1. Introduction
Aluminum phosphide (AlP) is an effective pesticide for preserving stored grain, especially rice, from weevil damage.[
1] AlP is known as the “rice tablet” in Iran and is widely used in Iran and some other countries, such as India, Thailand, Pakistan,
etc., to protect stored grains. However, acute and lethal toxicity is a common complication associated with AlP.[
1] There is a concerning record of suicidal attempts using AlP in Iran.[
1,
2,
3,
4,
5,
6] Unfortunately, there is no efficient therapeutic intervention against AlP poisoning so far, and the mortality rate of this compound is very high (up to 80%).[
7,
8]
AlP releases a significant amount of phosphine gas (PH
3) upon interacting with moisture or acids (
e.g., gastric HCl).[
9] Mechanistically, phosphine robustly impairs cellular energy metabolism. Phosphine inhibits the cytochrome
c oxidase complex and consequently impairs mitochondrial ATP production.[
9,
10] The phosphine-induced energy crisis is perilous for high energy-demanding organs such as the heart and brain,[
8,
11,
12]. However, many other complications and multi-organ failure could also occur in AlP toxicity, resulting in patient death.[
8,
13,
14]
AlP poisoning is an emergency state that requires immediate and rigorous therapeutic intervention. In addition to supportive care, previous studies suggested that the administration of olive/castor/coconut oils and antioxidant molecules such as N-acetyl cysteine, melatonin, vitamin C, and vitamin E are effective in the treatment of phosphine poisoning and significantly decreased AlP toxicity-associated complications.[
15,
16,
17,
18,
19,
20,
21,
22] These interventions should be considered along with rigorous monitoring of vital signs, blood biochemistry, and brain/heart function. However, there is still a high mortality rate despite the treatment of AlP poisoning based on the mentioned routine protocols. The mortality rate of patients largely depends on many factors, including the amount of AlP ingested, time of admission to the hospital, concurrent poisoning with other agents, and the quality of therapeutic strategies and hospital care. Therefore, developing effective therapeutic options for managing AlP poisoning is critical.
Dihydroxyacetone (1, 3-dihydroxypropanone; DHA) is a simple saccharide freely soluble in water. DHA has been investigated for its pharmacological and cosmetic effects. It is used for skin tanning, increasing exercise capacity in athletes and the elderly, reducing weight, and mitigating the complications of fatty liver disease.[
23,
24,
25,
26,
27,
28] Previously, we found that DHA is an effective antidote against the toxicity induced by lethal mitochondrial toxin cyanide in both
in vitro and
in vivo experiments.[
29,
30,
31] It was found that DHA significantly improved mitochondrial function and prevented cellular ATP depletion during cyanide exposure.[
29,
30,
31] In other
in vitro and
in vivo studies, we also found that DHA significantly prevented the toxicity induced by phosphine.[
32,
33,
34] It was found that DHA significantly prevented PH
3 cytotoxicity in cultured HePG2 cells and significantly decreased the mortality of phosphine-poisoned mice.[
32,
33,
34] Since DHA is produced in the body in the glycolysis pathway and is a very safe compound with an LD
50 of more than 8 g/kg in mice,[
35] the current study was designed to evaluate the effect of DHA in AlP-poisoned human cases.
In some recent animal studies, it has been reported that oral DHA administration significantly decreased cardiovascular complications of AlP toxicity.[
36] However, as AlP poisoning is an emergency state that rapidly deteriorates vital signs, a big challenge in managing AlP toxicity is the speed of delivering the protective agents to the body. On the other hand, it is preferred not to administer these agents by oral rote in AlP poisoning because of its complications (
e.g., increasing the chance of phosphine gas release). Hence, the parenteral delivery of protective agents is ideal.
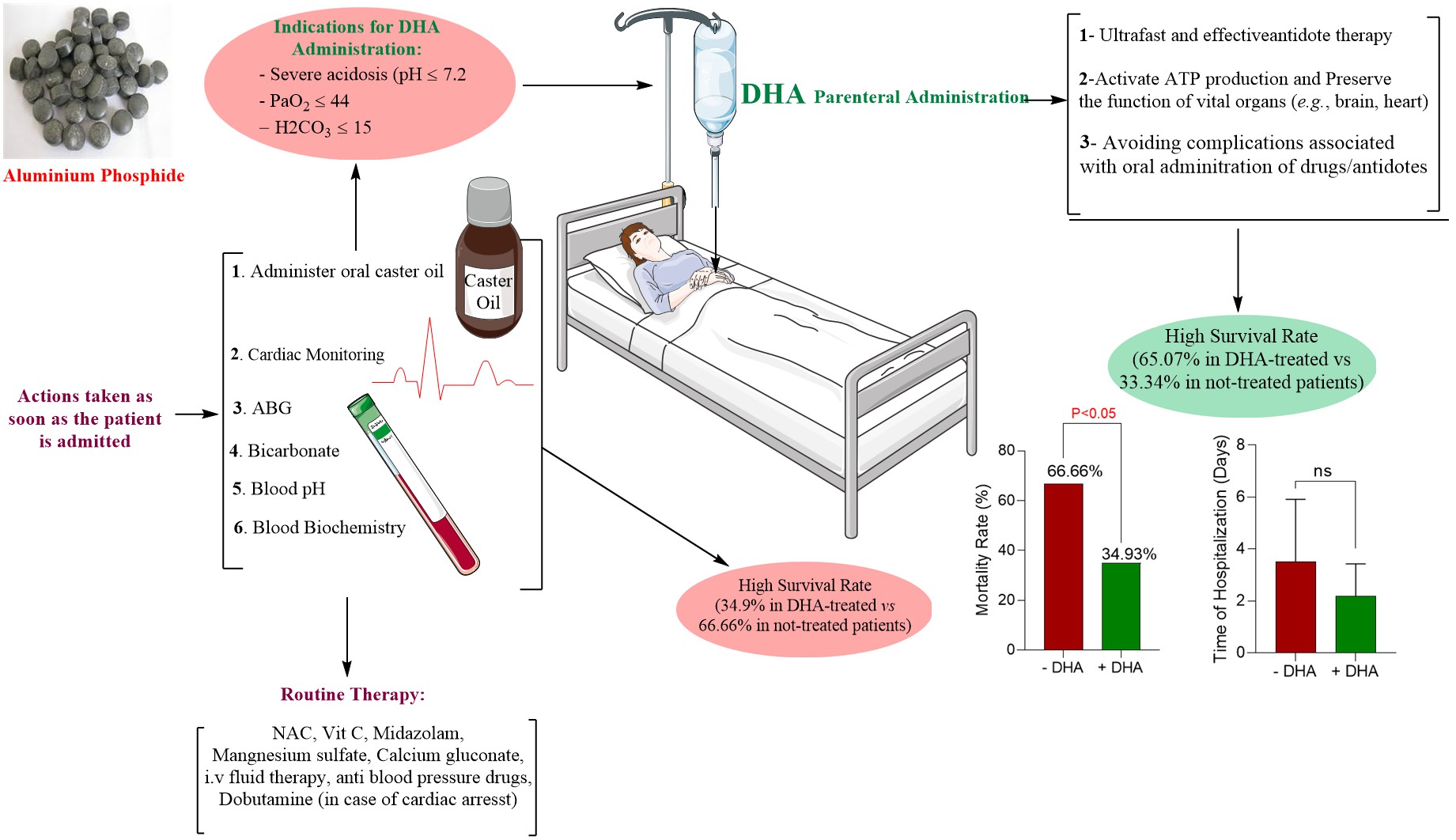
In the current study, patients with confirmed AlP poisoning received a parenteral formulation of DHA (at least one dose of 500 ml of 5% w: v of DHA solution, i.v.) in addition to systematic AlP poisoning treatment strategies. Several biomarkers and patients’ survival were evaluated and compared with AlP-poisoned patients without DHA therapy. This data could pave the way to effectively treating AlP poisoning and decreasing the high mortality rate induced by this lethal toxin.
2. Methods
2.2. Study Design and Patients
This study enrolled patients aged 17-60 (male and female) diagnosed with confirmed AlP poisoning with significant blood acidosis, decreased PaO
2, and increased PaCO
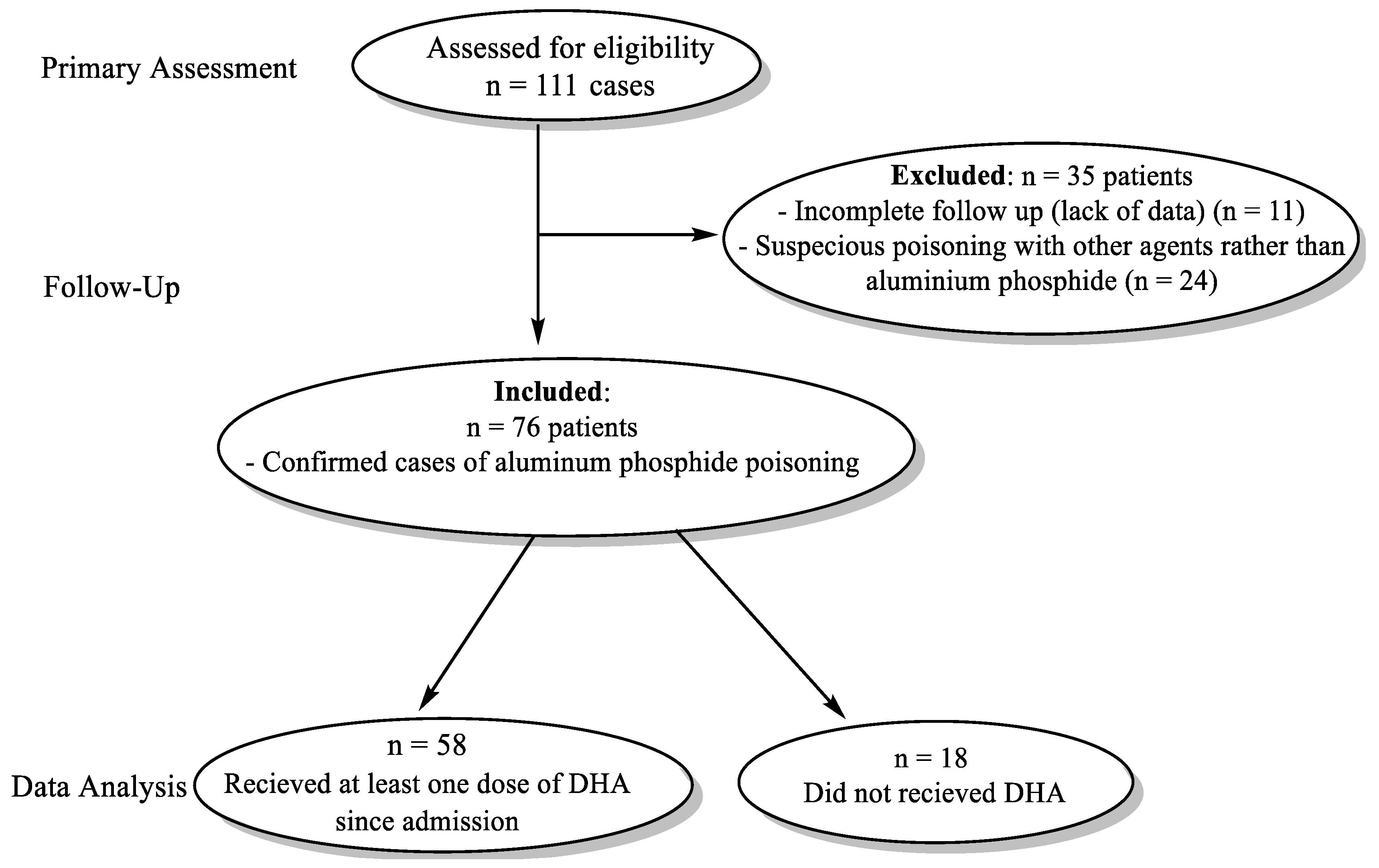
2 in arterial blood gas analysis from October 2016 to December 2021. The study was conducted on patients referred to the Emergency wards of Shahid Faghihi, Aliasghar, and Nemazi Hospitals, Shiraz University of Medical Sciences, Shiraz, Iran, and Loghman and Maharlooie Hospitals, Tehran, Iran. Patients were excluded if there was an incomplete follow-up during hospitalization or suspicious poisoning with other agents rather than AlP. The flow chart for selecting patients for receiving DHA and standard routine treatments for AlP-poisoned patients is shown in
Figure 1. In the primary assessment, n = 111 patients were evaluated for eligibility criteria. Among these patients, n = 35 cases were excluded due to incomplete data (n = 11) and suspicion of poisoning rather than AlP (n = 24). Meanwhile, n = 76 cases with confirmed AlP poisoning were included in the study. AlP-poisoned patients who did not receive DHA (n =18) were used as the control group. Other patients (n = 58) received at least one dose of DHA (500 ml of 5% DHA solution w/v, i.v.) (
Figure 1). Arterial blood gas (ABG), blood pH, bicarbonate levels, and other vital signs and biochemical measurements were monitored. Moreover, the mortality rate and time of hospitalization were evaluated in both DHA-treated (n = 58) and AlP-poisoned patients without DHA administration (n = 18) (
Figure 1). Several biomarkers were assessed before (upon hospitalization) and after DHA treatment. The routine tests for AlP-poisoned patients in this study were the measurement of electrolytes (K
+ and Na
+), WBC, RBC, hemoglobin, INR, carbonate (HCO
3), blood pH, PaCO
2, and PaO
2 and SGPT, SGOT, BUN, Cr.
2.3. DHA Treatment Protocol
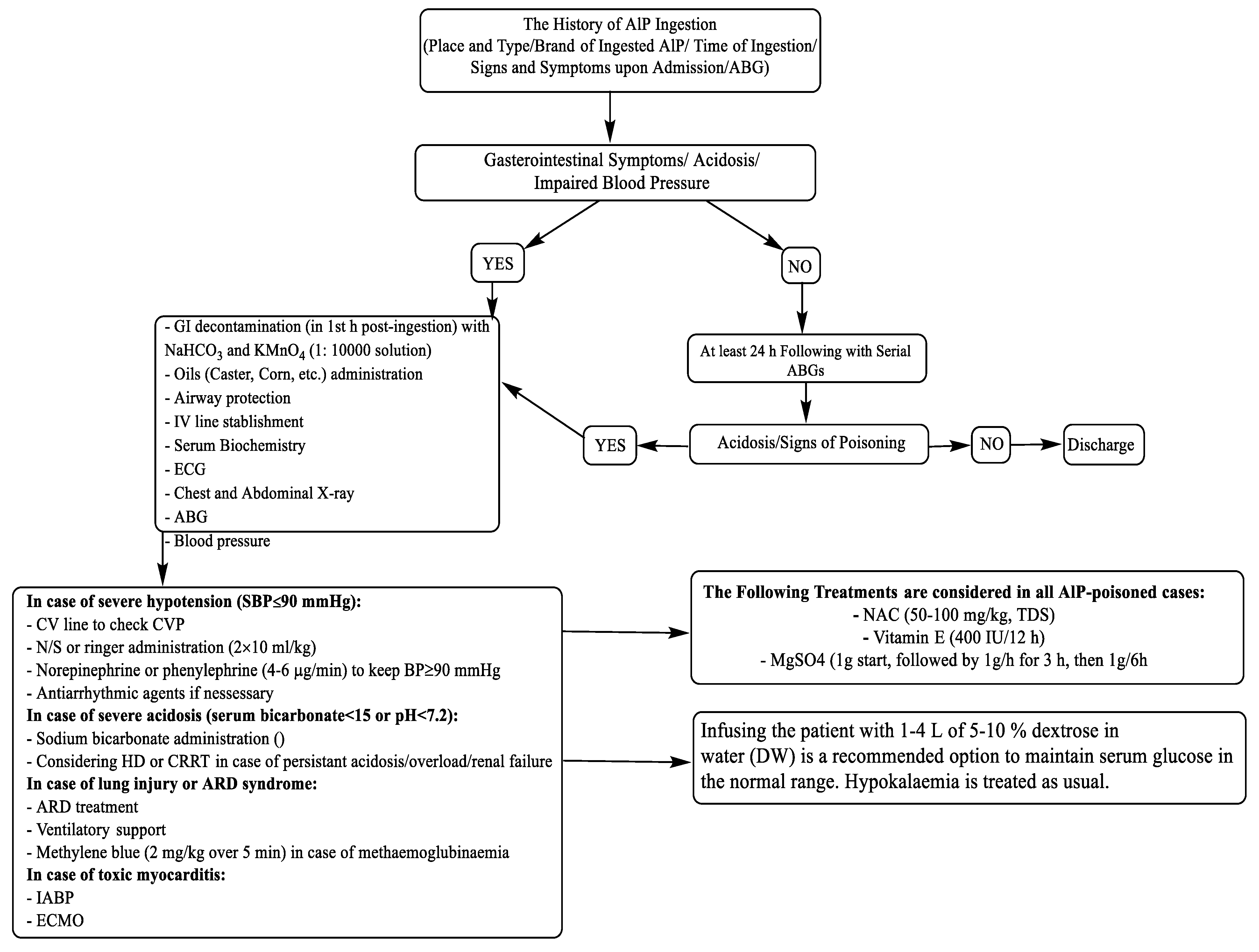
In addition to routine treatments for AlP poisoning (
Figure 2), the first 500 ml of 5% DHA was infused over 1 hour. Afterward, each 500 ml bottle was infused over 3 to 4 hours, if needed, till the patients' condition became normal. Usually, 2000 to 2500 ml of DHA was needed for each patient.
2.4. Statistical Analysis
Data are represented as mean±SD. The data comparison for biomarkers assessed before and after DHA administration was carried out using a two-tailed student T-test. The data distribution for hospitalization time was not parametric, and the Wilcoxon test was used for data comparison.
3. Results
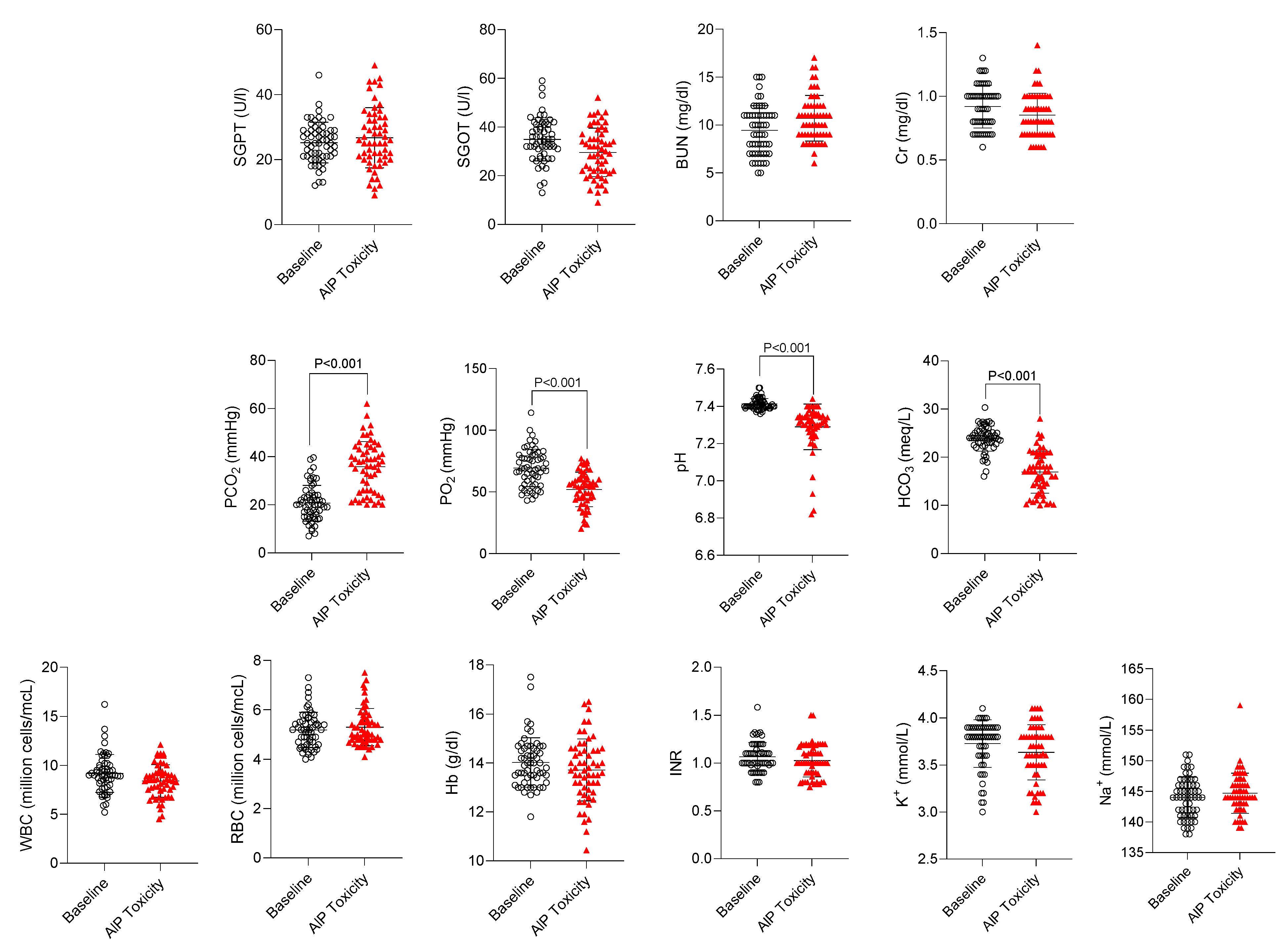
Several parameters, including biomarkers of organ injury (SGPT, SGOT, BUN, Cr), electrolytes (K
+ and Na
+), WBC, RBC, hemoglobin, INR, carbonate (HCO
3), blood pH, PaCO
2, and PaO
2 were assessed in confirmed cases of AlP-poisoned patients (n = 58) (
Figure 2). No significant changes in biomarkers such as organ injury markers (SGPT, SGOT, BUN, Cr), CBC, hemoglobin, hematocrit, blood coagulation, and electrolytes were detected in AlP-intoxicated cases included in the current study (
Figure 3). On the other hand, it was found that blood pH, PaO
2, and HCO
3 were significantly decreased, whereas PaCO
2 was increased in AlP-poisoned patients (
Figure 3). Therefore, these markers (pH, PaO
2, PaCO
2, and HCO
3) were selected as hallmarks of AlP poisoning and monitored in patients treated with DHA (
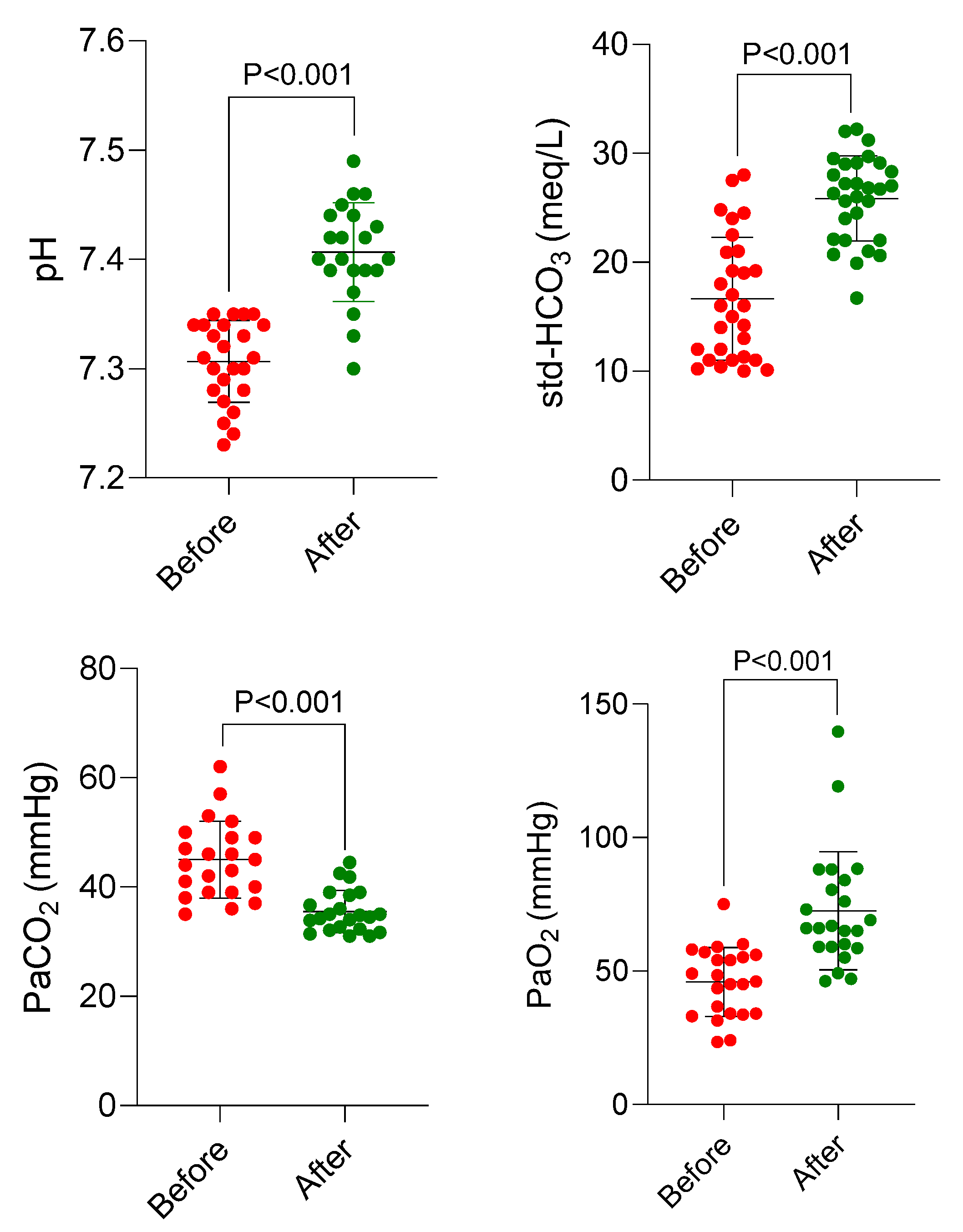
Figure 4).
The effects of DHA on these parameters before and after treatment with DHA are shown in
Figure 4. It was found that acidosis was blunted, PaO
2 was increased, HCO
3 was normalized, and PaCO
2 was significantly decreased in AlP-poisoned patients treated with DHA (
Figure 4).
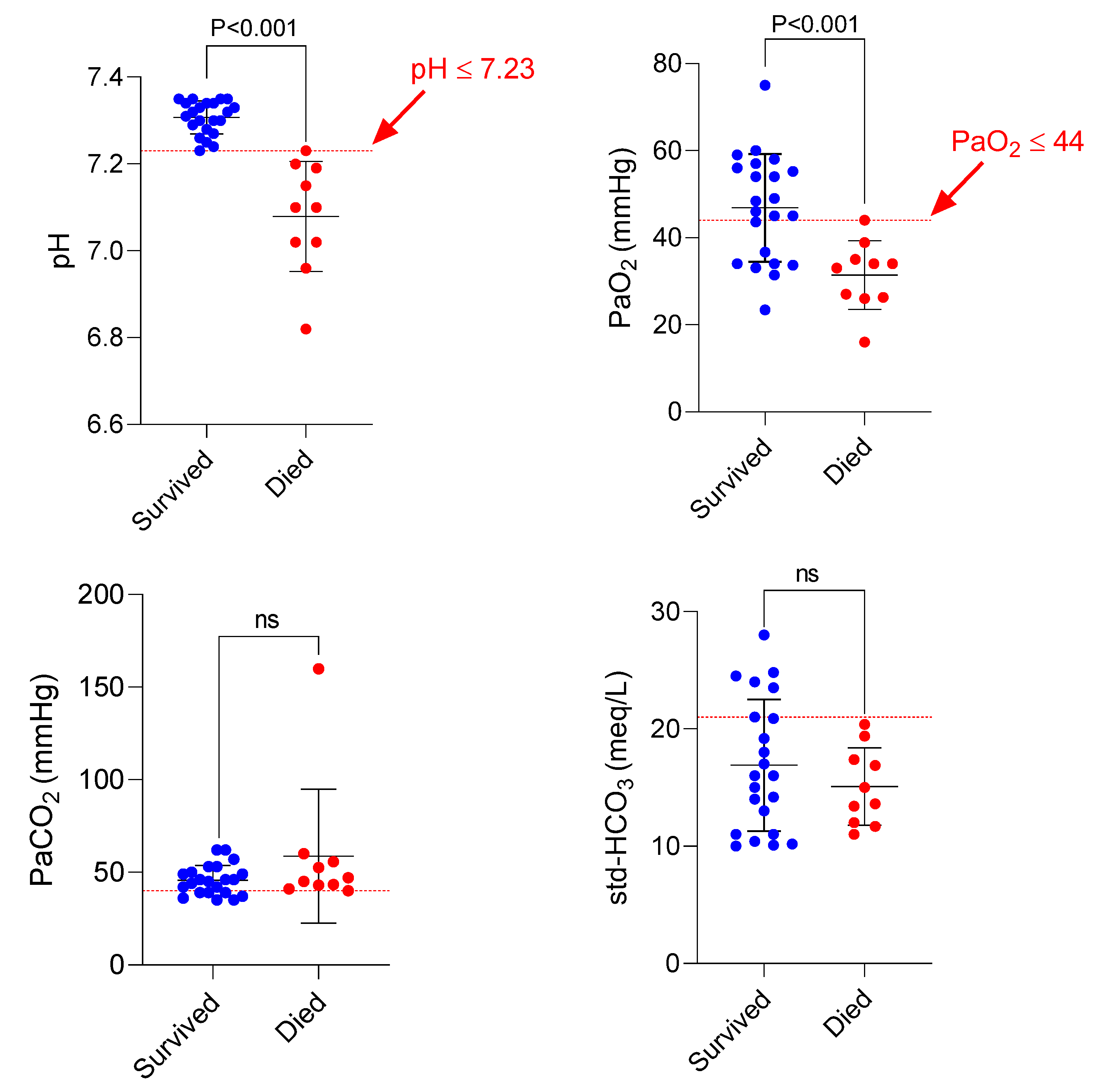
A critical finding of the current study was associated with factors related to poor prognosis in AlP-intoxicated patients (
Figure 5). It was found that patients with blood pH ≤ 7.23 or PaO
2 ≤ 44 mmHg died despite receiving DHA (
Figure 5).
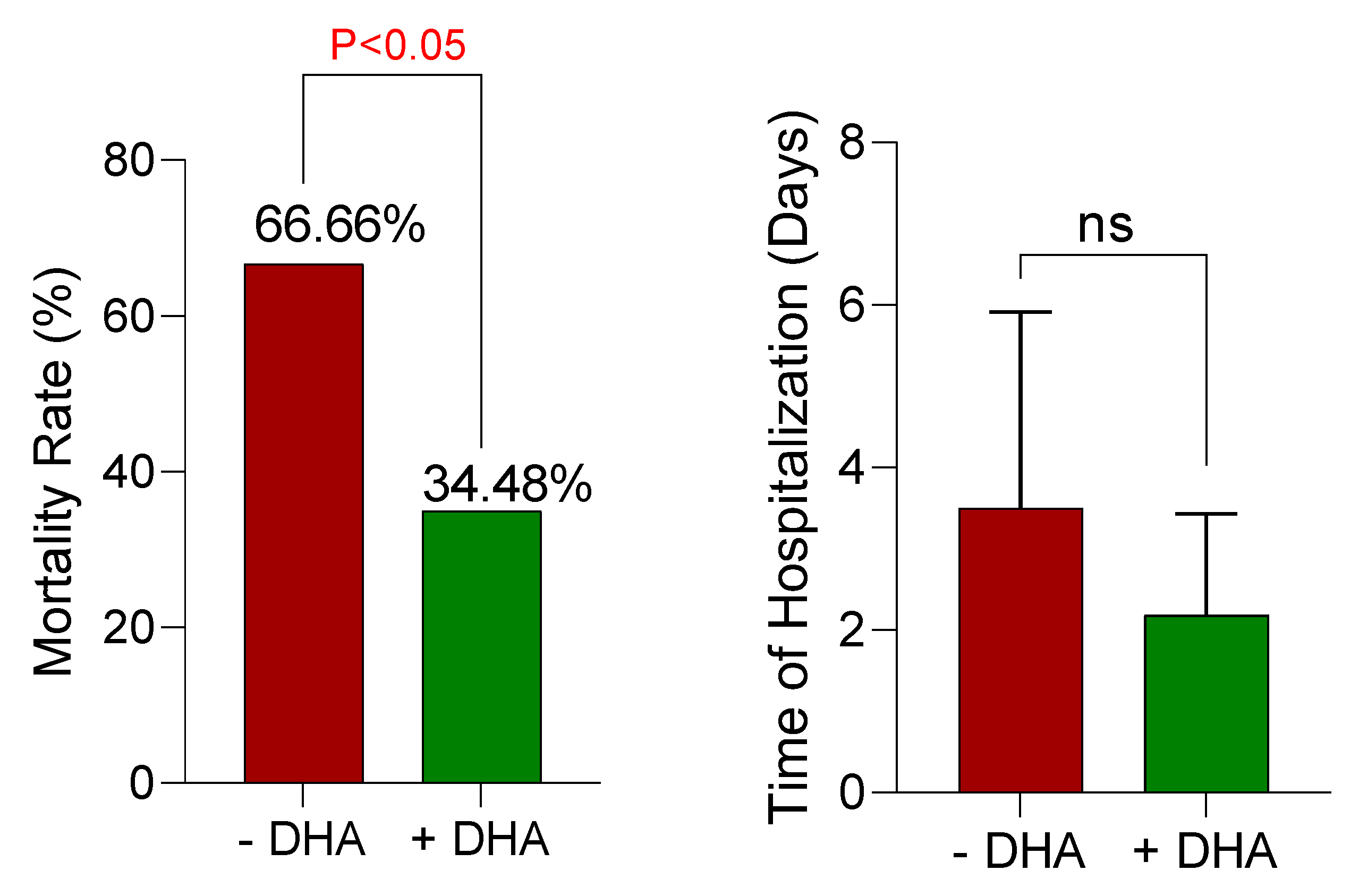
The mortality rate was another critical factor evaluated in DHA-treated patients and patients with routine AlP treatment protocols (
Figure 6). It was found that DHA administration significantly decreased the mortality rate (about 67% in non-treated vs. about 35% in DHA-treated cases) (
Figure 6). Although it was not statistically significant, the hospitalization time also tended to decrease in DHA-treated patients (
Figure 6).
4. Discussion
AlP is widely used in Iran as a pesticide for preserving stored grain, especially rice, from weevil damage.[
1] A significant number of accidental or intentional (suicidal attempts) cases of AlP poisoning are recorded annually, and its toxicity is associated with a high mortality rate.[
2,
37,
38] Unfortunately, there is no specific therapeutic intervention for AlP poisoning, and treatments are primarily supportive care. In the current study, we assessed the antidotal effect of a novel formulation of DHA for intravenous administration in AlP-intoxicated patients. The results indicate prominent antidotal effects of DHA against AlP poisoning as it decreased the mortality rate and normalized biochemical alterations in patients.
Our previous studies on the antidotal effects of DHA revealed that this saccharide could effectively prevent toxicity induced by lethal doses of the mitochondrial toxin cyanide in mice and rabbits
in vivo, primarily by reversibly binding to CN ion.[
29,
30,
31] We also previously showed that DHA could antagonize the toxicity of phosphine in cell culture[
34] and
in vivo in mice,[
32,
33] also by reversible binding to PH
3. DHA is produced in the form of phosphate in the glycolysis pathway and plays a role in the synthesis of ATP in the cell. Since the primary mechanism of phosphine toxicity is mediated through the inhibition of ATP production in the cell, the entry of DHA into the glycolysis pathway also can prevent the energy (ATP) crisis in cells and inhibit cell death. This is critical for preventing phosphine-induced organ damage, especially in high energy-demanding organs such as the heart and brain. In a recent preclinical animal study, we found that DHA could significantly decrease the mortality rate in phosphine-treated mice.[
33] It also significantly restored the cytochrome c oxidase enzyme activity as the primary target for phosphine toxicity in various tissues of phosphine-exposed animals,[
33] possibly by reversibly binding to PH
3 and partially restoring cytochrome c oxidase activity.[
33] Therefore, the direct effect of DHA on mitochondrial function and energy metabolism plays a central role in its protective properties against AlP toxicity. On the other hand, as mentioned, DHA could also ease ATP production through the glycolysis process in a mitochondria-independent manner.[
31] This unique characteristic of DHA could save the functionality of vital organs such as the brain and heart until appropriate ATP production is restored upon increased cytochrome c oxidase activity.
An interesting finding in the current study was related to some biomarkers, such as acidosis and PaO
2 in AlP-poisoned cases (
Figure 4 and
Figure 5). Our data indicated that significant acidosis and decreased PaO
2 are severe clinical manifestations of AlP toxicity associated with a poor prognosis (Figs 4, 5). We found that all of the poisoned patients who died had a pH≤7.23 and a PaO
2≤44 (
Figure 5). Based on this data, it could be better to start DHA therapy as soon as possible before pH or PaO
2 levels fall below the above-mentioned values. This simple point might significantly increase the survival rate of AlP-intoxicated patients.
On the other hand, it was found that DHA significantly improved severe acidosis and PaO
2 in AlP-poisoned patients (
Figure 4). Based on these data, the effects of DHA on these critical factors could play a vital role in its antidotal mechanisms in AlP toxicity. Unfortunately, although treatment protocols including gastric decontamination (
e.g., caster oil), correcting blood acidosis by bicarbonate, administration of drugs for the management of patient agitation, and antihypertensive drugs are routinely followed (
Figure 2), still the mortality rate of AlP is very high (up to 80%), depending on factors such as the quality of supportive care, the dose of ingested AlP, and the time of hospitalization.[
39,
40] Therefore, there is a need for an effective and rapidly acting agent. This novel DHA i.v. formulation, in addition to its significant antidotal effect, has an accelerated action time, which is its great strength. Also, the administration of oral oils in the cases of AlP poisoning only can partially inhibit the absorption of PH
3 created in the GI tract but cannot affect the already absorbed PH
3. Administration of i.v. DHA can bind the PH
3 circulating in the blood and present in the cell, as DHA can readily enter the cell.
Recently, a case report of DHA administration in AlP poisoning has been described, in which DHA was orally administered to two cases of AlP-poisoned patients.[
41] It should be mentioned that oral administration of DHA in water could increase the risk of PH
3 release from ingested AlP, and it may worsen the patient's condition. As mentioned, AlP poisoning is an emergency state that needs restriction and rapid intervention. Moreover, systemic administration (i.v.) of DHA could counteract the effects of already-absorbed PH
3 in AlP-poisoned patients.
5. Conclusion
Decreasing the mortality rate of AlP-poisoned patients is the tremendous clinical achievement of the current study. We found that parenteral DHA administration tremendously decreased DHA-poisoned patients’ mortality rate by about 65%. Interpreting the results of the current study and reaching conclusions are matters of assessing the integrity of the trial's design and the validity of the findings in a more controlled environment as well as on more patients. Finally, this novelty-designed formulation could ultimately find its application on a large scale.
Author Contributions
HN comprehended and designed this study. VA was involved in DHA formulation. RH performed data visualization, manuscript draft preparation, and statistical analysis. AJ was involved in DHA administration to poisoned patients and data collection. MR was involved in data collection and interpretation. All authors were involved in the initial drafting and editing of the manuscript and approved the final version before submission. The corresponding author is the guarantor and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences (Grant#7363).
Institutional Review Board Statement
The study was approved by an institutional review board and ethics committee at investigational sites at Shiraz University of Medical Sciences and conducted under the supervision of the Iranian Registry for Clinical Trials (IRCT; Trial registration: IR.SUMS.REC.1394.102), Ministry of Health, and Medical Education guidelines. After a complete description of the study to the patients or their families, written informed consent was obtained.
Data Availability Statement
Deidentified raw data are available upon reasonable request.
Acknowledgments
The authors would like to gratefully thank the physicians and medical personnel of Shahid Faghihi, Aliasghar, Nemazi, Loghman, and Maharlooie Hospitals for their cooperation in the administration of DHA formulation and patient monitoring as well as for supplying the data of the AlP-poisoned patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moghadamnia, AA. An update on toxicology of aluminum phosphide. DARU J Pharm Sci 2012, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Nosrati A, Karami M, Esmaeilnia M. Aluminum phosphide poisoning: A Case series in north Iran. Asia Pacific J Med Toxicol 2013, 2, 111–3. [Google Scholar]
- Mehrpour O, Singh S. Rice tablet poisoning: a major concern in Iranian population. Human Exp Toxicol 2010, 29, 701. [Google Scholar] [CrossRef] [PubMed]
- Kordrostami R, Akhgari M, Ameri M, Ghadipasha M, Aghakhani K. Forensic toxicology analysis of self-poisoning suicidal deaths in Tehran, Iran; trends between 2011-2015. DARU J Pharm Sci 2017, 25, 1–10.
- Kassiri H, Feiz-Haddad M-H, Ghasemi F, Rezaei M, Ghanavati F. An epidemiologic and demographic survey of poisoning in Southwest of Iran. Middle-East J Sci Res 2012, 12, 990–6. [Google Scholar]
- Navabi SM, Navabi J, Aghaei A, Shaahmadi Z, Heydari R. Mortality from aluminum phosphide poisoning in Kermanshah province, Iran: Characteristics and predictive factors. Epidemiol Health 2018, 40, e2018022. [Google Scholar] [CrossRef]
- Chugh SN, Ram S, Arora B, Malhotra KC. Incidence & outcome of aluminium phosphide poisoning in a hospital study. Ind J Med Res 1991, 94, 232–5. [Google Scholar]
- Mathai A, Bhanu MS. Acute aluminium phosphide poisoning: Can we predict mortality? Ind J Anaesthes 2010, 54, 302. [Google Scholar] [CrossRef]
- Mostafazadeh, B. Aluminium phosphide poisoning. Toxic Drug Testing 2012, 15, 345–60. [Google Scholar]
- Dua R, Sunkaria A, Kumar V, Gill KD. Impaired mitochondrial energy metabolism and kinetic properties of cytochrome oxidase following acute aluminium phosphide exposure in rat liver. Food and chemical toxicology 2010, 48, 53–60. [Google Scholar] [CrossRef]
- Petrovic M, Otero D, Leigh A, Singh V. Acute heart failure due to aluminum phosphide poisoning. Methodist Debakey Cardiovasc J 2021, 17, 6. [Google Scholar] [CrossRef]
- Hosseini SF, Forouzesh M, Maleknia M, Valiyari S, Maniati M, Samimi A. The molecular mechanism of aluminum phosphide poisoning in cardiovascular disease: Pathophysiology and diagnostic approach. Cardiovasc Toxicol 2020, 20, 454–61. [Google Scholar] [CrossRef] [PubMed]
- Anand R, Binukumar BK, Gill KD. Aluminum phosphide poisoning: an unsolved riddle. J Appl Toxicol 2011, 31, 499–505. [Google Scholar] [CrossRef]
- Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: a review of literature. Forensic Sci Int 2012, 214, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karimani A, Mohammadpour AH, Zirak MR, et al. Antidotes for aluminum phosphide poisoning–an update. Toxicol Report 2018, 5, 1053–9. [Google Scholar] [CrossRef] [PubMed]
- Shadnia S, Rahimi M, Pajoumand A, Rasouli M-H, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol 2005, 24, 215–8. [Google Scholar] [CrossRef]
- Hossien M, Zohoorian P, Foroughian M, Awli SH, Teimouri A. Successful treatment of acute aluminum phosphide poisoning: Possible benefit of olive oil-A case report. Update Emerg Med 2021, 1, 6–10. [Google Scholar]
- Tawfik, HM. Recent advances in management of aluminium phosphide poisoning. QJM 113(Supplement_1): hcaa049.-02.
- Katwal S, Malbul K, Mandal SK, et al. Successfully managed aluminum phosphide poisoning: A case report. Annal Med Surg 2021, 70, 102868. [Google Scholar] [CrossRef]
- Naddafi M, Mehrizi AA, Eghbal MA, et al. Reducing the risk of death induced by aluminum phosphide poisoning: The new therapies. Chemosphere 2022, 294, 133800. [Google Scholar] [CrossRef]
- Asghari MH, Moloudizargari M, Baeeri M, et al. On the mechanisms of melatonin in protection of aluminum phosphide cardiotoxicity. Arch Toxicol 2017, 91, 3109–20. [Google Scholar] [CrossRef]
- Asghari MH, Abdollahi M, de Oliveira MR, Nabavi SM. A review of the protective role of melatonin during phosphine-induced cardiotoxicity: focus on mitochondrial dysfunction, oxidative stress and apoptosis. Journal of Pharmacy and Pharmacology 2017, 69, 236–43. [Google Scholar] [CrossRef] [PubMed]
- Petersen AB, Na R, Wulf HC. Sunless skin tanning with dihydroxyacetone delays broad-spectrum ultraviolet photocarcinogenesis in hairless mice. Mutat Res 2003, 542, 129–38. [Google Scholar] [CrossRef] [PubMed]
- Draelos, ZD. Self-tanning lotions. Am J Clin Dermatol 2002, 3, 317–8. [Google Scholar] [CrossRef] [PubMed]
- Cortez MY, Torgan CE, Brozinick JT, Miller RH, Ivy JL. Effects of pyruvate and dihydroxyacetone consumption on the growth and metabolic state of obese Zucker rats. Am J Clin Nutr 1991, 53, 847–53. [Google Scholar] [CrossRef] [PubMed]
- Stanko RT, Diven WF, Robertson RJ, et al. Amino acid arterial concentration and muscle exchange during submaximal arm and leg exercise: the effect of dihydroxyacetone and pyruvate. J Sports Sci 1993, 11, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rao GA, Riley DE, Larkin EC. Fatty liver caused by chronic alcohol ingestion is prevented by dietary supplementation with pyruvate or glycerol. Lipids 1984, 19, 583–8. [Google Scholar] [CrossRef] [PubMed]
- Goheen SC, Pearson EE, Larkin EC, Rao GA. The prevention of alcoholic fatty liver using dietary supplements: Dihydroxyacetone, pyruvate and riboflavin compared to arachidonic acid in pair-fed rats. Lipids 1981, 16, 43–51. [Google Scholar] [CrossRef]
- Niknahad H, O'Brien PJ. Antidotal effect of dihydroxyacetone against cyanide toxicityin vivo. Toxicology and Applied Pharmacology 1996, 138, 186–91. [Google Scholar] [CrossRef] [PubMed]
- Niknahad H, Ghelichkhani E. Antagonism of cyanide poisoning by dihydroxyacetone. Toxicol Lett 2002, 132, 95–100. [Google Scholar] [CrossRef]
- Niknahad H, Khan S, Sood C, Obrien PJ. Prevention of cyanide-induced cytotoxicity by nutrients in isolated rat hepatocytes. Toxicology and Applied Pharmacology 1994, 128, 271–9. [Google Scholar] [CrossRef]
- Niknahad H, Hashemi A, Jamshidzade A. Antidotal effect of dihydroxyacetone against phosphine poisoning in vivo in mice. Toxicol Lett 2012, 211, S169–S70. [Google Scholar] [CrossRef]
- Niknahad H, Heidari R, Hashemi A, Jamshidzadeh A, Rashedinia M. Antidotal effect of dihydroxyacetone against phosphine poisoning in mice. Journal of Biochemical and Molecular Toxicology 2021, 35, e22897. [Google Scholar]
- Rashedinia M, Jamshidzadeh A, Mehrabadi AR, Niknahad H. Prevention of phosphine-induced cytotoxicity by nutrients in HepG2 cells. Indian J Med Res 2016, 144, 560. [Google Scholar]
- Laborit H, inventor Centre dEtudes Experimentals et Cliniques de Phisiobiologie de Pharmacologie et dEutonologie CEPBEPE, assignee. Treatment of hemorrhagic shock patent US4049795A. 1977 1977/09/20/.
- Ahmadi J, Joukar S, Anani H, Karami-Mohajeri S. Dihydroxyacetone as a definitive treatment for aluminium phosphide poisoning in rats. Arch Indust Hyg Toxicol 2018, 69, 169–77. [Google Scholar] [CrossRef] [PubMed]
- Dorooshi G, Mirzae M, Fard NT, Zoofaghari S, Mood NE. Investigating the outcomes of aluminum phosphide poisoning in khorshid referral hospital, Isfahan, Iran: A retrospective study. J Res Pharm Pract 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Etemadi-Aleagha A, Akhgari M, Iravani FS. Aluminum phosphide poisoning-related deaths in Tehran, Iran, 2006 to 2013. Medicine 2015, 94, e1637.
- Goel A, Aggarwal P. Pesticide poisoning. Natl Med J India 2007, 20, 182–91. [Google Scholar]
- Etemadi-Aleagha A, Akhgari M, Iravani FS. Aluminum phosphide poisoning-related deaths in Tehran, Iran, 2006 to 2013. Medicine 2015, 94. [Google Scholar]
- Oghabian Z, Ahmadi J, Pakravan SD, F, Heidari MR, Tajaddini S, Karami-Mohajeri S. Successful treatment of aluminium phosphide poisoning by dihydroxyacetone: A two-case report study. J. Clin. Pharm. Ther. 2020, 45. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).