Submitted:
25 March 2023
Posted:
27 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
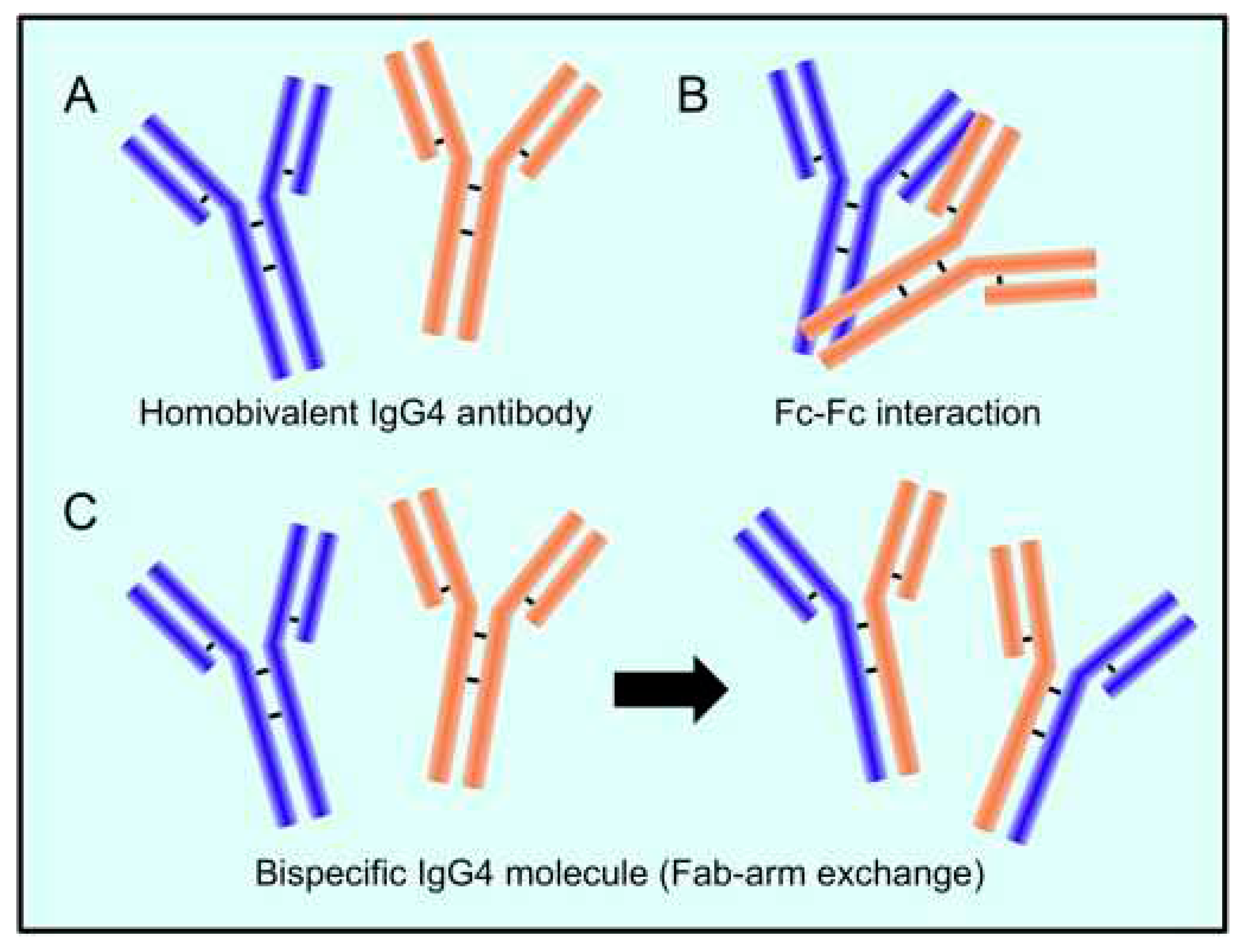
2. Characteristics of the unusual IgG4 antibody
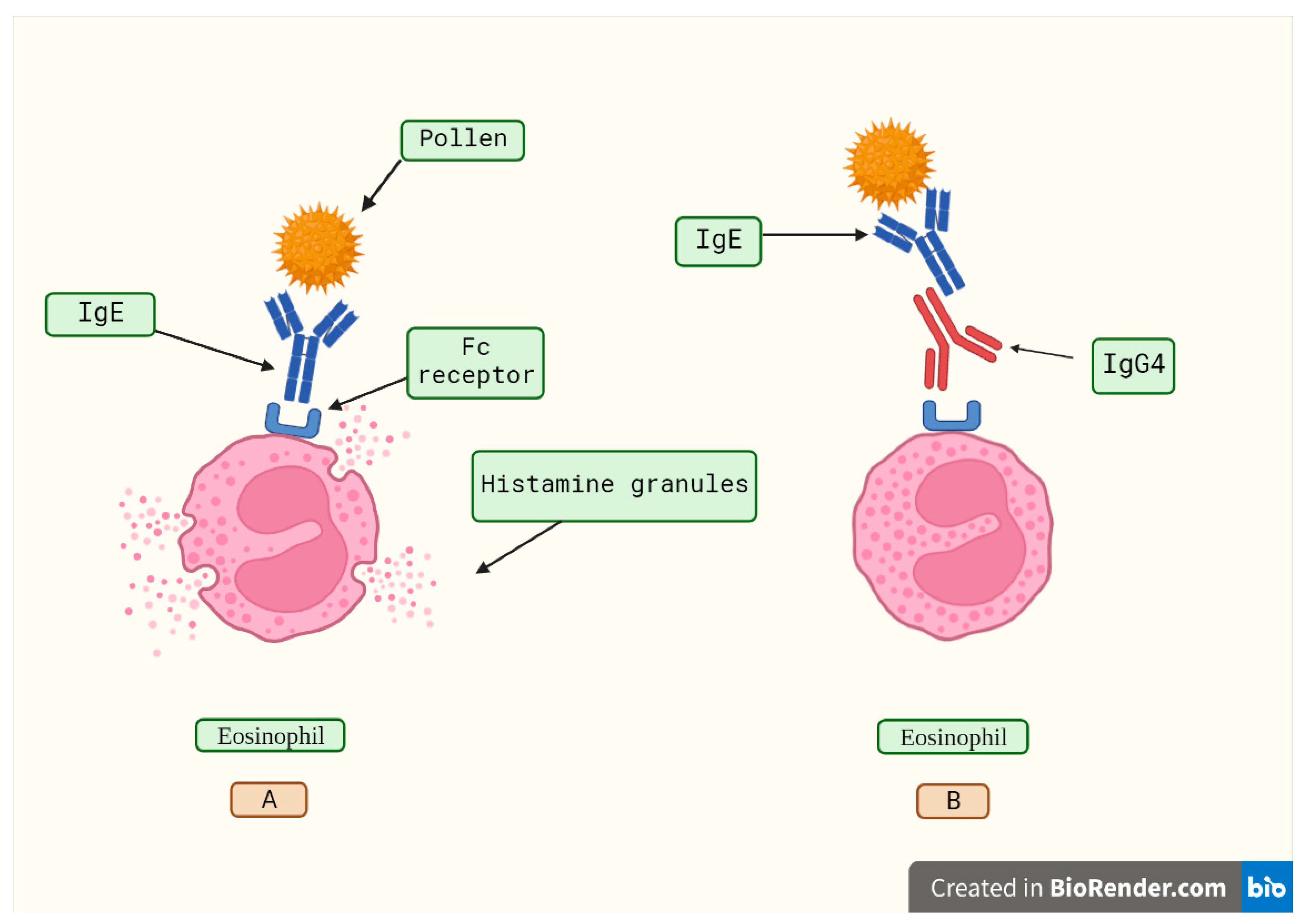
2.1. IgG4: a protective or pathogenic antibody?
2.1.1. Protective role of IgG4 in allergy immunotherapy
2.1.2. IgG4-related disease and its pathogenesis
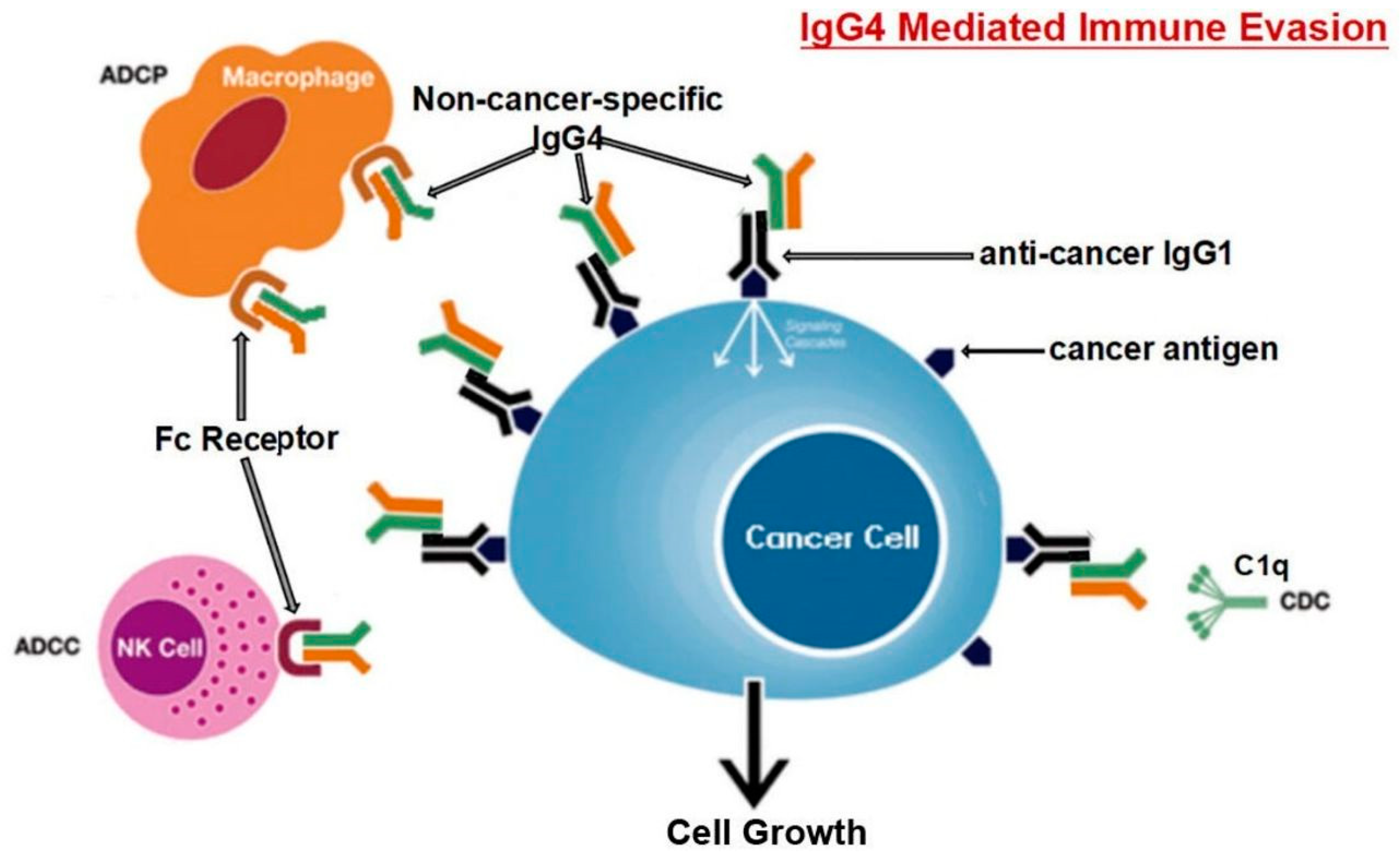
2.1.2.1. IgG4 role in cancer
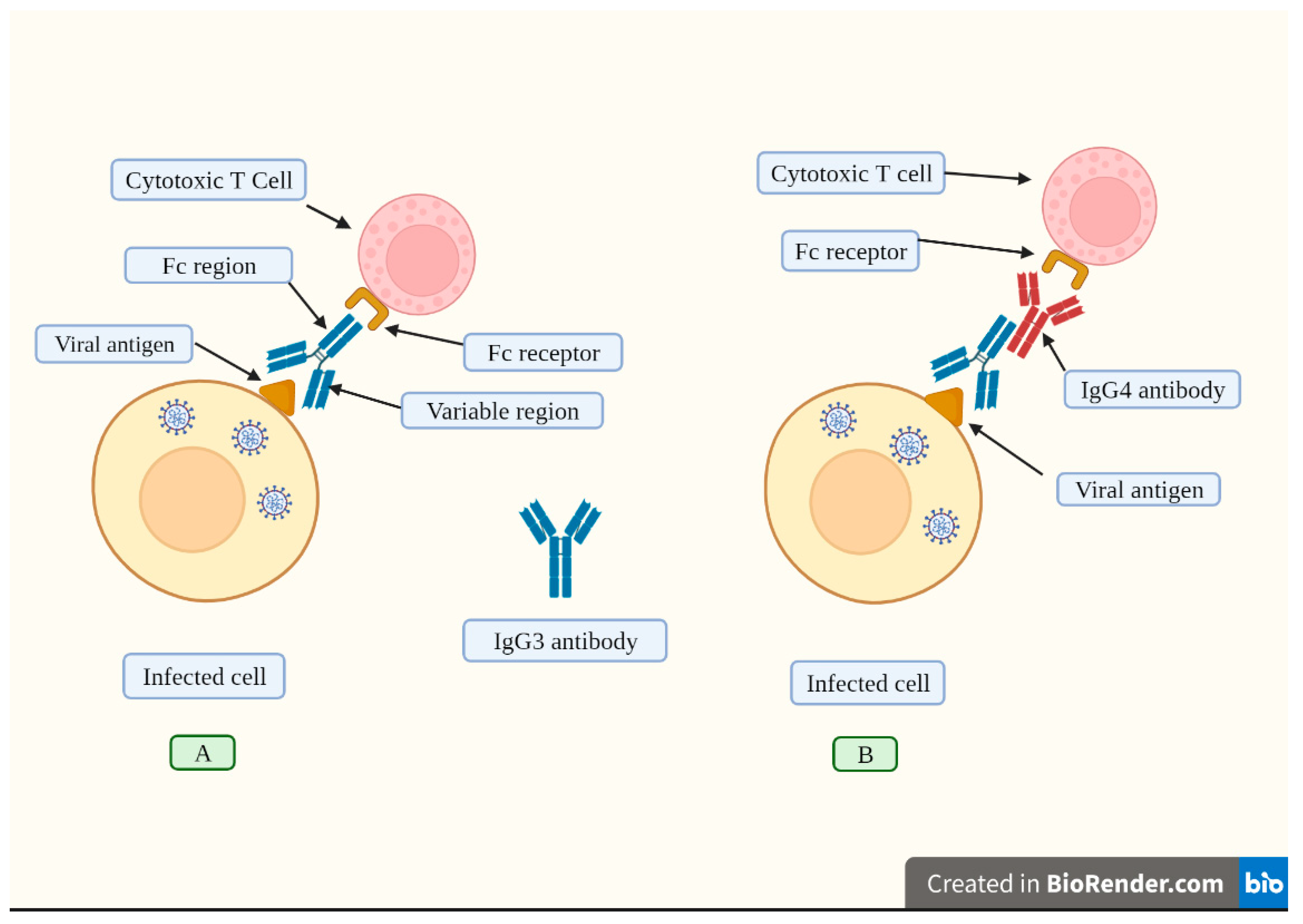
3. The role of IgG4 antibodies induced by mRNA vaccines
3.1. Lessons from the HIV vaccine trial
3.2. Lessons from the Malaria vaccine trial
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. Bmj 2022, 376. [Google Scholar] [CrossRef]
- 3 Tenforde, M.W.; Self, W.H.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; McNeal, T. Effectiveness of mRNA vaccination in preventing COVID-19–associated invasive mechanical ventilation and death—United States, March 2021–January 2022. Morbidity and Mortality Weekly Report 2022, 71, 459. [Google Scholar] [CrossRef]
- Plumb, I.D.; Feldstein, L.R.; Barkley, E.; Posner, A.B.; Bregman, H.S.; Hagen, M.B.; Gerhart, J.L. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19–associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. Morbidity and Mortality Weekly Report 2022, 71, 549. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Frontiers in Public Health 2022, 10, 2738. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Jama 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England journal of medicine 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature microbiology 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Tong, X.; Kang, J.; Avendaño, M.J.; Serrano, E.F.; García-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Cai, Y.; Renzi, I. Omicron variant Spike-specific antibody binding and Fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines. Science Translational Medicine 2022, 14, eabn9243. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Science immunology 2022, eade2798. [Google Scholar]
- Subramanian, S.; Kumar, A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. European journal of epidemiology 2021, 36, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: a master of immune evasion. Biomedicines 2022, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.D.; da Costa, H.H.; Correa, V.A.; de, S. Lima, A.K.; Lindoso, J.A.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Scientific Reports 2021, 11, 17642. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.; Strollo, M.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Serum IgG4 level predicts COVID-19 related mortality. European Journal of Internal Medicine 2021, 93, 107–109. [Google Scholar] [CrossRef]
- Grey, H.M.; Kunkel, H.G. H chain subgroups of myeloma proteins and normal 7S γ-globulin. The Journal of experimental medicine 1964, 120, 253. [Google Scholar] [CrossRef] [PubMed]
- Terry, W.D.; Fahey, J.L. Subclasses of human γ2-globulin based on differences in the heavy polypeptide chains. Science 1964, 146, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, M.; Kuritani, T.; Kubagawa, H.; Cooper, M. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. Journal of immunology (Baltimore, Md.: 1950) 1983, 130, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pober, J.S. Cellular and molecular immunology; Saunders: 1991.
- Meulenbroek, A. Human IgG subclasses: useful diagnostic markers for immunocompetence; CLB: 2002.
- Aucouturier, P.; Danon, F.; Daveau, M.; Guillou, B.; Sabbah, A.; Besson, J.; Preud'homme, J.L. Measurement of serum IgG4 levels by a competitive immunoenzymatic assay with monoclonal antibodies. Journal of immunological methods 1984, 74, 151–162. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Schuurman, J. IgG4 breaking the rules. Immunology 2002, 105, 9–19. [Google Scholar] [CrossRef]
- Aalberse, R.; Stapel, S.; Schuurman, J.; Rispens, T. Immunoglobulin G4: an odd antibody. Clinical & Experimental Allergy 2009, 39, 469–477. [Google Scholar]
- Nirula, A.; Glaser, S.M.; Kalled, S.L.; Taylor, F.R. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Current opinion in rheumatology 2011, 23, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Tanaka, A.; Maehara, T.; Furukawa, S.; Nakashima, H.; Nakamura, S. T helper subsets in Sjögren's syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. Journal of autoimmunity 2014, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Stone, J.H. A clinical overview of IgG4-related systemic disease. Current opinion in rheumatology 2011, 23, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Zhang, Y.; Wang, L.; Antonenko, S.; Zhang, S.; Zhang, Y.W.; Tabrizifard, M.; Ermakov, G.; Wiswell, D. Comprehensive analysis of the therapeutic IgG4 antibody pembrolizumab: hinge modification blocks half molecule exchange in vitro and in vivo. Journal of Pharmaceutical Sciences 2015, 104, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, J.; Van Ree, R.; Perdok, G.a.; Van Doorn, H.; Tan, K.; Aalberse, R. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 1999, 97, 693–698. [Google Scholar] [CrossRef]
- Van Der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.G.; Plomp, J.J.; van der Maarel, S.M.; Verschuuren, J.J. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Annals of the New York Academy of Sciences 2018, 1413, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Daveau, M.; Fischer, J.P.; Rivat, L.; Rivat, C.; Ropartz, C.; Peter, H.H.; Cesarini, J.-P.; Kourilsky, F.M. IgG4 subclass in malignant melanoma. Journal of the National Cancer Institute 1977, 58, 189–192. [Google Scholar] [CrossRef]
- Karagiannis, P.; Gilbert, A.E.; Josephs, D.H.; Ali, N.; Dodev, T.; Saul, L.; Correa, I.; Roberts, L.; Beddowes, E.; Koers, A. IgG4 subclass antibodies impair antitumor immunity in melanoma. The Journal of clinical investigation 2013, 123, 1457–1474. [Google Scholar] [CrossRef]
- Akdis, C.; Blaser, K. Mechanisms of allergen-specific immunotherapy. Allergy 2000, 55, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Current opinion in immunology 2006, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology 2006, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O'Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. New England Journal of Medicine 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.a.; Doglioni, C. Immunology of IgG4-related disease. Clinical & Experimental Immunology 2015, 181, 191–206. [Google Scholar]
- Aparisi, L.; Farre, A.; Gomez-Cambronero, L.; Martinez, J.; De Las Heras, G.; Corts, J.; Navarro, S.; Mora, J.; Lopez-Hoyos, M.; Sabater, L. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 2005, 54, 703–709. [Google Scholar] [CrossRef]
- Nishi, H.; Tojo, A.; Onozato, M.L.; Jimbo, R.; Nangaku, M.; Uozaki, H.; Hirano, K.; Isayama, H.; Omata, M.; Kaname, S. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrology Dialysis Transplantation 2007, 22, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Miyaji, E.; Morimoto, K.; Nagao, K.; Kamada, M.; Onishi, S. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 2005, 54, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Nishio, A.; Uchida, K.; Kido, M.; Ueno, S.; Uza, N.; Kiriya, K.; Inoue, S.; Kitamura, H.; Ohashi, S. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 2006, 33, 20–26. [Google Scholar] [CrossRef]
- Endo, T.; Takizawa, S.; Tanaka, S.; Takahashi, M.; Fujii, H.; Kamisawa, T.; Kobayashi, T. Amylase α-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 2009, 58, 732–737. [Google Scholar] [CrossRef]
- Frulloni, L.; Lunardi, C.; Simone, R.; Dolcino, M.; Scattolini, C.; Falconi, M.; Benini, L.; Vantini, I.; Corrocher, R.; Puccetti, A. Identification of a novel antibody associated with autoimmune pancreatitis. New England Journal of Medicine 2009, 361, 2135–2142. [Google Scholar] [CrossRef]
- Hart, P.A.; Topazian, M.D.; Witzig, T.E.; Clain, J.E.; Gleeson, F.C.; Klebig, R.R.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; Smyrk, T.C. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 2013, 62, 1607–1615. [Google Scholar] [CrossRef]
- Shiokawa, M.; Kodama, Y.; Kuriyama, K.; Yoshimura, K.; Tomono, T.; Morita, T.; Kakiuchi, N.; Matsumori, T.; Mima, A.; Nishikawa, Y. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–1332. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Frontiers in immunology 2014, 5, 520. [Google Scholar] [CrossRef]
- Plomp, J.J.; Huijbers, M.G.; van der Maarel, S.M.; Verschuuren, J.J. Pathogenic IgG4 subclass autoantibodies in MuSK myasthenia gravis. Annals of the New York Academy of Sciences 2012, 1275, 114–122. [Google Scholar] [CrossRef]
- Beck Jr, L.H.; Salant, D.J. Membranous nephropathy: recent travels and new roads ahead. Kidney international 2010, 77, 765–770. [Google Scholar] [CrossRef]
- Anhalt, G.J.; Labib, R.S.; Voorhees, J.J.; Beals, T.F.; Diaz, L.A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. New England Journal of Medicine 1982, 306, 1189–1196. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ohara, M.; Suzuki, C.; Naishiro, Y.; Yamamoto, H.; Takahashi, H.; Imai, K. Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scandinavian journal of rheumatology 2004, 33, 432–433. [Google Scholar] [CrossRef]
- Okazaki, K.; Uchida, K.; Koyabu, M.; Miyoshi, H.; Takaoka, M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. Journal of gastroenterology 2011, 46, 277–288. [Google Scholar] [CrossRef]
- Dahlgren, M.; Khosroshahi, A.; Nielsen, G.P.; Deshpande, V.; Stone, J.H. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis care & research 2010, 62, 1312–1318. [Google Scholar]
- Zen, Y.; Inoue, D.; Kitao, A.; Onodera, M.; Abo, H.; Miyayama, S.; Gabata, T.; Matsui, O.; Nakanuma, Y. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. The American journal of surgical pathology 2009, 33, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Zen, Y.; Abo, H.; Gabata, T.; Demachi, H.; Kobayashi, T.; Yoshikawa, J.; Miyayama, S.; Yasui, M.; Nakanuma, Y. Immunoglobulin G4–related lung disease: CT findings with pathologic correlations. Radiology 2009, 251, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Saito, A.; Yamazaki, H.; Emura, I.; Imai, N.; Ueno, M.; Nishi, S.; Miyamura, S.; Gejyo, F. Tubulointerstitial nephritis associated with IgG4-related systemic disease. Clinical and experimental nephrology 2007, 11, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Nishi, S.; Imai, N.; Ito, T.; Yamazaki, H.; Kawano, M.; Yamamoto, M.; Takahashi, H.; Matsui, S.; Nakada, S. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney international 2010, 78, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kojima, M.; Takata, K.; Morito, T.; Asaoku, H.; Takeuchi, T.; Mizobuchi, K.; Fujihara, M.; Kuraoka, K.; Nakai, T. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Modern Pathology 2009, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Notohara, K.; Kojima, M.; Takata, K.; Masaki, Y.; Yoshino, T. IgG4-related disease: Historical overview and pathology of hematological disorders. Pathology international 2010, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hamanou, H.; Kawa, S.; Ochi, Y.; Unno, H.; Shiba, N.; Wajiki, M.; Nakazawa, K.; Shimojo, H.; Kiyosawa, K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. The Lancet 2002, 359, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Onodera, M.; Inoue, D.; Kitao, A.; Matsui, O.; Nohara, T.; Namiki, M.; Kasashima, S.; Kawashima, A.; Matsumoto, Y. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. The American journal of surgical pathology 2009, 33, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Khosroshahi, A.; Hilgenberg, A.; Spooner, A.; Isselbacher, E.M.; Stone, J.R. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis and rheumatism 2009, 60, 3139–3145. [Google Scholar] [CrossRef]
- Evoli, A.; Tonali, P.A.; Padua, L.; Monaco, M.L.; Scuderi, F.; Batocchi, A.P.; Marino, M.; Bartoccioni, E. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003, 126, 2304–2311. [Google Scholar] [CrossRef]
- Farrugia, M.E.; Robson, M.D.; Clover, L.; Anslow, P.; Newsom-Davis, J.; Kennett, R.; Hilton-Jones, D.; Matthews, P.M.; Vincent, A. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006, 129, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- McConville, J.; Farrugia, M.E.; Beeson, D.; Kishore, U.; Metcalfe, R.; Newsom-Davis, J.; Vincent, A. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Annals of neurology 2004, 55, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Niks, E.; Van Leeuwen, Y.; Leite, M.; Dekker, F.; Wintzen, A.; Wirtz, P.; Vincent, A.; van Tol, M.; Jol-van der Zijde, C.; Verschuuren, J. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. Journal of neuroimmunology 2008, 195, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Shigemoto, K.; Fujinami, A.; Maruyama, N.; Konishi, T.; Ohta, M. Clinical and experimental features of MuSK antibody positive MG in Japan. European journal of neurology 2007, 14, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.N.; Reddel, S.W.; Gervásio, O.L.; Phillips, W.D. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2008, 63, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Ghazanfari, N.; Ngo, S.; Gervasio, O.; Reddel, S.; Phillips, W. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. The Journal of Physiology 2010, 588, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.H.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpen, K.; Verrips, A.; Losen, M. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Selcen, D.; Fukuda, T.; Shen, X.-M.; Engel, A.G. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology 2004, 62, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine 2016, 8, 328rv324–328rv324. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New England Journal of Medicine 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. New England Journal of Medicine 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O'day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T. , Togashi, Y., Tay, C., Ha, D., Sasaki, A., Nakamura, Y.,... & Nishikawa, H. (2019). PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proceedings of the National Academy of Sciences, 116(20), 9999-10008.
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1Hyperprogressive Disease with Anti-PD-1/PD-L1 Therapy. Clinical Cancer Research 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth RateGenomics of Immunotherapy-Associated Hyperprogressors. Clinical Cancer Research 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Champiat, S.; Ferrara, R.; Massard, C.; Besse, B.; Marabelle, A.; Soria, J.-C.; Ferté, C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nature Reviews Clinical Oncology 2018, 15, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shimoda, S.; Kimura, Y.; Sato, Y.; Ikeda, H.; Igarashi, S.; Ren, X.S.; Sato, H.; Nakanuma, Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 2012, 56, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Inada, H.; Kanayama, K.; Shiraishi, T. A case of pancreatic ductal adenocarcinoma with marked infiltration with IgG4-positive cells. Journal of Cytology 2013, 30, 46. [Google Scholar] [CrossRef] [PubMed]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; van Deventer, S.J.; van der Poll, T. Proinflammatory effects of IL-10 during human endotoxemia. The Journal of Immunology 2000, 165, 2783–2789. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in mast cell-mediated immune responses: anti-inflammatory and proinflammatory roles. International journal of molecular sciences 2021, 22, 4972. [Google Scholar] [CrossRef]
- Saxton, R.A.; Tsutsumi, N.; Su, L.L.; Abhiraman, G.C.; Mohan, K.; Henneberg, L.T.; Aduri, N.G.; Gati, C.; Garcia, K.C. Structure-based decoupling of the pro-and anti-inflammatory functions of interleukin-10. Science 2021, 371, eabc8433. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Q.; Zhao, C.; Zhu, Z.; Zhu, X.; Zhou, J.; Zhang, S.; Yang, T.; Zhang, B.; Li, J. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. Journal for ImmunoTherapy of Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future oncology 2015, 11, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Current medicinal chemistry 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. Journal for immunotherapy of cancer 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L. Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Balmelli, C.; Bossard, M.; Pfister, O.; Glatz, K.; Zippelius, A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for immunotherapy of cancer 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Matson, D.R.; Accola, M.A.; Rehrauer, W.M.; Corliss, R.F. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. Journal of forensic sciences 2018, 63, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.B.; Martínez, L.; Giordano, M.; Passeggi, C.; de Wolff, M.C.; Nates, S. Comparison of immunoglobulin G subclass profiles induced by measles virus in vaccinated and naturally infected individuals. Clinical and Vaccine Immunology 2002, 9, 693–697. [Google Scholar] [CrossRef]
- Urban, M.; Winkler, T.; Landini, M.; Britt, W.; Mach, M. Epitope-specific distribution of IgG subclasses against antigenic domains on glycoproteins of human cytomegalovirus. Journal of Infectious Diseases 1994, 169, 83–90. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Frontiers in Immunology 2023, 13, 7835. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Beikzadeh, B.; Berzofsky, J.A. The effect of antigen dose on T cell-targeting vaccine outcome. Human vaccines & immunotherapeutics 2019, 15, 407–411. [Google Scholar]
- Keck, S.; Schmaler, M.; Ganter, S.; Wyss, L.; Oberle, S.; Huseby, E.S.; Zehn, D.; King, C.G. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proceedings of the National Academy of Sciences 2014, 111, 14852–14857. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; McCormack, P.L. Reduced-Antigen, Combined Diphtheria, Tetanus, and Acellular Pertussis Vaccine, Adsorbed (Boostrix®) A Guide to Its Use as a Single-Dose Booster Immunization Against Pertussis. BioDrugs 2013, 27, 75–81. [Google Scholar] [CrossRef]
- McCormack, P.L. Reduced-Antigen, Combined Diphtheria, Tetanus and Acellular Pertussis Vaccine, Adsorbed (Boostrix®) A Review of its Properties and Use as a Single-Dose Booster Immunization. Drugs 2012, 72, 1765–1791. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Science translational medicine 2014, 6, 228ra238–228ra238. [Google Scholar] [CrossRef] [PubMed]
- Karnasuta, C.; Akapirat, S.; Madnote, S.; Savadsuk, H.; Puangkaew, J.; Rittiroongrad, S.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS research and human retroviruses 2017, 33, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clinical Infectious Diseases 2022, 75, e545–e551. [Google Scholar] [CrossRef] [PubMed]
- Shitrit, P.; Zuckerman, N.S.; Mor, O.; Gottesman, B.-S.; Chowers, M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Eurosurveillance 2021, 26, 2100822. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clinical Microbiology and Infection 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. The Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Quelhas, D.; Quintó, L.; Puyol, L.; Serra-Casas, E.; Mayor, A.; Nhampossa, T.; Macete, E.; Aide, P.; Mandomando, I. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clinical and Vaccine Immunology 2012, 19, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M. Immunity to blood stages of Plasmodium falciparum is dependent on a specific pattern of immunoglobulin subclass responses to multiple blood stage antigens. Medical hypotheses 2007, 69, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Mewono, L.; Maya, D.W.M.; Matsiegui, P.-B.; Agnandji, S.T.; Kendjo, E.; Barondi, F.; Issifou, S.; Kremsner, P.G.; Mavoungou, E. Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of Plasmodium falciparum in Gabonese children with acute falciparum malaria. European cytokine network 2008, 19, 30–36. [Google Scholar]
- Oeuvray, C.; Bouharoun-Tayoun, H.; Gras-Masse, H.; Bottius, E.; Kaidoh, T.; Aikawa, M.; Filgueira, M.-C.; Tartar, A.; Druilhe, P. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. 1994.
- Roussilhon, C.; Oeuvray, C.; Müller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.-F.; Theisen, M.; Balde, A.; Pérignon, J.-L.; Druilhe, P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS medicine 2007, 4, e320. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Richards, J.S.; McCallum, F.J.; Michon, P.; King, C.L.; Schoepflin, S.; Gilson, P.R.; Murphy, V.J.; Anders, R.F.; Mueller, I. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and immunity 2009, 77, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Aucan, C.; Traoré, Y.; Tall, F.; Nacro, B.; Traoré-Leroux, T.; Fumoux, F.; Rihet, P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infection and immunity 2000, 68, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Trampert, D.C.; Hubers, L.M.; van de Graaf, S.F.; Beuers, U. On the role of IgG4 in inflammatory conditions: lessons for IgG4-related disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2018, 1864, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clinical & Experimental Allergy 2001, 31, 1392–1397. [Google Scholar]
- Angioni, R.; Sanchez-Rodriguez, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell death & disease 2020, 11, 1–12. [Google Scholar]
- Della-Torre, E.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Napolitano, A.; La Marca, S.; Boffini, N.; Da Prat, V.; Di Terlizzi, G.; Lanzillotta, M. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Annals of the rheumatic diseases 2020, 79, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Della-Torre, F.; Kusanovic, M.; Scotti, R.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Treating COVID-19 with colchicine in community healthcare setting. Clinical Immunology (Orlando, Fla.) 2020, 217, 108490. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P. Respiratory impairment predicts response to IL-1 and IL-6 blockade in COVID-19 patients with severe pneumonia and hyper-inflammation. Frontiers in Immunology 2021, 12, 675678. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Sukhova, K.; Newman, J.; Kugathasan, R.; Yan, A.W.; Furnon, W.; De Lorenzo, G. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. biorxiv 2022, 15, e0241955. [Google Scholar]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Jain, N. Reduced pathogenicity of the SARS-CoV-2 Omicron variant in hamsters. Med 2022, 3, 262–268.e264. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nature microbiology 2022, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.-W.; Hu, B.; Chai, Y.; Yuen, T.T.-T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.-C.; Liu, H. Attenuated replication and pathogenicity of SARS-CoV-2 B. 1.1. 529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. The Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. Iscience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell discovery 2021, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.N.; Devlin, J.C.; Buus, T.B.; Koide, A.; Shwetar, J.; Cornelius, A.; Samanovic, M.I.; Herrera, A.; Mimitou, E.P.; Zhang, C. SARS-CoV-2 mRNA vaccine elicits a potent adaptive immune response in the absence of IFN-mediated inflammation observed in COVID-19. MedRxiv 2021. [Google Scholar]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and Chemical Toxicology 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Moorman, J.P.; Yao, Z.Q.; Jia, Z.S. Viral (hepatitis C virus, hepatitis B virus, HIV) persistence and immune homeostasis. Immunology 2014, 143, 319–330. [Google Scholar] [CrossRef]
- Tsumiyama, K.; Miyazaki, Y.; Shiozawa, S. Self-organized criticality theory of autoimmunity. PLoS One 2009, 4, e8382. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, S. Cause of systemic lupus erythematosus: a novel self-organized criticality theory of autoimmunity. Expert Review of Clinical Immunology 2011, 7, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Gadi, S.R.; Brunker, P.A.; Al-Samkari, H.; Sykes, D.B.; Saff, R.R.; Lo, J.; Bendapudi, P.; Leaf, D.E.; Leaf, R.K. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion 2021, 61, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Bril F, Al Diffalha S, Dean M, Fettig DM. 2021. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? Journal of Hepatology 75:222–224. [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Portuguese, A.J.; Sunga, C.; Kruse-Jarres, R.; Gernsheimer, T.; Abkowitz, J. Autoimmune-and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Advances 2021, 5, 2794–2798. [Google Scholar] [CrossRef]
- Ghielmetti, M.; Schaufelberger, H.D.; Mieli-Vergani, G.; Cerny, A.; Dayer, E.; Vergani, D.; Beretta-Piccoli, B.T. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? Journal of autoimmunity 2021, 123, 102706. [Google Scholar] [CrossRef]
- Vuille-Lessard, É.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. Journal of autoimmunity 2021, 123, 102710. [Google Scholar] [CrossRef] [PubMed]
- Chamling, B.; Vehof, V.; Drakos, S.; Weil, M.; Stalling, P.; Vahlhaus, C.; Mueller, P.; Bietenbeck, M.; Reinecke, H.; Meier, C. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clinical Research in Cardiology 2021, 110, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Chubb, D.; Schneider, D.; Freeman, E.; Kemp, W.; Roberts, S. Comment to the letter of Bril F et al.“Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty?”. J Hepatol 2021, 75, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Minocha, P.K.; Better, D.; Singh, R.K.; Hoque, T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. The Journal of pediatrics 2021, 238, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Elrashdy, F.; Tambuwala, M.M.; Hassan, S.S.; Adadi, P.; Seyran, M.; Abd El-Aziz, T.M.; Rezaei, N.; Lal, A.; Aljabali, A.A.; Kandimalla, R. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmunity reviews 2021, 20, 102941. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Lopes, S.; Simões, M.S.; Liberal, R.; Lopes, J.; Carneiro, F.; Macedo, G. Autoimmune hepatitis after COVID-19 vaccine–more than a coincidence. Journal of autoimmunity 2021, 125, 102741. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Reece, B.R.; Moore, J.S.; Means Jr, R.T. Autoimmune hemolytic anemia after mRNA COVID vaccine. Journal of Investigative Medicine High Impact Case Reports 2022, 10, 23247096211073258. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Lavine, N.; Ohayon, A.; Seida, R.; Alrais, M.; Zoubi, M.; Bragazzi, N.L. COVID-19 vaccines and the rate of autoimmune adverse events: insights from a literature review. Frontiers in immunology 2022, 3221. [Google Scholar]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurologica Scandinavica 2022, 145, 5–9. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurological Sciences 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Kaulen, L.D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H.M.; Nagel, S.; Wildemann, B. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. European journal of neurology 2022, 29, 555–563. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T. Autoimmune encephalitis following ChAdOx1-S SARS-CoV-2 vaccination. Neurological Sciences 2022, 43, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.; Giovanellla, L.; Campennì, A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: Real-life experience and review of the literature. Journal of Endocrinological Investigation 2022, 45, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




