1. Introduction
Health behaviours, specifically diet and exercise, are a crucial part of maintaining human health and a key strategy in cancer prevention [
1,
2]. Yet cancer rates continue to rise, with an estimated 233,900 Canadians being diagnosed with cancer in 2022 [
3], and 1.9 million new cancer cases and 609,360 cancer deaths for Americans that same year [
4]. Two factors that can impact cancer prevention and survival include modifiable health behaviours, specifically exercise and nutrition. However, despite the growing body of evidence demonstrating numerous beneficial effects of exercise and healthy dietary behaviours [
5,
6,
7], many individuals continue to engage in a sedentary lifestyle and poor nutritional habits.
Diet, Exercise, and Cancer
Health behaviours are actions that can directly affect one’s health outcomes. Smoking tobacco, excessive consumption of alcohol, unhealthy diet, and inactivity are prime examples of adverse health behaviours that increase one’s risk of developing cancer [
2]. Concerning diet, studies have found that healthy dietary behaviours exert protective effects against cancer risk and mortality [
8,
9]. A “healthy diet” has previously been defined as “health-promoting and disease-preventing. It provides adequacy without excess, of nutrients and health-promoting substances from nutritious foods and avoids the consumption of health-harming substances” [
10]. In addition to the detrimental effects of unhealthy dietary behaviours on physical health, implications for mental health are also evident. Specifically, dietary patterns with high consumption of processed sugars and meats, which are known carcinogens [
11,
12], and limited fruit and vegetable intake, such as the Standard Western diet, is characterized by local inflammation of the GI tract and increased intestinal permeability [
13], and associated with greater incidence and severity of mental health conditions and symptom burden, including depression [
14].
The American Institute of Cancer Research [
15], the American Cancer Society [
1], and other organizations have published numerous, evidence-based resources and guidelines to support clinicians in counselling patients, and individual patients in making informed decisions about their dietary choices. However, there exists a paucity of evidence describing dietary health behaviours among survivors of cancer, particularly in comparison to healthy peers. Moreover, evidence-based nutritional guidance from health care providers throughout the cancer care continuum has been identified as one of the main unmet needs for patients and their families [
16]. This is an important gap in the literature and clinical practice, since survivors’ dietary behaviours hold implications for cancer recovery, and establishing and maintaining health and longevity after a cancer diagnosis.
Physical inactivity has also been linked to increased risk for cancer and poorer survival post-diagnosis [
8]. The American Cancer Society recommends that adult cancer survivors engage in 150 to 300 minutes of moderate-intensity, or 75 to 150 minutes of vigorous-intensity, physical activity per week with an emphasis on aiming to meet or exceed the upper limit of 300 minutes [
1]. However, research indicates that relative to healthy peers with no history of cancer, survivors of cancer tend to engage in less physical activity [
17] that does not meet the recommended guidelines. This is despite a plethora of evidence showing that survivors’ engagement in physical activity exerts protective effects against cancer recurrence, improved physical function, and overall quality of life [
1,
18].
Several mechanisms have been elucidated that link the beneficial effects of both healthy diet and exercise on health outcomes. These include maintenance of homeostasis and glucose response [
19], reduced systemic inflammation and expression of pro-inflammatory cytokines [
20], and improved regulation of the stress response [
21]. Additionally, in recent years an expansion of evidence from both human and animal studies strongly implicates the role of health behaviours, and especially diet, in modulating the composition and function of the gut microbiota. This has implications for physical and mental health in individuals and may be especially crucial in supporting the recovery in survivors of cancer post anti-cancer therapy.
Health Behaviours Impact the Gut Microbiota
Exercise, and especially diet, have been implicated as important health behaviour factors impacting the human gut microbiota, which consists of the community of microorganisms, notably bacteria, fungi, archaea and eukaryotes, that colonize the gastrointestinal (GI) tract [
22]. Previous studies with rodents and humans using randomized controlled trials and observational designs, have shown that regular exercise can impact the composition of the gut microbiota [
6,
13]. Furthermore, several recent, comprehensive reviews drawing on clinical and preclinical evidence have detailed how specific dietary behaviours, such as the Mediterranean diet, can impact the gut microbiota [
13,
23,
24].
While health behaviours are clearly implicated in the health and function of the gut microbiota, adverse events such as long-term or repeated high-dose exposure to antibiotics can negatively impact the composition and function of the gut microbiome [
25]. Recent evidence also suggests that exposure to chemotherapy treatment for cancer adversely affects the gut microbiota acutely and may also result in chronic gut microbial perturbations, with potential effects on GI and mental health [
26,
27].
Primary work from our Chemo-Gut study found that within the first 6 months to 1-year post-chemotherapy, survivors had significantly reduced gut microbiota alpha diversity compared to survivors greater than 1-year post-treatment and healthy peers [
28]. Moreover, compared to healthy peers with no history of cancer, the relative abundances of specific taxa remained different in survivors up to 5-years post-treatment; Certain bacterial taxa were correlated with psychosocial outcomes, including increased depression and poorer cognitive function [
28]. Our previous work also found that survivors experience chronic, moderate to severe, GI symptoms, lasting for an average of 2.5 years post-treatment, and that higher GI symptom burden is associated with poorer mental health [
29]. However, the potential impact of diet and exercise behaviours on survivors’ gut microbiota remains to be elucidated.
Present Study
The present study is an analysis of secondary outcomes from the Chemo-Gut project. The objectives of this study were to: (i) describe health behaviours related to diet and exercise in a cohort of cancer survivors; (ii) investigate associations between health behaviours, specifically diet and exercise, and GI symptoms in a cohort of cancer survivors; and (ii) explore associations between exercise and dietary health behaviours and the gut microbiota in a subset of our sample that provided stool samples for analysis.
2. Materials and Methods
Participants
Participants for this secondary analysis were combined from both the Chemo-Gut Pilot study [
28] and the Chemo-Gut Survey study [
29]. The demographic, clinical, and GI outcome related data were similar between the two studies, thus allowing us to pool the results for the health behaviour and GI outcome data. The full methodology including sample size calculations are previously described [
28,
29]. Briefly, participants for the survey study cohort were recruited virtually via social media channels, and the sample was comprised of Canadians who had previously been diagnosed with cancer, were currently aged 18 years or older, and had received and completed anti-cancer therapies (e.g. chemotherapy, radiotherapy). Participants for the pilot study were recruited from the Tom Baker Cancer Centre in Calgary, Canada and via social media and local cancer support groups. Participants in this cohort were between 18 to 39 years of age, diagnosed with a blood cancer or solid tumor, had previously received chemotherapy, and were within 5 years from their final cancer treatments. These studies were approved by the Health Research Ethics Board of Alberta Cancer Committee (HREBA.CC-19-0018).
Procedure
All demographic, clinical, and GI outcome measures were administered via a link made available to participants through Remote Electronic Data Capture (REDCap), a secure browser-based application designed to support Electronic Data Capture for research studies provided through the Clinical Research Unit (CRU) in the University of Calgary Cumming School of Medicine. Full data and stool collection procedures are previously described [
28,
29,
30]. Briefly, participants were instructed to use the investigator-provided home stool collection kit. Samples were collected in a sterile conical tube, placed in a biohazard bag, and stored in the participant’s freezer until pick-up, no more than 3 days from time of collection. Samples were picked-up by research personnel and transported on ice directly to the University of Calgary Faculty of Kinesiology and stored at -80
oC degrees until analysis.
Demographic, clinical and GI measures
Details regarding patient reported outcomes have previously been published [
29,
30]. The demographic and clinical health outcomes questionnaire was a locally designed survey that was developed based on patient partner input, and coauthor expertise. Data collected and used for this secondary analysis included dietary and exercise behaviours. Dietary behaviours related use of antibiotics or probiotics within the last 2 years, dummy coded as No (0) or Yes (1). We also inquired about the number of times per week participants consumed processed foods/meals, and their self-rated diet healthiness. Specifically, we asked, “on average, how many times per week do you consume ready-made/processed meals (e.g. macaroni and cheese, pizza from a box, etc.)” and “In general, how would you rate your current diet?”. Response options are detailed in
Table 1.
For daily macronutrient consumption, we inquired about participants average intake of vegetables, fruits, proteins, and whole grains. Examples were provided to help participants quantify their intake. For instance, regarding vegetable consumption the question stated: “a standard serving of vegetables is about 75g (24 – 84 calories) (e.g. ½ cup cooked green or orange vegetables (e.g. broccoli, carrots), ½ cup cooked dried or canned beans, peas or lentils, or 1 cup green leafy or raw salad vegetables). On average, how many servings of vegetables do you consume each day?”. Response options are shown in
Table 1.
Exercise behaviours pertained to the average frequency of exercise per week, specifically we asked, “on average, how many hours each week of exercise do you get?”, as well as intensity, inquiring, “on average, how would you rate the intensity of your exercise?”. Response options are noted in
Table 1. Regarding intensity, examples were provided for each (i.e. “low intensity” is walking at a normal pace; “moderate intensity” could be jogging or weight lifting; “high intensity” includes high intensity interval training (HIIT), spin class, etc.) to help participants quantify the intensity of their exercise.
GI symptoms included patient-reported outcomes for constipation, diarrhea, gas/bloating, and abdominal pain as measured using the National Institutes for Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS) [
31]. PROMIS measures are person-centered, validated, and reliable tools used to evaluate a variety of health-related outcomes in people with health conditions, including cancer and GI disorders [
32,
33,
34].
Data analysis
To address objective 1, descriptive statistics and frequency analyses were used to describe health behaviours related to diet and exercise in this cohort of cancer survivors. For objective 2, investigating associations between health behaviours and GI symptoms, linear regression analyses were used. Antibiotic and probiotic use, and diet (i.e. self-rated diet healthiness, processed foods, and macronutrients including fruits, vegetables, whole grains, and protein), and exercise (i.e. frequency and intensity) behaviours were entered into the regression models to explore whether these behaviours were associated with GI symptoms (i.e. gas/bloating, belly pain, constipation, and diarrhea). Beta values are reported as unstandardized. To address objective 3, Spearman’s rho correlation analyses for non-parametric data were used to explore associations between antibiotic and probiotic use, dietary, and exercise health behaviours and the gut microbiota. Only the first 30 most abundant ASV’s with n >10 cases were used for analysis [
37]. As this objective was exploratory and utilized a small sample, corrections for multiple comparisons were not conducted which increases the potential risk for Type I errors. Statistical analyses were completed using IBM Statistical Package for the Social Sciences (SPSS) version 28, with alpha set at p< 0.05.
3. Results
3.1. Objective 1: Describing the health behaviours of cancer survivors
Our sample consisted of N=334 survivors of cancer. The mean current age of survivors was 46.2 (SD= 14.8) years, while the average age at diagnosis was 40.3 (SD= 15.2) years old. The sample was primarily female (83.2%) and comprised mainly of survivors of breast (41%), haematological (19.5%), gynecological (11.1%), and colorectal (6.6%) cancers. Cancer stages ranged from I through IV, although the majority of participants had been diagnosed with stage II (30.2%) or III (26.9%) malignancies. Most (86.8%) participants had received chemotherapy treatment, followed by surgery (76.9%) and radiation (61.1%). Complete details of participant and treatment related outcomes are previously reported elsewhere [
28,
29].
Data regarding diet and exercise related health-related behaviours are summarized in
Table 1. Within the last 2 years 34.7% of survivors reported using probiotics, while 47.3% reported using antibiotics. Overall, survivors tended to rate their diet as moderately healthy (55.7%), and reported consuming none (50.9%) or few (39.8%) processed foods or meals per week. Regarding macronutrients, 38.6% report consuming 2 or fewer servings of vegetables or fruits (52.1%) per day, while about half (48.8%) reporting consuming 2 to 4 servings of protein per day. Daily consumption of 2 or fewer servings of whole grains were reported by 48.2% of participants. Most survivors reported engaging in exercise for 2 or less (32.3%) or 3 to 5 (36.8%) hours per week. A low exercise intensity was most frequently reported by 53.9% of participants.
3.2. Objective 2: Associations between health behaviours and GI symptoms
For GI symptoms, participants ranged from minimum outcome values indicating few to no symptoms that are within “normal” range, to scores >70, indicative of severe GI symptoms associated with poorer health (see
Table 2) [
38]. However, the only symptom reported with a mean value higher than the reference population was gas/bloating (M= 56.2, SD= 8.1), indicating a clinically meaningful difference from the healthy population normative score of 50 [
39].
Table 3 details the results of the regression analysis examining whether consumption of antibiotics or probiotics was predictive of GI symptoms. Antibiotic use within the past 2 years was associated with more symptoms of belly pain (
B= 4.02,
SE= 1.30,
p< .00), constipation (
B= 2.71,
SE= 1.03,
p< .00), and diarrhea (
B= 2.91,
SE= 1.16,
p< .01). Probiotic use was associated with more belly pain (
B= 4.09,
SE= 1.35,
p< .00) and gas/bloating (
B= 3.64,
SE= 1.00,
p< .00) symptoms.
Table 4 reports the results of the regression analyses for dietary behaviours and GI symptoms. We found that lower protein consumption at 2 or fewer servings daily was associated with more belly pain symptoms (
B= -2.12,
SE= 1.06,
p< .05), while lower consumption of whole grains was associated with fewer symptoms of constipation (
B= 1.77,
SE= .88,
p< .05). Better self-rated diet healthiness was associated with fewer symptoms of gas/bloating (
B= -2.01,
SE= .89,
p< .02).
Table 5 shows associations between exercise behaviours and GI symptoms. The regression analysis revealed no significant relationships between exercise frequency or intensity and any of the GI symptoms.
3.3. Objective 3: Correlations between health behaviours and the gut microbiota
Spearman’s rho correlation analysis for non-parametric data were used to examine relationships between bacterial taxonomic composition and dietary and exercise health behaviours in a subset of 17 survivors.
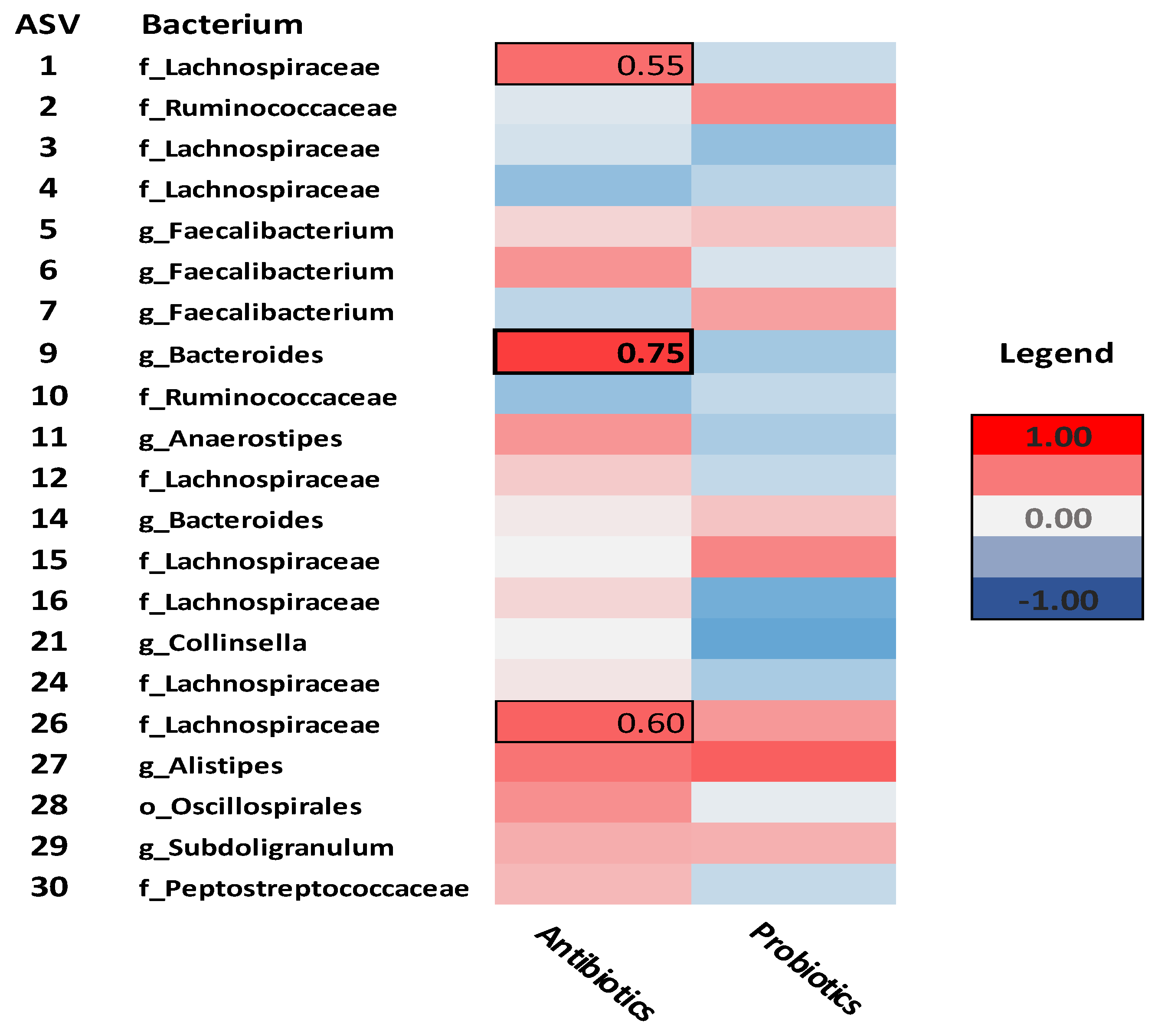
Figure 1 illustrates correlations between antibiotic and probiotic use and specific taxa. Only antibiotic use significantly correlated with any of the microbial taxa. Specifically,
Lachnospiraceae (ASV-1) (rho= .55,
p= .02),
Bacteroides (ASV-9) (rho= .75,
p< .01), and
Lachnospiraceae (ASV-26) (rho= .60,
p= .02) correlated positively with antibiotic use.
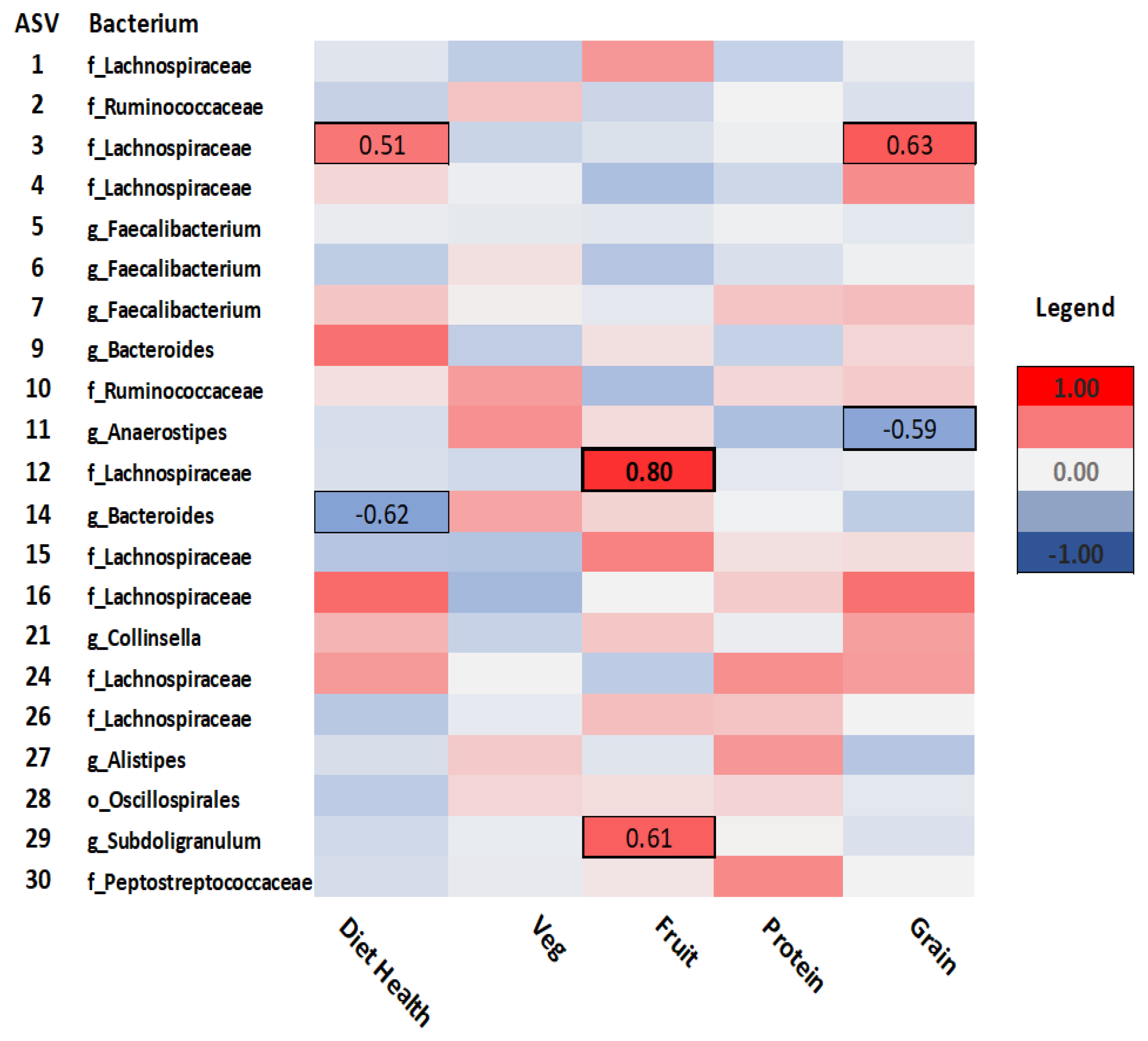
As seen in
Figure 2, for dietary behaviours,
Lachnospiraceae (ASV-3) was positively correlated with self-rated diet health (rho= .51,
p= .05), while
Bacteroides (ASV-14) correlated negatively (rho= -.62,
p= .02) with diet healthiness. Fruit consumption was positively correlated with both
Lachnospiraceae (ASV-12) (rho=.80,
p<.001) and
Subdoligranulum (ASV-29) (rho= .61,
p= .02). Consumption of whole grains was positively correlated with
Lachnospiraceae (ASV-3) (rho= .63,
p= .01), but negatively correlated with
Anaerostipes (ASV-11) (rho= -.59,
p= .02).
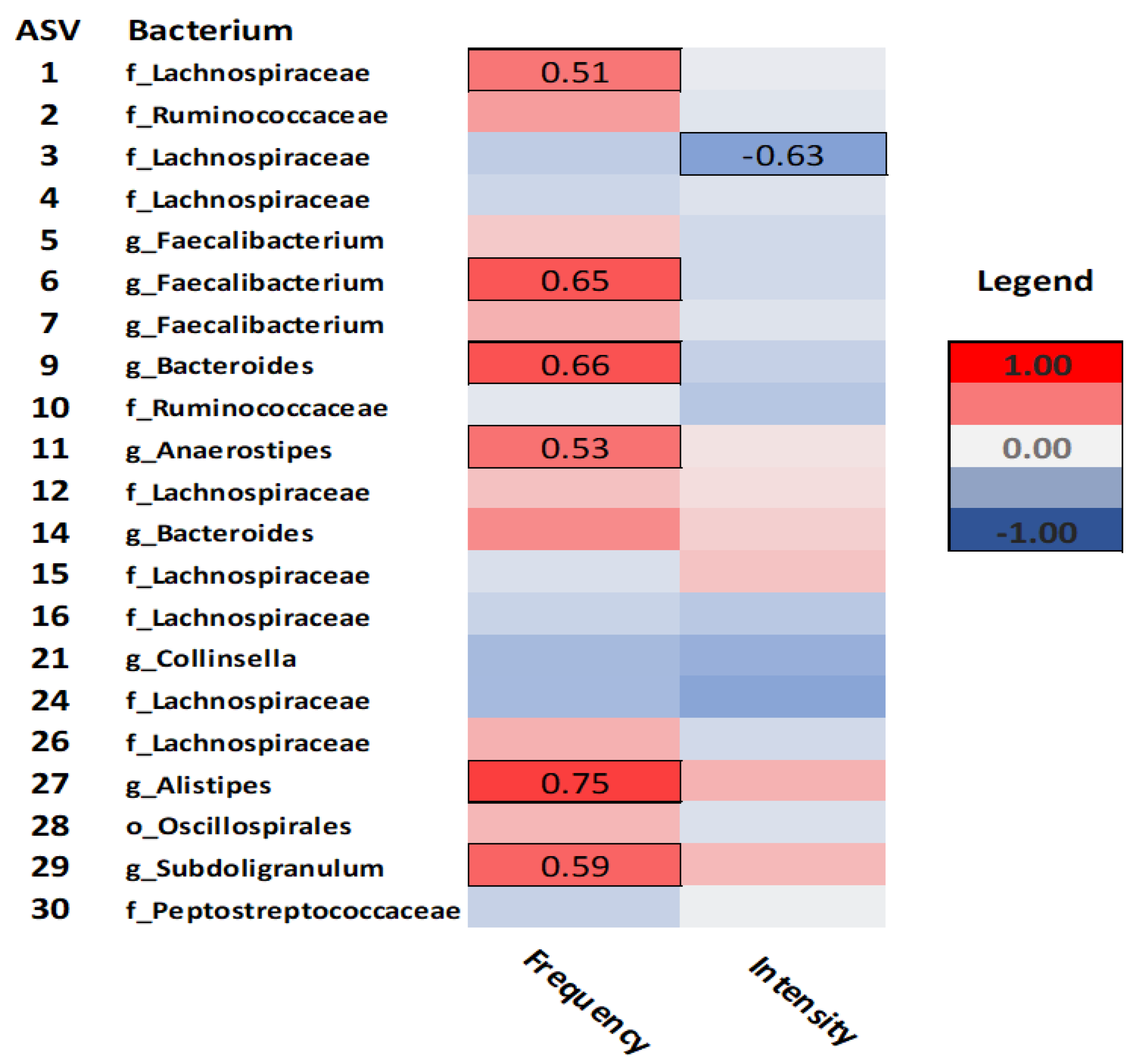
Figure 3 illustrates the correlations between exercise related health behaviours, specifically exercise frequency and intensity, and bacterial taxa. Exercise frequency was positively correlated with
Lachnospiraceae (ASV-1) (rho= 51,
p= .04),
Faecalibacterium (ASV-6) (rho= .65,
p= .01),
Bacteroides (ASV-9) (rho= .66,
p= .01),
Anaerostipes (ASV-11) (rho= .53,
p= .04),
Alistipes (ASV-27) (rho= .75,
p= .01), and
Subdoligranulum (ASV-29) (rho= .59,
p= .03). Meanwhile, only
Lachnospiraceae (ASV-3) was negatively correlated with exercise intensity (rho= -.63,
p= .01).
4. Discussion
Health behaviours in survivors of cancer
In this cohort of cancer survivors, self-rated diet healthiness was reported by over half of the participants as being moderately healthy. Participants indicated that they consumed no (50.9%), or few (39.8%) processed foods or meals per week, which is consistent with recommendations from the American Cancer Society [
1]. These data provide new evidence describing specific dietary health behaviours in a diverse sample of cancer survivors. Considering the established link between diet and good health, previous studies have investigated the effects of various dietary interventions in survivors of cancer [
40], yet there are a limited number of studies describing specific dietary behaviours in cancer cohorts. Further research is needed to characterize a comprehensive range of dietary behaviours in cancer survivors that may impact health outcomes.
Participants’ consumption of macronutrients, and particularly vegetables and fruits, which are known to beneficially support gut microbial health and overall health more generally, was concerningly low. Over one-third (38.6%) of survivors reported consuming 2 or fewer servings of vegetables per day, while about half (52.1%) reported consuming 2 or fewer servings of fruit per day. Canada’s Food Guide recommends 7 to 10 daily servings of fruits and vegetables for adults [
41], indicating that survivors’ in our study do not meet the guidelines for healthy fruit and vegetable consumption. Vegetables contain crucial vitamins and nutrients to support cancer prevention and recovery, in addition to fibre necessary for optimal gut microbial function. For instance, cruciferous vegetables like broccoli and cabbage contain isothiocyanates and a potent phytochemical called sulforaphane, which evidence suggests is effective in the prevention and treatment of several cancers including breast, colorectal, bladder, prostate, and oropharyngeal cancers [
42]. Studies have also shown that diets characterized by higher vegetable and fruit intake are associated with reduced risk and incidence of chronic diseases, such as diabetes and cancer [
43]. Higher consumption of vegetables has also been associated with an attenuated white blood cell profile, suggesting reduced markers of systemic inflammation [
44].
Most survivors reported engaging in exercise at a frequency of 2 or fewer (32.3%) or 3 to 5 (36.8%) hours per week, with low exercise intensity (i.e. vacuuming, walking) most frequently reported by over half (53.9%) of participants. This is consistent with previous research suggesting that in general survivors tend to engage in physical activity of lower intensity such as walking [
45], and that engagement in exercise post-treatment tends to be lower relative to pre-diagnosis [
17]. However, it must be noted that these data were collected during the COVID pandemic, when frequent closures of gyms and other exercise facilities occurred. As such, survivors may have reported engaging in less physical activity than they normally would do to the challenging circumstances brought on by the pandemic.
Health behaviours are associated with specific GI symptoms
The data revealed that antibiotic use within the past 2 years was predictive of more severe belly pain, constipation, and diarrhea GI symptoms. These findings are consistent with research suggesting that antibiotic treatment is frequently associated with a myriad of GI symptoms and functional GI disorders [
46]. Interestingly, probiotic use was associated with more symptoms of belly pain and gas/bloating. Although probiotic use has sometimes been associated with GI symptoms, such as gas or flatulence, it is also possible that survivors are consuming probiotics to help address these pre-existing GI symptoms. Further research is needed to understand the context within which survivors of cancer decide to use probiotic supplements.
About half (48.8%) of the participants reporting consuming 2 to 4 servings of protein per day. Our regression analysis found that lower protein consumption at 2 or fewer servings daily was actually predictive of more belly pain symptoms, suggesting that adequate protein consumption may be important for supporting gut health. However, the types of protein (e.g. milk, whey, poultry, plant sources, etc.) consumed by participants in our study are unknown and could be an important factor mediating the effects of protein consumption on GI symptomology.
Concerning whole grain consumption, nearly half (48.2%) of participants reported consuming 2 or fewer servings per day, and lower consumption of whole grains was predictive of fewer symptoms of constipation. This was surprising given that fibre from whole grains is typically associated with improved gut health and motility. For instance, Jung et al. compared the effects of a 4-week brown rice-based diet, white rice-based diet, or wheat-based diet in a sample of 39 women with functional constipation. Both the brown-rice and wheat-based diets were shown to improve symptoms of constipation, specifically total colon transit time [
47]. In contrast, studies suggest that following chemotherapy treatment, breast cancer survivors may have challenges digesting whole grains and higher consumption of whole grains may in fact have adverse implications for survivors’ health [
48]. Since 41% of our study sample was comprised of breast cancer survivors, this is an important consideration. Moreover, since whole grain consumption was self-reported, it is difficult to ascertain the sources and quality of grains consumed, which could also be a factor influencing affects on GI symptoms.
Better self-rated diet healthiness was predictably associated with fewer GI symptoms of gas/bloating. These data provide needed evidence regarding specific dietary behaviours and the implications for survivors in experiencing, and potentially managing, GI symptoms after treatments for cancer.
Gut microbiota are associated with health behaviours
In our small sample of participants who provided stool for gut microbiota analyses, only three bacterial taxa were significantly, positively correlated with antibiotic use. Specifically,
Lachnospiraceae (ASV-1 and 26) and
Bacteroides correlated positively with antibiotic use, such that a higher abundance of taxa was present in participants who had taken antibiotics. Although
Bacteroides species are abundant within the human gut microbiota and can be symbiotic with the host, under certain conditions
Bacteroides can also become opportunistic pathogens. For instance, overabundance of
Bacteroides vulgatus has been associated with Crohn’s disease, while
Bacteroides fragilis is implicated in appendicitis and inflammatory bowel disease [
49]. Considering that antibiotic treatment was identified as a predictor of GI symptoms in the present study, it is possible that antibiotic-associated changes in the gut microbiota may be a contributing factor to GI symptomology.
Concerning dietary behaviours,
Lachnospiraceae was positively correlated with self-rated diet health, while
Bacteroides correlated negatively with diet healthiness. Those who reported better diet health had a higher abundance of
Lachnospiraceae, one of the main producers of short-chain fatty acids [
50], and a lower abundance of
Bacteroides. Fruit consumption was positively correlated with both
Lachnospiraceae and
Subdoligranulum, such that those reporting a higher (3 to 5 servings) consumption of fruit per day had a higher abundance of these bacterium.
Subdoligranulum is an important species that in humans with overweight or obesity has been correlated with reduced fat mass, insulin resistance, leptin, and insulin levels, as well as attenuated levels of proinflammatory biomarkers including C-reactive protein and Interleukin-6 [
51]. Higher consumption (3 to 5 servings per day) of whole grains was positively correlated with a higher abundance of
Lachnospiraceae, while fewer servings (2 or less) of whole grains per day was associated with greater
Anaerostipes abundance. This is consistent with previous studies reporting higher abundances of
Anaerostipes among Chinese adults who consumed a lower intake of refined grains [
52].
Regarding exercise behaviour, our data suggest that it may be the frequency, rather than the intensity, impacting the gut microbiota the most. Specifically, correlation analyses found that higher exercise frequency was significantly correlated with a greater abundance of
Lachnospiraceae,
Faecalibacterium,
Bacteroides,
Anaerostipes,
Alistipes, and
Subdoligranulum. Previous studies suggest that
Faecalibacterium prausnitzii has beneficial effects in the human microbiome and may be a novel probiotic bacterium to treat functional GI disorders, like irritable bowel syndrome [
53]. Genus
Alistipes is another example of a bacterium that may be symbiotic, or pathogenic, depending on the gut microbial conditions. Previous studies suggested that
Alistipes may have potential protective effects against colitis, but when pathogenic it has also been associated colorectal cancer and depression [
54]. While our associations between specific microbial taxa and health behaviours are intriguing, further research is needed to contextualize these findings, clarify the direction of causality, and understand the implications for cancer survivors’ health.
Limitations and Future Directions
One of the main limitations of this study is that it relied primarily on participants’ self-report measures, which tend to be subjective and prone to bias. Participants may have over or underestimated certain health behaviours, such as self-rated diet healthiness or the amount or type of whole grains consumed daily. Further, while some participants reported taking both a probiotic and antibiotic within the past 2 years, we are unable to determine whether these were taken at the same time points and for how long, which are factors that may affect GI symptoms. For objective 3, a small sub-sample that provided stool samples was used. Due to the small sample size, no control for multiple comparisons was applied. Hence, there is an elevated risk for potential Type I errors. Additionally, as 16s rRNA gene sequencing analysis was used, only the composition of the gut microbiota could be quantified, limiting our understanding of the potential functional capacity of the gut microbiota and how this may relate to health behaviours. As well, the data used for this study was collected during the COVID pandemic between December 2019 to April 2021, which may have affected diet and exercise behaviours, especially during periods where lockdowns were implemented. Participants may have been engaging in less exercise due to inaccessibility to the gym and other fitness activities they would typically engage in. Given this, our results must be interpreted with caution and future trials with larger samples and a control group for comparison are warranted.
Despite the limitations of this study, our findings provide context regarding dietary and exercise health behaviours in a diverse group of cancer survivors, and how these behaviours relate to GI symptoms. Moreover, associations between specific dietary behaviours, exercise frequency, and the gut microbiota are revealed. Our findings can inform future research aimed at using dietary or exercise-based interventions to improve GI symptoms in survivors of cancer, potentially via modulation of the microbiota-gut-brain axis. Moreover, results can be used by clinicians to support recommendations for diet and exercise in survivorship care plans, and to educate patients on the importance of healthy nutrition and the integration of physical activity. Survivors of cancer can use this information to make informed decisions about their health, in conjunction with numerous other evidence-based resources and recommendations for safe and effective use of dietary strategies and exercise after cancer [
15,
16,
55,
56].
5. Conclusions
Overall, most survivors in this study reported consuming a moderately healthy diet and engaging in low intensity exercise a few times a week. Moreover, results provide evidence for associations between antibiotic use and more severe symptoms of belly pain, constipation, and diarrhea. Dietary behaviours including self-rated diet health, and average daily servings of protein and whole grains were associated with GI symptoms. Antibiotic use, self-rated diet healthiness, fruit, whole grain consumption, and exercise frequency behaviours correlated with specific types of bacterial taxa, but the direction of causality requires clarification. Behavioural and dietary-based interventions may be optimally suited to address survivors’ adverse GI symptoms by influencing the gut microbiota. However, larger, prospective trials are needed.
Author Contributions
Study conceptualization and methodology was completed by J.D., L.C., and R.R. Administrative and recruitment support was provided by M.B. and K.A.P. All data collection was completed by J.D., while sample processing was done by J.D., F.C., and D.L. Data analysis was completed by J.D., while all bioinformatics were completed by F.C. The original manuscript draft preparation was done by J.D. All other authors contributed to review and revision of the final manuscript and intellectual content. L.C. was responsible for supervision and funding acquisition.
Funding
This work was funded by the Enbridge Research Chair in Psychosocial Oncology, co-funded by the Canadian Cancer Society Alberta/NWT Division and the Alberta Cancer Foundation, awarded to L.E. Carlson, and by the Killam Foundation in the form of a scholarship awarded to J.M. Deleemans.
Data Availability Statement
Data is available upon reasonable written request to the corresponding author.
Acknowledgments
We wish to acknowledge and thank the participants who took the time to participate in this study, and research staff in the Reimer lab for assisting with sample processing procedures.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
Gastrointestinal (GI); Remote Electronic Data Capture (REDCap); Electronic Data Capture (EDC); High intensity interval training (HIIT); National Institutes for Health (NIH); Patient-Reported Outcomes Measurement Information System (PROMIS); Amplicon sequence variants (ASV); Statistical Package for the Social Sciences (SPSS); Mean (M); Standard Deviation (SD); Standard Error (SE); Unstandardized Beta Coefficient (Unstd.B).
References
- Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, Neuhouser ML, Bandera EV, Wang Y, Robien K et al: American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 2022, 72(3):230-262. [CrossRef]
-
Reduce Your Risk [https://cancer.ca/en/cancer-information/reduce-your-risk].
-
Canadian Cancer Statistics special report 2022 [https://cancer.ca/en/about-us/media-releases/2022/canadian-cancer-statistics-special-report-2022#:~:text=Today%2C%20it%20is%20estimated%20that,the%20importance%20of%20cancer%20prevention.].
-
Cancer Facts & Figures 2022 [https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html].
- Suriano F, Nystrom EEL, Sergi D, Gustafsson JK: Diet, microbiota, and the mucus layer: The guardians of our health. Front Immunol 2022, 13:953196. [CrossRef]
- Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S, Monda M et al: Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev 2017, 2017:3831972. [CrossRef]
- Penedo FJ, Dahn JR: Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry 2005, 18(2):189-193.
- Ding D, Van Buskirk J, Nguyen B, Stamatakis E, Elbarbary M, Veronese N, Clare PJ, Lee IM, Ekelund U, Fontana L: Physical activity, diet quality and all-cause cardiovascular disease and cancer mortality: a prospective study of 346 627 UK Biobank participants. Br J Sports Med 2022.
- Moazzen S, Cortes-Ibanez FO, van der Vegt B, Alizadeh BZ, de Bock GH: Diet quality indices and gastrointestinal cancer risk: results from the Lifelines study. Eur J Nutr 2022, 61(1):317-327. [CrossRef]
- Neufeld LM, Hendriks, M., Hugas, M.: Healthy diet: A definition for the United Nations Food Systems Summit 2021. In. Edited by Summit TSGftUFS; 2021. [CrossRef]
- Domingo JL, Nadal M: Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem Toxicol 2017, 105:256-261. [CrossRef]
- Epner M, Yang P, Wagner RW, Cohen L: Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers (Basel) 2022, 14(24). [CrossRef]
- Campaniello D, Corbo MR, Sinigaglia M, Speranza B, Racioppo A, Altieri C, Bevilacqua A: How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14(12). [CrossRef]
- Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, Li B: Dietary patterns and depression risk: A meta-analysis. Psychiatry Res 2017, 253:373-382. [CrossRef]
-
Cancer Survival [https://www.aicr.org/cancer-survival/].
- Frenkel M, Sapire KJ, Lacey J, Zollman C, Sierpina VS: "What Should I Eat?"-Addressing Questions and Challenges Related to Nutrition in the Integrative Oncology Setting. Curr Oncol Rep 2022, 24(11):1557-1567. [CrossRef]
- Murnane A, Gough K, Thompson K, Holland L, Conyers R: Adolescents and young adult cancer survivors: exercise habits, quality of life and physical activity preferences. Support Care Cancer 2015, 23(2):501-510. [CrossRef]
- Capozzi LC, Nishimura KC, McNeely ML, Lau H, Culos-Reed SN: The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med 2016, 50(6):325-338. [CrossRef]
- Mazidi M, Kengne AP, Mikhailidis DP, Toth PP, Ray KK, Banach M: Dietary food patterns and glucose/insulin homeostasis: a cross-sectional study involving 24,182 adult Americans. Lipids Health Dis 2017, 16(1):192. [CrossRef]
- Giugliano D, Ceriello A, Esposito K: The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol 2006, 48(4):677-685.
- Tsatsoulis A, Fountoulakis S: The protective role of exercise on stress system dysregulation and comorbidities. Ann N Y Acad Sci 2006, 1083:196-213. [CrossRef]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC: What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7(1). [CrossRef]
- Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, Jacka F, Dinan TG, Cryan JF: Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv Nutr 2021, 12(4):1239-1285. [CrossRef]
- Zmora N, Suez J, Elinav E: You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019, 16(1):35-56. [CrossRef]
- Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H: Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol 2020, 10:572912. [CrossRef]
- Bajic JE, Johnston IN, Howarth GS, Hutchinson MR: From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation. Front Behav Neurosci 2018, 12:104. [CrossRef]
- Loman BR, Jordan KR, Haynes B, Bailey MT, Pyter LM: Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci Rep 2019, 9(1):16490. [CrossRef]
- Deleemans JM, Chleilat, F., Reimer, R.A., Baydoun, M., Piedalue, K-A., Lowry, D., Henning, J-W., Carlson, L.E.: The Chemo-Gut Pilot Study: Associations between Gut Microbiota, Gastrointestinal Symptoms, and Psychosocial Health Outcomes in a Cross-Sectional Sample of Young Adult Cancer Survivors. Current Oncology 2022, 29:2973–2994. [CrossRef]
- Deleemans JM, Toivonen K, Reimer RA, Carlson LE: The Chemo-Gut Study: A Cross-Sectional Survey Exploring Physical, Mental, and Gastrointestinal Health Outcomes in Cancer Survivors. Glob Adv Health Med 2022, 11:2164957X221145940. [CrossRef]
- Deleemans JM, Chleilat F, Reimer RA, Henning JW, Baydoun M, Piedalue KA, McLennan A, Carlson LE: The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19(1):1243. [CrossRef]
-
PROMIS® Patient-Reported Outcomes Measurement Information System® [https://www.promishealth.org/57461-2/].
- Cessna JM, Jim HS, Sutton SK, Asvat Y, Small BJ, Salsman JM, Zachariah B, Fishman M, Field T, Fernandez H et al: Evaluation of the psychometric properties of the PROMIS Cancer Fatigue Short Form with cancer patients. J Psychosom Res 2016, 81:9-13. [CrossRef]
- Kochar B, Martin CF, Kappelman MD, Spiegel BM, Chen W, Sandler RS, Long MD: Evaluation of Gastrointestinal Patient Reported Outcomes Measurement Information System (GI-PROMIS) Symptom Scales in Subjects With Inflammatory Bowel Diseases. Am J Gastroenterol 2018, 113(1):72-79. [CrossRef]
- Spiegel BM, Hays RD, Bolus R, Melmed GY, Chang L, Whitman C, Khanna PP, Paz SH, Hays T, Reise S et al: Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol 2014, 109(11):1804-1814. [CrossRef]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP: DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13(7):581-583. [CrossRef]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO: The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013, 41(Database issue):D590-596. [CrossRef]
- May JO, Looney, S.W.: Sample Size Charts for Spearman and Kendall Coefficients. Biometrics & Biostatistics 2020, 11(2):1 - 7.
-
PROMIS® Score Cut Points [https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/promis-score-cut-points].
- Hays RD, Spritzer KL, Thompson WW, Cella D: U.S. General Population Estimate for "Excellent" to "Poor" Self-Rated Health Item. J Gen Intern Med 2015, 30(10):1511-1516. [CrossRef]
- Pekmezi DW, Demark-Wahnefried W: Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol 2011, 50(2):167-178. [CrossRef]
- Canada H: Eating Well with Canada Food Guide. In. Edited by Canada H. Ottawa, Canada; 2023.
- Nandini DB, Rao RS, Deepak BS, Reddy PB: Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J Oral Maxillofac Pathol 2020, 24(2):405. [CrossRef]
- van der Merwe M: Gut microbiome changes induced by a diet rich in fruits and vegetables. Int J Food Sci Nutr 2021, 72(5):665-669. [CrossRef]
- Menni C, Louca P, Berry SE, Vijay A, Astbury S, Leeming ER, Gibson R, Asnicar F, Piccinno G, Wolf J et al: High intake of vegetables is linked to lower white blood cell profile and the effect is mediated by the gut microbiome. BMC Med 2021, 19(1):37. [CrossRef]
- Wong JN, McAuley E, Trinh L: Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act 2018, 15(1):48. [CrossRef]
- Karakan T, Ozkul C, Kupeli Akkol E, Bilici S, Sobarzo-Sanchez E, Capasso R: Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13(2). [CrossRef]
- Jung SJ, Oh MR, Park SH, Chae SW: Effects of rice-based and wheat-based diets on bowel movements in young Korean women with functional constipation. Eur J Clin Nutr 2020, 74(11):1565-1575. [CrossRef]
- Ernest DK, Lemus H, Hsu FC, Pierce JP, Wu T: The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients 2022, 14(16). [CrossRef]
- Zafar H, Saier MH, Jr.: Gut Bacteroides species in health and disease. Gut Microbes 2021, 13(1):1-20. [CrossRef]
- Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M: The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8(4). [CrossRef]
- Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker JD, Delzenne NM, Muccioli G, Clement K, Cani PD: From correlation to causality: the case of Subdoligranulum. Gut Microbes 2020, 12(1):1-13. [CrossRef]
- Zhang Y, Chen H, Lu M, Cai J, Lu B, Luo C, Dai M: Habitual Diet Pattern Associations with Gut Microbiome Diversity and Composition: Results from a Chinese Adult Cohort. Nutrients 2022, 14(13). [CrossRef]
- Miquel S, Martin R, Rossi O, Bermudez-Humaran LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P: Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013, 16(3):255-261. [CrossRef]
- Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A: The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol 2020, 11:906. [CrossRef]
-
Cook For Your Life [https://www.cookforyourlife.org/].
-
Diet, activity and cancer [https://www.wcrf.org/diet-activity-and-cancer/].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).