1. Introduction
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) was the first reported pest in 1797 in Georgia, America (Smith & Abbott, 1797). Furthermore, this pest in 2016 was first reported in West and Central Africa (Goergen et al., 2016). Also, S. frugiperda populations were discovered in 2018 in many locations in India (Sharanabasappa et al., 2018) and Southeast Asia in 2019 (Li et al., 2020). In early 2019, it was reported to be discovered in Indonesia (Nonci et al., 2019).
The migration ability of S. frugiperda imago is high up to 1600 km in 30 hours (Johnson, 1987). Hence, it spread rapidly to several areas in Indonesia. For example, it was reported in West Sumatra in March 2019 (Nonci et al., 2019) and in May 2019 S. frugiperda was confirmed in Lampung (Trisyono et al., 2019), Maharani et al. (2019) reported that in June 2019 this insect was spread at Bandung, Garut, and Sumedang district. The pests attack corn plants from the vegetative to generative phases.
S. frugiperda is highly polyphagous, causing economic impacts on various plants (Prasanna et al., 2018). Moreover, 353 different host plant species have been reported from 76 plant families based on a thorough literature review, especially the Poaceae, Asteraceae, and Fabaceae families (Montezano et al., 2018) as well as the Brassicaceae family as other hosts (CABI, 2020). The availability of abundant hosts makes S. frugiperda populations able to create new food preferences on various plants (Barrros et al., 2010).
In line with Subramaniam and Mohankumar (2006), the ability of insects to survive on diverse host plants is an adaptive mechanism for survival in ecosystems. However, the availability and quality of host plants plays an important role in pest population dynamics by influencing larval and imago stage performance (Roy, 2015). Furthermore, according to Chapman (2013), the amount and quality of food consumed by insects affects development, reproduction, and life span.
Based on the pest management perspective, the life table is essential to determine the most vulnerable of pest stage for pest control (Kakde et al., 2014). Therefore, this research was conducted to examine the development, reproduction, nutritional indices, and life table of S. frugiperda in several plant species. In addition to knows the potency of this insect pest to exploit various plant as host. As implication we can known the potency of this insect as a pest of plant tested.
2. Materials and Methods
The research was conducted at the Pesticide and Environmental Toxicology Laboratory, Department of Plant Pests and Diseases, Faculty of Agriculture, Universitas Padjadjaran, West Java, Indonesia. The research was conducted from July to November 2020.
2.1. Rearing of test insect
S. frugiperda obtained from corn plantations in Jatinangor, Sumedang, West Java. Indonesia. Furthermore, the larvae were kept in plastic boxes measuring 34 x 28 x 7 cm. In plastic boxes, early instar larvae were fed using baby corn fruit (Zea mays). Before pupation, the larvae were transferred to a plastic container with a lined of paper and given sawdust as the medium for pupation. Furthermore, the pupae that had been formed were transferred to the cage (measuring 44.5 x 44.5 x 49.5 cm) where the imago was reared until became an imago.
The imago was fed 10% liquid honey absorbed on a lump of cotton. After which, pesticide-free corn leaves were put in bottles filled with water and placed in plastic cages as a place to lay eggs. The eggs laid by imago on corn leaves were collected daily and placed in a ventilated plastic box measuring 10 x 9 x 4.5 cm, lined with paper at the bottom. Moreover, insect were maintenance every day, hence, the larvae were available for testing.
2.2. Feed plant cultivation
The feed plants used in the test included leaves and young fruit (baby) of sweet corn (Zea mays L. (Poaceae); F1 Hybrid Talent, PT. Agri Makmur Pertiwi), broccoli (Brassica oleracea L. var. Italica (Brassicaceae); F1 Hybrid Broccoli Bonanza, Known-You Seed), rice (Oryza sativa L cv. Ciherang (Gramineae)) and oil palm (Elaeis guineensis Jacq (Arecaceae)). Corn and broccoli seeds were planted using polybags with a capacity of 5 kg containing a mixture of soil and manure (3:1). Planting of rice plants starts from seeds sown on plastic trays and then, after two weeks, transferred to plastic buckets with mixed soil conditions. Moreover, watering was performed regularly, and then fertilization was carried out seven days after planting (DAP) with a dose of NPK fertilizer of 3g per plant for corn and broccoli plants, as well as fertilization was carried out 14 days after transplanting for rice plants.
Replanting was carried out once a week to obtain uniform and sufficient plants to feed the larvae. Plants can be used as feed after they have more than 5 leaves or more than 2 months old. Meanwhile, oil palm leaves were obtained from young plants in Jatinangor, West Java.
2.3. Effect of Feed Types on Biology of S. frugiperda
Larvae that emerge from newly hatched eggs (< 24 hours) were selected to be placed in plastic cups as many as 50 larvae for each feed type. The larvae were placed separately in plastic cups (diameter of 2.5 cm, height of 4 cm) with leaves of corn, rice, broccoli, oil palm, and baby corn fruit as control. Fresh feed leaves and baby corn fruit were replaced daily with fresh ones.
Observations were made every day to determine the mortality, and development time of larvae. In addition, the length of larval development was observed by recording the time required for S. frugiperda larvae to develop from a certain instar to the next, marked by the molting of the larval cuticle. After the larvae became pupae, observations were made, including the development time and weight of pupae, normal and abnormal condition, as well as mortality. Weight of pupae was performed on the third day after pupation using analytical balance.
The imago that emerge from pupae were paired in a cage (diameter of 13.5 cm, height of 13 cm) where a 10% honey solution was absorbed into the cotton as food of imago. Furthermore, the corn leaves are placed in the cage to laid eggs according to the larval feed. The number of eggs laid by each female and mortality of imagos was recorded daily. Moreover, dead female imagos were dissected to reveal ovaries. A lateral incision was made in the abdomen following the midline of the thorax from the anterior to the posterior end to expose the internal organs. Furthermore, the abdomen was opened using a surgical needle to remove the ovaries carefully. Observations were carried out under a microscope on the number of eggs in the ovarioles.
The data was compiled in the form of a life table. The parameters observed were as follows (Birch, 1948; Ning et al., 2017):
Net reproduction rate (R0) (individual/parent/generation) = ∑IXmx
Intrinsic growth rate (r) (individual/parent/day) = (ln R0) / T
Average generation period (T) (days) =
Population doubled DT (days) = ln (2)/r
Gross reproduction rate (GRR) (individual/generation) = ∑mx
Description: x: cohort age class (days); Ix: the individual probability of each individual at age x; mx: fecundity per individual at age x; IXmx: the number of offspring born in the x age class.
2.4. Effect of Feed Plant Type on Food Utilization and Larval Growth of S. frugiperda
Measurement of food utilization and larval growth of S. frugiperda refers to the gravimetric method (Waldbauer, 1968). Each treatment consisted of 10 S. frugiperda instar V larvae that had just changed cuticles. The treatment types of feed included leaves of corn, rice, broccoli, oil palm, and baby corn fruit as control. The experiment was arranged using a Randomized Block Design with five replications. The experiment was started by weighing the larvae and feed to determine the initial wet weight, then placed individually in a plastic cup with each type of treatment feed. Each piece type of feed used was to 4 cm x 4 cm, while corn wass cut with a diameter of 2 cm and a thick of 1 cm. Feeding periods of the larvae were two days and the treatment was ended. Furthermore, larvae, food residue, and feces from each plastic cup were wrapped in aluminum foil and then dried in an oven at 90oC for 48 hours.
The correction factor was first calculated to obtain the initial dry weight of experimental larvae. Furthermore, the correction factor was obtained by weighing the wet and dry weights of 10 larvae. The correction factor is calculating the dry weight of the larvae divided by the wet weight and then multiplied by 100%. The percentage of biomass content of the correction factor was then multiplied by the initial wet weight of the experimental larvae to obtain the initial dry weight of the experiment larvae. The initial dry weight of each feed was calculated in the same way as the larvae dry weight. Finally, all values were converted into dry weight values for analysis.
Furthermore, the nutritional indices was calculated by the gravimetric method using the following formula (Waldbauer, 1968; Hasyim et al., 2010):
Consumption Rate/CR
CR (g / hari) =
Relative Consumption Rate/ RCR
RCR (g / g body weight/day) =
Growth rate (GR) (g/day)= G/T
Relative Growth Rate/ RGR
RGR (g / g body weight/day) =
The efficiency of Conversion of Digested Food / ECD
ECD (%) =
The efficiency of Conversion of Ingested food/ ECI
ECI =
Approximate Digestibility / AD
AD =
Description:
G: The weight gain of larvae during the feeding period, obtained from the final dry weight of the larvae minus the initial dry weight; F: The amount of feed consumed, obtained by subtracting the initial with the final dry weight of the feed; f: dry weight of feces; T: feeding period; A: The average weight of larvae during treatment, obtained from the addition of the initial to the final dry weight of the larvae divided by two.
2.5. Data analysis
Data from biological observations were processed using analysis of variance, followed by Duncan's multiple interval test. The life table data was processed and analyzed using Microsoft Office Excel 2010 Worksheet.
3. Results
3.1. Effects of plant species on development and reproduction S. frugiperda
The developmental phase of
S. frugiperda consisted of eggs, larvae, pupae, and imago. The duration of the egg stage on baby corn fruit, corn, rice, and broccoli leaves ranged from 2.31-2.98 days. The duration of the egg stage that laid by imago, whose larvae were fed with rice leaves, were at least 2.31 days, while corn leaves were 2.98 days longer than the other four types. The type of feed used did not affect the hatching time. Unfortunately, observation of palm leaf feed could not be conducted because only one abnormal pupae could develop at the immature stage (
Table 1).
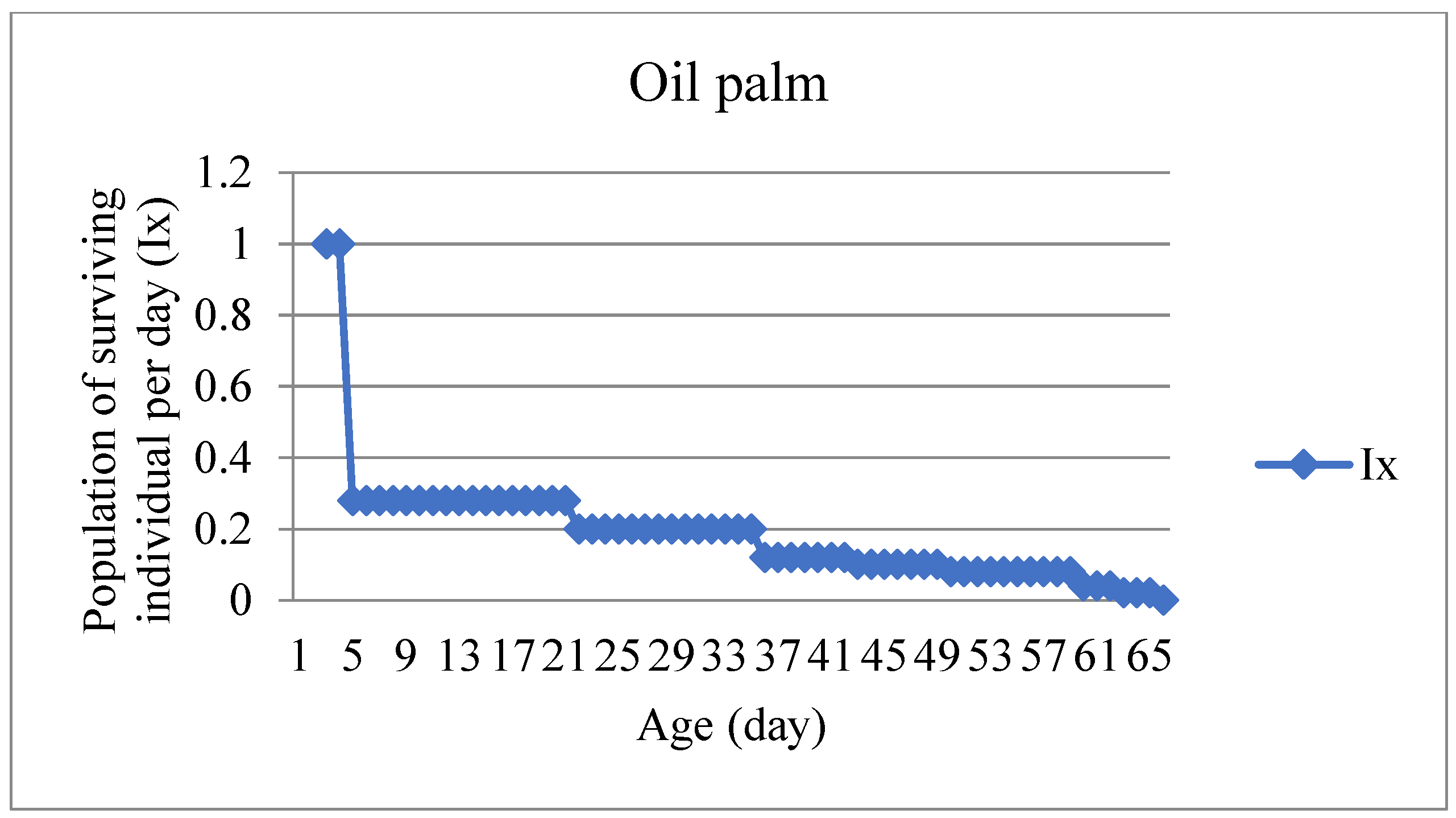
S. frugiperda passed six instars in the five types of feed. The development length of the first to the sixth instar on the five types of feed was significantly different. The shortest total duration of larval development was in the baby corn fruit feed treatment (14.68 days), followed by broccoli (17.60 days), corn (19.12 days), rice (20.73 days), and oil palm leaves (61.28 days). Meanwhile, the mortality of first instar larvae was relatively high in rice and oil palm leaf feed (
Table 1).
In oil palm leaf treatment, larvae that lived up to the sixth instar experienced long development and small larval bodies. Larvae in the sixth instar that failed to enter the prepupae stage died with symptoms of shortened and dry bodies. Oil palm leaf showed unsuitable hosts for the development of S. frugiperda compared to the treatment of baby corn fruit, corn, rice, and broccoli leaves. Larvae that feed on oil palm leaves have a long larval period indicating compensation when feeding on low-quality hosts.
The duration of the prepupae stage in the five types of feed was significantly different between treatments. Moreover, the difference in the feed type used did not affect the duration of the prepupae, which ranged from 1.98 to 2.08 days. Baby corn feed plants have a shorter time, while corn and broccoli leaves have a longer time.
Generally the duration and weight of pupae showed significantly different values among the five feed types. The duration of pupae ranged from 9.26-10.54 days (
Table 1). Baby corn plants have a shorter time, while rice leaf has a long time. Moreover, different plant species affected the pupae's weight. The five feed types tested showed significantly different values (
Table 2). The pupae weight ranged from 0.1249-0.1879 grams, highest in baby corn fruit, then broccoli, baby corn leaves, and rice leaves respectively (
Table 2).
The sex ratio of males and females
S. frugiperda emerged from the feed types of baby corn fruit, which include (1: 1.5), broccoli leaf (1.35: 1), corn leaf (1: 1.04), and rice leaf (1: 1.91) (
Table 2). The female imago appears 2-3 days earlier than the male imago. Imago copulates at the age of 1 to 3 days. However, the female imago of
S. frugiperda that emerged from the pupae in all feed treatments that did not copulate for more than five days was died. Hashim
et al. (2013) reported that in the female imago of
Helicoverpa armigera, calling the male to copulate peaked at the age of four days which was thought to be related to the sexual maturity of the female imago.
The life span of female imago in all treatments also showed a shorter time than male imago. The life span of males and females showed the longest on rice leaf followed by broccoli leaf, baby corn fruit, and corn leaf (
Table 3). However, the long life span in the rice leaf treatment was not followed by the number of eggs produced (
Table 3). In this test, the number of eggs per female and fertility produced by female imago in the baby corn fruit treatment was higher (1088.33 eggs; 97.95%) than the other three types of feed treatments (no imago emerged from oil palm treatment), while the lowest was in the corn leaf treatment (544, 07 eggs, 54.60%).
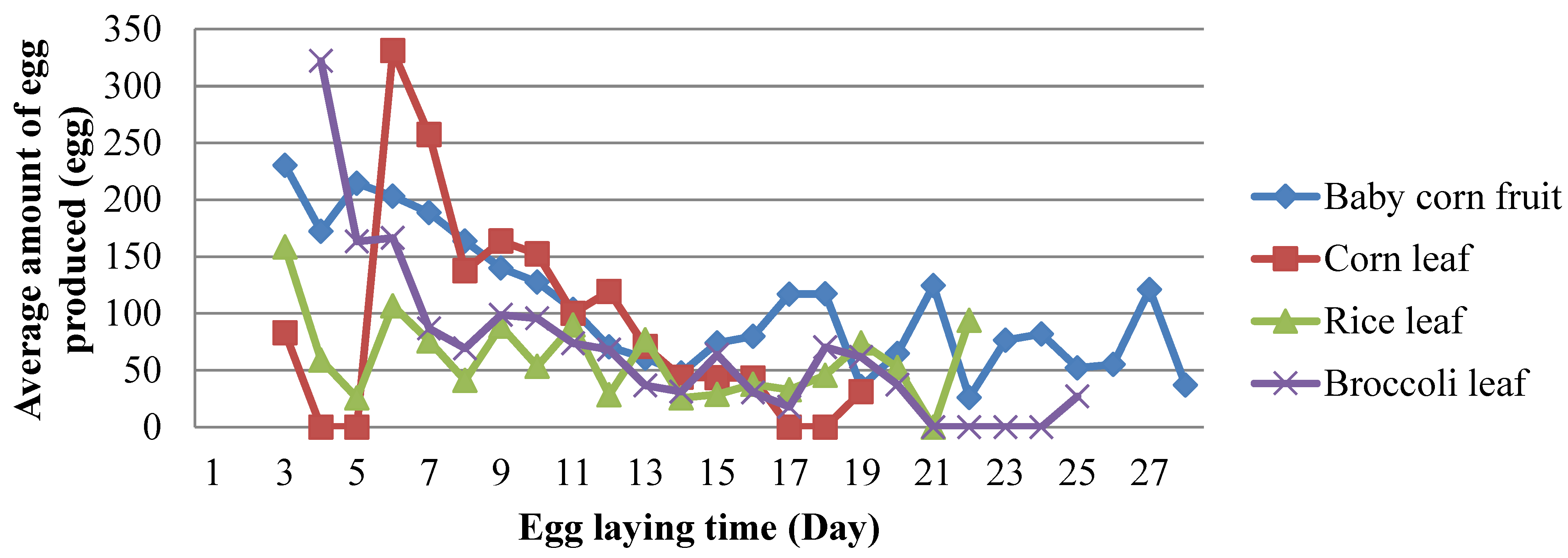
The number of eggs laid by female imago fluctuated in each type of feed treatment. In the treatment of baby corn fruit, broccoli, and rice leaves, the number of eggs laid peaked on the first day and then decreased. Furthermore, it experienced an increase again in the final stage before the female imago died. In contrast to the corn leaf feed treatment, eggs peaked on the seventh day after emerge from pupae or the fourth day after the preoviposition period. Moreover, in the final stage, it increases again before the female imago died (
Figure 1). In all treatments tested, the remaining eggs were found in ovary. The number of eggs remaining in ovary was highest in the rice leaf treatment was 18.4 eggs, and the lowest was 1.6 eggs in the broccoli leaf treatment (
Table 3). This indicated that broccoli preferenced and suitable for oviposition of the female.
3.2. Effect of feed plant species on survival and fecundity of S. frugiperda
The life table of
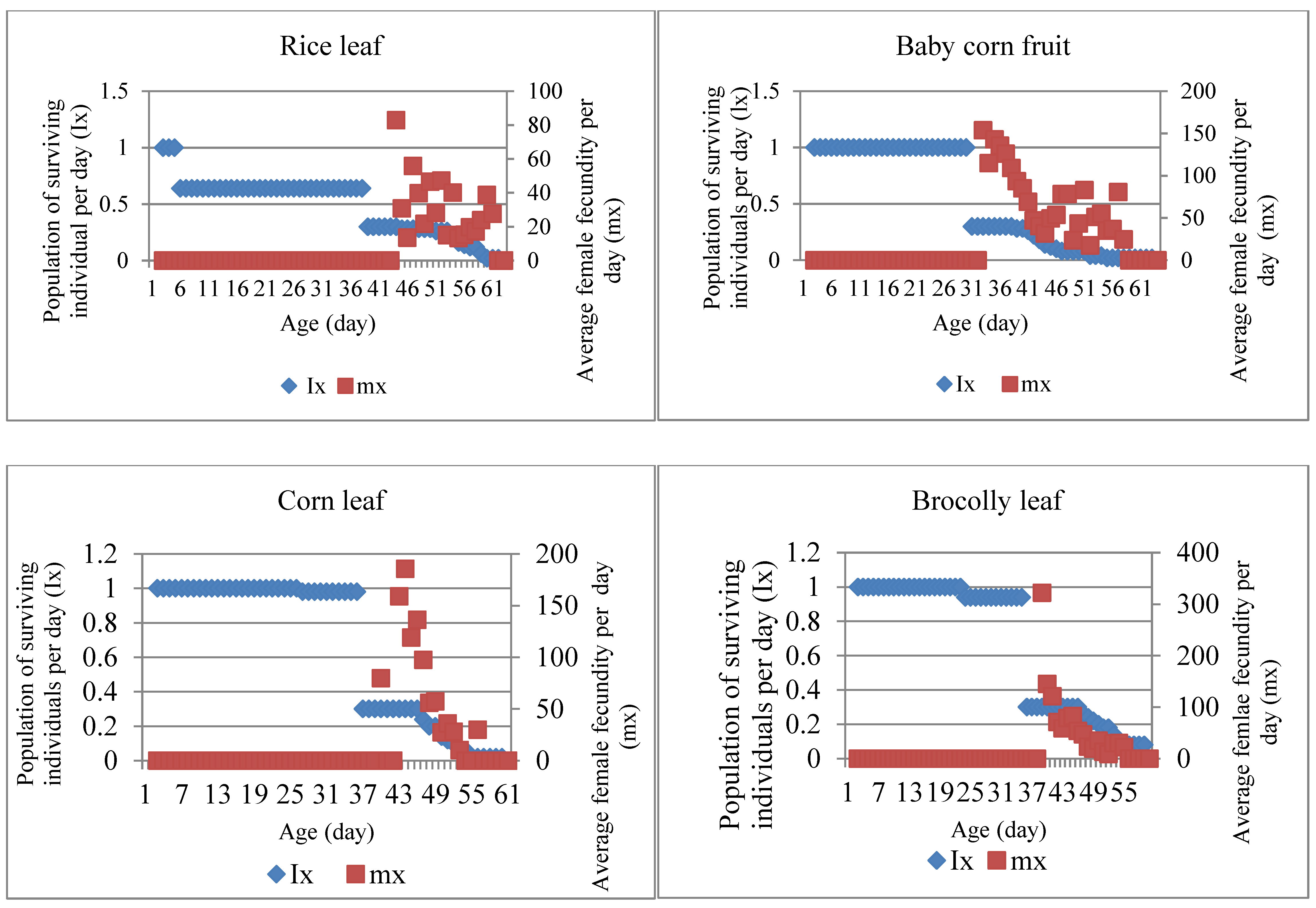
S. frugiperda was used to determine population development. The probability of individuals living at all stages starting from eggs, larvae, pupae, and imago (Ix) and the fecundity of female imago (mx) were shown from the survival curve. The survival curve illustrates that the individual's chances of survival decreased since the age of 5 days after infestation (
Figure 2) in the treatment of rice leaf feed. Meanwhile, for the treatment of corn and broccoli leaf feed, the chance of survival decreased at the final stages of development, namely the pre-pupae and pupae stages. In the treatment of baby corn fruit feed, it did not decrease at the early and late stages of development.
The average fecundity (mx) of the female imago of S. frugiperda that develop from treatment of feed baby corn fruit showed high numbers at the beginning of laying eggs (153.56 eggs), rice leaf (82.87 eggs), and broccoli leaf (322 eggs). Meanwhile, in the rice leaf treatment, the female imago laid a few eggs in the early stages of the imago, and the number increased in the middle age of the imago (576.2 eggs). It continued to fluctuate with increasing age of imago and decreased before imago died in all feed treatments.
Table 1.
Development of S. frugiperda in several feeds.
Table 1.
Development of S. frugiperda in several feeds.
| Development phase |
|
Average time required (days ± standard deviation)a
|
|
| n |
Baby corn fruit |
n |
Corn leaf |
n |
Rice leaf |
n |
Palm leaf |
n |
Broccoli leaf |
|
| Eggb
|
50 |
2.88 ± 0.19 b |
50 |
2.98 ± 0.23 b |
50 |
2.31 ± 0.23 a |
- |
- |
50 |
2.50 ± 0.26 a |
|
| Larva I |
50 |
3.22 ± 0.42 a |
50 |
4.1 ± 0.30 b |
32 |
5.14 ± 0.91 c |
27 |
11.32 ± 1.08 e |
50 |
5.54 ± 0.50 d |
|
| Larva II |
50 |
2.1 ± 0.30 a |
50 |
2.94 ± 0.24 b |
32 |
3.18 ± 0.72 b |
19 |
9.63 ± 0.74 c |
50 |
2.14 ± 0.35 b |
|
| Larva III |
50 |
2.06 ± 0.24 a |
50 |
2.06 ± 0.24 a |
32 |
2.46 ± 0.62 a |
14 |
8.78 ± 0.84 b |
50 |
2.04 ± 0.20 a |
|
| Larva IV |
50 |
1.92 ± 0.27 b |
50 |
2.08 ± 0.27 ab |
32 |
2.58 ± 0.50 b |
6 |
8.60 ± 0.89 c |
50 |
1.72 ± 0.46 a |
|
| Larva V |
50 |
2.04 ± 0.20 a |
50 |
2.8 ± 0.45a |
32 |
2.74 ± 0.67 a |
4 |
10.75 ± 3.30 b |
50 |
2.10 ± 0.27 a |
|
| Larva VI |
50 |
3.34 ± 0.66 a |
50 |
5.14 ± 0.76 a |
32 |
4.66 ± 1.04 a |
3 |
12.33 ± 4.62 b |
50 |
4.10 ± 0.75 a |
|
| Prepupae |
50 |
1.78 ± 0.42 b |
50 |
2.08 ± 0.44 b |
32 |
1.96 ± 0.93 b |
1 |
2.0 ± 0.0 a |
50 |
2.08 ± 0.35 a |
|
| Pupae |
50 |
9.26 ± 0.99 a |
49 |
9.9 ± 1.19 b |
32 |
10.54 ± 1.02 c |
- |
- |
47 |
10.47 ± 0.93 c |
|
Table 2.
Pupae weight and sex ratio of S. frugiperda imago.
Table 2.
Pupae weight and sex ratio of S. frugiperda imago.
| Feed type |
n |
Pupae weight average ± SDa (gram) |
Imago sex ratio (Male : Female) |
| Baby corn fruit |
50 |
0.1879±0.0284 d |
1.0 : 1.5 |
| Corn leaf |
49 |
0.1442±0.0269 b |
1.0 : 1.0 |
| Rice Leaf |
32 |
0.1249±0.2020 a |
1.0 : 1.9 |
| Palm leaf |
- |
- |
- |
| Broccoli leaf |
47 |
0.1656±0.0273 c |
1.4 : 1.0 |
Figure 1.
Effect of several feed types on egg-laying time and number of eggs in the female imago of S. frugiperda.
Figure 1.
Effect of several feed types on egg-laying time and number of eggs in the female imago of S. frugiperda.
Figure 2.
S. frugiperda survival curve for five types of feed; arrows indicate the time of imago appearance.
Figure 2.
S. frugiperda survival curve for five types of feed; arrows indicate the time of imago appearance.
Table 3.
Effect of several feed types on oviposition period, fecundity, fertility, and imago life span of S. frugiperda.
Table 3.
Effect of several feed types on oviposition period, fecundity, fertility, and imago life span of S. frugiperda.
| Feed Type |
Pre-oviposition period (days) |
Oviposition period (days) |
∑ Egg/female (egg) |
∑ Eggs/female/day (egg) |
Fertility (%) |
∑ Ovarian eggs (egg) |
The lifespan of imago ± S.D. (days) (n) |
| Male |
Female |
| Baby corn fruit |
3.0±0.6 a |
11.3±5.4 b |
1088.3±326.0 b |
138.42± 37.6 b |
98.0± 4.6 b |
5.7±20.9 a |
19.9±2.7(15) ab |
15.3±6.1(15) b |
| Corn leaf |
3.5±0.7 ab |
5.2±2.9 a |
544.1±289.7 a |
137.6± 58.0 b |
95.4±13.4 b |
3.3± 10.2 a |
18.7±3.3(15) a |
9.9±3.8(15) a |
| Rice Leaf |
4.1±1.4 b |
11.5±3.8 b |
713.3±257.9 a |
70.3±35.2 a |
54.6± 27.0 a |
18.4± 38.3 a |
22.6±5.9(11) b |
17.9±3.8(15) b |
| Palm leaf |
- |
- |
- |
- |
- |
- |
- |
- |
| Broccoli leaf |
3.3±1.0 a |
8.1±4.3 a |
684.4± 379.3 a |
81.7± 45.4 a |
86.4±35.1 b |
1.6±3.4 a |
22.5±4.8(15) b |
16.7±5.7(15) b |
3.3. Effect of plant species on demographic statistics of S. frugiperda
The value of the net reproduction rate (R
0) was higher in the baby corn fruit feed treatment of 368.60, followed by broccoli, corn, and rice leaves. The value of the intrinsic growth rate showing the reproductive potential (r) in the baby corn fruit feed treatment was 0.16, followed by broccoli, corn, and rice leaves. The smaller the mean value of the generation period (T) indicates, the faster an organism reproduces, as shown by the 36.29 baby corn fruit treatment followed by broccoli, corn, and rice leaf feed treatment. The gross reproduction rate (GRR) of S. frugiperda was 1856.41 on baby corn fruit feed, while the gross reproduction rate (GRR) was 576.16 on rice leaf feed (
Table 4).
The higher intrinsic growth rate in the baby corn fruit feed treatment resulted in faster development (shorter generation time), higher endurance, and high fecundity. A high value indicates the susceptibility of the host plant to insect food, while a low value indicates that the host plant species is slightly resistant or tolerant to pests. It was indicated that broccoli suitable for S. frugiperda to grow and development as well as in baby corn fruit as feed.
3.4. Effect of plant species on the nutrient indices of S. frugiperda
The consumption rate, growth rate, and feeding efficiency of
S. frugiperda larvae instar V were shown in
Table 5. The analysis of variance showed that all test parameters affected the analysis of the nutritional indices of
S. frugiperda larvae. The larvae consumption rate was higher in the treatment of baby corn fruit and broccoli leaves, while the lowest was in the palm and rice leaves. The increased consumption rate (CR) indicated that the larvae ate more parts of the baby corn and broccoli leaves. Furthermore, the increase in the relative consumption rate (RCR) on the treatment of baby corn fruit and broccoli leaves was in line with the high consumption rate (CR).
The value of low consumption rate correlates with low digestibility (AD), which also causes lower production of feces in palm leaves. Meanwhile, high digestibility (AD) values were shown in broccoli leaf and baby corn fruit treatment. In addition, larvae consuming baby corn fruit and broccoli leaves showed softer or watery feces, presumably some of the water that came out with feces.
The palm leaf treatment showed the lowest growth rate (GR), while the highest growth rate was in the baby corn fruit treatment. Furthermore, the growth rate value correlated with the relative growth rate (RGR). The growth rate is affected by the larvae consumption rate, where the low feed consumption caused less feed converted into biomass, therefore a lower growth rate. In line with Hwang et al. (2008), the suitability of larval feeds containing high nutrients increased the growth rate and development period more quickly than larvae fed with low nutrition diets.
The efficiency conversion of food ingested (ECI) showed the lower on palm leaf treatment, while the highest was in the rice leaf treatment. Likewise, the efficiency conversion of digested food (ECD) showed the lower on broccoli leaf treatment, while the highest was on the corn leaf treatment. Thus, high ECI and ECD values indicate that the feed effectively converts ingested and digested feed into body biomass.
The low ECD and ECI values in the treatment of baby corn fruit and broccoli leaf were thought to contain secondary metabolites in the feed, therefore the larvae compensated. In line with (Rahman & Rosli, 2014), the content of polyphenols and flavonoids in baby corn fruit is higher than that of ripe corn, while broccoli leaf is thought to be due to the presence of secondary plant metabolites in the form of glucosinolate compounds. Furthermore, it is also in line with Li et al. (2000) that Plutella xylostella treated with artificial feed containing high glucosinate was toxic to larvae and reduced the relative growth rate (RGR).
The nutritional needs of various stages of larval development, and the unavailability of a variety of foods, the increase in the amount of food digested (ECD) and consumed (ECI) must be allocated in the metabolic process for optimal growth. (Simpson & Simpson, 1990). High ECI and ECD values indicate higher efficiency of ingested and digested food conversion into body biomass with a high increase in larval weight. Silva et al. (2017) also stated that different host plants affected the growth, weight gain, and efficiency of digested food conversion in S. frugiperda.
4. Discussion
S. frugiperda was tested on several host plants, including corn (leaf and fruit), broccoli, rice, and palm. The type of host plant has a significant effect on the development, survival, and reproduction of S. frugiperda. S. frugiperda reared on baby corn fruit feed showed faster larval development and reproduction and high egg fertility. In contrast, corn leaf feed treatment showed longer larval development time and low reproduction.
The larval development time in treatment of broccoli leaf was shorter than the corn and rice leaf feed treatment, in line with the higher female imago reproduction than corn leaves and rice leaves. At the beginning of the development of S. frugiperda, larvae treated with rice leaf feed showed high mortality, while in the oil palm leaf, the larvae did not develop as in the other four types of feed. In our study oil palm was not host for this insect. In Indonesia, Herlinda et al., 2022 report that in feeding test S. frugiperda can eat oil palm leaf. Unfortunately Herlida’s research observation does not continued until the insect develop to imago, so that it can not be concluded the oil palm suitable for growth and development for this insect pest. Basaed on literature study, Montezano et al. (2018) report that S. frugiperda is find on oil palm, but no report the insect survival on oil palm. So that, the term of host plant must be difined acuratelly and carefully.
Differences in the length of development, survival, and reproduction are thought to be due to secondary metabolites, plant characteristics, and poor nutritional content. For example, in leaf of corn plants contain abundant benzoxazinoid as a chemical defense against herbivores (Wouters et al., 2014). This allows the effect of development time of larvae on corn leaf feed longer than baby corn fruit due to the presence of secondary metabolites in the leaf.
The secondary metabolite content in rice includes oxalic acid, tricin, schaftoside, and apigenin-C-glycoside compounds, which function as deterrence, antifeeding, and are toxic to brown planthoppers (Iswanto et al., 2016). The stunted development of S. frugiperda larvae due to disturbed feeding activity resulted in lower pupal weight loss and affected egg reproduction in rice leaf but did not directly affect the length of larval development. This was thought to be due to adjustments to rice plants' nutritional content and secondary metabolites.
The high mortality in the early stages and the failure of larvae to reach the pre-adult stage are thought to be due to an imbalance in the composition of nutrients and secondary metabolites found in a palm leaf. In line with Nurhajijah (2018), palm leaves contain high levels of fiber, lignin, and ash content, which have antifeedant properties for Spodoptera litura.
Differences in plant characteristics such as water content in baby corn fruit with higher water content (90.57%) than the four types of feed used, namely broccoli leaf (85.24%), palm leaf (84.69%), corn leaf (80.75%), and rice leaf (71.32%) will affect S. frugiperda. Furthermore, in line with Wei et al. (2000), the higher water content in a feed will positively correlate herbivorous insects for host preference.
It is essential to understand the nutritional status of the different plant food types as herbivorous insect preferences. The different nutritional requirements at the early and late stages of larval development correspond to the damage caused. This is also supported by the study of S. frugiperda life table in our research, which shown that corn and broccoli plants were suitable for the development of S. frugiperda. However, the high mortality of larvae in the early stages of rice and oil palm indicates that these plants are unsuitable as host plants.
The suitability of host plant for larval feed which contains high nutrients, increased the growth rate and development period more quickly than larvae fed with low nutrition feeds (Hwang et al., 2008). Result of this experiment showed that corn and broccoli had a significant effect on the survival, development, and reproduction of S. frugiperda. In general, shorter development time and high reproduction represent higher suitability of host plant. The test results showed that broccoli plants were first reported to be suitable for the survival of S. frugiperda. So far, the Broccoli plant (Brassica oleracea L. var. Italica) has never been reported as a host for this insect. As an implication, S. frugiperda has the potential to become a pest on other vegetable plants. Result of this research in line with Wang et al. (2020) state that old larvae very voracious and can cause serious lose on Chinese Cabbage (Brassica pekinensis (Lour.) Rupr) var. Qinza 2] (Brassicaceae) althought young larvae has hight mortality and only produce of 5.3% imago with sex ratio of 2:1 (male:female).
5. Conclusion
Different types of feed affect developmental time, imago life span, fecundity, and fertility of S. frugiperda, therefore it affects higher net reproductive value (R0), intrinsic growth rate (r), gross reproduction rate (GRR), mean generation period (T), and population doubling time (D.T.) were shorter in corn fruit and broccoli leaf than in corn, rice, and oil palm leaves. Furthermore, the effect of nutritional quality on several feed types tested provided information that when there was no corn plant, broccoli becomes the preferred host for S. frugiperda. The implication of the research is S. frugiperda can be a potential pest on other vegetable plants in the future.
Author contribution
The authors contributed equal for this research work.
Acknowledgements
The research was funded by Universitas Padjadjaran Internal Grant Program (HIU) through the Research of Doctor Disertation (Numbers : 1427/UN6.3.1/LT/2020 and 1595/UN6.3.1/PT.00/2021) with Danar Dono as principal investigator.
Conflicts of Interest
All author writers who play a role in research, funding, and writing this manuscript actually state that they have no conflict of interest.
References
- Barros, E.M., Torres, J.B., Ruberson, J.R., Oliveira, M.D., 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata 137: 237–245.
- Birch, L.C. 1948. The intristic rate of natural increase of an insect population. The Journal of Animal Ecology. 17:15–26.
- CABI. 2020. Invasive Species Compendium. Wallingford. U.K.: CAB International. www.cabi.org/isc.
- Chapman, R.F [ed.]. 2013. The Insects: Structure and Function. Cambridge University Press, New York, USA.
- Goergen, G., Kumar, P.L., Sankung, S.B., Togola, A., Tamo, M., 2016. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae): a New Alien Invasive Pest in West and Central Africa. Journal P One. [CrossRef]
- Hasyim, A., Setiawati, W., Murtiningsih R., & Sofiari R., 2010. Effication and persistance of Cymbopogon oil as biopesticide against Helicoverpha armigera Hubn. (Lepidoptera: Noctuidae). J. Hort. 20(4):377-386.
- Hasyim, A., Setiawati, W., Murtiningsih, R., 2013. Calling behavior of female moths and evaluation of male moth response to sex pheromone gland extract in red chili plants. J Hort. 23(1): 72-79.
- Hwang, S.Y., Li, C.H., Shen, T.C., 2008. Effects of plant nutrient availability and host plant species on the performance of two Pieris butterflies (Lepidoptera: Pieridae). Biochemical Systematics and Ecology, 36(7): 505–513. [CrossRef]
- Iswanto, E.H., Praptana, R.H., Guswara, A., 2016. Role Rice Secondary Metabolites to Brown Planthopper (Nilaparvata lugens) Resistance. Iptek Tanaman Pangan, 11 (2): 127-132.
- Johnson S.J., 1987. Migration and life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. International Journal of Tropical Insect Science. 8: 543- 549.
- Kakde, A.M., Patel K.G., Tayade S., 2014. Role of life table in insect pest management-A review. IOSRJAVS 7: 40-43. [CrossRef]
- Li, Q., Eigenbrode, S.D., Stringam G.R., Thiagarajah, M.R., 2000. Feeding and Growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with Varying Glucosinolate Concentrations and Myrosinase Activities. J Chem Ecol 26: 2401–2419. [CrossRef]
- Li, X.J., Wu, M.F., Ma, J., Gao, B.Y., Wu, Q.L., Chen, A.D., Liu, J., Jiang, Y.Y., Zhai, B.P., Early, R., Chapman J.W., Hu, G., 2020. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest management science, 76(2): 454–463. [CrossRef]
- Maharani, Y., Dewi, V.K., Puspasari, L.T., Rizkie, L., Hidayat Y., Dono, D., 2019. Cases of Fall Army Worm Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) Attack on Maize in Bandung, Garut, and Sumedang District, West Java. Cropsaver. 2(1): 38-46. [CrossRef]
- Montezano, D.G., Specht, A., Sosa-Gómez, D.R., Roque-Specht, V.F., Sousa-Silva, J.C., Paula-Moraes, S.V., Peterson, J.A., Hunt, T.E. 2018. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas," African Entomology, 26(2): 286-300.
- Ning, S., Zhang, W., Sun, Y. & Feng, J. 2017. Development of insect life tables: comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. www.nature. com/scientificreports. Published: 06 July 2017. [CrossRef]
- Nonci, N., Kalqutny, S.H., Mirsam, H., Muis, A., Azrai, M., Aqil, M. 2019. Introduction of Fall Armyworm (Spodoptera frugiperda J.E. Smith) a New Pest on Corn Plants in Indonesia. Jakarta: Indonesian Ministry of Agriculture, Research centre of Cerealia. Balai Penelitian Tanaman Serealia. 64 p.
- Nurhajijah, 2018. Preferences and biology of Spodoptera litura (Lepidoptera: Noctuidae) on legumes, oil palm plantations on peat and minerals in the laboratory. [Thesis] Departemen agroteknologi. UniversitasSumatera Utara.
- Prasanna, B.M., Huesing, J.E., Eddy, R., Peschke, V.M., 2018. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: Mexico.
- Rahman, N.A., Rosli, W.I.W., 2014. Nutritional compositions and antioxidative capacity of the silk obtained from immature and mature corn. Journal of King Saud University - Science, 26, Issue 2. [CrossRef]
- Roy, N., 2015. Life table and population parameters of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae) on jute, Chorchorus capsularis (cv. Sonali; JRC-321), leaves. International Journal of Fauna and Biological Studies, 2: 23-29.
- Sharanabasappa, Kalleshwaraswamy, C.M., Asokan, R., Mahadevaswamy, H.M., Maruthi, M.S., Pavithra, H.B., 2018. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Management in Horticultural Ecosystems, 24(1):23-29.
- Silva, D.M.D.,. Bueno, A.D.F., Andrade, K., Stecca C.D.S., Neves, P.M.O.J., Oliveira, M.C.N.D., 2017. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Scientia Agricola, 74(1), 18-31.
- Simpson, S.J., Simpson, C.L., 1990. The mechanism of nutritional compensation by phytophagus insect. Pages 111-160. In: Insect-Plant Interaction. Vol.2. CRC Press, Florida.
- Smith, J.E., Abbott, J., 1797. The natural history of the rarer lepidopterous insects of Georgia. V. 2 illus. London.
- Subramanian, S., Mohankumar, S., 2006. Genetic variability of the bollworm, Helicoverpa armigera, occurring on different host plants. Journal of Insect Science. [CrossRef]
- Trisyono Y.A., Suputa, Aryuwandri, V.E.F., Hartaman, M., Jumari, 2019. Occurrence of Heavy Infestation by the Fall Armyworm Spodoptera frugiperda, a New Alien Invasive Pest, in Corn in Lampung Indonesia. Jurnal Perlindungan Tanaman Indonesia.
- Wang, W., He, P., Zhang, Y., Liu, T., Jing, X., Zhang, S., 2020. The Population Growth of Spodoptera frugiperda on Six Cash Crop Species and implications for Its Occurrence and Damage Potential in China. Insects, 11, 639. [CrossRef]
- Wei, J., Zou L., Kuang R.P., He, L., 2000. Influence of leaf tissue structure on host feeding selection by pea leafminer Liriomyza huidobrensis (Diptera: Agromyzidae). Zool. Studies 39 (4): 295 – 300.
- Wouters, F.C., Reichelt, M., Glauser, G., Bauer, E., Erb, M., Gershenzon, J., Vassão, D.G., 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Zuschriften Chemical Ecology 126: 11502–1. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).