1. Introduction
Clinical diagnosis and management of heart failure (HF) are currently linked to ejection fraction (EF). HF with preserved ejection fraction (HFpEF) is now considered a distinct disease from HF with reduced ejection fraction (HFrEF). Although the morbidity and mortality rates of HFpEF and HFrEF are comparable, therapeutic options for HFpEF patients remain elusive. HFpEF was initially thought to be due to diastolic dysfunction but now recognized as a complex interplay of impairments in cardiac reserve, as well as altered renal metabolism, vascular, pulmonary, and renal nerve activation [
1,
2,
3,
4]. Thus, there is a genuine interest in studying the association of HFpEF with extra-cardiac features, including disorders of renal feedback and associated metabolic regulation. Currently, there is no effective cure or therapy for HFpEF. Hence, the incidence and prevalence of HFpEF continue to increase [
2,
5,
6,
7,
8,
9,
10]. In that very context, finding potential novel targets is an important unmet need.
The reciprocal contribution by other organs to redistribute blood volume has been observed during myocardial infarction during hind-limb remote ischemic condition [
11]. The contribution of skeletal muscle myokines, sympathetic inactivation, bone marrow induction along with exercise and muscle myokine/musclin HF has been suggested; however, the reciprocal contribution in HFpEF to HFpEF is unclear [
12,
13,
14,
15,
16]. Our recent study shows that the remote hind-limb ischemia releases exosome containing musclin that contributes to the HFpEF [
17]. Joslin et al. have shown interrelation between heart failure with preserved ejection fraction and renal impairment [
18]. Renal denervation has been shown to be cardioprotective [
19,
20,
21]. Angiopoietins release from kidney play protective role in heart failure [
22]. Also, the congenital heart disease in adults is associated with autosomal dominant polycystic kidney disease [
23]. The mitochondrial implication as potential mediator of systemic inflammation, and organ crosstalk in the causation of acute kidney injury has been suggested [
24]. Although during volume overload the kidneys sense the channels to release extracellular ATP, and thus compensate the systemic organs [
25]. However, it is unclear whether volume overload activates the renal sympathetic nerve, helps excrete the exosomes containing erythropoietin (EPO), and increase the regeneration capacity of the heart. Recently, Marban and colleagues have shown that cell therapy preserves ejection fraction (EF) via the vascular endothelium [
26]. Here we show that renal denervation preserves ejection fraction via endocardial endothelium. Erythropoietin is released from kidneys that in turn induces bone marrow parenchyma in releasing the regenerating cell population [
27,
28]. Therefore, it is novel to propose that increased EPO release via the exosomes enhances endothelial nitric oxide synthase (eNOS), and cardiac regeneration by anti-apoptotic effects via caspases.
2. Materials and Methods
2.1. Creation of the Aorta Vena Cava Fistula (AVF) in a Mouse Model
To create chronic/congestive (cardio-pulmonary) heart failure (CHF) in mice, aorta vena cava fistula (AVF) below the kidneys was performed as narrated earlier between the aorta and caudal vena cava approximately 0.5 cm below the kidneys using a 30-gauge sterile needle under aseptic surgical condition [
29,
30,
31,
32]. The sham surgery was used as control in a separate group of mice. Why AVF model of HFpEF to HFrEF works better? As mentioned earlier there are basically four ways to create the experimental CHF procedure
(vide supra). By creating fistula, the red blood enters the vena cava without an injury to the heart. In fact, this model mimics the real CHF phenotype such as the aging process wherein we age, and our preload keeps increasing than what our heart can help pump out because of the weakened cardiomyocytes in the aging heart. This technique of creating the AVF also involves right ventricle to lung to left ventricle, thus enforcing the congestive heart failure (CHF) phenotype. After the AVF process, the heart transitions from the HFpEF to HFrEF overtime. Again, our lab is one of the original labs that is instrumental in creating and perfecting the AVF procedure. To determine the protective role of renal nerve denervation (RDN), the RDN procedure was performed in one group of the AVF mice, and in a sham mice group side by side. The mice were housed in the core animal care facility at the University of Louisville School of Medicine. To study the role(s) of renal nerve in volume sensing, experiments were carried out in renal “in-nerve and de-nerve” animals. The denervation procedure was performed by surgically removing all the nerves ending around renal artery and subsequently treated with phenol reagent, as described earlier [
20,
21,
33]. These are novel experiments because the cardiac vascular reactivity in volume overload denervated condition is not studied in detail before.
2.2. Serial and Longitudinal Echocardiograms (ECHOs) in Mice
To determine HFpEF and HFrEF phenotypes, serial and longitudinal ECHOs in mice at 1-6 weeks (HFpEF), and 12-16 weeks (HFrEF) were carried out. The concentric cardiac hypertrophy, a marker of HFpEF, was assessed via the long-axis ECHO as described earlier [
32]. After the mice were sacrificed, the concentric hypertrophy was assessed by histology as we have described previously [
34]. CHF precedes chronic volume overload; therefore, aorta-vena cava fistula (AVF) procedure was created below the kidney in the male wild type (WT, C57BL/6J) mice with and without renal denervation (RDN) intervention. To differentiate the HFpEF vs HFrEF, serial/longitudinal ECHOs were performed to determine the ejection fraction (EF) from 1-6 weeks after the AVF procedure by Vevo 2100 echocardiography equipment. The EF > 50% was denotes as HFpEF, and the EF < 50% was taken as HFrEF. The changes in the left ventricle (LV) filling volume, and the early diastolic velocity were measured. A pulse-wave pressure of >40 mmHg was considered as the chronic congestive cardiopulmonary heart failure (CHF) phenotype.
2.3. Measurement of Renal and Blood Exosomal Levels of EPO
In addition, to focus on the exosomes containing the erythropoietin (EPO) regulated vascular density and cardiac function in HFpEF versus HFrEF, the role(s) of the circulating exosomal EPO in the context of the expression levels of molecules such as eNOS, ACE1, ACE2, SGLT1/2 and nephrin were assessed. To carry out the assessment, the renal and cardiac EPO, eNOS, Wnt1 and β-catenin and respective levels of the renal (cortical), and medullary expression amounts of the caspases were also studied and quantified in sham, and AVF at 4-6 weeks following the AVF procedure. Circulating exosomes containing the EPO were analyzed by Western blotting (WB) experiments. The fibrosis was assessed by trichrome (collagen) and van-Giessen (elastin) staining procedure. A quantitative collagen estimation was carried out biochemically by the help of hydroxy proline measurements. Because glomerular function rate (GFR) elicits overall function, reactivity to acetylcholine (ACH) was measured via the
ex-vivo preparation as described earlier [
35].
2.4. Statistical Analysis
Data obtained from the same samples were used to express results in terms of percentage change(s) relative to the controls (prior to treatment/procedure). Experiments using commercial biochemical assays were performed in triplicate. Statistical significance was determined by student’s
t-test for two groups, or 2-way ANOVA (analysis of variance) was used to compare the respective data with wild type, with RDN or without RDN according to the Bonferroni correction [
36]. The differences were considered statistically significant at p<0.05.
3. Results
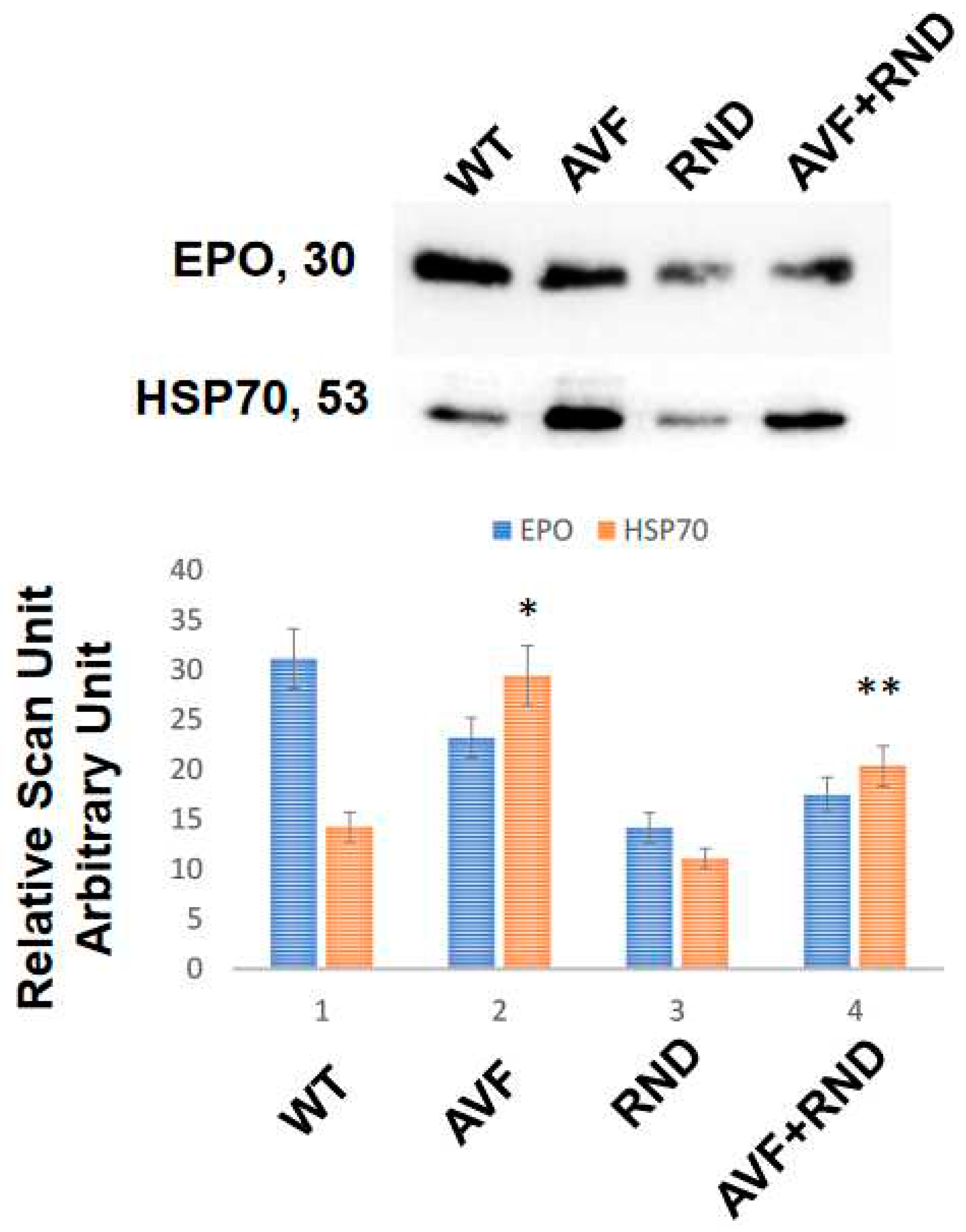
To determine whether the chronic volume overload causes increase in exosomes’ release in the blood of C57BL/6J experimental mice, we created AVF and at 16 weeks measured the blood levels of exosomes by marking heat shock protein 70 (HSP70). The results revealed an increase in Exosomal amount in AVF mice group compared to WT mice. To mitigate AVF induced volume receptors we created renal denervation (RDN). The results suggested that RDN has tendency to increase exosomes in AVF mice compared with that of the RDN mice alone, but it was lower than the AVF group. Similarly, the levels of erythropoietin (EPO) were lower in the exosomes released during RDN intervention. Interestingly, the levels of exosomes secreted in AVF was significantly higher than the WT mice group. These results suggested that volume stress does cause an increase in the overall exosomes released independently to that of the renal denervation (Figure 1).
Figure 1.
Blood levels of circulating exosomes in the C57BL/6J (WT-controls), AVF, RND and AVF-RND male mice at 16 weeks. The upper gel panel represents levels of erythropoietin (EPO), and lower panel depicts exosomal marker heat shock protein 70 (HSP70). The bar graph represents mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 1.
Blood levels of circulating exosomes in the C57BL/6J (WT-controls), AVF, RND and AVF-RND male mice at 16 weeks. The upper gel panel represents levels of erythropoietin (EPO), and lower panel depicts exosomal marker heat shock protein 70 (HSP70). The bar graph represents mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
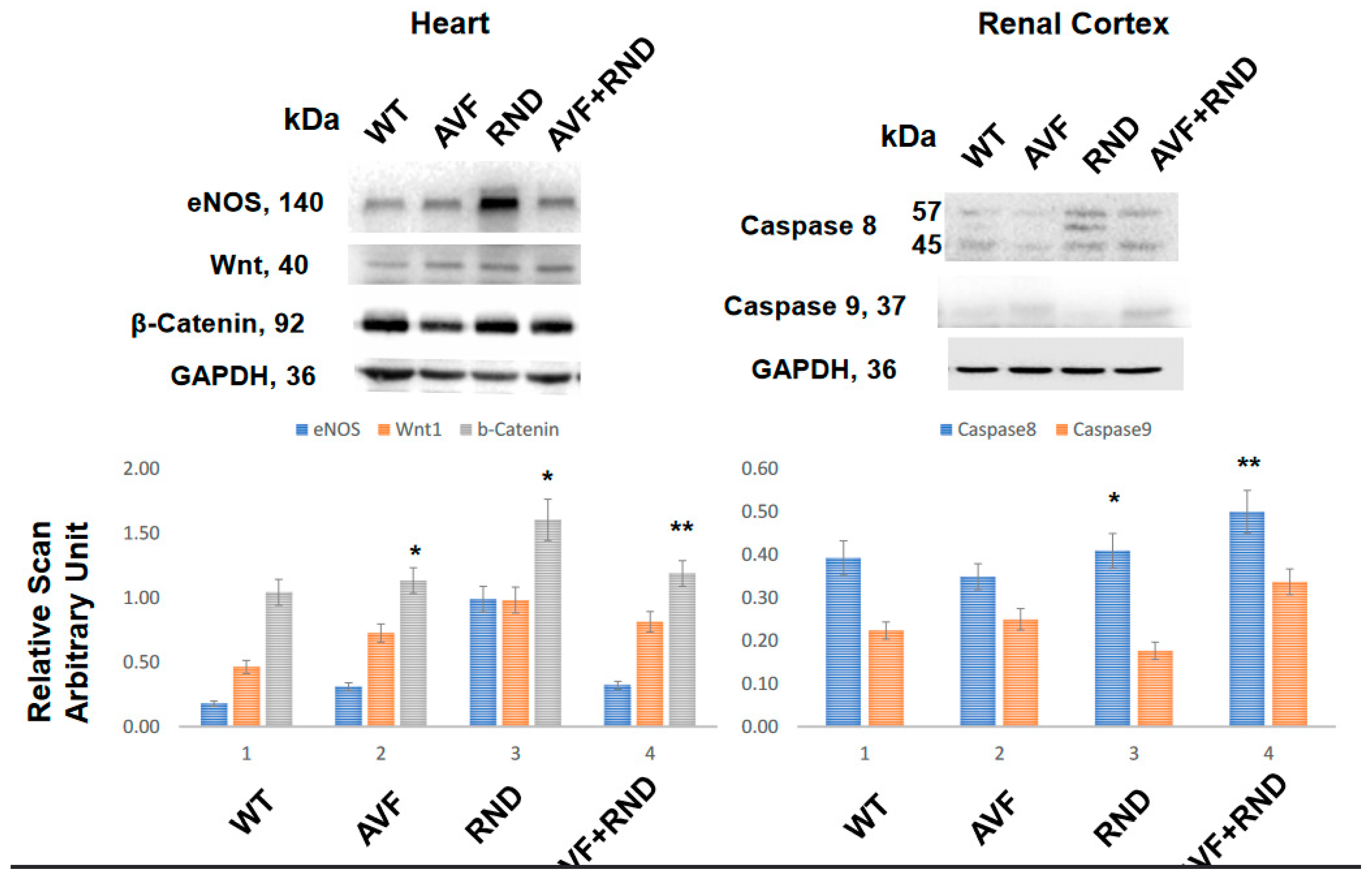
To determine whether the AVF suppresses the regeneration and RDN procedure improves cardiac regeneration, we measured the levels of Wnt1 and β-catenin. The results demonstrated the increase in Wnt1 and β-catenin expression post RDN procedure in the mice, thus suggesting an improvement of cardiac regeneration by RDN during HFrEF to maintain HFpEF phenotype (
Figure 2). Interestingly, the expression levels eNOS was found to be robust in the renal denervation mice group thus indicating the involvement in the cardiac endothelial function by RDN during HFrEF (
Figure 2). To determine whether RDN causes renal apoptosis and intracellular renal remodeling, we measured the levels of caspase 8 and caspase 9. The results suggested increase in caspase 8 levels in RDN and AVF with RDN mice groups. These findings revealed that renal denervation causes renal intracellular remodeling and thereby improves cardiac function during HFrEF (
Figure 2).
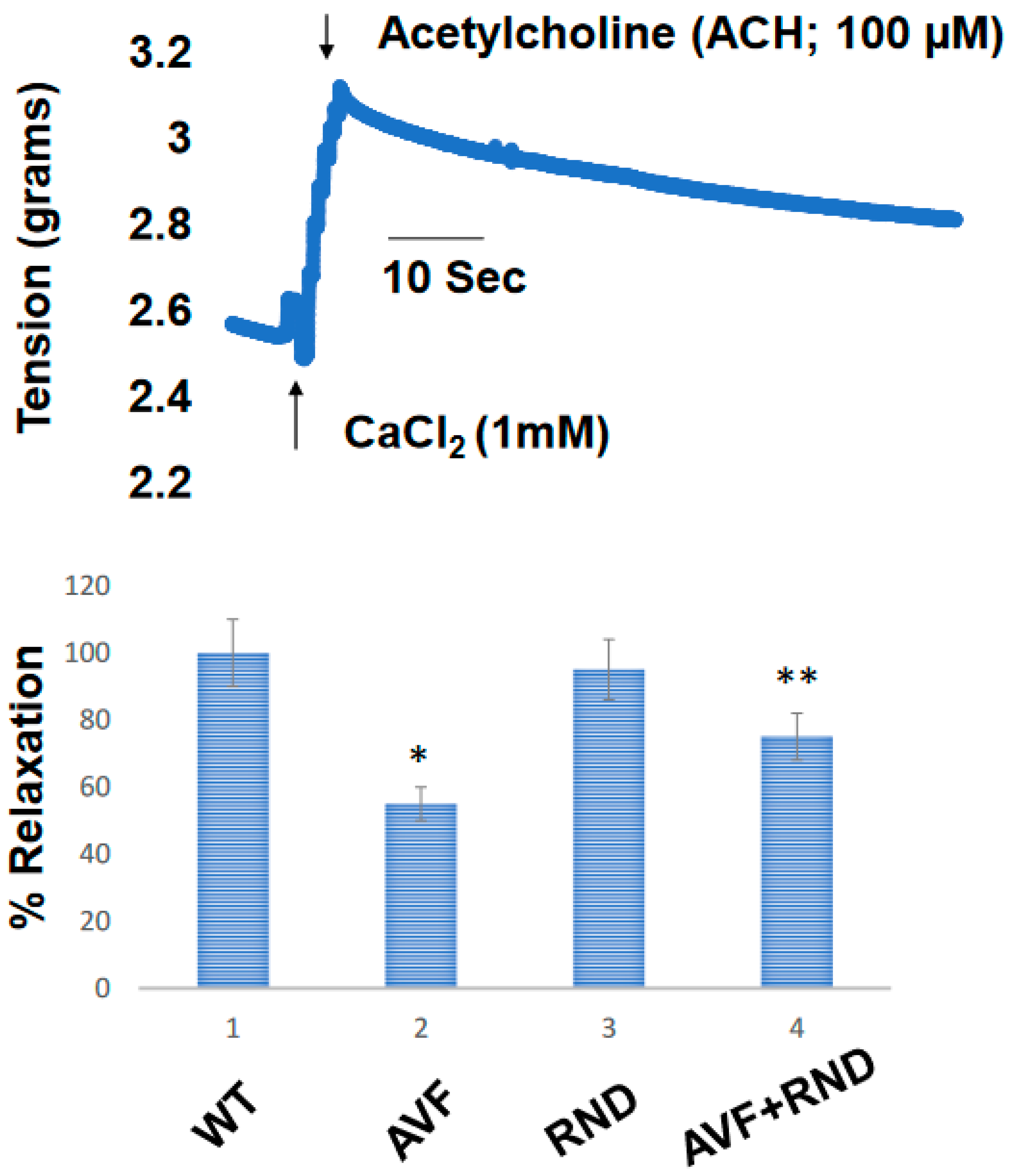
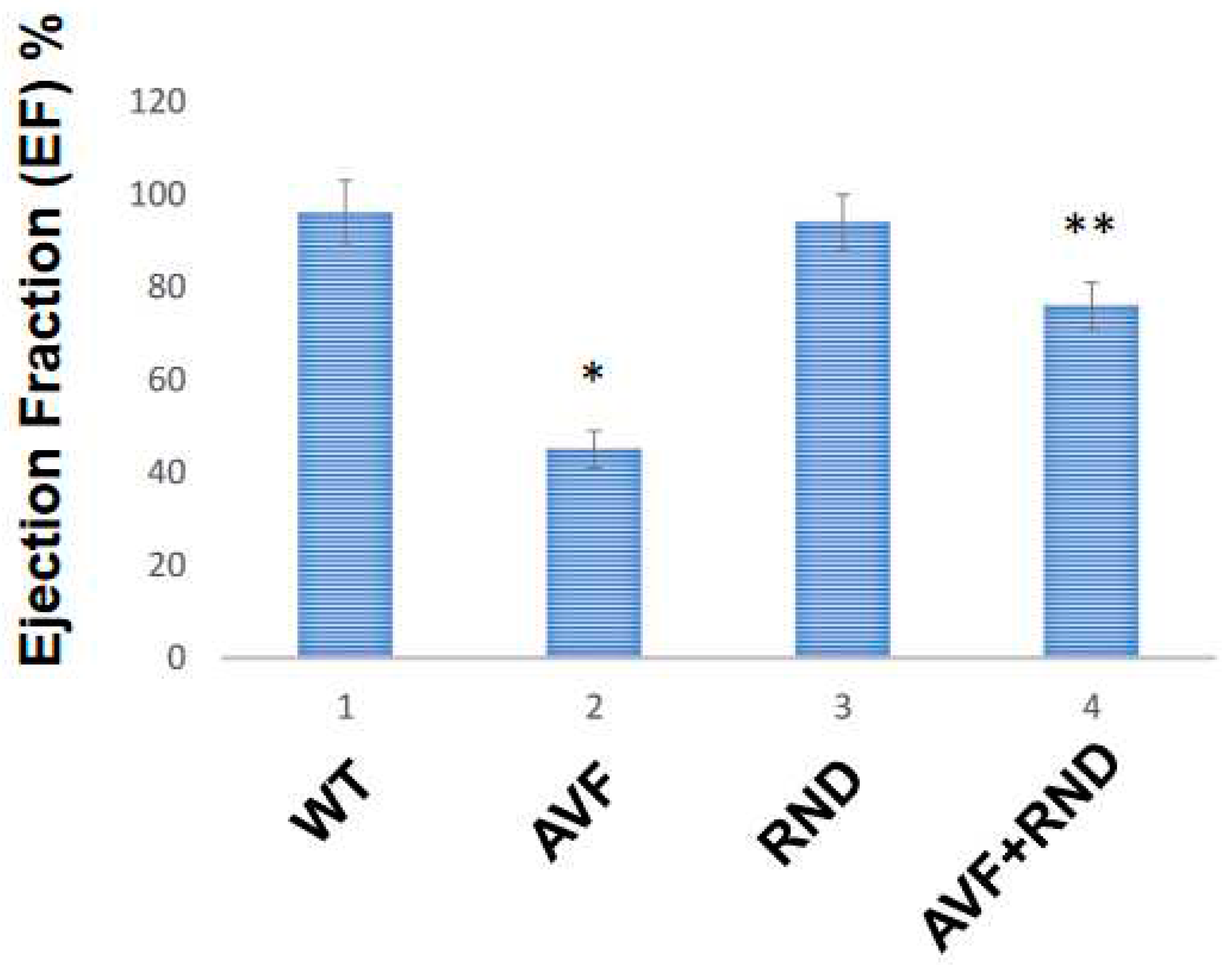
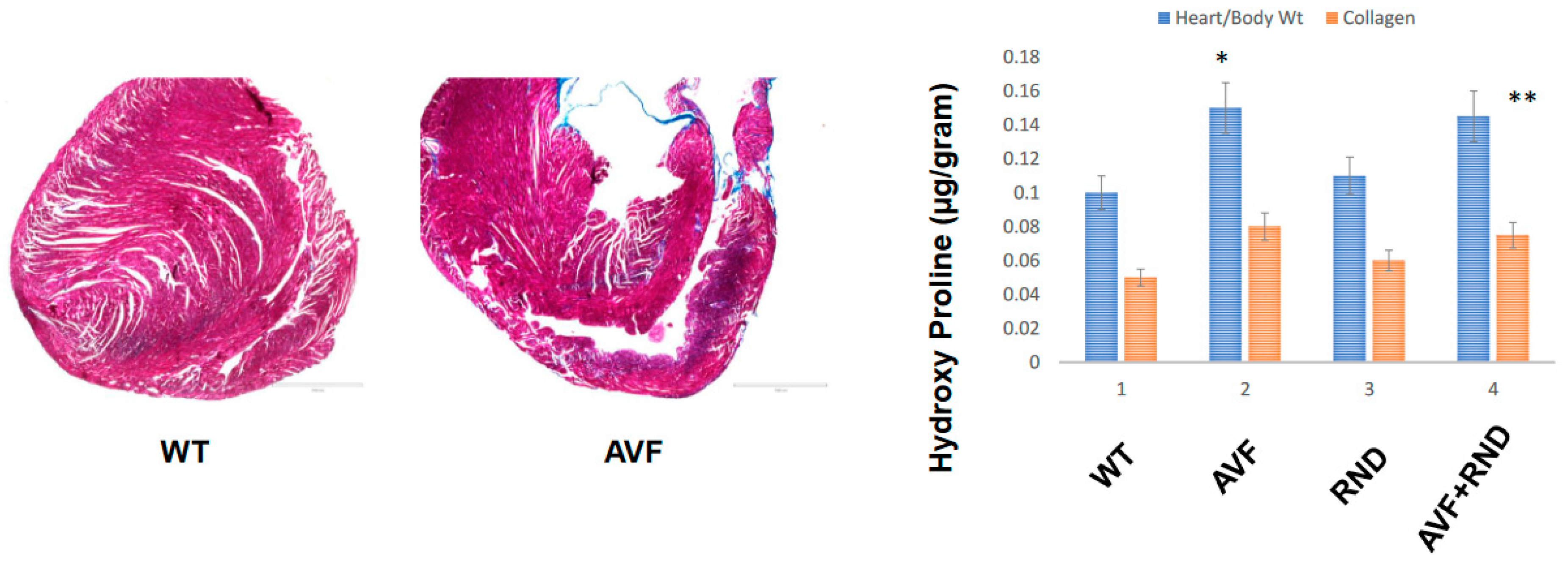
Next, we wanted to determine whether a putative increase in eNOS during RDN procedure will improve the endocardial-endothelial dependent cardiac function, hence we measured the endocardial ring response (prepared from the cardiac left ventricle (LV) from C57BL/6J WT-control, AVF, RND and AVF-RND mice groups) to acetylcholine (ACH). The results demonstrated an attenuation in endothelial dependent cardiac function in AVF group of mice; however, the RDN procedure was able to improve the cardiac endothelial function in experimental animals (Figure 3). Further, to investigate whether the RDN intervention maintains HFpEF phenotype rather than the HFrEF status, we decided to measure the cardiac echocardiographic (ECHO) parameters in the mice groups to quantitate the changes in percentage of the ejection fraction (EF). Our results suggested that RDN procedure successfully improved the cardiac EF percentage in the AVF mice group as shown in Figure 4. Finally, to determine whether the RDN procedure also influences the changes like cardiac hypertrophy and fibrosis, we set out to measure the heart/body weight ratio and collagen contents by measuring hydroxy proline. The results suggested that there were strong indications of cardiac hypertrophy and fibrosis in the AVF hearts, but the RDN procedure did not have any significant effect(s) on the cardiac hypertrophy and fibrosis (Figure 5).
Figure 3.
Cardiac endothelial dependent endocardial endothelial function measured in cardiac left ventricle (LV) ring prepared from in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The rings were contracted with CaCl2 (1 mM), and acetylcholine was added to relax the cardiac rings. The bar graph represents the percent relaxation from CaCl2 (1mM) contraction by 100 µMole acetylcholine (ACH). The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 3.
Cardiac endothelial dependent endocardial endothelial function measured in cardiac left ventricle (LV) ring prepared from in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The rings were contracted with CaCl2 (1 mM), and acetylcholine was added to relax the cardiac rings. The bar graph represents the percent relaxation from CaCl2 (1mM) contraction by 100 µMole acetylcholine (ACH). The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 4.
The cardiac ECHO data from in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The bar graph represents percent (%) ejection fraction of the male mice hearts. The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 4.
The cardiac ECHO data from in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The bar graph represents percent (%) ejection fraction of the male mice hearts. The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 5.
Left panel highlights the collagen trichrome-blue staining for the C57BL/6J WT and AVF male mice at 16 weeks while the right panel indicates cardiac hypertrophy as estimated by heart weight/body weight ratios and collagen fibrosis via hydroxy proline measurements in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The bar graph represents hypertrophy in ratio (g/g) and hydroxyproline (µg/g of the tissue). The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
Figure 5.
Left panel highlights the collagen trichrome-blue staining for the C57BL/6J WT and AVF male mice at 16 weeks while the right panel indicates cardiac hypertrophy as estimated by heart weight/body weight ratios and collagen fibrosis via hydroxy proline measurements in C57BL/6J WT-control, AVF, RND and AVF-RND male mice at 16 weeks. The bar graph represents hypertrophy in ratio (g/g) and hydroxyproline (µg/g of the tissue). The mean +/- SD from n=5; *, p<0.05 compared to WT; **, p<0.05 compared with AVF.
4. Discussion
Although skeletal muscle denervation increases the exosomes (
Figure 1) that often contain miRNAs; however, it is unclear whether renal denervation increases the Exosomal output during HFpEF phenotype [
37]. In this study, our results suggested that chronic volume overload causes an increase in exosomes that are released in the blood of the experimental mice. The results revealed that there was a significant increase in the Exosomal contents in the AVF mice group in comparison to the C57BL/6J WT mice. Further, it implies that the RDN procedure tends to increase the exosomes in AVF compared with RDN intervention, but interestingly it was lower than the AVF procedure alone. Although the levels of erythropoietin (EPO) were lower in the exosomes released during RDN; however, it is possible that the respective cargo levels such as miRNAs related to hypertrophy and fibrosis are increased as well.
Others have shown an increase in eNOS levels and a concomitant decrease in hypertrophy by renal denervation procedure [
38,
39]. Our study corroborates and show that the levels of eNOS increases and in fact were found to be robust in renal denervation, thus suggesting its involvement in the cardiac endothelial function by RDN procedure during HFrEF (
Figure 2). Whether RDN process is responsible for renal parenchymal cells’ apoptosis and the renal intracellular remodeling, we demonstrated the role of caspases (caspase 8 and -9). An increase in caspase 8 level in the RDN procedure and AVF specially with RDN intervention was notable as shown in
Figure 2 meaning that renal denervation most likely causes renal intracellular remodeling and helps improve the cardiac function during HFrEF phenotype (
Figure 2). In addition, RDN procedure might also be participating in promoting the cardiac tissue regeneration.
It is known that renal denervation improves the vascular endothelial function, but it is unclear whether it also assists in the endocardial endothelial function during HFrEF phenotype [
40]. From our study it appears that the RDN intervention also improves the endocardial-endothelial dependent cardiac function as seen from our results that employed the cardiac LV ring responses to acetylcholine (ACH). The attenuation in endothelial dependent cardiac function were expected post AVF procedure; however, the RDN intervention was able to improve the cardiac endothelial function as depicted in
Figure 3. our results were further supported by echocardiography (ECHO) measurements stemming from the RDN procedure towards maintaining the HFpEF phenotype from HFrEF one. Although the renal denervation procedure helps improve the cardiac fibrosis and hypertrophy, it is unclear whether it does too in HFrEF phenotype [
41,
42].
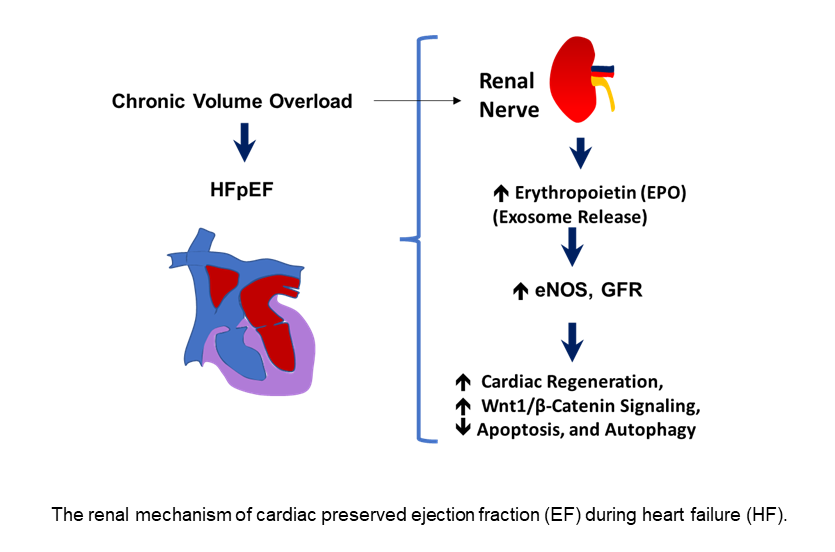
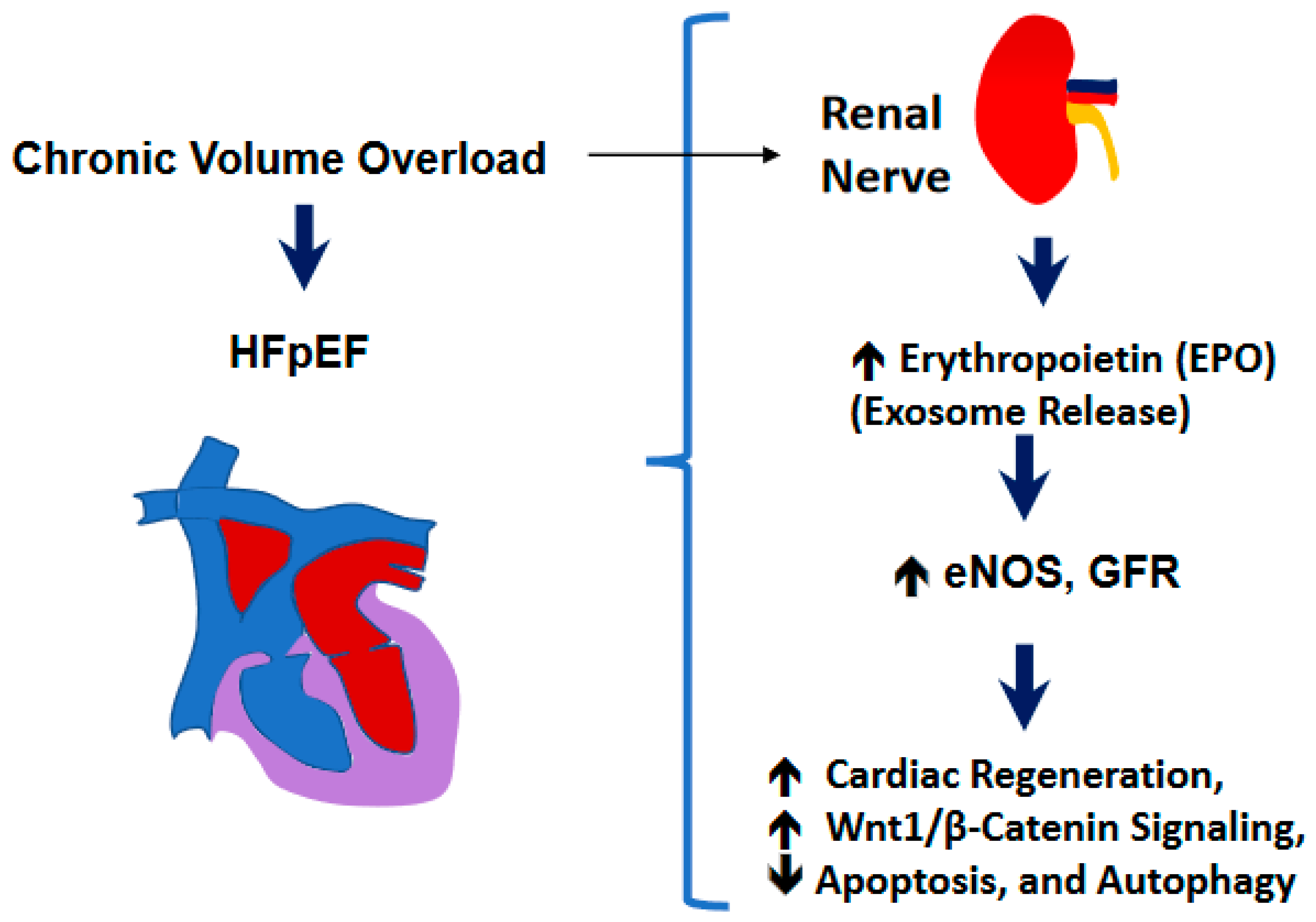
In this work we show that the RDN procedure influences the cardiac hypertrophy and fibrosis that were obvious after the AVF procedure in the hearts, but RDN intervention did not have much significant effects on cardiac hypertrophy and fibrosis (Figure 5) thus suggesting the preservation of ejection fraction (EF) despite not influencing the cardiac hypertrophy and fibrosis processes. Although more work needs to be performed in validating our observations but based upon our results in the experimental mice, we do opine that RDN intervention could certainly assist in decreasing the activity of the sympathetic nerves (i.e., the renal sympathetic afferent nerve-hypothalamus-renal sympathetic efferent nerve circuit system) as schematized in Figure 6 and that could help improve the left ventricular governed heart function in patients who remain prone to failure, and cardiac death.
Figure 6.
A schematic showing the renal mechanism of cardiac preserved ejection fraction (EF) during heart failure (HF).
Figure 6.
A schematic showing the renal mechanism of cardiac preserved ejection fraction (EF) during heart failure (HF).
Author Contributions
M.S. and S.C.T. designed the study and wrote the manuscript’s initial draft. Experiments were performed by S.P., O.E.A. and Y.Z. and U.S. conducted statistical analysis and drafted the figures for the manuscript. M.S., S.C.T., S.P.L.M. and D.K. helped finalize the manuscript before its submission. All the authors read and approved the final manuscript.
Funding
A part of this work was supported by HL-139047, DK116591 and AR-71789.
Acknowledgments
We thank other lab members for their continued support and help.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Owan, T. E.; Hodge, D. O.; Herges, R. M.; Jacobsen, S. J.; Roger, V. L.; Redfield, M. M., Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine 2006, 355, (3), 251-9. [CrossRef]
- Sharma, K.; Kass, D. A., Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circulation research 2014, 115, (1), 79-96. [CrossRef]
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A. P.; Nathan, S.; Butler, J.; Davidson, C. J.; Fearon, W. F.; Shah, S. J.; Blair, J. E. A., Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. American journal of physiology. Heart and circulatory physiology 2018, 314, (5), H1033-h1042. [CrossRef]
- Shah, S. J.; Borlaug, B. A.; Kitzman, D. W.; McCulloch, A. D.; Blaxall, B. C.; Agarwal, R.; Chirinos, J. A.; Collins, S.; Deo, R. C.; Gladwin, M. T.; Granzier, H.; Hummel, S. L.; Kass, D. A.; Redfield, M. M.; Sam, F.; Wang, T. J.; Desvigne-Nickens, P.; Adhikari, B. B., Research Priorities for Heart Failure With Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020, 141, (12), 1001-1026. [CrossRef]
- Tsao, C. W.; Lyass, A.; Enserro, D.; Larson, M. G.; Ho, J. E.; Kizer, J. R.; Gottdiener, J. S.; Psaty, B. M.; Vasan, R. S., Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC. Heart failure 2018, 6, (8), 678-685. [CrossRef]
- Beale, A. L.; Nanayakkara, S.; Kaye, D. M., Impact of Sex on Ventricular-Vascular Stiffness and Long-Term Outcomes in Heart Failure With Preserved Ejection Fraction: TOPCAT Trial Substudy. Journal of the American Heart Association 2019, 8, (13), e012190. [CrossRef]
- Goyal, P.; Paul, T.; Almarzooq, Z. I.; Peterson, J. C.; Krishnan, U.; Swaminathan, R. V.; Feldman, D. N.; Wells, M. T.; Karas, M. G.; Sobol, I.; Maurer, M. S.; Horn, E. M.; Kim, L. K., Sex- and Race-Related Differences in Characteristics and Outcomes of Hospitalizations for Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association 2017, 6, (4). [CrossRef]
- Sharma, K.; Mok, Y.; Kwak, L.; Agarwal, S. K.; Chang, P. P.; Deswal, A.; Shah, A. M.; Kitzman, D. W.; Wruck, L. M.; Loehr, L. R.; Heiss, G.; Coresh, J.; Rosamond, W. D.; Solomon, S. D.; Matsushita, K.; Russell, S. D., Predictors of Mortality by Sex and Race in Heart Failure With Preserved Ejection Fraction: ARIC Community Surveillance Study. Journal of the American Heart Association 2020, 9, (19), e014669. [CrossRef]
- Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Okada, K.; Dohi, T.; Kitamura, T.; Sunaga, A.; Kida, H.; Oeun, B.; Sato, T.; Komukai, S.; Tamaki, S.; Yano, M.; Hayashi, T.; Nakagawa, A.; Nakagawa, Y.; Yasumura, Y.; Yamada, T.; Sakata, Y., Sex Differences in Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association 2021, 10, (5), e018574. [CrossRef]
- Pfeffer, M. A.; Shah, A. M.; Borlaug, B. A., Heart Failure With Preserved Ejection Fraction In Perspective. Circulation research 2019, 124, (11), 1598-1617. [CrossRef]
- Heusch, G.; Bøtker, H. E.; Przyklenk, K.; Redington, A.; Yellon, D., Remote ischemic conditioning. Journal of the American College of Cardiology 2015, 65, (2), 177-95.
- Ciccarelli, M.; Dawson, D.; Falcao-Pires, I.; Giacca, M.; Hamdani, N.; Heymans, S.; Hooghiemstra, A.; Leeuwis, A.; Hermkens, D.; Tocchetti, C. G.; van der Velden, J.; Zacchigna, S.; Thum, T., Reciprocal organ interactions during heart failure: a position paper from the ESC Working Group on Myocardial Function. Cardiovascular research 2021, 117, (12), 2416-2433. [CrossRef]
- Málek, F.; Gajewski, P.; Zymliński, R.; Janczak, D.; Chabowski, M.; Fudim, M.; Martinca, T.; Neužil, P.; Biegus, J.; Mates, M.; Krüger, A.; Skalský, I.; Bapna, A.; Engelman, Z. J.; Ponikowski, P. P., Surgical ablation of the right greater splanchnic nerve for the treatment of heart failure with preserved ejection fraction: first-in-human clinical trial. European journal of heart failure 2021, 23, (7), 1134-1143. [CrossRef]
- D’Assante, R.; Arcopinto, M.; Rengo, G.; Salzano, A.; Walser, M.; Gambino, G.; Monti, M. G.; Bencivenga, L.; Marra, A. M.; Åberg, D. N.; De Vincentiis, C.; Ballotta, A.; Bossone, E.; Isgaard, J.; Cittadini, A., Myocardial expression of somatotropic axis, adrenergic signalling, and calcium handling genes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. ESC heart failure 2021, 8, (2), 1681-1686. [CrossRef]
- Wolfien, M.; Klatt, D.; Salybekov, A. A.; Ii, M.; Komatsu-Horii, M.; Gaebel, R.; Philippou-Massier, J.; Schrinner, E.; Akimaru, H.; Akimaru, E.; David, R.; Garbade, J.; Gummert, J.; Haverich, A.; Hennig, H.; Iwasaki, H.; Kaminski, A.; Kawamoto, A.; Klopsch, C.; Kowallick, J. T.; Krebs, S.; Nesteruk, J.; Reichenspurner, H.; Ritter, C.; Stamm, C.; Tani-Yokoyama, A.; Blum, H.; Wolkenhauer, O.; Schambach, A.; Asahara, T.; Steinhoff, G., Hematopoietic stem-cell senescence and myocardial repair - Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3. EBioMedicine 2020, 57, 102862. [CrossRef]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S. R.; Reyes, S.; Stepniak, E.; Walsh, S. A.; Acevedo, M. R.; Perez-Terzic, C. M.; Hodgson-Zingman, D. M.; Zingman, L. V., Musclin is an activity-stimulated myokine that enhances physical endurance. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, (52), 16042-7. [CrossRef]
- Homme, R. P.; Zheng, Y.; Smolenkova, I.; Singh, M.; Tyagi, S. C., Remote Hind-Limb Ischemia Mechanism of Preserved Ejection Fraction During Heart Failure. Frontiers in physiology 2021, 12, 745328. [CrossRef]
- Joslin, J. R.; Lioudaki, E.; Androulakis, E., Interrelation between heart failure with preserved ejection fraction and renal impairment. Reviews in cardiovascular medicine 2022, 23, (2), 69. [CrossRef]
- Nejima, J.; Uemura, N.; Vatner, D. E.; Homcy, C. J.; Hintze, T. H.; Vatner, S. F., Role of intact cardiac nerves and reflex mechanisms in desensitization to catecholamines in conscious dogs. The Journal of clinical investigation 1990, 86, (6), 2046-53. [CrossRef]
- Maranon, R. O.; Lima, R.; Mathbout, M.; do Carmo, J. M.; Hall, J. E.; Roman, R. J.; Reckelhoff, J. F., Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. American journal of physiology. Regulatory, integrative and comparative physiology 2014, 306, (4), R248-56. [CrossRef]
- Moreno, C.; Mistry, M.; Roman, R. J., Renal effects of glucagon-like peptide in rats. European journal of pharmacology 2002, 434, (3), 163-7. [CrossRef]
- Mansour, S. G.; Bhatraju, P. K.; Coca, S. G.; Obeid, W.; Wilson, F. P.; Stanaway, I. B.; Jia, Y.; Thiessen-Philbrook, H.; Go, A. S.; Ikizler, T. A.; Siew, E. D.; Chinchilli, V. M.; Hsu, C. Y.; Garg, A. X.; Reeves, W. B.; Liu, K. D.; Kimmel, P. L.; Kaufman, J. S.; Wurfel, M. M.; Himmelfarb, J.; Parikh, S. M.; Parikh, C. R., Angiopoietins as Prognostic Markers for Future Kidney Disease and Heart Failure Events after Acute Kidney Injury. Journal of the American Society of Nephrology : JASN 2022, 33, (3), 613-627. [CrossRef]
- Chedid, M.; Hanna, C.; Zaatari, G.; Mkhaimer, Y.; Reddy, P.; Rangel, L.; Zubidat, D.; Kaidbay, D. N.; Irazabal, M. V.; Connolly, H. M.; Senum, S. R.; Madsen, C. D.; Hogan, M. C.; Zoghby, Z.; Harris, P. C.; Torres, V. E.; Johnson, J. N.; Chebib, F. T., Congenital Heart Disease in Adults with Autosomal Dominant Polycystic Kidney Disease. American journal of nephrology 2022, 53, (4), 316-324. [CrossRef]
- Hepokoski, M.; Singh, P., Mitochondria as mediators of systemic inflammation and organ cross talk in acute kidney injury. American journal of physiology. Renal physiology 2022, 322, (6), F589-f596. [CrossRef]
- Libby, A. E.; Jones, B.; Levi, M., Learning the ABCs of ATP release. The Journal of biological chemistry 2020, 295, (16), 5204-5205. [CrossRef]
- de Couto, G.; Mesquita, T.; Wu, X.; Rajewski, A.; Huang, F.; Akhmerov, A.; Na, N.; Wu, D.; Wang, Y.; Li, L.; Tran, M.; Kilfoil, P.; Cingolani, E.; Marbán, E., Cell therapy attenuates endothelial dysfunction in hypertensive rats with heart failure and preserved ejection fraction. American journal of physiology. Heart and circulatory physiology 2022, 323, (5), H892-h903. [CrossRef]
- Fliser, D.; Bahlmann, F. H.; deGroot, K.; Haller, H., Mechanisms of disease: erythropoietin--an old hormone with a new mission? Nature clinical practice. Cardiovascular medicine 2006, 3, (10), 563-72. [CrossRef]
- Gok, M. G.; Paydas, S.; Boral, B.; Onan, E.; Kaya, B., Evaluation of eryptosis in patients with chronic kidney disease. International urology and nephrology 2022, 54, (11), 2919-2928. [CrossRef]
- Guyton, A. C.; Sagawa, K., Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. The American journal of physiology 1961, 200, 1157-63. [CrossRef]
- Brower, G. L.; Henegar, J. R.; Janicki, J. S., Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. The American journal of physiology 1996, 271, (5 Pt 2), H2071-8. [CrossRef]
- Cox, M. J.; Sood, H. S.; Hunt, M. J.; Chandler, D.; Henegar, J. R.; Aru, G. M.; Tyagi, S. C., Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. American journal of physiology. Heart and circulatory physiology 2002, 282, (4), H1197-205. [CrossRef]
- Tyagi, S. C.; Kumar, S. G.; Haas, S. J.; Reddy, H. K.; Voelker, D. J.; Hayden, M. R.; Demmy, T. L.; Schmaltz, R. A.; Curtis, J. J., Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. Journal of molecular and cellular cardiology 1996, 28, (7), 1415-28. [CrossRef]
- Lim, G. B., Sustained BP reduction with renal denervation. Nature reviews. Cardiology 2022, 19, (6), 352. [CrossRef]
- Tyagi, S. C.; Kumar, S.; Voelker, D. J.; Reddy, H. K.; Janicki, J. S.; Curtis, J. J., Differential gene expression of extracellular matrix components in dilated cardiomyopathy. Journal of cellular biochemistry 1996, 63, (2), 185-98. [CrossRef]
- Passmore, J. C.; Joshua, I. G.; Rowell, P. P.; Tyagi, S. C.; Falcone, J. C., Reduced alpha adrenergic mediated contraction of renal preglomerular blood vessels as a function of gender and aging. Journal of cellular biochemistry 2005, 96, (4), 672-81. [CrossRef]
- Tarone, R. E., A modified Bonferroni method for discrete data. Biometrics 1990, 46, (2), 515-22. [CrossRef]
- De Gasperi, R.; Hamidi, S.; Harlow, L. M.; Ksiezak-Reding, H.; Bauman, W. A.; Cardozo, C. P., Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Scientific reports 2017, 7, (1), 12888. [CrossRef]
- Lu, D.; Wang, K.; Wang, S.; Zhang, B.; Liu, Q.; Zhang, Q.; Geng, J.; Shan, Q., Beneficial effects of renal denervation on cardiac angiogenesis in rats with prolonged pressure overload. Acta physiologica (Oxford, England) 2017, 220, (1), 47-57. [CrossRef]
- Ye, F.; Shi, G.; Wang, X.; Tu, S.; Zhang, Z.; Zeng, L., [Renal sympathetic denervation can significantly reduce blood pressure and improve arterial stiffness in hypertensive beagles]. Nan fang yi ke da xue xue bao = Journal of Southern Medical University 2021, 41, (11), 1609-1615. [CrossRef]
- Eikelis, N.; Hering, D.; Marusic, P.; Sari, C.; Walton, A.; Phillips, S.; Lambert, E.; Duval, J.; Krum, H.; Lambert, G.; Esler, M.; Schlaich, M., The effect of renal denervation on endothelial function and inflammatory markers in patients with resistant hypertension. International journal of cardiology 2015, 188, 96-8. [CrossRef]
- Doltra, A.; Messroghli, D.; Stawowy, P.; Hassel, J. H.; Gebker, R.; Leppänen, O.; Gräfe, M.; Schneeweis, C.; Schnackenburg, B.; Fleck, E.; Kelle, S., Potential reduction of interstitial myocardial fibrosis with renal denervation. Journal of the American Heart Association 2014, 3, (6), e001353. [CrossRef]
- Xiao, B.; Liu, F.; Jin, Y. H.; Jin, Y. Q.; Wang, L.; Lu, J. C.; Yang, X. C., Renal sympathetic denervation attenuates left ventricle hypertrophy in spontaneously hypertensive rats by suppressing the Raf/MEK/ERK signaling pathway. Clinical and experimental hypertension (New York, N.Y. : 1993) 2021, 43, (2), 142-150. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).