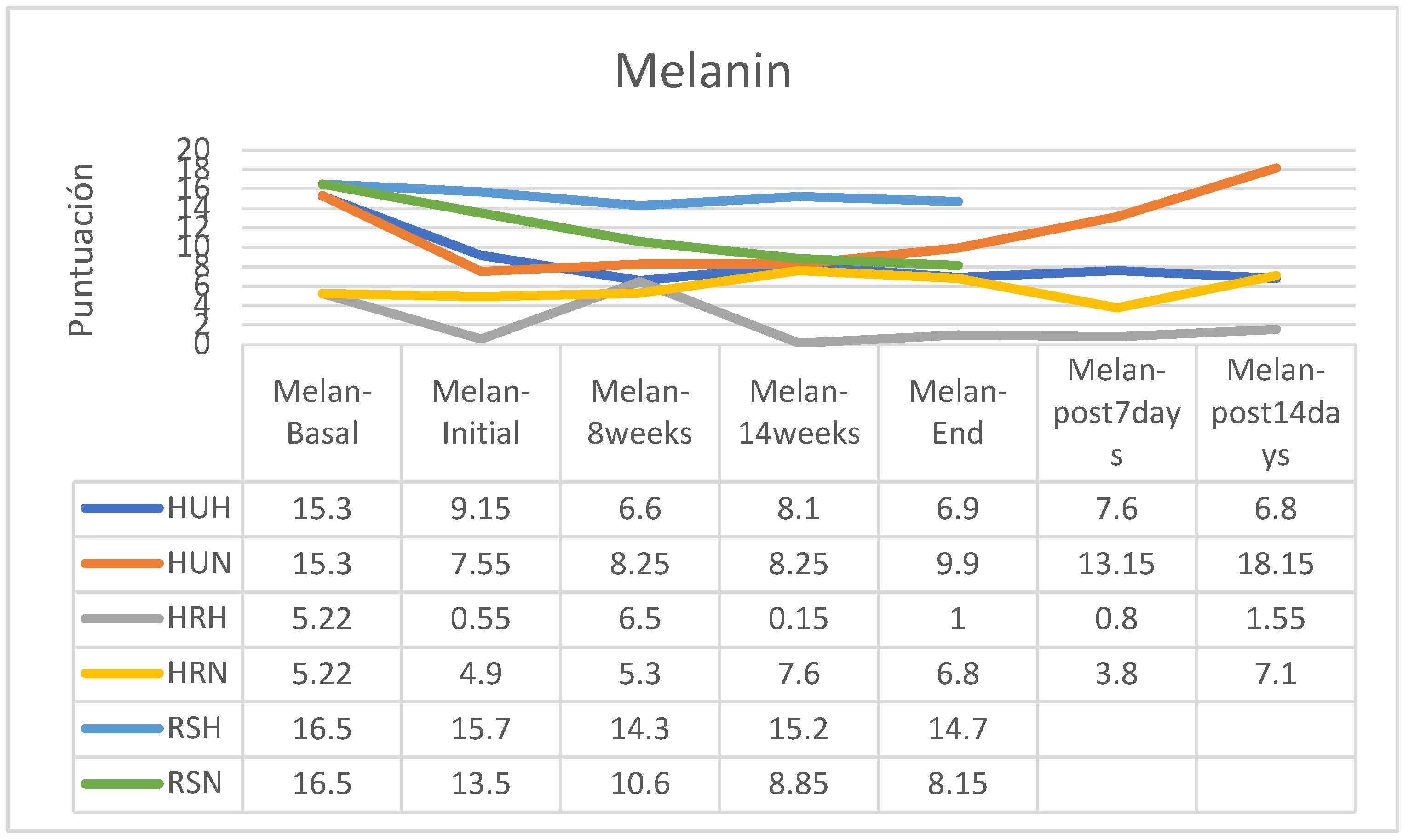
Regarding skin pigmentation, an increase was observed in the group of patients following normofractionated therapy and HU cream and a reduction in melanin in the RS group following normofractionated therapy; however, in the other groups’ the differences were small (
Figure 12).
Without considering the RT regimen followed by the patients, all groups saw their skin pigmentation reduced throughout the treatment, although the differences were very small in the groups that followed HU and HR creams while the patients in the RS group reduced their melanin more significantly (
Figure 13).
In the case of patients who followed a hypofractionated regimen, melanin decreased in the groups that applied HU and HR creams, while the group that applied RS creams was practically stable (
Figure 14). In the group of normofractionated regimen, melanin decreased at the beginning of treatment and at the end of this it increased in patients who followed HU cream, was practically stable in patients who applied HR cream and decreased significantly in patients who followed applications with RS cream (
Figure 15).
Percentage-wise there were increases in skin pigmentation in the groups that followed a normofractionated regimen (HU: +31.13%; HR: + 38.78%) except in participants who followed RS cream applications (-39.63%); in those who followed the hypofractionated regimen, an increase was observed only in patients in the HR group (+81.82%) while pigmentation decreased in the other two groups (HU: -24.59%; RS: -39.63%). In the retrospective control group, which had followed applications of other commercial preparations, on average pigmentation had increased by 11% (between 7 and 15%, without data on applied fractionation) (
Table 8).
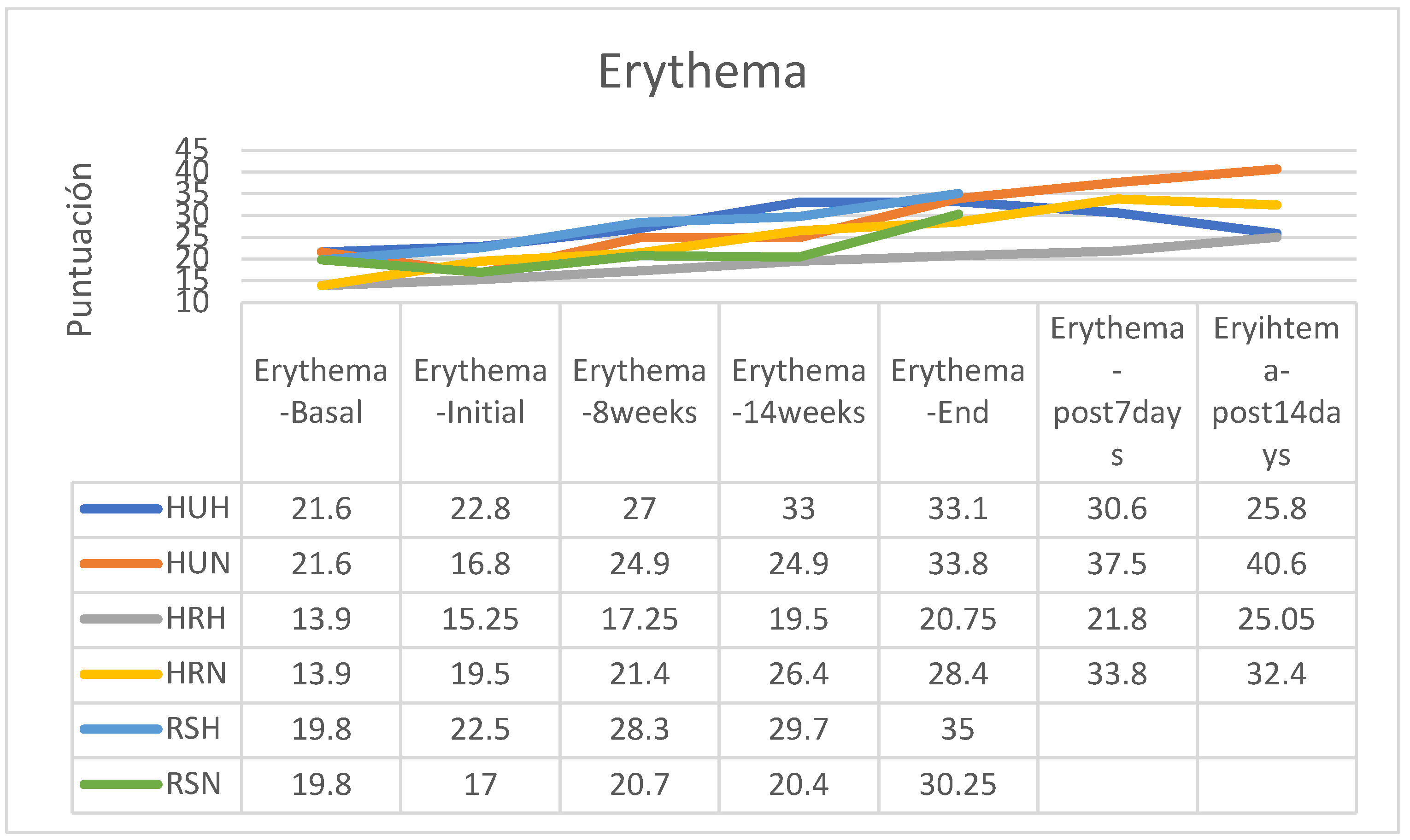
ERYTHEMA
About the apparition of cutaneous erythema secondary to RT treatment, there was an erythema increase in all groups (
Figure 16). Without considering the RT regimen, the group of participants who applied HU cream at the end of the RT erythema tended to stabilize, while those who followed HR cream continued to see their erythema increase during the first two weeks (
Figure 17).
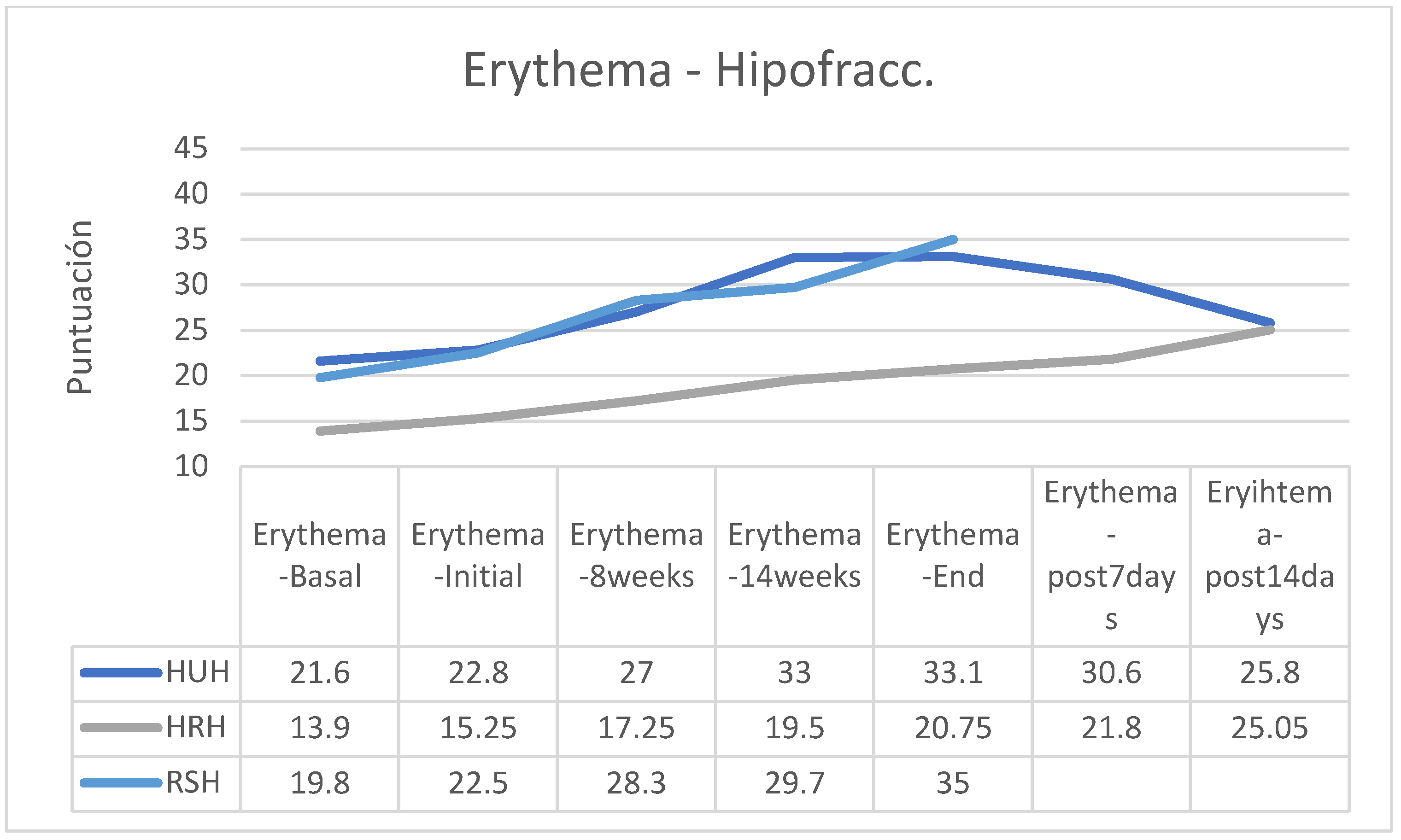
In the group of patients treated with a hypofractionated regimen and following HU cream, erythema attended to disappear rapidly during the two weeks after RT, while in the other groups erythema remained stable or increased slightly (there are no post-RT data from the group following the RS cream) (
Figure 17).
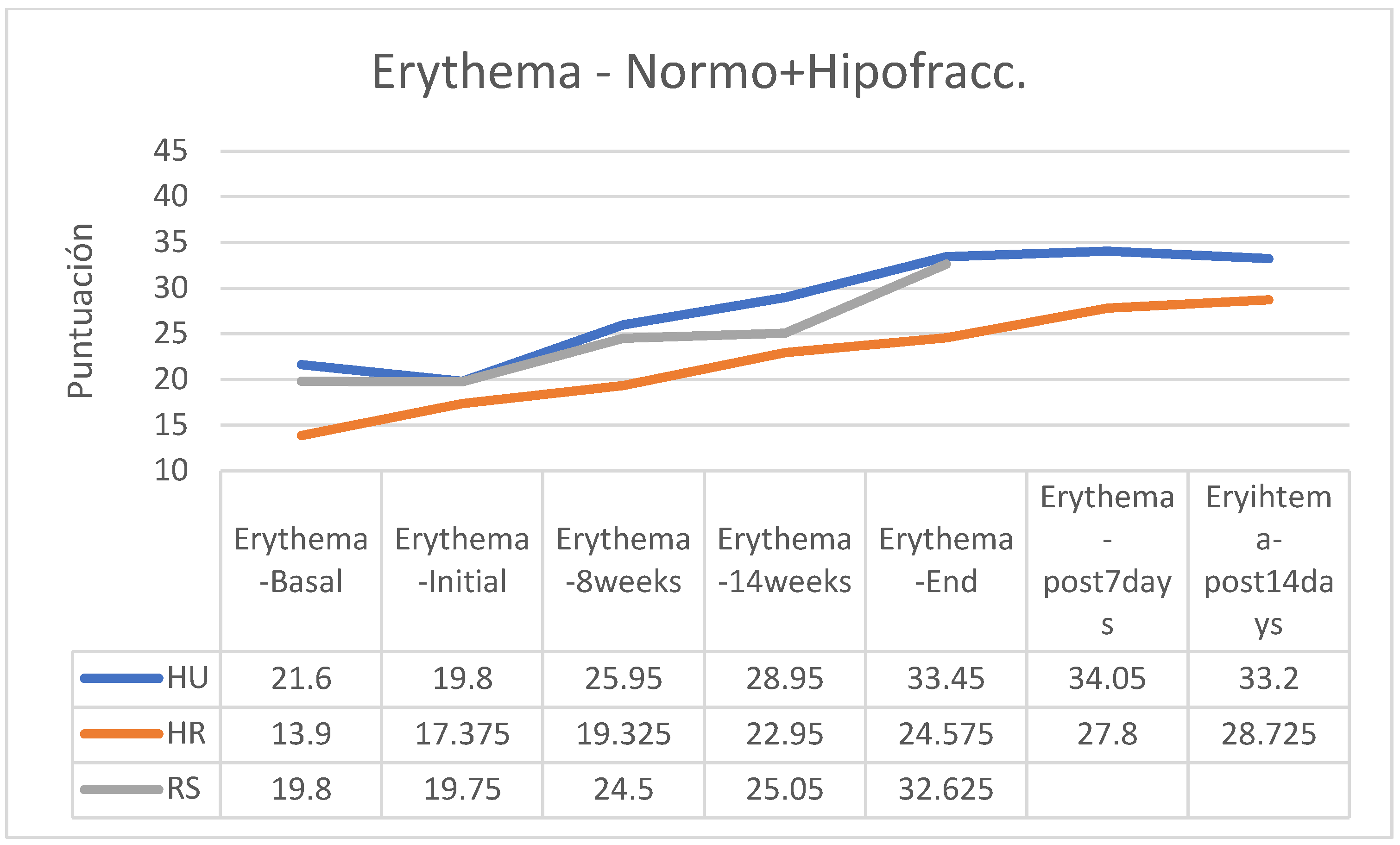
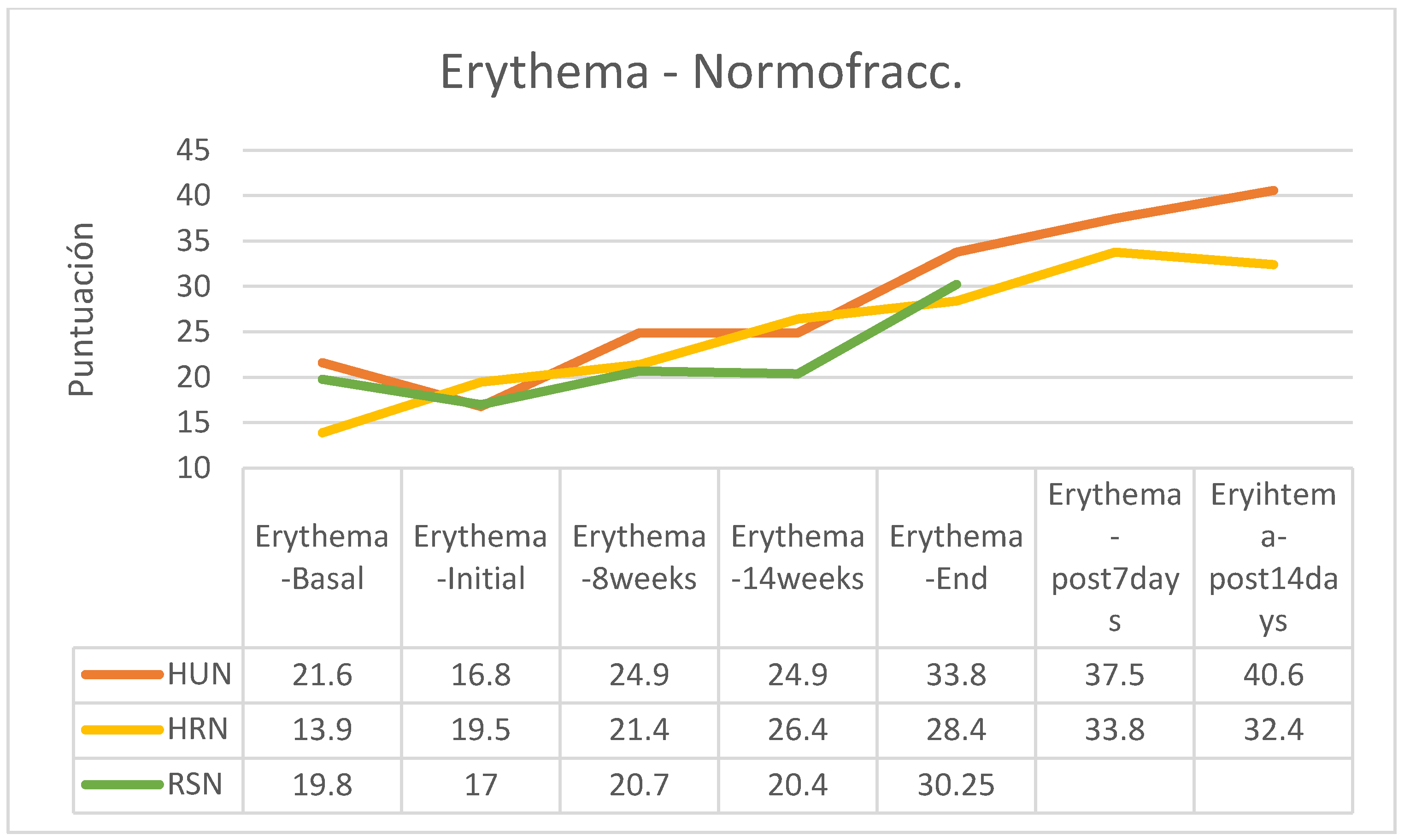
In both the normofractionated and hypofractionated regimens, erythema increased with the passage of the sessions and at the end of the treatment, except in the hypofractionated regimen group that followed applications of HU cream in which erythema was reduced from the end of the application of RT (
Figure 18,
Figure 19).
Between the beginning and the end of the intervention program of application of the creams there were increases in erythema in all groups, these being more important in those patients who received normofractionated regimens (HU cream group 101.19%; HR 45.64%, SR 77.94%), while patients who received hypofractionated therapy showed a smaller increase in erythema (HU: 45.18%; HR: 36.07%; RS: 55.56%%). In the retrospective control group, which had followed applications of other commercial preparations, on average erythema had increased by 16% on average (between 7 and 25%, without data on applied fractionation) (
Table 9).
5. Discussion
In the Patient-Observer scale, the evolution of the symptoms derived from the effect of RT can be analyzed, in which the group that followed treatment with the HU cream has greater symptoms than the rest of the groups. It is also noteworthy that the group that used the RS cream presented fewer symptoms at the beginning of treatment, but after the end of treatment the symptoms tend to increase more than the remaining groups (
Figure 1). Therefore, larger studies could analyze the convenience of using HR cream throughout the period or RS cream during RT and HU or HR cream after completing cancer treatment.
As for the analysis of the skin using Maxidex, it can be seen that the cream that provides the greatest elasticity and hydration is the HU cream, while the RS cream is the one that can reduce the production of melanin, probably due to its EGF content. As for erythema, the cream probably controls it more effectively, because of its neutral composition (
Table 10).
Analyzing together the creams without EGF compared to the RS cream, the cream containing the factor can further improve hydration compensating to a greater extent for the reduction of elasticity produced by RT; this fact is also considered consistent with the composition of the cream RS and its content in EGF. In the same sense, the cream containing EGF can maintain hydration and compensate for the decrease in it, while tending to decrease the overproduction of melanin. However, cream with EGF does not have as much effect in terms of controlling erythema (inflammation) (
Table 11), which may be beneficial for better tissue repair after RT.
Comparing the available data 2 weeks after the end of the RT regimen (there are no data on the RS cream group in this period), it can be seen that all the parameters are more satisfactory with HR cream: elasticity and hydration have been reduced less, melanin production has increased less and erythema is slightly lower (
Table 12).
These results, as discussed above, are not statistically significant, but can be observed as clinical experience and could serve as a basis for larger studies.
Separating the results according to the fractionation of the RT, it is observed that in hypofractionated regimens the RS cream is the one that achieves less reduction of elasticity, greater hydration and lower production of melanin but less control of erythema, results consistent with the analysis without separation by fractionation (
Table 13). Regarding the behavior of the control group, it should be noted that it had the best control of erythema, although the results are not completely valuable due to the possible great heterogeneity of this group. As for elasticity, in the normal evolution of a radiotherapy process there is a loss of elasticity due to the effect of radiation. The loss of elasticity at significant levels produces more fibrosis. The most recommended cream would be one that allows losing the least elasticity from the basal level until the end of treatment; In our series, it would be the HR cream. During RT there is also a decrease in skin hydration. The most interesting cream would be the one that lost less hydration and kept the skin more hydrated. In this case, the RS cream even increases basal hydration. In general, and depending on the phenotype of the patient, RT can produce an increase in skin pigmentation (melanin production); this increase is usually more increased in people with phenotypes 1-2 and greater in people with phenotypes 3-4. The most aesthetically interesting cream will be the one that better controls the tendency to hyperpigmentation For this purpose, the HU cream is the one that provides the greatest control of melanin, followed by the RS cream. RT erythema is inevitable and is part of tissue repair: the higher this blood supply is provided to the tissue. Erythema should decrease significantly 15 days after the end of RT, manifesting as Mild Grade 1 of the ORTC. In this case the one that most increases erythema, and therefore skin repair, is RS cream.
Regarding the study of normofractionated patterns (
Table 14), it is observed that the cream that achieves better control of elasticity, erythema and melanin is RS cream, which also achieves good control of erythema; in this group of patients, erythema was even higher by HU cream. We conclude. The expected evolution of elasticity during RT is a decrease in elasticity because of radiation. The most recommended cream would be the one that decreases the least elasticity from the basal level to the end of treatment, in our series the RS cream. As for the loss of hydration during RT, the most indicated cream would be the one that lost less hydration and kept the skin more hydrated; in our pilot study it would be the RS cream. Concerning pigmentation (melanin), a lower increase in pigmentation would be the most recommended, and the RS cream seems to be the one that best allows controlling the tendency to hyperpigmentation produced by RT. Erythema, and its consequent increase in blood supply, as a factor linked to tissue repair may be beneficial in the absence of unpleasant symptoms such as pruritus, burning or pain; erythema is expected to decrease significantly 2 weeks after completion of RT to Grade 1 (Mild) ORTC treatment; in our study, the cream that allows the greatest increase in erythema is HU.
6. Conclusions
All creams provide their special characteristics with improvements in specific concepts and the recommendation of one or the other compositions will depend on clinical criteria.
In the Patient-Observer scale, which shows the symptoms as undesirable effects of RT, it is observed that the RS cream may be more effective in controlling the symptoms of RT during it and that the HU cream may be more effective after the end of treatment.
In the analysis of the skin using Maxidex, in hypofractionated therapies the RS cream may be the one that most retains skin elasticity during RT, the HU and RS creams the ones that most conserve hydration, the RS cream the one that allows less pigmentation and the HU and RS creams those that allow a greater blood supply.
In normofractionated therapies, all the creams included allow preserved elasticity and hydration, and e RS cream allows better control of hyperpigmentation and erythema.
However, due to the sample size being a pilot study, these conclusions should be analyzed through a study with a larger sample size and experimental design.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Funding
Hydroskin OncologyTM provided all study materials.
References
- Ramseier JY, Ferreira MN, Leventhal JS. Dermatologic toxicities associated with radiation therapy in women with breast cancer. Int J Womens Dermatol. 2020 Sep 30;6(5):349-356. [CrossRef]
- Biswal SG, Mehta RD. Cutaneous Adverse Reactions of Chemotherapy in Cancer Patients: A Clinicoepidemiological Study. Indian J Dermatol. 2018 Jan-Feb;63(1):41-46. [CrossRef]
- Barrios DM, Phillips GS, Freites-Martinez A, Hsu M, Ciccolini K, Skripnik Lucas A, Marchetti MA, Rossi AM, Lee EH, Deng L, Markova A, Myskowski PL, Lacouture ME. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study. J Eur Acad Dermatol Venereol. 2020 Jun;34(6):1340-1347. [CrossRef]
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019 Aug;81(2):558-567. [CrossRef]
- Yee C, Wang K, Asthana R, Drost L, Lam H, Lee J, Vesprini D, Leung E, DeAngelis C, Chow E. Radiation-induced Skin Toxicity in Breast Cancer Patients: A Systematic Review of Randomized Trials. Clin Breast Cancer. 2018 Oct;18(5):e825-e840. [CrossRef]
- Kole AJ, Kole L, Moran MS. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer (Dove Med Press). 2017 May 5;9:313-323. [CrossRef]
- Leventhal J, Young MR. Radiation Dermatitis: Recognition, Prevention, and Management. Oncology (Williston Park). 2017 Dec 15;31(12):885-7, 894-9.
- Esquirol Caussa J, Herrero Vila E. Epidermal growth factor, innovation and safety. Med Clin (Barc). 2015 Oct 5;145(7):305-12. Spanish. [CrossRef]
- Lurge G, Lenz HJ. EGFR signalling and drug discovery. Oncology. 2009;77:400-10.
- Berlanga J, Álvarez S, de la Fuente J, López P. Considerations on the transforming potential of the epidermal growth factor. Biotecnol Apl. 1998;15(2):65-9.
- Berlanga-Acosta J, Gavilondo-Cowley J, López-Saura P, González-López T, Castro-Santana MD, López-Mola E, Guillén-Nieto G, Herrera-Martinez L. Epidermal growth factor in clinical practice—a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009 Oct;6(5):331-46. [CrossRef]
- Kang HC, Ahn SD, Choi DH, Kang MK, Chung WK, Wu HG. The safety and efficacy of EGF-based cream for the prevention of radiotherapy-induced skin injury: results from a multicenter observational study. Radiated Oncol J. 2014 Sep;32(3):156-62. two: 10.3857/roj.2014.32.3.156.
- Kong M, Hong SE. Topical use of recombinant human epidermal growth factor (EGF)-based cream to prevent radiation dermatitis in breast cancer patients: a single-blind randomized preliminary study. Asian Pac J Cancer Prev. 2013;14(8):4859-64. [CrossRef]
- Seral-Gajón AL, Carreras-Sospedra A, López-Jimeno C, Hijano- Berberia V, Viedma-Pastor M, Russo G, Mayayo-Falo T, Martínez-Martínez F, Fernández-Rodríguez M. Treatment of skin toxicity secondary to radiotherapy treatment with a cream based on sH oligopeptide-1 (epidermal growth factor-egf): a pilot study. Current. Med. 2018; 103: (804): 76-81. [CrossRef]
- Kang HC, Ahn SD, Choi DH, Kang MK, Chung WK, Wu HG. The safety and efficacy of EGF-based cream for the prevention of radiotherapy-induced skin injury: results from a multicenter observational study. Radiat Oncol J. 2014 Sep;32(3):156-62. [CrossRef]
- EU Regulation No 358/2014 COMMISSION OF 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Available in: https://www.boe.es/buscar/doc.php?id=DOUE-L-2014-80745.
- Formaldehyde allergic contact dermatitis and formaldehyde releasers. N. Latorre, J.F. Silvestre, A.F. Monteagudo. Actas Dermosifiliográficas 2011;102:86-97 - Vol. 102 Num.2.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council, of 30 November 2009 on cosmetic products.
- Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006 Dec 1;80(3):217-27. [CrossRef]
- Halden RU, Lindeman AE, Aiello AE, Andrews D, Arnold WA, Fair P, Fuoco RE, Geer LA, Johnson PI, Lohmann R, McNeill K, Sacks VP, Schettler T, Weber R, Zoeller RT, Blum A. The Florence Statement on Triclosan and Triclocarban. Environ Health Perspect. 2017 Jun 20;125(6):064501. [CrossRef]
- Lee GA, Choi KC, Hwang KA. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ Toxicol Pharmacol. 2017 Jan;49:48-57. [CrossRef]
- Promotion of Thyroid Carcinogenesis by para-aminobenzoic Acid in Rats Initiated with N-bis(2-hydroxypropyl)nitrosamine. Toxicological Sciences, Volume 86, Issue 1, 1 July 2005, Pages 61–67.
- U.S. EPA: Report of the Food Quality Protection Act (FQPA) Tolerance Reassessment Progress and Risk Management Decision (TRED) for Bitertanol. [http://www.epa.gov/oppsrrd1/REDs/bitertanol_tred.pdf].
- Committee for Human Medicinal Products (CHMP). Background review for sodium laurilsulfate used as an excipient EMA/CHMP/351898/2014. European Medicines Agency. 2015. Disponible en: https://www.ema.europa.eu/en/documents/report/background-review-sodium-laurilsulfate-used-excipient-context-revision-guideline-excipients-label_en.pdf.
- Directive 2003/15/ec of the European Parliament and of the Council, Annex III. Official Journal of the European Union. Feb 2003. Disponible en: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF.
- Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009 Nov 15;69(22):8784-9. [CrossRef]
- LEAP-On Study, Funded by Food Allergy Research & Education and the National Institute of Allergy and Infectious Diseases, Continues Earlier Work to Determine Impact of Peanut Avoidance vs. Consumption. New Research Reinforces Early Introduction of Peanuts May Be Key to Preventing Children from Developing Peanut Allergy. PRWeb Disponible en: http://www.prweb.com/releases/2016/03/prweb13248836.htm.
- Jones FW. Multiresidue Analysis of Pesticides in Wool Wax and Lanolin Using Gel Permeation and Gas Chromatography. J. Agric. Food Chem. 1996, 44, 10, 3197–3201.
- Ngan V, Writer S. Allergy to wool alcohols. DermNet NZ. 2002. Available in: https://dermnetnz.org/topics/allergy-to-wool-alcohols.
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017 Jan 5;541(7635):41-45. [CrossRef]
- Ramos JJ, Esteban M, Castaño A. Phthalate exposure in children and adults. Rev. Environmental Health. [Internet]. 21 June 2015 [cited 29 June 2022];15:80-3. Available in: https://ojs.diffundit.com/index.php/rsa/article/view/751.
- 1,4-Dioxane. International Agency for Research on Cancer (IARC) - Summaries & Evaluations. Disponible en: https://inchem.org/documents/iarc/vol71/019-dioxane.html.
- 1,4-Dioxane (1,4-Diethyleneoxide). U.S. Environmental Protection Agency. Available in: https://www.epa.gov/sites/default/files/2016-09/documents/1-4-dioxane.pdf.
- Fifteenth Commission Directive 92/86/EEC of 21 October 1992 adapting to technical progress Annexes II, III, IV, V, VI and VII to Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Available in: https://www.boe.es/buscar/doc.php?id=DOUE-L-1992-81824.
Figure 1.
Evolutionary graph of cutaneous symptoms during and after RT, by treatment groups in terms of creams applied.
Figure 1.
Evolutionary graph of cutaneous symptoms during and after RT, by treatment groups in terms of creams applied.
Figure 2.
Graphical representation of the increase in the score on the Patient-Observer scale between the groups of creams applied between the beginning and end of RT treatment.
Figure 2.
Graphical representation of the increase in the score on the Patient-Observer scale between the groups of creams applied between the beginning and end of RT treatment.
Figure 3.
Graphical representation of the increase in the score on the Patient-Observer scale among the groups of creams applied between the beginning and 14 days after the end of the RT treatment.
Figure 3.
Graphical representation of the increase in the score on the Patient-Observer scale among the groups of creams applied between the beginning and 14 days after the end of the RT treatment.
Figure 4.
Evolution of skin elasticity by treatment groups, without considering the fractionation of the RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 4.
Evolution of skin elasticity by treatment groups, without considering the fractionation of the RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 5.
Evolution of skin elasticity by groups (HUH: HU cream in hypofractionated pattern; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 5.
Evolution of skin elasticity by groups (HUH: HU cream in hypofractionated pattern; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 6.
Evolution of skin elasticity in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 6.
Evolution of skin elasticity in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 7.
Evolution of skin elasticity in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 7.
Evolution of skin elasticity in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 8.
Evolution of skin hydration by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 8.
Evolution of skin hydration by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 9.
Evolution of skin hydration by treatment groups, without considering the fractionation of RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 9.
Evolution of skin hydration by treatment groups, without considering the fractionation of RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 10.
Evolution of cutaneous hydration in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 10.
Evolution of cutaneous hydration in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 11.
Evolution of skin hydration in the group of normofractionated regimens (HUN: cream HU in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 11.
Evolution of skin hydration in the group of normofractionated regimens (HUN: cream HU in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 12.
Evolution of cutaneous melanin by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 12.
Evolution of cutaneous melanin by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 13.
Evolution of skin pigmentation (melanin) by treatment groups, without taking into account the fractionation of RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 13.
Evolution of skin pigmentation (melanin) by treatment groups, without taking into account the fractionation of RT guidelines (HU: HU cream; HR: HR cream; RS: RS cream).
Figure 14.
Evolution of cutaneous melanin in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 14.
Evolution of cutaneous melanin in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 15.
Evolution of cutaneous melanin in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 15.
Evolution of cutaneous melanin in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 16.
Evolution of cutaneous erythema by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 16.
Evolution of cutaneous erythema by groups (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern).
Figure 17.
Evolution of cutaneous erythema by treatment groups, without taking into account the fractionation of the RT guidelines (HU: cream HU; HR: HR cream; RS: RS cream).
Figure 17.
Evolution of cutaneous erythema by treatment groups, without taking into account the fractionation of the RT guidelines (HU: cream HU; HR: HR cream; RS: RS cream).
Figure 18.
Evolution of cutaneous erythema in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 18.
Evolution of cutaneous erythema in the hypofractionated regimen group (HUH: HU cream in hypofractionated regimen; HRH: HR cream in hypofractionated regimen; RSH: RS cream in hypofractionated regimen).
Figure 19.
Evolution of cutaneous erythema in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Figure 19.
Evolution of cutaneous erythema in the normofractionated regimen group (HUN: HU cream in normofractionated regimen; HRN: HR cream in normofractionated pattern; RSN: RS cream in normofractionated pattern).
Table 1.
Results of the Patient-Observer scale during the study (SD: Standard Deviation).
Table 1.
Results of the Patient-Observer scale during the study (SD: Standard Deviation).
| |
Initial |
Session 8 |
Session 14 |
Final |
Post 7 days |
Post 14 days |
| Cream |
Mean |
DS |
Mean |
DS |
Mean |
DS |
Mean |
DS |
Mean |
DS |
Mean |
DS |
| HU |
0,40 |
0,548 |
2,40 |
1,949 |
3,20 |
2,775 |
4,80 |
2,775 |
3,60 |
2,302 |
2,33 |
2,517 |
| HR |
0,20 |
0,447 |
1,20 |
2,168 |
2,00 |
2,345 |
2,40 |
2,408 |
2,60 |
2,074 |
0,67 |
0,577 |
| RS |
0,00 |
0,000 |
0,80 |
0,837 |
1,20 |
1,304 |
2,40 |
1,817 |
3,75 |
2,500 |
5,50 |
2,121 |
Table 2.
Table of the differences in the Patient-Observer scale between the beginning and end of the RT regimen, and between the beginning and 14 days after the end of it.
Table 2.
Table of the differences in the Patient-Observer scale between the beginning and end of the RT regimen, and between the beginning and 14 days after the end of it.
| |
N |
Mean |
Standard deviation |
Confidence interval for the mean to 95% |
Minimal |
Maximum |
| Lower limit |
Upper limit |
| First-End Difference |
HR |
5 |
4,4000 |
2,96648 |
,7166 |
8,0834 |
,00 |
8,00 |
| HU |
5 |
2,2000 |
2,16795 |
-,4919 |
4,8919 |
,00 |
5,00 |
| RS |
5 |
2,4000 |
1,81659 |
,1444 |
4,6556 |
,00 |
5,00 |
| Difference Onset-14 days post-treatment |
HR |
3 |
2,0000 |
2,00000 |
-2,9683 |
6,9683 |
,00 |
4,00 |
| HU |
3 |
,6667 |
,57735 |
-,7676 |
2,1009 |
,00 |
1,00 |
| RS |
2 |
5,5000 |
2,12132 |
-13,5593 |
24,5593 |
4,00 |
7,00 |
Table 3.
Percentages of variation between the three treatment groups in the 4 skin characteristics analyzed without taking into account the type of radiotherapy regimen followed (HU: application of HU cream; HR, application of HR cream; RS: application of RS cream).
Table 3.
Percentages of variation between the three treatment groups in the 4 skin characteristics analyzed without taking into account the type of radiotherapy regimen followed (HU: application of HU cream; HR, application of HR cream; RS: application of RS cream).
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-end |
HU |
-9,8 |
-5,625 |
0,05 |
13,65 |
| |
HR |
-6,65 |
-1,125 |
1,175 |
7,2 |
| |
RS |
-3,45 |
2,375 |
-3,175 |
12,875 |
Table 4.
Percentages of variation between the treatment groups with cream without or without Epidermal Growth Factor (EGF), in the 4 skin characteristics analyzed without taking into account the type of radiotherapy regimen followed (HU+HR: HU and HR cream application groups united; RS: application of RS cream with EGF).
Table 4.
Percentages of variation between the treatment groups with cream without or without Epidermal Growth Factor (EGF), in the 4 skin characteristics analyzed without taking into account the type of radiotherapy regimen followed (HU+HR: HU and HR cream application groups united; RS: application of RS cream with EGF).
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-end |
HU+HR |
-8,225 |
-3,375 |
0,6125 |
10,425 |
| |
RS |
-3,45 |
2,375 |
-3,175 |
12,875 |
Table 5.
HR cream, in the 4 skin characteristics analyzed (without taking into account the type of radiotherapy regimen followed) between the beginning and 14 days three the end of the RT regimen (HU: application of HU cream; HR: application of HR cream).
Table 5.
HR cream, in the 4 skin characteristics analyzed (without taking into account the type of radiotherapy regimen followed) between the beginning and 14 days three the end of the RT regimen (HU: application of HU cream; HR: application of HR cream).
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-post14d |
HU |
-11,65 |
-7,725 |
4,125 |
13,4 |
| |
HR |
-0,275 |
-3,075 |
1,6 |
11,35 |
Table 6.
Elasticity of skin variation between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
Table 6.
Elasticity of skin variation between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
| Cream |
RT regimen |
Group |
beginning |
end |
% Change |
| HU |
Hipofracc |
HUH |
64,80 |
53,70 |
-17,13 |
| |
Normofracc |
THEIR |
80,00 |
71,50 |
-10,63 |
| HR |
Hipofracc |
HRH |
67,50 |
56,50 |
-16,30 |
| |
Normofracc |
HRN |
65,30 |
63,00 |
-3,52 |
| RS |
Hipofracc |
RSH |
70,70 |
64,20 |
-9,19 |
| |
Normofracc |
RSN |
66,60 |
66,20 |
-0,60 |
| Control |
Hipofracc |
CH |
|
|
-12,00 |
| |
Normofracc |
CN |
|
|
Table 7.
Variation of skin hydration between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
Table 7.
Variation of skin hydration between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
| Cream |
RT regimen |
Group |
beginning |
end |
% Change |
| HU |
Hipofracc |
HUH |
39,60 |
34,80 |
-12,12 |
| |
Normofracc |
THEIR |
37,15 |
30,70 |
-17,36 |
| HR |
Hipofracc |
HRH |
29,40 |
32,25 |
9,69 |
| |
Normofracc |
HRN |
37,80 |
32,70 |
-13,49 |
| RS |
Hipofracc |
RSH |
33,40 |
40,50 |
21,26 |
| |
Normofracc |
RSN |
35,60 |
33,25 |
-6,60 |
| Control |
Hipofracc |
CH |
|
|
-9,00 |
| |
Normofracc |
CN |
|
|
Table 8.
Variation of skin pigmentation between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
Table 8.
Variation of skin pigmentation between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
| Cream |
RT regimen |
Group |
beginning |
end |
% Change |
| HU |
Hipofracc |
HUH |
9,15 |
6,90 |
-24,59 |
| |
Normofracc |
THEIR |
7,55 |
9,90 |
31,13 |
| HR |
Hipofracc |
HRH |
0,55 |
1,00 |
81,82 |
| |
Normofracc |
HRN |
4,90 |
6,80 |
38,78 |
| RS |
Hipofracc |
RSH |
15,70 |
14,70 |
-6,37 |
| |
Normofracc |
RSN |
13,50 |
8,15 |
-39,63 |
| Control |
Hipofracc |
CH |
|
|
11,00 |
| |
Normofracc |
CN |
|
|
Table 9.
Variation of erythema between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
Table 9.
Variation of erythema between the beginning and end of treatment, according to the cream applied and the fractionation of the RT regimen including data from the retrospective control group (HUH: HU cream in hypofractionated regimen; HUN: HU cream in normofractionated pattern; HRH: HR cream in hypofractionated regimen; HRN: HR cream in normofractionated pattern; RSH: RS cream in hypofractionated regimen; RSN: RS cream in normofractionated pattern; CH, control in hypofractionated regimen; NC: control in normofractionated pattern).
| Cream |
RT regimen |
Group |
beginning |
end |
% Change |
| HU |
Hipofracc |
HUH |
22,80 |
33,10 |
45,18 |
| |
Normofracc |
THEIR |
16,80 |
33,80 |
101,19 |
| HR |
Hipofracc |
HRH |
15,25 |
20,75 |
36,07 |
| |
Normofracc |
HRN |
19,50 |
28,40 |
45,64 |
| RS |
Hipofracc |
RSH |
22,50 |
35,00 |
55,56 |
| |
Normofracc |
RSN |
17,00 |
30,25 |
77,94 |
| Control |
Hipofracc |
CH |
|
|
16,00 |
| |
Normofracc |
CN |
|
|
Table 10.
Evolution of the skin parameters analyzed, by type of cream applied.
Table 10.
Evolution of the skin parameters analyzed, by type of cream applied.
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-end |
HU |
-9,8 |
-5,625 |
0,05 |
13,65 |
| |
HR |
-6,65 |
-1,125 |
1,175 |
7,2 |
| |
RS |
-3,45 |
2,375 |
-3,175 |
12,875 |
Table 11.
Comparison of skin results for creams with and without EGF (HU and HR, RS respectively) between the beginning and end of the RT program.
Table 11.
Comparison of skin results for creams with and without EGF (HU and HR, RS respectively) between the beginning and end of the RT program.
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-end |
HU+HR |
-8,225 |
-3,375 |
0,6125 |
10,425 |
| |
RS |
-3,45 |
2,375 |
-3,175 |
12,875 |
Table 12.
Comparison of skin results for creams without EGF between baseline and 2 weeks after the end of RT.
Table 12.
Comparison of skin results for creams without EGF between baseline and 2 weeks after the end of RT.
| |
|
Elasticity |
Hydration |
Melanin |
Erythema |
| Difference first-post14d |
HU |
-11,65 |
-7,725 |
4,125 |
13,4 |
| |
HR |
-0,275 |
-3,075 |
1,6 |
11,35 |
Table 13.
Comparison of initial-final RT data for participants with the hypofractionated regimen.
Table 13.
Comparison of initial-final RT data for participants with the hypofractionated regimen.
| % Change |
|
|
|
|
|
| RT regimen |
Cream |
Elasticity |
Hydration |
Melanin |
Erythema |
| Hipofracc |
HU |
-17,13 |
-12,12 |
-24,59 |
45,18 |
| Hipofracc |
HR |
-16,30 |
9,69 |
81,82 |
36,07 |
| Hipofracc |
RS |
-9,19 |
21,26 |
-6,37 |
55,56 |
| Hipofracc |
Control |
-12,00 |
-9,00 |
11,00 |
16,00 |
Table 14.
Comparison of initial-final RT data for participants with normofractionated regimen.
Table 14.
Comparison of initial-final RT data for participants with normofractionated regimen.
| % Change |
|
|
|
|
|
| RT regimen |
Cream |
Elasticity |
Hydration |
Melanin |
Erythema |
| Normofracc |
HU |
-10,63 |
-17,36 |
31,13 |
101,19 |
| Normofracc |
HR |
-3,52 |
-13,49 |
38,78 |
45,64 |
| Normofracc |
RS |
-0,60 |
-6,60 |
-39,63 |
77,94 |
| Normofracc |
Control |
-12,00 |
-9,00 |
11,00 |
16,00 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).