1. Introduction
According to the US Centers for Disease Control (CDC), more than 42% of adults are classified as obese, and approximately 74% of US adults are classified as overweight (body mass index of 25.0 to < 30 kg/m2) or obese (>30 kg/m2) (Ow/Ob) [
1]. The costs on human health are profound, increasing the risk of heart disease, stroke, type 2 diabetes, and certain cancers, and ultimately increasing morbidity associated with these chronic diseases. Obesity and the negative sequalae inflict staggering economic costs, estimated at
$261 billion, and that was in 2016 [
2]. On the other hand, the weight loss or weight management market, on one account, is estimated
$192 billion, suggesting there is a desire for commercially available dietary products, many of which are not regulated or assessed for efficacy. Therefore, understanding and developing novel dietary interventions that can help address Ow/Ob can reduce the risk of chronic disease, reduce healthcare costs, and likely improve quality of life.
Obesity is associated with physiological dysfunction, specifically metabolic dysfunction such as impaired ability to use fat or metabolic inflexibility [
3]. Caloric restriction and/or fasting may induce favorable adaptations increasing the ability to utilize fat as fuel [
4], thereby aiding in reducing adiposity. This occurs likely through the “metabolic switch” which subsequently transforms liver metabolism “switching” from predominately carbohydrate/glucose in the liver/muscle to ketones derived from adipocytes, and further adaptations such as improved ability to cope with cellular or metabolic stress and glucose handling [
5]. Interventions beyond alteration of diet typically involves alterations to physical activity and/or exercise. However, prior systematic review and meta-analysis suggests that when comparing diet alone vs. exercise alone, dietary interventions may result in ~3-fold greater weight loss (11 vs 3 Δkg) [
6]. While the additional benefits of exercise are not be discounted [
7], engaging dietary approaches can be a highly effective way to promote weight loss. Caloric restriction (CR) has long been touted as an effective method of inducing weight loss [
8]. Caloric restriction, and the potential health benefits, is not a new concept or experience for humankind. Some of the earliest data on health outcomes with CR are from the Dutch famine during World War II [
9], which was reduced cardiovascular mortality [
10]. Since then, cultural comparisons of the US and Okinawa, Japan [
11] and more recently the case study of Biosphere2 study [
8] are other examples that have documented that caloric restriction on the order of ~15-40% improves cardiometabolic risk profile (e.g. blood pressure, blood lipids, etc.). However, the data from these studies is observational or correlative in nature, where other factors are likely involved. A recent controlled interventional study, namely the prominent Comprehensive Assessment of Long-term Effects of Reducing Calorie Intake (CALERIE) trial, is a multicenter longitudinal study of caloric restriction [
12]. Phase 1 documented that 20-25% caloric restriction reduced body weight and adiposity, and further study of the CALERIE trial documented that chronic 25% reduction in caloric intake (vs. ad libitum) lowered systolic and diastolic blood pressure, decreased total and low-density lipoprotein (LDL), lowered triglycerides, lowered total cholesterol/high density lipoprotein (HDL) cholesterol ratio, and increased HDL in “non-obese” healthy individuals (defined as body mass index 22 to 27.9 m2) [
12]. Though long term sustained CR may induce improvements (e.g. body weight, adiposity, blood pressure and lipid profile), it doesn’t present without potential physiological issues (e.g. risk of osteoporosis, reproductive dysfunction, etc.) [
13] but also issues with adherence and maintenance [
14]. Thus, more investigation into possible benefit of short-term CR is warranted.
Though the simple act of reducing caloric intake over time with CR, or intermittent fasting (IF), results in caloric deficit and contributes to weight loss in Ow/Ob [
15,
16]. Such caloric deficit engages the “metabolic switch” [
5], which may be beneficial in Ow/Ob given the impaired ability to use fat or metabolic inflexibility [
3], increasing the ability to utilize fat [
4], and therefore reducing fat mass. Caloric restriction benefits likely extend beyond simple loss of weight [
16], also likely influencing hormonal regulators of metabolism (PYY, NPY[
17], Thyroid Hormones, Ghrelin, and leptin [
18]), which are known to be disrupted in Ow/Ob [
19]. To date, no studies have tested 3 days of caloric restriction (~590kcal/day intake) using a standardized diet in Ow/Ob individuals, as might be included in a long term intermittent fasting diet, to see it improves weight/adiposity, metabolic switching (from utilizing carbohydrates to fats [
20]), and potential alterations in the hormonal regulators of appetite and metabolism (e.g. protein YY (PYY), neuropeptide Y(NPY), ghrelin, leptin, thyroid stimulating hormone (TSH), insulin, cortisol).
Accordingly, the purpose of this study was to document the effects of a 3-day CR diet on body weight/body composition, measures of cardiometabolic health as well as circulating regulatory factors that regulate hunger, satiety, and metabolism. We hypothesized that the 3-day CR diet would induce favorable changes in body weight, body composition, metabolic function, via increased fat use, blood pressure, and blood glucose/lipid profile. This study could provide evidence on the acute efficacy of 3-day CR but also identify the potential underlying physiological mechanisms through which such dietary intervention may elicit cardiometabolic improvements. Collectively, this may provide a window into the possible adaptations with a longer-term or repeated dietary intervention.
2. Materials and Methods
Subjects and General Procedures
Participants were recruited via email and publicly posted flyers from the Saratoga Springs, NY community. To be included participants must have been overweight or obese (BMI > 27.5 kg/m2), and weight stable (±4.4 lb) for > 6 months prior, and 25 to 65 years of age. Participants were otherwise relatively healthy without uncontrolled chronic disease (e.g. cardiovascular, metabolic, or pulmonary) and 2 or fewer positive risk factors for cardiovascular disease (e.g. high blood pressure, high cholesterol, etc.) as described by the American College of Sports Medicine/American Heart Association Criteria [
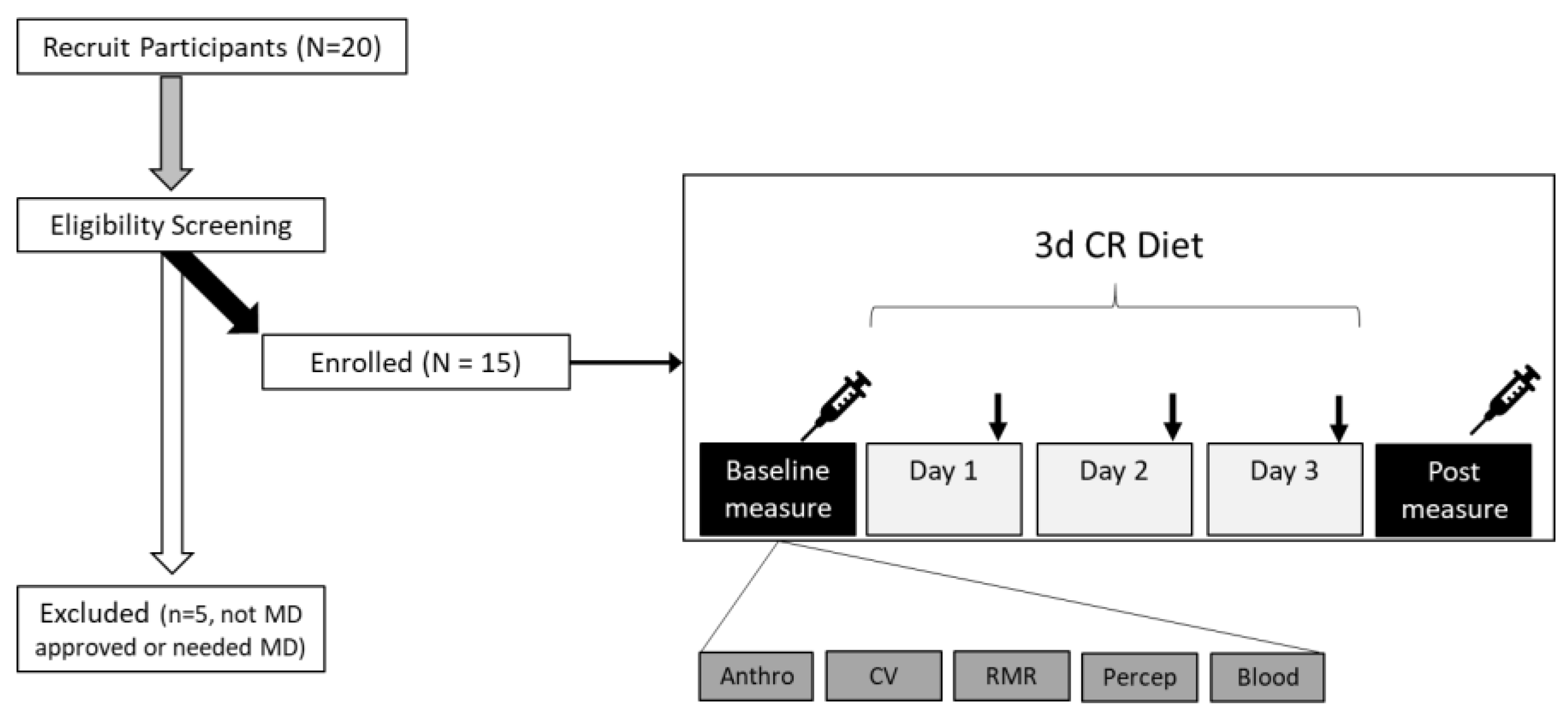
21]. Participants were screened for eligibility by health history form in person prior to baseline measurements (
Figure 1). Additionally, considering the use of CR, we sought clearance from participants’ physicians regarding an individual’s suitability for participation. Thus, to be included participants also must have been cleared by their physician. Subjects with more than 2 CVD risk factors or have uncontrolled/overt cardiovascular, pulmonary, or metabolic disease (Diabetes Mellitus), recent blood donation (<8 weeks), who have cancer or are being treated for cancer, or history of an eating disorder, or food allergies were excluded. To ensure greater ecological validity and representation, women were included in this study; however, we did not control for menstrual cycle phase, as done previously [
22]. Women who were currently pregnant, breastfeeding, attempting to conceive, or amenorrheic (not associated with menopause) were excluded from the study given the use of CR. Subjects were asked to continue any currently prescribed medications, but avoid any supplements (e.g. vitamins, nutraceuticals [herbs, extracts, etc.], weight loss pills, etc.) prior to and during the study. Participants were also asked to avoid strenuous exertion, and/or alcohol intake throughout the duration of the study, and to avoid strenuous exercise for 24 hours and caffeine/alcohol intake for 12 hours preceding the pre-post visits. Participants provided written informed consent prior to participation. This study was reviewed and approved by the Skidmore College Institutional Review Board (#2204-1028) and registered with clinicaltrials.gov (NCT05422391).
Procedures
This study was an open label, single arm study, see overview of the study procedures in
Figure 1. Once deemed eligible and cleared by their physician participants were then scheduled to come into the lab for baseline testing, prior to starting the diet. Participants came into the Human Nutrition and Metabolism laboratory at Skidmore College between 06:30-07:30 am (Monday or Tuesday), under standardized conditions described above, and were asked to arrive hydrated with water only. Upon arrival, height, weight, waist/hip circumference (waist at level of umbilicus/hip widest portion of buttocks) [
23] and total body composition (fat and lean mass, body water) were assessed using a stadiometer (Seca, Mt. Pleasant, SC, USA), Gulick tape measure (Gulick II Plus), and body composition scale (RD-545, Tanita, Arlington Heights, IL, USA) [
24], respectively. The Tanita BIA is known to be reliable and valid approach [
24], and in-house testing yielded an average coefficient of variation of 0.4% across 3 trials.
Participants were then positioned supine, and allowed to rest for 10 minutes, during this time we instrumented them with an oscillometric blood pressure cuff. Peripheral blood pressure, estimated central blood pressure and pulse wave analysis (Augmentation Index, AIx) [
25] were assessed using oscillometric cuff technique, in duplicate and then averaged. To determine the potential influence of the 3-day CR on metabolism, we measured resting metabolic rate (volume of oxygen consumed, VO2) using indirect calorimetry and ventilated hood technique [
26] with a metabolic cart (TrueOne2400, Parvomedics, Sandy, UT, USA) [
27]. From the metabolic cart data, we then estimated relative substrate utilization (%fat and % carbohydrate) [
28]. After completing metabolic testing, participants completed visual analog scales (0-100mm line) to assess perceptions of hunger, satiety, fullness, and desire to eat [
29], using online survey software (QualtricsXM, Boston, MA, USA). These perceptual measures were repeated nightly each day over the study and to assess compliance participants were also asked to report what time consumed each of diet as part of this survey. Participants were asked to complete the consensus sleep diary (CSD) core form [
30], again on the Qualtrics online platform.
Finally, participants were transported to the Health Services department to have a blood sample taken from an upper extremity. A small aliquot (40 uL) of fresh blood was used for blood lipid and glucose measurement using the cholestech LDX analyzer (Abbott, Lake Forest, IL, USA), which has been validated against standard clinical testing [
31]. In brief, total cholesterol, high density lipoprotein (HDL), low-density lipoprotein (LDL), HDL/total cholesterol ratio, triglycerides, and glucose were assessed. Remaining blood in the EDTA tubes was centrifuged and plasma aliquoted for storage at -80C for later analysis of biomarkers.
Participants were then given the standardized diet, which was the Plexus 3-day Reset diet program (Plexus Worldwide LLC, Scottsdale, AZ), and this marked the start of day 1 (Monday or Tuesday). Details of the diet are provided in
Table 1. Participants followed the diet on this day and 2 subsequent days, and then came in on the fourth day (Thursday or Friday) to repeat the same set of tests as baseline (“post-diet”). The diet itself approximates 590 kcal/day (when prepared as directed), is low fat (<30% of Kcal from fat), and is taken with 68-84 ounces of fluid (~10 cups or 2.5L of fluid, mostly from water). The diet also provides 3 “meals” or boluses of protein in the recommended 20-40g range to sustain muscle protein synthesis [
32], but is also associated with improvements in body weight/composition [
33]. Additional clear liquids were allowed ad libitum.
Biomarker Analysis
Plasma samples were analyzed using commercially available assay kits for ketone bodies (b-hydroxybutyric acid, BOH; and Acetoacetic acid, AcAc; Sigma Aldrich, Burlington, MA), factors that regulate metabolism (insulin, thyroid stimulating hormone, cortisol; RayBiotech, Peachtree, GA), factors that influence hunger/satiety (leptin, ghrelin, protein YY, neuropeptide Y; RayBiotech, Peachtree, GA), and inflammation (tumor necrosis factor alpha, TNFa; RayBiotech, Peachtree, GA). From glucose, mentioned above, and insulin the homeostatic model of assessment of insulin resistance (HOMA-IR) was calculated using published formula [
34]. All assays were completed in duplicate with standard curve linearity r
2>0.9, and coefficient of variation (cv) of <8%.
Data and Statistical Analysis
In a paired samples t-test model, using a one-tail approach, α=0.05, large effect size (Cohen’s d = 0.8), to yield an acceptable power of 0.8, an estimated 12 subjects would be needed (G*Power, Dusseldorf, Germany). We assumed that by recruiting 20, this could allow for possible dropout/non-compliance ensuring beyond the minimally effective sample size. Data were analyzed using open source software (JASP v.16.4.0, Amsterdam, Netherlands). The data were analyzed using paired samples t-tests and a complementary estimate of effect size, Cohen’s d, where 0.2, 0.5, and >0.8 represent small, medium, and large effect sizes, respectively. Perceptual data over time were analyzed using a one-way repeated measures analysis of variance. Assumptions for these tests were run and if a violation to normality occurred, a non-parametric alternative was employed, or an adjustment to the degrees of freedom was made. Alpha was set to 0.05. Data are presented as means ± standard deviation, unless otherwise noted.
3. Results
3.1. Participants
The fifteen individuals who completed the study were nearly middle aged, with a relatively even split of men and women (
Table 2). According to the body mass index (BMI), they indeed met the criteria of being classified as overweight or obese, but appeared otherwise healthy. Using the CR-diet protein intake (77g) and the average body weight, the relative protein intake was 0.82 g/kg of body weight/day which meets the recommended daily allowance (RDA). Though men and women were both included in this study, aside from the expected differences at baseline in height/weight, exploratory statistical analysis suggested no difference in how men and women responded to the 3-d CR diet, thus the data are combined.
3.2. Impact of 3-Day CR Diet on Body Weight, Body Composition, and Waist/Hip Circumferences
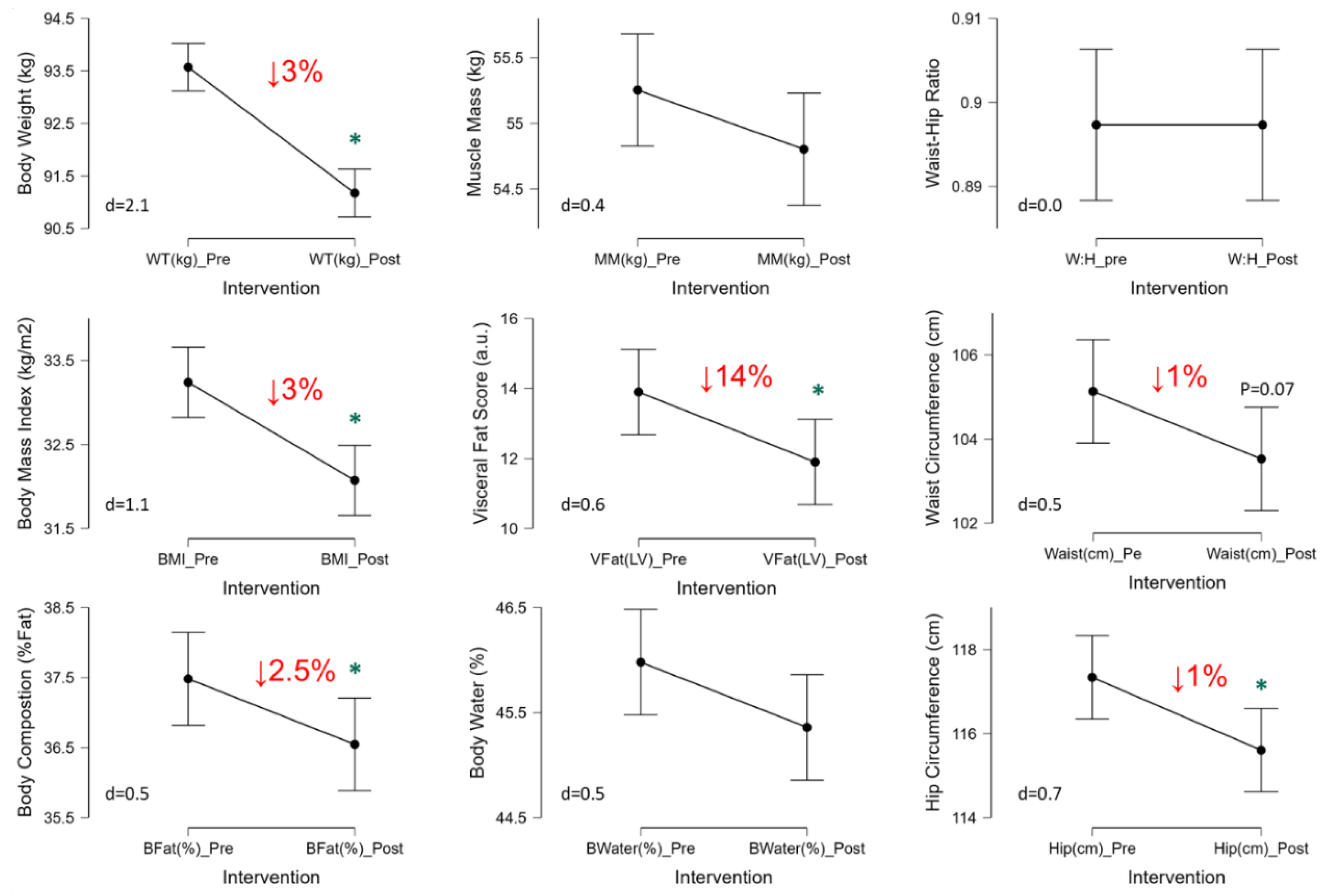
The 3-Day CR diet significantly lowered body weight by 3% (p<0.001, d=1.9), body mass index by 3% (p<0.001, d=1.4), body fat (p=0.002, d=1.1), and visceral fat score by 14% (p=0.002, d=1.1) (
Figure 2). Waist (p=0.07, d=0.5) and hip (p=0.02, d=0.7) circumferences were lower as well, by ~1% each, thus waist-hip ratio was unaffected (p=1.0, d=0,
Figure 2). No significant changes were observed in muscle mass (p=0.13, d=0.4) or body water (p=0.29, d=0.3) in response to the 3-Day CR diet (
Figure 2).
3.3. Impact of 3-Day CR Diet on Blood Pressure and Pulse Wave Analysis
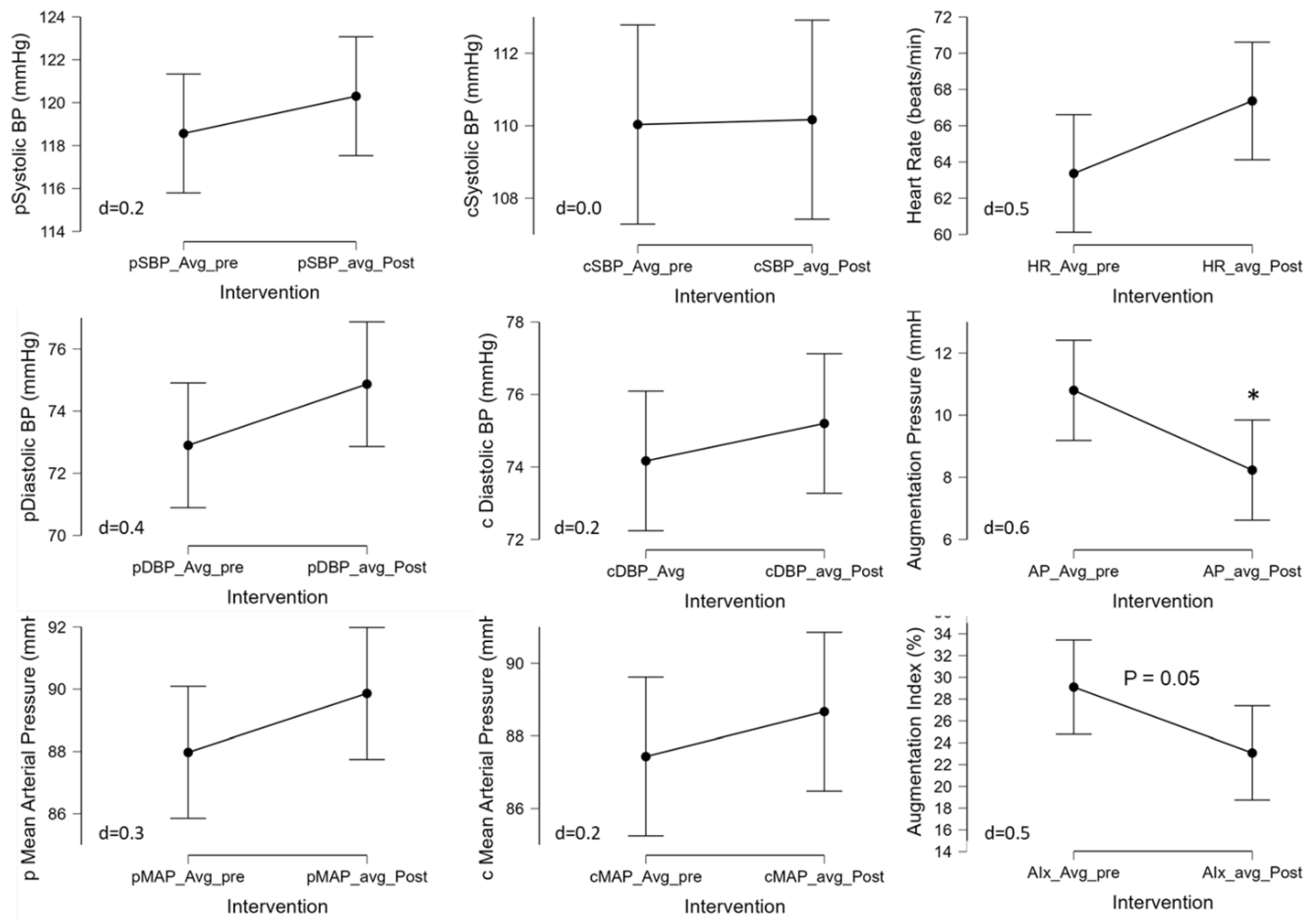
The 3-Day CR diet had no significant effect on peripheral systolic (pSBP; p=0.36, d=0.2), diastolic (pDBP; p=0.16, d=0.4), or mean (pMAP; p=0.23, d=0.3) blood pressures (
Figure 3, left panel). This was also true for estimated central systolic (cSBP; p=0.94, d=0.0), diastolic (cDBP; p=0.43, d=0.2), or mean (cMAP; p=0.41, d=0.2) blood pressures (
Figure 3, central panel). Heart rate was not significantly different with the 3-Day CR diet (HR; p=0.08, d=0.2). The pulse wave analysis revealed that while the 3-Day CR diet had no effect on pulse pressure (p=0.56, d=0.1), it did significantly alter augmentation pressure (AP; p=0.03, d=0.6) and augmentation index (29.8±17.5 to 21.5±14.5%, p=0.05, d=0.6) (
Figure 3, right panel).
3.4. Impact of 3-Day CR Diet on Blood Lipids and Glucose
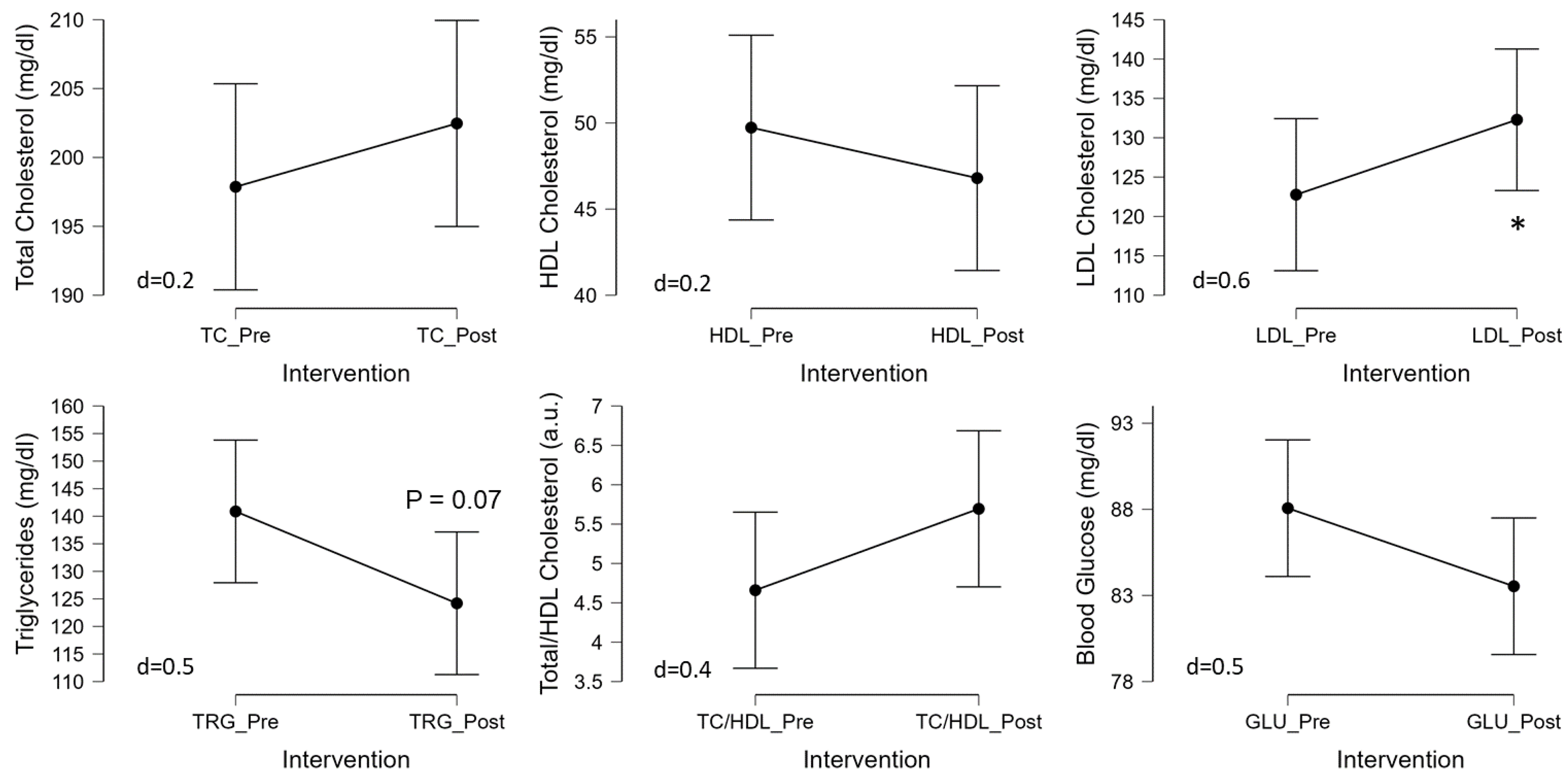
The 3-d CR diet had no significant effect on blood glucose (p=0.11, d=0.4), total cholesterol (p=0.37, d=0.2), HDL cholesterol (p=0.42, d=0.2), or TC/HDL (p=0.14, d=0.4) (
Figure 4). Although triglycerides tended to decrease (p=0.07, d=0.5) and LDL cholesterol tended to increase (p=0.05, d=0.6) (
Figure 4) in response to the 3-d CR diet in Ow/Ob men and women.
3.5. Impact of 3-Day CR Diet on Metabolism and Relative Substrate Utilization
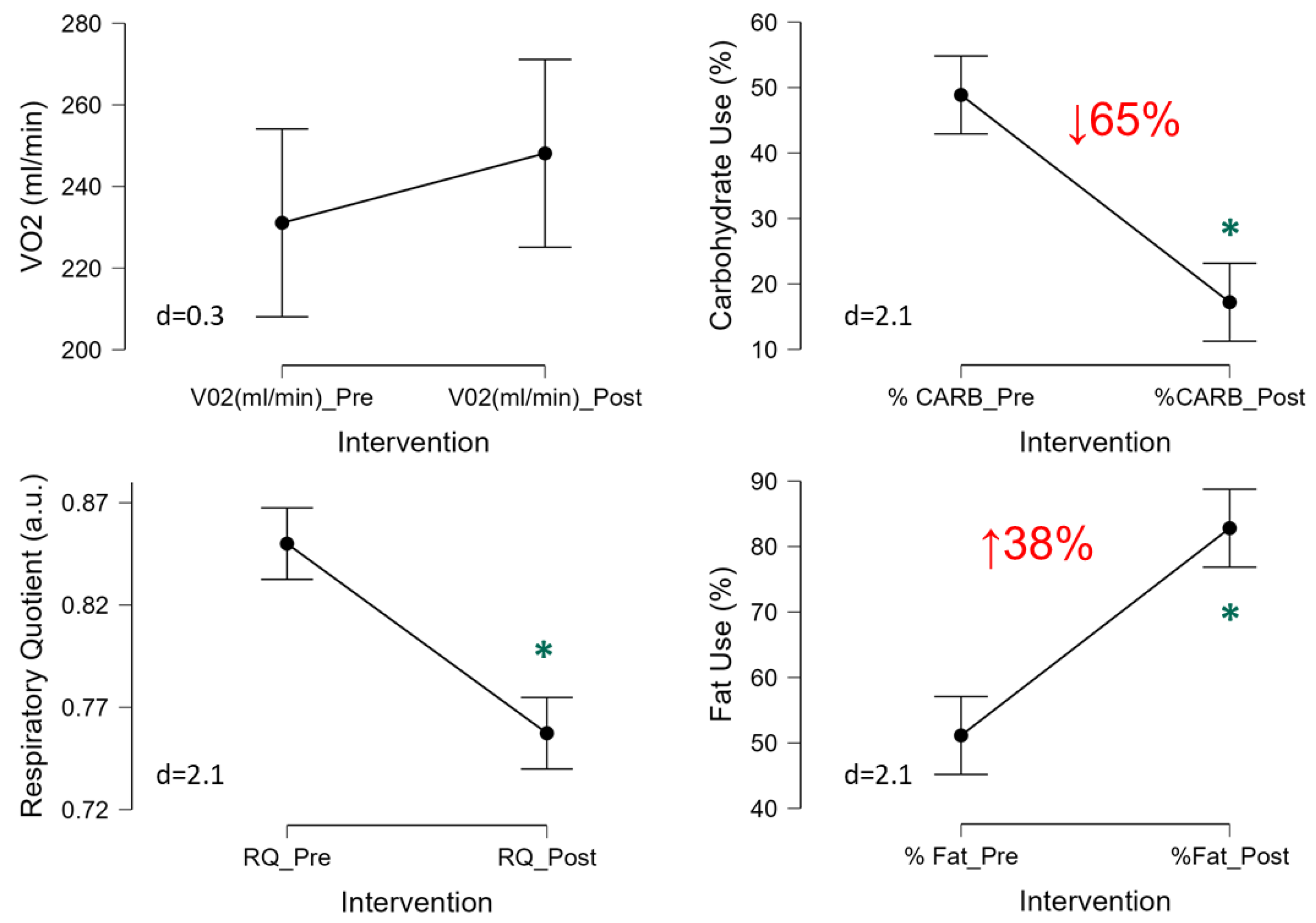
The 3-Day CR diet had no significant effect on VO2 (p=0.28, d=0.3,
Figure 5) and thus resting energy expenditure (1718±274 to 1722±317 kcal/d, p=0.83, d=0.1), which is derived from the VO2. Though a significant reduction in the respiratory quotient (RQ) was observed (p<0.001, d=2.1) suggesting metabolic shift from using carbohydrates to fats (
Figure 5). Indeed, further quantification of relative substrate utilization indicated a ~65% reduction in the relative use of carbohydrates (p<0.001, d=2.1) and corresponding 38% increase in the amount of fat oxidation (p<0.001, d=2.1) (
Figure 5).
3.6. Impact of 3-Day CR Diet on Circulating Metabolic Factors
The 3-Day CR diet had no significant effect on ketone bodies (acetoacetic acid and 3-hydroxybutryic acid), cortisol, insulin, neuropeptide Y, ghrelin, protein YY, TNFa, or on sirtuin 1 (all: p>0.05, d<0.4,
Table 3). Leptin was significantly increased (p=0.02, d=0.6) in response to the 3-day CR diet in Ow/Ob men and women (
Table 3). Using the glucose and insulin parameters, HOMA-IR was calculated although was not significantly changed following the 3-d CR diet (p=0.49, d=0.2,
Table 3).
3.7. Impact of 3-Day CR Diet on Perceptions of Hunger, Fullness, and Self-Reported Sleep
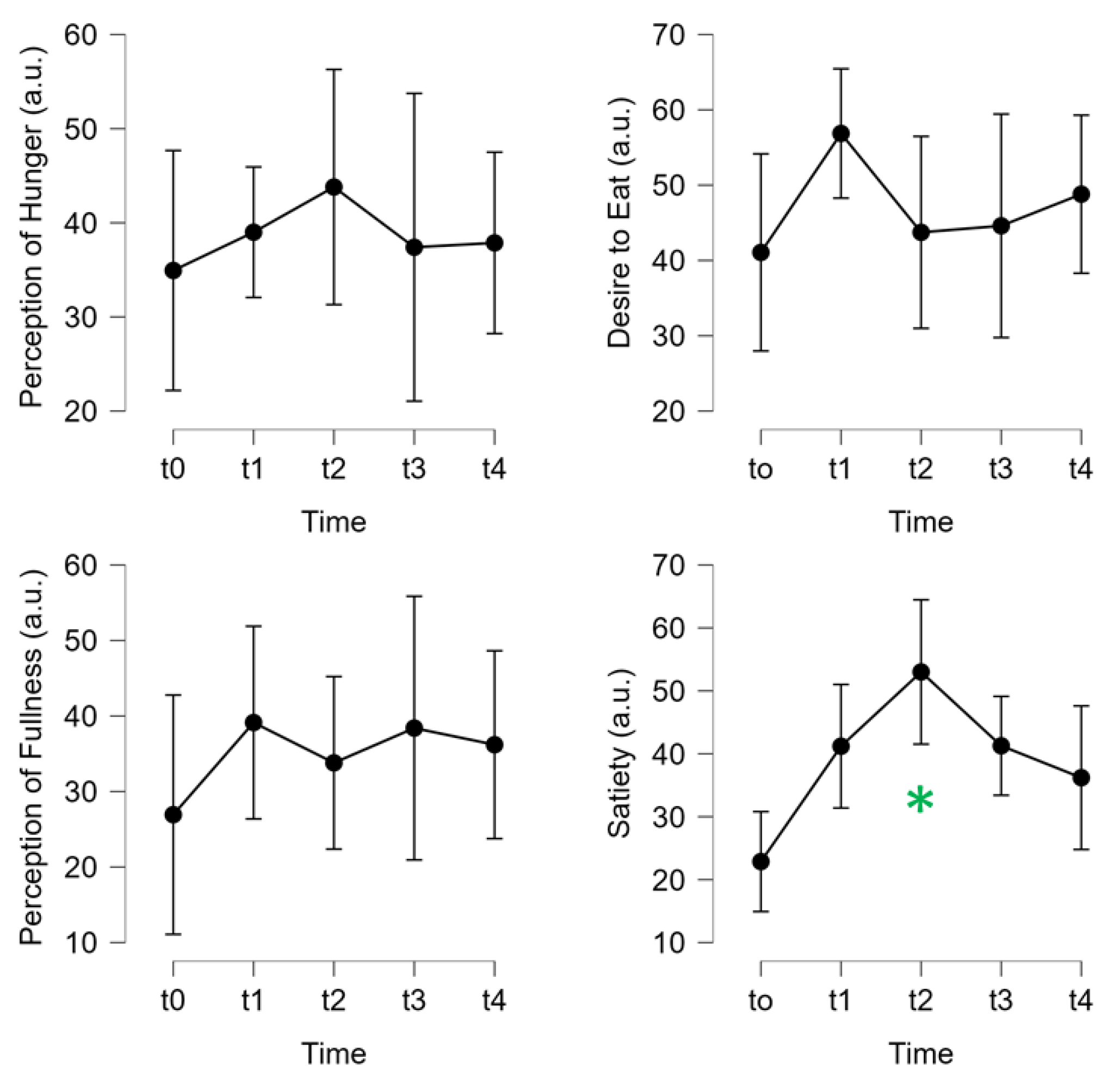
The 3-Day CR diet had no significant effect on self-reported perceptions or feelings of hunger, desire to eat, or fullness (all: p>0.05,
Figure 6). Satiety did vary over time and seemed to reach a high point at the end of day 2, which then decreased at day 3, and to post-diet testing on day 4 (
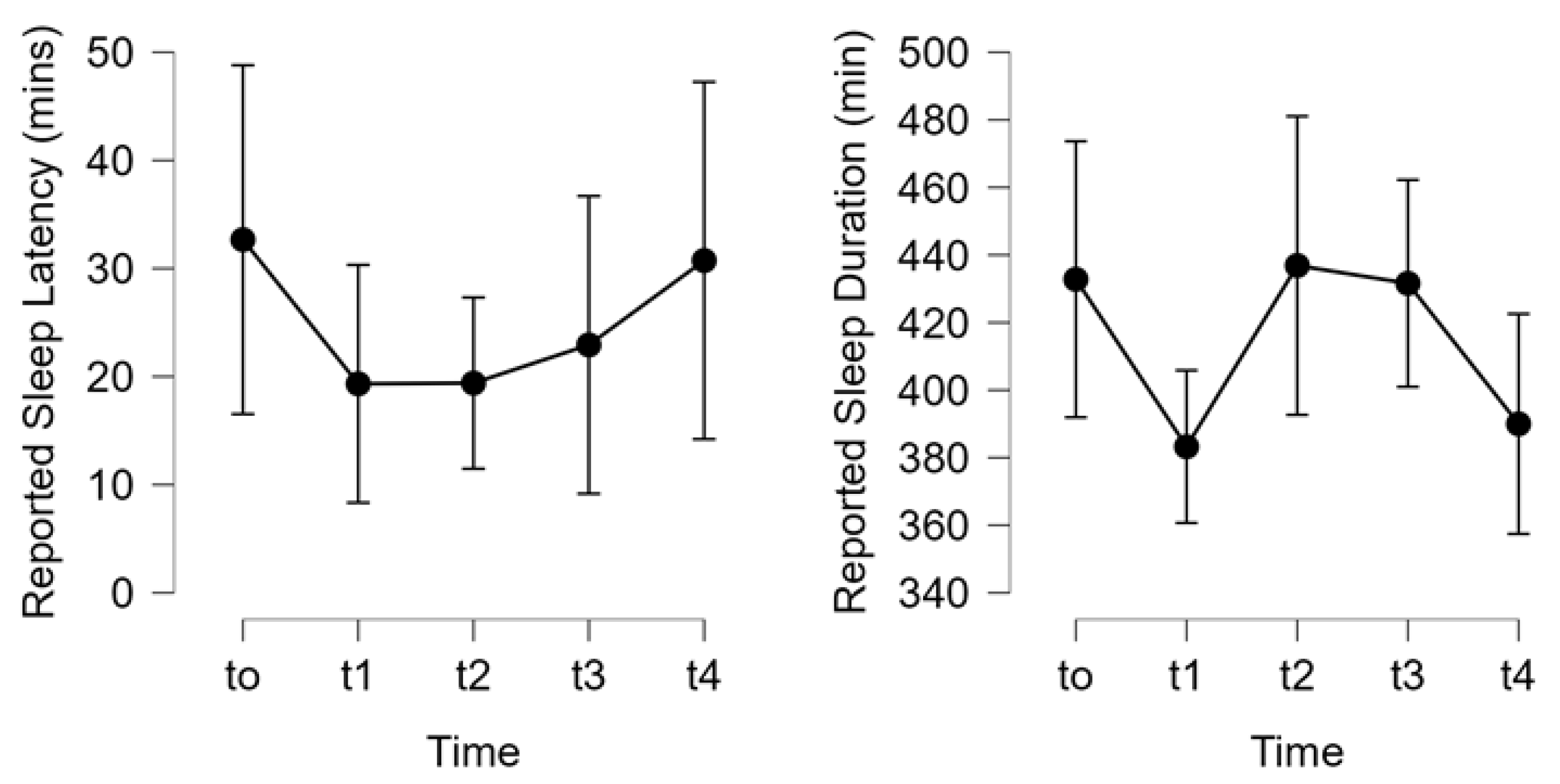
Figure 6). The 3-Day CR diet had no significant effect on self-reported sleep latency, or the total sleep duration over the course of the study (both p>0.05,
Figure 7).
4. Discussion
In the current study we aimed to see how an acute 3-Day CR diet might affect body weight/ composition, metabolism, cardiovascular health, and circulating metabolic factors that might explain changes in these parameters in overweight and obese (Ow/Ob) men and women. Three days of CR resulted in: significant weight loss, loss of total body fat /visceral fat, and loss in waist and hip circumference, without significant changes in body water or muscle mass. These anthropometric changes might be supported by switching from reliance on carbohydrates to oxidizing more fat as fuel, which could explain, along with increased circulating leptin, the loss of fat mass. There were minimal changes in blood pressure and blood lipids/glucose, suggesting nominal effects, although augmentation pressure and index, indicators of aortic stiffness responded favorably to the intervention. In terms of individual perceptions of the effects of short-term CR on hunger or fullness, there was not much effect, other than satiety which peaked on day 2 of the diet. Self-reported sleep latency and duration were also unaffected. Collectively, the findings from the current study suggest that 3-days of CR acutely induces weight and fat loss, increases circulating leptin and resting fat oxidation, without much change to cardiovascular health, feelings of hunger, or sleep in adult men and women who are overweight or obese. Considering these findings, such a short-term CR diet could be an effective way to induce weight loss or be incorporated as part of an intermittent fasting diet approach to weight loss, but further studies are warranted to determine efficacy.
Impact of Short-Term CR on Body Weight and Composition
Caloric restriction is not a novel approach of losing weight, in those that desire to do so, and has even been purported to induce health benefits beyond weight loss [
12,
35]. The CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Calorie Intake, CALERIE) trial might be one of the more prominent controlled trials of long CR, achieving 12% reduction in caloric intake, yielded an average weight loss 7.5kg over two years in healthy weight men and women [
12]. In overweight and obesity, a review of CR (15-60% reduction in intake) indicated that daily CR resulted in reductions of body weight and fat mass loss were 5-8%, and 10-20%, respectively [
36]. However, less is known about the temporal nature of these responses. One study that explored the acute and chronic impact of caloric restriction (~1000kcal/d) in obese women, observed significant reductions in waist circumference and tendency for lower fat mass in the first week, though body weight was not reported [
37]. This prior study examined the temporal response using 1-week epochs and only measured women. In the current study of men and women, using 3 days of more aggressive CR or very low-calorie diet (VLCD, ~600 kcal) we observed a 3% reduction in body weight, 3% reduction in %body fat, a 14% reduction in visceral fat score, and 1% reduction in waist/hip circumferences (
Figure 2). Thus, VLCD CR may induce benefits earlier on than might be expected. Interestingly, muscle mass and body water were not significantly impacted by the 3-Day CR. This is perhaps due to the protein pacing (multiple protein intakes over the day) in the range of 20-40g/meal that is recommended to sustain muscle protein synthesis [
32], which has been shown to be effective in weight loss and lean mass maintenance [
33,
38,
39]. Further, prescribed fluid intake with this standardized diet and is taken with 68-84 ounces of fluid (~10 cups or 2.5L of fluid, mostly from water), with possible additional
ad libitum fluid intake, which may explain the lack of significant change in body water. These aspects of the diet, protein pacing and sufficient fluid intake, may be able to sustain body water and lean mass, at least over this 3-day duration.
This 3-day CR diet may be a way to commence an individual’s weight loss in a distinct but effective way, but perhaps more importantly could be part of an intermittent CR or intermittent fasting dietary approach to weight loss and body composition improvement. Consumers without knowledge or training may struggle to identify adequate dietary approaches to weight loss, such as IF, and the dietary planning associated with them, therefore an “off the self” product or suite of products could make adopting such a weight loss strategy easier. Thus, findings from the current study may provide insight into a new paradigm of IF (4:3, 4 days ad libitum, 3 days caloric restriction using the 3-day diet) and provide evidence on an “off the shelf” way of carrying out a version of IF, as many individuals may struggle to plan and effectively carry out such dietary interventions. Such an IF approach might avoid complications associated with chronic/continuous CR [
13], especially very low-calorie diets over the long term, which may be inadvisable. This low-fat diet which maintains minimally recommended protein intake, ensures adequate intake of fluids and electrolytes, and leverages various supplements, such as fiber and other plant-derived factors (e.g. green coffee beans) could be beneficial for those that are looking to lose weight. Future studies should explore this in larger, and more diverse, groups to confirm these findings.
Impact of Short-Term CR on Cardiovascular Health
Long term caloric restriction has been demonstrated to induce favorable cardiovascular effects, namely reductions in blood pressure and improved lipid profile [
8,
35,
37,
40,
41], though intermittent fasting (IF) may elicit similar effects [
42,
43]. Long term CR of ~25% in magnitude lowered systolic and diastolic blood pressure, decreased total and LDL cholesterol, lowered triglycerides, lowered total cholesterol/ HDL cholesterol ratio, and increased HDL in “non-obese” healthy individuals (BMI of 22 to 27.9 m
2) [
12]. Although these reductions with modest CR [
12], and those reported with IF [
43], are minimal, on the order of a couple mmHg reduction in BP or fractions of 1 mmol/L cholesterol or triglyceride, only with more extreme CR are more dramatic reductions in these parameters observed. For example, in humans upwards of 40% reduction in caloric intake, over many years, can reduce systolic and diastolic BP by ~20mmHg and improve lipid profile by >30% in normal weight individuals [
41]. Focusing on those who are overweight or obese, and a more acute perspective, on a 1000kcal/d diet, obese women saw reductions in systolic BP of -10mmHg, diastolic BP of -4 mmHg in a 4-week time span, with only fractional changes in triglycerides [
37]. Interestingly, systolic BP was significantly reduced by ~6 mmHg with a week of CR onset, suggesting that CR may induce rapid changes in systolic BP.
Although, in the present study, using a short-term 3-day CR down to ~590kcal/d we observed no significant changes in systolic, diastolic, or mean arterial blood pressure measured peripherally or estimated centrally at the aorta (
Figure 3). This extension of prior work, examining more acute response to CR, and using oscillometric cuff technique and generalized transfer function to estimate central pressures and pulse wave analysis provides novel insight. Especially considering that central pressures are independent predictors of cardiovascular events and mortality [
44], and provide more detailed investigation into possible effects on the heart better than peripheral BP [
45]. Interestingly, the 3-day CR lowered augmentation pressure and index (
Figure 3), suggestive of a cardioprotective or beneficial effect [
44], although prior investigations have found that longer bouts of CR had no such effect on augmentation index or other parameters of vascular stiffness [
46,
47]. Thus, this reduction in augmentation pressure and index may be beneficial but also might be an acute phase response that may normalize over time or may be specific to the macro and micronutrient content of the diet employed in CR or initial weight status of the study population.
In terms of lipid profile, while longer term CR may induce reductions in total cholesterol, low density lipoprotein cholesterol, triglycerides, and glucose with concomitant increases in high density lipoprotein cholesterol, thereby improving the total/HDL ratio [
8,
37,
43] the effects with low intensity CR are modest. In the current study, we observed no significant changes in total, HDL, total/HDL ratio, or in blood glucose, but we did observe a significant increase in LDL cholesterol (
Figure 4). Although prior studies have indicated that acute fasting or CR may increase LDL, which may be related to weight loss [
48,
49]. Examination of insulin and glucose independently and together in the HOMA-IR (
Table 3) indicated no effect of the 3-day CR on insulin sensitivity and glucose homeostasis. Further analysis of the blood for cardiovascular health relevant parameters, namely the pro-inflammatory marker tumor necrosis factor alpha (TNFa) and CR-sensitive but vasoprotective sirtuin-1 (SIRT1) [
50], revealed no significant effects of the 3-day CR diet on inflammation or SIRT1 levels (
Table 3). Thus, the 3-day CR diet had a minimal or perhaps predictable effect on parameters germane to cardiovascular health (e.g. blood pressure and lipids/glucose), but tended to reduce estimates of central arterial stiffness in Ow/Ob men and women.
Impact of Short-Term CR on Perceptions of Hunger, Satiety, and Sleep
There is less known about the effects of acute or short-term CR on perceptions hunger/desire to eat or satiety/fullness, although longer term CR is known to induce psychological predilection to hunger and less so to feelings of satiety or fullness [
54]. In the present study we found no significant effects of the short-term 3-day CR diet on feelings of hunger, desire to eat, or fullness; although, satiety seemed change peaking on the second day of the diet (
Figure 6). Although the appetite and hunger related hormones NPY, PYY, and ghrelin were all unchanged with the 3-day CR diet in Ow/Ob, the elevation in leptin, though temporally or kinetically misaligned, may be related to this self-reported increase in satiety [
16]. While perhaps unrelated to hunger/fullness, we sought to determine the potential effects of short-term CR on sleep, and using self-reported sleep parameters, latency and duration, we observed no significant effects of the 3-day CR diet on sleep in Ow/Ob men and women.
5. Conclusions
In summary, the findings from the current study highlight that 3-days of standardized CR (~590 kcal/d intake) induces significant weight and fat loss, increases circulating leptin, increases fat oxidation, without much change to blood lipid profile, blood pressure, feelings of hunger, or sleep in adult men and women who are overweight or obese. These findings suggest that such a diet could be beneficial as an approach to initiating weight loss in those that are overweight or obese, or could be utilized in an intermittent fasting diet (e.g. alternate day or 5:2) approach to weight loss, but further studies are warranted to determine efficacy and whether such weight loss is sustained.
Author Contributions
Conceptualization, JD and SI; methodology, JD and SI.; formal analysis, AC, BY, JD, SI.; investigation, JD, AC, BY, CK, AC, SI.; resources, SI AC; data curation, AC, BY, JD, SI.; writing—original draft preparation, JD, SI, CK; writing—review and editing, JD, SI, AC, BY CK AC.; visualization, AC BY SI; supervision, SI JD; project administration, SI.; funding acquisition, SI. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Plexus Worldwide, grant number 2204-1028.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Skidmore College (protocol # 2204-1028 and 05/31/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the participants for volunteering and completing the study. We would also like to thank the health services nursing team at Skidmore for their assistance in completing the study.
Conflicts of Interest
Plexus Worldwide provided funding and product for the current study although the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fryar CD, C.M., Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United states, 1960–1962 through 2017–2018. NCHS Health E-Stats 2020.
- Cawley, J.; Biener, A.; Meyerhoefer, C.; Ding, Y.; Zvenyach, T.; Smolarz, B.G.; Ramasamy, A. Direct medical costs of obesity in the united states and the most populous states. Journal of managed care & specialty pharmacy 2021, 27, 354–366. [Google Scholar]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999, 277, E1130–1141. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent fasting and obesity-related health outcomes: An umbrella review of meta-analyses of randomized clinical trials. JAMA network open 2021, 4, e2139558–e2139558. [Google Scholar] [CrossRef]
- Miller, W.C.; Koceja, D.M.; Hamilton, E.J. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int. J. Obes. 1997, 21, 941–947. [Google Scholar] [CrossRef]
- Giallauria, F.; Strisciuglio, T.; Cuomo, G.; Di Lorenzo, A.; D’Angelo, A.; Volpicelli, M.; Izzo, R.; Manzi, M.V.; Barbato, E.; Morisco, C. Exercise training: The holistic approach in cardiovascular prevention. High Blood Pressure & Cardiovascular Prevention 2021, 28, 561–577. [Google Scholar]
- Walford, R.L.; Mock, D.; Verdery, R.; MacCallum, T. Calorie restriction in biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J. Gerontol. A. Biol. Sci. Med. Sci. 2002, 57, B211–224. [Google Scholar] [CrossRef]
- Elias, S.G.; Peeters, P.H.; Grobbee, D.E.; van Noord, P.A. The 1944-1945 dutch famine and subsequent overall cancer incidence. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1981–1985. [Google Scholar] [CrossRef]
- Strøm, A.; Jensen, R.A.; Oslo, M.D.; Oslo, M.D. Mortality from circulatory diseases in norway 1940-1945. The Lancet 1951, 257, 126–129. [Google Scholar] [CrossRef]
- Everitt, A.V.; Le Couteur, D.G. Life extension by calorie restriction in humans. Ann. N. Y. Acad. Sci. 2007, 1114, 428–433. [Google Scholar] [CrossRef]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (calerie): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Dirks, A.J.; Leeuwenburgh, C. Caloric restriction in humans: Potential pitfalls and health concerns. Mech. Ageing Dev. 2006, 127, 1–7. [Google Scholar] [CrossRef]
- Wadden, T.A. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann. Intern. Med. 1993, 119, 688–693. [Google Scholar]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brun, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database System Rev Implement Rep 2018, 16, 507–547. [Google Scholar] [CrossRef]
- Most, J.; Redman, L.M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide y, peptide yy and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obesity reviews : an official journal of the International Association for the Study of Obesity 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? International journal of obesity (2005) 2016, 40, 622–632. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell metabolism 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Wu, Y.; Panza, G.A.; Zaleski, A.; Guidry, M. Development of a novel clinical decision support system for exercise prescription among patients with multiple cardiovascular disease risk factors. Mayo Clinic Proceedings: Innovations, Quality & Outcomes 2021, 5, 193–203. [Google Scholar]
- Dudar, M.D.; Bode, E.D.; Fishkin, K.R.; Brown, R.A.; Carre, M.M.; Mills, N.R.; Ormsbee, M.J.; Ives, S.J. Pre-sleep low glycemic index modified starch does not improve next-morning fuel selection or running performance in male and female endurance athletes. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Brown, R.E.; Randhawa, A.K.; Canning, K.L.; Fung, M.; Jiandani, D.; Wharton, S.; Kuk, J.L. Waist circumference at five common measurement sites in normal weight and overweight adults: Which site is most optimal? Clinical obesity 2018, 8, 21–29. [Google Scholar] [CrossRef]
- Vasold, K.L.; Parks, A.C.; Phelan, D.M.L.; Pontifex, M.B.; Pivarnik, J.M. Reliability and validity of commercially available low-cost bioelectrical impedance analysis. International journal of sport nutrition and exercise metabolism 2019, 29, 406–410. [Google Scholar] [CrossRef]
- Shoji, T.; Nakagomi, A.; Okada, S.; Ohno, Y.; Kobayashi, Y. Invasive validation of a novel brachial cuff-based oscillometric device (sphygmocor xcel) for measuring central blood pressure. J. Hypertens. 2017, 35, 69–75. [Google Scholar] [CrossRef]
- Haugen, H.A.; Melanson, E.L.; Tran, Z.V.; Kearney, J.T.; Hill, J.O. Variability of measured resting metabolic rate. The American Journal of Clinical Nutrition 2003, 78, 1141–1144. [Google Scholar] [CrossRef]
- Crouter, S.E.; Antczak, A.; Hudak, J.R.; DellaValle, D.M.; Haas, J.D. Accuracy and reliability of the parvomedics trueone 2400 and medgraphics vo2000 metabolic systems. Eur. J. Appl. Physiol. 2006, 98, 139–151. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Carey, M.; Markham, C.; Gaffney, P.; Boran, C.; Maher, V. Validation of a point of care lipid analyser using a hospital based reference laboratory. Ir. J. Med. Sci. 2006, 175, 30–35. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. Journal of the International Society of Sports Nutrition 2017, 14, 33. [Google Scholar] [CrossRef]
- Arciero, P.J.; Baur, D.; Connelly, S.; Ormsbee, M.J. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: The prise study. Journal of applied physiology (Bethesda, Md. : 1985) 2014, 117, 1–10. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Fontana, L. Caloric restriction in humans. Exp. Gerontol. 2007, 42, 709–712. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obesity Reviews 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Jakobsdottir, S.; van Nieuwpoort, I.C.; van Bunderen, C.C.; de Ruiter, M.B.; Twisk, J.W.; Deijen, J.B.; Veltman, D.J.; Drent, M.L. Acute and short-term effects of caloric restriction on metabolic profile and brain activation in obese, postmenopausal women. International journal of obesity (2005) 2016, 40, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Edmonds, R.; He, F.; Ward, E.; Gumpricht, E.; Mohr, A.; Ormsbee, M.J.; Astrup, A. Protein-pacing caloric-restriction enhances body composition similarly in obese men and women during weight loss and sustains efficacy during long-term weight maintenance. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Ives, S.J.; Norton, C.; Escudero, D.; Minicucci, O.; O'Brien, G.; Paul, M.; Ormsbee, M.J.; Miller, V.; Sheridan, C.; et al. Protein-pacing and multi-component exercise training improves physical performance outcomes in exercise-trained women: The prise 3 study. Nutrients 2016, 8, 332. [Google Scholar] [CrossRef]
- Nicoll, R.; Henein, M.Y. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: A review of the evidence. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Poe, M.; Mohr, A.E.; Ives, S.J.; Arciero, A.; Sweazea, K.L.; Gumpricht, E.; Arciero, K.M. Intermittent fasting and protein pacing are superior to caloric restriction for weight and visceral fat loss. Obesity 2022, n/a.
- Yang, F.; Liu, C.; Liu, X.; Pan, X.; Li, X.; Tian, L.; Sun, J.; Yang, S.; Zhao, R.; An, N.; et al. Effect of epidemic intermittent fasting on cardiometabolic risk factors: A systematic review and meta-analysis of randomized controlled trials. Front Nutr 2021, 8, 669325. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; O'Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Lagou, S.; Zeniodi, M.E.; Boubouchairopoulou, N.; Stergiou, G.S. Association of central versus brachial blood pressure with target-organ damage. Hypertension 2016, 67, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Clifton, P.M.; Lister, N.; Keogh, J.B. Effect of weight loss induced by energy restriction on measures of arterial compliance: A systematic review and meta-analysis. Atherosclerosis 2016, 247, 7–20. [Google Scholar] [CrossRef]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; McDaniel, J.L.; Klein, S.; Villareal, D.T. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: A randomized intervention trial. The American Journal of Clinical Nutrition 2016, 104, 576–586. [Google Scholar] [CrossRef]
- Sävendahl, L.; Underwood, L.E. Fasting increases serum total cholesterol, ldl cholesterol and apolipoprotein b in healthy, nonobese humans. J. Nutr. 1999, 129, 2005–2008. [Google Scholar] [CrossRef]
- Akaberi, A.; Golshan, A.; Moojdekanloo, M.; Hashemian, M. Does fasting in ramadan ameliorate lipid profile? A prospective observational study. Pakistan journal of medical sciences 2014, 30, 708–711. [Google Scholar]
- Csiszar, A.; Labinskyy, N.; Jimenez, R.; Pinto, J.T.; Ballabh, P.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Ungvari, Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and sirt1. Mech. Ageing Dev. 2009, 130, 518–527. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Abdullah, K.; Al-Habori, M.; Al-Eryani, E. Ramadan intermittent fasting affects adipokines and leptin/adiponectin ratio in type 2 diabetes mellitus and their first-degree relatives. BioMed Research International 2020, 2020, 1281792. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Han, H.; York, E.; Martin, C.K.; Ravussin, E.; Williamson, D.A. Effect of calorie restriction on subjective ratings of appetite. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association 2009, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).