1. Introduction
Neuroendocrine tumors (NETs), are a rare group of epithelial neoplasms with a presence of single or nests of neuroendocrine cells (NECs) (neuroendocrine differentiation) [
1]. According to the American Society of Clinical Oncology (ASCO), NET is found in gastrointestinal tract in (67.5%) and bronchopulmonary tree in (25.3-30%) of cases but in 15% of cases the primary site could not be identified. [
2] The small intestine (19%), large intestine (20%), stomach (8.7%), and pancreas (7%) are common sites in the gastrointestinal tract [
2]. In the United States, the annual incidence of newly diagnosed NET is approximately 2 per 100,000 [
3,
4]. The increased rates of NETs over the last few decades are mostly attributed to advancements in diagnostic abilities.[
3] Dasari et al., a retrospective study using nationally representative data from the Surveillance, Epidemiology, and End Results (SEER) program had identified 6.4-fold increase (1.09 to 6.98 per 100 000) in age-adjusted incidence rates between 1973 and 2012 for early-stage tumors. [
5] Through methods such as endoscopic screening and improvements in pathological techniques, in addition to an expansion of data pertaining to safety and efficacy on treatment modalities, early detection has shown to be clinically beneficial as survival rates of patients with a NET have increased over time [
1,
5,
6].
Although diagnostic approaches and survival rates are improving, NETs still present medical challenges which lead to worse overall outcomes compared to other types of tumors. NETs first exhibit vague, non-specific symptoms that can make an initial diagnosis difficult. The tumors are typically found incidentally during other surgeries, such as those for bowel wall obstruction or pancreatitis. [
4] Because of their nondescript presentation, and the fact that more specific symptoms, including those of carcinoid syndrome (flushing, wheezing, diarrhea, and heart valve issues), appear only at the time of metastasis, the average length of time from tumor onset to diagnosis is nine years. [
7] Because metastasis is generally associated with poorer outcomes, the challenge of diagnosing NETs is hypothesized to be the primary reason for its low survival rates (with a 5-year survival rate of 67 percent). [
4]
There are a number of published studies that have looked at survival outcomes and prognostic factors for patients diagnosed with an NET. For example, multiple studies have found negative correlations between worsened clinical outcomes (increased rate of recurrence and shorter disease-free survival times) and tumor size, staging, and grading. [
8,
9,
10] Other prognostic factors such as age and gender are less well-defined and present conflicting data. For example, a study conducted by Folkstead et al. found age, but not gender, to be associated with diminished outcomes while a study by Rosenblum et al. obtained opposite findings. [
9,
11] Although we restricted our search and analysis to gastric neuroendocrine tumors, we found that there is a high degree of heterogeneity even within this limited group.
Overall, we aimed to evaluate the predictive roles of various prognostic factors from a wide array of studies for GI Neuroendocrine tumors on overall survival. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) is based on the World Health Organization's Ninth Revision, International Classification of Diseases (ICD-9). ICD-9-CM is the official system of assigning codes to diagnoses and procedures associated with hospital utilization in the United States
2. Methods
2.1. Aim and Literature search strategy
The primary aim for the study is to evaluate the association of prognostic factors with overall survival in Gastroenteric pancreatic neuroendocrine tumors (GEP NETs). We followed PRISMA guidelines [
12] and MOOSE checklist [
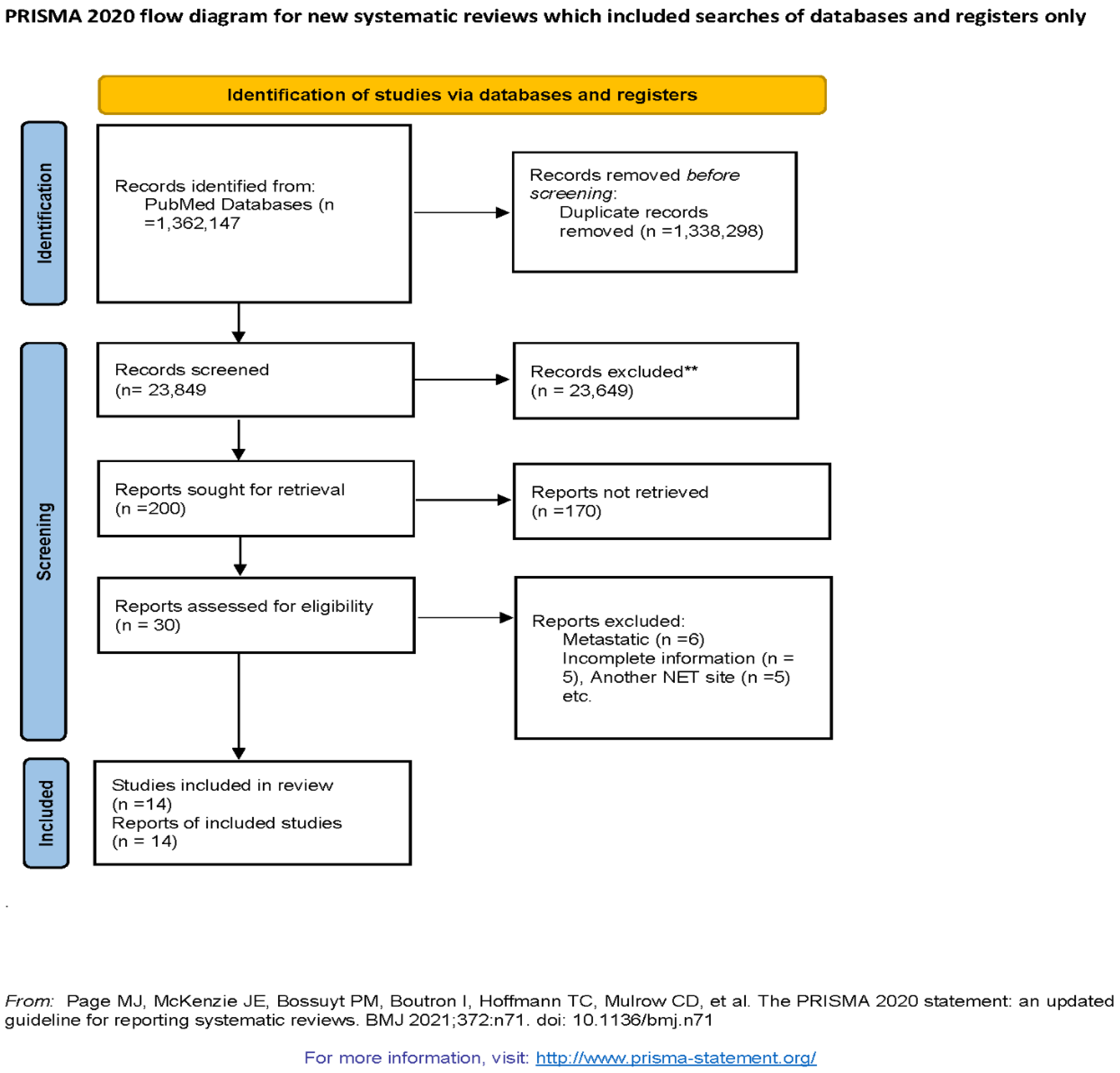
13] in conducting the systematic review and meta-analysis of observational studies evaluating the association of prognostic factors in overall survival of GEP NETs. Observational studies were searched using PubMed with keywords (((((Neuroendocrine Tumors[Title/Abstract]) OR (NET[Title/Abstract])) OR (Gastropancreatic NET[Title/Abstract])) OR (Midgut NET[Title/Abstract])) OR (pNET[Title/Abstract])) OR (pancreatic NET[Title/Abstract]) AND "magnetic"[All Fields] AND (((Survival[Title/Abstract]) OR (Overall Survival[Title/Abstract])) OR (Prognosis[Title/Abstract])) OR (Outcomes[Title/Abstract])) OR (Recurrence[Title/Abstract(((Survival[Title/Abstract]) OR (Overall Survival[Title/Abstract])) OR (Prognosis[Title/Abstract])) OR (Outcomes[Title/Abstract])) OR (Recurrence[Title/Abstract]) from May 2011- May 202. Flow diagram of literature search and study selection process is described in Figure 1.
2.2. Study Selection
We reviewed abstracts and full-length articles for observational studies which have availability of data on overall survival in GEP NETs and collected them for quantitative analysis. AA, MA, AK, and RJ independently screened all of the identified studies and assessed full texts to determine eligibility. Any disagreement was resolved through consensus with the PM and AA.
Observational studies which have described the overall survival or prognostic factors of primary GEP NETs were included. We have excluded the studies which have metastatic NET and tumor location other than gastrointestinal pancreatic and also, studies not in English language. Prognostic factors included in our meta-analysis were age, gender, tumor grading, KI67 index and positive lymph nodes.
2.3. Data extraction
Data was extracted by MA, AK, and RJ. The descriptive variables extracted were the author's name, study year, country, sample size, study period, NET site, Overall Survival and prognostic factors
(Table 1)
| Study name, year |
Country |
Study period |
Sample size |
NET site |
Overall Survival |
Prognostics factors |
| Folkestead et al., 2020 |
Norway |
Jan 1998– May 2018 |
186 |
Intestinal |
5-year survival: 75.8%
9.7 years (95% CI 7.7–11.6) |
Age, gender and Positive Lymph node |
| Tan et al., 2020 |
China |
January 2009 to December 2017 |
88 |
Pancreatic |
NA |
Age, Ki-67 and positive lymph nodes |
| Liu et al. 2020 |
China |
Feb 2003 - Feb 2014 |
155 |
Gastroenteropancreatic
(GEP) NENs |
1-year=82%, 3-year=72%, 5-year=51% |
Grade and positive lymph nodes |
| Kim et al, 2015 |
Korea |
1996-2014 |
175 |
Gastric |
NA |
Age, gender, grade and positive lymph node |
| Ptasnuka et al, 2019 |
Latvia |
2006-2018 |
205 |
Gastropancreatic |
1-year: 88.0% (95% CI 83.3–92.7)
3 year: 77.1% (95% CI 70.4–83.8) |
NA |
| Pellat A et al, 2019 |
France |
2000-2016 |
73 |
GI pancreatic |
5-year OS 50% (25-50%) |
Age, gender, Ki-67 and positive lymph nodes |
| Yang et al., 2018 |
China |
1973- 2014 |
3740 |
Gastric |
NA |
Age, gender and grade |
| Fathi et al., 2020 |
USA |
1988-2012 |
1787 |
Pancreatic |
5-year OS: 24.4%. |
Age, gender, grade and positive lymph nodes |
| Cetinkaya et al., 2014 |
Norway |
1982-2010 |
114 |
Pancreatic |
5-year OS: 53.9% (95% CI: 43.4-63.3) |
NA |
| Zang et al., 2014 |
China |
2003-2012 |
168 |
Gastroenteropancreatic
(GEP- NENs) |
8.94 years (95% confidence interval (CI): 8.40-9.48) |
Age and gender |
| fang et al., 2017 |
South China |
2005-2015 |
1183 |
GEP- NENs |
28 months (range, 4–135 months) |
Age, gender, grade
and positive lymph nodes |
| Foubert, et al., 2018 |
France |
October 1994-October 2013 |
151 |
Intestinal and Pancreatic NET |
NA |
Age, grade and Ki-67 |
| Sakin et al., 2018 |
Turkey |
2000- 2016 |
85 |
GEP NET |
NA |
Age and Ki-67 |
| Rosenblum et al., 2020 |
USA |
1990-2017 |
501 |
Pancreatic |
NA |
Age, gender and grade |
2.4. Statistical Analysis
Meta-analysis was conducted using review manager software (v5.4). We have used random effects models to calculate pooled Hazard ratio and their respective 95% confidence interval (95%CI). I2 of 25%, 50% and 75% was considered as low, medium and high heterogeneity respectively. We have assessed the publication bias using funnel plots. Also, the risk of bias was assessed using the NOS scale. P value <0.05 is considered statistically significant.
3. Results
Out of 200 articles screened, we have 30 articles that were eligible for study after considering our inclusion and exclusion criteria. During the second round of eligibility, 16 more studies were excluded due to incomplete information, metastatic NET, location of NET other than GEP NETs. Hence, final 14 observation studies were selected for systematic review and meta-analysis. (Figure 1)
3.1. Prognostic factors
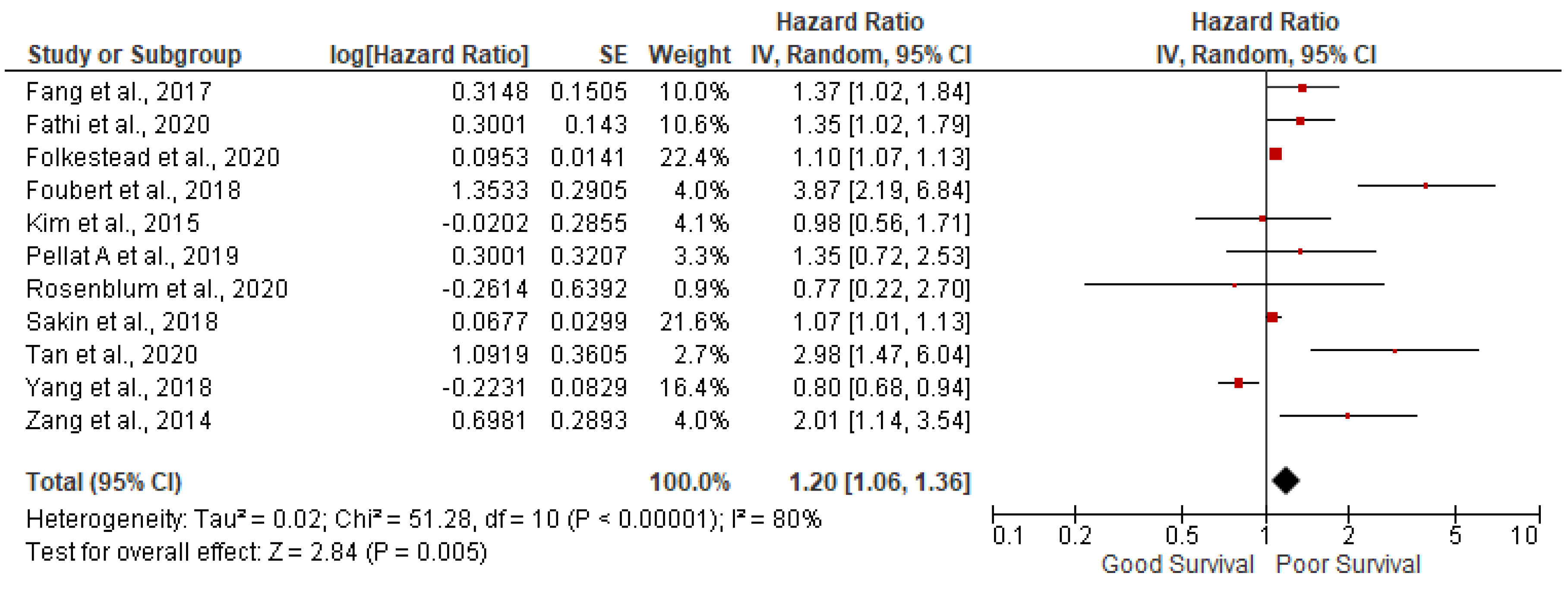
AGE: 11 studies out of 14 have reported the association of age with overall survival in GEP NETs. In our meta-analysis we found that increasing age is associated with poor survival (pooled HR: 1.20; 95%CI:1.06-1.36; p=0.005) with 80% heterogeneity (p<0.00001) (
Figure 2). In order to account for heterogeneity, we have conducted the sensitivity analysis by removing outlying studies of Foubert et al., Tan et al., and Yang et al., Results after sensitivity analysis are still significant (pooled HR: 1.11; 95%CI:1.05-1.18; p=0.0004) with 31% heterogeneity (p=0.17).
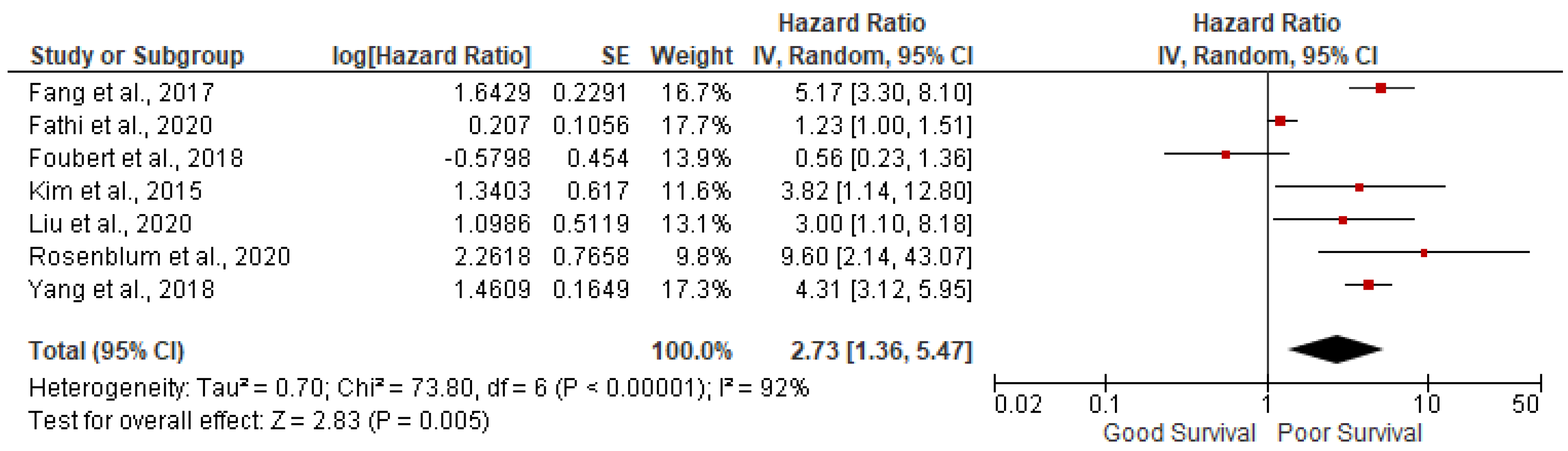
Grade 3: 7 studies out of 14 have reported the association of grade 3 tumor with overall survival in GEP NETs. We found grade 3 tumors were associated with poor survival (pooled HR: 2.73; 95%CI:1.36-5.47; p=0.005) with 92% heterogeneity (p<0.00001) (
Figure 3). In sensitivity analysis, after removing outlying studies of Fathi et al. and Foubert et al., analysis was significant with pooled HR: 4.53; 95%CI: 3.54- 5.58; p<0.00001) with 0% heterogeneity (p=0.72)
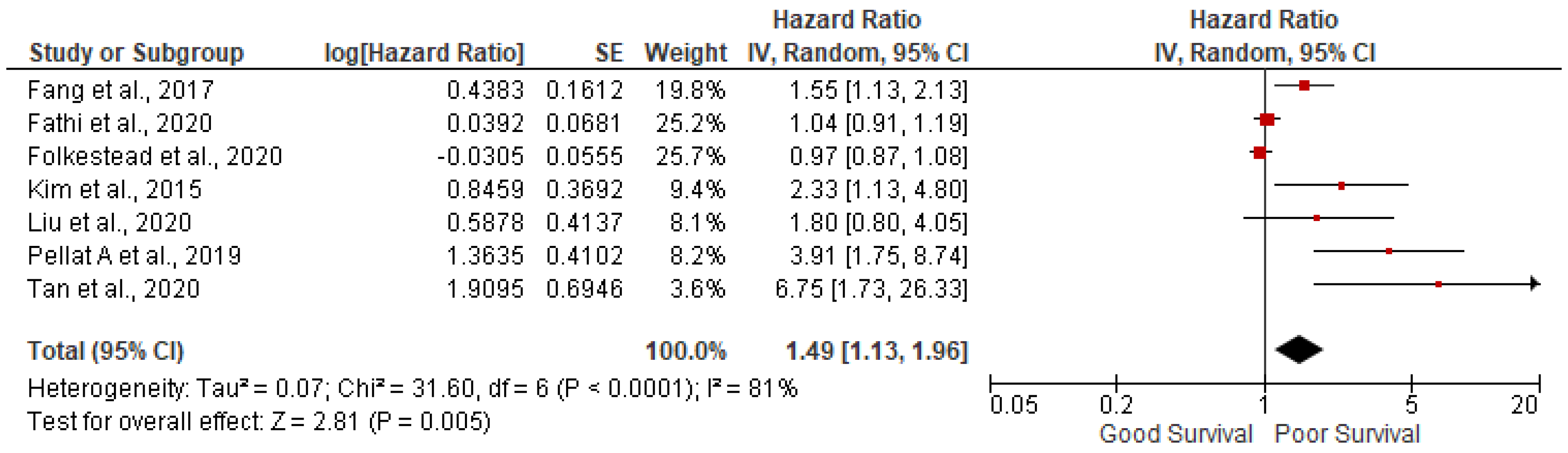
3.2. Lymph node positivity
7 studies out of 14 have reported the association of lymph node positivity and found lymph node positivity were associated with poor survival (pooled HR: 1.49; 95%CI:1.13-1.96; p=0.005) with 81% heterogeneity (p<0.00001) (
Figure 4).
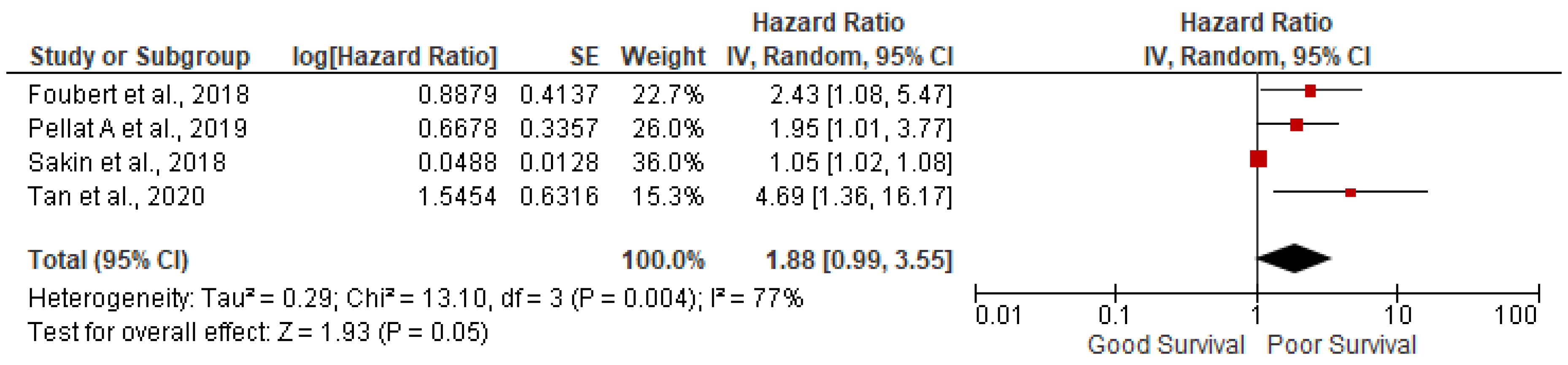
3.3. Ki-67 more than 5%
5 studies out of 14 have found that Ki67 index more than 5% were not significantly associated with poor survival (pooled HR: 1.88; 95%CI:0.99-3.55; p=0.05) with 77% heterogeneity (p=0.004) (
Figure 5).
4. Discussion
As we age the immune system weakens and responds more slowly. This allows for tumors to better escape the host immune defenses with a decreased ability of immune cells to suppress tumor growth. [
14] A study done by Niederele et al. found that benign GI tumors were far more common in younger patients while malignant ones were more prevalent in older populations. [
15] In addition, one study found that those above 80 years of age with a GI tumor (pancreatic) have three times higher mortality rate than those less than 40, and those between the ages of 40-80 years have a two times higher mortality rate than those below 40 years of age. Since decline of the immune system, with age, is a well-established fact it is surprising to see the heterogeneity in the studies included in our analysis. This could partially be accounted for by geographical distribution, in addition to limitations in sample size. For example, a study conducted by Zhu et al. noted that there are significant differences between populations in the United States and China with respect to NETs. Patients in China were found to be older and to have larger tumors. [
16] While a study has not been conducted to observe regional differences of gastric NETs in the United States, it is plausible that there is also significant regional diversity, which may be able to account for some of the discrepancies in outcomes across studies.
The grading system of NETs and neuroendocrine neoplasms (NEN) was updated in 2010 by the World Health Organization (WHO). [
17] Essentially, grade 1 tumors have the lowest mitotic rates with Ki-67 Index (<3%) , grade 3 tumors have the highest mitotic rate with Ki-67 Index (>20%), and grade 2 tumors are found to be intermediate with Ki-67 (3% to 20%). Moreover, there is an extra component to this classification based on the degree of differentiation of the tumor which ranges from well differentiated to poorly differentiated. [
18] The majority of GI NET tumors fall between grade 1 and grade 2 (accounting for 84 percent of all GI NETs), while grade 3 are much rarer (6-8% of GI NETs). [
17] This is perhaps due to advances in medicine allowing for tumors to be diagnosed and treated at an earlier stage. Our results again fall in line with the expectations based on grading of tumors. Tumors with higher proliferative rates, and hence higher grades, tend to be more aggressive and spread faster. This leads to more rapid onset symptoms and a narrower window for curative therapeutic intervention before only palliative care can be given. Furthermore, in most cases grade 1 tumors can be excised while grade 2 and 3 tumors are far more challenging to treat. [
19] It is of interest to note that because grade 2 tumors span a wide range, our results indicate that a threshold of five percent Ki-67 index for a tumor is associated with a poor survival prognosis. Hence, while it is not surprising that our meta-analysis revealed a negative correlation between grading and survival outcomes, the five percent marker may serve a threshold with relevant clinical significance.
Furthermore, we found that metastasis to lymph nodes leads to poor survival outcomes. In most cases, the lymph nodes are among the first places a metastatic cancer travels to reach subsequent tissues. As a result, our data falls in line with the expected outcome since metastasis is associated with later stages of cancer. For example, stage 0 cancer is defined as a cancer that has not spread to any tissues, while stages 1-4 which are subsequently associated with worse clinical outcomes, refer to a cancer that has metastasized. [
20] In addition, it is important to note the relationship between number of lymph nodes affected and survival outcomes. A study conducted by Zaidi et al. found that a metastasis to four or more lymph nodes increases the risk of cancer recurrence post treatment. [
21] The number of lymph nodes affected also affects overall survival rates, with data showing a negative correlation between 10-year survival rates and the number of lymph nodes affected. [
22]
In our study we found that female gender is associated with a worse survival outcome, however the mechanisms behind this remain unclear. It is interesting to note, that NETs are slightly more common in females than males. [
23] It has been hypothesized that differences in hormonal regulation may play a role in this relationship, but more studies are needed before being able to make any definitive conclusions. Moreover, another possibility that may explain our findings is survival rate differences between genders may be attributed to differences in tumor locations. For example, appendiceal tumors are more common in females, while small intestine tumors are more common in males. [
24] Certain GI tumor locations may lead to worse clinical outcomes, however more studies are needed investigating location-survival relationships.
Our findings not only serve to corroborate relationships that already existed, but also have important clinical significance. For instance, our analysis revealed a five percent Ki-67 index threshold for poor clinical outcomes. This benchmark may have an influence on future clinical decision making and treatment options. Because grade 2 tumors are so variable, this percentage marker may be able to draw a distinction between administering more and less aggressive forms of treatment. Moreover, although we established a relationship between female gender and worse survival outcomes, we understand that more research needs to be conducted in this domain to develop an understanding of the underlying mechanisms. While the relationships of age and lymph node positivity were already well established, they serve as important findings that help to corroborate the overall quality of our study.
5. Conclusions
Overall, the results of our study fall in line with the normal expectation of all tumors, not just those limited to the GI system. Essentially, we found that older age, higher grade tumors, lymph node positivity, and female gender are associated with worse clinical outcomes and lower survival rates. Although the basic science mechanism for most types of cancers and associated prognostic factors are well established, our study reveals more nuanced relationships on GI NETs than are present in the current literature. Our subcategory analysis, such as the specific Ki67 index percentage and number of lymph nodes affected for poor survival, are new findings with important clinical relevance. While we acknowledge that there are still needs for further research to evaluate prognostic factors to help corroborate our findings, we hope that our study serves as an initial guide that may help with future clinical decision making.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Aysha Aslam, Preeti Malik and Urvish Patel; Formal analysis, Preeti Malik and Urvish Patel; Investigation, Vikramaditya Venkata; Methodology, Preeti Malik; Project administration, Neel Patel, Vikramaditya Venkata and Urvish Patel; Resources, Vikramaditya Venkata; Software, Preeti Malik and Urvish Patel; Supervision, Vikramaditya Venkata and Urvish Patel; Validation, Neel Patel, Sriram Chowdary and Urvish Patel; Visualization, Neel Patel; Writing – original draft, Aysha Aslam, Azadeh Khayat, Muhammed Asad, Rizwan Rabbani, Sameer Dawodi, Sangeetha Chandramohan, Nkechi Unachukwu, Bibimariyam Nasyrlaeva, Laseena Vaisyambath and Sriram Chowdary; Writing – review & editing, Neel Patel, Sriram Chowdary, Vikramaditya Venkata and Urvish Patel.
Funding
This research received no external funding.
Institutional Review Board Statement
“The study was conducted according to the guidelines of the Declaration of Helsinki, and Ethical review and approval were waived for this study, due to meta-analysis of already published studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data included in this study is available from previously published studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang Z, Wang W, Lu J, et al. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018, 45, 389–396. [CrossRef]
- Cancer.net: Neuroendocrine Tumors/Introduction [Internet]. Available from: https://www.cancer.net/cancer-types/neuroendocrine-tumors/introduction.
- Neuroendocrine Tumors: Statistics [Internet]. Available from: https://www.cancer.net/cancer-types/neuroendocrine-tumors/statistics#:~:text=Overall%2C it is estimated that,has been increasing for years.
- Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician. 2006, 74, 429–434.
- Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [CrossRef] [PubMed]
- Kaderli RM, Spanjol M, Kollár A, et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol 2019, 5, 480–489. [CrossRef] [PubMed]
- Horton KM, Kamel I, Hofmann L, et al. Carcinoid Tumors of the Small Bowel: A Multitechnique Imaging Approach. Am J Roentgenol 2004, 182, 559–567. [CrossRef] [PubMed]
- Chung CS, Tsai CL, Chu YY, et al. Clinical features and outcomes of gastric neuroendocrine tumors after endoscopic diagnosis and treatment a digestive endoscopy society of tawian (dest). Med (United States) 2018, 97. [CrossRef]
- Folkestad O, Wasmuth HH, Mjønes P, et al. Survival and disease recurrence in patients operated for small intestinal neuroendocrine tumors at a referral hospital. Surg Oncol 2020, 35, 336–343. [CrossRef] [PubMed]
- Kim BS, Park YS, Yook JH, et al. Differing Clinical Courses and Prognoses in Patients with Gastric Neuroendocrine Tumors Based on the 2010-WHO Classification Scheme. Med (United States) 2015, 94, e1748. [CrossRef]
- Rosenblum RE, Harris CK, Baeg KJ, et al. Predictors of Recurrence and Survival in Patients with Surgically Resected Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 249–254. [CrossRef] [PubMed]
- Prisma guidelines [Internet]. Available from: http://www.prisma-statement.org/Extensions/NetworkMetaAnalysis.
- IJNS. MOOSE ( Meta-analyses Of Observational Studies in Epidemiology ) Checklist. 2019.Available from: https://www.elsevier.com/__data/promis_misc/ISSM_MOOSE_Checklist.pdf.
- Aging Changes in Immunity [Internet]. Available from: https://medlineplus.gov/ency/article/004008.htm#:~:text=As you grow older%2C your,your risk of getting sick.
- Niederle MB, Hackl M, Kaserer K, et al. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr Relat Cancer 2010, 17, 909–918. [CrossRef]
- Zhu LM, Tang L, Qiao XW, et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in China and the United States: A multicenter study. Med (United States) 2016, 95, e2836. [CrossRef]
- Coriat R, Walter T, Terris B, et al. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist 2016, 21, 1191–1199. [CrossRef]
- Oronsky B, Ma PC, Morgensztern D, et al. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia (United States) 2017, 19, 991–1002. [CrossRef]
- Mcgrath PC, Neifeld JP, Lawrence W, et al. Gastrointestinal sarcomas: Analysis of prognostic factors. Ann Surg 1987, 206, 706–710. [CrossRef]
- NIH: Cancer Staging [Internet]. Available from: https://www.cancer.gov/about-cancer/diagnosis-staging/staging.
- Zaidi MY, Lopez-Aguiar AG, Dillhoff M, et al. Prognostic Role of Lymph Node Positivity and Number of Lymph Nodes Needed for Accurately Staging Small-Bowel Neuroendocrine Tumors. JAMA Surg 2019, 154, 186–187. [CrossRef]
- Blair, R. 乳鼠心肌提取 HHS Public Access. Physiol Behav 2017, 176, 139–148. [Google Scholar]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol 2020, 12, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: Trends in incidence in England since 1971. Am J Gastroenterol 2010, 105, 2563–2569. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).