1. Introduction
One challenge in managing tropical forests is reconciling the extraction needs of the local people with conservation of intact ecosystems. Many tropical forests are in regions with high population density coupled with relative poverty giving rise to a pervasive reliance on subsistence harvest of forest products (e.g. fuel wood) [
1] (Meyer and Turner 1992). This common dilemma to reconcile the human needs of forest adjacent communities with the needs of forest biodiversity conservation and preservation of ecosystem services is the primary challenge facing conservationists.
There is growing consensus that trees on farms can play an important role in conserving biodiversity, improving agricultural production, providing fuel and incomes, delivering and extending ecosystem services, and ultimately halting the loss and degradation of forests [
2,
3,
4,
5] (Schroth 2004, Jerneck and Olsson 2013, Nyambane 2014, Mbow et al. 2014). In the modern fragmented landscape, it is commonly recognized that the human matrix (e.g. farms and agroforestry) will be increasingly important for biodiversity conservation [
6] (Perfecto and Vandermeer 2008), and studies in Africa have shown that successful agrofrorestry systems can contribute to biodiversity conservation of fragmented forest and woodland ecosystems while providing socio-economic benefits to farmers [
7,
8,
9] (Sileshi et al 2007, Kalaba et. al. 2010, Jose 2012).
The FAO defines agroforestry as “land-use systems and technologies where woody perennials are deliberately used on the same land-management units as agricultural crops and/or animals, in some form of spatial arrangement or temporal sequence”. Trees on farms can provide multiple benefits to both the farmer and the ecosystem at large [
9,
10,
11,
12] (Mattison 2005, Bhagwat et al. 2008, Jose 2012, Tscharntkea et. al. 2012) but balancing optimal conditions for crops and the extractive needs of the farmer with biodiversity and ecosystem benefits is a challenge [
9,
13,
14] (van Noordwijk et. al. 2016, Jose 2011, Jose 2012). Despite the potential of agroforestry, it remains rarely systematically implemented [
13] (van Noordwijk et. al. 2016) and its contribution to livelihoods and forest biodiversity are relatively undescribed. Reasons for this stem from multiple causes, including a myriad of policy adjustments [
13] (van Noordwijk et. al. 2016), a constant shift of our conceptualization of agroforestry, and the complex inter-connections and feedbacks between the social and natural dimensions inherent in its implementation. The almost ubiquitous tendency arising from this lack of clarity is to separate the human and natural dimensions; with policy dictating that agricultural development and forestry end up under different dockets, and ecologists ignoring or underestimating the ecological value of agricultural landscapes in conservation [
15] (de Foresta et al. 2013). Recently, increasing recognition is given to the value of micro-reserves outside of the primary reserve network [
16] (Laguna et al. 2016) giving rise to recommendations for a “top-down” approach to regional reserve planning [
17] (Margules et al. 1988) that allows for incorporation of private land holdings into conservation planning. This gives a new impetus to better understand and implement agroforestry systems.
Though trees on farms can simultaneously provide economic value while conserving biodiversity [
18,
19,
20,
21](Bugayong 2003, Schroth 2004, Swihart and Moore 2004, Sodhi and Ehrlich 2010), the attributes of trees on farms required for these benefits may conflict: exotic species (e.g.
Eucalyptus spp. in the African setting) may provide the most economic value while providing the least ecosystem benefits, while indigenous tree mixtures may be more valuable for biological conservation but provide the least immediate economic value to farmers. The actual composition of trees on farms will depend on a number of factors, but ultimately for agroforestry to be effective, thereby ensuring its adoption, trees in the forest and on farms must complement each other in terms of maintaining biodiversity and providing forestry resources [
8,
22] (Cannell et. all. 1996, Kalaba et. al. 2010), or at the very least, not be in direct conflict. This is a key challenge in building sustainable and viable agroforestry systems.
Wood fuel continues to be the primary energy source in Africa, with data showing that not only is it used in 77% of African households, but use in rural households in some African countries is as high as 90% and may be increasing [
23,
24,
25] (Mugo and Gathui 2010, Legros et al. 2009, Bonjour et al. 2013). In the western ecoregion of Kenya, for example, there are over 500,000 households each consuming over 4,000 kilograms of wood annually, predominately from forests, but also from trees on farms [
26,
27] (KNBS 2015, Lung and Espira 2019). While not responsible for large scale forest loss, there is considerable evidence this subsistence wood fuel use is causing complex changes in forest diversity and structure and is leading to irreversible degradation [
28,
29,
30,
31] (Lung and Schaab 2007, Kefa 2015, Lung and Espira 2015, Kefa et al. 2017).
The objective of this study was to explore whether agroforestry practices in a moist tropical forest ecosystem can simultaneously provide adequate resources for timber and fuelwood extraction by farmers while extending forest tree biodiversity conservation. First, we described the agroforestry attributes on farms in terms of tree numbers, biomass and diversity. Second, we assessed the relationships between farm attributes (i.e. land size, crop diversity, and presence of cash crop) and agroforestry attributes (i.e. number and biomass of timber/fuelwood trees and tree biodiversity). Third, controlling for the effects of farm attributes, we assessed the relationship between number and biomass of timber/fuelwood trees and tree biodiversity. Lastly, we assessed the relationships between forest tree diversity attributes and farm tree diversity attributes on a landscape scale using spatial analyses. If agroforestry practices can simultaneously provide timber and fuelwood while conserving tree biodiversity, then we should see no changes in tree biodiversity per farm as the number and biomass of timber/fuelwood trees varies. Conversely, if these two are in contrast, then biodiversity should decrease with increasing number of biomass of timber/fuelwood trees.
2. Materials and Methods
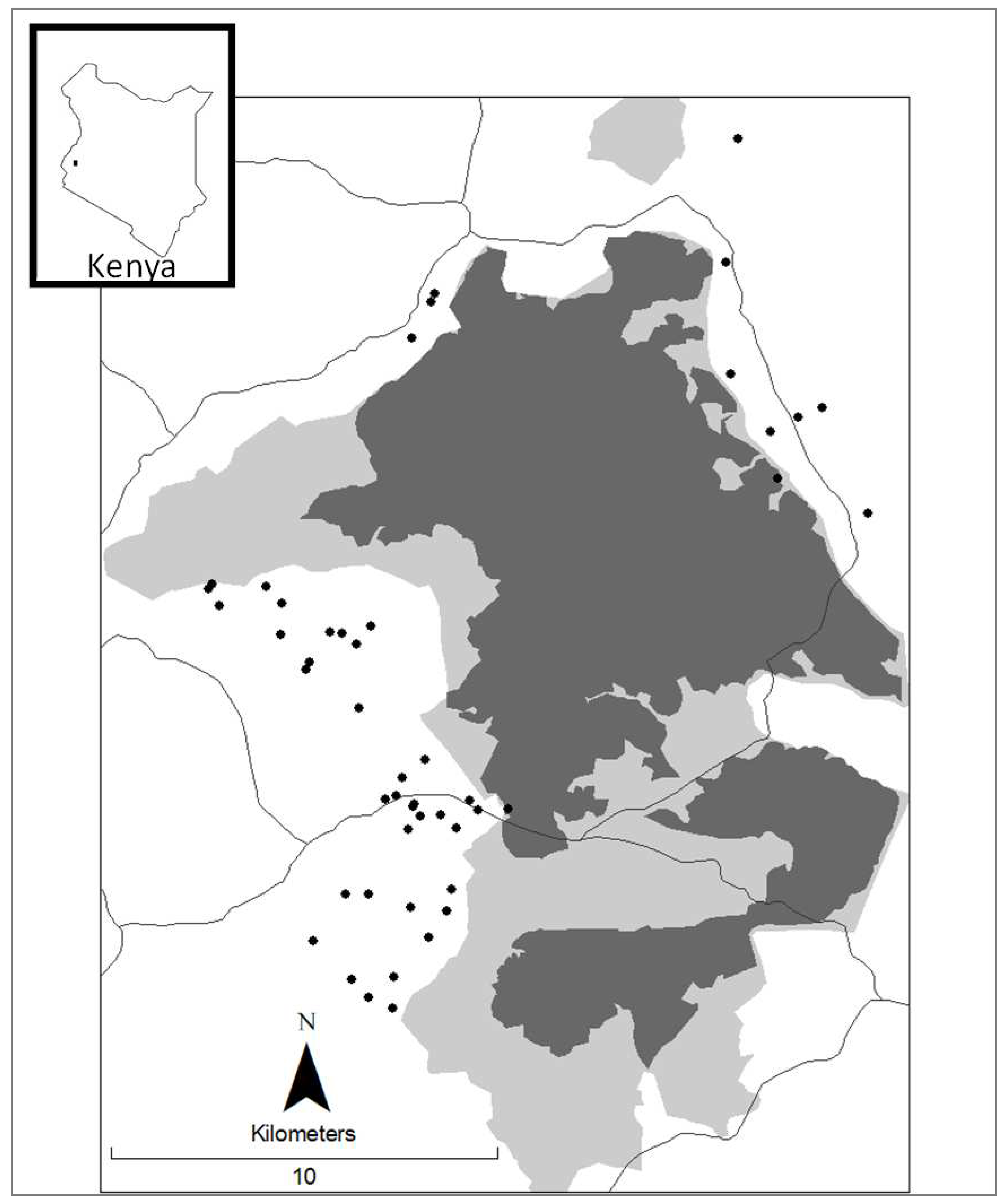
The study was conducted on small-holder farms in communities bordering the Kakamega forest in western Kenya (34°75’ E; 0°15’ N) (
Figure 1). A large majority (>90%) of the rural population are subsistent farmers with few formal employment opportunities, and with greater than 50% of the population being unemployed and living below the poverty line [26}(KNBS 2015). Most farmers grow a combination of cash crops and subsistence food crops, with the ratio varying depending on season and size of land holding. The primary cash crops are sugar cane and tea, and the primary food crop is maize usually grown with a variety of vegetables and pulses. Almost all rural households use wood for cooking [
27] (Lung and Espira 2019). Many farmers maintain trees on their land for a variety of reasons, including cultural beliefs, shade, and fuelwood among others.
On-farm tree assessment was done on the farms of 50 households in the study area. The households were randomly selected from an existing database of over 15,000 households located within 3 kilometers of the forest edge. Each household farm was assessed by a trained field technician and assistant who worked with the farm owner to identify and measure all trees on the farm, to elucidate their use and origin, and to answers questions about farm size, presence of cash crops and crop diversity. All trees were identified to species level (where possible), counted, and their diameter at breast height (DBH) measured. Each tree was also categorized by potential use (e.g. fuelwood, timber, fruit, etc.) and origin (exotic or indigenous). To corroborate use, we also surveyed 20 people collecting fuelwood regarding their preferred fuelwood species. Using DBH we created 5 age-classes of trees (<10 cm, 10-20 cm, 21-30 cm, 21-40 cm, and >40 cm). DBH, along with wood density (ƥ) and an estimate of environmental stress (E) were then used in a pan-tropical allometric equation [
32](Chave et al., 2014) to estimate above ground biomass (AGB) for each tree:
Wood density (ƥ) was obtained from the global wood density database [
32] (Chave et al., 2009). When multiple values were available for a species, we used the average the value. Environmental stress (E) values for the regions were obtained from a gridded global layer available at
http://chave.ups-tlse.fr/pan-tropical_allometry.htm [
32](Chave et al., 2014). For each farm we calculated the total number of trees and total biomass for (1) all trees, (2) all indigenous trees, (3) all trees used for fuelwood/timber, and (4) all indigenous trees used for fuelwood/timber.
To provide a more comprehensive account of forest diversity on farms than any one metric could provide, we calculated four indices of species diversity (species richness, Simpson’s index, Shannon’s index, and evenness) for all tree species (including exotic species) and for just indigenous tree species per farm.
We used a modified point quarter count method to measure species richness, rarity, and diversity at 35 randomly selected 0.5-acre survey locations within the Kakamega Forest [
31] (Kefa et al. 2017). At forest survey locations, the standard USDA Forest Service Point Quarter Count Method for surveying forest points was modified to include measurement of a central tree and up to the two largest trees in the plot if those trees were not included in the initial survey. This means that each site included measurements and identities for up to six total trees. On-farm surveys of diversity included a complete census of all trees, and included up to 200 measured trees. Therefore, prior to analysis we rarefied the farm biomass and tree diversity data to the median forest diversity and biomass measurements of 5.4 trees per site using the R Package iNEXT [
33] (Hsieh et al. 2016). We then calculated biodiversity using the Shannon Diversity index H’, Species Richness, and RWR on the forest data and rarefied farm data. Biomass for each tree was calculated following the same allometric biomass equations for trees on farms and then summed for each site or rarefied site set of trees.
There were two primary components statistically analyzed within farms – the relationship between farm attributes and tree attributes, and the influence of timber/fuelwood tree numbers on tree biodiversity. We used a multivariate regression general linear model to explore the relationship between three farm attributes (land size, presence of a cash crop, and crop diversity) and five tree attributes (number of total species, number of indigenous species, number of all trees, number of exotic timber/fuelwood trees, and number of all timber/fuelwood trees). We also used a multivariate regression general linear model to analyze the influence of the number of timber/fuelwood species on each diversity index (richness, Simpson’s, Shannon’s, and evenness) calculated for all tree species and for only indigenous tree species. For both statistical components, all predictor variables were compared in a correlation matrix to check for autocorrelation, and all variables in resulting models were checked for multicollinearity using VIF, tolerance calculations, and eigenvalues [
34](Field 2013). We used the Durbin-Watson estimation to check for independence of residuals, a case-wise diagnosis to look for bias due to extreme values, residual plots and partial plots to assess homogeneity of variance. All statistical analyses were conducted in SPSS. Unless otherwise stated, all values are reported as mean (+/- standard error).
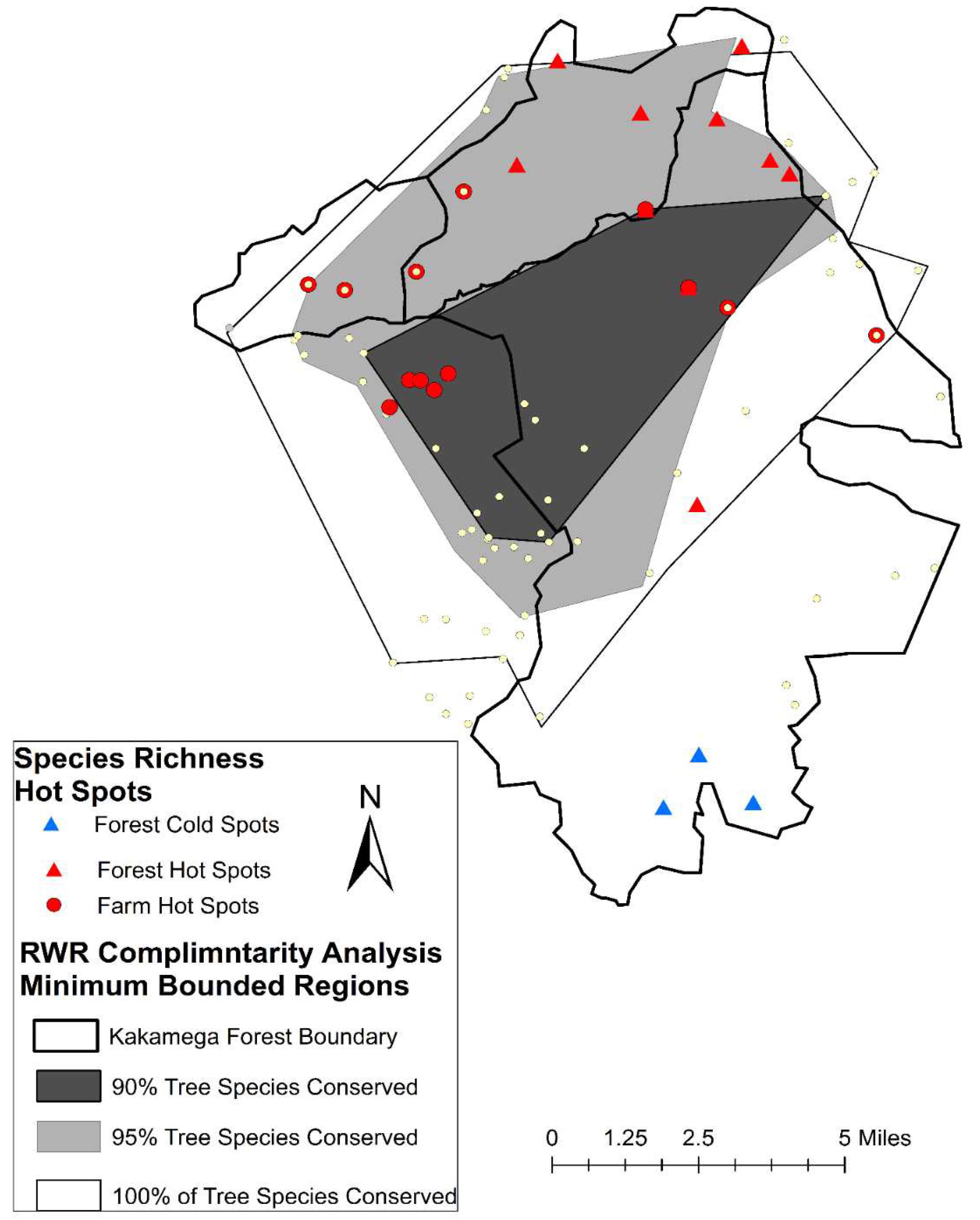
Complementarity and HotSpot Analyses. We used a complementarity analysis based on rarity weighted richness scores to identify priority conservation areas within our set of forest and farmed sites [
35,
36,
37] (Kirkpatrick 1983, Margules et al. 1988, Albuqurque and Gregory 2017). Complementarity analyses use an iterative process to identify priority sites for conservation in an effort to save all species in the system, or as many species as is possible, in as small an area as possible. For our complementarity analysis we ordered surveyed farm or forest sites in descending order by their RWR scores. We then chose sites with the highest RWR scores until 90%, 95%, and 100% of all tree species identified in the system were included in the set of chosen sites. [
38] Albuquerque and Beier (2015) have shown that this application of RWR scores to choose reserve designs is highly effective and efficient at identifying priority areas for conserving species, and [
37] Albuquerque and Gregory (2017) have further shown that this method can be applied to large geographic areas and is effective at evaluating gaps in species coverage by existing reserve networks.
We used ArcGIS 10.4 minimum bounding geometry to identify the spatial area encompassed by the sets identified as conserving 90%, 95% and 100% of the species identified in our field surveys. We then calculated the area encompassed by the minimum bounding geometry of each region, and the number of forest and farm sites included in each area. Some farm and forest sites included within the spatial area encompassed by the minimum bounded areas were of lower complementarity (RWR) rank and therefore not used to identify the minimum conservation extent needed to reach species conservation coverage of 90%, 95%, 100% species representation. These areas represent potential redundancy in species coverage.
We used ArcGIS 10.4 to conduct a Gi* HotSpot analysis on the species richness data and the biomass data. Hotspot analysis identifies areas of significantly clustered high × high spatial autocorrelation (HotSpots) and areas of significantly clustered low x low spatial autocorrelation [
39] (ColdSpots; Ord and Getis 1995). We performed the Hotspot analysis independently on the farm and forest species richness data sets, and on a pooled dataset that included both the farm and forest diversity data in a single HotSpot analysis. Similarly, we performed HotSpot analyses on the forest and farm biomass datasets and a combined forest and farm dataset. We retained all sites that were clustered at >90% confidence as Hotspots or ColdSpots. We used a Geographically Weighted Regression to test for significant correlations among species richness and biomass, and rarity (RWR) and biomass. For GWR we used an adaptive kernel and search radius.
4. Discussion
Our results suggest that the tradeoffs between agroforestry and conservation may not be as pronounced as casual observations may suggest. On farms surrounding Kakamega Forest, we found a considerable abundance of trees in terms of numbers, biomass, and diversity, although the variability was large. This is consistent with prior research which documented agroforestry interventions in the region in the 1990’s in response to concerns about forest timber/fuelwood supply in relation to demand [
40,
41,
42,
43](Tengnas 1994, Scherr 1995, Paterson 1998, Mercer 2004) in the context of a very dense rural population [
26](>500 persons/km2 – KNBS 2015) almost entirely dependent on forest-sourced fuelwood as their primary fuel [
27](Lung and Espira 2019). Three descriptive observations from our study provide insight regarding the potential tradeoff between the wood value to farmers and the conservation value to society. First, timber/fuelwood tree species accounted for 90% of tree numbers and biomass. Second, while two exotic timber/fuelwood tree species (
Eucalyptus and
Cuppressus) dominate numbers and biomass on farms, there are at least five indigenous tree species used for fuelwood commonly found on farms (
Croton macrostachyus, Markhamia lutea,
Harungana madagascariensis, Maesopsis eminii, Strychnos usambarensis). Third, tree richness averaged 8 species per farm (50% indigenous) and about one third of farms had multiple age-classes of indigenous trees, usually including the largest tree per farm. As such, despite the reliance on fuelwood and the trend towards using trees on farms for fuelwood supply, the presence of large relic indigenous trees on farms and the use of a diversity of trees for timber/fuelwood suggest the possibility that wood value and conservation value are not at complete odds. This is further supported by the observation that indigenous tree diversity on farms (averaging 4 species but as high as 12 species per farm) can be as high as average tree diversity in forest plots [
30](7.4 species per 0.31-acre plot: Lung and Espira 2015).
We predicted that if timber/fuelwood and conservation values were at odds, then we would see a negative relationship between the number of timber/fuelwood trees on a farm and tree diversity variables. In other words, planting trees for timber/fuelwood would be at the expense of tree diversity. For most diversity variables, we detected no significant relationship between the number of timber/fuelwood trees and overall tree diversity. Specifically, Shannon diversity index and tree species richness index did not covary with number of timber/fuelwood trees. This effect was present regardless of if we included exotic species or not. Based on calculated coefficients and effect sizes it is unlikely that we failed to detect differences. For example, we detected no significant relationship between the number of all timber/fuelwood trees and the number of indigenous species per farm and the coefficient and effect size for this relationship was 0.009 and 0.030, respectively. These findings, which suggest that wood and conservation values are not always at odds, may be explained by the high diversity of trees used for timber/fuelwood. [
31]Kefa et al. (2017) found 47 tree species (34 indigenous) in fuelwood head bundles in the same study region, and of the ten most common trees found on farms, seven were the most commonly mentioned fuelwood species by surveyed wood gatherers (
Table 2).
Lastly, we found that on a landscape level, trees on farms added to the biodiversity of natural forests and could be important components in conservation management. The total diversity of trees on farms (64 total species, 42 indigenous) was similar to the tree diversity in adjacent forest. In the 33 plots used in this study, we recorded 58 tree species. [
30]Lung and Espira (2015) recorded 65 tree species from 95 plots in adjacent forest. However, this has to be taken in the context that a significant portion of tree diversity on farm land was accounted for by exotic species. Of the 65 species encountered on farms, 25 were exotic (38%) and 6 (9%) were indigenous non-forest trees, and only 34 species of forest trees were found on farms. This is significantly fewer than the 65 species recorded in the forest [
30](Lung and Espira 2015). This raises the question whether on-farm tree diversity, consisting of a large assemblage of exotic and non-forest trees, is able to provide the same biodiversity and ecosystem services as indigenous forest.
Our spatial analysis provides some interesting insights. First, the geographically weighted regression results reinforce the notion of no tradeoff in terms of biodiversity and biomass, even taking spatial heterogeneity into account. It also suggests there is no spatial heterogeneity in biomass. We also found no Hotspots when we pooled the farm and forest data sets into one dataset but found offsetting hotspots when the farm and forest data were analyzed separately. In other words, in forest regions that contained Hotspots we detected no corresponding Hotspots in adjacent farm regions, while in forest regions that contained no Hotspots, we detected farm Hotspots in adjacent farm regions. This observation suggests that spatial autocorrelation in one data set offsets the spatial autocorrelation in the other data set. While it is an anecdotal observation it could be explained through the lens of market economics. We know that there is a very high diversity of trees used for fuelwood and timber [
29,
31] (Kefa 2015, Kefa et al. 2017). In areas where the forest lacks high diversity, we found farms with high diversity of trees. This could be explained as a response by farmers to grow a diversity of trees in response to a low supply by forests. However, this could also be explained by farmers merely conserving high diversity forest remnants. Regardless it further highlights the conservation value of farms in maintaining regional tree species richness.
The complementarity analysis with RWR also allowed us to identify the minimum spatial area necessary to conserve landscape species richness. It is interesting that even when conserving only 90% of the total species richness farm sites were required. This highlights that farms and areas outside of the forest are important components in conserving regional biodiversity. This supports a growing body of literature about the value of micro reserves and areas outside of reserve areas in conservation [
44,
45] (Laguna et al. 2016, Arenas et al. 2017).
One of the challenges of agroforestry is getting farmers to both plant and maintain trees on their actively used farmland. Though the theory of agroforestry suggests that farmers should seek complementarity of resource capture by trees and crops [
46](Cannell et. all. 1996) and provision of forestry resources [
8](Kalaba et. al. 2010), a number of our findings suggest that farmers are not actively selecting or maintaining tree biodiversity on their farms, but more likely passively letting biodiversity remain on their farmland. First, the majority of indigenous trees on farms are likely to be relic species or self-dispersed species that are not planted by the farmer. Other than
Markhamia lutea, and
Zanthoxylum gilletii, species that are of cultural significant (Espira, personal observation), the opportunistic species
Croton macrostachyus, Harungana madagascariensis and
Bridelia micrantha, that dominate forest regeneration regions, are the most common indigenous trees on farm land. In addition, over 73% of all trees counted were exotic species, with
Eucalyptus saligna alone comprising 46% of all trees on farms. This suggests that farmers are making their choices on which trees to plant based primarily on their economic value (Eucalyptus being the quintessential example of such a species) and on cultural considerations rather through an active choice to maintain diversity or to provide agroforestry benefits (e.g. fruit, nitrogen fixing, etc.).
Eucalyptus is both fast growing and easily converted to revenue at multiple stages of its growth phase (from firewood to poles to timber). The fact that the largest trees on farms are indigenous forest species suggests that relic trees (left over from forest clearing) also play a vital role in contributing to farmland diversity, and may form the nexus around which pioneer species such as
Croton macrostachyus, Harungana madagascariensis and
Bridelia micrantha may seed themselves. These results suggest that farmers may be willing to devote a substantial area of their farmland to trees as long as they see a specific benefit of these trees, with cultural and economic benefits being good examples.
If agroforestry is to play an increasingly active role in conserving biodiversity in human-dominated landscapes, particular in areas of dense subsistence farmer populations, increase recognition needs to be given to farmer’s perception of the value of trees and their selection of what trees to plant or maintain. In this study, we found that farmers choose to include trees within their farm landscape even though there is currently no active agroforestry initiative in the area. This suggests that a drive to increase on-farm biodiversity should target species that farmers already prefer or species that can serve the same purpose that farmers perceive their on-farm trees serving. Furthermore, as much as exotics like Eucalyptus spp. are not seen as being ideal for agroforestry, an insistence on their exclusion from any drive for increased trees on farmers is likely to alienate farmers.
Although agroforestry initiatives have historically targeted resource use complementarity (e.g. shade, water use), alternate farm uses (e.g. fodder, nitrogen fixing), biodiversity benefits (e.g. pollination), and food production (e.g. fruit, berries), a further consideration, namely farmer perception, should be given a high priority as well. For this to be successful, agroforestry needs to be approached from an increasingly multi-disciplinary approach, giving increasing weight to economic and social factors. In the meantime, ecologists should continue to study how much biodiversity can benefit from various agroforestry initiatives, what roles farmland can play in conservation, and just how much of ecosystem function can be maintain by heavily altered and fragmented, but nevertheless biologically rich, landscapes.