1. Introduction
In the recent years, the most typical durability design method to make concrete structures more resistant to chemical attack is the implementation of other cementitious materials as partial replacement of OPC in order to make the cement matrix denser. The most commonly used materials are fly ash (PFA), ground granulated blast furnace slag (GGBS) and silica fume (SF). This method has gained much popularity because by using industrial by-products it can also reduce the initial cost of construction and still improve concrete properties (Siddique, 2007). Therefore, considering the surge in constructions made from this binary mix designs, it is important to study the corrosion control methods on those situations.
Electrochemical chloride extraction (ECE), referred as desalination as well, has been trending on the last 40 years as a promising treatment against corrosion of steel in concrete, since it is a non-destructive method and can be applied in relatively short time. The main goal of the treatment is to remove chloride ions from the concrete, mitigating the corrosion process and therefore reestablishing the alkalinity on the vicinity of steel. The chloride removal process is driven by a direct current of high density for a limited period passing through a temporary anode fixed to the concrete surface, an electrolyte and the steel acting as the cathode. In the end of treatment, the anode and the electrolyte are removed and the concrete surface is cleaned.
Considering previous researches it is possible to see that the binder type has considerable influence on the efficiency of treatment due to different binding capacities. For instance, it is from common knowledge that chlorides are present in concrete as free and bound chlorides, which can be divided into physically and chemically bound. In this manuscript, the main reference for the definitions of chloride types is Glass et al. (2000), which classified the chloride ions taking into consideration mainly the mobility level, leading to four types of chloride ions – free, bound, physically bound and chemically bound. According to Glass et al. the physically bound chlorides can also be called “loosely bound chlorides” because they can be easily extracted from C-S-H gel, by physical adsorption. On the other hand, chemically bound chlorides are seen in two types, the “strongly bound” by chemical reactions (Friedels salt), that cannot be extracted under physical changes of the environment, and the ones bound to cement hydrates. In this study, as the main focus is chloride removal, the chlorides chemically bound to crystalline salts are not of great importance. Kim et al. (2016) studied the application of ECE for OPC, GGBS and PFA in different concrete mix proportions. The removal of chlorides at the depth of the steel was more significant in GGBS and ternary mixes, presumably due to a release of adsorbed chlorides on the hydration surface into free. However, the extraction rates in terms of different chloride types was not accounted for. Yu et al. (2019) stated that in the situation of coexisting attack from sulfate and chloride ions, cement paste experienced destabilization of bound chlorides under ECE. By increasing GGBS content from 0% to 30%, the content of bound chlorides in the vicinity of the steel bars first increased and then decreased. Norton et al. (2018) showed that cement containing fly ash has higher capacities to bind chlorides and thus PFA cement replacement requires a longer time to extract a significant amount of chlorides. It is possible to see that ECE should be conducted with caution depending on the binder type, considering that certain cementitious materials may present better performance of electrochemical treatment but higher corrosion damage as well (Lee et al., 2017).
The overarching aim of this study is to analyze the different chloride removal rates according to chloride types, to identify more precisely, until which extent the binding capacity can influence the treatment, following suggestions of further works from previous papers (Kim et al., 2016). On that sense, the application of ECE considering 3 different binders, namely, GGBS, PFA and SF is discussed. Concrete specimens were used to assess characteristics such as compressive strength and resistance to chloride penetration on different binders. Meanwhile, mortar samples were used for ECE treatment and chloride profiling, in order to avoid unnecessary influence of coarse aggregates on the chloride distributions over depth. Ryou and Ann (2008) stated that the aggregate itself does not affect the chemistry between cement hydration and chloride ions, which is a key factor of corrosion risk, despite the influence on the pore structure, validating the use of mortar specimens on ECE instead of concrete for the chloride removal. Additionally, microscopic examination was performed to quantitatively confirm the formation of C-S-H gel in the cement matrix, which is subsequently indicative of which type of chloride binding is more expected. The X-ray diffraction analysis was used to define composites of raw products and mainly quantify the phases of C-S-H gel. ECE was applied to the specimens at a current density of 2A/m2 for 4 weeks, in order to achieve a charge passed of 1344 A-h/m2. Moreover, titanium mesh was used as the anode and calcium hydroxide as the electrolyte. All experiments were carried out in ambient temperature (23 to 25 Celsius degrees). Once all information was collected, the feasibility of ECE application to concrete with different binders was determined.
2. Experimental procedure
2.1. Sample preparation
In the current study, concrete, mortar and paste specimens were used to assess characteristics related to different binders. The oxide composition of each binder was obtained by X-ray fluorescence (XRF) analysis and is given in
Table 1. It is seen that OPC and GGBS mainly consist of CaO, SiO
2 and Al
2O
3, while SF and PFA have mainly high proportions of only SiO
2. It can then be assumed that GGBS has higher reactivity (Babu and Kumar, 2000). On the same sense, PFA as a by-product generated by coal raw materials in thermal power plants showed on its chemical composition high contents of silicon with minor amounts of aluminum oxide, being classified as class F (Fauzi et al., 2016). Important to mention that the author acknowledges the recommendation from the American Concrete Institute (ACI) Committee 211-91 (1998) guidelines that a maximum replacement rate of Class F PFA is 25%, however for easier calculation of the mix proportion, the maximum replacement ratio was defined as 30% in this study.
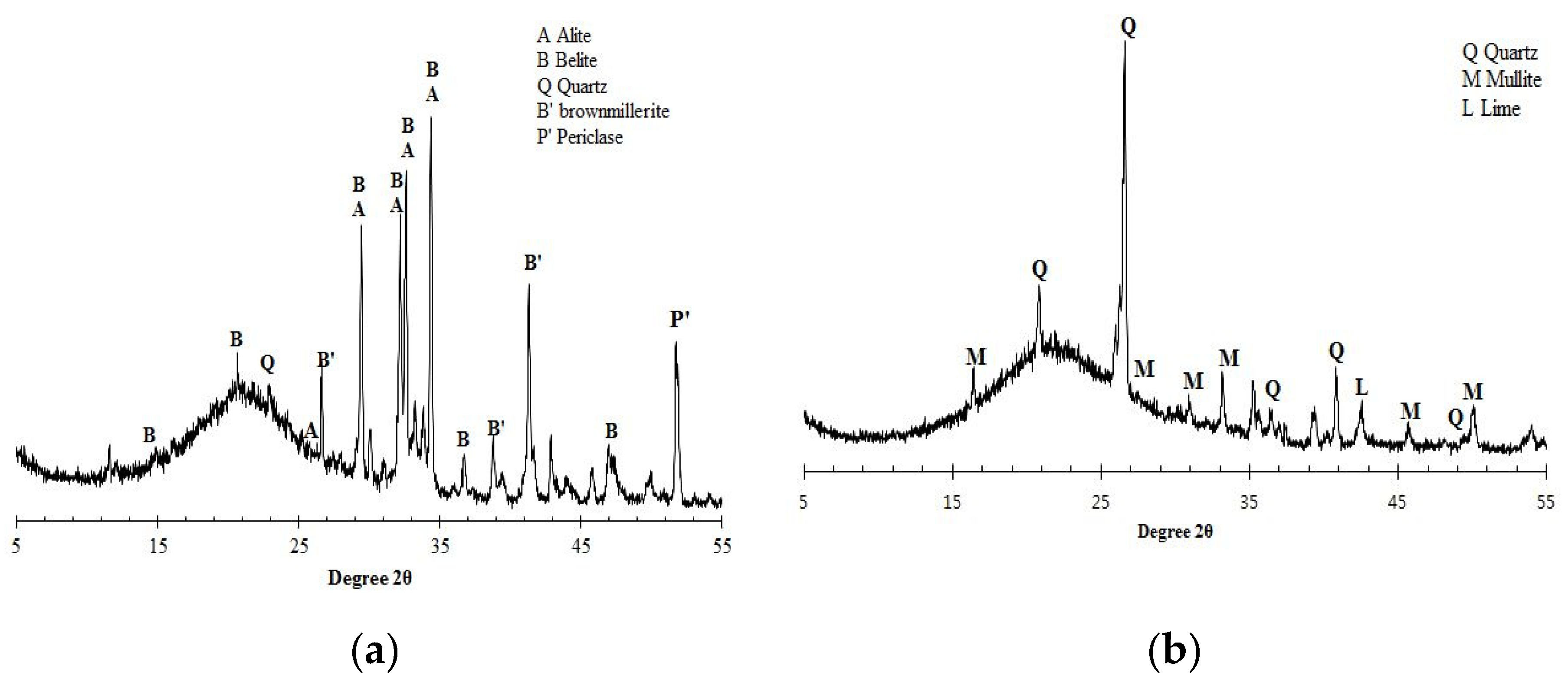
Prior to designing the concrete mix, the chemical characteristics of raw materials were preliminarily examined by the X-ray diffraction (XRD) as given in
Figure 1. In the case of OPC, alite (3CaO∙SiO
2) and belite (2CaO·SiO
2) are the main clinker phases while others such as gypsum, periclase, brownmillerite(4CaO∙Al
2O
3∙Fe
2O
3) appear in lower quantities. XRD patterns of pozzolanic materials show that all GGBS, SF and PFA present high peaks of silica content. In fabricating specimens containing partial replacement of the different binders, the replacement ratio was accordingly 65% for GGBS, 30 % for PFA or 10% of SF in the total binder. The replacement ratios are in accordance with previously published works, such as Kim et al. (2016), which concluded that those values represent optimal quantities. The specimen preparation consisted of casting the specimens, curing, and applying a direct current (DC) in case of chloride removal. The mixture proportions used in manufacturing the concrete specimens for binder: water: sand (Grade M): gravel (maximum size of 25mm) was 1.00: 0.45: 2.64: 1.76. The quantities of materials per m
3 of concrete in each mix design are shown in
Table 2; a free water-binder ratio was always kept at 0.45, while sand and gravel were added at the given ratio in the process of fabricating each type of mix. Concrete specimens were used to assess the development of compressive strength and resistance to chloride penetration on different binders. Mortar samples were used for ECE treatment and chloride profiling, in order to avoid unnecessary influence of coarse aggregates on the chloride distributions over depth. Paste specimens were used for X-ray diffraction analysis.
Table 3 shows the proportions of materials and experiments conducted according to each kind of sample. Specimens were demolded in 24 hours after casting, and then cured in a damp chamber at 23±2°C, Relative Humidity 50±5%, of which the condition was sustained until measuring properties of the specimens.
2.2. Compressive strength
In order to provide supplementary information on the mixes with OPC replacement from different binders, the compressive strength was measured by using fabricated cylinders (Ø 100 mmⅹ200 mm) according to C192 at 7, 28, 56 and 91 days following the standards on ASTM C39 (2020). Samples were cured as following the process of curing explained above. Before the load was given at the speed of 3N/sec, each end of the specimen was ground off to eliminate the interference of the uneven surface.
2.3. Hydration characteristic
The microscopic assessment was used to characterize hydrations of supplementary cementitious materials mixed paste, employing the X-ray diffraction (XRD) technique coupled with the MDI JADE package software. For sample preparation, paste was fabricated and cured for 28 days in a damp chamber at 23±2°C. The matured paste specimens were crushed and immersed in acetone for 48 hours. Then, they were dried in a desiccator for 48 hours at 25±2°C to halt the further hydrations and eliminate the moisture inside the pores. The scanning was carried out in the diffraction range (2θ) of 5-60° at a rate of 4.0°/min with 40 kV voltage and 100 mA current with a wavelength of l.5405 A° (1 A°= 0.1 nm). The count per second (CPS) intensity was plotted at every 0.02° within the cover range to obtain a diffraction pattern. The software package of MDI JADE was used to identify solid phases at different diffraction angles in the XRD curve. Once the phases were determined, their occupation in paste was calculated by rating the CPS intensity. In this study, C-S-H gel was mainly identified.
2.4. Resistance to chloride penetration
Rapid chloride permeability test (RCPT) was conducted according to ASTM C 1202 (2000). The electrical current passed through the concrete slices (Ø 100 mmⅹ50 mm) cured for 365 days and was subsequently measured for 6 h until the total charge (C, columb) was obtained. The voltage of 60 V DC was maintained across the ends of the concrete slice which were immersed between two chambers one with 3% NaCl solution and the other with 0.3 M NaOH solution.
2.5. Electrochemical chloride removal
In order to promote the corrosion onset from the beginning on specimens used for ECE, sodium chloride was added to the mixing water (3% Cl- by mass of cement). Specimens were cast in a 150 mm cubic mold with a centrally located 10 mm diameter mild plain steel bar, containing 3 replications each to guarantee accuracy of results. The ends of the steel bars were masked off using a cementitious coating and then covered with heat-shrink insulation to avoid corrosion under the masking material. The specimens were then cured for 28 days at 21 ± 2°C.
The chloride extraction treatment was performed using an impressed constant current density of 2 A/m2 of mortar surface for 4 weeks. Saturated Ca(OH)2 solution at 0.1 mol concentration was used as electrolyte. Specimens were treated after approximately 28 days of curing, which is standard for most experiments, as an alternative to optimize the experimental schedule. During application of the electric charge, the specimens were immersed in an acrylic box filled with the electrolyte. A 15 × 15 cm2 titanium mesh plate with a thickness of 2 mm was used as the external electrode (anode) and placed inside the acrylic box as well close to the mortar surface in all directions. A direct electric potential was applied from the regulator between the reinforcement bars and the external electrode. Voltage was accordingly increased up to 20 ± 1 V in the beginning of the treatment to maintain the current density for 4 weeks. The total charge passed substantially accounted for 1344 Ah/m2. After treatment was completed, specimens were removed from the acrylic box and kept in constant relatively humidity and temperature.
To measure the chloride ion concentration, methods were similar to the ones used in another publication by Tofeti Lima and Ann (2020). Mortar powder was drilled from the specimens at the core obtaining dust samples. Specimens were ground by a diamond-grit plate from the surface in the direction of steel rebar at regular increments. Samples were diluted in solvents to extract chloride ions., The chloride concentration in solution was obtained by potentiometric titration against silver nitrate before and after treatment. Different solvents were used, in the case, water for determining the concentration of free chlorides, and, water plus nitric acid for total chlorides. In fact, the water soluble chloride measured by the following method was regarded as free, whilst acid soluble as total. Simultaneously, the concentration of bound chlorides was determined by subtracting free from total values.
3. Results
3.1. Development of strength and permeability
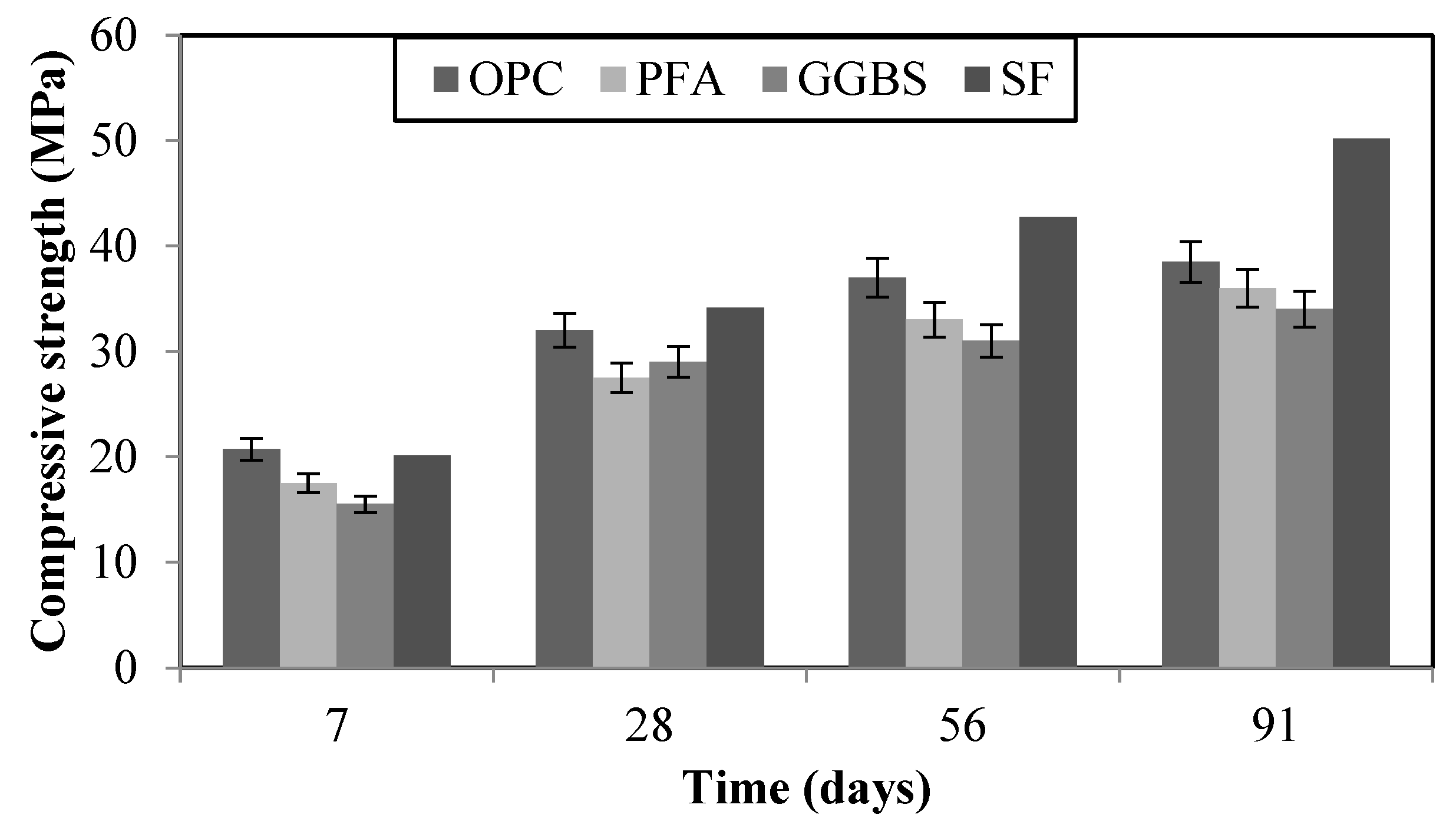
In order to provide supplementary information on the mixes with OPC replacement from different binders, the compressive strength was measured at 7, 28, 56 and 91 days (
Figure 2). OPC had better results on compressive strength for the early age, 7 days, as expected due to the latent hydration characteristics of the supplemented cementitious materials. However, at later stages, SF showed higher values. The reached compressive strength for 30% PFA, 65% GGBS and 10% SF was in the acceptable range for all mixes, being 36, 34, and 50.18 MPa, respectively.
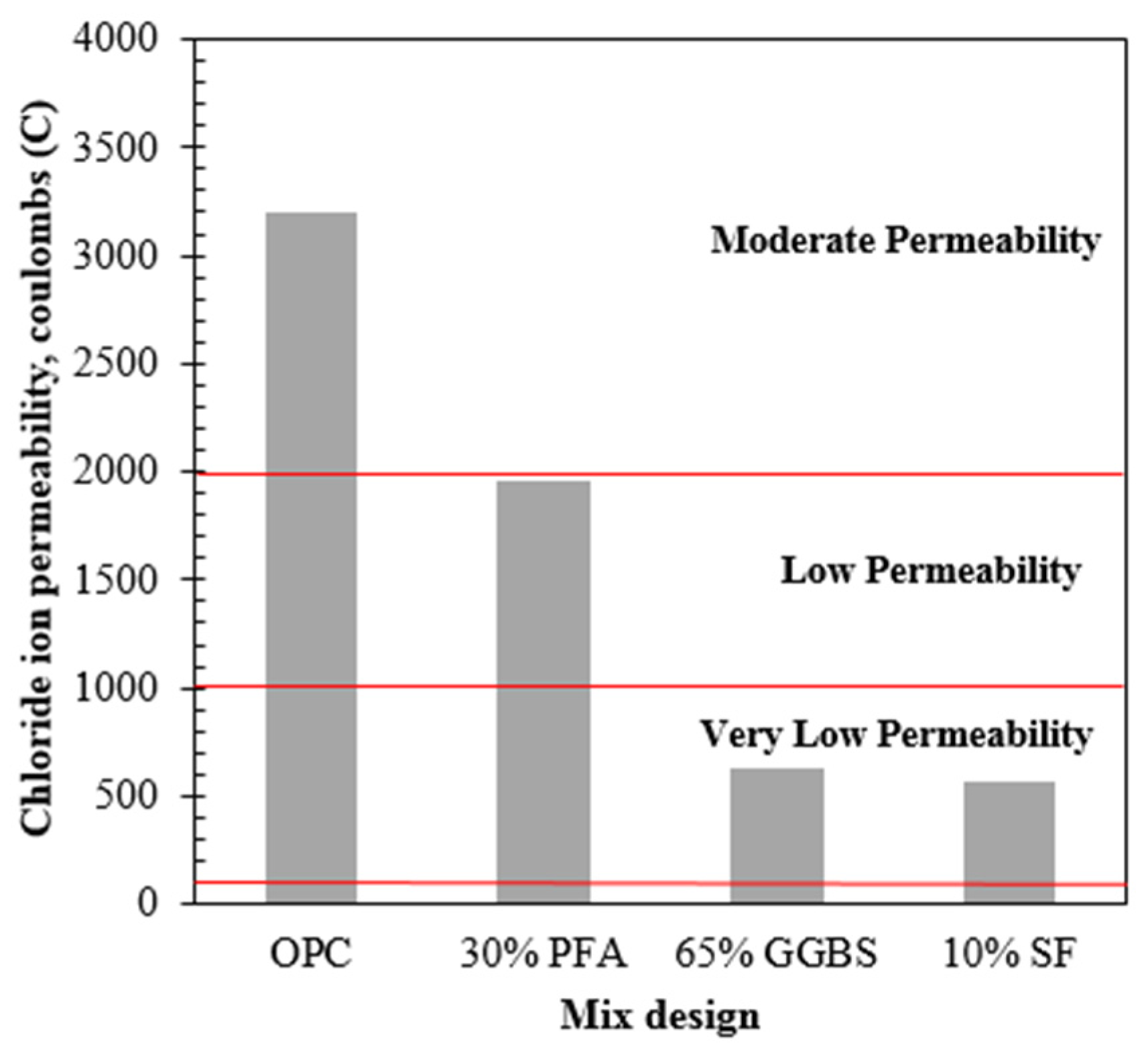
RCPT results are given in
Figure 3. As it is expected, samples made with only OPC had the highest level of chloride ion penetrability, while the others partially replaced showed lower levels at identical conditions. This result is explained by the fact that mineral admixtures in the concrete mix reduce the critical pore size on the matrix leading to reduced ionic penetrability. It is also remarkable that mixes using GGBS and SF were expressively resistant to chloride ions penetration, more than PFA, having results of 626 and 570 C of total charge passed, respectively, which is tabulated as very low permeability (Stanish et al., 1997). Notwithstanding results for all specimens did not lead to values in the negligible range, which justifies the studies of ECE application to those different binders.
3.3. Removal of different chloride types by ECE
3.3.1. Free chlorides removal
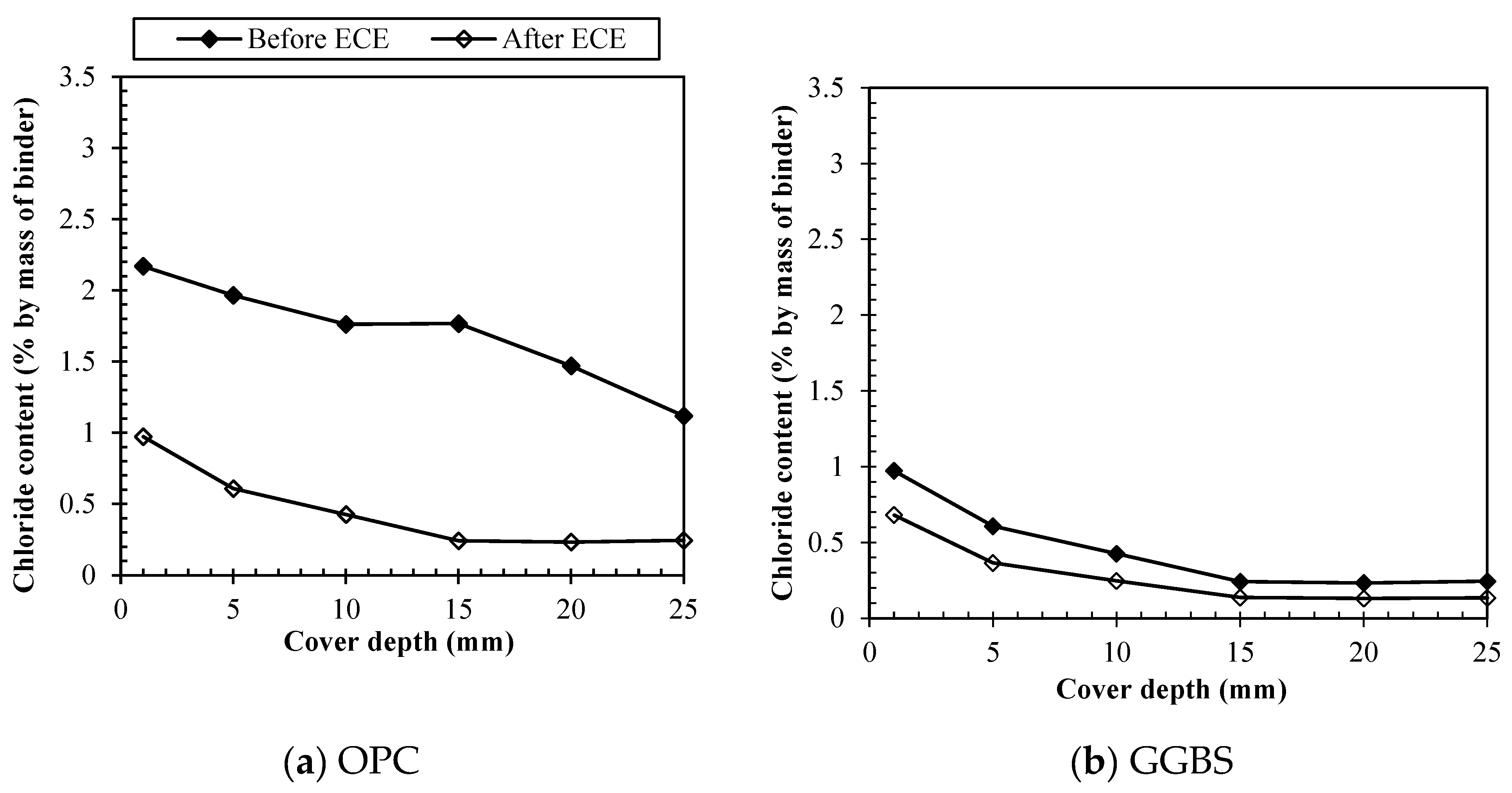
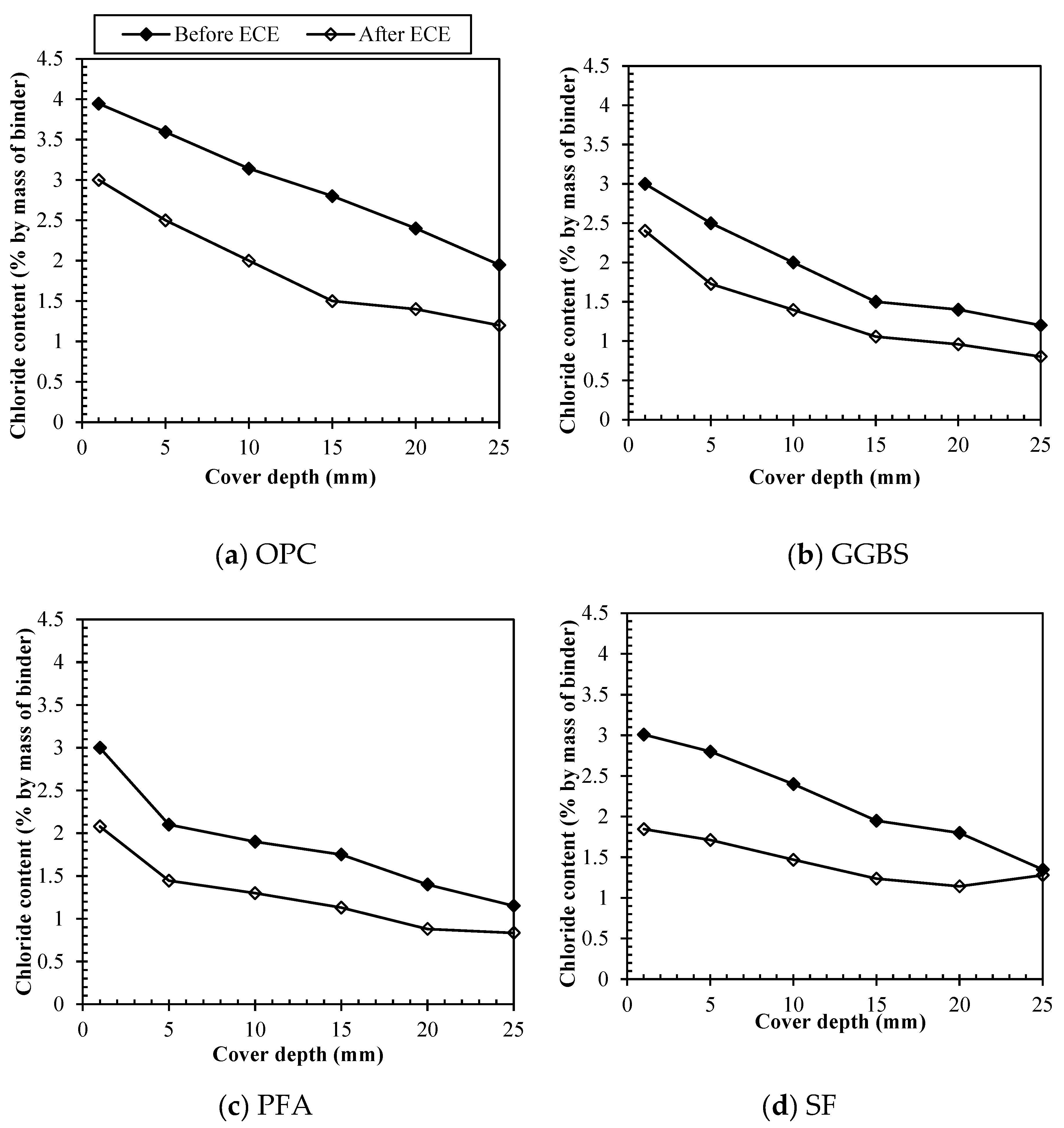
Figure 4 represents profiles for free chlorides before and after
ECE considering the different binders. For samples made with 100% OPC,
Figure 4 (a) shows that the concentration of free chlorides before treatment ranged from 0.72 to 1.77% by weight of cement, increasing for shallower depths and being above the chloride threshold at all levels. After treatment, the free chlorides concentration was decreased at all depths accounting for 0.23 – 0.97% by weight of cement, removing free chlorides at levels lower than the chloride threshold to initiate corrosion. Meanwhile for samples made with 65% of GGBS replacement, as it is shown on
Figure 4 (b), the concentration of free chlorides before treatment ranged from 0.24-0.97% by weight of binder, increasing for shallower depths, however still in a lower range than for OPC. After treatment, the free chlorides concentration was also decreased at all depths accounting for 0.13 – 0.68% by weight of binder.
Figure 4 (c), in turn, represents profiles for free chlorides before and after treatment when using 30% of PFA. The concentration of free chlorides was found to be in the range of 0.19-1.13% by weight of cement immediately after mixing, similarly to GGBS at lower rates when compared to OPC samples. After treatment, the free chlorides concentration was further decreased to lower levels of 0.08 – 0.67%, being the values at the increasing depths (>20mm) almost insignificant, which was perceived for GGBS as well. Concerning samples with 10% replacement of SF
Figure 4 (d) shows that the concentration of free chlorides was found to be in the range of 0.6-1.63% by weight of cement immediately after mixing, increasing for the shallower depths. SF, however, presented a higher portion of free chlorides before treatment, still at lower rates than OPC samples, but remarkably more than the other pozzolanics. This can be explained because among all materials silica fume is the material that imposes the lowest hydration rates, therefore, more free chlorides would be present due to less hydration products to adsorb them. Moreover, chemically bound chlorides can be formed by the reaction with unhydrated aluminate phases to form Friedel's salt, and, as it was seen by the XRF results, the content of alumina oxides in silica fume is remarkably lower than for other materials, accounting for only 0.27%, which would also point to less chloride binding. Either way, after treatment, the free chlorides concentration was decreased to lower levels of 0.33 – 1.06%, however those values are still very high.
3.3.2. Bound chlorides removal
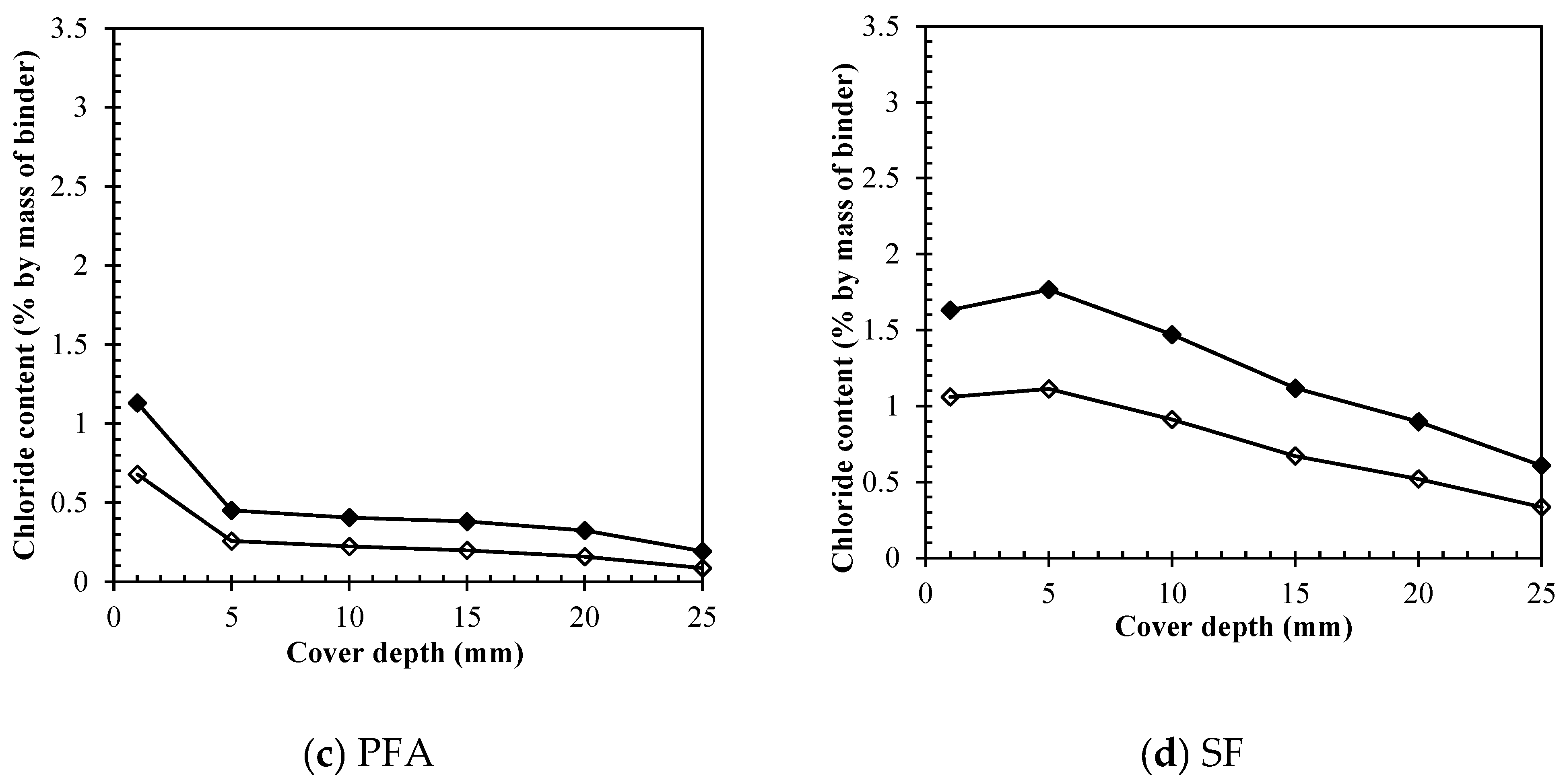
Figure 5 (a) represents the results obtained for bound chlorides. For OPC, the quantity of bound chlorides ranged from 1.26 to 2.33% by weight of cement in untreated specimens, while after treatment a considerable reduction is perceived and the range of bound chlorides was limited to about 0.83-1.78%. Moreover, at increasing depths the concentration of bound chlorides converged to a specific range between 1.38 and 1.78%.
Figure 5 (b) presents the results obtained for 65% GGBS replacement. The quantity of bound chlorides ranged from 0.95 to 2.02% by weight of binder in untreated specimens, while after treatment a considerable reduction is also perceived and the range of bound chlorides was limited distributed to about 0.47-1.31%. It implies that bound chlorides were removed at greater rates than for OPC, meaning that the electric field worked better for GGBS in terms of weakening the chloride binding. In turn,
Figure 5 (c) represents the results obtained for 30% replacement of PFA. The removal of bound chlorides did not happen at so expressive rates as for GGBS although it was also accountable. The quantity of bound chlorides ranged from 0.95 to 1.86% by weight of cement in untreated specimens, while after treatment, again the reduction is perceived and the range of bound chlorides was distributed to about 0.74-1.40% depending on the depth. For depths greater than 10.0 mm, the residual bound chloride also converged to a limited range of 0.74-1.07%.
Figure 5 (d) presents the results obtained for bound chlorides when silica fume is used. The quantity of bound chlorides ranged from only 0.74 to 1.37% by weight of cement in untreated specimens. These results confirm the affirmation stated above, about the lower binding capacity of silica fume when compared to the other binders. The lower rate of bound chlorides occurred, accompanying the higher presence of free chlorides, as it was expected. However, the results for silica fume after treatment had a much unexpected trend considering that at the steel depth (25mm) the contents of bound chlorides increased instead of reducing, accounting to 0.74% before ECE and 0.94% after. In general, after treatment the reduction is perceived and the range of bound chlorides was distributed to about 0.78-1.94% depending on the depth, implying a considerably lower efficiency when compared to other binders.
3.3.3. Total chlorides removal
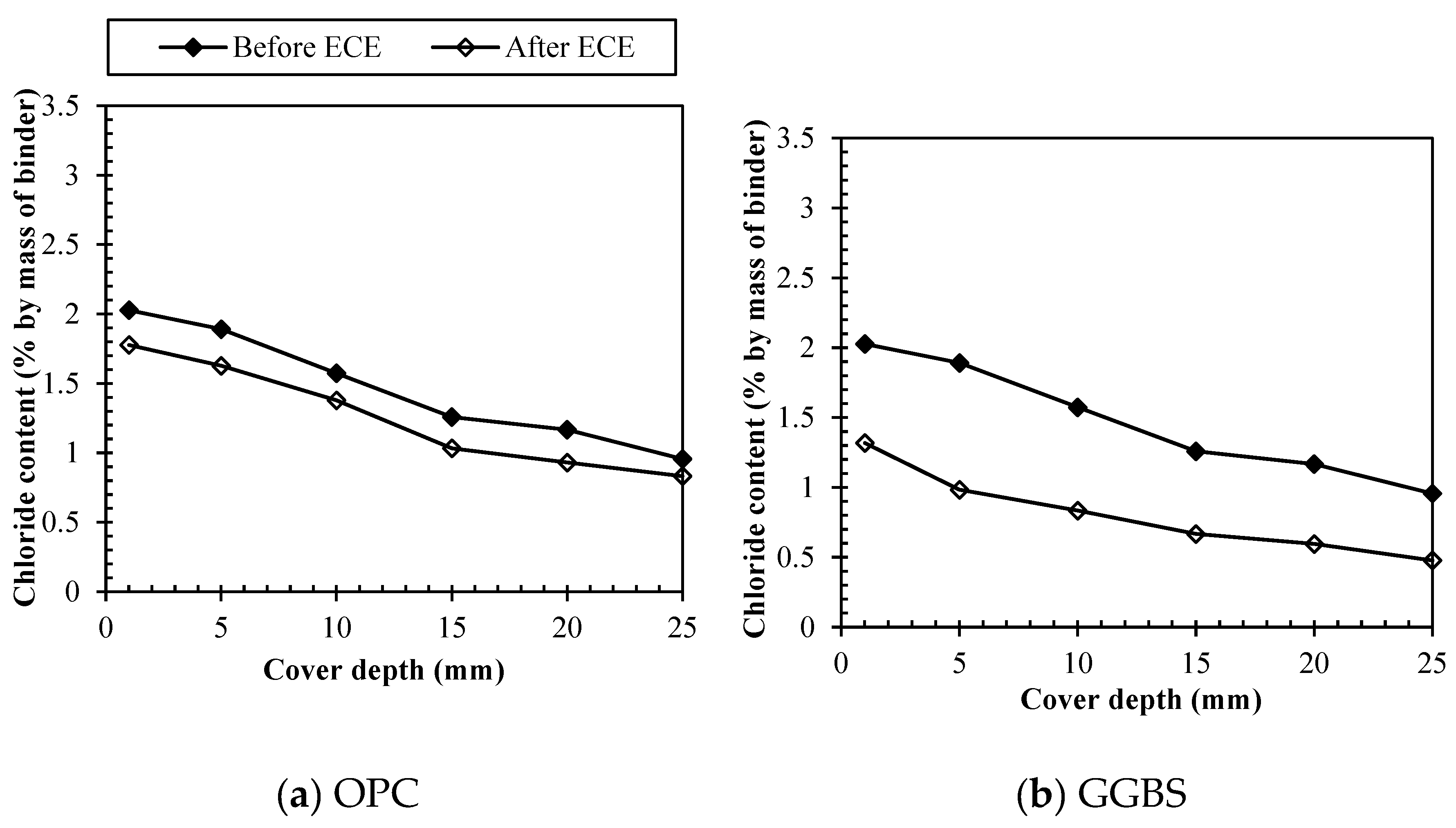
Figure 6 (a) shows the profiles for total chlorides before and after treatment for all binders. For OPC the contents of total chlorides ranged from 1.97 – 4.10% (by weight of cement) decreasing with the increase in depth immediately after mixing. After treatment the total chloride concentration was reduced to 1.20 – 3.00% at all depths. For GGBS,
Figure 6 (b) shows that total chlorides before treatment ranged from 1.20 – 3.0% (by weight of binder) decreasing with the increase in depth. After treatment the total chloride concentration was reduced to 0.61 – 1.99% at all depths, resulting on a percentage removal efficiency of 33.3 – 48.9% depending on the cover depth.
Figure 6 (c) shows that for PFA, the concentration of total chlorides in the specimens initially ranged from 1.15 - 3.00% (by weight of binder) at all depths. After treatment, these values were reduced to 0.83 - 2.08% depending on the cover depth. In particular, for PFA, the chloride removal occurred at lower rates than for GGBS. Moreover, for depths exceeding 15.0 mm, the total chloride content was reduced into a similar range of values being around 0.87%. Meanwhile, for SF
Figure 6 (d) shows that the concentration of total chlorides in the specimens initially ranged from 1.34 - 3.00% (by weight of binder) at all depths. After treatment, these values were reduced to 1.27 – 1.84% depending on the cover depth. Therefore, in particular, as expected for SF the chloride removal was not as efficient as for the other binders.
3.3.4. Direct comparison among binders
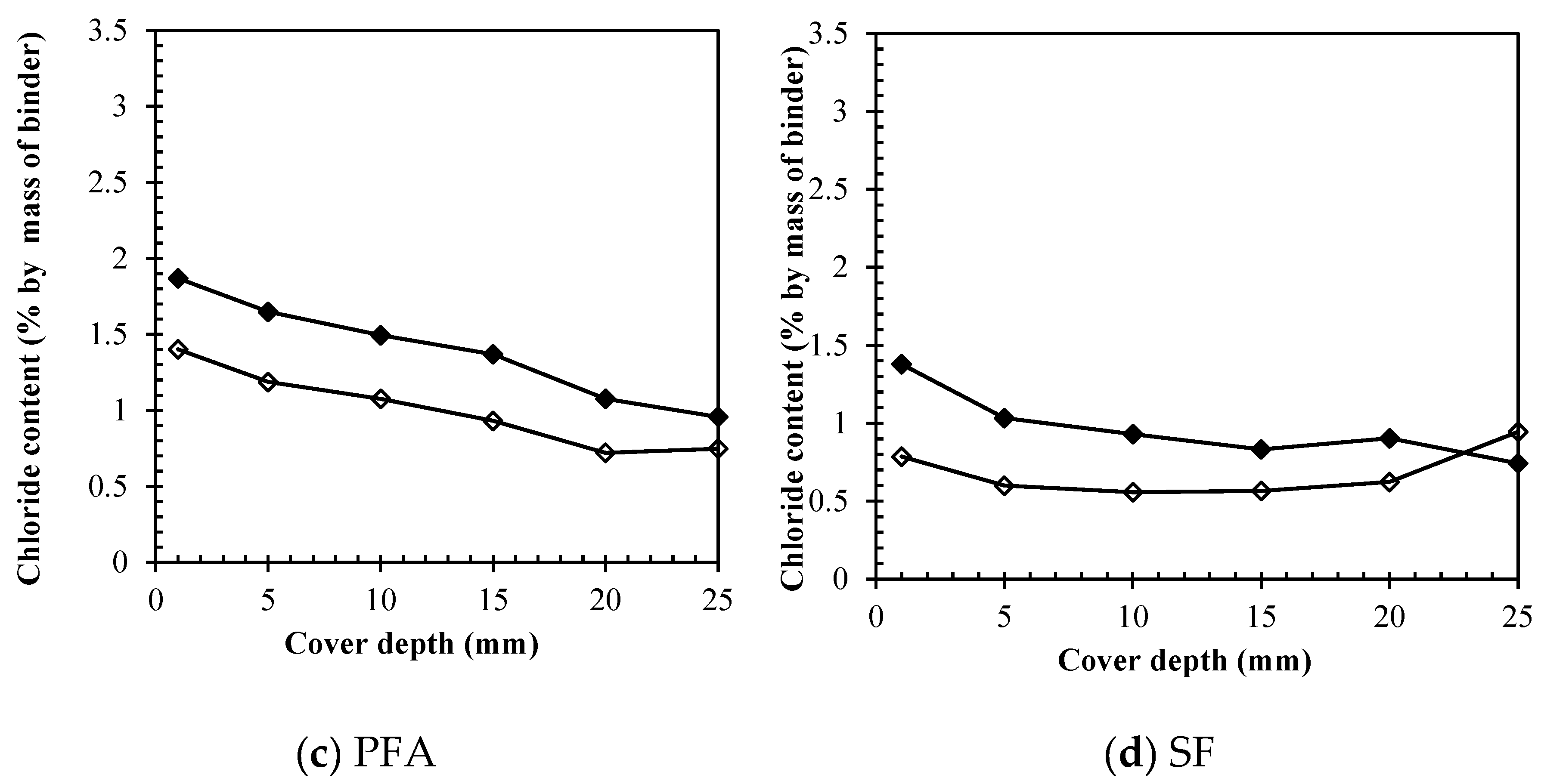
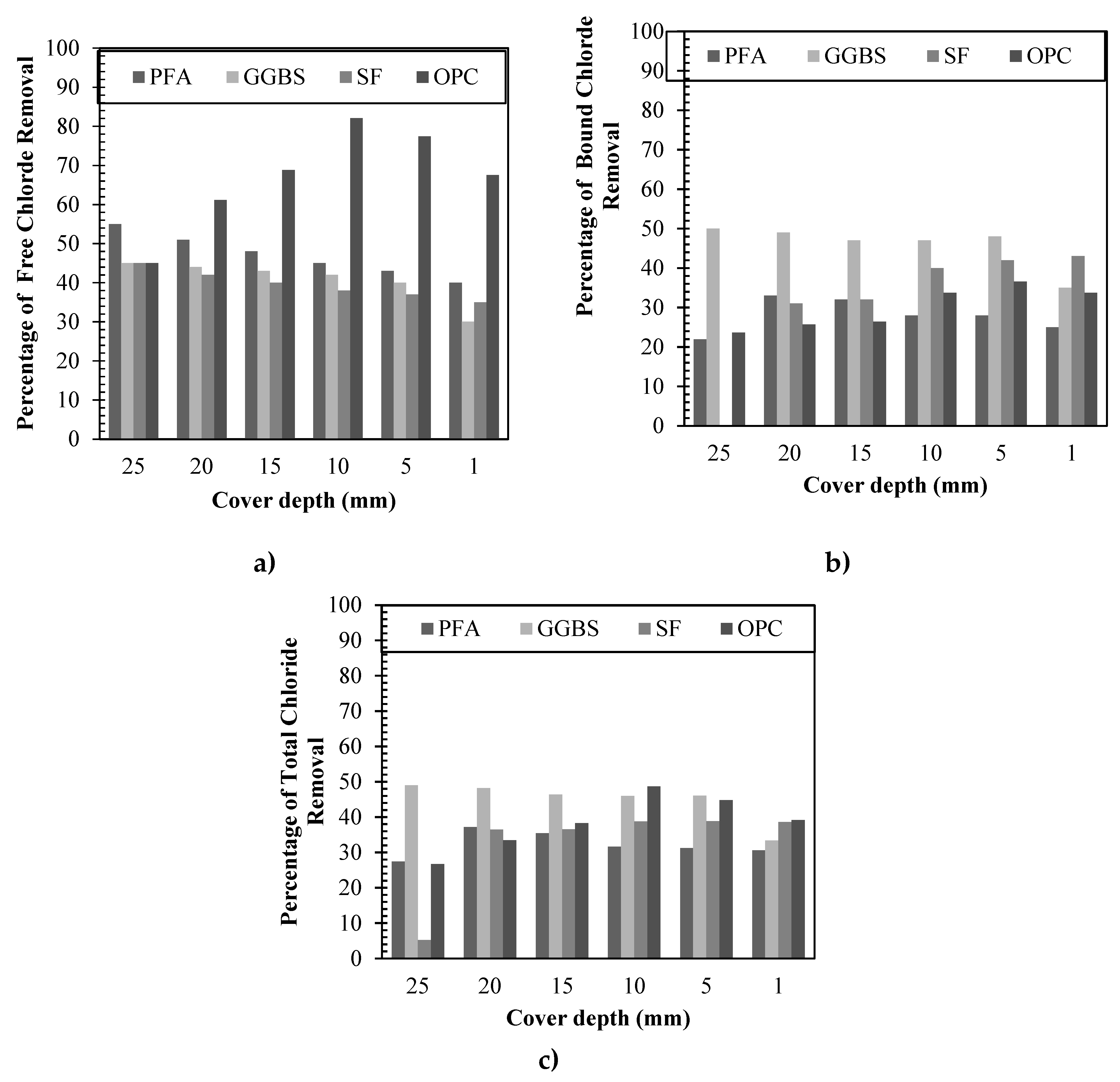
Figure 7 summarizes the results for the chloride removal rates in percentages, making a direct comparison among all binders for each depth. As it is shown on
Figure 7 (a) the removal efficiency of free chlorides ranged from 45 – 67.0% depending on the cover depth for specimens made with only OPC, being the most efficient on the free chlorides removal. For PFA, GGBS and SF, the removal occurred at similar values, resulting on percentage removal efficiencies of 40 – 55%, 35 – 45%, and 30 - 45%, respectively. The chloride removal was always greater at increasing depths for reasons previously mentioned. The concentration of chlorides at shallower depths tends to be always higher, considering that near the core of the specimen hydration of cement is expected to be at a higher degree.
In
Figure 7 (b), only the removal of bound chlorides is taken in consideration, and an important trend could be perceived. The percentage removal efficiency of bound chlorides for GGBS was considerably higher than for all others, accounting from 35 – 50%. For OPC, SF and PFA, the values were 23 -36%, 30 – 43% and 21 – 35%, respectively. The removal was slightly more significant at greater depths. This result proves that for pozzolanic materials, it is easier to extract bound chlorides, due to a higher percentage of physically adsorbed chlorides (higher amounts of silicate hydrates). Moreover, the more significant removal at greater depths again comes in agreement with the hypothesis, because as the hydration degree is higher in the core, more C-S-H gel is expected to be formed and in consequence more adsorbed chlorides. Additionally, it is important to mention that for silica fume, the increasing in the bound chloride contents at the steel depth after treatment may be due to its latent hydration characteristics, which means more possibilities of adsorbing chlorides, even during treatment. On the same line,
Figure 7 (c) represents the direct comparison of results obtained for total chlorides. A considerable removal could be perceived for all cases. In fact, different percentage removal was found for different binders being the maximum 48% for OPC and GGBS, 37% for PFA, and 36% for silica fume. The fact that even though GGBS had greater rates of bound chloride removal but still same percentage as OPC for total efficiency lays on the fact that in OPC the removal of free chlorides is more expressive, therefore, in the end, performances are somehow similar.
4. Discussions
4.1. Influence of oxide composition of different binders
In the present study, by imposing same conditions for treatment (e.g. same current density and duration, same setup concerning electrolyte and anode and initial chloride contamination), the chloride removal was strongly influenced by the binder type, confirming previous hypothesis on the consequent influence of chloride binding. In the recent years, the idea that only free chlorides could be removed during electrochemical treatment was outdated by numerous researchers that pointed to a partial mobility of bound chlorides (Glass et al.1996; Pargar et al., 2017; Stoop and Polder, 1996; Sun et al., 2016). Different binding mechanisms are present in the cement matrix. For example, in one hand, chloride ions may chemically react with C3A and C4AF in cement compounds to form chloroaluminate and chloroferrite hydrates, forming Friedel’s salt, an immobile crystalline compound. These salts could solely be dissolved into mobile, releasing free chlorides, by acidification of the cement matrix, which is not the case of concrete under electrochemical treatments. On the other hand, chlorides can be bound within C–S–H gel and adsorbed on the surface of silicate hydrates, which does not involve any chemical reaction. The absence of chemical reactions in this case, brought their classification as “loosely bound chlorides”, that can be easily decomposed into free by electric charge (Arya et al. 1987; Lambert et al. 1985).
Those binding mechanisms have important implications on the different performance perceived for all binders, OPC, GGBS, PFA and SF. For example, a remarkably lower rate of bound chlorides accompanied by a higher percentage of free chlorides before treatment was found for samples with 10% replacement of silica fume, when compared to the other three binders. Silica fume is a byproduct of producing silicon metal or ferrosilicon alloys, while GGBS is a byproduct of steel and pulverized fly ash is produced by coal-fired electric and steam generating plant. The difference on the original products for each material imposed different compositions and reactivity (Qureshi et al., 2020). Therefore, silica fume has a considerably lower percentage of CaO, Al2O3, and Fe2O3 than GGBS, PFA, and OPC. Substantially, a lower percentage of C3A and C4AF is expected to be formed on the hydration process, and, consequently, on the chemical binding of chlorides, less chloroaluminate and chloroferrite hydrates can be formed. However, at the same time, silica fume presents a considerably higher percentage of SiO2, which could induce the formation of more silicate hydrates available to adsorb chlorides. However, that does not happen due to the low presence of CaO, limiting the production of C-S-H gel for example (Bonen and Diamond, 1992). Therefore, the oxide composition explain the trend found on the experimental results, that SF is expected to present less bound chlorides when compared to other binders.
4.2. Influence of hydration products
The greater removal of bound chlorides perceived for GGBS, mainly, implies that the electric field worked better for GGBS in terms of weakening the chloride binding. This result confirmed the previous hypothesis in other works that the higher level of chloride removal for GGBS and ternary mixes at the steel depth may be attributed to the lower immobility of bound chlorides in those mixes, which would mean a higher portion of physically adsorbed chlorides by hydration products and those can turn into mobile easily under electric charge. Kim et al. (2016) came to similar results but the ratio of chemical and physical removed chlorides was not dealt with in their study.
It would be common to expect that a higher binding capacity of chlorides in concrete, imposed by pozzolanic materials, would imply a lower removal of chlorides. However, the difference between chloride binding and mobility under electric charge may be attributed to different binding mechanism. As it was previously discussed, chloride ions may chemically react with C
3A and C
4AF in cement compounds to form chloroaluminate and chloroferrite hydrates respectively, immobile crystalline salts. This type of chemically bound ions could only by mobile under acidification of the cement matrix. However, the physically adsorbed chlorides on the surface of
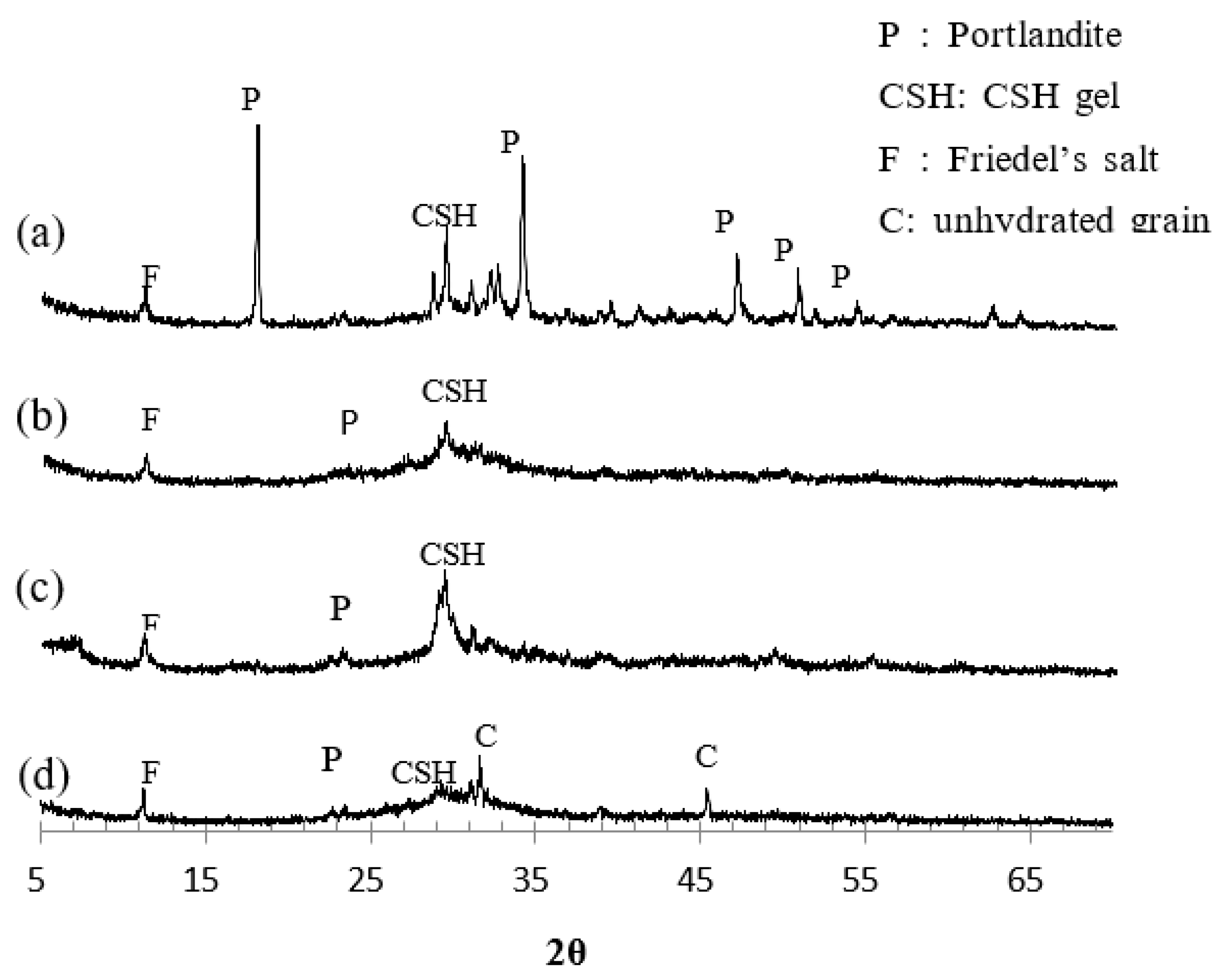
silicate hydrates and C–S–H gel can be easily decomposed under electric charge. Therefore, the results found on this dissertation are plausible. In order to provide better support for this statement, the X-ray diffractogram of the different binders is presented in
Figure 8. As it was expected, the main peak of C-S-H gel was higher for GGBS, while for PFA and SF, those peaks were smaller, in that order. Therefore, the proportion of hydration products followed the same trend as the removal of bound chlorides, being higher for GGBS. Those results are in agreement with statements by previous researchers, such as Wang and Scrivener (1995) that confirmed that C-S-H gel is the main reaction product of alkali activated slag, and, Alonso and Puertas (2000) that studied the mixture of fly ashes and slag, having reported that C-S-H gel was the main reaction product. As well as for Song et al. (1999; 2000), whom mentioned that the main reaction product of blast furnace slag hydration is C-S-H gel with minor amounts of hydrotalcite.
The results found imply as well that the driving force has predominant influence on the chloride transport on this case. Generally, the steel-concrete interface (SCI) has relevant influence on the structural durability and performance of concrete, being even more crucial when it comes to chloride induced corrosion behavior (Mohammed et al., 2015). Usually chlorides can penetrate the concrete through capillary pores, since they make a network that enables ionic transport (Mehta and Monteiro, 2014). Therefore, the pore structure of concrete also has influence on the electrochemical extraction of chloride ions from concrete. For concrete containing those different cementitious, generally the total pore volume tends to be similar to 100% OPC mix (de Gutierrez et al., 2000), however the biggest percentage of the pores in the matrix concerns smaller capillary pores. Following this line, concrete mixes with pozzolanics would be expected to have adverse effects on chloride removal, since the chloride ions would find a difficult path of smaller capillary pores (Ismail and Muhammad, 2011; Kim et al., 2016). However, there was no adverse effect; in fact, the total chloride extraction was similar for OPC and GGBS, around 48%. In addition, on the case of bound chlorides, GGBS had even better results at the steel surface. Thus, it can be inferred that the very high driving force imposed by the electric current minimize the effects of pore structure on chloride transport, turning electromigration predominant compared to the diffusion process.
5. Conclusion
In this study, the electrochemical chloride extraction treatment for concrete structures under chloride contamination was performed using an impressed current density of 2 A/m2 of concrete surface for 4 weeks. Saturated Ca(OH)2 at 0.1 molar concentration was used as electrolyte. Specimens were manufactured using 100% OPC or partial replacements of 65% GGBS, 30 % PFA or 10% SF. As a general conclusion, it is possible to see that ECE should indeed be conducted with caution depending on the binder type, considering that certain cementitious materials may present better performance of electrochemical treatment but higher initial corrosion damage as well. Moreover, the following conclusions are important to be highlighted from the experimental results:
Different percentages of total chloride removal were found according to each binder, being the maximum of 48% for OPC and GGBS, 37% for PFA and 36% for silica fume. The chloride removal rate was always greater at increasing depths. In terms of free chlorides, more expressive results were achieved, where the efficiency ranking was OPC, followed by PFA, GGBS and SF.
The chloride binding mechanisms were found to have important implications on the different performance perceived for all binders. For instance, the remarkably lower rate of bound chlorides accompanied by a higher percentage of free chlorides before treatment found for samples with 10% replacement of silica fume could be explained partially by the oxide composition of this material. Silica fume has a considerably lower percentage of oxides such as CaO, Al2O3 and Fe2O3 when compared to the others. Consequently, a lower percentage of C3A and C4AF is expected to be formed on the cement hydration process, and, thus, a lower percentage of chemically bound chlorides in form of chloroaluminate and chloroferrite hydrates is noticed.
The greater removal of bound chlorides by ECE perceived for GGBS, mainly, implies that the electric field worked better for GGBS in terms of weakening the chloride binding. This result confirmed the previous hypothesis in other works that the higher level of chloride removal for GGBS and ternary mixes at the steel depth may be attributed to the lower immobility of bound chlorides in those mixes, which would mean a higher portion of physically adsorbed chlorides by hydration products.
In order to prove that pozzolanic materials have higher rates of bound chloride removal, the X-ray diffractogram was analyzed for all binders. As expected, the main peak of C-S-H gel was higher for GGBS. A higher percentage of this hydrate influences on a higher percentage of physically adsorbed chlorides, since the chloride ions easily attach to the surface of the C-S-H particles. Physically bound chlorides can be easily turn into free due to the presence of an electrical field, as in the case for ECE. In addition, the chloride removal was greater at increasing depths, considering that, near the core of the specimen, hydration of cement happens at a higher degree.
The fact that even though GGBS had greater rates of bound chloride removal but still same percentage as OPC for total efficiency lays on the finding that in OPC the removal of free chlorides is more expressive, therefore, in the end, performances are equalized.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
We acknowledge support by the Open Access Publication Funds of Technische Universität Braunschweig.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Puertas, F.; Martı́nez-Ramı́rez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: strength behaviour and hydration products. Cement and concrete research 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Assessment of simple methods of determining the free chloride ion content of cement paste. Cement and Concrete Research 1987, 17, 907–918. [Google Scholar] [CrossRef]
- ASTM C 1202—Standard Test Method for Electrical Indication of Concrete's Ability to Resist Chloride Ion Penetration.; Annual Book of American Society for Testing Materials Standards, vol. C04.02. 2000.
-
ASTM C39 / C39M-20; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, 2020. Available online: www.astm.
- Babu, K.G.; Kumar, V.S.R. Efficiency of GGBS in concrete. Cement and Concrete Research 2000, 30, 1031–1036. [Google Scholar] [CrossRef]
- Bonen, D.; Diamond, S. Occurrence of large silica fume-derived paticles in hydrated cement paste. Cement and Concrete Research 1992, 22, 1059–1066. [Google Scholar] [CrossRef]
- Alonzo, O.; Barringer, W.L.; Barton, S.G.; Bell, L.W.; Bennett, J.E.; Boyle, M.; Dixon, D.E. Guide for selecting proportions for high-strength concrete with portland cement and fly ash. ACI Mater J 1993, 90, 272–283. [Google Scholar]
- De Gutierrez, R.; Delvasto, S.; Talero, R. Permeability properties of cement mortars blended with silica fume, fly ash, and blast furnace slag. ASTM SPECIAL TECHNICAL PUBLICATION 2000, 1399, 190–196. [Google Scholar]
- Fauzi, A.; Nuruddin, M.F.; Malkawi, A.B.; Abdullah, M.M.A.B. Study of fly ash characterization as a cementitious material. Procedia Engineering 2016, 148, 487–493. [Google Scholar] [CrossRef]
- Glass, G.K.; Wang, Y.; Buenfeld, N.R. An investigation of experimental methods used to determine free and total chloride contents. Cement and concrete research 1996, 26, 1443–1449. [Google Scholar] [CrossRef]
- Ismail, M.; Muhammad, B. Electrochemical chloride extraction effect on blended cements. Advances in cement research 2011, 23, 241–248. [Google Scholar] [CrossRef]
- Kim, K.B.; Hwang, J.P.; Ann, K.Y. Influence of cementitious binder on chloride removal under electrochemical treatment in concrete. Construction and Building Materials 2016, 104, 191–197. [Google Scholar] [CrossRef]
- Lambert, P.; Page, C.L.; Short, N.R. Pore solution chemistry of the hydrated system tricalcium silicate/sodium chloride/water. Cement and Concrete Research 1985, 15, 675–680. [Google Scholar] [CrossRef]
- Lee, K.H.; Jung, Y.H.; Hwang, J.P.; Sim, J.S. Evaluation of Electrochemical Treatment of Chloride Contaminated Mortar Containing GGBS. Advances in Materials Science and Engineering. 2017. [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: microstructure, properties, and materials. McGraw-Hill Education. 2014.
- Mohammed, T.U.; Hamada, H.; Hasnat, A.; Al Mamun, M.A. Corrosion of steel bars in concrete with the variation of microstructure of steel-concrete interface. Journal of Advanced Concrete Technology 2015, 13, 230–240. [Google Scholar] [CrossRef]
- Norton, B.; Bond, S.; Osmani, C.; Holmes, N. Influence of cement type on the efficiency of electrochemical chloride extraction. 2018. [Google Scholar]
- Pargar, F.; Koleva, D.A.; Van Breugel, K. Determination of chloride content in cementitious materials: from fundamental aspects to application of Ag/AgCl chloride sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef]
- Qureshi, L.A.; Ali, B.; Ali, A. Combined effects of supplementary cementitious materials (silica fume, GGBS, fly ash and rice husk ash) and steel fiber on the hardened properties of recycled aggregate concrete. Construction and Building Materials 2020, 263, 120636. [Google Scholar] [CrossRef]
- Ryou, J.S.; Ann, K.Y. Variation in the chloride threshold level for steel corrosion in concrete arising from different chloride sources. Magazine of Concrete Research 2008, 60, 177–187. [Google Scholar] [CrossRef]
- Siddique, R. Waste materials and by-products in concrete. Springer Science & Business Media. 2007.
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cement and concrete research 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. Journal of materials science 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Stanish, K.D.; Hooton, R.D.; Thomas, M.D.A. Testing the chloride penetration resistance of concrete: a literature review. 1997.
- Stoop, B.T.; Polder, R.B. Redistribution of chloride after electrochemical chloride removal from reinforced concrete prisms. Heron 1999, 44. [Google Scholar]
- Sun, W.; Liu, J.; Yan, J.; Dai, Y. Study on the influence of chloride ions content on the sea sand concrete performance. American Journal of Civil Engineering 2016, 4, 50–54. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cement and Concrete Research 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Yu, L.B.; Jiang, L.H.; Chu, H.Q.; Guo, M.Z.; Zhu, Z.Y.; Dong, H. Effect of electrochemical chloride removal and ground granulated blast furnace slag on the chloride binding of cement paste subjected to NaCl and Na2SO4 attack. Construction and Building Materials 2019, 220, 538–546. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The inhibitive effects of electrochemical treatment applied to steel in concrete. Corrosion Science 2000, 42, 923–927. [Google Scholar] [CrossRef]
- Tofeti Lima, T.; Ann, K.Y. Efficiency of Different Electrolytes on Electrochemical Chloride Extraction to Recover Concrete Structures under Chloride-Induced Corrosion. Advances in Materials Science and Engineering 2020. [CrossRef]
Figure 1.
- X-ray diffractogram of raw materials for a) OPC, b) PFA, c) SF and d) GGBS.
Figure 1.
- X-ray diffractogram of raw materials for a) OPC, b) PFA, c) SF and d) GGBS.
Figure 2.
- Development of compressive strength at 7, 28, 56, and 91 days of curing for different binders.
Figure 2.
- Development of compressive strength at 7, 28, 56, and 91 days of curing for different binders.
Figure 3.
- Rapid chloride permeability test results for different binders.
Figure 3.
- Rapid chloride permeability test results for different binders.
Figure 4.
– Free* chloride profiles before and after treatment using Ca(OH)2 as electrolyte for at 2A/m2 constantly for 4 weeks, for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF *Free: water soluble chlorides dissolved in 50°C distilled water.
Figure 4.
– Free* chloride profiles before and after treatment using Ca(OH)2 as electrolyte for at 2A/m2 constantly for 4 weeks, for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF *Free: water soluble chlorides dissolved in 50°C distilled water.
Figure 5.
Bound* chloride profiles before and after treatment for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF * Bound: value obtained by subtracting the free chlorides concentration from the total at each depth.
Figure 5.
Bound* chloride profiles before and after treatment for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF * Bound: value obtained by subtracting the free chlorides concentration from the total at each depth.
Figure 6.
– Total* chloride profiles before and after treatment using Ca(OH)2 as electrolyte for at 2A/m2 constantly for 4 weeks, for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF *Total: acid soluble titration against silver nitrate at the ambient temperature.
Figure 6.
– Total* chloride profiles before and after treatment using Ca(OH)2 as electrolyte for at 2A/m2 constantly for 4 weeks, for a)100% OPC b) 65% GGBS c) 30% PFA and d) 10% SF *Total: acid soluble titration against silver nitrate at the ambient temperature.
Figure 7.
– Direct comparison between different binder compositions on the efficiency on chloride removal for each designated cover depth in terms of a) total b) free and c) bound chlorides.
Figure 7.
– Direct comparison between different binder compositions on the efficiency on chloride removal for each designated cover depth in terms of a) total b) free and c) bound chlorides.
Figure 8.
– XRD patterns for hydration products of paste samples made with a) 100% OPC, b) 30% PFA, c) 65% GGBS and d) 10% SF.
Figure 8.
– XRD patterns for hydration products of paste samples made with a) 100% OPC, b) 30% PFA, c) 65% GGBS and d) 10% SF.
Table 1.
- Chemical composition obtained by X-ray fluorescence and loss of ignition of binder materials.
Table 1.
- Chemical composition obtained by X-ray fluorescence and loss of ignition of binder materials.
| Oxide Composition (%) |
|---|
| Binder |
CaO |
SiO2
|
Al2O3
|
Fe2O3
|
MgO |
SO3
|
Ignition Loss (%) |
Fineness (cm2/g) |
| OPC |
60.0 |
23.0 |
5.0 |
2.0 |
1.0 |
2.0 |
0.8 |
3120 |
| PFA |
4.22 |
55.0 |
21.1 |
10.9 |
1.2 |
0.06 |
5.0 |
|
| GGBS |
33.5 |
44.2 |
14.0 |
0.8 |
4.9 |
1.4 |
0.1 |
|
| SF |
0.54 |
94.9 |
0.27 |
0.8 |
0.9 |
0.8 |
1.0 |
|
Table 2.
- Quantities of materials per m3 of concrete in each mix design.
Table 2.
- Quantities of materials per m3 of concrete in each mix design.
| Mix proportion |
Binder (kg/m3) |
Water(kg/m3) |
Sand(kg/m3) |
Gravel(kg/m3) |
| 100% OPC |
OPC |
400 |
180 |
1056 |
704 |
| GGBS 65% |
OPC
GGBS |
140
260 |
178.8 |
1049.1 |
699.4 |
| PFA 30% |
OPC
PFA |
280
120 |
178.9 |
1049.4 |
699.6 |
| SF 10% |
OPC
SF |
360
40 |
179 |
1050.2 |
700.1 |
Table 3.
- Mix design of cement paste, mortar and concrete containing OPC, GGBS, SF and PFA and applied experiments on the mix.
Table 3.
- Mix design of cement paste, mortar and concrete containing OPC, GGBS, SF and PFA and applied experiments on the mix.
| Mix type |
Water |
Binder |
Sand |
Gravel |
Chloride source |
Experiments applied |
| Paste |
0.45 |
OPC |
GGBS |
SF |
PFA |
- |
- |
- |
XRD |
| 1.0 |
- |
- |
- |
| 0.35 |
0.65 |
- |
- |
| 0.7 |
- |
- |
0.3 |
| 0.9 |
- |
0.1 |
- |
| Mortar |
0.45 |
Same for all |
2.64 |
- |
In cast 3%Immersion |
ECE
Chloride Profile
Chloride Profile |
| Concrete |
0.45 |
Same for all |
2.64 |
3.12 |
- |
Compressive strength
RCPT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).