Submitted:
06 March 2023
Posted:
07 March 2023
You are already at the latest version
Abstract
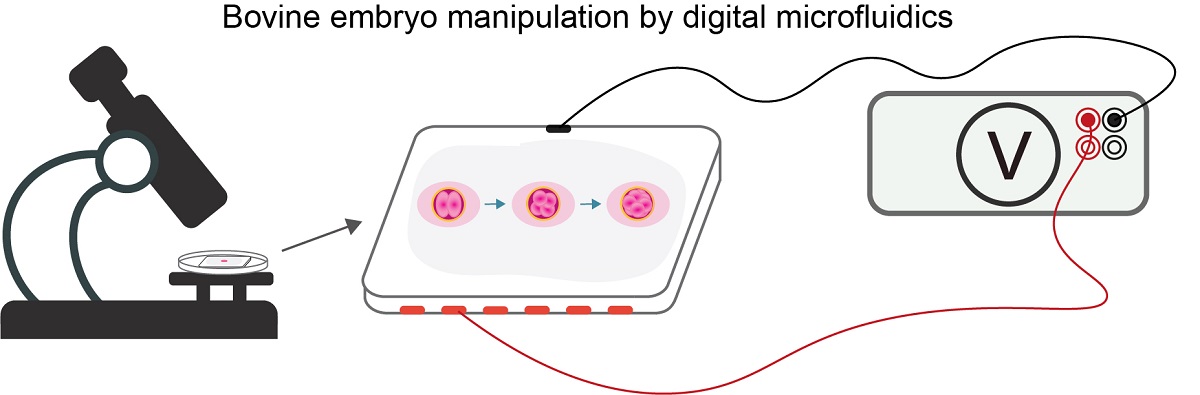
Keywords:
1. Introduction
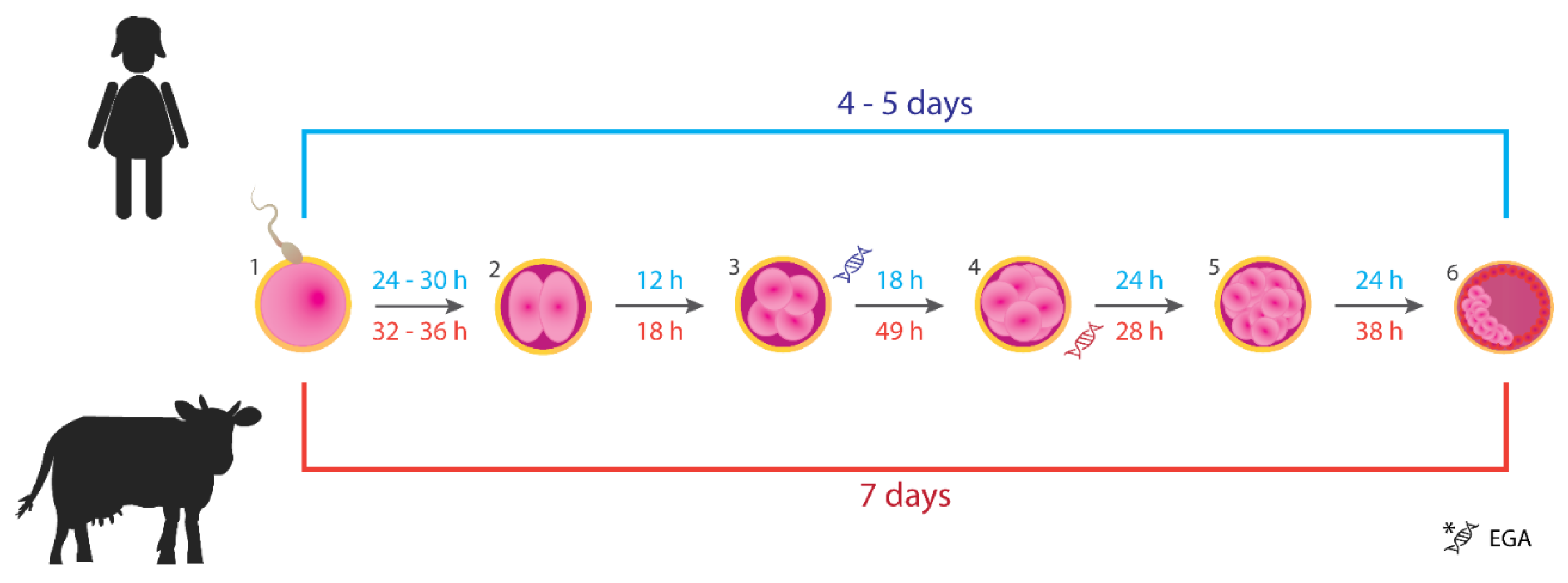
2. Materials and Methods
2.1. Materials and chemicals
2.1.1. Microdevice fabrication
2.1.2. In vitro production of bovine embryos
2.2. Methods
2.2.1. Fabrication of EWOD chips
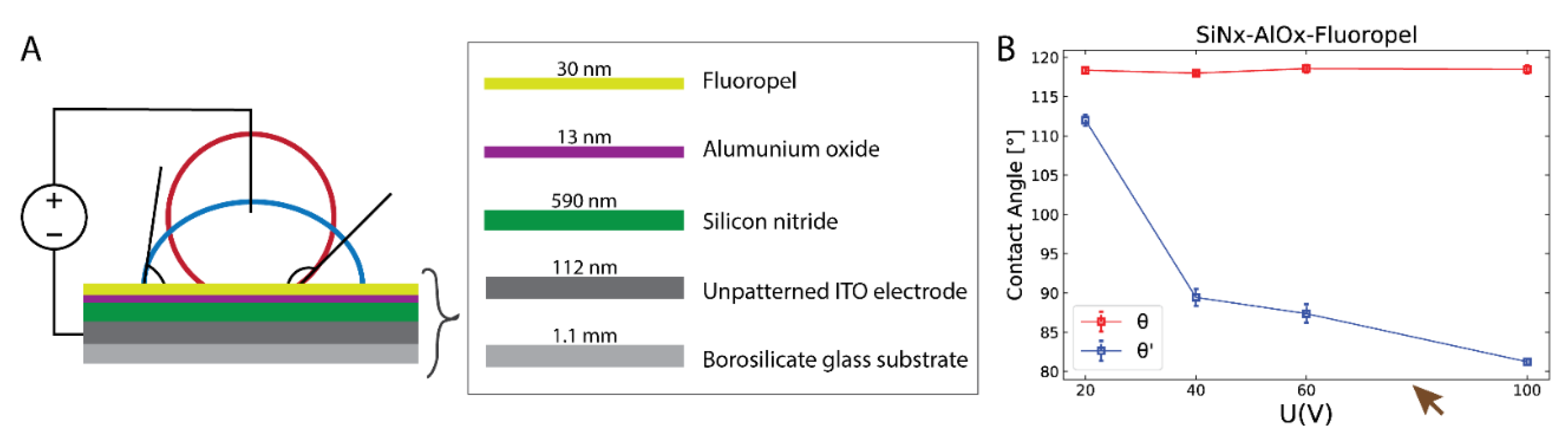
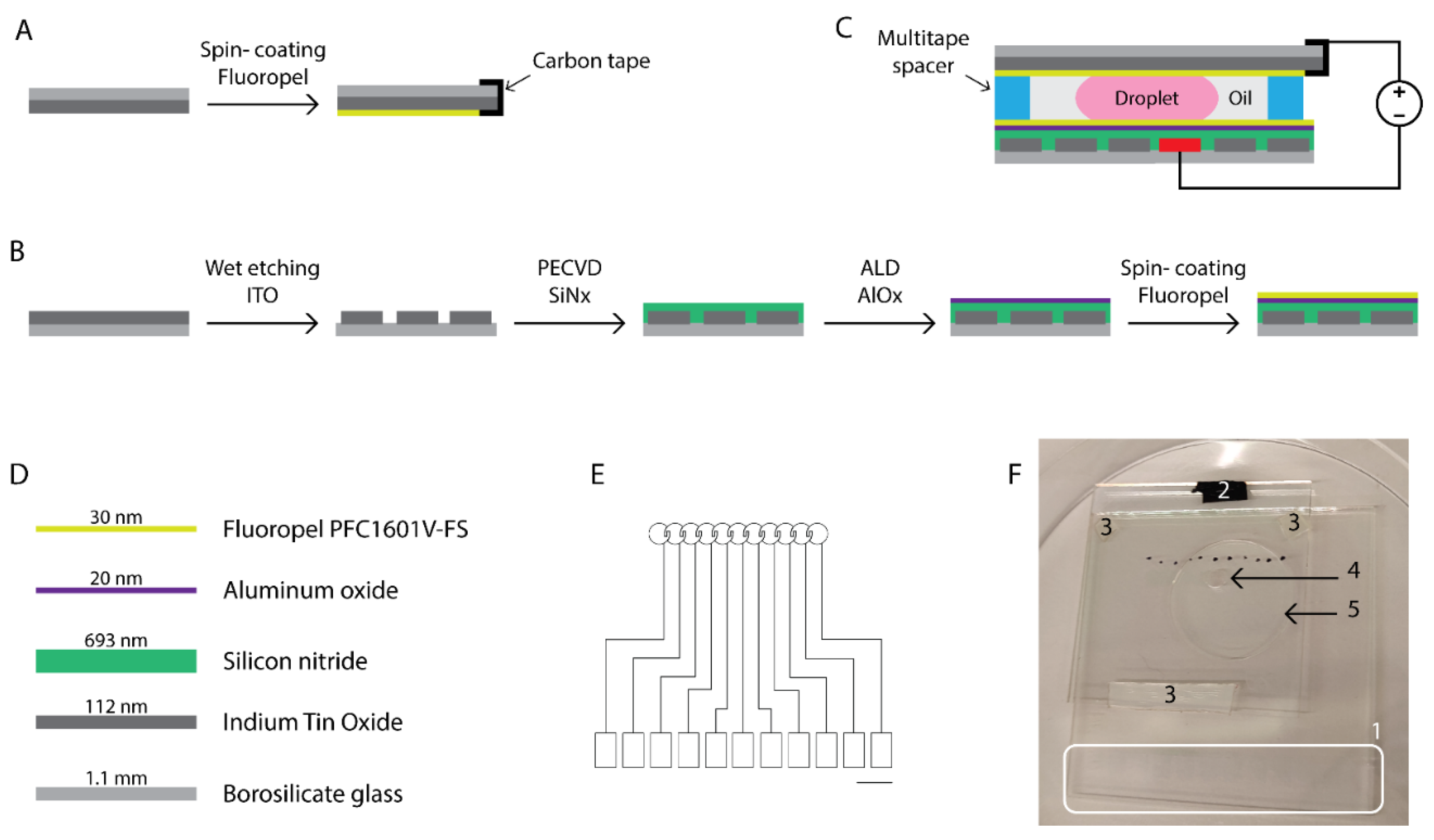
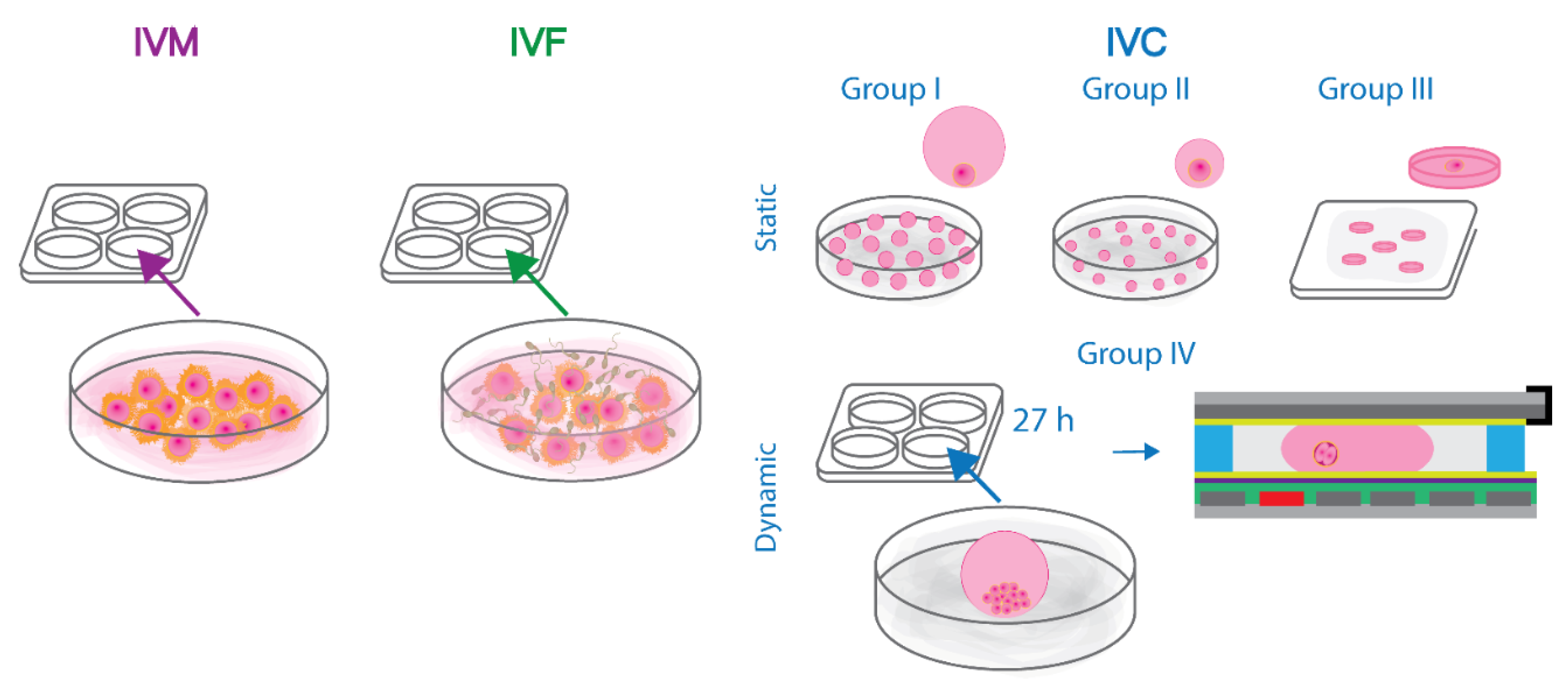
2.2.2. In vitro production of bovine embryos
3. Results
3.1. Development of EWOD microdevices with multilayered insulators
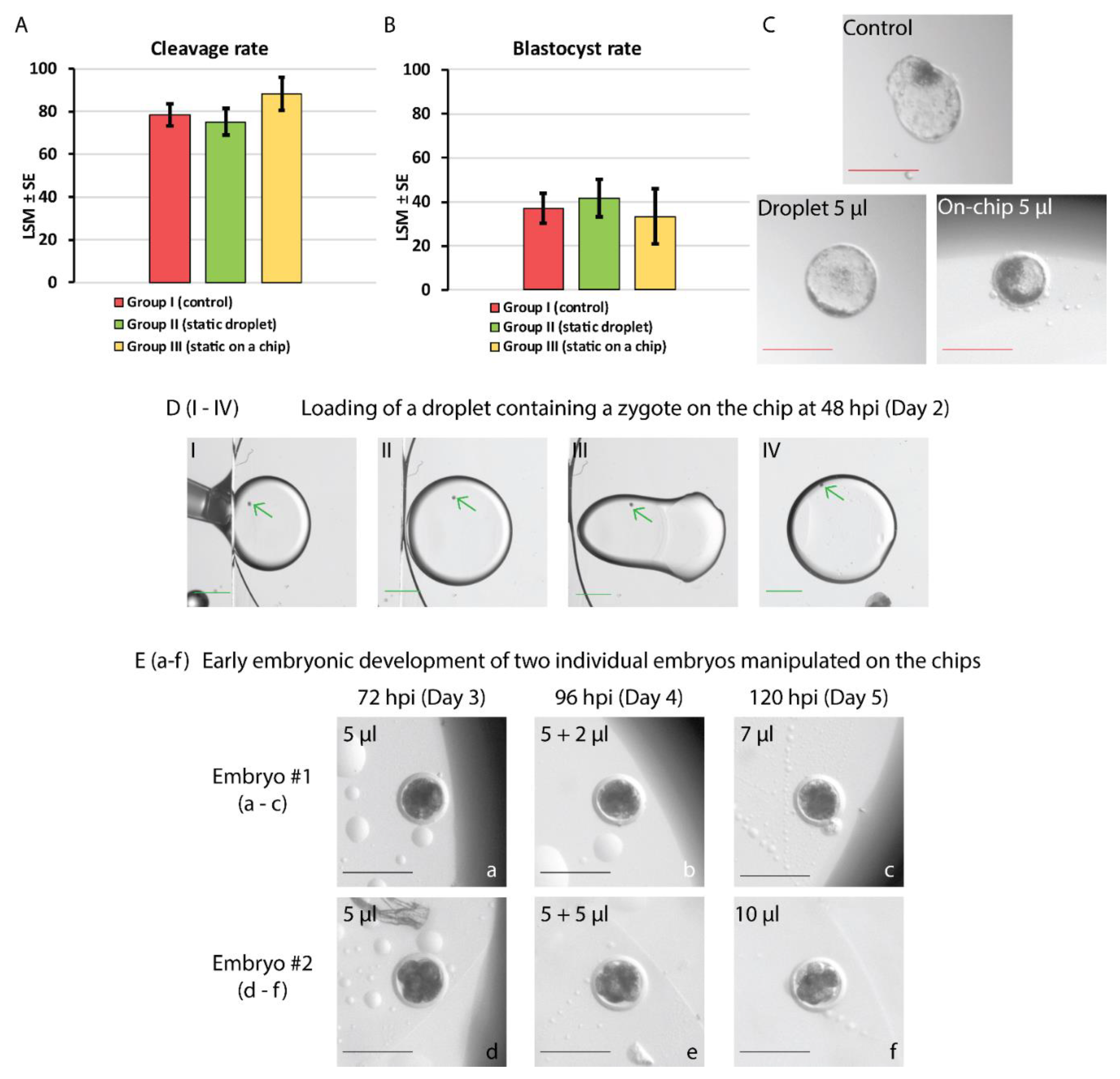
3.2. In vitro development of bovine embryos: static culture and dynamic manipulation on the developed chips
4. Discussion
4.1. Development of EWOD chips using multilayered dielectric approach
4.2. Culture medium modification with Tween 80
4.3. In vitro culture and on-chip manipulation of bovine embryos
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the Globe: New Thinking on Gender, Reproductive Technologies and Global Movements in the 21st Century. Hum. Reprod. Update 2014, 21, 411–426. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A. Genetics of Male Infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Yatsenko, S.A.; Rajkovic, A. Genetics of Human Female Infertility. Biol. Reprod. 2019, 101, 549–566. [Google Scholar] [CrossRef]
- Thurston, L.; Abbara, A.; Dhillo, W.S. Investigation and Management of Subfertility. J. Clin. Pathol. 2019, 72, 579–587. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gelbaya, T. , Potdar, N., Jeve, Y., N.L. CME Review Article. Pediatr. Emerg. Care 2017, 33, 792–793. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male Infertility as a Window to Health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Johnstone, E.; Dorais, J.; Silver, B.; Peterson, C.M.; Hotaling, J. Female Infertility, Infertility-Associated Diagnoses, and Comorbidities: A Review. J. Assist. Reprod. Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef]
- Rooney, K.L.; Domar, A.D. The Relationship between Stress and Infertility. Dialogues Clin. Neurosci. 2018, 20, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fact sheet on ART. https://www.eshre.eu/Europe/Factsheets-and-infographics (accessed 2022-01-12).
- Biggers, J.D. IVF and Embryo Transfer: Historical Origin and Development. Reprod. Biomed. Online 2012, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Speyer, B.; O’Neill, H.; Saab, W.; Seshadri, S.; Cawood, S.; Heath, C.; Gaunt, M.; Serhal, P. In Assisted Reproduction by IVF or ICSI, the Rate at Which Embryos Develop to the Blastocyst Stage Is Influenced by the Fertilization Method Used: A Split IVF/ICSI Study. J. Assist. Reprod. Genet. 2019, 36, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Schulte, K.; Ehmcke, J.; Schlatt, S.; Boiani, M.; Nordhoff, V. Lower Total Cell Numbers in Mouse Preimplantation Embryos Cultured in Human Assisted Reproductive Technique (ART) Media Are Not Induced by Apoptosis. Theriogenology 2015, 84, 1620–1630. [Google Scholar] [CrossRef]
- Isobe, T.; Ikebata, Y.; Do, L.T.K.; Tanihara, F.; Taniguchi, M.; Otoi, T. In Vitro Development of OPU-derived Bovine Embryos Cultured Either Individually or.Pdf. 2015, pp 661–665. [CrossRef]
- Lacham-Kaplan, O.; Trounson, A. Reduced Developmental Competence of Immature, in-Vitro Matured and Postovulatory Aged Mouse Oocytes Following IVF and ICSI. Reprod. Biol. Endocrinol. 2008, 6, 1–13. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent Advances in Bovine in Vitro Embryo Production: Reproductive Biotechnology History and Methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Rossant, J.; Tam, P.P.L. Exploring Early Human Embryo Development. Science 2018, 360, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Cevik, M. Correlation between the Cryosurvival, Cell Number and Diameter in Bovine in Vitro Produced Embryos. Cryobiology 2016, 73, 203–208. [Google Scholar] [CrossRef]
- Richter, K.S.; Harris, D.C.; Daneshmand, S.T.; Shapiro, B.S. Quantitative Grading of a Human Blastocyst: Optimal Inner Cell Mass Size and Shape. Fertil. Steril. 2001, 76, 1157–1167. [Google Scholar] [CrossRef]
- An, L.; Liu, Y.; Li, M.; Liu, Z.; Wang, Z.; Dai, Y.; Presicce, G.A.; Du, F. Site Specificity of Blastocyst Hatching Significantly Influences Pregnancy Outcomes in Mice. FASEB J. 2021, 35, 1–15. [Google Scholar] [CrossRef]
- Svoboda, P. Seminars in Cell & Developmental Biology Mammalian Zygotic Genome Activation. Semin. Cell Dev. Biol. 2018, 84, 118–126. [Google Scholar]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A.R. Human Pre-Implantation Embryo Development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J. Usefulness of Bovine and Porcine IVM/IVF Models for Reproductive Toxicology. Reprod. Biol. Endocrinol. 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asami, M.; Lam, B.Y.H.; Ma, M.K.; Rainbow, K.; Braun, S.; VerMilyea, M.D.; Yeo, G.S.H.; Perry, A.C.F. Human Embryonic Genome Activation Initiates at the One-Cell Stage. Cell Stem Cell 2022, 29, 209–216. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. The ART of Studying Early Embryo Development: Progress and Challenges in Ruminant Embryo Culture. Theriogenology 2014, 81, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.A. 40 Years of Bovine IVF in the New Genomic Selection Context. Reproduction 2018, 156, R1–R7. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board Invited Review: Post-Transfer Consequences of in Vitro-Produced Embryos in Cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Booth, P.J.; Callesen, H. Kinetics of Early in Vitro Development of Bovine in Vivo- and in Vitro-Derived Zygotes Produced and/or Cultured in Chemically Defined or Serum-Containing Media. Reproduction 2002, 123, 553–565. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gimeno, I.; Cañón-Beltrán, K.; Gutiérrez-Adán, A.; Rizos, D.; Gómez, E. Senescence and Apoptosis During in Vitro Embryo Development in a Bovine Model. Front. Cell Dev. Biol. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Heras, S.; De Sutter, P.; Deforce, D.; Peelman, L.; De Schauwer, C.; Van Soom, A. Autocrine Embryotropins Revisited: How Do Embryos Communicate with Each Other in Vitro When Cultured in Groups? Biol. Rev. 2017, 92, 505–520. [Google Scholar] [CrossRef]
- Asaadi, A.; Dolatabad, N.A.; Atashi, H.; Raes, A.; Damme, P. Van; Hoelker, M.; Hendrix, A.; Pascottini, O.B.; Soom, A. Van; Kafi, M.; et al. Extracellular Vesicles from Follicular and Ampullary Fluid Isolated by Density Gradient Ultracentrifugation Improve Bovine Embryo Development and Quality. Int. J. Mol. Sci. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the Oviduct in Mammalian Fertilization. Reproduction 2012, 144, 649–660. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics toward a Lab-on-a-Chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Ahmadkhani, N.; Hosseini, M.; Saadatmand, M.; Abbaspourrad, A. The Influence of the Female Reproductive Tract and Sperm Features on the Design of Microfluidic Sperm-Sorting Devices. J. Assist. Reprod. Genet. 2022, 39, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, S.; Nordhoff, V. Microfluidics for Mammalian Embryo Culture and Selection: Where Do We Stand Now? Mol. Hum. Reprod. 2017, 23, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Shuchat, S.; Yossifon, G.; Huleihel, M. Perfusion in Organ-on-Chip Models and Its Applicability to the Replication of Spermatogenesis In Vitro. Int. J. Mol. Sci. 2022, 23, 1–24. [Google Scholar] [CrossRef]
- Alias, A.; Huang, H.; Yao, D.-J. A Review on Microfluidics : An Aid to Assisted. Molecules 2021, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Xiao, Z.; Wang, Y.; Wang, H. Human Embryonic Development: From Peri-Implantation to Gastrulation. Trends Cell Biol. 2022, 32, 18–29. [Google Scholar] [CrossRef] [PubMed]
- el Azhar, Y.; Sonnen, K.F. Development in a Dish—In Vitro Models of Mammalian Embryonic Development. Front. Cell Dev. Biol. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karcz, A.; Van Soom, A.; Smits, K.; Verplancke, R.; Van Vlierberghe, S.; Vanfleteren, J. Electrically-Driven Handling of Gametes and Embryos: Taking a Step towards the Future of ARTs. Lab on a Chip. Royal Society of Chemistry. 2022, pp 1852–1875. [CrossRef]
- Kothamachu, V.B.; Zaini, S.; Muffatto, F. Role of Digital Microfluidics in Enabling Access to Laboratory Automation and Making Biology Programmable. SLAS Technol. 2020, 25, 411–426. [Google Scholar] [CrossRef]
- Alistar, M. Mobile Microfluidics. Bioengineering 2019, 6, 1–19. [Google Scholar] [CrossRef]
- Suman Chakraborty. Electrocapillary. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2008; pp. 460–469. [Google Scholar]
- Lin, Y.Y.; Welch, E.R.F.; Fair, R.B. Low Voltage Picoliter Droplet Manipulation Utilizing Electrowetting-on- Dielectric Platforms. Sensors Actuators, B Chem. 2012, 173, 338–345. [Google Scholar] [CrossRef]
- Nikapitiya, N.Y.J.B.; Nahar, M.M.; Moon, H. Accurate, Consistent, and Fast Droplet Splitting and Dispensing in Electrowetting on Dielectric Digital Microfluidics. Micro Nano Syst. Lett. 2017, 5. [Google Scholar] [CrossRef]
- Nahar, M.M.; Nikapitiya, J.B.; You, S.M.; Moon, H. Droplet Velocity in an Electrowetting on Dielectric Digital Microfluidic Device. Micromachines 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Wang, Y. Fundamentals and Applications of Electrowetting: A Critical Review. Rev. Adhes. Adhes. 2013, 1, 114–174. [Google Scholar] [CrossRef]
- Choi, K.; Ng, A.H.C.; Fobel, R.; Wheeler, A.R. Digital Microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A Review of Digital Microfluidics as Portable Platforms for Lab-on a-Chip Applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, N.T. Magnetic Digital Microfluidics - a Review. Lab Chip 2017, 17, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, H.; Li, W.; Li, M. Recent Advances in Magnetic Digital Microfluidic Platforms. Electrophoresis 2021, 42, 2329–2346. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Song, S.; Wang, W.; Zhou, J. Applications of Electrowetting-on-Dielectric (EWOD) Technology for Droplet Digital PCR. Biomicrofluidics 2020, 14, 1–17. [Google Scholar] [CrossRef]
- Barman, S.R.; Khan, I.; Chatterjee, S.; Saha, S.; Choi, D.; Lee, S.; Lin, Z.H. Electrowetting-on-Dielectric (EWOD): Current Perspectives and Applications in Ensuring Food Safety. J. Food Drug Anal. 2020, 595–621. [Google Scholar] [CrossRef]
- Lee, M.S.; Hsu, W.; Huang, H.Y.; Tseng, H.Y.; Lee, C.T.; Hsu, C.Y.; Shieh, Y.C.; Wang, S.H.; Yao, D.J.; Liu, C.H. Simultaneous Detection of Two Growth Factors from Human Single-Embryo Culture Medium by a Bead-Based Digital Microfluidic Chip. Biosens. Bioelectron. 2020, 150, 111851. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Shen, H.H.; Tien, C.H.; Li, C.J.; Fan, S.K.; Liu, C.H.; Hsu, W.S.; Yao, D.J. Digital Microfluidic Dynamic Culture of Mammalian Embryos on an Electrowetting on Dielectric (EWOD) Chip. PLoS One 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Karcz, A.A.; Schaubroeck, D.; Verplancke, R.; Van Soom, A.; Vanfleteren, J. Introduction of a High Quality Nanofilm of Aluminum Oxide Enhances the Performance of Ewod Microfluidic Platforms. MicroTAS 2021 - 25th Int. Conf. Miniaturized Syst. Chem. Life Sci. 2021; 1395–1396. [Google Scholar]
- Wydooghe, E.; Vaele, L.; Piepers, S.; Dewulf, J.; Van Abbeel, E. Den; De Sutter, P.; Van Soom, A. Individual Commitment to a Group Effect: Strengths and Weaknesses of Bovine Embryo Group Culture. Reproduction 2014, 148, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Yota, J.; Shen, H.; Ramanathan, R. Characterization of Atomic Layer Deposition HfO2, Al2O3, and Plasma-Enhanced Chemical Vapor Deposition Si 3 N 4 as Metal–Insulator–Metal Capacitor Dielectric for GaAs HBT Technology. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2013, 31, 01A134. [Google Scholar] [CrossRef]
- Robertson, J. High Dielectric Constant Oxides. Eur. Phys. JournalApplied Phys. 2004, 28, 265–291. [Google Scholar] [CrossRef]
- Https://Cytonix.Com/Products/1601v. Page Accessed on 21.10.2022.
- Schultz, A.; Chevalliot, S.; Kuiper, S.; Heikenfeld, J. Detailed Analysis of Defect Reduction in Electrowetting Dielectrics through a Two-Layer “barrier” Approach. Thin Solid Films 2013, 534, 348–355. [Google Scholar] [CrossRef]
- Gisby, T.A.; Xie, S.Q.; Calius, E.P.; Anderson, I.A. Leakage Current as a Predictor of Failure in Dielectric Elastomer Actuators. Electroact. Polym. Actuators Devices 2010, 7642, 764213. [Google Scholar] [CrossRef]
- Popova, E.; Bader, M.; Krivokharchenko, A. Effects of Electric Field on Early Preimplantation Development in Vitro in Mice and Rats. Hum. Reprod. 2011, 26, 662–670. [Google Scholar] [CrossRef]
- Cross, M.H.; Cross, P.C.; Brinster, R.L. Changes in Membrane Potential during Mouse Egg Development. Dev. Biol. 1973, 416, 412–416. [Google Scholar] [CrossRef]
- Funk, R.H.W. Endogenous Electric Fields as Guiding Cue for Cell Migration. Front. Physiol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Tai, G.; Tai, M.; Zhao, M. Electrically Stimulated Cell Migration and Its Contribution to Wound Healing. Burn. Trauma 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous Electric Fields in Embryos during Development, Regeneration and Wound Healing. Radiat. Prot. Dosimetry 2003, 106, 375–383. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. Endogenous Electrical Currents and Voltage Gradients in Xenopus Embryos and Consequences of Their Disruption. Dev. Biol. 1994, No. 166, 789–800. [Google Scholar] [CrossRef]
- Koo, J.; Lee, J.; Kim, S.; Kim, Y. Do; Jeon, H.; Kim, D.S.; Kim, Y. Characteristics of Hafnium-Aluminum-Oxide Thin Films Deposited by Using Atomic Layer Deposition with Various Aluminum Compositions. J. Korean Phys. Soc. 2005, 47, 501–507. [Google Scholar]
- Ylivaara, O.M.E.; Liu, X.; Kilpi, L.; Lyytinen, J.; Schneider, D.; Laitinen, M.; Julin, J.; Ali, S.; Sintonen, S.; Berdova, M.; et al. Aluminum Oxide from Trimethylaluminum and Water by Atomic Layer Deposition: The Temperature Dependence of Residual Stress, Elastic Modulus, Hardness and Adhesion. Thin Solid Films 2014, 552, 124–135. [Google Scholar] [CrossRef]
- Wydooghe, E.; Heras, S.; Dewulf, J.; Piepers, S.; Van Den Abbeel, E.; De Sutter, P.; Vandaele, L.; Van Soom, A. Replacing Serum in Culture Medium with Albumin and Insulin, Transferrin and Selenium Is the Key to Successful Bovine Embryo Development in Individual Culture. Reprod. Fertil. Dev. 2014, 26, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L. Albumin and Mammalian Cell Culture: Implications for Biotechnology Applications. Cytotechnology 2010, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and Qualitative Evaluation of Adsorption/Desorption of Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces. Langmuir 2009, 25, 11614–11620. [Google Scholar] [CrossRef] [PubMed]
- Luk, V.N.; Mo, G.C.; Wheeler, A.R. Pluronic Additives: A Solution to Sticky Problems in Digital Microfluidics. Langmuir 2008, 24, 6382–6389. [Google Scholar] [CrossRef]
- Zadymova, N.M.; Yampol’skaya, G.P.; Filatova, L.Y. Interaction of Bovine Serum Albumin with Nonionic Surfactant Tween 80 in Aqueous Solutions: Complexation and Association. Colloid J. 2006, 68, 162–172. [Google Scholar] [CrossRef]
- Ruiz-Peña, M.; Oropesa-Nuñez, R.; Pons, T.; Louro, S.R.W.; Pérez-Gramatges, A. Physico-Chemical Studies of Molecular Interactions between Non-Ionic Surfactants and Bovine Serum Albumin. Colloids Surfaces B Biointerfaces 2010, 75, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Magnero, K.H.; Valiente, P.A.; Ruiz-Peña, M.; Pérez-Gramatges, A.; Pons, T. Unraveling the Binding Mechanism of Polyoxyethylene Sorbitan Esters with Bovine Serum Albumin: A Novel Theoretical Model Based on Molecular Dynamic Simulations. Colloids Surfaces B Biointerfaces 2014, 116, 720–726. [Google Scholar] [CrossRef]
- Baltz, J.M.; Tartia, A.P. Cell Volume Regulation in Oocytes and Early Embryos: Connecting Physiology to Successful Culture Media. Hum. Reprod. Update 2009, 16, 166–176. [Google Scholar] [CrossRef]
- Gardner, D.K.; Hamilton, R.; McCallie, B.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Human and Mouse Embryonic Development, Metabolism and Gene Expression Are Altered by an Ammonium Gradient in Vitro. Reproduction 2013, 146, 49–61. [Google Scholar] [CrossRef]
- Hammon, D.S.; Wang, S.; Holyoak, G.R. Effects of Ammonia during Different Stages of Culture on Development of in Vitro Produced Bovine Embryos. Anim. Reprod. Sci. 2000, 59, 23–30. [Google Scholar] [CrossRef]
- Gardner, D.K.; Kelley, R.L. Impact of the IVF Laboratory Environment on Human Preimplantation Embryo Phenotype. J. Dev. Orig. Health Dis. 2017, 8, 418–435. [Google Scholar] [CrossRef]
- Lopes, A.S.; Lane, M.; Thompson, J.G. Oxygen Consumption and ROS Production Are Increased at the Time of Fertilization and Cell Cleavage in Bovine Zygotes. Hum. Reprod. 2010, 25, 2762–2773. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L. Oxidative Stress in Oocytes and Embryo Development: Implications for in Vitro Systems. Antioxidants Redox Signal. 2021, 34, 1394–1406. [Google Scholar] [CrossRef]
- Nagao, Y.; Iijima, R.; Saeki, K. Interaction between Embryos and Culture Conditions during in Vitro Development of Bovine Early Embryos. Zygote 2008, 16, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ottosen, L.D.M.; Hindkjær, J.; Ingerslev, J. Light Exposure of the Ovum and Preimplantation Embryo during ART Procedures. J. Assist. Reprod. Genet. 2007, 24, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, K.O.; Reed, M.L. The Effect of Light on Embryos and Embryo Culture. J. Reprod. Stem Cell Biotechnol. 2012, 3, 46–54. [Google Scholar] [CrossRef]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the Culture Environment and Embryo Manipulation to Help Maintain Embryo Developmental Potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Fesenko, I.; Christensen, D.R.; Parry, K.L.; Lowen, P.; Wellstead, S.J.; Harris, S.F.; Calder, P.C.; Macklon, N.S.; Houghton, F.D. Incubator Type Affects Human Blastocyst Formation and Embryo Metabolism : A Randomized Controlled Trial. 2022, 0, 1–11.
- Xiong, L.; Chen, P.; Zhou, Q. Adhesion Promotion between PDMS and Glass by Oxygen Plasma Pre-Treatment. J. Adhes. Sci. Technol. 2014, 28, 1046–1054. [Google Scholar] [CrossRef]
| Coating | Material | Thickness | Dielectric constant εr* | |
|---|---|---|---|---|
| Open platform (Figure 2) | Chip for embryo manipulation (Figure 3) | |||
| Dielectric 1 | Silicon nitride | 590 nm | 693 nm | 6-8 [59] |
| Dielectric 2 | Aluminum oxide | 13 nm | 20 nm | 9 [60] |
| Hydrophobic** | Fluoropel PFC1601V-FS | 30 nm | 30 nm | 1.9 [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





