Submitted:
12 March 2023
Posted:
13 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Historical background
3. Definition and diagnostic criteria
| Clinical parameters | Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Central obesity | FBS | ↑ TG | ↓ HDL-C | ↑ BP | Other | Diagnosed as MS, if | |
| WHO (1998) [19] |
Waist/hip ratio: Male:>0.9 cm Female:>0.85 or BMI>30kg/m2 |
≥110mg/dl or IR or T2DM or Rx |
≥150mg/dl | Male: <40mg/dl Female: <50mg/dl |
Diastolic≥140 and systolic ≥90 mmHg | Microalbuminuria | Absolutely required IR plus ≥2 criteria |
| EGIR (1999) [20] |
WC Male: ≥94cm Female:≥80cm |
≥108.11 mg/dl | ≥150mg/dl | <39mg/dl | Diastolic≥140 and/or systolic ≥90 mmHg or Rx | Absolutely required IR plus ≥2 criteria | |
| NCEP: ATP III (2005) [40] |
WC Male:≥102cm Female:≥88cm |
≥100 mg/dl or Rx | ≥150 mg/dl | Male: <40mg/dl Female: <50mg/dl |
Diastolic≥130 and/or Systolic≥85 mmHg or Rx |
≥3criteria | |
| IDF (2005) [22] |
WC defined in terms of Ethnicity specific valuesǂ |
≥100 mg/dl or Rx | ≥150 mg/dl or Rx | Male: <40mg/dl Female: <50mg/dl |
Diastolic≥130 and/or Systolic≥85 mmHg or Rx |
Absolutely required central obesity plus ≥ 2 criteria | |
| AHA/NHLBI (2005) [24] |
WC Male: ≥102 cm Female:≥88 cm |
≥100 mg/dl or Rx | ≥150mg/dl or Rx | Male: <40mg/dl Female: <50mg/dl |
Diastolic≥130 and/or Systolic≥85 mmHg or Rx |
≥3criteria | |
| AHA/NHLBI and IDF:2009 [25] |
WC defined in terms of population and country based specific definitionǂǂ | ≥100 mg/dl or Rx | ≥150mg/dl or Rx | Male: <40mg/dl Female: <50mg/dl |
Diastolic≥130 and/or Systolic≥85 mmHg or Rx |
≥3criteria | |
| Country/Ethnic group | Waist Circumference | |
|---|---|---|
| Male | Female | |
| Europids | ≥ 94cm | ≥ 80cm |
| South Asians Based on Chinese, Malay and Asian-Indian population |
≥ 90 cm | ≥ 80 |
| Chinese | ≥ 90cm | 80 cm |
| Japanese | ≥ 90cm | ≥ 80cm |
| Ethnic South and Central Americans | Use South Asian recommended until more specific data are available | |
| Sub-Saharan Africans | Use European data until more specific data are available. | |
| Eastern Mediterranean and Middle East (Arab) population | Use European data until more specific data are available. | |
| Population | Organization | Recommended waist circumference | |
|---|---|---|---|
| Male | Female | ||
| Europid | “IDF | ≥94 cm | ≥80 cm |
| Caucasian | WHO | ≥94 cm (increased risk) ≥102 cm (still higher risk) |
≥80 cm (increased risk) ≥88 cm (still higher risk) |
| United States | AHA/NHLBI(ATP III”) | ≥102 cm | ≥88 cm |
| Canada | Health Canada | ≥102 cm | ≥88 cm |
| European | European Cardiovascular Societies | ≥102 cm | ≥88 cm |
| Asian (including Japanese) | IDF | ≥90 cm | ≥ 80 cm |
| Asian | WHO | ≥90 cm | ≥ 80 cm |
| Japanese | Japanese Obesity Society |
≥85 cm | ≥90 cm |
| China | CooperativeTask Force | ≥85 cm | ≥80 cm |
| Middle East, Mediterranean | IDF | ≥94 cm | ≥80 cm |
| Sub-Saharan African | IDF | ≥94 cm | ≥80 cm |
| Ethnic Central and South American | IDF | ≥90 cm | ≥80 cm |
4. Prevalence of Metabolic Syndrome:
5. Pathophysiology
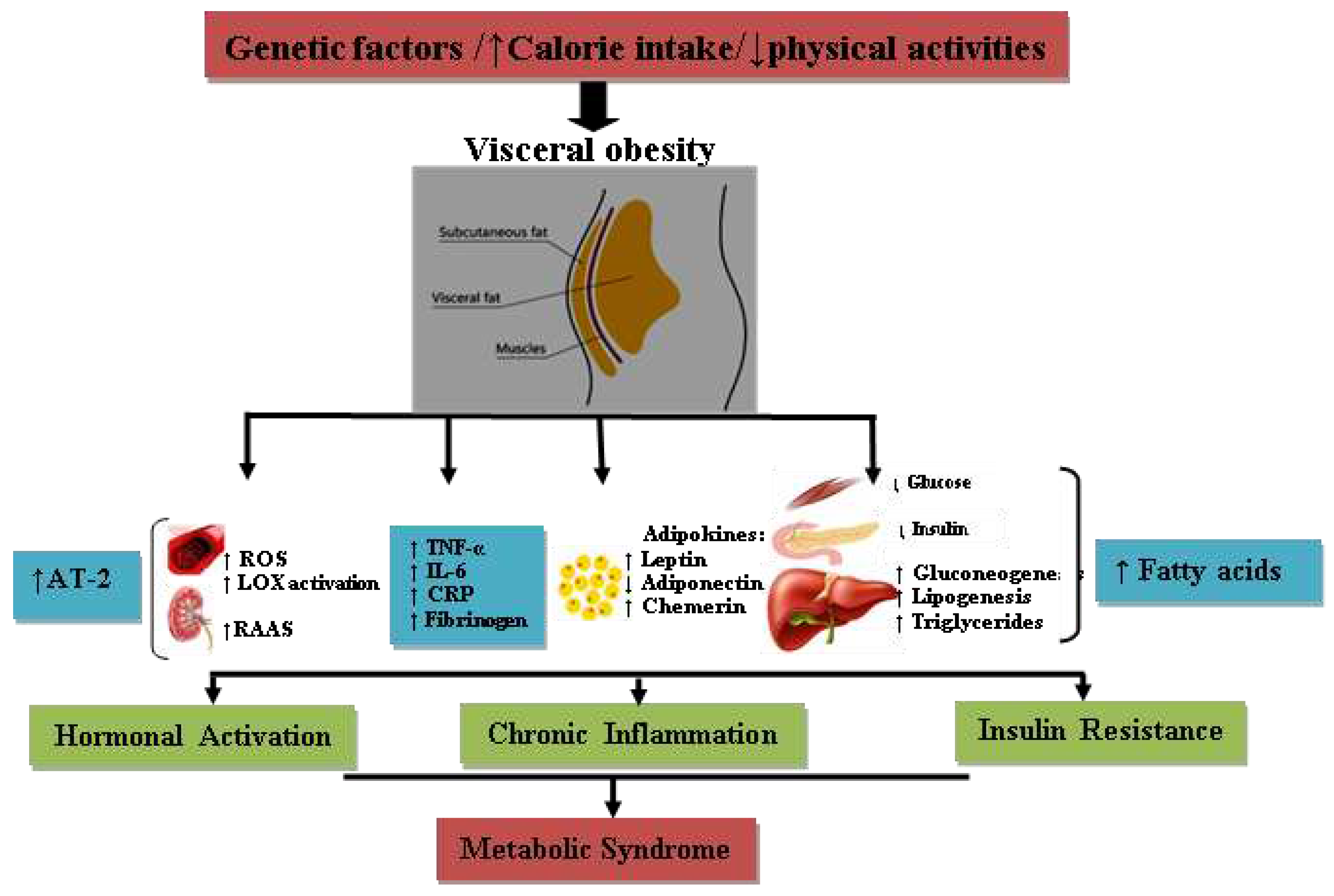
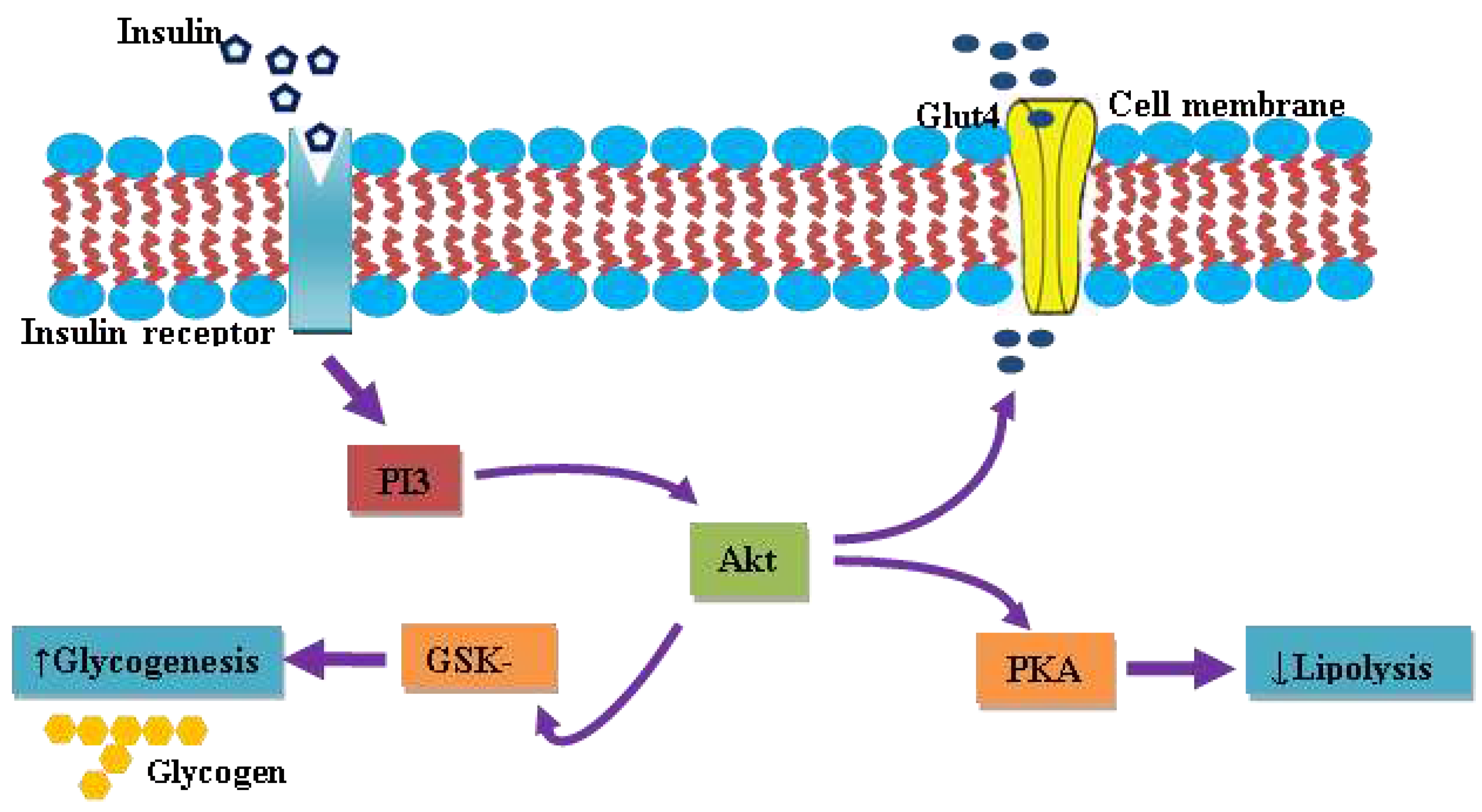
6. Insulin and Insulin resistance
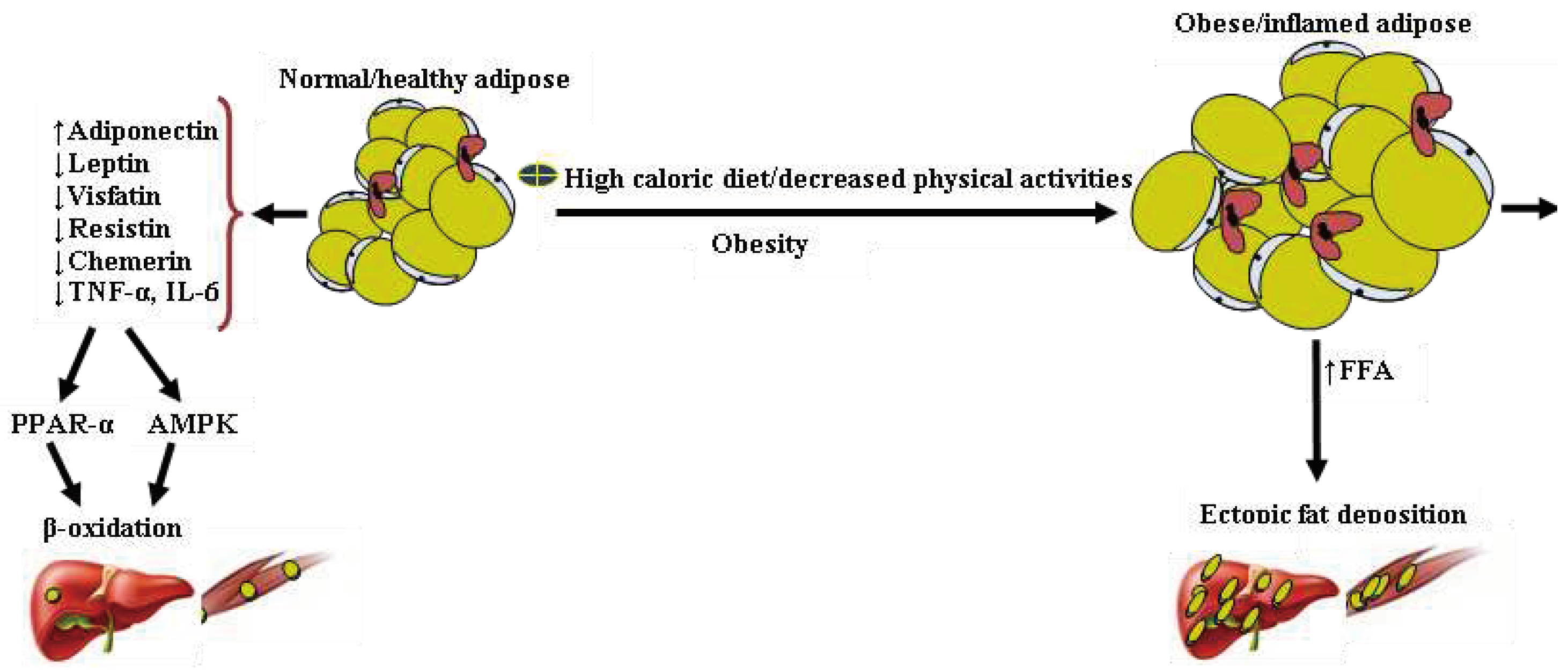
7. Adipose tissue and its endocrine activities
8. Leptin
9. Adiponectin
10. Resistin
11. Visfatin
12. Chemerin
13. Inflammatory status and oxidative stress
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M. Joint Scientific Statement, Harmonizing the Metabolic Syndrome Circulation. 2009. [Google Scholar]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Olijhoek, J.K.; Van Der Graaf, Y.; Banga, J.-D.; Algra, A.; Rabelink, T.J.; Visseren, F.L.J. The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur. Heart J. 2004, 25, 342–348. [Google Scholar] [CrossRef] [PubMed]
- A Comprehensive Review on Metabolic Syndrome. Available online: https://www.researchgate.net/publication/261445761_A_Comprehensive_Review_on_Metabolic_Syndrome (accessed on 21 August 2022).
- Apridonidze, T.; Essah, P.A.; Iuorno, M.J.; Nestler, J.E. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metabolism. 2005, 90, 1929–1935. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef]
- Haller, H.; Hanefeld, M. Synoptische betrachtung metabolischer risikofaktoren. In Lipidstoffwechselstörungen; Gustav Fischer Verlag: Jena, Germany, 1975; pp. 254–264. [Google Scholar]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, G.; Maggi, S. The metabolic syndrome: A historical context. Diabetes Voice 2006, 51, 8–10. [Google Scholar]
- Vague, J. Sexual differentiation; Factor determining forms of obesity. La Presse Médicale 1947, 55, 339. [Google Scholar]
- Paulescu, N. Traité de Physiologie Medicale; Cartea Românească: Bucharest, Romania, 1920. [Google Scholar]
- Avogaro, P.; Crepaldi, G.; Enzi, G.; Tiengo, A. Associazione di iperlipemia, diabete mellito e obesita'di medio grado. Acta Diabetol. Lat. 1967, 4, 572–590. [Google Scholar] [CrossRef]
- Avogaro, P.A.; Crepaldi, G. Essential hyperlipidemia, obesity and diabetes. Diabetologia 1965, 1, 137. [Google Scholar]
- Kaplan, N.M. The deadly quartet and the insulin resistance syndrome: An historical overview. Hypertens. Res. 1996, 19 (Suppl. I), S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Valdez, R.A.; Hazuda, H.P.; Mitchell, B.D.; Morales, P.A.; Stern, M.P. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992, 41, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tîrgovişte, C. Tratat de Diabet Paulescu; Academiei Romane: Bucharest, Romania, 2004; pp. 321–335. [Google Scholar]
- Alessi, M.C.; Juhan-Vague, I. Contribution of PAI-1 in cardiovascular pathology. Arch. Des Mal. Du Coeur Et Des Vaiss. 2004, 97, 673–678. [Google Scholar]
- Hollman, G.; Kristenson, M. The prevalence of the metabolic syndrome and its risk factors in a middle-aged Swedish population—Mainly a function of overweight? Eur. J. Cardiovasc. Nurs. 2008, 7, 21–26. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B. Comment Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999, 16, 442–443. [Google Scholar] [PubMed]
- Expert Panel on Detection, E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- IDF Epidemiology Task Force Consensus Group. International Diabetes Federation: The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2005. (accessed on). Available online: http://www. idf. org/web data/docs/Metabolic syndrome def. pdf.
- Anderson, P.J.; Critchley, J.A.; Chan, J.C.; Cockram, C.S.; Lee, Z.S.; Thomas, G.N.; Tomlinson, B. Factor analysis of the metabolic syndrome: Obesity vs insulin resistance as the central abnormality. Int. J. Obes. 2001, 25, 1782–1788. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef]
- Alberti, K.G. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Hansen, B.C.; Bray, G.A. (Eds.) The Metabolic Syndrome: Epidemiology, Clinical Treatment, and Underlying Mechanisms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Desroches, S.; Lamarche, B. The evolving definitions and increasing prevalence of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hollman, G.; Kristenson, M. The prevalence of the metabolic syndrome and its risk factors in a middle-aged Swedish population—Mainly a function of overweight? Eur. J. Cardiovasc. Nurs. 2008, 7, 21–26. [Google Scholar] [CrossRef]
- IDF Epidemiology Task Force Consensus Group. International Diabetes Federation: The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2005. (accessed on). Available online: http://www.idf.org/webdata/docs/Metabolic_syndrome_def. pdf.
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl. Health Stat. Report 2009, 5, 1–7. [Google Scholar]
- Nestel, P.; Lyu, R.; Low, L.P.; Sheu, W.H.; Nitiyanant, W.; Saito, I.; Tan, C.E. Metabolic syndrome: Recent prevalence in East and Southeast Asian populations. Asia Pac. J. Clin. Nutr. 2007, 16. [Google Scholar]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. 2004, 33, 351–375. [Google Scholar] [CrossRef]
- Yeh, W.T.; Weng, L.C. Epidemiology of metabolic syndrome in Asia. Asia Pac. J. Clin. Nutr. 2008, 17, 37–42. [Google Scholar]
- Nematic, M.; Ahmadpour, F.; Rassouli, Z.B.; Ardabili, H.M.; Azimi-Nezhad, M. A review on underlying differences in the prevalence of metabolic syndrome in the Middle East, Europe and North America. J. Mol. Genet. Med. 2014, 2, 019. [Google Scholar]
- Ko, G.T.; Cockram, C.S.; Chow, C.C.; Yeung, V.; Chan, W.B.; So, W.Y.; Chan, N.N.; Chan, J.C. High prevalence of metabolic syndrome in Hong Kong Chinese—Comparison of three diagnostic criteria. Diabetes Res. Clin. Pract. 2005, 69, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.K.; Sherpa, M.L.; Dahal, B.K.; Singh, J.K. Prevalence of Metabolic Syndrome and Its Components in Adults with Central Obesity at Janakpur Zone, Nepal. J. Nepal. Health Res. Counc. 2021, 18, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Deedwania, P.C.; Gupta, A.; Rastogi, S.; Panwar, R.B.; Kothari, K. Prevalence of metabolic syndrome in an Indian urban population. Int. J. Cardiol. 2004, 97, 257–261. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 93 (11 Suppl. 1), s9–s30. [Google Scholar] [CrossRef]
- Sawant, A.; Mankeshwar, R.; Shah, S.; Raghavan, R.; Dhongde, G.; Raje, H.; D'souza, S.; Subramanium, A.; Dhairyawan, P.; Tour, S.; et al. Prevalence of metabolic syndrome in urban India. Cholesterol 2011, 2011, 920983. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Wainwright, G.; Mascitelli, L. Is the metabolic syndrome caused by a high fructose, and relatively low fat, low cholesterol diet? Arch. Med. Sci. 2011, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The concept of metabolic syndrome: Contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar]
- Handbook of Diabetes, 4th ed.; Excerpt #4: Normal Physiology of Insulin Secretion and Action; Diabetes In Control; A free weekly diabetes newsletter for Medical Professionals 2014-07-28. Retrieved 2017-06-01; Wiley-Blackwell: Hoboken, NJ, USA, 2010.
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, J.M.; Alessi, D.R. The insulin signalling pathway. Curr. Biol. 2002, 12, R236–R238. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.R. Mechanisms regulating phosphoinositide 3-kinase signaling in insulin-sensitive tissues. Acta Physiol. Scand. 2005, 183, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E.; Hannemann, M.M. Adverse endothelial function and the insulin resistance syndrome. J. Intern. Med. 2000, 247, 425–431. [Google Scholar] [CrossRef]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and β-cell dysfunction. Eur. J. Clin. Investig. 2002, 32, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Bernlohr, D.A.; Jenkins, A.E.; Bennaars, A.A. Adipose tissue and lipid metabolism. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 263–289. [Google Scholar]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; George, S.R.; O’Dowd, B.F. Unravelling the roles of the apelin system: Prospective therapeutic applications in heart failure and obesity. Trends Pharmacol. Sci. 2006, 27, 190–194. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Muoio, D.M.; Dohn, G.L.; Fiedorek, F.T., Jr.; Tapscott, E.B.; Coleman, R.A. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 1997, 46, 1360–1363. [Google Scholar] [CrossRef]
- Wallace, A.M. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2004, 44, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Ohishi, M.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nagaretani, H.; Kumada, M.; Ohashi, K.; Okamoto, Y.; Nishizawa, H.; et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003, 42, 231–234. [Google Scholar] [CrossRef]
- Pischon, T.; Girman, C.J.; Hotamisligil, G.S.; Rifai, N.; Hu, F.B.; Rimm, E.B. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004, 291, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Hammer, R.E.; Ikemoto, S.; Brown, M.S.; Goldstein, J.L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999, 401, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, K.; Ogawa, Y.; Masuzaki, H.; Shintani, M.; Miyanaga, F.; Aizawa-Abe, M.; et al. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes 2001, 50, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, F.; García-Macedo, R.; Alarcón-Aguilar, F.; Cruz, M. Adipocinas, tejido adiposo y su relación con células del sistema inmune. Gac. Méd. Méx. 2005, 141, 505–512. [Google Scholar] [PubMed]
- Ukkola, O.; Santaniemi, M. Adiponectin: A link between excess adiposity and associated comorbidities? J. Mol. Med. 2002, 80, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Lee, G.Y.; Chung, J.J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor α. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Funahashi, T.; Hanson, R.L.; Matsuzawa, Y.; Tanaka, S.; Tataranni, P.A.; Knowler, W.C.; Krakoff, J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002, 360, 57–58. [Google Scholar] [CrossRef]
- Sánchez, J.C.; López, D.F.; Pinzón, Ó.A.; Sepúlveda, J.C. Adipocinas y síndrome metabólico: Múltiples facetas de un proceso fisiopatológico complejo. Rev. Colomb. De Cardiol. 2010, 17, 167–176. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Way, J.M.; Görgün, C.Z.; Tong, Q.; Uysal, K.T.; Brown, K.K.; Harrington, W.W.; Oliver, W.R.; Willson, T.M.; Kliewer, S.A.; Hotamisligil, G.S. Adipose tissue resistin expression is severely suppressed in obesity and stimulated by peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 2001, 276, 25651–25653. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, A.K.; Gupta, V.; Kumar, S.; Srivastava, N.; Jafar, T.; Pant, A.B. Association of circulating resistin with metabolic risk factors in Indian females having metabolic syndrome. Toxicol. Int. 2011, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.R.; Lazar, M.A. Human resistin: Found in translation from mouse to man. Trends Endocrinol. Metab. 2011, 22, 259–265. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- Jurdana, M.; Petelin, A.; Bizjak, M.Č.; Bizjak, M.; Biolo, G.; Jenko-Pražnikar, Z. Increased serum visfatin levels in obesity and its association with anthropometric/biochemical parameters, physical inactivity and nutrition. Clin. Nutr. ESPEN 2013, 8, E59–E67. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with pro-inflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Wu, Q.-F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Muruganandan, S.; Dranse, H.J.; McMullen, N.M.; Signal, C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 2014, 222, 201–215. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.N.; Imbault, V.; Robert, V.; De Poorter, C.; Mcheik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Chakaroun, R.; Raschpichler, M.; Kloting, N.; Oberbach, A.; Fleming, G.; Kern, M.; Schön, M.R.; Shang, E.; Lohmann, T.; Dreßler, M.; Fasshauer, M. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B. Oxidative stress and inflammation: What polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor-alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Deshmukh, A.; GuruMurthy, G.S.; Pothineni, N.V.; Watts, T.E.; Romeo, F.; Mehta, J.L. Inflammation and atherosclerosis—Revisited. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor-alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Laclaustra, M.; Corella, D.; Ordovas, J.M. Metabolic syndrome pathophysiology: The role of adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 125–139. [Google Scholar] [CrossRef]
- Bernlohr, D.A.; Jenkins, A.E.; Bennaars, A.A. Adipose tissue and lipid metabolism. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 263–289. [Google Scholar]
- Wisse, B.E. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





