1. Introduction
The average lifespan of people across all racial backgrounds is increasing at a fast pace[
1]. The average life expectancy in the nineteenth century was much lower than in the twentieth century, and now in the 21st century, there is an incongruity between age expectancies in developed and developing nations [
2,
3,
4]. Multiple factors have contributed to this, but the most crucial ones are technological advancements like industrialization and surplus food production and availability due to mechanization in the agriculture sector, which is an influence of industrialization. Contributing to less menial work, better nutrition, and overall health. But there is another factor called the quality-adjusted life-year (QALY)[
5], which determines the quality of life due to medical interventions in the elderly population because a big part of life that adds to the life expectancy is the life after retirement when the elderly are the most prone to disabling conditions. QALY scores from 1 and 0, where 1 means perfect health and 0 indicates death. The elderly population is expected to rise in the coming future as the projected population of people above age 65 is expected to be more than 1.5 billion in 2050 [
6]. IoT interventions need to be implemented to increase the quality of their lives. With declining birth rates and the average number of children born per couple, the elderly population is and will, unfortunately, be pushed towards living independently; hence, IoT-based assistance will become helpful in accompanying their needs. Compared to other age groups across demographics, elderly people are the most prone to chronic diseases which require continuous monitoring and attention, like diabetes, Parkinson's, asthma, etc. One of the major constraints in availing better healthcare is its cost which also is rising. Also, the inability of the elderly population to generate income unless they are covered or insured by the governments or third-party companies constricts their potential to access good healthcare. Elderlies also have difficulty going long distances and being physically present at healthcare centers. These issues have inspired the scientific community to look for assistive technologies in the healthcare sector, specifically for the geriatric population.
With rising advancements in the IoT domain in the last decade, along with AI, data analytics, automation, and low-powered electronics, the arena is open for research to thrive concerning the aging population. IoT is defined as a network of physical objects with embedded technology that can sense and interact with the surrounding environment and provide autonomous communication. IoT is a platform for flawless integration between the physical world and computer-based systems. The concept of IoT was created in 1999 as a part of the RFID ( Radio-frequency identification) development community; however, the idea of linked devices has been around since the 1970s. The IoT concept expands into different domains based on applications. Such as IoRT, IoMT, a-IoT, and IoT-C. IoRT (Internet of Robotic Things) refers to the integration of IoT technology with robots and automation systems, enabling them to communicate and share data with other devices and systems. This can include industrial robots, drones, and autonomous vehicles. IoMT (Internet of Medical Things) refers to the use of IoT technology in the healthcare industry, such as connected medical devices, remote patient monitoring, and telemedicine. This includes wearable devices, smart pillboxes, and other medical devices that can transmit data to healthcare providers for analysis and treatment. a-IoT (Artificial Intelligence of Things) refers to the integration of IoT technology with artificial intelligence (AI) and machine learning (ML) algorithms, enabling devices to make decisions and take actions based on the data they collect. IoT-C (Internet of Things for Consumers) refers to IoT devices and applications that are used in the consumer space, such as smart home devices, wearables, and connected cars. This is a subtype of the IoT that is focused on the consumer market and is designed to make everyday life more convenient and efficient.
IoMT blends medical devices with associated applications into a medical data processing framework. The application of IoT in healthcare has reduced the repetitive manual examinations of various physiological parameters such as heart rate, BP, blood oxygen level, body temperature, etc. moreover, these devices produce data that get stored in a central server or the cloud and get processed and analyzed for further process of making decisions.
Smart healthcare is one factor driving the population's motivation to integrate healthcare devices and applications into their lives for better well-being. With today's era integrating conventional medicine with biotechnology, smart healthcare has emerged as a tool for preventative healthcare and disease or disorder treatment. The concept of a “smart planet” in 2009 by IBM in Armonk, New York, led to the emergence of smart healthcare. The smart planet, as defined by them, is a system with an intelligent infrastructure using sensors to gather information, transmit it via IoT, and procprocessthrough supercomputers and cloud computing. Smart healthcare is a healthcare service that utilizes the Internet of Medical Things (IoMT), mobile internet, and wearable devices to connect people, materials, and institutions related to healthcare.
IoT devices can potentially improve quality services to the elderly population living with chronic diseases[
7]. IoT refers to a worldwide network infrastructure that connects both physical and virtual objects and is characterized by dynamic interactions [
8]. Several techniques have emerged to help and manage big data, providing personalized medicine and improving business processes in the healthcare domain[
9]. With the costs of low-powered electronics coming down[
10], batteries being safer and more efficient than ever[
11], and the elderly population's healthcare expenses can be reduced through the use of IoT [
12]. The next movement of computing would be outside the realm of traditional desktops, as stated by [
13], and that's evident in today's environment since the sales of mobile phones and tablets have surpassed the sale of desktops[
14] and the boundaries of conventional desktop computers are being erased by portable laptops including even smaller devices. [
15] reported that the IoMT enables devices to collect and share data between themselves. This sharing of data will help healthcare providers and patients as well. In addition to that, wearable devices and mobile applications are increasingly capturing patients' healthcare and well-being data.[
16] states that in the healthcare industry, AI and IoT help improve patients’ care by using sensors from smart devices, fitness trackers, healthcare applications, and digital medical records to produce and store patients' data. This data then further can be used to predict preventive disease maintenance[
17]. Several studies, including clinical trials, have already proven the efficacy of IoT in healthcare concerning elderly patients.
2. Need for health monitoring in geriatric population
Aging is a natural process, and aging-associated diseases are frequently seen with increasing biological aging [
18]. Diseases that occur with age include conditions such as atherosclerosis, glaucoma, macular degeneration, heart disease, cancer, arthritis, cataracts, osteoporosis, type 2 diabetes, high blood pressure, loss of muscle mass (sarcopenia), and Alzheimer's[
19]. The occurrence of these diseases also increases exponentially with age[
20]. The share of deaths related to aging per day across the globe is around 100,000, which is roughly 2/3rd of all deaths. Surprisingly, in industrialized nations, the proportion is even higher at nine age-related deaths out of every ten deaths. In the case of the elderly population, the dis faster when compared to a young adult. This faster progression leads to complications early and requires more prolonged treatment to get them to recover. Since continuous monitoring is needed to keep the disease progression in check, the process of frequent visits to labs and clinics makes elderly people exhausted both physically as well as mentally.
2.1. Auditory and visual impairments
Vision and hearing are considered essential senses in humans, and it is estimated that they account for approximately 80-90% and 10-20%, respectively, of our overall perception of the world. The ability to see allows us to navigate our environment, recognize and identify objects, and perceive visual information such as color, shape, and movement while the ability to hear allows us to communicate with others, detect and locate sounds in our environment, and perceive auditory information such as pitch, volume, and timbre. Visual and auditory impairments limit individuals' ability to sense the world around them. With the decreased ability to listen and see, the ability to correctly comprehend information also gets affected, resulting in irritability and frustration. When left untreated for a long, these issues get in the way of sociability resulting in isolation, depression, loneliness, compromised physical mobility, and cognitive impairment. For example, an older person may appear timid, hesitant, confused, or all of them when placed in an unfamiliar situation[
21]. Similarly, an older individual with hearing issues might miss the critical part of a conversation and may appear confused, making his or her peers impatient[
22]. A solution to this will empower the patient's ability to interact with his environment effectively and carry out daily tasks and the relationship with family and peers. Restored ability to see and hear positively affects the quality of life in elderly people. Visual impairment reports are associated with an increased risk related to social isolation, self-image, depression, and physical disability[
23].
2.2. Falls
Almost one-third of the geriatric population, every year, suffers from falls. Out of these, up to 50% are unable to walk normally again[
24]. Often, when the elderly fall, they remain to lie on the floor for a significant amount of time, often in the cold, before they are discovered by others. Moreover, due to the higher mortality rate in the elderly male population, it's often elderly females who constitute the majority of those who fall. Geriatric patients with movement disorders have the highest rate of falling[
25]. Falling has serious risks associated with concussion, traumatic brain injury, fractures, and joint dislocation; worldwide, it is the second-most cause of mortality[
26]. Home is the most common place for falls, and the bathroom is the most common place[
27]. Falling causes mental stress and damages self-confidence and self-trust[
28]. The self-esteem and independence of geriatric individuals are also affected by injuries associated with falls. Around 40% of the individuals who fall get prescribed bed rest after reporting pain and loss of stamina, which also adds to their mental stress[
29].
2.3. Osteoporosis
Osteoporosis means “porous bone.” It is a condition that weakens bones due to decreased bone mineral density It makes the bones extremely fragile to the point where even normal activities such as coughing can cause a break. Osteoporosis is also an age-related disease, and nearly half of the population above seventy-five and one-third of the women post-menopause get affected by it, which poses a mental as well as a socioeconomic risk[
30]. On a global scale, millions of elderly suffer from vertebral, hip, and wrist fractures[
31]. Most often, the fractures associated with falling have osteoporosis as the major factor in fractured bones. Around one-fourth of the women aged 50 and older having fracture-related incidents have osteoporosis. Osteoporosis-related hip fractures cause patients to undergo long-term hospitalization, resulting in irritability, frustration, and chronic bedridden diseases like pneumonia and thromboembolic disease [
30]. The inability of older people to do outdoor activities and get sufficient sunlight for the production of vitamin D also reduces calcium absorption, further worsening the condition[
32]. Since bone loss occurs insidiously and is asymptomatic, the diagnosis doesn't happen until a clinical fracture occurs[
33].
2.4. Malnutrition
Proper functioning of the human body requires a well-balanced amount of minerals, vitamins, proteins, carbohydrates, and macro-nutrients. Malnutrition happens when there is a deficiency or a severe imbalance in the amounts of essential substances required. In the geriatric population, the inability to think well of themselves and other factors like loneliness, depression, and cognitive decline results in malnutrition. Although only one percent of the older adults who live independently and are healthy are malnourished, the health and nutrition examination survey's data concluded that 16 percent of the Americans living in communities consume less than a thousand calories per day which on the scale reads undernutrition[
34]. The government-backed drive to have smaller families in the 80s and the emergence of white collared jobs in the early 2010s saturating the metropolitans made most of the college-educated single children move away from their families to earn and support. This made the parents back at home lonely, and they lived in solitude [
35]. Old age homes flourished, as a result, bounding elderly people to live in them. Elderlies who are in assisted living rely on others for their meals and medications, and it is found that irresponsibility by caregivers causes malnutrition in the elderly. Poor nutrition negatively affects the immunity of the elderly and causes a delay in their recovery from diseases[
36].
2.5. Depression
Depression, a mental disorder, is one of the prevalent illnesses in the geriatric population[
37]. The symptoms include fatigue, low energy levels, sleep disturbances, weight loss, mood swings, demotivation, and expressive inability[
38]. Depression manifests itself with comorbidities coexisting with other medical conditions like Alzheimer's disease, Parkinson's disease, malnutrition, cancer, stroke, HIV, hepatitis, hypothyroidism, hyperparathyroidism, arthritis, and skin problems that can lead to depression[
39]. Depression in the geriatric population causes problems associated with daily activities, including standing and walking. Moreover, depression makes individuals lose hope in leading a quality life making its treatment prolonged with the inclusion of medications and therapy sessions like CBT(Cognitive behavioral therapy). Depression further influences the health of the gut microbiome, and this bi-directional influence can cause both actress and poor absorption of nutrition[
40]. The rate of mortality due to suicide has increased, as with other diseases in the elderly population[
41].
2.6. Derilium and Dementia
Delirium comes from the Latin root word “dēlīrāre,” which means derangement. Cognitive impairments like delirium and dementia are quite prevalent in the geriatric population[
42]. Delirium is caused by chronic or severe illness, changes in metabolic balance and medication, prior cognitive impairments, and infection and carries a high mortality rate [
43]. Delirium is a neuropsychiatric syndrome that negatively affects emotional, sensory, and behavioral functioning [
44]. Delirium causes hallucinations and most often at night time. As much as half of the elderly patients at the hospital have at least one episode of delirium. Elderly patients with multiple health problems are most susceptible to delirium. Delirium of the hyperactive kind in older patients expresses itself with agitated or aggressive behaviors, incoherent speeches, disorganized thoughts, delusions, hallucinations, and disorientation[
44]. Episodes of delirium are often scary for inexperienced caretakers. The other variant of delirium is hypoactive, with symptoms like sluggishness, drowsiness, sullen behavior, and withdrawal. Delirium is unexpected and causes huge stress in the patient's life, disrupting their sleep cycle, making them sleep during the day and wide awake at night[
45]. Delirium negatively affects the social life of elderly patients, making them even more isolated from their peers. Delirium is often misdiagnosed with dementia due to common symptoms[
46]. Dementia is a disorder with problems in memory, thinking, problem-solving, language, and perception[
47]. Elderly patients with dementia have memory problems making them forget recent events. This causes them to repeatedly ask the same question, negatively affecting their relationships with peers and self-esteem. Dementia and delirium in geriatric patients are intricately linked [
48] and cause their social life to suffer, pushing them into social withdrawal.
3. Influence of IoT on geriatric health monitoring
The aging population can be facilitated to lead a better life with the employment of IoT-based products and services using technologies such as big data analytics, artificial intelligence, cloud computing, mobile computing, and real-time operating systems[
49]. These technologies are used to develop interconnected low-powered electronic devices using sensors interfacing with smartphones and computers. With increased processing power, resolution, and data throughput, wearable sensors can promote public health care services at remote locations or for elderly patients with locomotor disabilities[
50]. Using these devices based on IoT in a network system can ease the stress on overworked medical and health facilities, enhancing the quality and services provided by healthcare providers... An IoT-based healthcare system would help in the real-time monitoring of vitals such as blood pressure, temperature, heart rate, sodium content, and daily activities in geriatric patients[
51]. The healthcare monitoring domains are discussed in the subsections below.
3.1. Wearable devices and sensors
Sensors are the electronic modules that collect and translate analog physiological information into digital data, which gets filtered, processed, and forwarded by a microprocessor to a communicating module. The enclosed module with a power source and a base to interface all the modules for intentional working is called a device. Wearable devices are the ones that are attached to the human body. In the case of geriatric patients, wearable devices find their utility significant as the available sensors can detect falls [
52], monitor sleep patterns[
53], monitor cardiac health[
53], blood oxygen levels[
54], blood pressure[
55], body temperature[
54], and sedentary behaviors[
56]. The connected wearable devices have the ability to alert healthcare providers or caretakers when a situation worth attention arises. Real-time monitoring also can be programmed for continuous 24/7 monitoring using the internal storage or the cloud server. Over the years, with improvements in the fabrication technology and prevalence in the usage of RISC and ARM-based microprocessors, wearables have become both reliable and affordable, and hence in recent years, the market has been flooded with smart bands, fitness trackers, and smartwatches. Researchers have surveyed various available wearable devices in the market from most of the manufacturers, including Apple, Fitbit, Samsung, and Pebble. Regarding challenges, security threats and information confidentiality are serious issues[
57]. Wearables integrated with clothing have been studied, which lets patients monitor their health data[
58]. Studies regarding the interest of the geriatric population regarding wearables positions the factor “ease of use” at the top[
59]. Older people have technology anxiety as they were not growing up around technology as GenZ has; hence, it becomes challenging for them to get used to it [
60]. The devices available today are made keeping the youth and the general population in mind; hence it is a little tricky for the older population to use them. At the world level, the Covid 19 pandemic has worked as a catalyst in realizing the challenges faced by the older population[
61]. It is evident in the disparities regarding how older adults are considered and treated in different parts of the world[
62]. This pandemic has intensified the discrimination and digital divide against older adults worldwide [
63]. On the brighter side, the pandemic has made people talk about technological tools, mobile applications, and telehealth opportunities[
64].
3.2. Ambient Assisted Living (AAL)
Ambient assisted living, or AAL, is a trend where AI enables the use of new technology, products, and services, providing a high-quality, safe and independent living environment for the elderly population for as long as possible[
65,
66]. AAL is a multidisciplinary field that creates an ecosystem of connected sensors, computers, and portable devices with applications to provide healthcare monitoring[
67]. AAL aims to minimize the elderly population's worries about using electronic devices and, at the same time, utilize technology to support assisted living. Examples of AAL technology would be an ultrasonic sensor-based gait speed measurement system[
68], haptic enhanced telerehabilitation system[
69], a smart home simulator using semantic and virtual reality[
70], a wearable device pain management system[
71] and physiological data analysis system[
72] just to name a few. AAL as an info-communication technology(ICT) is proposed as “ElderCare,” which provides an interactive TV-based platform for geriatric care[
73]. AAL systems can also assess the variables of the environment, like air quality, sunlight intensity, and seasonal information, to calculate the probability of various illnesses[
73]. AAL can also include robotic assistance for elderly patients[
74] and can be integrated with the smart home infrastructure[
75], wearables, and mobile technologies[
76,
77] to provide a functional support system, as illustrated in
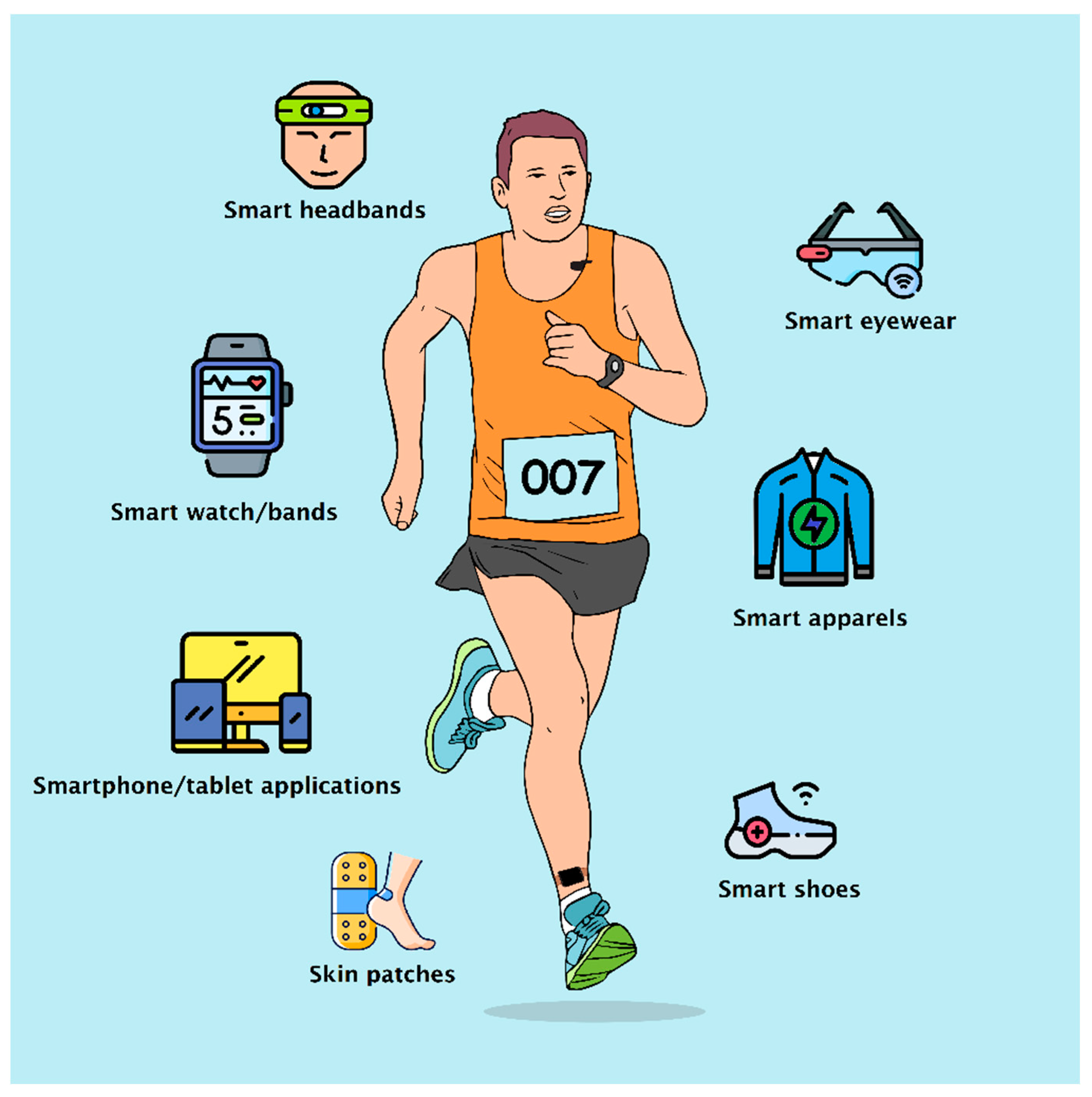
Figure 1.
Figure 1.
Illustration of various IoMT based wearable devices.
Figure 1.
Illustration of various IoMT based wearable devices.
3.3. Telemedicine
Telemedicine is the use of technology to remotely diagnose and treat patients by means of a technology-based virtual platform, and hence it proves to be substantial in providing healthcare in remote and low-income regions. Telemedicine is an umbrella term that encompasses the involvement in any medical activity and an element of distance in between. The term goes back to the time when sea captains received medical advice on the ship over radio communication from the shore[
78]. The world healthcare organization defines telemedicine as “
the delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities”[
79,
80]. According to the American Telemedicine Association (ATA), telemedicine is seen as the progression of healthcare in the digital world. [
81]. The term “telemedicine” literally stands for ‘healing from a distance’ and is not only for healthcare delivery but also for education, research, health surveillance, and public health promotion[
82]. If the term “medical care” involves consultation, diagnosis, treatment, and prescription of medicines, the term “telemedication” stands for providing all the services mentioned above with the involvement of sensors, wearables, and communication technologies[
234]. The first instance of telemedicine dates back to the early 1900s when electrocardiogram (ECG) information was transmitted through telephone lines [
78], and later telephone became the instrument that helped avail the telemedicine services such as availing emergency medical care services by dialing 100, 901, 911 and so forth. Several telemedicine systems have been developed, and one of them is cloud-based, where an anaesthesiologist can monitor sedated patients through a mobile application[
83]. Regarding geriatric patients, an interactive telemedicine system is developed where caregivers can monitor and evaluate their patients[
84].
3.4. Mobile healthcare services
Under the umbrella term of mobile healthcare services, there is a mobile healthcare system and mobile healthcare units. In mobile healthcare systems, the patient’s vital information is securely stored in an electronic record as a medical report and lets healthcare professionals access this information electronically over the air. This is done by employing a body sensor network (BSN) and mobile ad hoc network(MANET)[
85]. Most of these services use the cloud to store data and mobile applications to access the data[
86]. The mobile application also acts as the gateway to the interface connecting patients, doctors, caregivers, and additional service providers. Since smart mobile devices are easier to use and carry than laptop computers, elderly people prefer smartphones; many applications provided by the hospitals have already been in use to allow registration, book an appointment, pay and receive lab reports and notifications[
87]. There have been geriatric care systems proposed to have the capability to monitor falls, heart rate, and video surveillance using IoT and mobile technology[
88]. Other areas where the geriatric mobile health system has proved to be substantial are cardiac diseases[
89,
90], mental health[
91,
92], diabetes[
93], and other chronic disorders[
94,
95].
Mobile health clinics are innovative healthcare delivery models that could alleviate the disparity between vulnerable populations, including populations with chronic diseases like the elderly. MHCs deliver healthcare directly to communities using the existing community assets and offer urgent care, preventative health screenings, and chronic disease management[
96]. This way of providing healthcare helps with real-time modeling parameters and health trends, reducing the burden on health clinics and hospitals.
3.5. Robotic Technology
Robotic technology concerning geriatric care integrates the use of robotic devices made primarily to assist sensorimotor functions[
97]. Robotics are classified into three types; household, companion, and assistive [
98]. The idea of using machines as a human aid dates back to 1910 when Theodor Budingen patented an electric motor-powered apparatus to guide and support stepping movements in patients with heart diseases[
235]. In situations where physical assistance is required, robotic technology can fill the gap between elderly care and human caretaking facilities[
99]. The amalgamation of IoT and robotics help the elderly with their daily physical tasks efficiently, making the chances of physiological issues not worsening further [
100]. Assistive robots can undertake the position of a human presence for the elderly when it is connected with their actual caretakers and doctors virtually[
101,
102,
103]. IoT robot technology and automation integrated with a wheelchair were made for real-time monitoring[
104]; the system captured the environment with an array of cameras to achieve efficient and safe navigational capability[
105]. IoT-based wearables integrated with robotics can take in all the variables and process the data to understand in real-time the patients' needs and cater in a more adaptive manner[
74,
106]. The prosthesis has benefitted control systems and bionic limb patients with paralysis and motor control issues get rehabilitation[
107,
108]. During the covid-19, to mitigate the involvement of humans, robots were used to keep the hospital areas sanitized and disinfected 24/7[
109,
110].
4. IoT applications in geriatric care
The advent of IoT has made a huge and lasting impact on the conventional healthcare industry[
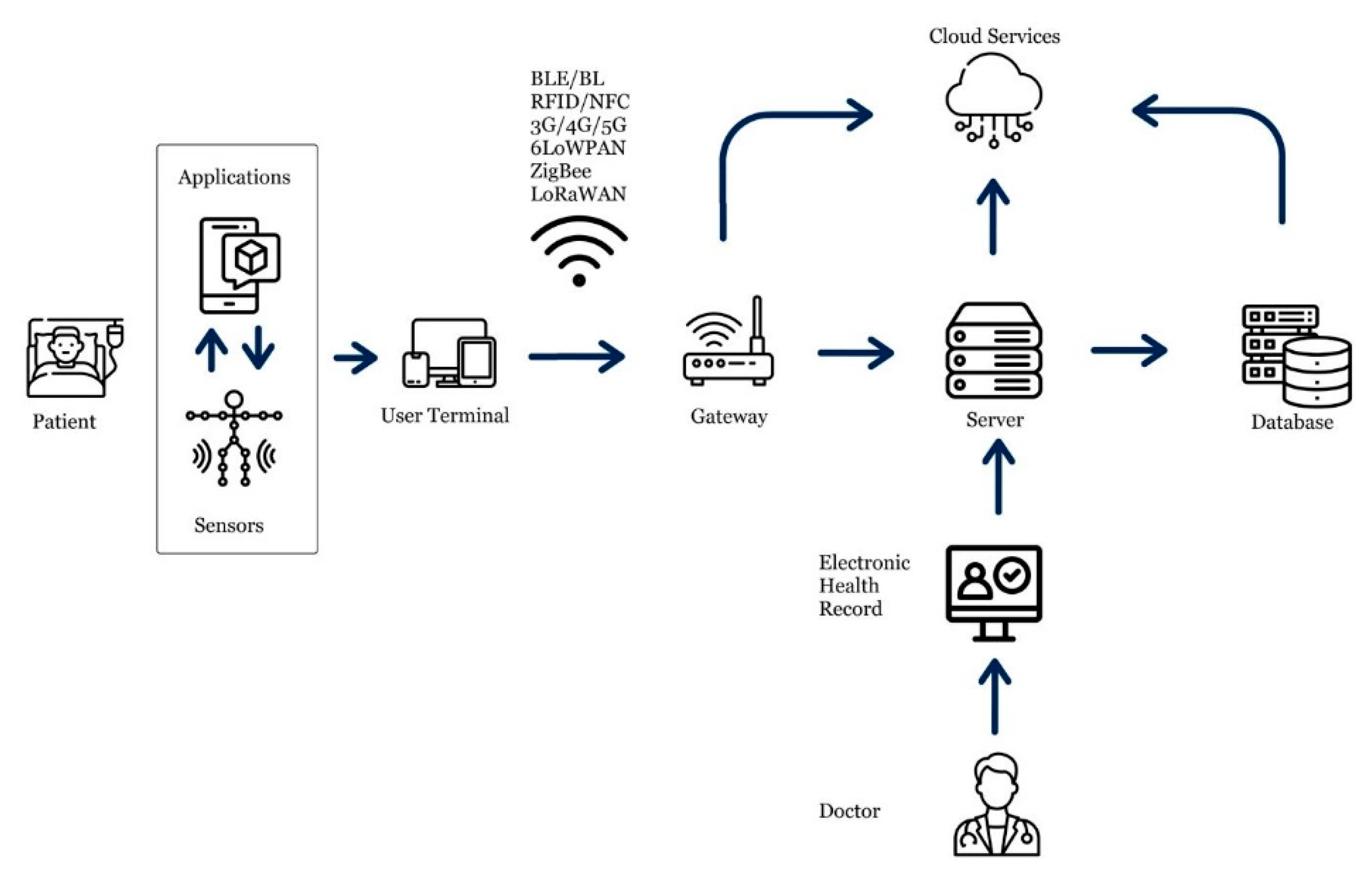
111]. Thanks to cutting-edge sensing technologies, communication protocols, and data analysis techniques, the quality of life for elderly patients and people across the age spectrum has been enhanced. In the following sections, some of the essential applications of IoT in the healthcare industry concerning geriatric care have been discussed. A schematic illsutration of IoHT architecture is shown in
Figure 2.
Figure 2.
IoHT architecture illustration.
Figure 2.
IoHT architecture illustration.
4.1. Monitoring clinical healthcare parameters
Clinical healthcare parameters such as blood oxygen levels, blood pressure, pulse rate, sugar levels, body temperature, gait, balance, and lipid profile act as vital signs for having or predicting to be having in future various health abnormalities[
112,
113]. In the case of elderlies, the situation is even more concerning, and attempts are required to be made early to get information regarding their health status. The timely access to information can be the difference since the information lets the doctors diagnose the disease and prescribe the measures to avoid later complications. Many studies have been done to assess the role of IoT in studying different health conditions[
114,
115,
116,
117].
Blood pressure or hypertension is emerging to be a major health concern in developing countries[
118]. It is infamously called “the silent killer” as it doesn’t have apparent symptoms, even though it affects an estimated 1.28 billion people worldwide and accounts for 7.5 million deaths annually [
119,
120]. One of the factors that participate in hypertension among working-class or middle-class people, which constitutes an estimated 3.8 billion people, roughly half of the world population, is their poor judgment towards health[
121]. This, accompanied by consuming salty foods, lack of regular exercise, and obesity, leads to organ failures[
122,
123]. Prolonged Hypertension negatively affects the health and performance of the brain[
124], liver[
125], lungs[
126], and kidney[
127]. In today's world, where stress is hyped as a sign of performance and a way to climb up the career ladder, people have little concern for the early signs of hypertension[
128]. The influence of pop culture like “work hard and play hard,” “busyness,” “money never sleeps,” and “early retirement” instills the notion of working overtime and competition ultimately dehumanizing the workplace environments[
129]. The first step to creating healthy working environments is to monitor stress levels[
130]. [
131] made a working prototype using PPG (Photoplethysmography) sensors to collect blood pressure data and upload it to the cloud using a proprietary application and MATLAB for real-time monitoring. Another research[
132] uses an implantable lab-on-chip platform for continuous monitoring of blood pressure data. A proposed solution is a low-cost IoT-based remote HRV monitoring system using the MQTT protocol using ZigBee and Arduino over the cloud [
133].
Older people are more prone to diabetes[
134]. According to WHO, the prevalence of diabetes is rising in low and middle-income countries[
135,
136]. Elevated sugar levels in the blood welcome a myriad of complications like blindness, cardiac arrest, stroke, and lower limb amputation[
137,
138,
139]. As we don’t have a cure for diabetes, a reliable blood glucose monitor becomes crucial. In severely diabetic individuals, a system that continuously monitors blood glucose levels could work wonders[
140]. Currently, the most prevalent glucose measurement method is the strips and readers. Although the costs of these devices have come down a lot, they still are not as accessible to low-income individuals. However, there have been other noninvasive methods to sense glucose levels in the blood using sensors[
141]. One such method proposed in [
142] uses a visible laser and the physics of reflection to measure glucose levels. In another, Ali et al. [
143] proposed a technology that uses a Bluetooth low-energy implantable glucose monitoring sensor that communicates with a smartphone. The data received can be studied in text and visualization form. In 2016, Ascencia developed a wearable sensor-based glucose monitoring system that can work for 180 days.]. Menon et al. proposed a noninvasive glucose monitoring system that uses near-infrared light and sensors to sense the glucose levels in the blood[
236].
Prevalently, most of the devices that sense vital human health parameters are situated in the intensive care unit (ICU)s. Due to their application-based product development, they are rather dull-looking, power-hungry, and huge, and they induce anxiety among technology-ignorant geriatric patients[
144]. Due to these factors, they have not been able to garner much acceptability among the older population to be used at home. Also, because they are expensive, they leave a void filled by low-energy and low-cost IoT-based sensing devices in the market.
4.2. Activity Recognition
One of the issues faced by the geriatric population is the decline in their physical activity[
145]. Since we are evolved to be healthy with our bodies moving and doing physical activities[
146], there is a need to make the elderly motivated to at least do some physical activity. “The active grandparents' hypothesis” [
147] suggests the participation of grandparents far past their prime in supporting and succor their grandchildren in the stone age. The bilateral relationship between the young and old ones helped the active hunter-gatherers survive. Contrasting to present times, where food can be delivered to the doorsteps, the motivation of the elderly can be induced by IoT devices [
148]. Hence the role of an IoT-based activity detector device comes into play. The data regarding activity can let doctors assess the health status of patients and can pinpoint the effect of being stationary on other chronic diseases. IoT devices with a sensor like an accelerometer, gyroscope, and GPS let collect a wide range of data to detect falls, movement, and the distance moved. According to the WHO, more than 28% of the aged world population is expected to get affected by falls every year[
149]. Approximately 50% of people above 80 suffer accidental falls yearly [
26]. A severe consequence of falling is when an individual lies dormant on the floor unaccompanied for a longer time[
150]. This brings serious consequences like hypothermia, pneumonia, pressure sores, rhabdomyolysis, and dehydration[
151,
152]. There are various categorized solutions for detecting falls in the elderly, including non-wearable systems (NWS), wearable systems (WS), and fusion or hybrid systems (FS). [
153].
NWS-based systems use either vision-based devices or sensors attached to the environment where the elderly lives and are, at present, the most reliable and resilient at detecting falls[
154,
155,
156]. These include techniques like camera triangulation[
157], infrared thermography[
158], time of flight(TOF)[
159], or floor sensors[
160]. However, they must be strategically placed in the environment, draw more power, and be intrusive on the individual’s privacy. To overcome these limitations, WS [
161] is proposed, which uses inertia sensors like triaxial accelerometers and gyroscopes to detect falls and are placed on body parts like the lower torso and lower limbs. Due to their low power consumption, small size, and ease of attachment and use, they offer more advantages[
162,
163]. A threshold-based algorithm is used to distinguish between a fall and activities of daily life. However, it's a complex task to fine-tune the algorithm to accurately detect a fall.
WS systems have been using machine learning techniques to improve accuracy in these algorithms and thus deal with the shortcomings of WS systems[
164,
237]. Machine learning is a technique where statistical inference from a data-based model is used to make automated predictions[
165]. Models built by ML techniques are used for prediction or to refine prediction models even further. In many such works[
166,
167], researchers have used ML techniques for fall detection. Mezghani et al. [
168] used the data captured by the accelerometer attached to the fabric with a non-linear support vector machine technique to obtain meaningful information. In another study [
169], data collected from the triaxial sensors attached to the human trunk was converted into time series, and the Hidden Markov Model (HMM) detected and predicted falls. The experiment yielded an ideal success rate in falls detection with cent percent sensitivity and cent percent specificity. However, the system does not alert when a fall occurs. Finally, Aguiar et al. [
170] used smartphones' built-in accelerators for continuous movement monitoring. The data captured was then fed to test offline three different learning classifiers. Namely, decision trees, K nearest neighbors, and naive Bayes, out of which the decision tree-based algorithm produced the best results. Nevertheless, smartphones consume relatively high power and need to be recharged every other day, if not daily.
Akash et al. [
171] proposed a low-power six-axis MPU-6050 accelerometer and gyro sensor module with an onboard digital motion processor (DMP) to process 9-axis motion fusion algorithms. This module is connected to a Raspberry Pi single-board computer (SBC), Google Cloud, and Google Sheet API, along with an android app performed as an interconnected system for detecting and informing about a fall.
4.3. Chronic disease monitoring
Worldwide, chronic diseases are one of the major causes of elderly deaths[
172]. As per the medical surveys[
173], around 4/5th of the geriatric population above 65 suffer from at least one chronic disease. The most chronic diseases associated with elderlies are Alzheimer's, osteoporosis, kidney and lung disease, Parkinson's, and dementia.[
174] while suffering from even one of these chronic diseases, it is difficult for elderlies to live without assisted care, resulting in poor quality of life and less satisfaction with present conditions[
175]. Since chronic diseases require continuous monitoring and frequent intervention of medical professionals, IoT-driven devices show a potential to deal positively with these diseases[
176]. Many studies have proposed IoT-based solutions to improve the conditions of geriatric patients regarding the previously mentioned chronic diseases. Diego Gachet Paez et al. discussed the use of big data along with IoT to manage chronic diseases[
177]. Li et al. proposed a pervasive monitoring system that measures the physiological vitals of a person like BP, ECG, HR, and spo2 and uploads the information to the remote server[
178]. The uploaded data over time gets huge, and big data can be employed to process and provide helpful information regarding the disease status. [
179] Sinnapolu and Alawneh developed a wearable-based HR monitoring ios application that, in emergency conditions, could prompt the emergency services to route toward the patient.
Chronic Kidney Disease (CKD) is a condition that compromises the excretory functions of the kidneys. CKD causes an imbalance in urine output and creates an imbalance of the fluids in the affected person's body. Chronic diseases like diabetes, hypertension, and cardiovascular disease, as well as genetics and environment, play a role in kidney disorders' effects [
180]. This makes the diagnosis process of kidney-related issues complex and time-consuming. The application of IoT and other healthcare technologies can help doctors with diagnosing and monitoring[
181]. Hosseinzadeh et al. [
182] developed a predictive model to detect CKS using the IoT multimedia model. Factors like salt intake, water intake, and sleep patterns measured by IoT devices can be used to diagnose and monitor kidney issues. The conventional method of diagnosing CKD involves collecting urine periodically for monitoring and is susceptible to human error. Even with many methods involving IoT for CKD monitoring and diagnosis, the allowing agencies haven't granted approvals. Hence, there is a lack of commercially available IoT-based monitoring - cum diagnosis systems for CKDs.
In situations where real-time data processing is essential, with factors like a huge volume of data and network unreliability at remote locations, latency becomes an issue with cloud computing, fog or edge computing can be taken for help. Developed by the networking giant Cisco, fog or edge computing sits between the device and the cloud. Acting as an intermediator and an extension to cloud computing, fog computing can solve the issues relating to latency by processing large chunks of data rather than sharing and retrieving data from the cloud.[
183]
Blockchain is a decentralized system that creates and keeps records of data by using a digital ledger of interconnected blocks, which manage how data is shared, accessed, or altered on its peer-to-peer networks[
184,
185]. Blockchain was introduced by Bitcoin, and since then, there has been researching on making use of it in applications in addition to finance. Since blockchain is decentralized, it finds its application in electronic medical records (EMR) [
186,
187]. The medical industry has dramatically been concerned with sending and receiving patients' sensitive and confidential data. With blockchain’s robustness and immunity against data breach and theft, it gives confidence to people, including geriatric patients, with their confidential information while facilitating customized openness and interoperability [
188]. Several of such projects have been implemented at a relatively large scale. One such is in Estonia, where a company named guard time used blockchain technology to secure over one million patients' EMRs [
189,
190]. HealthChain is another EMR application using IBM blockchain’s Hyperledger Fabric[
191] deployed on Bluemix. Ancile [
192] is a blockchain-based framework that utilizes smart contracts built on Ethereum's blockchain platform for EMR applications.
4.4. Drug/Pharmaceutical supply chain
With improving manufacturing technologies, counterfeit products are more likely to get into the market. Many drugs for chronic diseases are manufactured by big pharma companies under strict standards and guidelines and based on that, they are prescribed by doctors to patients. Many of these drugs have complicated release mechanisms, making them specifically dispatched by a licensed pharmacist. The supply chain is often not strictly monitored, resulting in the flourishing of low-grade counterfeit drugs. IoT systems can be used to tackle this issue [
193]. Using unique identifiers UIDs, every step of the drug manufacturing process can be monitored along with the delivery of raw materials, warehouse activities, and the supply chain [
194]. Sensors like RFID, GPS biometric authentication, and automated tracking, along with a central data storage database system like a cloud, can be used to ensure that the right drug is delivered to geriatric patients[
195].
4.5. Monitoring mental health and cognitive diseases
Mental illnesses come with many comorbidities and can severely impact the cognitive and social well-being of elderly people. With aging, the process of neurogenesis decreases [
196], making elderly people more prone to mental disorders and neurodegeneration [
197]. The proportion of people worldwide with mental disorders is increasing, with more than 620 million suffering from anxiety, bipolar disorder, depression, and dementia [
198]. The commonly observed psychiatric and neurodegenerative disorders among the older population are Alzheimer's disease, Parkinson's disease, dementia, depression, and schizophrenia. Multiple studies have been done [
105,
199] that have used IoT-based technology concerning these conditions. Tanaka [
206], Faedda [
207], [
208] have used IoT-based wrist-worn accelerometers to measure the physical activities of participants with major depressive disorders, bipolar disorders, borderline personality disorders, attention deficit hyperactivity disorders, and healthy controls to distinguish between the activity patterns. The results helped understand how differences in activity models could be used to make IoT-based wearable devices for diagnosis and motivating individuals with mental disorders to engage more with the world and not let depressive thoughts kick in. Researchers like Valenza[
238], Nardelli [
209], and Gentili [
210] used T-shirt-embedded ECG with electrodes and sensors to study patients with bipolar one and bipolar two disorders. The experiments aimed to study the heartbeat dynamics to predict mood states. Wearables like these with IoT-based e-health systems can help independently living elderly people keep track of their mental states and psychiatrists to be notified if they see an abnormal pattern with change in individuals’ activity or heartbeat. One example of such a system is the "Alzheimer's Assistant" app, developed by researchers at the University of California, San Francisco (UCSF). The app uses a combination of location-based services, sensors, and machine-learning algorithms to provide personalized assistance and reminders for daily tasks such as taking medication, eating, and exercising.
Another example is "Elderly Care," which is a mobile-based IoT system; this system includes a mobile application, a smartwatch, and a smart home sensor. The system helps the patient to remind them of their daily activities and medication schedule, helps track their location, and sends an alert to the caretaker if the patient wanders off.[
239]
Enshaeifar and colleagues [
211] developed a device for elderly people living independently with Alzheimer's. The device utilized a safety assistant called the Path Tracking and Fall Detection System (PTFaD). The system used the smartphone's Camera and GPS modules to gather data regarding the whereabouts of the patients. The data is dumped into the cloud and can then be used to monitor and dispatch emergency services if required. Brodie et al. proposed to analyze information extracted from gait as a biomarker for Parkinson’s disease. Variables like a jerk, harmonic stability, and oscillation range collected from accelerometers can help form a model for diagnosing PD [
212].
The available assessments for motor issues in Parkinson's disease are clinical. Out of various scales, the Unified Parkinson's Disease Rating Scale (UPDRS) is the most widely utilized. Unfortunately, the application of scale is limited to hospitals, hence using different IoT-based techniques. Several research studies have proposed objective methods for sensing and measuring tremors and postural instability. These methods typically involve using sensors, such as accelerometers and gyroscopes, which can be worn on the body or integrated into devices such as smartphones or smartwatches. One example of such a method is the use of wearable sensors to measure tremor amplitude and frequency. This approach involves attaching accelerometers to the skin at the site of the tremor and then analyzing the sensor data to determine the amplitude and frequency of the tremor.
Another technique involves using inertial measurement units (IMUs) to assess postural instability. IMUs consist of a combination of accelerometers, gyroscopes, and magnetometers that can be worn on the body to measure the 3D orientation and movement of the body. These devices can be used to measure the displacement of the body’s center of mass, which can indicate postural instability. Paslousta et al. [
213] proposed a novel technique to measure the postural instability by the pull test using inertial sensors attached to the patient's shoes.
Some researchers also proposed to use of machine learning algorithms to analyze the sensor data; this approach can help to classify the type of tremors, for example, essential tremor or Parkinson's disease tremor, and also to detect the severity of the postural instability. [
240] used accelerator sensor and neural network to diagnose and assess tremors in Parkinson’s patients. Another research [
241] proposed smartphone-based, custom-made glove case systems to record accelerometer data. Braybrook et al. [
242] proposed the Parkinson's kinetigraph system, a tremor-detecting wrist-worn device that collects data continuously. With multiple sensors for improving the resolution for detecting abnormal patterns continuously daily, the issue regarding processing a large amount of data arises. This huge data needs processing to deliver healthcare services[
214].
Biochemically, the markers of PD are proteins and macromolecules, which, theoretically, when detected, can give information regarding the status of mental disorders over time[
215]. Biochemical sensors can be used to detect the biomarkers of PD which have been identified[
216].
Comatose is another area where IoT has been used as a healthcare service. An altered mental state is a universal term for conditions of the spectrum from confusion to deep unresponsiveness. Comatose is at the end of that spectrum. Brain-dead subjects lack total awareness of their surroundings and cannot voluntarily receive or convey information. Coma has multiple causes, including traumatic brain injury, strokes, tumors, diabetes, infection, and seizures, but drug toxicity causes 40 percent of coma cases. Other issues like irregular heartbeat, sweating, blood pressure, lack of oxygen, cardiac arrest, and opioid misuse indirectly cause coma. Since there is a negligible possibility of verbal or nonverbal communication, the importance of vitals to convey information becomes crucial. IoT-based sensors like body temperature, BP, heartbeat, and EEG are used to detect state changes to confirm if the patient needs something [
217]. As comatose patients have no sense of nature's calls, like urination and excretion, ultrasound sensors are used to determine urine levels [
243]. To monitor consciousness, David Burton [
244] presented methods and apparatus. The invention provides the functionality to detect, analyze, monitor, and alert the states with consciousness using surface-attached electrodes. Gang Liu et al. [
245] A proposed method called EEG-R has been introduced for the early diagnosis of comatose patients.
4.6. Telerehabilitation
Rehabilitation is essential to treating the symptoms and helping with recovery in geriatric patients. It plays the role of counteracting the Ill effects caused by a heart attack, fracture, replacement surgery, and muscular dystrophy. Several researchers [
218,
219] have used wearable sensors, virtual reality (VR), and sensor-integrated systems to aid in the rehabilitation process for geriatric patients. [
220] Smartphones have facilitated in-house rehabilitation for geriatric patients, using device-integrated accelerometers to monitor movements like sitting, walking, running, or cycling and motions like twisting arm, shoulder roll, arm press, and so on. Different machine learning algorithms are used to differentiate between different motions and movements. Nave and Postolache [
221] created a smart walker rehabilitation system that is centered on geriatric patients and uses IoT to monitor gait metrics during rehabilitation sessions. The data collected by many sensors, including inertial management, load cell, and ultrasound sensors, was recorded and transmitted through a smartphone application to the cloud. There are other researches [
222,
223] where the data was collected and monitored in post-operative rehabilitative therapy sessions. Studies proposed the idea of developing smart homes as an add-on to IoT-based geriatric healthcare where Integrated sensors like PIR for occupancy and ambient light sensors can be used for in-house monitoring and rehabilitation.
One of the prominent features of IoT-based telerehabilitation is to erase the physical physiotherapists’ visits and associated costs. However, the available assistive rehabilitation devices are costly, and the need for low costs widely accessible rehabilitation assistive devices is high in the future.
Another aspect where telerehabilitation can use the features of high-speed video streaming and 5G networks [
224] is face-to-face video calls to connect therapists to geriatric patients. The therapist can guide the patient to exercise and can use image processing to monitor movements and progress quantifiably. A 5G enabled network can interconnect devices, doctors, and emergency services to participate in telerehabilitation. Although the solution is beneficial but not viable in all cases of telerehabilitation.
4.7. Monitoring nutrition and medication
Nutrition plays a significant role in ensuring the person is healthy. The aspect of nutrition becomes even more critical in geriatric patients whose bodies are less capable of absorbing nutrition than young adults. A deficiency in a healthy diet leads to malnutrition generating serious consequences in geriatric patients. Cancer is associated with body weight and fat loss [
225,
226]. In addition, chronic conditions like depression, Alzheimer's, and dementia can cause a loss of memory and appetite, leading to malnutrition [
227]. Certain drugs may also have side effects causing a decreased appetite. Hence, there is a concern regarding following a time-based schedule to have ideal delays between medications and meals in geriatric patients. An IoT-based solution tracking activities can help overcome or prevent malnutrition. Lin et al. [
228] proposed a system using a single-board computer SBC, LEDs, and RFID cards as a diet control measure for the elderly. The system with preloaded lists of foods that might worsen the symptoms in geriatric patients helps avoid the intake of those specific meals. Several researchers have proposed using smartphone applications as a Medication reminder to geriatric patients. Sundaravadivel et al. (2018) [
229] proposed "Smart-Log'', a deep learning-based nutrition monitor. The system uses an IoT-based weighing scale connected to a smartphone. The food placed on the weighing scale, along with the camera sensor of the mobile phone, is used to predict the nutritional value with an accuracy of 98.6%. Another research [
230] proposed an IoT-based diet monitoring system. The system uses a weighing scale, RFID, and a smart dining table with cameras and microphones connected to Wi-Fi for effective diet monitoring. The data collected in the cloud is used to identify a nutrition-rich food that gets recommended for intake.
4.8. Emergency healthcare services
Emergency healthcare services get called upon in emergency situations where a trained professional's immediate intervention is needed. Sudden and unpredictable health crises like falls, injuries, concussions, heart attacks and strokes require emergency healthcare services. The emergency Medical Technician or EMT's goal is to save the patient as soon as possible. The IoT can help them be better prepared and equipped with detailed information from the database through the cloud regarding the patient's health conditions in transit to the patient's location. Wearables and patients' health-focused smart homes can also relay information about the vitals in real-time to the EMTs while they are on their way. Having advanced knowledge of the patient will allow them to efficiently handle the situation. A real-world implementation of IoT is from New Orleans. Microsoft developed an IoT-based single platform that integrated data from all branches of emergency services like fire, police, and EMT. This resulted in increased safety for both patients and responders. Researchers have pointed out that the implementation of EMR has the potential to improve the delivery of healthcare services [
231,
232,
233].
5. Current issues, challenges, and future scope
There are three crucial aspects upon which the development of an efficient diagnosing and monitoring healthcare system depends. The first is a fast response time during the first aid service, the second is an effective communication system, and the third is a user-friendly interface. The first important aspect depends upon the human limits; since it has been for a long time, the protocols are refined. The second aspect is regarding the processing speed and networking efficiency. Since most of the IoT-based devices run on powerful and efficient single-board computers with multiple processing cores and high-speed modems following the latest standard, the only concerning aspect is the third one. With age sets in neurodegeneration, elderlies who haven't grown up around electronic devices have difficulty getting their head wrapped around the user interface. So the challenge revolves around making the UI as simple as possible with a background functionality to learn the user input dynamics through AI. The collected data should then be made to make the UI intuitive and hassle-free based on the mental dexterity of the geriatric individual. Developing adaptive systems concerning the UI will make IoT-based systems better and more efficient overall.
Most of the vendors of IoT-based healthcare devices have a proprietary UI, and there's no compulsion on these vendors to follow a protocol. This variation in the communication protocols, standards, and design creates issues, and the lack of a healthcare-centric foundation hurdles the development of interoperability among different devices. From a security standpoint, having a standard operating system for recording, storing, transmitting, and receiving data will also help develop advanced data-securing protocols. Establishing a standardization agency would pave the path for a homogeneous foundation for different manufacturers to develop devices. Two examples of such standardization agencies are SNOMED and LOINC. With the ongoing advancements in IoT-based healthcare devices, there will be a situation where proprietary UI-based devices will require a standard platform for doctors to analyze the trends based on history. Not having enough publications talking about the standardization of IoT-based healthcare systems, there is a need.
6. Conclusions
The paper has reviewed the application and potential of IoT-based solutions for the elderly population. It has highlighted how these solutions can be utilized to improve healthcare and quality of life for older adults, from remote monitoring and telemedicine to fall detection and medication management. The study also discussed how IoT impacts other interdisciplinary domains such as robotics, supply chain management, information technology, electronics, biomedical sensors, and Ambient Assisted Living (AAL). Up-to-date information on the current status of IoT-based solutions in healthcare was provided, specifically addressing issues related to mental health, physiological problems, and chronic diseases. The study also examined the outcomes and limitations of various healthcare systems and devices. Overall, the paper has presented a comprehensive overview of the current state of IoT-based solutions in geriatric healthcare and its future prospects.
This paper will help future researchers have a piece of current comprehensive knowledge in the fields mentioned above, which they can use to analyze gaps in the current research and develop better and more advanced healthcare systems and solutions.
References
- United Nations, Department of Economic and Social Affairs, and Population Division, World population ageing, 2019 highlights. 2020.
- S. H. Preston, “The changing relation between mortality and level of economic development. Population Studies, Vol. 29, No. 2, July 1975,” Int. J. Epidemiol., vol. 36, no. 3, pp. 484–490, Jun. 2007. [CrossRef]
- T. P. Schultz, “Health Human Capital and Economic Development,” J. Afr. Econ., vol. 19, no. suppl_3, pp. iii12–iii80, Nov. 2010. [CrossRef]
- M. Jetter, S. Laudage, and D. Stadelmann, “The Intimate Link Between Income Levels and Life Expectancy: Global Evidence from 213 Years*,” Soc. Sci. Q., vol. 100, no. 4, pp. 1387–1403, 2019. [CrossRef]
- S. Schwartz, J. Richardson, and P. P. Glasziou, “Quality-adjusted life years: origins, measurements, applications, objections,” Aust. J. Public Health, vol. 17, no. 3, pp. 272–278, Sep. 1993. [CrossRef]
- “Our world is growing older: UN DESA releases new report on ageing | UN DESA | United Nations Department of Economic and Social Affairs.” https://www.un.org/development/desa/en/news/population/our-world-is-growing-older.html (accessed Jul. 06, 2022).
- B. Farahani, F. Firouzi, V. Chang, M. Badaroglu, N. Constant, and K. Mankodiya, “Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare,” Future Gener. Comput. Syst., vol. 78, pp. 659–676, Jan. 2018. [CrossRef]
- Z. H. Ali, H. A. Ali, and M. M. Badawy, “Internet of Things (IoT): Definitions, Challenges and Recent Research Directions,” Int. J. Comput. Appl., vol. 128, no. 1, pp. 37–47, Oct. 2015.
- S. Peek et al., “Older Adults’ Reasons for Using Technology while Aging in Place,” Gerontology, vol. 62, Jun. 2015. [CrossRef]
- C. A. Mack, “Fifty Years of Moore’s Law,” IEEE Trans. Semicond. Manuf., vol. 24, no. 2, pp. 202–207, May 2011. [CrossRef]
- M. S. Ziegler and J. E. Trancik, “Re-examining rates of lithium-ion battery technology improvement and cost decline,” Energy Environ. Sci., vol. 14, no. 4, pp. 1635–1651, Apr. 2021. [CrossRef]
- R. Kodali, G. Swamy, and B. Lakshmi, “An implementation of IoT for healthcare,” 2015 IEEE Recent Adv. Intell. Comput. Syst. RAICS, 2015. [CrossRef]
- J. Gubbi, R. Buyya, S. Marusic, and M. Palaniswami, “Internet of Things (IoT): A vision, architectural elements, and future directions,” Future Gener. Comput. Syst., vol. 29, no. 7, pp. 1645–1660, Sep. 2013. [CrossRef]
- B. Smith, “ARM and Intel Battle over the Mobile Chip’s Future,” Computer, vol. 41, no. 5, pp. 15–18, May 2008. [CrossRef]
- D. V. Dimitrov, “Medical Internet of Things and Big Data in Healthcare,” Healthc. Inform. Res., vol. 22, no. 3, pp. 156–163, Jul. 2016. [CrossRef]
- S. A. Goswami, B. P. Padhya, and K. D. Patel, “Internet of Things: Applications, Challenges and Research Issues,” in 2019 Third International conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Dec. 2019, pp. 47–50. [CrossRef]
- M. Chen, Y. Hao, K. Hwang, L. Wang, and L. Wang, “Disease Prediction by Machine Learning Over Big Data From Healthcare Communities,” IEEE Access, vol. 5, pp. 8869–8879, 2017. [CrossRef]
- T. B. L. Kirkwood, “Evolution of ageing,” Mech. Ageing Dev., vol. 123, no. 7, pp. 737–745, Apr. 2002. [CrossRef]
- T. Fulop et al., “Aging, frailty and age-related diseases,” Biogerontology, vol. 11, no. 5, pp. 547–563, Oct. 2010. [CrossRef]
- A. V. Belikov, “Age-related diseases as vicious cycles,” Ageing Res. Rev., vol. 49, pp. 11–26, Jan. 2019. [CrossRef]
- J. A. Leja, “Review of Vision and Aging: Crossroads for Service Delivery. Albert L. Orr. Reviewed by James A. Leja, Western Michigan University.,” J. Sociol., p. 5.
- J. E. Crews and V. A. Campbell, “Vision Impairment and Hearing Loss Among Community-Dwelling Older Americans: Implications for Health and Functioning,” Am. J. Public Health, vol. 94, no. 5, pp. 823–829, May 2004. [CrossRef]
- J. Tetteh et al., “Visual impairment and social isolation, depression and life satisfaction among older adults in Ghana: analysis of the WHO’s Study on global AGEing and adult health (SAGE) Wave 2,” BMJ Open Ophthalmol., vol. 5, no. 1, p. e000492, Jun. 2020. [CrossRef]
- “Falls in the Elderly.” https://www.aafp.org/pubs/afp/issues/2000/0401/p2159.html?referer=www.clickfind.com.au (accessed Jul. 07, 2022).
- N. Dahodwala, C. Nwadiogbu, W. Fitts, H. Partridge, and J. Karlawish, “Parkinsonian signs are a risk factor for falls,” Gait Posture, vol. 55, pp. 1–5, Jun. 2017. [CrossRef]
- “Falls.” https://www.who.int/news-room/fact-sheets/detail/falls (accessed Jul. 07, 2022).
- T. Rosen, K. A. Mack, and R. Noonan, “Slipping and tripping: fall injuries in adults associated with rugs and carpets,” J. Inj. Violence Res., vol. 5, no. 1, Art. no. 1, 2013. [CrossRef]
- N. Jayasinghe et al., “Posttraumatic stress symptoms in older adults hospitalized for fall injury,” Gen. Hosp. Psychiatry, vol. 36, no. 6, pp. 669–673, Nov. 2014. [CrossRef]
- I. of M. (US) D. of H. P. and D. Prevention, R. L. Berg, and J. S. Cassells, Falls in Older Persons: Risk Factors and Prevention. National Academies Press (US), 1992. Accessed: Jul. 07, 2022. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK235613/.
- T. D. Rachner, S. Khosla, and L. C. Hofbauer, “Osteoporosis: now and the future,” The Lancet, vol. 377, no. 9773, pp. 1276–1287, Apr. 2011. [CrossRef]
- “An overview and management of osteoporosis.” http://eurjrheumatol.org/en/an-overview-and-management-of-osteoporosis-132921 (accessed Jul. 07, 2022).
- S. Christakos, P. Dhawan, A. Porta, L. J. Mady, and T. Seth, “Vitamin D and Intestinal Calcium Absorption,” Mol. Cell. Endocrinol., vol. 347, no. 1–2, pp. 25–29, Dec. 2011. [CrossRef]
- P. Vestergaard, L. Rejnmark, and L. Mosekilde, “Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark,” Osteoporos. Int., vol. 16, no. 2, pp. 134–141, Feb. 2005. [CrossRef]
- “Malnutrition in the Elderly: A Multifactorial Failure to Thrive | The Permanente Journal.” https://www.thepermanentejournal.org/doi/10.7812/TPP/05-056 (accessed Jul. 07, 2022). [CrossRef]
- “Living Alone, Loneliness, and Psychological Well-Being of Older Persons in Singapore.” https://www.hindawi.com/journals/cggr/2011/673181/ (accessed Jul. 07, 2022).
- B. Lesourd and L. Mazari, “Nutrition and immunity in the elderly,” Proc. Nutr. Soc., vol. 58, no. 3, pp. 685–695, Aug. 1999. [CrossRef]
- J. Angst and K. Merikangas, “The depressive spectrum: diagnostic classification and course,” J. Affect. Disord., vol. 45, no. 1, pp. 31–40, Aug. 1997. [CrossRef]
- K. I. Shulman, “Conceptual problems in the assessment of depression in old age,” Psychiatr. J. Univ. Ott. Rev. Psychiatr. Univ. Ott., vol. 14, no. 2, pp. 364–366; discussion 370-371, Jun. 1989.
- K. R. R. Krishnan et al., “Comorbidity of depression with other medical diseases in the elderly,” Biol. Psychiatry, vol. 52, no. 6, pp. 559–588, Sep. 2002. [CrossRef]
- M. H. Mohajeri, G. La Fata, R. E. Steinert, and P. Weber, “Relationship between the gut microbiome and brain function,” Nutr. Rev., vol. 76, no. 7, pp. 481–496, Jul. 2018. [CrossRef]
- M. Gebretsadik, S. Jayaprabhu, and G. T. Grossberg, “Mood Disorders in the Elderly,” Med. Clin. North Am., vol. 90, no. 5, pp. 789–805, Sep. 2006. [CrossRef]
- Z. J. Lipowski, “Delirium in the Elderly Patient,” N. Engl. J. Med., vol. 320, no. 9, pp. 578–582, Mar. 1989. [CrossRef]
- J. GEORGE, S. BLEASDALE, and S. J. SINGLETON, “Causes and prognosis of delirium in elderly patients admitted to a district general hospital,” Age Ageing, vol. 26, no. 6, pp. 423–427, Nov. 1997. [CrossRef]
- T. G. Fong, S. R. Tulebaev, and S. K. Inouye, “Delirium in elderly adults: diagnosis, prevention and treatment,” Nat. Rev. Neurol., vol. 5, no. 4, Art. no. 4, Apr. 2009. [CrossRef]
- J. E. Wilson et al., “Delirium,” Nat. Rev. Dis. Primer, vol. 6, no. 1, Art. no. 1, Nov. 2020. [CrossRef]
- S. C. Armstrong, K. L. Cozza, and K. S. Watanabe, “The Misdiagnosis of Delirium,” Psychosomatics, vol. 38, no. 5, pp. 433–439, Sep. 1997. [CrossRef]
- N. Gupta, J. de Jonghe, J. Schieveld, M. Leonard, and D. Meagher, “Delirium phenomenology: What can we learn from the symptoms of delirium?,” J. Psychosom. Res., vol. 65, no. 3, pp. 215–222, Sep. 2008. [CrossRef]
- T. G. Fong, D. Davis, M. E. Growdon, A. Albuquerque, and S. K. Inouye, “The interface between delirium and dementia in elderly adults,” Lancet Neurol., vol. 14, no. 8, pp. 823–832, Aug. 2015. [CrossRef]
- M. M. Baig, S. Afifi, H. GholamHosseini, and F. Mirza, “A Systematic Review of Wearable Sensors and IoT-Based Monitoring Applications for Older Adults – a Focus on Ageing Population and Independent Living,” J. Med. Syst., vol. 43, no. 8, p. 233, Jun. 2019. [CrossRef]
- “Wearable devices for remote vital signs monitoring in the outpatient setting: an overview of the field | BMJ Innovations.” https://innovations.bmj.com/content/6/2/55 (accessed Jul. 07, 2022).
- K. Guk et al., “Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare,” Nanomaterials, vol. 9, no. 6, Art. no. 6, Jun. 2019. [CrossRef]
- S. Patel, H. Park, P. Bonato, L. Chan, and M. Rodgers, “A review of wearable sensors and systems with application in rehabilitation,” J. NeuroEngineering Rehabil., vol. 9, no. 1, p. 21, Apr. 2012. [CrossRef]
- J. Heikenfeld et al., “Wearable sensors: modalities, challenges, and prospects,” Lab. Chip, vol. 18, no. 2, pp. 217–248, Jan. 2018. [CrossRef]
- S. Imani et al., “A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring,” Nat. Commun., vol. 7, no. 1, Art. no. 1, May 2016. [CrossRef]
- “Management of Hypertension in the Digital Era | Hypertension.” https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.120.14742 (accessed Jul. 07, 2022). [CrossRef]
- E. Kańtoch, “Recognition of Sedentary Behavior by Machine Learning Analysis of Wearable Sensors during Activities of Daily Living for Telemedical Assessment of Cardiovascular Risk,” Sensors, vol. 18, no. 10, Art. no. 10, Oct. 2018. [CrossRef]
- “JMIR mHealth and uHealth - Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review.” https://mhealth.jmir.org/2020/9/e18694/ (accessed Jul. 07, 2022).
- A. S. Muhammad Sayem, S. Hon Teay, H. Shahariar, P. Luise Fink, and A. Albarbar, “Review on Smart Electro-Clothing Systems (SeCSs),” Sensors, vol. 20, no. 3, Art. no. 3, Jan. 2020. [CrossRef]
- T. G. Stavropoulos, A. Papastergiou, L. Mpaltadoros, S. Nikolopoulos, and I. Kompatsiaris, “IoT Wearable Sensors and Devices in Elderly Care: A Literature Review,” Sensors, vol. 20, no. 10, Art. no. 10, Jan. 2020. [CrossRef]
- R. R. Gudur, A. Blackler, V. Popovic, and D. Mahar, “Ageing, Technology Anxiety and Intuitive Use of Complex Interfaces,” in Human-Computer Interaction – INTERACT 2013, Berlin, Heidelberg, 2013, pp. 564–581. [CrossRef]
- “Challenges Experienced by Older People During the Initial Months of the COVID-19 Pandemic | The Gerontologist | Oxford Academic.” https://academic.oup.com/gerontologist/article/61/1/48/5909436 (accessed Jul. 07, 2022).
- N. D. Yanez, N. S. Weiss, J.-A. Romand, and M. M. Treggiari, “COVID-19 mortality risk for older men and women,” BMC Public Health, vol. 20, no. 1, p. 1742, Nov. 2020. [CrossRef]
- “JMIR Aging - Impact of the COVID-19 Pandemic on Older Adults: Rapid Review.” https://aging.jmir.org/2021/2/e26474/ (accessed Jul. 07, 2022).
- A. Jnr. Bokolo, “Application of telemedicine and eHealth technology for clinical services in response to COVID-19 pandemic,” Health Technol., vol. 11, no. 2, pp. 359–366, Mar. 2021. [CrossRef]
- A. Dohr, R. Modre-Opsrian, M. Drobics, D. Hayn, and G. Schreier, “The Internet of Things for Ambient Assisted Living,” in 2010 Seventh International Conference on Information Technology: New Generations, Apr. 2010, pp. 804–809. [CrossRef]
- P. Rashidi and A. Mihailidis, “A Survey on Ambient-Assisted Living Tools for Older Adults,” IEEE J. Biomed. Health Inform., vol. 17, no. 3, pp. 579–590, May 2013. [CrossRef]
- D. Calvaresi, D. Cesarini, P. Sernani, M. Marinoni, A. F. Dragoni, and A. Sturm, “Exploring the ambient assisted living domain: a systematic review,” J. Ambient Intell. Humaniz. Comput., vol. 8, no. 2, pp. 239–257, Apr. 2017. [CrossRef]
- X. Ferre et al., “Gait Speed Measurement for Elderly Patients with Risk of Frailty,” Mob. Inf. Syst., vol. 2017, p. e1310345, Dec. 2017. [CrossRef]
- E. Ivanova, J. Krüger, R. Steingräber, S. Schmid, H. Schmidt, and S. Hesse, “Design and concept of a haptic robotic telerehabilitation system for upper limb movement training after stroke,” in 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Aug. 2015, pp. 666–671. [CrossRef]
- D. Spoladore, S. Arlati, and M. Sacco, “Semantic and Virtual Reality-Enhanced Configuration of Domestic Environments: The Smart Home Simulator,” Mob. Inf. Syst., vol. 2017, p. e3185481, Dec. 2017. [CrossRef]
- “Wearable Devices: Current Status and Opportunities in Pain Assessment and Management - FullText - Digital Biomarkers 2021, Vol. 5, No. 1 - Karger Publishers.” https://www.karger.com/Article/FullText/515576 (accessed Jul. 07, 2022).
- G. Acerbi et al., “A Wearable System for Stress Detection Through Physiological Data Analysis,” in Ambient Assisted Living, Cham, 2017, pp. 31–50. [CrossRef]
- D. López-de-Ipiña, S. Blanco, X. Laiseca, and I. Díaz-de-Sarralde, “ElderCare: An Interactive TV-based Ambient Assisted Living Platform,” in Activity Recognition in Pervasive Intelligent Environments, L. Chen, C. D. Nugent, J. Biswas, and J. Hoey, Eds. Paris: Atlantis Press, 2011, pp. 111–125. [CrossRef]
- D. Loza-Matovelle, A. Verdugo, E. Zalama, and J. Gómez-García-Bermejo, “An Architecture for the Integration of Robots and Sensors for the Care of the Elderly in an Ambient Assisted Living Environment,” Robotics, vol. 8, no. 3, Art. no. 3, Sep. 2019. [CrossRef]
- H. Ghayvat, S. Mukhopadhyay, B. Shenjie, A. Chouhan, and W. Chen, “Smart home based ambient assisted living: Recognition of anomaly in the activity of daily living for an elderly living alone,” in 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), May 2018, pp. 1–5. [CrossRef]
- K. Mandarić, P. Skočir, M. Vuković, and G. Ježić, “Anomaly Detection Based on Fixed and Wearable Sensors in Assisted Living Environments,” in 2019 International Conference on Software, Telecommunications and Computer Networks (SoftCOM), Sep. 2019, pp. 1–6. [CrossRef]
- G. Marques and R. Pitarma, “Promoting Health and Well-Being Using Wearable and Smartphone Technologies for Ambient Assisted Living Through Internet of Things,” in Big Data and Networks Technologies, Cham, 2020, pp. 12–22. [CrossRef]
- R. Wootton, “Telemedicine,” BMJ, vol. 323, no. 7312, pp. 557–560, Sep. 2001. [CrossRef]
- A. Dasgupta and S. Deb, “Telemedicine: A new horizon in public health in India,” Indian J. Community Med., vol. 33, no. 1, p. 3, Jan. 2008. [CrossRef]
- S. W. Strode, S. Gustke, and A. Allen, “Technical and Clinical Progress in Telemedicine,” JAMA, vol. 281, no. 12, pp. 1066–1068, Mar. 1999. [CrossRef]
- V. G. Chellaiyan, A. Y. Nirupama, and N. Taneja, “Telemedicine in India: Where do we stand?,” J. Fam. Med. Prim. Care, vol. 8, no. 6, pp. 1872–1876, Jun. 2019. [CrossRef]
- L. S. Wilson and A. J. Maeder, “Recent Directions in Telemedicine: Review of Trends in Research and Practice,” Healthc. Inform. Res., vol. 21, no. 4, pp. 213–222, Oct. 2015. [CrossRef]
- N. Kamdar and L. Jalilian, “Telemedicine: A Digital Interface for Perioperative Anesthetic Care,” Anesth. Analg., vol. 130, no. 2, pp. 272–275, Feb. 2020. [CrossRef]
- N. Salles et al., “Global geriatric evaluation is feasible during interactive telemedicine in nursing homes,” Eur. Res. Telemed. Rech. Eur. En Télémédecine, vol. 6, no. 2, pp. 59–65, Jul. 2017. [CrossRef]
- Y. Ren, R. Werner, N. Pazzi, and A. Boukerche, “Monitoring patients via a secure and mobile healthcare system,” IEEE Wirel. Commun., vol. 17, no. 1, pp. 59–65, Feb. 2010. [CrossRef]
- D. G. Korzun, A. V. Borodin, I. V. Paramonov, A. M. Vasilyev, and S. I. Balandin, “Smart Spaces Enabled Mobile Healthcare Services in Internet of Things Environments,” Int. J. Embed. Real-Time Commun. Syst., vol. 6, no. 1, pp. 1–27, Jan. 2015. [CrossRef]
- C. L. Ventola, “Mobile Devices and Apps for Health Care Professionals: Uses and Benefits,” Pharm. Ther., vol. 39, no. 5, pp. 356–364, May 2014.
- K. Saraubon, K. Anurugsa, and A. Kongsakpaibul, “A Smart System for Elderly Care using IoT and Mobile Technologies,” in Proceedings of the 2018 2nd International Conference on Software and e-Business, New York, NY, USA, Dec. 2018, pp. 59–63. [CrossRef]
- D. Kauw et al., “Mobile health in cardiac patients: an overview on experiences and challenges of stakeholders involved in daily use and development,” BMJ Innov., vol. 6, no. 4, Oct. 2020. [CrossRef]
- R. Miramontes et al., “PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables,” Sensors, vol. 17, no. 1, p. 176, Jan. 2017. [CrossRef]
- “Advances in mobile mental health: opportunities and implications for the spectrum of e-mental health services - Hilty - mHealth.” https://mhealth.amegroups.com/article/view/16192/16352 (accessed Jul. 07, 2022).
- M. Bauer et al., “Smartphones in mental health: a critical review of background issues, current status and future concerns,” Int. J. Bipolar Disord., vol. 8, no. 1, p. 2, Jan. 2020. [CrossRef]
- S. Izahar et al., “Content Analysis of Mobile Health Applications on Diabetes Mellitus,” Front. Endocrinol., vol. 8, 2017, Accessed: Jul. 07, 2022. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fendo.2017.00318. [CrossRef]
- M. Jones, J. Morris, and F. Deruyter, “Mobile Healthcare and People with Disabilities: Current State and Future Needs,” Int. J. Environ. Res. Public. Health, vol. 15, no. 3, Art. no. 3, Mar. 2018. [CrossRef]
- Y. Y. Shieh, F. Y. Tsai, A. Anavim, M. D. Wang, and C.-M. C. Lin, “Mobile Healthcare: Opportunities and Challenges,” in International Conference on the Management of Mobile Business (ICMB 2007), Jul. 2007, pp. 50–50. [CrossRef]
- S. W. Y. Yu, C. Hill, M. L. Ricks, J. Bennet, and N. E. Oriol, “The scope and impact of mobile health clinics in the United States: a literature review,” Int. J. Equity Health, vol. 16, no. 1, p. 178, Oct. 2017. [CrossRef]
- R. Gassert and V. Dietz, “Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective,” J. NeuroEngineering Rehabil., vol. 15, no. 1, p. 46, Jun. 2018. [CrossRef]
- R. Bogue, “Robots to aid the disabled and the elderly,” Ind. Robot Int. J., vol. 40, no. 6, pp. 519–524, Jan. 2013. [CrossRef]
- R. Bemelmans, G. J. Gelderblom, P. Jonker, and L. de Witte, “Socially Assistive Robots in Elderly Care: A Systematic Review into Effects and Effectiveness,” J. Am. Med. Dir. Assoc., vol. 13, no. 2, pp. 114-120.e1, Feb. 2012. [CrossRef]
- G. Morone, I. Cocchi, S. Paolucci, and M. Iosa, “Robot-assisted therapy for arm recovery for stroke patients: state of the art and clinical implication,” Expert Rev. Med. Devices, vol. 17, no. 3, pp. 223–233, Mar. 2020. [CrossRef]
- S. Koceski and N. Koceska, “Evaluation of an Assistive Telepresence Robot for Elderly Healthcare,” J. Med. Syst., vol. 40, no. 5, p. 121, Apr. 2016. [CrossRef]
- L. Almeida, P. Menezes, and J. Dias, “Telepresence Social Robotics towards Co-Presence: A Review,” Appl. Sci., vol. 12, no. 11, Art. no. 11, Jan. 2022. [CrossRef]
- “ExCITE Project: A Review of Forty-Two Months of Robotic Telepresence Technology Evolution | PRESENCE: Virtual and Augmented Reality | MIT Press.” https://direct.mit.edu/pvar/article-abstract/25/3/204/58931/ExCITE-Project-A-Review-of-Forty-Two-Months-of?redirectedFrom=fulltext (accessed Jul. 07, 2022).
- S. Chatterjee and S. Roy, “A low-cost assistive wheelchair for handicapped & elderly people,” Ain Shams Eng. J., vol. 12, no. 4, pp. 3835–3841, Dec. 2021. [CrossRef]
- V. K. L. Ha, R. Chai, and H. T. Nguyen, “A Telepresence Wheelchair with 360-Degree Vision Using WebRTC,” Appl. Sci., vol. 10, no. 1, Art. no. 1, Jan. 2020. [CrossRef]
- M. Luperto et al., “Integrating Social Assistive Robots, IoT, Virtual Communities and Smart Objects to Assist at-Home Independently Living Elders: the MoveCare Project,” Int. J. Soc. Robot., Feb. 2022. [CrossRef]
- A. Betlej, “Designing Robots for Elderly from the Perspective of Potential End-Users: A Sociological Approach,” Int. J. Environ. Res. Public. Health, vol. 19, no. 6, Art. no. 6, Jan. 2022. [CrossRef]
- M. KATSUMURA, S. OBAYASHI, K. YANO, A. HAMADA, T. NAKAO, and K. TORII, “Retractor-Type Robotic Knee Prosthesis to Prevent Fall,” in 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Nov. 2020, pp. 94–99. [CrossRef]
- M. Diab-El Schahawi et al., “Ultraviolet disinfection robots to improve hospital cleaning: Real promise or just a gimmick?,” Antimicrob. Resist. Infect. Control, vol. 10, no. 1, p. 33, Feb. 2021. [CrossRef]
- C. McGinn et al., “Exploring the Applicability of Robot-Assisted UV Disinfection in Radiology,” Front. Robot. AI, vol. 7, 2021, Accessed: Jul. 07, 2022. [Online]. Available: https://www.frontiersin.org/articles/10.3389/frobt.2020.590306. [CrossRef]
- R. Shah and A. M. Chircu, “IOT AND AI IN HEALTHCARE: A SYSTEMATIC LITERATURE REVIEW,” 2018. [CrossRef]
- I. N. Soyiri and D. D. Reidpath, “An overview of health forecasting,” Environ. Health Prev. Med., vol. 18, no. 1, pp. 1–9, Jan. 2013. [CrossRef]
- A. Ahmed, M. M. Khan, P. Singh, R. S. Batth, and M. Masud, “IoT-based real-time patients vital physiological parameters monitoring system using smart wearable sensors,” Neural Comput. Appl., Apr. 2022. [CrossRef]
- L. A. Durán-Vega et al., “An IoT System for Remote Health Monitoring in Elderly Adults through a Wearable Device and Mobile Application,” Geriatrics, vol. 4, no. 2, Art. no. 2, Jun. 2019. [CrossRef]
- S. Banuleasa, R. Munteanu, A. Rusu, and G. Tonţ, “IoT system for monitoring vital signs of elderly population,” in 2016 International Conference and Exposition on Electrical and Power Engineering (EPE), Oct. 2016, pp. 059–064. [CrossRef]
- M. Talal et al., “Smart Home-based IoT for Real-time and Secure Remote Health Monitoring of Triage and Priority System using Body Sensors: Multi-driven Systematic Review,” J. Med. Syst., vol. 43, no. 3, p. 42, Jan. 2019. [CrossRef]
- S. J. Park et al., “Development of the Elderly Healthcare Monitoring System with IoT,” in Advances in Human Factors and Ergonomics in Healthcare, Cham, 2017, pp. 309–315. [CrossRef]
- “Hypertension in Developing Countries: A Major Challenge for the Future | SpringerLink.” https://link.springer.com/article/10.1007/s11906-018-0839-1 (accessed Jul. 07, 2022).
- “Indicator Metadata Registry Details.” https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3155 (accessed Jul. 07, 2022).
- “Hypertension.” https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed Jul. 07, 2022).
- K. Wong, A. H. S. Chan, and S. C. Ngan, “The Effect of Long Working Hours and Overtime on Occupational Health: A Meta-Analysis of Evidence from 1998 to 2018,” Int. J. Environ. Res. Public. Health, vol. 16, no. 12, Art. no. 12, Jan. 2019. [CrossRef]
- A. Grillo, L. Salvi, P. Coruzzi, P. Salvi, and G. Parati, “Sodium Intake and Hypertension,” Nutrients, vol. 11, no. 9, Art. no. 9, Sep. 2019. [CrossRef]
- G. A. Mensah, “Hypertension and Target Organ Damage: Don’t Believe Everything You Think!,” Ethn. Dis., vol. 26, no. 3, Art. no. 3, Jul. 2016. [CrossRef]
- “Hypertensive Target Organ Damage and Longitudinal Changes in Brain Structure and Function | Hypertension.” https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.115.06268 (accessed Jul. 07, 2022). [CrossRef]
- J. H. Henriksen and S. Møller, “Hypertension and liver disease,” Curr. Hypertens. Rep., vol. 6, no. 6, pp. 453–461, Nov. 2004. [CrossRef]
- J. Lee et al., “Hypertension Is Associated with Increased Risk of Diabetic Lung,” Int. J. Environ. Res. Public. Health, vol. 17, no. 20, Art. no. 20, Jan. 2020. [CrossRef]
- “Pathophysiology of Hypertensive Renal Damage | Hypertension.” https://www.ahajournals.org/doi/10.1161/01.HYP.0000145180.38707.84 (accessed Jul. 07, 2022). [CrossRef]
- A. Bannai and A. Tamakoshi, “The association between long working hours and health: A systematic review of epidemiological evidence,” Scand. J. Work. Environ. Health, vol. 40, no. 1, pp. 5–18, 2014.
- Y. Chen, P. Li, and C. Yang, “Examining the Effects of Overtime Work on Subjective Social Status and Social Inclusion in the Chinese Context,” Int. J. Environ. Res. Public. Health, vol. 17, no. 9, Art. no. 9, Jan. 2020. [CrossRef]
- S. Uday, C. Jyotsna, and J. Amudha, “Detection of Stress using Wearable Sensors in IoT Platform,” in 2018 Second International Conference on Inventive Communication and Computational Technologies (ICICCT), Apr. 2018, pp. 492–498. [CrossRef]
- N. I. Zainal, M. Z. Mohd Rodzi, S. Khan, M. H. Habaebi, and T. S. Gunawan, “Design and development of wireless PPG data acquisition for health monitoring application using Bluetooth module,” in 2016 IEEE Student Conference on Research and Development (SCOReD), Dec. 2016, pp. 1–6. [CrossRef]
- S. Gupta et al., “Modeling of On-Chip Biosensor for the in Vivo Diagnosis of Hypertension in Wireless Body Area Networks,” IEEE Access, vol. 9, pp. 95072–95082, 2021. [CrossRef]
- R. N. Kirtana and Y. V. Lokeswari, “An IoT based remote HRV monitoring system for hypertensive patients,” in 2017 International Conference on Computer, Communication and Signal Processing (ICCCSP), Jan. 2017, pp. 1–6. [CrossRef]
- K. Mordarska and M. Godziejewska-Zawada, “Diabetes in the elderly,” Menopause Rev. Menopauzalny, vol. 16, no. 2, pp. 38–43, 2017. [CrossRef]
- D. Flood et al., “The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults,” Lancet Healthy Longev., vol. 2, no. 6, pp. e340–e351, Jun. 2021. [CrossRef]
- “double burden of diabetes and global infection in low and middle-income countries | Transactions of The Royal Society of Tropical Medicine and Hygiene | Oxford Academic.” https://academic.oup.com/trstmh/article/113/2/56/5229286 (accessed Jul. 07, 2022).
- Mi. Mouri and M. Badireddy, “Hyperglycemia,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2022. Accessed: Jul. 07, 2022. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK430900/.
- “Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress.” https://www.wjgnet.com/1007-9327/full/v15/i33/4137.htm (accessed Jul. 07, 2022).
- G. Singh and S. Chawla, “Amputation in Diabetic Patients,” Med. J. Armed Forces India, vol. 62, no. 1, pp. 36–39, Jan. 2006. [CrossRef]
- J. Lawton et al., “Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study,” BMC Endocr. Disord., vol. 18, no. 1, p. 12, Feb. 2018. [CrossRef]
- T. Chang et al., “Highly integrated watch for noninvasive continual glucose monitoring,” Microsyst. Nanoeng., vol. 8, no. 1, Art. no. 1, Feb. 2022. [CrossRef]
- H. Ali, F. Bensaali, and F. Jaber, “Novel Approach to Non-Invasive Blood Glucose Monitoring Based on Transmittance and Refraction of Visible Laser Light,” IEEE Access, vol. 5, pp. 9163–9174, 2017. [CrossRef]
- M. Ali, L. Albasha, and H. Al-Nashash, “A Bluetooth low energy implantable glucose monitoring system,” in 2011 8th European Radar Conference, Oct. 2011, pp. 377–380.
- J. A. Tate, A. Devito Dabbs, L. A. Hoffman, E. Milbrandt, and M. B. Happ, “Anxiety and Agitation in Mechanically Ventilated Patients,” Qual. Health Res., vol. 22, no. 2, pp. 157–173, Feb. 2012. [CrossRef]
- Z. Milanović, S. Pantelić, N. Trajković, G. Sporiš, R. Kostić, and N. James, “Age-related decrease in physical activity and functional fitness among elderly men and women,” Clin. Interv. Aging, vol. 8, pp. 549–556, May 2013. [CrossRef]
- S. B. Eaton and S. B. Eaton, “An evolutionary perspective on human physical activity: implications for health,” Comp. Biochem. Physiol. A. Mol. Integr. Physiol., vol. 136, no. 1, pp. 153–159, Sep. 2003. [CrossRef]
- “The active grandparent hypothesis: Physical activity and the evolution of extended human healthspans and lifespans.” https://www.pnas.org/doi/10.1073/pnas.2107621118 (accessed Jul. 07, 2022). [CrossRef]
- E. Knippenberg, A. Timmermans, S. Palmaers, and A. Spooren, “Use of a technology-based system to motivate older adults in performing physical activity: a feasibility study,” BMC Geriatr., vol. 21, no. 1, p. 81, Jan. 2021. [CrossRef]
- “Examining sensor-based physical activity recognition and monitoring for healthcare using Internet of Things: A systematic review - ScienceDirect.” https://www.sciencedirect.com/science/article/pii/S153204641830176X?via%3Dihub (accessed Jul. 07, 2022).
- “Inability to get up after falling, subsequent time on floor, and summoning help: prospective cohort study in people over 90 | The BMJ.” https://www.bmj.com/content/337/bmj.a2227 (accessed Jul. 07, 2022).
- D. Wild, U. S. Nayak, and B. Isaacs, “How dangerous are falls in old people at home?,” Br Med J Clin Res Ed, vol. 282, no. 6260, pp. 266–268, Jan. 1981. [CrossRef]
- “Occult Elevation of CK as a Manifestation of Rhabdomyolysis in the Elderly - Marcus - 1992 - Journal of the American Geriatrics Society - Wiley Online Library.” https://agsjournals.onlinelibrary.wiley.com/doi/10.1111/j.1532-5415.1992.tb02010.x (accessed Jul. 07, 2022).
- K. Chaccour, R. Darazi, A. H. El Hassani, and E. Andrès, “From Fall Detection to Fall Prevention: A Generic Classification of Fall-Related Systems,” IEEE Sens. J., vol. 17, no. 3, pp. 812–822, Feb. 2017. [CrossRef]
- X. Ma, H. Wang, B. Xue, M. Zhou, B. Ji, and Y. Li, “Depth-Based Human Fall Detection via Shape Features and Improved Extreme Learning Machine,” IEEE J. Biomed. Health Inform., vol. 18, no. 6, pp. 1915–1922, Nov. 2014. [CrossRef]
- L. Yang, Y. Ren, and W. Zhang, “3D depth image analysis for indoor fall detection of elderly people,” Digit. Commun. Netw., vol. 2, no. 1, pp. 24–34, Feb. 2016. [CrossRef]
- G. Mastorakis and D. Makris, “Fall detection system using Kinect’s infrared sensor,” J. Real-Time Image Process., vol. 9, no. 4, pp. 635–646, Dec. 2014. [CrossRef]
- R. Phan Ba, S. Pierard, G. Moonen, M. Van Droogenbroeck, and S. Belachew, “Detection and Quantification of Efficiency and Quality of Gait Impairment in Multiple Sclerosis through Foot Path Analysis,” in Multiple Sclerosis Journal, Oct. 2012, vol. 18, no. S4. Accessed: Jul. 07, 2022. [Online]. Available: https://orbi.uliege.be/handle/2268/132779.
- Z. Xue, D. Ming, W. Song, B. Wan, and S. Jin, “Infrared gait recognition based on wavelet transform and support vector machine,” Pattern Recognit., vol. 43, no. 8, pp. 2904–2910, Aug. 2010. [CrossRef]
- “Time-of-Flight Sensors in Computer Graphics (State-of-the-art report).” https://www2.imm.dtu.dk/pubdb/pubs/5801-full.html (accessed Jul. 07, 2022).
- L. Middleton, A. A. Buss, A. Bazin, and M. S. Nixon, “A floor sensor system for gait recognition,” in Fourth IEEE Workshop on Automatic Identification Advanced Technologies (AutoID’05), Oct. 2005, pp. 171–176. [CrossRef]
- A. Ometov et al., “A Survey on Wearable Technology: History, State-of-the-Art and Current Challenges,” Comput. Netw., vol. 193, p. 108074, Jul. 2021. [CrossRef]
- B. Najafi, T. Khan, and J. Wrobel, “Laboratory in a box: Wearable sensors and its advantages for gait analysis,” in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Aug. 2011, pp. 6507–6510. [CrossRef]
- B. Mariani, M. C. Jiménez, F. J. G. Vingerhoets, and K. Aminian, “On-Shoe Wearable Sensors for Gait and Turning Assessment of Patients With Parkinson’s Disease,” IEEE Trans. Biomed. Eng., vol. 60, no. 1, pp. 155–158, Jan. 2013. [CrossRef]
- “Machine Learning for Healthcare Wearable Devices: The Big Picture.” https://www.hindawi.com/journals/jhe/2022/4653923/ (accessed Jul. 07, 2022).
- C. Janiesch, P. Zschech, and K. Heinrich, “Machine learning and deep learning,” Electron. Mark., vol. 31, no. 3, pp. 685–695, Sep. 2021. [CrossRef]
- P. Vallabh, R. Malekian, N. Ye, and D. C. Bogatinoska, “Fall detection using machine learning algorithms,” in 2016 24th International Conference on Software, Telecommunications and Computer Networks (SoftCOM), Sep. 2016, pp. 1–9. [CrossRef]
- A. Chelli and M. Pätzold, “A Machine Learning Approach for Fall Detection and Daily Living Activity Recognition,” IEEE Access, vol. 7, pp. 38670–38687, 2019. [CrossRef]
- N. Mezghani, Y. Ouakrim, Md. R. Islam, R. Yared, and B. Abdulrazak, “Context aware adaptable approach for fall detection bases on smart textile,” in 2017 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Feb. 2017, pp. 473–476. [CrossRef]
- L. Tong, Q. Song, Y. Ge, and M. Liu, “HMM-Based Human Fall Detection and Prediction Method Using Tri-Axial Accelerometer,” IEEE Sens. J., vol. 13, no. 5, pp. 1849–1856, May 2013. [CrossRef]
- B. Aguiar, T. Rocha, J. Silva, and I. Sousa, “Accelerometer-based fall detection for smartphones,” in 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jun. 2014, pp. 1–6. [CrossRef]
- A. Gupta, R. Srivastava, H. Gupta, and B. Kumar, “IoT Based Fall Detection Monitoring and Alarm System For Elderly,” in 2020 IEEE 7th Uttar Pradesh Section International Conference on Electrical, Electronics and Computer Engineering (UPCON), Nov. 2020, pp. 1–5. [CrossRef]
- R. Wong, C. González-González, and A. Palloni, “Mortality and its association with chronic and infectious diseases in Mexico: A panel data analysis of the elderly,” Salud Pública México, vol. 57, pp. S39–S45, Jan. 2015. [CrossRef]
- J. H. Fong, “Disability incidence and functional decline among older adults with major chronic diseases,” BMC Geriatr., vol. 19, no. 1, p. 323, Nov. 2019. [CrossRef]
- E. Jaul and J. Barron, “Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population,” Front. Public Health, vol. 5, 2017, Accessed: Jul. 08, 2022. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fpubh.2017.00335. [CrossRef]
- M. Smith, K. C. Buckwalter, H. Kang, V. Ellingrod, and S. K. Schultz, “Dementia Care in Assisted Living: Needs and Challenges,” Issues Ment. Health Nurs., vol. 29, no. 8, pp. 817–838, Jan. 2008. [CrossRef]
- S. Krishnamoorthy, A. Dua, and S. Gupta, “Role of emerging technologies in future IoT-driven Healthcare 4.0 technologies: a survey, current challenges and future directions,” J. Ambient Intell. Humaniz. Comput., May 2021. [CrossRef]
- “Big Data and IoT for Chronic Patients Monitoring | SpringerLink.” https://link.springer.com/chapter/10.1007/978-3-319-13102-3_68 (accessed Jul. 08, 2022).
- C. Li, X. Hu, and L. Zhang, “The IoT-based heart disease monitoring system for pervasive healthcare service,” Procedia Comput. Sci., vol. 112, pp. 2328–2334, Jan. 2017. [CrossRef]
- G. Sinnapolu and S. Alawneh, “Integrating wearables with cloud-based communication for health monitoring and emergency assistance,” Internet Things, vol. 1–2, pp. 40–54, Sep. 2018. [CrossRef]
- R. Thomas, A. Kanso, and J. R. Sedor, “Chronic Kidney Disease and Its Complications,” Prim. Care Clin. Off. Pract., vol. 35, no. 2, pp. 329–344, Jun. 2008. [CrossRef]
- A. Abdelaziz, A. S. Salama, A. M. Riad, and A. N. Mahmoud, “A Machine Learning Model for Predicting of Chronic Kidney Disease Based Internet of Things and Cloud Computing in Smart Cities,” in Security in Smart Cities: Models, Applications, and Challenges, A. E. Hassanien, M. Elhoseny, S. H. Ahmed, and A. K. Singh, Eds. Cham: Springer International Publishing, 2019, pp. 93–114. [CrossRef]
- A. M. Hosseinzadeh et al., “A diagnostic prediction model for chronic kidney disease in internet of things platform,” Multimed. Tools Appl., vol. 80, no. 11, pp. 16933–16950, May 2021. [CrossRef]
- M. Laroui, B. Nour, H. Moungla, M. A. Cherif, H. Afifi, and M. Guizani, “Edge and fog computing for IoT: A survey on current research activities & future directions,” Comput. Commun., vol. 180, pp. 210–231, Dec. 2021. [CrossRef]
- “Blockchain Technology in Business and Information Systems Research | SpringerLink.” https://link.springer.com/article/10.1007/s12599-017-0505-1 (accessed Jul. 08, 2022).
- F. K. Nishi et al., “Electronic Healthcare Data Record Security Using Blockchain and Smart Contract,” J. Sens., vol. 2022, p. e7299185, May 2022. [CrossRef]
- “A blockchain-based framework for electronic medical records sharing with fine-grained access control | PLOS ONE.” https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0239946 (accessed Jul. 08, 2022).
- H. S. A. Fang, T. H. Tan, Y. F. C. Tan, and C. J. M. Tan, “Blockchain Personal Health Records: Systematic Review,” J. Med. Internet Res., vol. 23, no. 4, p. e25094, Apr. 2021. [CrossRef]
- X. Zheng, R. R. Mukkamala, R. Vatrapu, and J. Ordieres-Mere, “Blockchain-based Personal Health Data Sharing System Using Cloud Storage,” in 2018 IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom), Sep. 2018, pp. 1–6. [CrossRef]
- A. Ojo and S. Adebayo, “Blockchain as a Next Generation Government Information Infrastructure: A Review of Initiatives in D5 Countries,” in Public Administration and Information Technology, Springer, 2017, pp. 283–298. Accessed: Jul. 08, 2022. [Online]. Available: https://ideas.repec.org/h/spr/paitcp/978-3-319-63743-3_11.html.
- M. Mettler, “Blockchain technology in healthcare: The revolution starts here,” in 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom), Sep. 2016, pp. 1–3. [CrossRef]
- Y. Xiao, B. Xu, W. Jiang, and Y. Wu, “The HealthChain Blockchain for Electronic Health Records: Development Study,” J. Med. Internet Res., vol. 23, no. 1, p. e13556, Jan. 2021. [CrossRef]
- G. G. Dagher, J. Mohler, M. Milojkovic, and P. B. Marella, “Ancile: Privacy-preserving framework for access control and interoperability of electronic health records using blockchain technology,” Sustain. Cities Soc., vol. 39, pp. 283–297, May 2018. [CrossRef]
- V. Ahmadi, S. Benjelloun, M. El Kik, T. Sharma, H. Chi, and W. Zhou, “Drug Governance: IoT-based Blockchain Implementation in the Pharmaceutical Supply Chain,” in 2020 Sixth International Conference on Mobile And Secure Services (MobiSecServ), Feb. 2020, pp. 1–8. [CrossRef]
- A. Sharma, J. Kaur, and I. Singh, “Internet of Things (IoT) in Pharmaceutical Manufacturing, Warehousing, and Supply Chain Management,” SN Comput. Sci., vol. 1, no. 4, p. 232, Jul. 2020. [CrossRef]
- K. M. Botcha, V. V. Chakravarthy, and Anurag, “Enhancing Traceability in Pharmaceutical Supply Chain using Internet of Things (IoT) and Blockchain,” in 2019 IEEE International Conference on Intelligent Systems and Green Technology (ICISGT), Jun. 2019, pp. 45–453. [CrossRef]
- Y. Kase, T. Shimazaki, and H. Okano, “Current understanding of adult neurogenesis in the mammalian brain: how does adult neurogenesis decrease with age?,” Inflamm. Regen., vol. 40, no. 1, p. 10, Jun. 2020. [CrossRef]
- P. M. Rossini, S. Rossi, C. Babiloni, and J. Polich, “Clinical neurophysiology of aging brain: From normal aging to neurodegeneration,” Prog. Neurobiol., vol. 83, no. 6, pp. 375–400, Dec. 2007. [CrossRef]
- “Mental disorders.” https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed Jul. 08, 2022).
- S. S. Aljehani, R. A. Alhazmi, S. S. Aloufi, B. D. Aljehani, and R. Abdulrahman, “iCare: Applying IoT Technology for Monitoring Alzheimer’s Patients,” in 2018 1st International Conference on Computer Applications & Information Security (ICCAIS), Apr. 2018, pp. 1–6. [CrossRef]
- R. J. Oskouei, Z. MousaviLou, Z. Bakhtiari, and K. B. Jalbani, “IoT-Based Healthcare Support System for Alzheimer’s Patients,” Wirel. Commun. Mob. Comput., vol. 2020, p. e8822598, Oct. 2020. [CrossRef]
- C. b. Sivaparthipan et al., “Innovative and efficient method of robotics for helping the Parkinson’s disease patient using IoT in big data analytics,” Trans. Emerg. Telecommun. Technol., vol. 31, no. 12, p. e3838, 2020. [CrossRef]
- S. F. Desyansah, M. N. Mohammed, S. Al-Zubaidi, H. Syamsudin, I. Abdullah, and E. Yusuf, “Bradykinesia Detection System Using IoT Based Health Care System for Parkinson’s Disease Patient,” in 2021 IEEE International Conference on Automatic Control & Intelligent Systems (I2CACIS), Jun. 2021, pp. 329–333. [CrossRef]
- H. Ishii, K. Kimino, M. Aljehani, N. Ohe, and M. Inoue, “An Early Detection System for Dementia Using the M2 M/IoT Platform,” Procedia Comput. Sci., vol. 96, pp. 1332–1340, Jan. 2016. [CrossRef]
- L.-P. Hung, W. Huang, J.-Y. Shih, and C.-L. Liu, “A Novel IoT Based Positioning and Shadowing System for Dementia Training,” Int. J. Environ. Res. Public. Health, vol. 18, no. 4, Art. no. 4, Jan. 2021. [CrossRef]
- W.-L. Chen, L.-B. Chen, W.-J. Chang, and J.-J. Tang, “An IoT-based elderly behavioral difference warning system,” in 2018 IEEE International Conference on Applied System Invention (ICASI), Apr. 2018, pp. 308–309. [CrossRef]
- T. Tanaka, K. Kokubo, K. Iwasa, K. Sawa, N. Yamada, and M. Komori, “Intraday Activity Levels May Better Reflect the Differences Between Major Depressive Disorder and Bipolar Disorder Than Average Daily Activity Levels,” Front. Psychol., vol. 9, 2018, Accessed: Jul. 08, 2022. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fpsyg.2018.02314.
- G. L. Faedda et al., “Actigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls,” J. Child Psychol. Psychiatry, vol. 57, no. 6, pp. 706–716, 2016. [CrossRef]
- J. T. O’Brien et al., “A study of wrist-worn activity measurement as a potential real-world biomarker for late-life depression,” Psychol. Med., vol. 47, no. 1, pp. 93–102, Jan. 2017. [CrossRef]
- M. Nardelli, A. Lanata, G. Bertschy, E. P. Scilingo, and G. Valenza, “Heartbeat Complexity Modulation in Bipolar Disorder during Daytime and Nighttime,” Sci. Rep., vol. 7, no. 1, Art. no. 1, Dec. 2017. [CrossRef]
- C. Gentili et al., “Longitudinal monitoring of heartbeat dynamics predicts mood changes in bipolar patients: A pilot study,” J. Affect. Disord., vol. 209, pp. 30–38, Feb. 2017. [CrossRef]
- S. Enshaeifar et al., “The Internet of Things for Dementia Care,” IEEE Internet Comput., vol. 22, no. 1, pp. 8–17, Jan. 2018. [CrossRef]
- M. A. Brodie et al., “Gait as a biomarker? Accelerometers reveal that reduced movement quality while walking is associated with Parkinson’s disease, ageing and fall risk,” in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Aug. 2014, pp. 5968–5971. [CrossRef]
- “Pasluosta, Cristian.” http://www5.cs.fau.de/index6653.html?id=2806&type=98&L= (accessed Jul. 08, 2022).
- S. Dash, S. K. Shakyawar, M. Sharma, and S. Kaushik, “Big data in healthcare: management, analysis and future prospects,” J. Big Data, vol. 6, no. 1, p. 54, Jun. 2019. [CrossRef]
- S. Cohen, L. R. Bataille, and A. K. Martig, “Enabling breakthroughs in Parkinson’s disease with wearable technologies and big data analytics,” mHealth, vol. 2, no. 5, Art. no. 5, May 2016, Accessed: Jul. 08, 2022. [Online]. Available: https://mhealth.amegroups.com/article/view/10369.
- A. Mobed, S. Razavi, Ali. Ahmadalipour, S. K. Shakouri, and G. Koohkan, “Biosensors in Parkinson’s disease,” Clin. Chim. Acta, vol. 518, pp. 51–58, Jul. 2021. [CrossRef]
- R. Subha, M. Haritha, B. Nithishna, and S. G. Monisha, “Coma Patient Health Monitoring System Using IOT,” in 2020 6th International Conference on Advanced Computing and Communication Systems (ICACCS), Mar. 2020, pp. 1454–1457. [CrossRef]
- S. Vukićević, Z. Stamenković, S. Murugesan, Z. Bogdanović, and B. Radenković, “A new telerehabilitation system based on internet of things,” Facta Univ. - Ser. Electron. Energ., vol. 29, no. 3, pp. 395–405, 2016.
- R. Lloréns, J.-A. Gil-Gómez, P. Mesa-Gresa, M. Alcañiz, C. Colomer, and E. Noé, “BioTrak: a comprehensive overview,” in 2011 International Conference on Virtual Rehabilitation, Jun. 2011, pp. 1–6. [CrossRef]
- “JMIR mHealth and uHealth - Smartphone Apps to Support Falls Rehabilitation Exercise: App Development and Usability and Acceptability Study.” https://mhealth.jmir.org/2020/9/e15460/ (accessed Jul. 08, 2022).
- C. Nave and O. Postolache, “Smart Walker based IoT Physical Rehabilitation System,” in 2018 International Symposium in Sensing and Instrumentation in IoT Era (ISSI), Sep. 2018, pp. 1–6. [CrossRef]
- M. Tousignant, P. Boissy, H. Corriveau, H. Moffet, and F. Cabana, “In-Home Telerehabilitation for Post-Knee Arthroplasty: A Pilot Study,” Int. J. Telerehabilitation, pp. 9–16, Sep. 2009. [CrossRef]
- G. F. de S. Miguel, A. A. R. de Sá, J. T. de Souza, and E. L. M. Naves, “Home-based telerehabilitation: A review of remote therapy frameworks,” Res. Soc. Dev., vol. 10, no. 6, Art. no. 6, May 2021. [CrossRef]
- D. Li, “5G and intelligence medicine—how the next generation of wireless technology will reconstruct healthcare?,” Precis. Clin. Med., vol. 2, no. 4, pp. 205–208, Dec. 2019. [CrossRef]
- “Pancreas Cancer-Associated Weight Loss | The Oncologist | Oxford Academic.” https://academic.oup.com/oncolo/article/24/5/691/6439151 (accessed Jul. 08, 2022).
- M. Ebadi and V. C. Mazurak, “Evidence and Mechanisms of Fat Depletion in Cancer,” Nutrients, vol. 6, no. 11, Art. no. 11, Nov. 2014. [CrossRef]
- A. Koyama et al., “Malnutrition in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Lobar Degeneration: Comparison Using Serum Albumin, Total Protein, and Hemoglobin Level,” PLOS ONE, vol. 11, no. 6, p. e0157053, Jun. 2016. [CrossRef]
- C.-C. Lin, P.-Y. Lin, P.-K. Lu, G.-Y. Hsieh, W.-L. Lee, and R.-G. Lee, “A Healthcare Integration System for Disease Assessment and Safety Monitoring of Dementia Patients,” IEEE Trans. Inf. Technol. Biomed., vol. 12, no. 5, pp. 579–586, Sep. 2008. [CrossRef]
- P. Sundaravadivel, K. Kesavan, L. Kesavan, S. P. Mohanty, E. Kougianos, and M. Ganapathiraju, “Smart-log: An automated, predictive nutrition monitoring system for infants through the IoT,” in 2018 IEEE International Conference on Consumer Electronics (ICCE), Jan. 2018, pp. 1–4. [CrossRef]
- S. Suganyadevi, D. Shamia, and K. Balasamy, “An IoT-Based Diet Monitoring Healthcare System for Women,” in Smart Healthcare System Design, John Wiley & Sons, Ltd, 2022, pp. 167–202. [CrossRef]
- “Electronic Health Records in Ambulatory Care — A National Survey of Physicians | NEJM.” https://www.nejm.org/doi/full/10.1056/nejmsa0802005 (accessed Jul. 08, 2022). [CrossRef]
- “IoT-Enabled Health Monitoring and Assistive Systems for in Place Aging Dementia Patient and Elderly | IntechOpen.” https://www.intechopen.com/chapters/67395 (accessed Jul. 08, 2022).
- A. Boonstra and M. Broekhuis, “Barriers to the acceptance of electronic medical records by physicians from systematic review to taxonomy and interventions,” BMC Health Serv. Res., vol. 10, no. 1, p. 231, Aug. 2010. [CrossRef]
- A. Dasgupta and S. Deb, “Telemedicine: A New Horizon in Public Health in India,” Indian J Community Med, vol. 33, no. 1, pp. 3–8, Jan. 2008. [CrossRef]
- B. THEODOR, “Movement-Cure Apparatus.,” 0964898, Jul. 19, 1910 Accessed: Jan. 21, 2023. [Online]. Available: https://www.freepatentsonline.com/0964898.html.
- K. A. Unnikrishna Menon, D. Hemachandran, and T. K. Abhishek, “A survey on non-invasive blood glucose monitoring using NIR,” in 2013 International Conference on Communication and Signal Processing, Apr. 2013, pp. 1069–1072. [CrossRef]
- M. E. H. Chowdhury et al., Machine Learning in Wearable Biomedical Systems. IntechOpen, 2020. [CrossRef]
- G. Valenza et al., “Wearable Monitoring for Mood Recognition in Bipolar Disorder Based on History-Dependent Long-Term Heart Rate Variability Analysis,” IEEE J. Biomed. Health Inform., vol. 18, no. 5, pp. 1625–1635, Sep. 2014. [CrossRef]
- “Elderly Care: health + protect – Apps on Google Play.” https://play.google.com/store/apps/details?id=com.levstone.mobility.trustedelderlycare&hl=en_IN&gl=US/.
- O. Bazgir, J. Frounchi, S. A. H. Habibi, L. Palma, and P. Pierleoni, “A neural network system for diagnosis and assessment of tremor in parkinson disease patients,” in 2015 22nd Iranian Conference on Biomedical Engineering (ICBME), Nov. 2015, pp. 1–5. [CrossRef]
- J.-F. Daneault, B. Carignan, C. É. Codère, A. Sadikot, and C. Duval, “Using a Smart Phone as a Standalone Platform for Detection and Monitoring of Pathological Tremors,” Frontiers in Human Neuroscience, vol. 6, 2013, Accessed: Jan. 22, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fnhum.2012.00357. [CrossRef]
- M. Braybrook, S. O’Connor, P. Churchward, T. Perera, P. Farzanehfar, and M. Horne, “An Ambulatory Tremor Score for Parkinson’s Disease,” Journal of Parkinson’s Disease, vol. 6, no. 4, pp. 723–731, Jan. 2016. [CrossRef]
- Terna, A. Ramtirthkar, J. Digge, V. R. Koli, “Iot based Healthcare System for Coma Patient,” IJEAT, vol. 9, no. 3, pp. 3327–3330, Feb. 2020. [CrossRef]
- D. Burton and E. Zilberg, “Methods and apparatus for monitoring consciousness,” US7774052B2, Aug. 10, 2010 Accessed: Jan. 22, 2023. [Online]. Available: https://patents.google.com/patent/US7774052B2/en.
- G. Liu et al., “Predicting Outcome in Comatose Patients: The Role of EEG Reactivity to Quantifiable Electrical Stimuli,” Evid Based Complement Alternat Med, vol. 2016, p. 8273716, 2016. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).