1. Introduction
The pathogenic agent of Coronavirus disease-2019 (COVID-19); Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), is an enveloped virion that contains a positive sense single strand of ribonucleic acid (RNA).1 Despite the unique feature of proofreading polymerase activity during replication, the RNA virus is associated with a high mutation rate due to the uncontrolled viral replication facilitated by interferons of the infected host. With every round of infection, the absolute quantity of mutations increases and clusters within populations before spreading across populations by way of global travel and migration activities. Per World Health Organization (WHO) and Center for Disease Control and Prevention (CDC), the declared Variants of Concern (VOCs) for SARS-CoV-2 include the Alpha, Beta, Gamma, Delta and Omicron isolates of SARS-CoV-2.2,3 Lineage B.1.1.7, Alpha, was designated as the first VOC by the WHO, and continues to diverge into a monophyletic clade. In context to these aforementioned VOCs, SARS-CoV-2 has defied a ladder paradigm of viral evolution,4 as these variants have not antigenically descended from another in a progressive fashion. The dominant consequences of emerging mutant variants of SARS-CoV-2 include the possibility of new variants that may potentially bypass the standard diagnostic investigation protocol, impact disease severity, have faster transmissibility, and alter vaccine effectiveness.
Immunization has been recognized as a key pillar of disease prevention since the advent of vaccines. The development of the first vaccine by Edward Jenner for prevention of smallpox is a historical landmark in immunology. Dr. Jenner’s published case studies on inoculation in 1798 demonstrated that artificially induced pathogenic exposure can effectively prevent disease when the body encounters the same pathogen in the future.5 Vaccination elicits a tailored adaptive immune response when the body encounters the target pathogen.6 The adaptive immune response progresses via T and B lymphocytes. Helper T cells recognize presenting pathogens and activate memory B cells which bring about rapid replication of existing antigen specific antibodies to target, contain, and signal for the destruction of the presented pathogen.6 Building on this knowledge, disease prevention was quickly recognized as one of the key strategies in curtailing the COVID-19 pandemic, resulting in the development and eventual approval of the first-ever messenger RNA (mRNA) vaccine, by the US Federal Drug Administration (FDA).7 Some key vaccines that were developed for COVID-19 are:
1. mRNA vaccines: BNT162b2/Pfizer and mRNA-1273/Moderna
2. SARS-CoV-2 spike protein and Matrix-M adjuvant vaccine: NVX-CoV2373 (Novavax)
3. Adenovirus vector vaccine: Janssen/Ad26.COV2.S, ChadOx1 nCoV-19/AZD1222 (Oxford/AstraZeneca)
These vaccines elicit a specific immune response against the spike protein of SARS-CoV-2 to prevent the virion from host cell binding, fusion and entry.8–12 In the case of mRNA vaccines, the Pfizer (BNT162b2) and Moderna (mRNA-1273) vaccine efficiencies were reported to show an overall reduction following the emergence of the Omicron variant (as compared to the delta variant) for adults who completed the primary immunization series, consisting of two doses.13 Additionally, both mRNA vaccines were found to have a continually decreasing rate of effectiveness over time, following the last administration of a booster dose.13 Following the onset and spread of the Omicron variant, Andrews et al. found that subjects who received two doses of the viral vector vaccine, ChADOx1(Oxford/AstraZeneca) had significantly reduced protective effect following the administration of a booster dose against the variant. The recombinant protein nanoparticle vaccine developed by Novavax (NVX-COV2373) was shown to similarly display a progressive reduction in neutralization antibody titers in adults administered with 2 and 3 doses, as the variant waves of SARS-CoV-2 progressed, from the Beta to Omicron variants.14
While the deployment of SARS-CoV-2 vaccines have significantly reduced the disease burden globally, efficacy of these vaccines has been steadily declining as new mutagenic sequences of the virus compete and spread. In this context, it is pertinent to understand viral evolution and its impact on vaccine efficacy.
2. Evolution of SARS-CoV-2 Variants
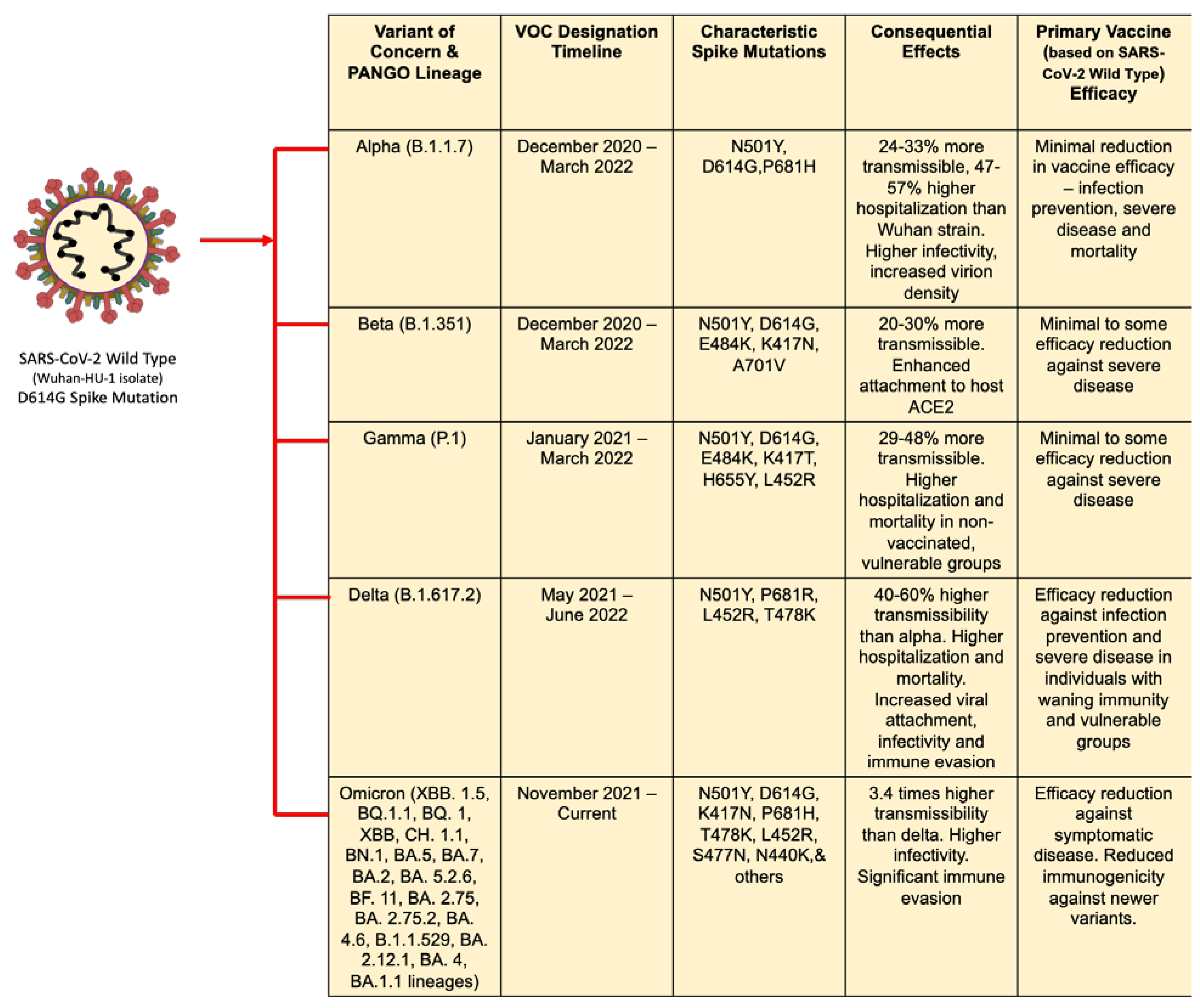
SARS-CoV-2, like other viruses, continues to evolve over time through genetic recombination or genetic mutations. While some of these changes do not affect the viral properties, other mutations affect the virulence, transmissibility, risk of reinfection or other factors, including vaccine efficacy, immune evasion and diagnosis. The global public health organizations WHO and SARS-CoV-2 Interagency group (SIG) have been monitoring these viral mutations through viral genetic sequence-based surveillance and epidemiological investigations. They characterize these variants as VOC and variants of interest (VOI), variant being monitored (VBM) and variant of high consequence (VOHC) to prioritize monitoring, research and response to the COVID-19 pandemic.3,15 The naming and tracking of the genetic variants for SARS-CoV-2 is based on greek alphabets and designated by ‘Technical Advisory Group on Virus Evolution’, representatives from WHO COVID-19 reference laboratory network, Global Initiative on Sharing All Influenza Data (GISAID), Nextstrain, Pango and experts in virological, microbial nomenclature and communication from several countries.15 A VOI is categorized by changes in genetic markers that affect the receptor binding and neutralization ability by antibodies from previous infection or vaccination.3,15 Currently no SARS-CoV-2 variants are designated as VOI. A VOC is categorized for variants that exhibit increase in transmissibility, significant reduction in neutralizing antibodies generated during previous infection and vaccination, reduced effectiveness of therapeutics or vaccines, diagnosis evasion and more severe disease.3,15 The Omicron variant is currently classified as a VOC. Previously circulating variants-alpha, beta, gamma, delta and epsilon were designated as VOC but later downgraded as a VBM based on emerging evidence suggesting that while being associated with severe disease and higher transmissibility, they are no longer detectable or circulating at very low levels, thus posing no risk to public health.3,15 A fourth designation termed VOHC is used to categorize strains with clear evidence of significantly reduced or failure in preventive and medical countermeasures compared to previous strains. Currently, no SARS-CoV-2 strain has been designated under this category.3 The progression of the SARS-CoV-2 pandemic has been delineated by five dominant variants, driven by the Alpha (B.1.1.7 and Q lineages), Beta (B.1.351 and descendants), Gamma (P.1 and descendent lineages), Delta (B.1.617.2 and AY lineages) and Omicron (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4, BA.5, XBB.1.5, B.Q1.1, B.Q.1, XBB, C.H.1.1, B.N.1 lineages) variants.16
2.1. Previous VOC (Alpha, Beta, Gamma and Delta)
The alpha variant (B.1.1.7 and Q lineages) emerged in September 2020, became the dominant variant in the United Kingdom (UK) by the end of 2020 and was designated a VOC from December 2020 until March 2022.15 The alpha variant was found to be 24-33% more transmissible,17 leading to 47-57% higher hospitalization and 44.74% higher mortality 18 than the wild-type SARS-CoV-2 (ancestral strain). It was found to have 17 mutations in the viral genome, 8 of which manifest in the spike protein-characteristically N501K, D614G, P681H, mutations, resulting in screening failures, immune escape, and a greater receptor-binding domain (RBD) binding affinity to angiotensin-converting enzyme 2 (ACE2) host receptor cells.19,20 The alpha, B.1.1.7 variant is a descendent of the early mutation D614G, found early in the pandemic as it emerged from China, allowing higher infectivity and increased virion density.21
The beta variant (B.1.351 and descendants) was originally documented in May 2020 in South Africa and designated a VOC from December 2020 until March 2022.15 The variant was found to be 20-30% more transmissible,17 with eight mutations in the spike regions, four of particular concern which enhanced the attachment of the variant virion to human cells. While the N501Y, D614G mutations were common across the alpha variant as well, E484K, K417N, A701V were associated with the beta variant.20,22
A third variant-gamma (P.1 and descendent lineages) was designated VOC around a similar timeline-January 2021, after being detected first in Brazil in November 2020, and was reclassified as a VBM by March 2022.15 It was noted to have seventeen mutations, with ten in the spike protein. Some notable mutations included the N501Y, D614G, E484K-common to the beta strain as well, and K417T, H655Y unique to the gamma variant.20 There were higher transmission rates (29-48%) associated with this variant, including among previously recovered individuals,17 and higher hospitalization and associated mortality relative to non-VOCs and the ancestral SARS-CoV-2 strain.23 Of the 4 lineages of gamma variant, P.4 was found to host a L452R mutation in the spike receptor binding domain (RBD) region, also key to the delta variant.24
The Delta (B.1.617.2) variant, first detected in Oct 2020 in India, was found to be highly transmissible (76-117%),17 and remained as the globally dominant variant of the latter half of 2021, displacing previous VOCs in the majority of regions. It was designated a VOC by May 2021,15 and hosted at least thirteen mutations, with four concerning mutations in the spike protein-L452R, P681R, D614G (shared with other highly transmissible VOCs as well) and T478K.20 The former two mutations significantly affected the viral attachment to the host cell, infectivity and decreased recognition by the host immune system.25 Studies from UK suggested 40-60% higher transmissibility compared to the alpha variant and reduced vaccine efficacy.26 The delta variant was downgraded as a previous VOC in June 2022, by the WHO.15
2.2. Current VOC (Omicron)
The Omicron variant (XBB.1.5, XBB, B.Q1.1, B.Q.1, C.H.1.1, B.N.1, BA.5, BA.7, BA.2, BA. 5.2.6, BF. 11, BA. 2.75, BA. 2.75.2, BA. 4.6, B.1.1.529, BA. 2.12.1, BA. 4, BA.1.1 lineages), currently the only designated VOC, has predominant worldwide circulation and is marked by a distinct clade from the ancestral branch of SARS-CoV-2, as its emergence did not evolve from the previously circulating delta variant. It is characterized with increased transmissibility and significant immune evasion compared with the early wild-type strain and the four previously identified VOCs.27 Five sub-lineages of Omicron have been identified, with the initial three sister lineages BA.1, BA.2, and BA.3, later followed by BA.4 and BA.5.28 The Omicron variant was first identified as a unique mutagenic sequence of SARS-CoV-2 in South Africa in November 2021, and rapidly spread to over 140 countries within 7 weeks.29 It is the most antigenically divergent of all variants, carrying over 50 unique characteristic mutations, over 30 of which result in changes to the spike protein, as compared to the alpha variant. 30,31
The first dominant Omicron variant sublineage, B.1.1.529 (BA.1), is characterized by containing 37 mutations.32 BA.1, BA.2, and BA.3 share 21 common mutations, 11 of which are contained in the RBD, theorized to contribute to a larger ACE2 affinity due to an overall increase in positive electrostatic surface potential.33 BA.2 and BA.3 sub-lineages branch off the same node of the B.1.1 subvariant, and went on to mutate independently along separate branches.28 The BA.2 sublineage genome contains 28 unique mutations not present in BA.1, four of which are located on the RBD.34 BA.4 and BA.5, which contain identical spike proteins, are marked by similar deletion in the spike, also found in the alpha and BA.1 lineage.28 As of December 2022, the XBB 1.5 VOC has become a predominant variant, out competing the other omicron sub variants. The relative effective reproduction number of XBB.1.5 is more than 1.2-fold higher than the parental XBB lineage.35 Additionally, the binding affinity to host ACE2 and infectivity of XBB.1.5 was found to be 4.3-fold and 3.3-fold higher, by acquiring the S:F486P substitution to augment ACE2 binding affinity.35 Overall the immune resistance, infectivity and transmissibility was enhanced with this variant.35,36 The Omicron variant is associated with higher infectivity, antigenic changes that mediate antibody escape from an existing pre-immune population, and was found to have a transmission rate 3.4 times higher than that of the Delta variant, and 2.1 times higher than other variants in the United States.37 Interestingly, the Omicron sub lineages are able to evade immunity in both convalescent and fully vaccinated individuals.36,38 Convalescent sera from individuals infected with BA.1 showed significant reduction in neutralizing antibodies against the BA.4 and BA.5 strains.39 They harbor the L452Q/R mutation that allows evasion from humoral immunity.20,40
The SARS-CoV-2 virion is surrounded by a lipid bilayer, decorated with glycosylated protruding class fusion proteins-spike proteins, which mediate virion profusion. The omicron mutation has been found to exhibit a favorable epistasis in the RBD. The presence of both Q498R and N501Y mutations in the Omicron variant has been found to result in a two-fold increase in spike protein binding affinity to the angiotensin-converting enzyme 2 (ACE2), as compared to Alpha variant. Studies have shown that Omicron’s structural gene variations affect infectivity. This assessment was made by analyzing all four structural proteins of the virion by developing SARS-CoV-2 virus-like particles (SC2-VLPs), indicating that the spike, nucleocapsid, membrane, and envelope structural proteins all contained notable mutations.41,42 The VLP-mediated study found that the spike and nucleocapsid protein mutations of omicron contribute to an increase in infectivity, while the membrane and envelope gene variants compromise infectivity relative to previous viral variants.41,42 Viral immune evasion can occur through three mechanisms. A major ramification of the emergence and evolution of Omicron is the vaccine-elicited immune and anti-spike monoclonal antibody escape. The Omicron variant is characterized by a cluster of mutations resulting in alternate structural conformations of the spike protein, the primary target for monoclonal antibody therapeutics.30 Monoclonal antibody therapeutics for SARS CoV-2 can be escaped by a single mutation, as they bind to a single epitope on the S protein of the virion. On the other hand, polyclonal antibodies are more resistant to mutation induced escape in principle, as they bind to multiple regions of the key viral proteins. Omicron variant escaped the neutralization activity of convalescent plasma and two doses of vaccine-induced serum more easily than the ancestral strain and other VOCs, including Beta and Delta.43
3. Impact of Viral Evolution on Vaccine Efficacy
Currently 50 vaccines for COVID-19 have been approved worldwide.44 The WHO has listed 9 vaccines for Emergency use, also accepted for travel to the United States (US)-Comirnaty (Pfizer-BioNTech), Spikevax (Moderna), Vaxzevria (AstraZeneca), Covaxin, Covishield, BIBP/Sinopharm, CoronaVac (Sinovac), Nuvaxovid (Novavax), Covovax.45,46 In the US, three major types of COVID-19 vaccines have been approved or authorized for use by the Food and Drug Administration (FDA)-1. mRNA vaccines: Cominarty (Pfizer-BioNTech) and Spikevax (Moderna), 2. protein subunit vaccine: Novavax and 3. viral vector vaccine: Jannsen/J&J.47,48 The antigenic target for the vaccines is the spike protein RBD on the virus surface. The host generated antibodies can attach to this target thus preventing the attachment to the host cell ACE2 receptor and neutralizing the virus.49 The focus of vaccine development has been to prevent severe disease and mortality, and while many of the vaccines have shown robust seroconversion towards this effect, studies have shown that the immune response wanes over time and is affected by the evolving viral strains. For this review we have only selected some key vaccines based on the available literature demonstrating the impact of viral evolution on vaccine efficacy.
3.1. Effectiveness of Primary Vaccination Series
While conventional vaccines such as live attenuated, killed and subunit vaccines have provided successful protection against a variety of pathogens, one of the obstacles associated with conferring protection against infectious agents is combating pathogens that have the ability to evade the adaptive immune system.50 Additionally, the rapid development and large-scale deployment of new vaccines has been limited by conventional methodology as witnessed during the early days of the COVID-19 pandemic, thus paving the way for mRNA vaccines. 51 mRNA based approaches had been promising alternatives and was first published when a reporter gene mRNA was injected into mice and protein production was detected.52 Subsequent studies furthered research in this area through animal studies, though high innate immunogenicity, mRNA instability, and inefficient in vivo delivery lead to concerns in human application.53
Technological inventions over the past decade lead to promising developments in application of nucleic acid therapeutics in humans.51 Research demonstrated that to achieve an antigen-specific immune response the synthetic mRNA in a vaccine would need to enter the cytosol through the plasma membrane, but that the exonuclease catalyzed decay of mRNA in the cytosol would be a challenge for mRNA vaccine development.54 In recent years, the potential for exogenous mRNA or mRNA synthesized in-vitro to become an expression vector for antigenic proteins have been recognized, but the mechanisms of mRNA delivery, immunity at the cellular level, and measurement of mRNA uptake into the cell have been ongoing subjects of study.55 Designs proposed for mRNA vaccines were modeled after eukaryotic mRNA and an open reading frame (ORF) with a cap, a poly(A) tail, and 5’ and 3’ untranslated regions (UTRs), which all contribute to mRNA stability.54 mRNA vaccines have some key advantages over live, attenuated and subunit vaccines. mRNA vaccines are non-integrating and non-infectious platform with minimal insertional mutagenesis risk, degraded by normal cellular processes. Additionally, the ease of delivery modifications can be used to modulate the safe profile and half life of vaccines. mRNA vaccines have a key advantage for inexpensive, scalable production in short timelines due to high yields through in vitro transcriptional processes.51 The COVID-19 mRNA vaccines are delivered to the deltoid muscle site, then transit to the myocyte cytosol and ribosomes undergoing translation to produce spike protein which induces host antibody and cell mediated immune response, including neutralizing antibodies, after entering circulation.56,57 To ensure safe and successful delivery of the mRNA for intracellular uptake they are encapsulated in lipid nanoparticles that facilitate the process.58,59
Two mRNA vaccines were developed to combat the COVID-19 pandemic. Moderna’s COVID-19 primary series monovalent vaccine (mRNA-1273) received emergency use authorization (EUA) from the FDA in December 2020, delivering 100 μg mRNA in each dose. The vaccination schedule is two doses given at a a 28-day interval, for individuals 18 years and older. Preliminary data from a phase 1, dose-escalation, open-label trial showed immune responses in all trial participants who were placed in three dosage groups (25, 100, and 250 micrograms) and received the vaccine60. CD4 T-cell responses and expression of Th1 cytokines were noted, and the authors concluded that the 100µg dose had a better reactogenicity profile while still maintaining a high level of Th1-based CD4 T-cell responses.60 Notably, the preliminary report addresses the issue of sufficient mRNA uptake and some of its mechanisms of action. The efficacy and safety assessment published 3 months later demonstrated that the vaccine efficacy for various demographic subgroups ranged from 86.4% to 97.5% efficacy with a CI of 95%.61 Simultaneously, Pfizer’s primary series monovalent vaccine (BNT162b2), received EUA during the same timeline for individuals 12 years and older, delivering 30 μg mRNA in each dose with a vaccination schedule of two doses to be administered with 21-day interval.61 Both BNT162b2 and mRNA-1273 utilize lipid nanoparticles (LNPs) for the delivery of the full length spike proline substitutions.61,62 According to the published data at the time for subgroups, vaccine efficacy for BNT162b2 ranged from 87.7% to 100% (95% CI).62 T follicular helper cells (TFH) and T helper 1 cells (TH1)-type CD4 T cell responses were reported in early experiments and data for BNT162b2, suggesting that a TH1-type CD4 T cell response may be a general effect of the mRNA COVID-19 candidate vaccines that use LNPs for delivery.63 These two candidate monovalent vaccines were subsequently also authorized for use in children younger than 12 years of age, two doses recommended to be given with four to eight weeks interval.64
The immune response associated with these vaccines was initially thought to be largely humoral, triggering B cells to promote the production of neutralizing antibodies. Although, a significant body of research has since shown that these vaccines reprogram both the innate and adaptive immune responses, including CD4+ and CD8+ T cells against SARS-CoV-2.8,65,66 Data from clinical trials of the primary series monovalent vaccination of mRNA-1273 and BNT162b2 showed 94.1 and 95 % efficacy, respectively, in preventing symptomatic and severe COVID-19 disease.61,62 However, this immune response is variable across different population groups (eg; underlying immunocompromising conditions or treatments etc)67, duration since last vaccination (waning immunity across all age groups over time)68 and the new variants of SARS-CoV-2 69–71 that have evolved. Both the vaccines were able to provide comparable efficacy (~91%) against the alpha variant of the virus when sera from vaccinated individuals was studied through virus-neutralization assays.70,72 Although there was a 6.4 fold reduction in neutralization antibody titers against the beta variant against infection, but effectiveness remains high against hospitalization or severe disease.70 By August 2021, the delta variant had become predominant and the vaccine effectiveness saw a reduction to about 66%.73–75 Studies show that a key feature of the new VOC was reduction in post vaccination protection, as a factor of duration since the last vaccine dose, underlying immunocompromised status and others factors.8,76 Based on significant body of research the CDC recommended a third and fourth doses for protection against breakthrough infections and severe disease owing to waning immunity associated with the aforementioned factors.8
3.2. Effectiveness of Bivalent Vaccines
In November 2021, the omicron variant emerged as the most antigenically diverse and quickly dominated new infections worldwide.77 In contrast to other variants the omicron variants and subvariants have outperformed and significantly evaded immunity induced by the monovalent vaccination; leading to a higher number of breakthrough infections.78,79 A study from December 2021 suggested that vaccine efficacy against hospitalization for COVID-19 caused by the omicron variant (B.1.1.529), for monovalent/primary two-dose dose series of BNT162b2, was reduced to 70%.80 Another study found that vaccine effectiveness of the monovalent/primary two-dose series of the BNT162b2 and a second (booster) dose of the Ad26.COV2.S vaccine against COVID-19 related hospitalization caused by the omicron variant (B.1.1.529) was 70 and 72% respectively, 1 to 2 months after the vaccine was administered.81 A recent study, from October 2022, showed that effectiveness and durability of the BNT162b2 vaccine (monovalent/primary two-dose series plus a booster) against hospitalization, caused by BA.1 or BA.2 and BA.4 or BA.5 Covid-19, was further diminished to 56.3%.82 The study also noted that boosting with a third dose of the monovalent vaccine was effective against severe disease caused by all four sub lineages at 1 to 2 months.82
The efficacy of a third or fourth dose of monovalent vaccine was much reduced and limited primarily to protection against severe disease while omicron specific breakthrough infections were observed in significant number of individuals.83,84 In a study of 274 healthcare workers a fourth dose of the monovalent mRNA vaccine yielded similar seroconversion and comparable levels of Omicron-specific neutralizing antibodies in contrast to the peak response one month after the third dose. Suggesting that mRNA vaccines confer optimal humoral immunogenicity after three doses and that antibody titers can be restored by a fourth dose.83
The primary series of vaccination have largely been ineffective against the omicron variants and sub lineages. The omicron variants also showed reduced neutralization by sera of individuals vaccinated with triple doses of ChAdOx1 (Oxford/AstraZeneca) and Ad26.COV2.S (Johnson & Johnson), among other primary series vaccines.85,86 Studies have shown that across vaccine types either primary homologous vaccination or heterologous boosting does not seem to affect the breakthrough infection incidence associated with omicron, although a heterologous boosting, when having received a primary live vector or attenuated vaccine, may allow for more robust humoral immune responses that may allow for better protection against severe disease.84,87–90
SARS-CoV-2 has a propensity to rapidly mutate and compete against the host immune system to evade neutralization and transmission.91 While the primary series of vaccines have been able to drive a robust immune response against the previous VOC, including being boosted by a 3rd or 4th dose (homologous or heterologous) in some individuals, the omicron variants have been largely evading this conferred protection. The BA.4 and BA.5 sub lineages were also seen to evade neutralizing immunity when sera from BA.1 infected vaccinated and unvaccinated individuals was tested.92 This low neutralization gap could significantly affect the unvaccinated and immunocompromised populations against symptomatic and severe disease.92 This prompted adjustments to the antigenic target in the monovalent mRNA vaccines that encodes the spike protein of the ancestral SARS-CoV-2 (Wuhan-HU-1 isolate), to address the mutational changes associated with the newer viral variants (discussed in section 2.B).93
A first version of the bivalent booster 50 ug containing gene encoding spike protein for BA.1 (25ug) and the ancestral strain (25ug) was authorized for use in multiple countries. It elicited strong neutralizing antibody responses against BA.1 and the epidemiologically dominant BA.4 and BA.5 sub variants.
94 Another study showed robust neutralizing antibody response against the BA.2.75 subvariant, regardless of previous SARS-CoV-2 infection.
95 By August 2022 FDA had authorized Moderna and Pfizer’s modified bivalent vaccines with equal amounts of the mRNA encoding the original/ancestral strain and omicron BA.4/BA.5 strains of SARS-CoV-2, to provide a broader, durable and potent protection.
96 This strategy can help incur greater combined protection against both earlier variants and the current VOCs including its sublineages, even as the virus continues to evolve, in contrast to the monovalent booster that targeted only the original viral strain.
97 Muik et al. demonstrated that sera from triple mRNA vaccinated individuals with subsequent breakthrough infection through omicron BA.4/BA.5 showed cross-neutralizing activity against previous Omicron variants BA.1, BA.2, BA.2.12.1, and BA.4/BA.5 itself. Additional studies in mice showed that when the BA.4/BA.5-adapted mRNA booster was administered after the primary series mRNA vaccine, a broader cross-neutralizing activity was noted than a BA.1-adapted booster.
98 Further, in naïve mice, primary immunization with the modified bivalent vaccine induces strong cross-neutralizing activity against Omicron VOCs and previous variants.
98 At the time of writing this manuscript a few papers have elucidated the efficacy of this updated/modified booster in humans. Tenforde et al., estimated the effectiveness of the updated booster in preventing severe disease among immunocompetent adults. The study found that in the described study cohort, administration of this booster dose provided additional protection against COVID-19 associated emergency department/urgent care encounters and hospitalizations in persons who previously received 2, 3, or 4 monovalent vaccine doses. Additionally, the study indicated that the effectiveness of the bivalent vaccine was evident relative to the waned immunity associated with previous monovalent vaccine doses (either 2 or 3 or 4 doses).
99 In a phase 2/3 clinical trial, Chalkias et al. studied the safety and immunogenicity of 50 µg updated bivalent vaccine compared against 50 µg mRNA-1273 (monovalent vaccine) as a second booster, in healthy adults, after having received the 2 dose primary series monovalent vaccination.
100 They found the neutralizing titer achieved against BA.4/BA.5 and ancestral strain at day 29 post-boost was higher in participants who received the updated bivalent booster compared to the monovalent booster.
100 Additionally, a random subset of participants were selected from the updated bivalent booster group, who exhibited cross-neutralization against the emerging omicron variants BQ.1.1 and XBB.1.
100 Kurhade et al. studied the effectiveness of the 2, 3, 4 dosed monovalent and updated bivalent vaccinated individuals in neutralizing the BA.4, BA.5 and newly emerged BA.2.75.2, BQ.1.1 or XBB.1.
101 The study found that while the sera from individuals receiving the updated bivalent vaccine was effective in producing high titers of neutralizing antibodies for BA.4/BA.5 (contrasting the monovalent vaccinated sera), they did not produce robust neutralization against the newer emerging variants BA.2.75.2, BQ.1.1 or XBB.1.
101 While further human studies will be required to confirm the robustness of the updated bivalent booster, it is pertinent to understand the unpredictability of viral evolution and its effect on vaccine efficacy. However, current data supports a vaccine upgrade strategy that matches newer emerging SARS-CoV-2 variants, bolstering protection against future VOC. A summary of the VOCs, key spike mutations and their effects and vaccine efficacy is described in
Figure 1.
4. Conclusions
Multiple vaccines that were developed in response to the COVID-19 pandemic and were based on the spike protein of the ancestral strain of SARS-CoV-2, have proven effectiveness at protecting from severe disease, caused by previous VOC such as alpha, beta, gamma and delta strains of SARS-CoV-2. However, a reduction in effectiveness was observed with the delta variant, prompting recommendation of a booster dose, of the primary homologous or heterologous vaccine, to circumvent the waning immune response associated with evolving viral strains, duration since last vaccine and individuals with immunocompromise, among others. With evolution of the omicron variant, immune evasion and significantly decreased vaccine effectiveness has become a key issue against effective disease management. The development of the bivalent vaccines have marked the beginning of a new paradigm towards pandemic response. This strategy, to regularly update the vaccine ingredient target antigens to match the dominant circulating strain, parallels an influenza-like situation, with yearly effective vaccines, while overcoming issues with fading immunity and creating robust protection particularly in vulnerable populations. To achieve optimal immunization and achieve vaccine development that parallels viral evolution, there will be a need for continued variant and seroprevalence surveillance and real-world vaccine effectiveness monitoring.
Conflicts of Interest
None.
References
- Ke Z, Oton J, Qu K, et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498-502. [CrossRef]
- WHO/BS/2022.2427: Establishment of the 2nd WHO International Standard for anti-SARS-CoV-2 immunoglobulin and Reference Panel for antibodies to SARS-CoV-2 variants of concern. Available online: https://www.who.int/publications/m/item/who-bs-2022.2427 (accessed on 7 November 2022).
- CDC. SARS-CoV-2 Variant Classifications and Definitions - Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Published February 11, 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 2 January 2023).
- Jacobs JL, Haidar G, Mellors JW. COVID-19: Challenges of Viral Variants. Annu Rev Med. 2023;74(1):31-53. [CrossRef]
- Gross CP, Sepkowitz KA. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. Int J Infect Dis. 1998;3(1):54-60. [CrossRef]
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509-517. [CrossRef]
- Lamb YN. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81(4):495-501. [CrossRef]
- CDC-COVID-19. Science Brief: SARS-CoV-2 Infection-induced and Vaccine-induced Immunity-Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention - Science Brief: SARS-CoV-2 Infection-induced and Vaccine-induced Immunity. Published February 11, 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 10 November 2022).
- Commissioner O of the. Pfizer-BioNTech COVID-19 Vaccines. FDA. Published online December 22, 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccines (accessed on 2 January 2023).
- Commissioner O of the. Moderna COVID-19 Vaccines. FDA. Published online December 15, 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccines (accessed on 2 January 2023).
- Commissioner O of the. Janssen COVID-19 Vaccine. FDA. Published online September 30, 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine (accessed on 2 January 2023).
- Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385(25):2348-2360. [CrossRef]
- Andrews N, Stowe J, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532-1546. [CrossRef]
- Bhiman JN, Richardson SI, Lambson BE, et al. Novavax NVX-COV2373 Triggers Potent Neutralization of Omicron Sub-Lineages. Immunology; 2022. [CrossRef]
- Tracking SARS-CoV-2 variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 2 January 2023).
- CDC. Summary of Variant Surveillance. Summary of Variant Surveillance-Centers for Disease Control and Prevention. Published March 28, 2020. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 1 March 2023).
- Campbell F, Archer B, Laurenson-Schafer H, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26(24):2100509. [CrossRef]
- Nyberg T, Twohig KA, Harris RJ, et al. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: cohort analysis. The BMJ. 2021;373:n1412. [CrossRef]
- Choi JY, Smith DM. SARS-CoV-2 Variants of Concern. Yonsei Med J. 2021;62(11):961. [CrossRef]
- Ou J, Lan W, Wu X, et al. Tracking SARS-CoV-2 Omicron diverse spike gene mutations identifies multiple inter-variant recombination events. Signal Transduct Target Ther. 2022;7(1):1-9. [CrossRef]
- Zhang L, Jackson CB, Mou H, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat Commun. 2020;11(1):6013. 6013. [CrossRef]
- Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438-443. [CrossRef]
- Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815-821. [CrossRef]
- Bittar C, Possebon FS, Ullmann LS, et al. The Emergence of the New P.4 Lineage of SARS-CoV-2 With Spike L452R Mutation in Brazil. Front Public Health. 2021;9. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2021.745310 (accessed on 3 January 2023).
- Mlcochova P, Kemp SA, Dhar MS, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114-119. [CrossRef]
- SPI-M-O: Consensus statement on COVID-19, 3 June 2021. GOV.UK. Available online: https://www.gov.uk/government/publications/spi-m-o-consensus-statement-on-covid-19-3-june-2021 (accessed on 6 January 2023).
- Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679-686. [CrossRef]
- Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022;28(9):1785-1790. [CrossRef]
- Weng S, Shang J, Cheng Y, et al. Genetic differentiation and diversity of SARS-CoV-2 Omicron variant in its early outbreak. Biosaf Health. 2022;4(3):171-178. [CrossRef]
- Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600(7887):21-21. [CrossRef]
- Sun Y, Lin W, Dong W, Xu J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J Biosaf Biosecurity. 2022;4(1):33-37. [CrossRef]
- Li Q, Zhang M, Liang Z, et al. Antigenicity comparison of SARS-CoV-2 Omicron sublineages with other variants contained multiple mutations in RBD. MedComm. 2022;3(2). [CrossRef]
- Kumar S, Karuppanan K, Subramaniam G. Omicron (BA.1) and Sub-Variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 Spike Infectivity and Pathogenicity: A Comparative Sequence and Structural-Based Computational Assessment. Bioinformatics; 2022. [CrossRef]
- Chen J, Wei GW. Omicron BA.2 (B.1.1.529.2): High Potential for Becoming the Next Dominant Variant. J Phys Chem Lett. 2022;13(17):3840-3849. [CrossRef]
- Uriu K, Ito J, Zahradnik J, et al. Enhanced transmissibility, infectivity and immune resistance of the SARS-CoV-2 Omicron XBB.1.5 variant. Published online January 17, 2023. [CrossRef]
- Qu P, Faraone JN, Evans JP, et al. Extraordinary Evasion of Neutralizing Antibody Response by Omicron XBB.1.5, CH.1.1 and CA.3.1 Variants. Published online January 17, 2023. [CrossRef]
- Xue L, Jing S, Zhang K, Milne R, Wang H. Infectivity versus fatality of SARS-CoV-2 mutations and influenza. Int J Infect Dis. 2022;121:195-202. [CrossRef]
- Zhang X, Wu S, Wu B, et al. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6(1):1-3. [CrossRef]
- Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593-602. [CrossRef]
- Xia S, Wang L, Zhu Y, Lu L, Jiang S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct Target Ther. 2022;7(1):1-7. [CrossRef]
- Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602(7898):664-670. [CrossRef]
- Syed AM, Ciling A, Taha TY, et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci. 2022;119(31):e2200592119. [CrossRef]
- Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94(6):2376-2383. [CrossRef]
- Vaccines–COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/vaccines/ (accessed on 3 January 2023).
- COVID-19 Vaccines with WHO Emergency Use Listing. WHO-Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). Published November 3, 2021. Available online: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (accessed on 3 January 2023).
- CDC. COVID-19 and Travel. Centers for Disease Control and Prevention. Published April 15, 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/travelers/proof-of-vaccination.html (accessed on 7 November 2022).
- CDC. Stay Up to Date with COVID-19 Vaccines Including Boosters - COVID-19 Vaccination. Centers for Disease Control and Prevention-COVID-19 Vaccination. Published December 30, 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html (accessed on 3 January 2023).
- Commissioner O of the. COVID-19 Vaccines. FDA. Published online December 19, 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 3 January 2023).
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516-527. [CrossRef]
- Rodrigues CMC, Pinto MV, Sadarangani M, Plotkin SA. Whither vaccines? J Infect. 2017;74 Suppl 1:S2-S9. 1. [CrossRef]
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261-279. [CrossRef]
- Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465-1468. [CrossRef]
- Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccines Immunother. 2017;13(12):2837-2848. [CrossRef]
- Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol. 2012;9(11):1319-1330. [CrossRef]
- Kirschman JL, Bhosle S, Vanover D, et al. Characterizing exogenous mRNA delivery, trafficking, cytoplasmic release and RNA–protein correlations at the level of single cells. Nucleic Acids Res. 2017;45(12):e113-e113. [CrossRef]
- Laczkó D, Hogan MJ, Toulmin SA, et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity. 2020;53(4):724-732.e7. [CrossRef]
- Sewell HF, Agius RM, Kendrick D, Stewart M. Covid-19 vaccines: delivering protective immunity. BMJ. Published online December 17, 2020:m4838. [CrossRef]
- Zhang NN, Li XF, Deng YQ, et al. A Thermostable mRNA Vaccine against COVID-19. Cell. 2020;182(5):1271-1283.e16. [CrossRef]
- Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021;29(3):645-649. [CrossRef]
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;383(20):1920-1931. [CrossRef]
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. [CrossRef]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615. [CrossRef]
- Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283-289. [CrossRef]
- Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC. Published November 14, 2022. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (accessed on 5 January 2023).
- Ivanova EN, Devlin JC, Buus TB, et al. SARS-CoV-2 mRNA vaccine elicits a potent adaptive immune response in the absence of IFN-mediated inflammation observed in COVID-19. Published online August 23, 2021. [CrossRef]
- Föhse FK, Geckin B, Overheul GJ, et al. The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses. Published online May 6, 2021. [CrossRef]
- Bergman P, Blennow O, Hansson L, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. eBioMedicine. 2021;74:103705. [CrossRef]
- Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. The Lancet. 2022;399(10328):924-944. [CrossRef]
- Andrews N, Stowe J, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386(16):1532-1546. [CrossRef]
- Wu K, Werner AP, Moliva JI, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. BioRxiv Prepr Serv Biol. Published online January 25, 2021:2021.01.25.427948. [CrossRef]
- Liu Y, Liu J, Xia H, et al. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med. 2021;384(15):1466-1468. [CrossRef]
- Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371(6534):1152-1153. [CrossRef]
- Fowlkes A. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance—Eight U.S. Locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70. [CrossRef]
- Tenforde MW. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70. [CrossRef]
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet. 2021;398(10309):1407-1416. [CrossRef]
- Rajsri KS, McRae MP, Simmons GW, et al. A Rapid and Sensitive Microfluidics-Based Tool for Seroprevalence Immunity Assessment of COVID-19 and Vaccination-Induced Humoral Antibody Response at the Point of Care. Biosensors. 2022;12(8):621. [CrossRef]
- Hastie KM, Li H, Bedinger D, et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: A global consortium study. Science. 2021;374(6566):472-478. [CrossRef]
- Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657-663. [CrossRef]
- Mohsin M, Mahmud S. Omicron SARS-CoV-2 variant of concern. Medicine (Baltimore). 2022;101(19):e29165. [CrossRef]
- Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386(5):494-496. [CrossRef]
- Gray G, Collie S, Goga A, et al. Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. N Engl J Med. 2022;386(23):2243-2245. [CrossRef]
- Collie S, Nayager J, Bamford L, Bekker LG, Zylstra M, Gray G. Effectiveness and Durability of the BNT162b2 Vaccine against Omicron Sublineages in South Africa. N Engl J Med. 2022;387(14):1332-1333. [CrossRef]
- Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N Engl J Med. 2022;386(14):1377-1380. [CrossRef]
- Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. Published online January 28, 2022. [CrossRef]
- Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422-2433.e13. [CrossRef]
- Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676-681. [CrossRef]
- Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. The Lancet. 2022;399(10324):521-529. [CrossRef]
- Wang X, Zhao X, Song J, et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microbes Infect. 2022;11(1):477-481. [CrossRef]
- Deshpande GR, Yadav PD, Abraham P, et al. Booster dose of the inactivated COVID-19 vaccine BBV152 (Covaxin) enhances the neutralizing antibody response against Alpha, Beta, Delta and Omicron variants of concern. J Travel Med. 2022;29(3):taac039. [CrossRef]
- Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481-485. [CrossRef]
- Markov PV, Katzourakis A, Stilianakis NI. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat Rev Microbiol. 2022;20(5):251-252. [CrossRef]
- Khan K, Karim F, Ganga Y, et al. Omicron sub-lineages BA.4/BA.5 escape BA.1 infection elicited neutralizing immunity. Published online May 1, 2022. [CrossRef]
- Zhou H, Møhlenberg M, Thakor JC, et al. Sensitivity to Vaccines, Therapeutic Antibodies, and Viral Entry Inhibitors and Advances To Counter the SARS-CoV-2 Omicron Variant. Clin Microbiol Rev. 35(3):e00014-22. [CrossRef]
- Chalkias S, Harper C, Vrbicky K, et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med. 2022;387(14):1279-1291. [CrossRef]
- Chalkias S, Feng J, Chen X, et al. Neutralization of Omicron Subvariant BA.2.75 after Bivalent Vaccination. N Engl J Med. 2022;387(23):2194-2196. [CrossRef]
- Commissioner O of the. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. FDA. Published August 31, 2022. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 5 January 2023).
- Scheaffer SM, Lee D, Whitener B, et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat Med. Published online October 20, 2022:1-11. [CrossRef]
- Muik A, Lui BG, Bacher M, et al. Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice. Sci Immunol. 2022;7(78):eade9888. [CrossRef]
- Tenforde MW. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19–Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults — VISION Network, Nine States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022;71. [CrossRef]
- Chalkias S, Whatley J, Eder F, et al. Safety and Immunogenicity of Omicron BA.4/BA.5 Bivalent Vaccine Against Covid-19. Published online December 13, 2022. [CrossRef]
- Kurhade C, Zou J, Xia H, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. Published online December 6, 2022:1-4. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).