INTRODUCTION
Covid-19 has unleashed a tsunami of research that has been further energized by the persistent and debilitating aftermath of Long Covid. Explaining the gender reversal from male to female preponderance is challenging and covering the range of symptoms intimidating. Although there have been a few hypotheses incorporating autoantibodies, nutrient deficiencies, reactivation of Epstein-Barr virus (EBV),…, resolution of the paradoxes remains frustratingly out of reach. Recent research has revealed that the BBB is not all inclusive and that some areas of the CNS are not so protected. These are called circumventricular organs (CVOs). Other research has revealed a close association between Covid-19 and a very common enzymatic polymorphism in the folate cycle involving methylenetetrahydrofolate reductase (MTHFR) 1,2. Most with this polymorphism are unaware of it. But it may play a large role in Long Covid and may provide unique insight into its pathogenesis. Incorporating the multiorgan involvement of LC and its bewildering range of symptoms into a single all encompassing model is a complex task. Receptor polymorphisms, enzyme polymorphisms, the impact of methylation on suppressor and promoter genes, …, create a maze. Broad strokes only are presented here.
HYPOTHESIS
The autonomic and neuroendocrine symptoms of LC, POTS, and CFS are primarily determined by CNS nuclei located in areas without a BBB. LC and ME/CFS microbiomes incriminate low fiber diets that support low bacterial diversity with production of minimal SCFAs, e.g., butyrates, GABA, and B vitamins. This collective shortfall drives the symptoms of these syndromes. POTS represents the intersection of LC and ME/CFS that are predominantly undiagnosed MTHFR 677TT, present in over 30-40% of the population. The oxidative stress of homocysteine in the MTHFR genotype, the residual spike protein S in LC, and reactivated viruses due to persistent lymphopenia conspire to overwhelm mitochondria lacking an antioxidant shield. Estrogen and BKN play prominent supporting roles.
1. MTHFR and Homocysteine
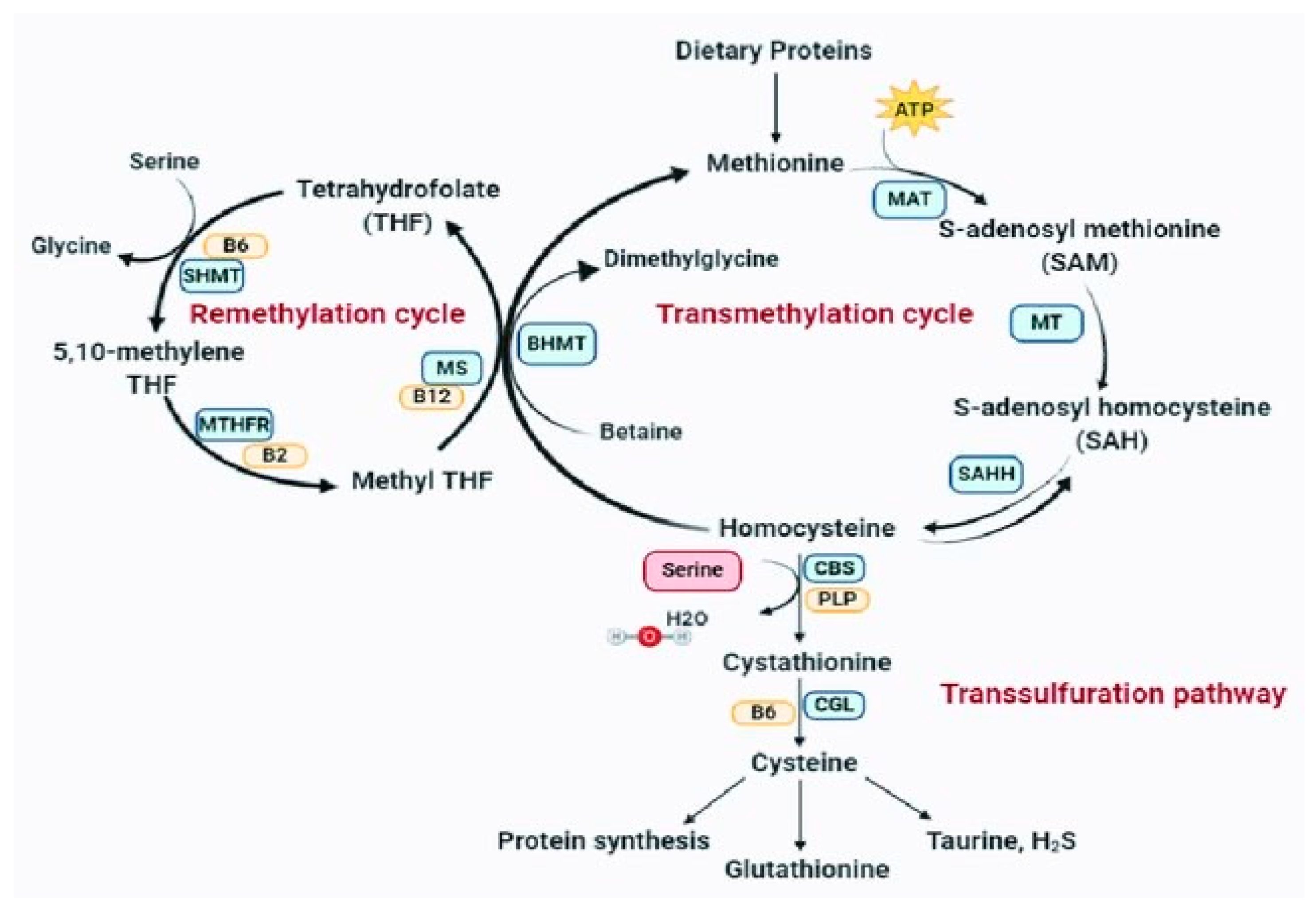
The MTHFR gene markedly compromises conversion of 5,10 -methylenetetrahydrofolate to 5-methyltetrahydrofolate. This is critical to the recycling of methyl groups (one carbon pathway or folate cycle). This defective gene leads to an increase in homocysteine, which can’t be sufficiently methylated via B12 to return to methionine (see
Figure 1). If the active form of B6 (P5P) is not sufficient, then glutathione, the master antioxidant, will not be produced. ROS will increase and mitochondrial function compromised.
The MTHFR 677T allele frequency is high in Europe (24.1-64.3%), and low in Africa (0-35.5%). The MTHFR 1298C allele frequency is approximately 20-70% in Asia, 24-46% in Europe, and 0-15% in America4. According to the CDC, there are more people in the United States who have one or two copies of the MTHFR C677T variant than people who do not have it. About 30 to 40 percent of the American population may have the C677T mutation. Roughly 25 percent of people of Hispanic descent and 10 to 15 percent of Caucasian descent are homozygous for this variant. So this is an exceedingly common polymorphism with incidence that somewhat reflects the percentage of individuals that develop LC.
Homocysteine is a strong marker for both LC2, the MTHFR gene5, and for ME/CFS6. High homocysteine may be a good marker for the MTHFR gene and low folate may be a good negative marker for homocysteine7. Homocysteine is a strong marker for thrombosis and may explain the oft encountered thrombosis in Covid-19. Homocysteine via CVOs causes brain fog in LC (see CVO section)8.
Wild type and heterozygous MTHFR gene variants characteristically exhibit hypomethylation9. But when the genotype is 677TT (homozygous), DNA overmethylation is encountered10. Those homozygous for 677T need higher doses of 5-methyltetrahydrofolate (methylfolate)11.
Methylation status in the body can be determined by measuring whole blood histamine, as histamine and methylation are inversely related. Low blood histamine levels indicate that the individual may be overmethylated; high levels indicate undermethylation12.
2. POSTURAL ORTHOSTATIC TACHYCARDIA SYNDOME
POTS represents a complex mixture of genetic and epigenetic inputs. It generally exhibits a 80%-90% female predominance, afflicts those 15-45, and is typically precipitated by a viral infection13. POTS is considered a disorder of the hypothalamus-pituitary-adrenal (HPA) axis that comes with a paradox - low renin and low aldosterone in the face of low blood volume. This was originally reported in POTS with ME/CFS and later with LC14. In addition to low renin and aldosterone ME/CFS patients have lower levels of oxytocin, corticotropin-releasing hormone (CRH), and vasopressin (ADH)15.
2.A. Low Flow POTS
Almost all POTS patients are hypovolemic. Most are low flow (neuropathic). All with low flow POTS have elevated Ang II and low PRA. Almost 70% of those with CFS have orthostatic intolerance16. These may represent those with concomitant but undiagnosed MTHFR 677TT17.
The tonic vasoconstriction shrinks blood volume and the juxtaglomerular apparatus (JGA) senses relative normotension. Renin is lower than would be otherwise expected. Neuropathic POTS patients have angiotensin II autoantibodies18 or beta adrenergic autoantibodies19,20. Autoantibodies to receptors can block or accentuate their actions21, but in neuropathic POTS autoantibodies accentuate expression22. Angiotensin II antibodies have also been found in LC23. ARBs can block both angiotensin II type 1 receptors and beta adrenergic receptors18,24.
Low flow or neuropathic POTS can also be seen in the absence of autoantibodies when the vasodilator nitric oxide (NO) has been oxidized25. This can also be blocked by ARBs26.
Vascular leakage induced by BKN might explain the lower limb cyanosis in neuropathic POTS. A preceding viral respiratory infection can upregulate BKN tenfold27.
Down regulation of ACE (ACE degrades BKN) by estrogen combined with a viral infection can supercharge BKN. This indirect inhibition of renin by BKN is greater than direct inhibition of renin by the RAS28. This might partially explain the decreased renin in the POTS paradox. An increased Ca/Mg might also contribute29,30.
2.B. High Flow POTS
There is a small subset of POTS that exhibits high flow, characterized by hypovolemia and reduction in renin and aldosterone activity. High flow POTS patients suffer from the same precipitating factors, viral infection, pregnancy,… POTS seen in MCAS and EDS may reflect this hyperadrenergic type31. BKN in this predominantly female subset may work in concert with MCAS to cause hypovolemia32. There is strong linkage between MCAS, POTS, and EDS33.
ARBs down regulate TGF-β1, increased in Marfan's34, i.e., type IV Ehlers–Danlos Syndrome (EDS)35. Further delineation awaits.
3. Gut Microbiome
A The neuropathic type of POTS seen in ME/CFS16 might be due to a CVO mediated glutamate/GABA imbalance that leads to decreased ADH, oxytocin, and CRH (see CVO section). It is both a neuroendocrine and an autonomic disorder. Gut microbiome studies of both LC36 and ME/CFS37 report deficiencies in butyrate and GABA producing bacteria, e.g., Bacteroides38, Bifidobacterium dentium39, and Lactobacillus brevium40. Bacteroides not only drives gut microbiota diversity but also is one of only six known species that produces the active P5P (PLP). B6 deficiency itself reduces the relative abundance of Bacteroides in the microbiota41.
Without P5P glutathione cannot be synthesized from homocysteine (see
Figure 1). A gut microbiome that lacks an abundance of Bifidobacteria that also degrades intestinal histamine leaves its allergic features unopposed
42.
This may be contributory to MCAS. GABA secreted by histaminergic neurons also downregulates histamine signaling43. The endogenous pathways for the degradation of histamine require either methylation (SAMe, Mg2+, and ATP) or mitochondrial ALDH. Alcohol intolerance is a primary complaint in LC, CFS, and MCAS with their dysfunctional mitochondria. Hepatic metabolism of alcohol in such individuals stops at acetaldehyde and a giant hangover.
LC, ME/CFS and by extension POTS share gut microbiome features that underscore the criticality of bacterial GABA synthesis to proper functioning of the HPA axis, mediated by CVOs. GABA receptor polymorphisms are linked with restless legs syndrome (RLS)44, which is increased in POTS45. This further underscores the role of GABA deficiency in the pathogenesis of POTS, LC, and CFS.
4. CVOs
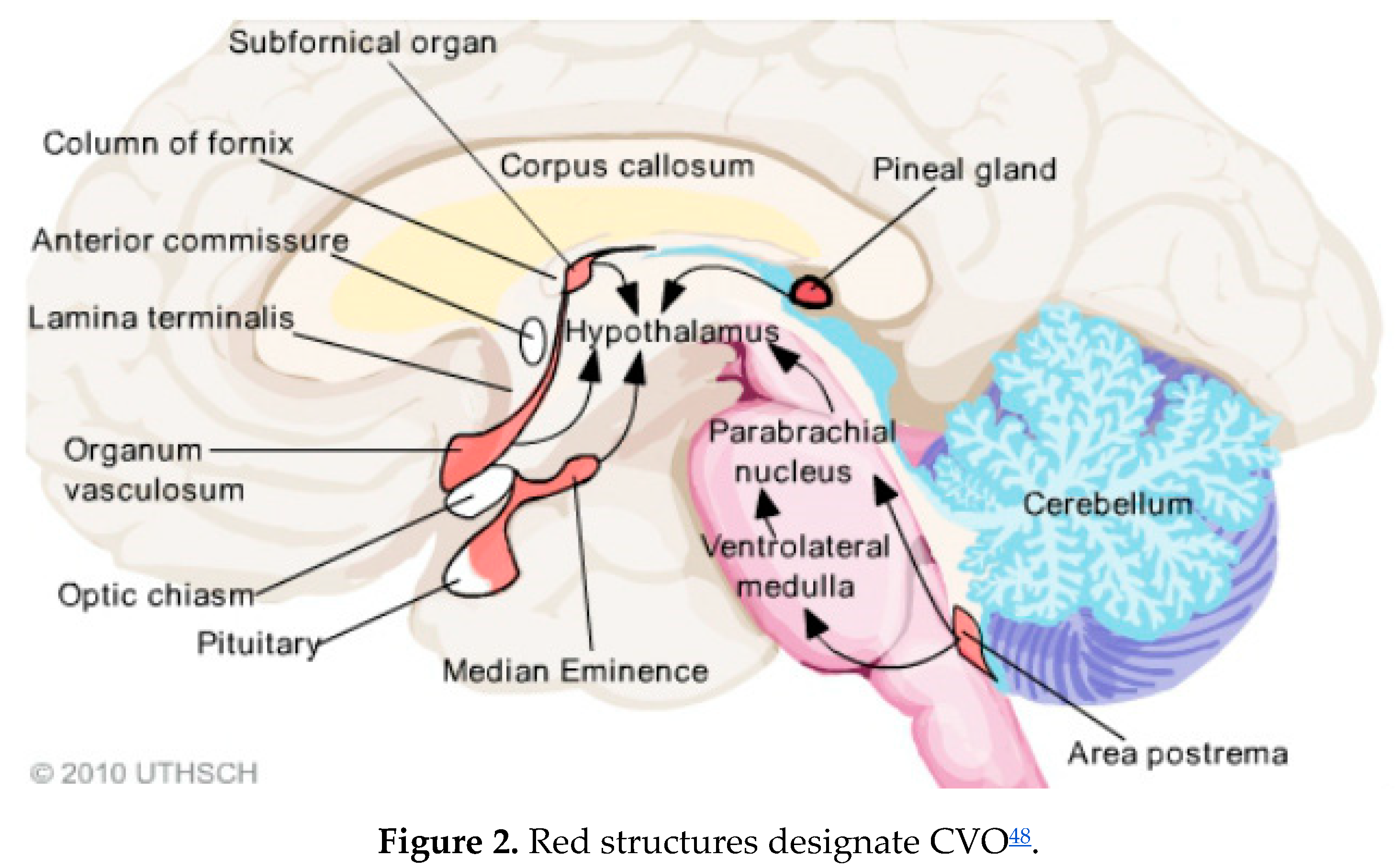
Several recent articles have highlighted CVOs46,47. There are several areas within the brain that abut ventricles and have no blood brain barrier. Several of these, which are called circumventricular organs (CVO), are quite relevant to the autonomic and neuroendocrine dysfunctions of LC.
These areas are the pineal gland, the posterior pituitary a.k.a. neurohypothesis, the paraventricular nucleus (PVN) in the median eminence, the area postrema (AP), the organum vasculosum of the lamina terminalis (OVLT) or supraoptic crest, and the subfornical organ (see
Figure 2).
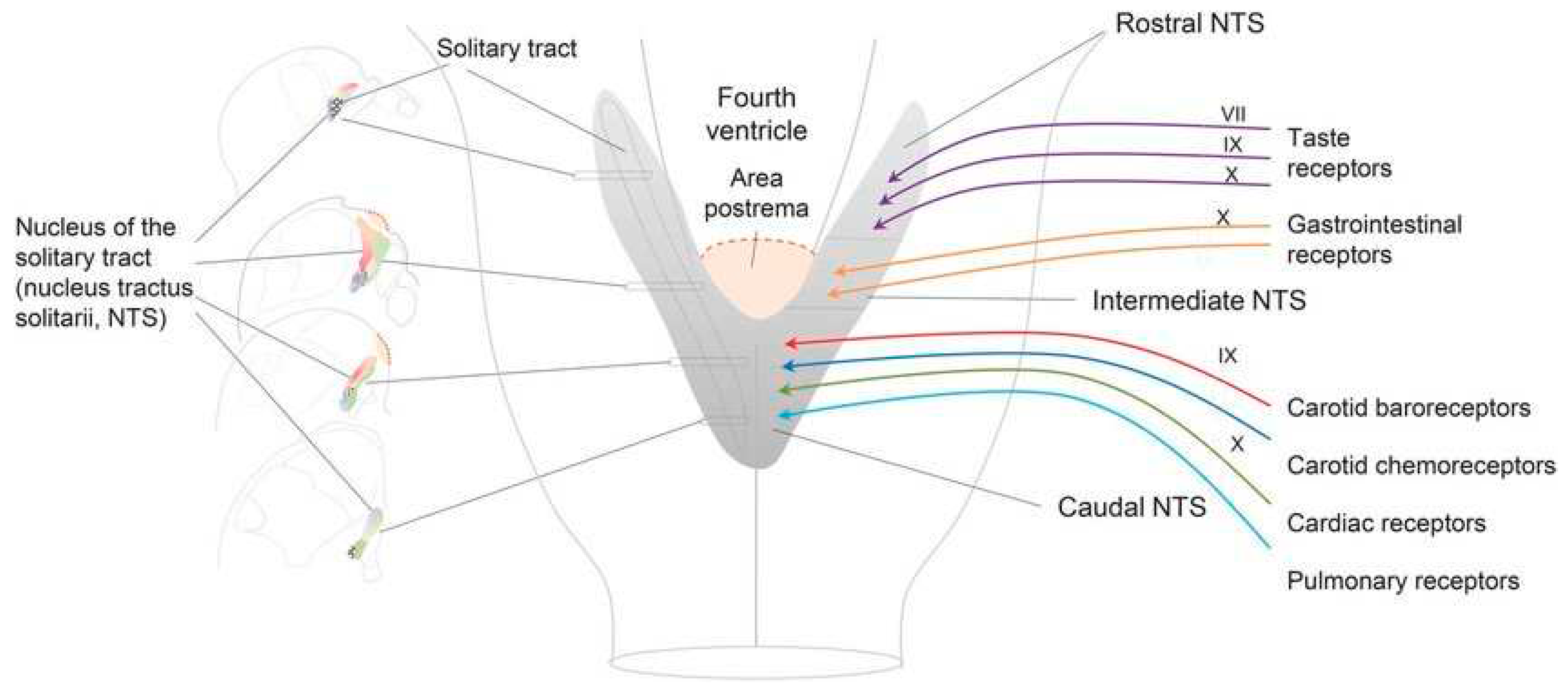
The AP is part of the dorsal vagal complex, closely associated with baroreceptors and chemoreceptors, as well as cranial nerves VII and IX (see
Figure 3).
CVOs enable a diet induced CNS glutamate (excitatory)/GABA (inhibitory) imbalance to precipitate not only POTS but also symptoms that involve decreased oxytocin, vasopressin (ADH), corticotropin releasing hormone (CRH for ACTH), and gonadotropin releasing hormone (GnRH for LH, FSH).
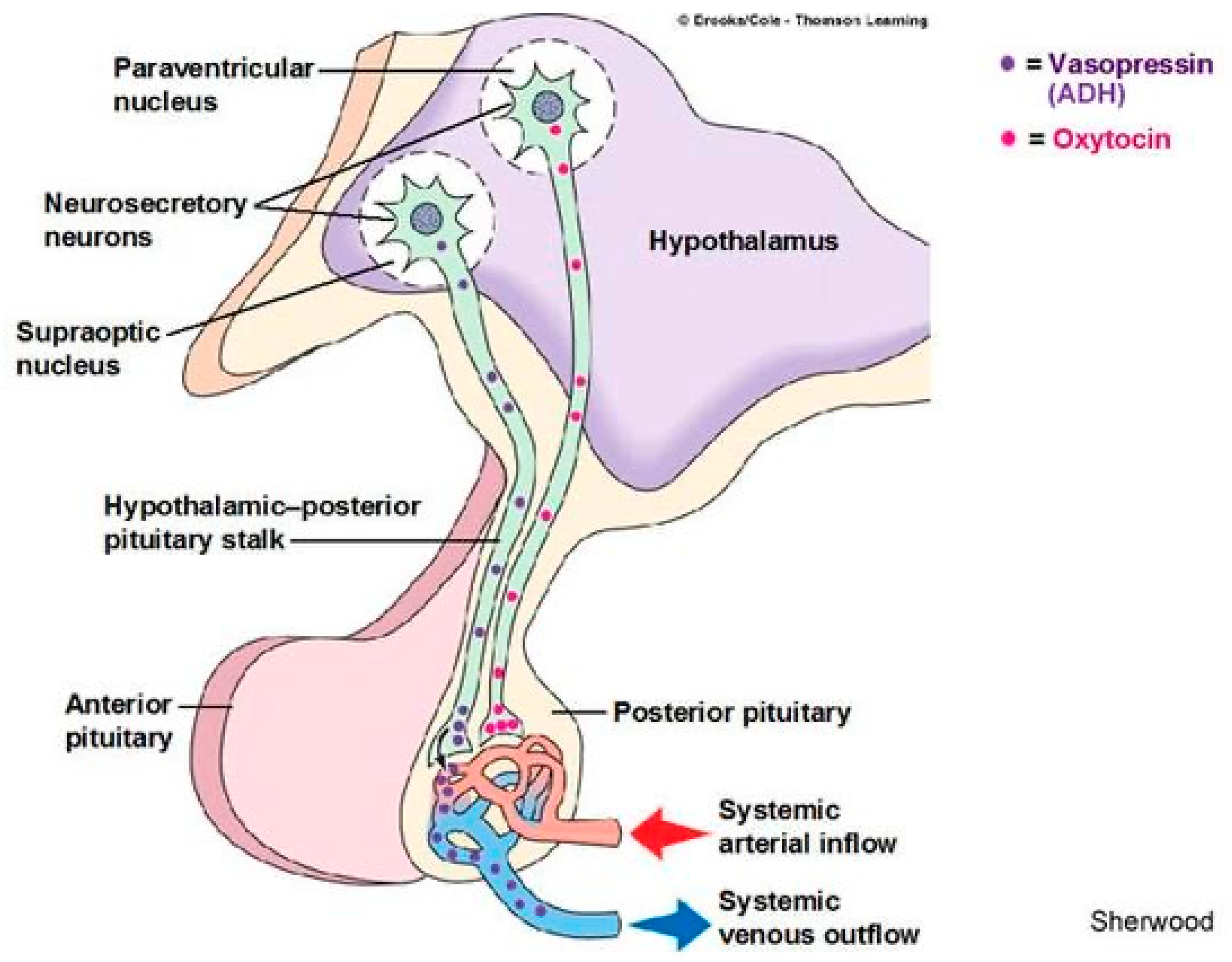
CVOs can conveniently explain the POTS paradox in the neuropathic hypovolemic low flow subtype. The PVN normally inhibits the baroreflex via tonic GABAergic inputs
49. GABAergic signals also dictate secretion of neurohypophyseal hormones, including vasopressin (ADH)
50, oxytocin
51 (see
Figure 4), CRH
53, and GnRH
54. GABA inhibits the secretion of TRH
55.
The lamina terminalis controls renal secretion of renin and angiotensin56. ME/CFS patients have experienced relief from desmopressin57 and CRF58. POTS and ME/CFS have benefited from exogenous CRF and vasopressin (ADH)59. Oxytocin in CFS increased stamina, decreased pain, improved cognitive function, decreased fibromyalgia pain, anxiety, and depression. Some LC patients have experienced relief with oxytocin60. Many POTS patients claim heat intolerance and this may be mediated by unopposed glutamate61.
5. Methylation and Shingles
In a recent review compared to controls, patients that experienced mild to moderate COVID-19 or LC exhibited hypomethylation and hypermethylation62 respectively. This reflects the methylation disposition of MTHFR wild type and 677TT respectively, i.e., wild type quickly recovered and 677TT devolved into LC. Hypermethylation in LC persisted for one year after hospitalization63. The hypermethylated have higher Hcy levels, which portend more serious Covid-19 and LC64.
Alcohol intolerance is almost universal among ME/CFS patients65. Acetaldehyde, produced by the liver, must then be metabolized in mitochondria. Post exertional malaise may then be joined by a persistent post alcohol hangover.
Shingles serves as a good example for modeling the pathogenesis of LC. VZV can emerge from its dorsal root ganglion (DRG) and invade its designated peripheral sensory nerve (shingles) or it can invade sensory fibers of the facial nerve (VII). These facial nerve sensory fibers emerge from the NTS and the AP (see
Figure 3). The AP is the first CVO encountered by any VZV migrating upstream through the subarachnoid CSF and the foramen of Magendie and into the fourth ventricle from its DRG origin. Justin Bieber encountered herpes zoster oticus affiliated with cranial nerve VII (Ramsay Hunt Syndrome) post vaccine. Shingles risk is definitely increased in those with LC or CFS
66. This may be due to hypermethylation that characterizes LC, as lysine is hypermethylated initiating cold sores
67.
6. Autoimmunity in Females
Increased autoimmune disease in females may be due to their enhanced innate immunity and production of pleiotropic IFN gamma. When combined with chronic low grade inflammation, this pro-inflammatory pleiotropic cytokine may switch from self recognition to autoimmune status68.
IFN gamma is primarily secreted by CD4+ and CD8+ T cells. These cells also induce production of C1 esterase inhibitors (C1INH) by hepatocytes. Lymphopenia is associated with persistence of COVID-19 symptoms69. CD147 (basigin) receptors but not ACE2 receptors are found on peripheral blood mononuclear cells (PBMCs)70. This receptor may mediate the lymphopenia. CD147 epitopes are also found on the spike protein S, as Justin Bieber discovered, and are responsible for this T cell lymphopenia and drop in surveillance. Any subsequent viral infection that challenges lymphocytes opens the door to the classic complement cascade, which cross talks with the kallikrein kinin system (KKS) and increases BKN71.
Estrogen affects more than BKN. It may play a role in neurogenic POTS by decreasing renin levels, ACE activity (increasing BKN), AT1 receptor density, and aldosterone72.
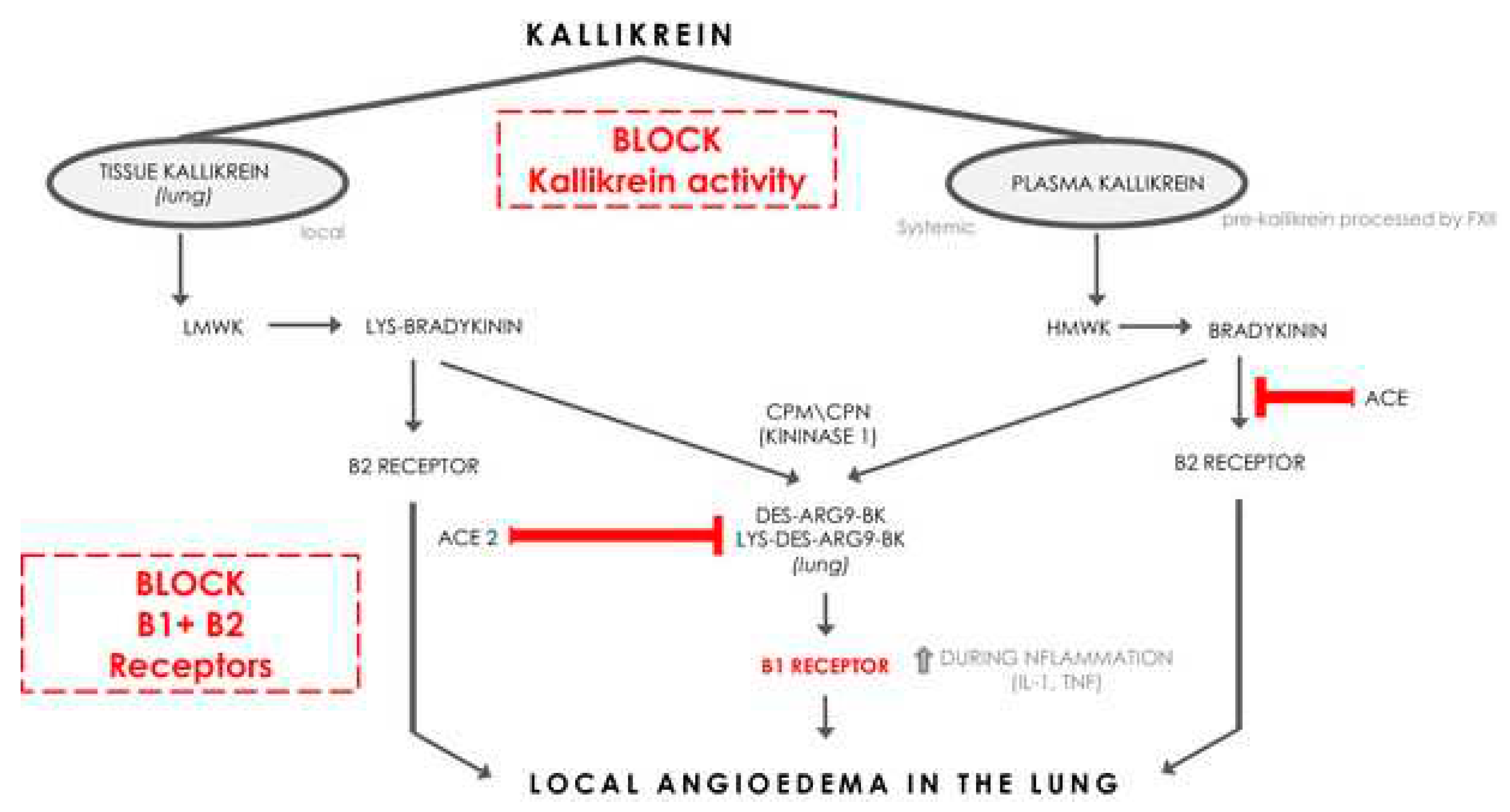
In addition estrogen appears to lower cortisol
73 and ACE2, at least in the lungs
74. Perhaps lower pulmonary ACE2 is Covid 19 protective, but the downregulated ACE2 may be associated with higher levels of des-Arg9-bradykinin (see
Figure 5). Des-arg-BKN appears to be a prominent kinin in Covid-19
75.
7. A. Mitochondria and Oxidative Stress
Mitochondrial health is especially important in maintaining optimal levels of active vitamin D. Men with the highest compared to lowest 1,25(OH)
2D and activation ratios (1,25(OH)
2D/25(OH)D) are more likely to possess butyrate-producing bacteria that are associated with favorable gut microbial health. Sun exposure may affect the storage form of vitamin D, but it exerts little influence on the active hormone
77. Oxidative stress (ROS due to inflammation, smoking, toxins, …) compromises mitochondrial oxidative phosphorylation. If these ROS are not quenched by onboard antioxidants, the cell risks lysis
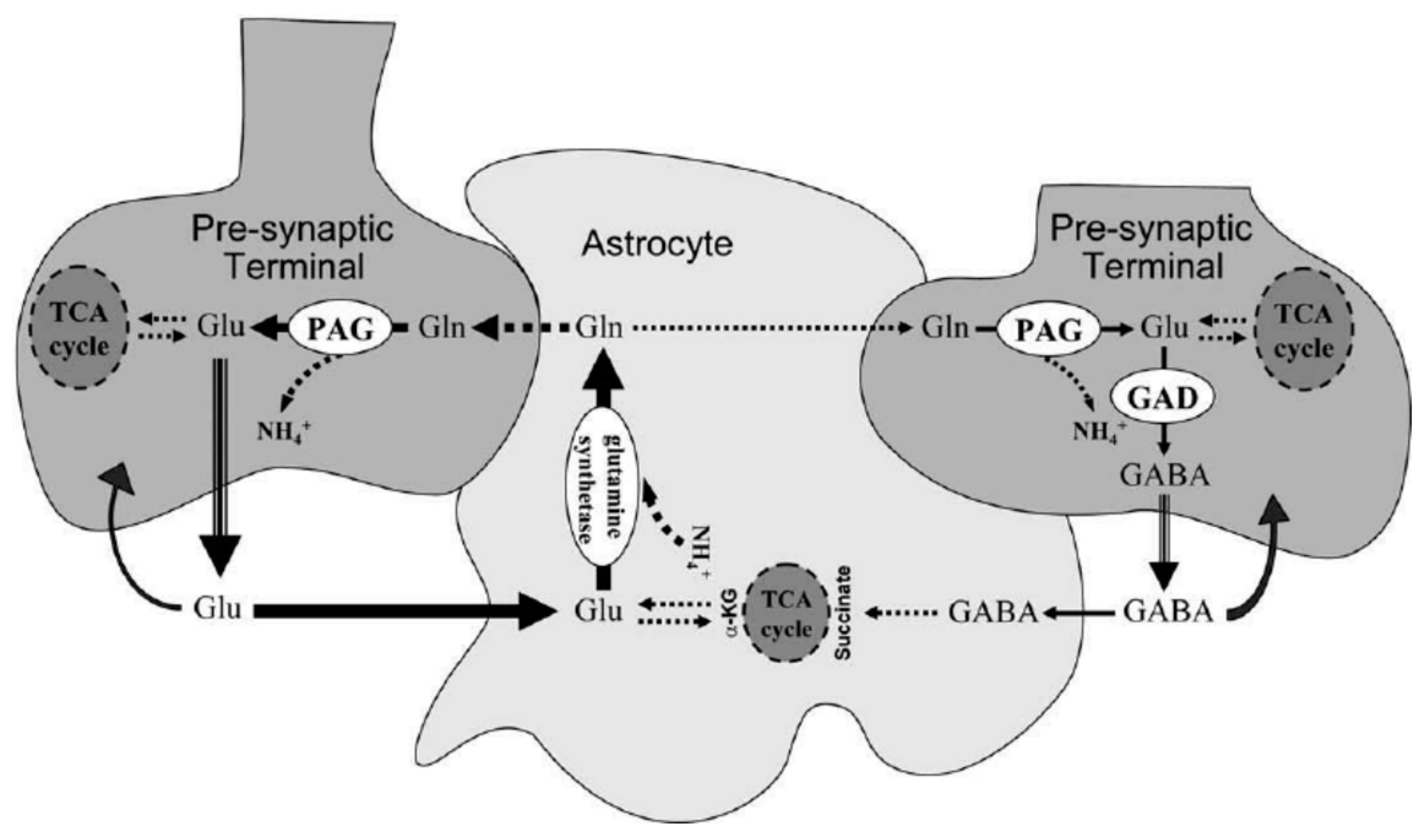
78. Many mitochondria are shut down to avoid this, diminishing ATP production. The Krebs or TCA cycle with its electron transport chain ETC also requires activated B2 and B3. ATP is required to synthesize glutamine (Gln) from glutamate (Glu) and to recycle GABA in astrocytes (see
Figure 6). Recycling of Glu is not so limited. Any shortage of active B6 (P-5-P), required by glutamate decarboxylase (GAD), further compromises production of GABA from glutamate.
7. B. Magnesium and Vitamins
Magnesium deficiency is an additional plausible explanation for low aldosterone and low cortisol. Synthesis of aldosterone requires aldosterone synthase and synthesis of cortisol requires 11beta hydroxylase. These two enzymes are part of the CYP450 system, all of which require Mg++. Furthermore these two reactions occur in the mitochondria
80, i.e., they also require ATP. If oxidative stress is sufficient and antioxidants are not, mitochondria are shut down and ATP down regulated, limiting synthesis of aldosterone and cortisol
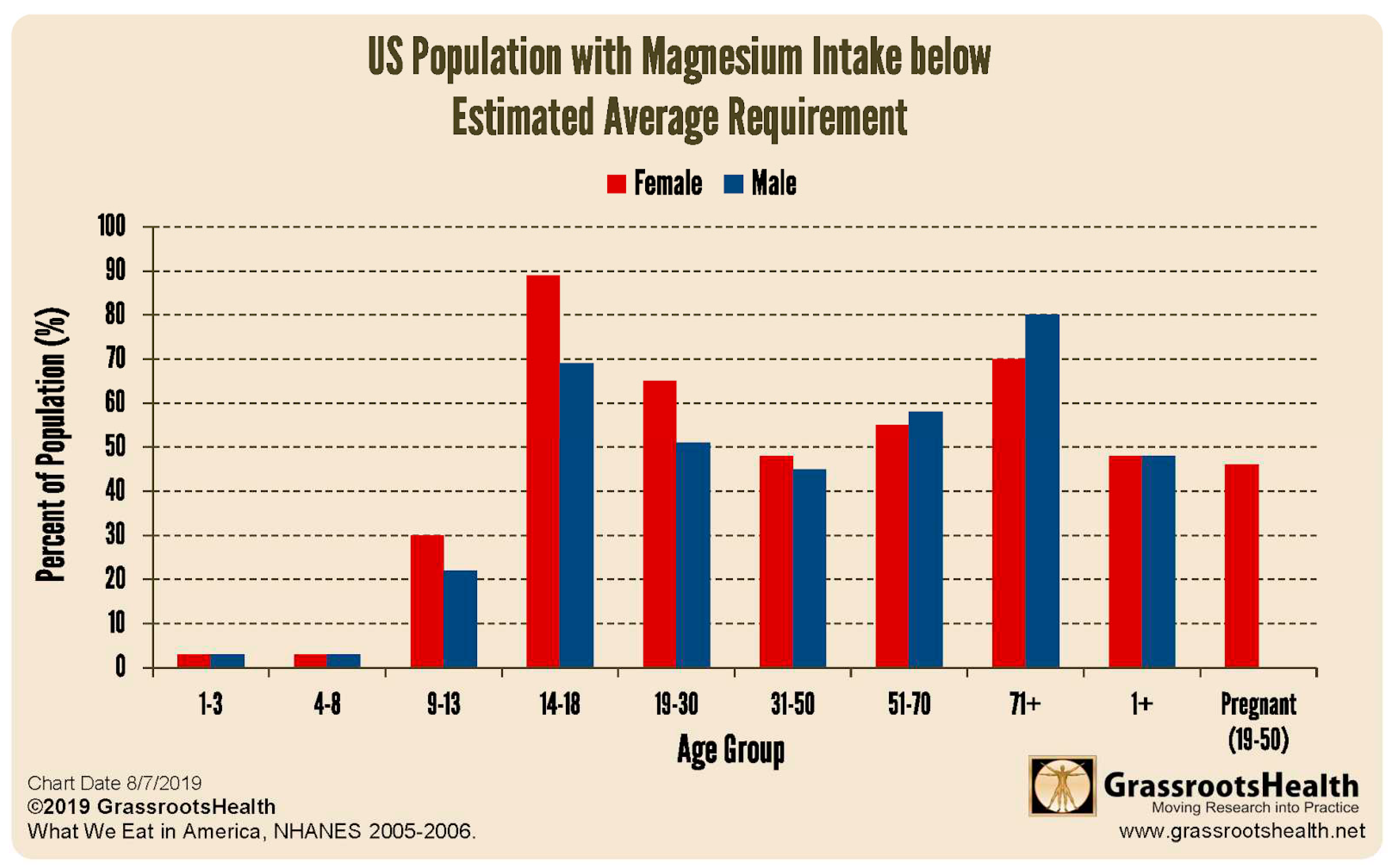
81. Females are more magnesium deficient than males before age 50 (see
Figure 7). An elevated Ca:Mg may also compromise synthesis of aldosterone and cortisol
29,30.
Magnesium is also required for the synthesis of all eight B vitamins, except biotin (B7). Mitochondrial function is dependent upon adequacy of all the B vitamins82.
If mitochondrial function is marginal, production of the active form of vitamin D may be marginal. Mitochondrial CYP27B1 is the only enzyme that hydroxylates C1 (activates vitamin D)83.
Thiamine (B1), riboflavin (B2), niacin (B3), pantothenate (B5), pyridoxine (B6) require phosphorylation for activation. Cyanocobalamin and folate require methylation (SAMe) for activation. Magnesium is required for both phosphorylation and methylation. Homocysteine is highly influenced by B vitamin status84.
The last enzymatic step to produce serotonin, dopamine, and GABA are decarboxylases that require active B6 (P5P), as a cofactor. Melatonin and glutathione synthesis also requires B6.
Norepinephrine synthesis requires magnesium. A shortfall in magnesium or B6 compromises monoamine neurotransmitter synthesis and leads to depression, a symptom shared by LC, POTS, ME/CFS. MCAD, and EDS. High doses of pyridoxine are associated with peripheral neuropathy, whereas high doses of the active form (P5P) are not85. Estrogens compete with B6 for some receptor sites including that for serotonin synthesis86.
8. Thoughts on Therapy
Changing one's diet is more difficult than changing one's religion. Attaining a blood Ca/Mg around 2.0 can also be challenging. But maintaining a 25(OH)D3 of 50 ng/mL with abundant antioxidants is critical. Other recommended epigenetic actions include supplementing with the methylated or phosphorylated forms of all the B vitamins, especially methyl folate. A good supplementary probiotic might be worth considering. However, under professional supervision angiotensin receptor blockers (ARBs) might be worth pursuing, but even a small dose can elicit mild hypoglycemia and/or hypotension.
The potential benefits of ARBs for LC include:
ARBs upregulate GABA, which displays anti-hypertension, anti-senescence, anti-diabetes, antioxidant, and anti-inflammatory properties87.
ARBs block AT1Rs (and hypertension induced dementia) and increase ACE88.
ARBs alleviate POTS26 and degrade BKN otherwise associated with AD89.
ARBs increase ACE290 that degrades amyloid β-peptide (Aβ) in AD91.
ARBs down regulate TGF-β1, increased in LC92, CFS93, AD94, Marfan Syndrome34, and Ehlers–Danlos Syndrome (EDS)35.
ARBs improve insulin sensitivity95 and are antidiabetic96.
ARBs are neuroprotective97.
Indeed, a post mortem study of brains demonstrated more neuropathology in non-hypertensives, than in hypertensives on ARBs98. Furthermore, angiotensin II–stimulating antihypertensives, e.g., ARBs exhibited lower dementia risk than angiotensin II inhibiting antihypertensive, e.g., ACEIs99.
CONCLUSION
In summary LC reveals undiagnosed MTHFR 677TT present in 30-40% of the population. These patients comprise the majority of those with LC and POTS. The integration of CVOs with the gut microbiome opens new insight into the POTS paradox and links LC, POTS, and ME/CFS. Homocysteine, a marker for MTHFR polymorphisms, is elevated in LC and ME/CFS. It can directly access the BBB less CNS and is directly proportional to brain fog severity and oxidative stress. In the absence of critical cofactors synthesis of the master antioxidant glutathione from homocysteine cannot proceed (see
Figure 1). HIV (low CD4/CD8) and SARS CoV2 (high CD4/CD8) specifically target CD4+ and CD8+ T cells respectively, disabling surveillance of intracellular pathogens and enabling the emergence of EBV and VZV, previously dormant within dorsal root ganglia. VZV also targets CD8+ T cells
100.
Loss of these T cells translates to a decrease in IFN gamma and with it a decrease in hepatic C1INH. The classic complement pathway is triggered and the KKS is activated. BKN is released. Any recent viral infection induces additional release of BKN. Estrogen, an ACE inhibitor, prolongs BKN half life. BKN combines with histamine to produce low volume low flow POTS. The LC and ME/CFS gut microbiomes lack biodiversity (insufficient bifidobacteria, lactobacilli, ..). These latter bacteria produce butyrates (SCFAs), GABA, B vitamins, and can degrade histamine.
Subsequent inflammation induced oxidative stress from homocysteine, residual spike S protein, and reactivated viruses conspire to overwhelm those with insufficient on board antioxidants. An additional magnesium shortfall in 15-50 year old females leads to fatigue and post exertional dyspnea
References
- Karst, M.; Hollenhorst, J.; Achenbach, J. Life-threatening course in coronavirus disease 2019 (COVID-19): Is there a link to methylenetetrahydrofolic acid reductase (MTHFR) polymorphism and hyperhomocysteinemia? Med. Hypotheses. 2020, 144, 110234. [CrossRef]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Iannella, R.; Tomasi, A. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [CrossRef]
- The Spectrum of Mutations of Homocystinuria in the MENA Region (2020). [CrossRef]
- Geographical Distribution of MTHFR C677T, A1298C and MTRR A66G Gene Polymorphisms in China: Findings from 15357 Adults of Han Nationality. [CrossRef]
- Homocysteine in coronavirus disease (COVID-19): a systematic literature review. (2022). [CrossRef]
- Increased concentrations of homocysteine in the cerebrospinal fluid in patients with fibromyalgia and chronic fatigue syndrome. [CrossRef]
- Homocysteine, folate, methylation, and monoamine metabolism in depression. [CrossRef]
- High Homocysteine Levels Are Associated with Cognitive Impairment in Patients Who Recovered from COVID-19 in The Long Term (2023). [CrossRef]
- MTHFR Gene Polymorphism Is Associated With DNA Hypomethylation and Genetic Damage Among Benzene-Exposed Workers in Southeast China 2018. [CrossRef]
- Fundamental Role of Methylenetetrahydrofolate Reductase 677 C → T Genotype and Flavin Compounds in Biochemical Phenotypes for Schizophrenia and Schizoaffective Psychosis. [CrossRef]
- Methylenetetrahydrofolate reductase 677C→T genotype modulates homocysteine responses to a folate-rich diet or a low-dose folic acid supplement: a randomized controlled trial. [CrossRef]
-
https://methyl-life.com/blogs/mthfr/mthfr-overmethylation-symptoms.
- Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management (2019). [CrossRef]
- Long COVID-19 and Postural Orthostatic Tachycardia Syndrome- Is Dysautonomia to Be Blamed? (2022). [CrossRef]
- Betrayal by the Brain: The Neurologic Basis of Chronic Fatigue Syndrome, Fibromyalgia Syndrome, and Related Neural Network 1st Edition (1996) Jay Goldstein).
- Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. [CrossRef]
- Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. [CrossRef]
- Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome (2018). [CrossRef]
- Antiadrenergic autoimmunity in postural tachycardia syndrome. [CrossRef]
- A functional cell-based bioassay for assessing adrenergic autoantibody activity in postural tachycardia syndrome (2018). [CrossRef]
- Receptors, antibodies, and disease https://pubmed.ncbi.nlm.nih.gov/6329552/.
- A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors (2020). [CrossRef]
- Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity (2022). [CrossRef]
- Dual Inhibition of β-Adrenergic and Angiotensin II Receptors by a Single Antagonist. [CrossRef]
- Decreased Microvascular Nitric Oxide–Dependent Vasodilation in Postural Tachycardia Syndrome. [CrossRef]
- Stewart, J.M., Taneja, I., Glover, J. and Medow, M.S. (2008) Angiotensin II type 1 Receptor Blockade Corrects Cutaneous Nitric Oxide Deficit in Postural Tachycardia Syndrome. American Journal of Physiology-Heart and Circulatory Physiology, 294, H466-H473. [CrossRef]
- Association between the level of Bradykinin and viral infection in patient suffering from respiratory infection, renal transplant, and renal failure (2020) https://www.researchgate.net/publication/345037567.
- Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. [CrossRef]
- Renin: origin, secretion and synthesis. [CrossRef]
- Effects of magnesium on the renin-angiotensin-aldosterone system in human subjects https://pubmed.ncbi.nlm.nih.gov/8228558/.
- Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. [CrossRef]
- Mast Cells Increase Vascular Permeability by Heparin-Initiated Bradykinin Formation In Vivo (2011). [CrossRef]
- The relationship between mast cell activation syndrome, postural tachycardia syndrome, and Ehlers-Danlos syndrome (2021). [CrossRef]
- Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. [CrossRef]
- Transforming Growth Factor-β and Inflammation in Vascular (Type IV) Ehlers–Danlos Syndrome. [CrossRef]
- Yeoh YK, Zuo T, Lui GC, et al Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19 Gut 2021;70:698-706 (2021) https://gut.bmj.com/content/70/4/698.
- The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) (2022). [CrossRef]
- GABA-modulating bacteria of the human gut microbiota (2019). [CrossRef]
- Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 2017;29. [CrossRef]
- Lin H-TV, Tsai GJ. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013;48:559–68. [CrossRef]
- Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats (2020). [CrossRef]
- Suppression of histamine signaling by probiotic Lac-B: a possible mechanism of its anti-allergic effect. [CrossRef]
- Wakefulness Is Governed by GABA and Histamine Cotransmission. [CrossRef]
- Gamma-aminobutyric acid (GABA) receptors genes polymorphisms and risk for restless legs syndrome (2018). [CrossRef]
- Restless legs syndrome is increased in postural orthostatic tachycardia syndrome (2021). [CrossRef]
- Investigating the possible mechanisms of autonomic dysfunction post-COVID-19 (March 2023). [CrossRef]
- The circumventricular organs (2017). [CrossRef]
- Cytokines Driving Sympathetic Nervous System Activation In The Subfornical Organ: Implications For Heart Failure And Hypertension (2015) Gabriel Bassi https://brainimmune.com/subfornical-organ-heart-failure-hypertension/).
- GABA in the paraventricular nucleus tonically suppresses baroreflex function: alterations during pregnancy (2011). [CrossRef]
- GABA Is Excitatory in Adult Vasopressinergic Neuroendocrine Cells. [CrossRef]
- GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat (2015). [CrossRef]
- Unnikrishnan P, Diabetes Insipidus, Dept of Neuroanesthesia SCTIMST, Trivandrum, Kerala, India https://www.uzhnu.edu.ua/uk/infocentre/get/23985.
- GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. [CrossRef]
- Mapping GABA and glutamate inputs to gonadotropin-releasing hormone neurons in male and female mice (2018). [CrossRef]
- Thyroid hormone and γ-aminobutyric acid (GABA) interactions in neuroendocrine systems. [CrossRef]
- The lamina terminalis and its role in fluid and electrolyte homeostasis. [CrossRef]
- Down-regulation of renin–aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome (2027). [CrossRef]
- Acute Corticotropin-Releasing Factor Receptor Type 2 Agonism Results in Sustained Symptom Improvement in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (2021). [CrossRef]
- Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. [CrossRef]
- Oxytocin, the panacea for long-COVID? a review (2021). [CrossRef]
- Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. [CrossRef]
- Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells (2022). [CrossRef]
- Persistent blood DNA methylation changes one year after SARS-CoV-2 infection (2022). [CrossRef]
- Is Homocysteine Associated with the Prognosis of Covid-19 Pneumonia (2023). [CrossRef]
- American Myalgic Encephalomyelitis and Chronic Fatigue Syndrome Society https://ammes.org/diet/.
- Could long COVID be linked to herpes viruses? Early data offer a hint (2022). [CrossRef]
- Trimethylation of Histone H3 Lysine 4 by Set1 in the Lytic Infection of Human Herpes Simplex Virus 1 (2006). [CrossRef]
- Targeting interferon-γ in hyperinflammation: opportunities and challenges (2021). [CrossRef]
- Persistent symptoms and lab abnormalities in patients who recovered from COVID-19 (2021). [CrossRef]
- T Lymphocytes as Targets for SARS-CoV-2 (2022). [CrossRef]
- Bossi, F., Peerschke, E.I., Ghebrehiwet, B. and Tedesco, F. (2011) Cross-Talk between the Complement and the Kinin System in Vascular Permeability. Immunology Letters, 140, 7-13. [CrossRef]
- The effect of estrogen in coronavirus disease 2019 (2021). [CrossRef]
- Estradiol Therapy After Menopause Mitigates Effects of Stress on Cortisol and Working Memory (2017). [CrossRef]
- Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells (2020). [CrossRef]
- The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19 (2022). [CrossRef]
- van De Veerdonk, F.L., Netea, M.G., van Deuren, M., van der Meer, J.W.M., De Mast, Q., et al. (2020) KKS Blockade in Patients with COVID-19 to Prevent Acute Respiratory Distress Syndrome (Apr 2020). ELife, 9, Article ID: E57555. [CrossRef]
- Vitamin D metabolites and the gut microbiome in older men (2020). [CrossRef]
- Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers’? (2021). [CrossRef]
- The micro-architecture of the cerebral cortex: Functional neuroimaging models and metabolism. [CrossRef]
- Atanassova, N. and Koeva, Y. (2012) Hydrohysteroid Dehydrogenases—Biological Role and Clinical Importance—Review. In: Canuto, R.A., Ed., Dehydrogenases, IntechOpen, London. [CrossRef]
- Antioxidants and Long Covid (2022). [CrossRef]
- Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. [CrossRef]
- Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. [CrossRef]
- Homocysteine: Its Possible Emerging Role in At-Risk Population Groups (2020). [CrossRef]
- Hadtstein, F., & Vrolijk, M. (2021). Vitamin B-6-induced neuropathy: exploring the mechanisms of pyridoxine toxicity. Advances in Nutrition, 12(5), 1911-1929. [CrossRef]
- The interactions between vitamin B6 and hormones. [CrossRef]
- Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry (2022). [CrossRef]
- Effects of Losartan on Angiotensin and Bradykinin Peptides and Angiotensin-Converting Enzyme. [CrossRef]
- Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer’s patients (2021). [CrossRef]
- Correlation of ACE2 with RAS components after Losartan treatment in light of COVID-19 (2021). [CrossRef]
- Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. [CrossRef]
- Long Covid and Neurodegenerative Disease (2023) https://www.researchgate.net/publication/367332223.
- The clinical value of cytokines in chronic fatigue syndrome (2019). [CrossRef]
- TGF-β1 factor in the cerebrovascular diseases of Alzheimer's disease https://pubmed.ncbi.nlm.nih.gov/28051272/.
- Improved Glucose-Stimulated Insulin Secretion by Selective Intraislet Inhibition of Angiotensin II Type 1 Receptor Expression in Isolated Islets of db/db Mice. [CrossRef]
- Chang, C.-H.; Chang, Y.-C.; Wu, L.-C.; Lin, J.-W.; Chuang, L.-M.; Lai, M.-S. Different angiotensin receptor blockers and incidence of diabetes: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2014, 13, 91. [CrossRef]
- Neuroprotective Effects of Angiotensin Receptor Blockers. [CrossRef]
- Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. [CrossRef]
- Association of Angiotensin II–Stimulating Antihypertensive Use and Dementia Risk (2021). [CrossRef]
- Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).