Submitted:
30 March 2023
Posted:
31 March 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
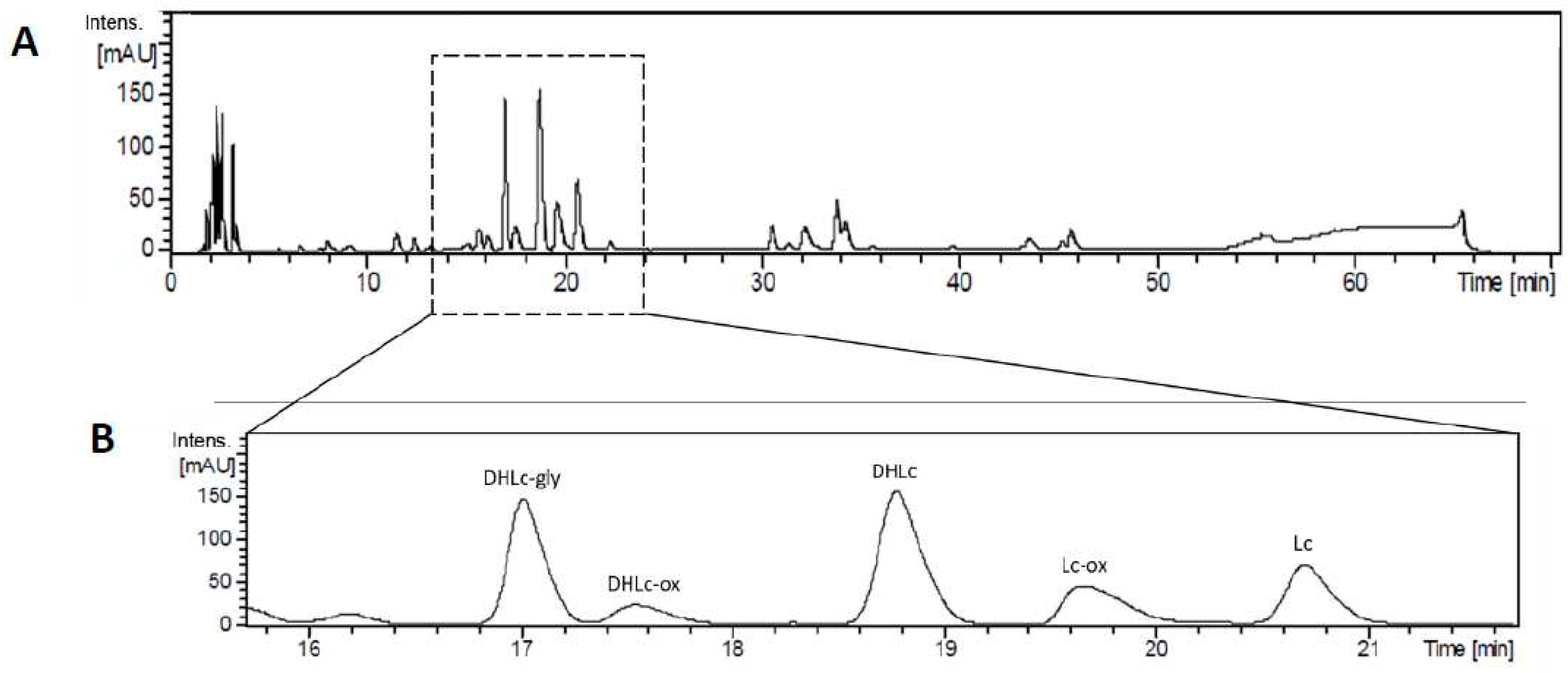
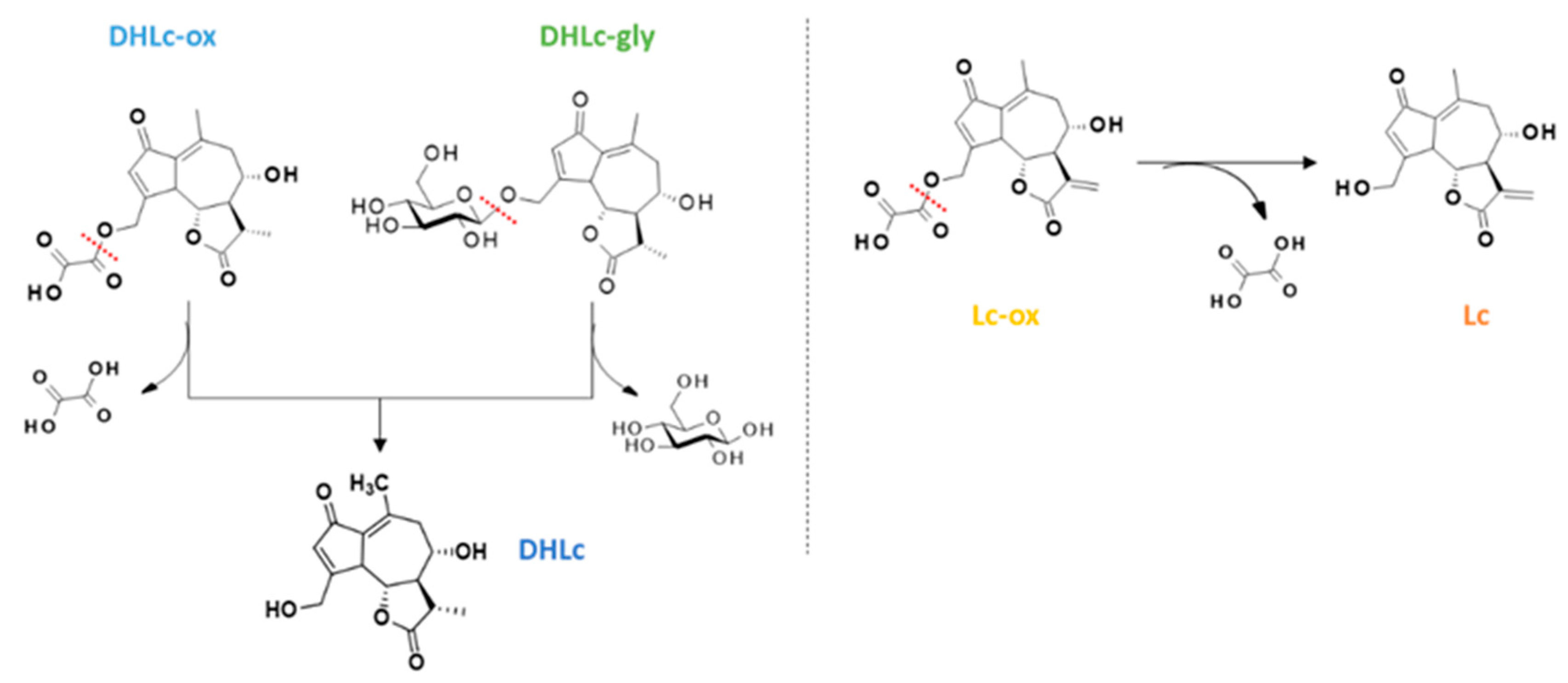
2.1. Identification of the Main STLs
2.2. Method Development
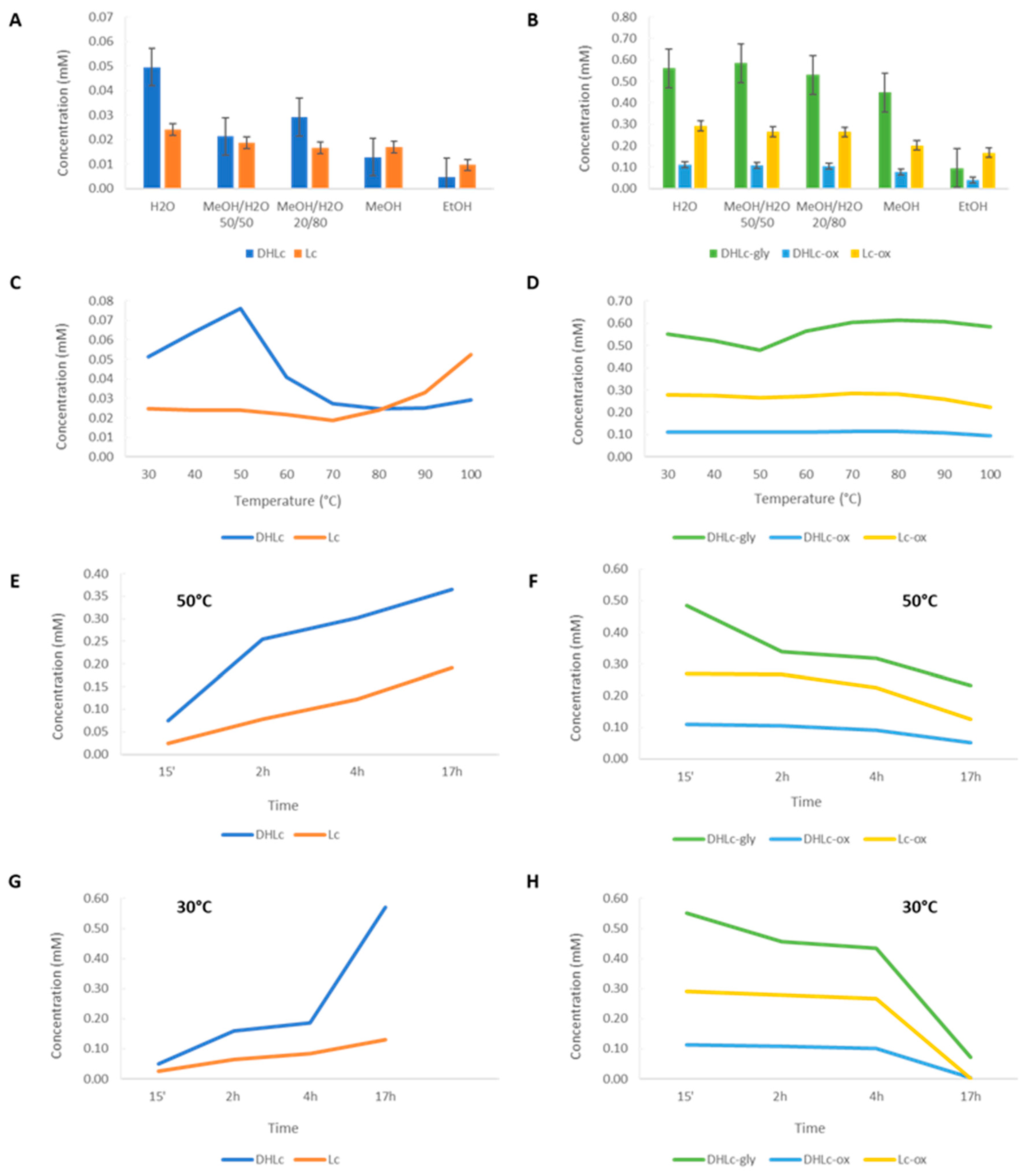
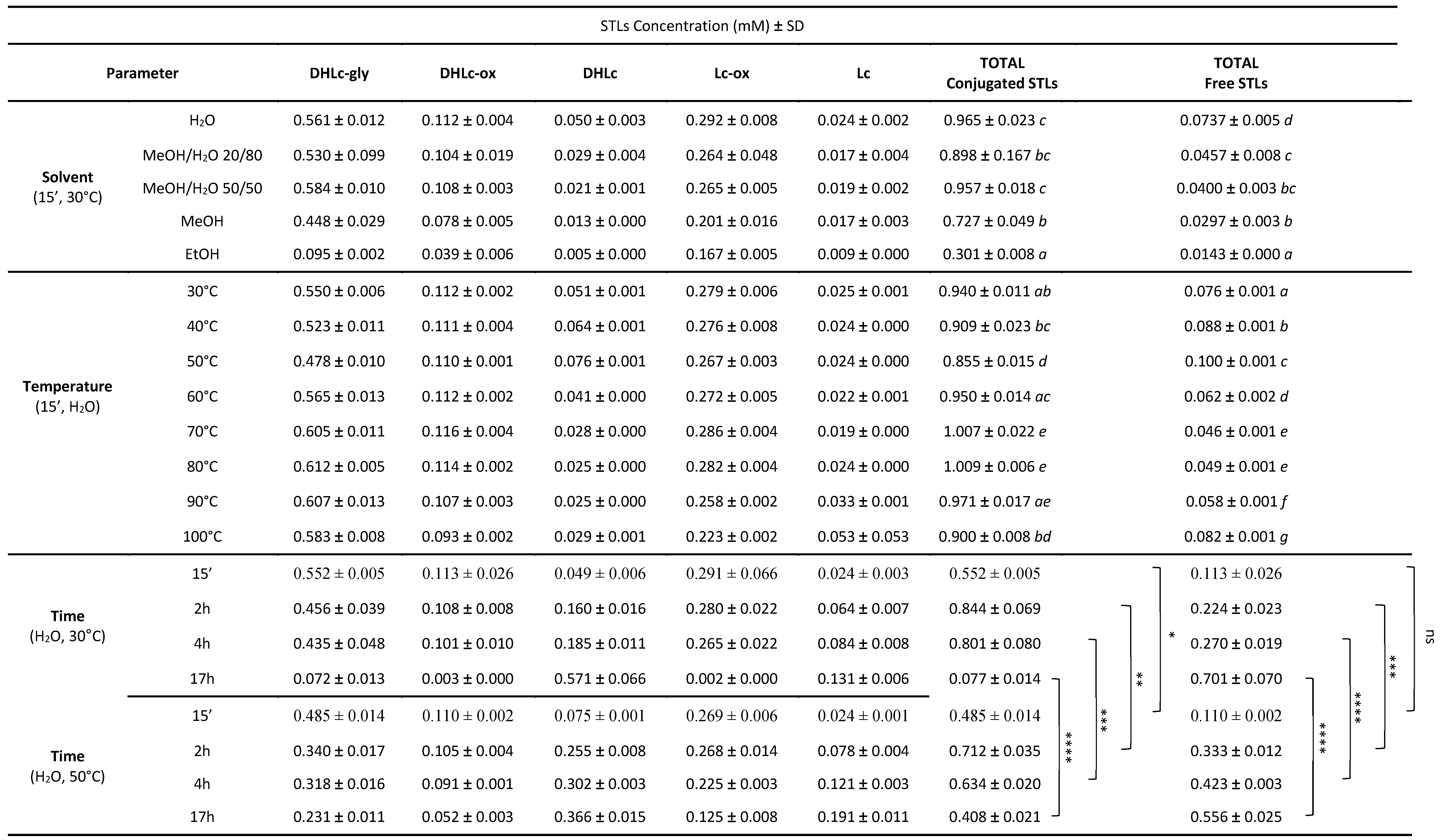
2.2.1. Extraction Conditions Determination
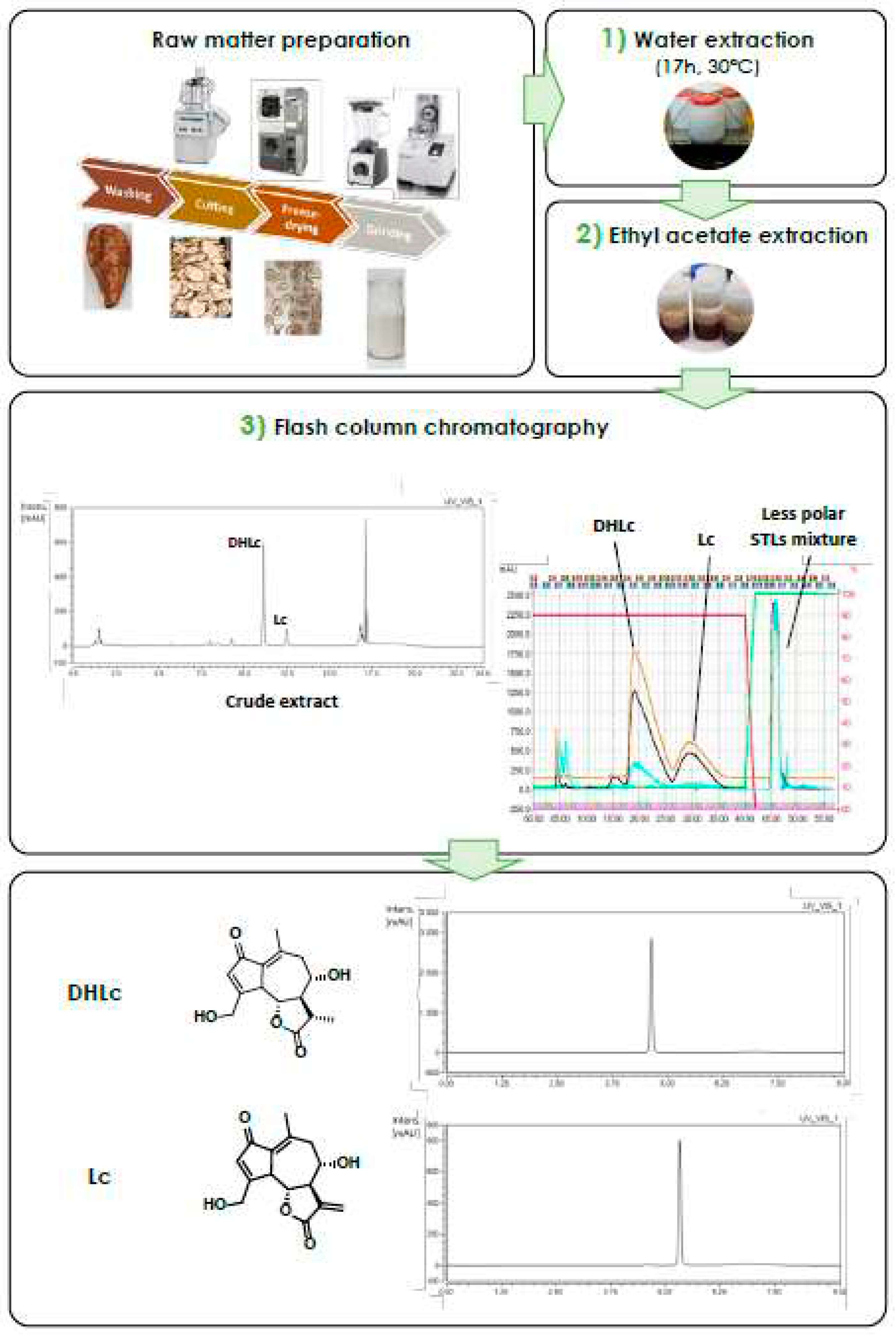
2.3. Scaling-up
| STLs Concentration (mM) ± SD | ||
| DHLc | Lc | |
| Initial H2O | 0.571 ± 0.066 | 0.134 ± 0.005 |
| EtOAc 1 | 0.345 ± 0.010 | 0.081 ± 0.002 |
| EtOAc 2 | 0.153 ± 0.004 | 0.033 ± 0.003 |
| EtOAc 3 | 0.066 ± 0.000 | 0.013 ± 0.011 |
| FinalH2O | 0.004 ± 0.016 | 0.005 ± 0.003 |
2.4. Analytical Validation of STLs
| DHLc: (CD3)2SO | Lc: (CD3)2SO | |||
| Position | 1H (mult, J Hz) | 13C | 1H (mult, J Hz) | 13C |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 |
- - 6.27 (s) - 3.76–3.64 (m) 3.76–3.64 (m) 2.17–2.05 (m) 3.61–3.49 (m) 9: 2.74–2.55 (m) 9’: 2.24 (dd, 13.6, 1.8) - 2.74–2.55 (m) - 1.25 (d, 7.0) 2.33 (s 15: 4.64 (ddd, 18.8, 5.8, 1.9) 15’: 4.22 (ddd, 18.9, 4.9, 1.5) |
132.0 194.9 132.5 175.8 48.4 80.9 60.5 68.4 48.7 147.5 40.9 178.3 15.7 21.5 61.8 |
- - 6.28 (d, 1.0) - 3.83–3.67 (m) 3.83–3.67 (m) 3.12–3.03 (m) 3.83–3.67 (m) 9: 2.74–2.55 (m) 9’: 2.24 (dd, 13.6, 1.8) - - - 13: 6.13 (dd, 3.0, 1.5) 13’: 6.01 (dd, 3.2, 1.5) 2.33 (s 15: 4.66 (d, 18.7) 15’: 4.25 (d, 18.8) |
132.3 194.8 132.7 169.2 48.3 81.1 56.8 66.9 48.8 147.0 138.4 175.4 121.9 21.5 61.8 |
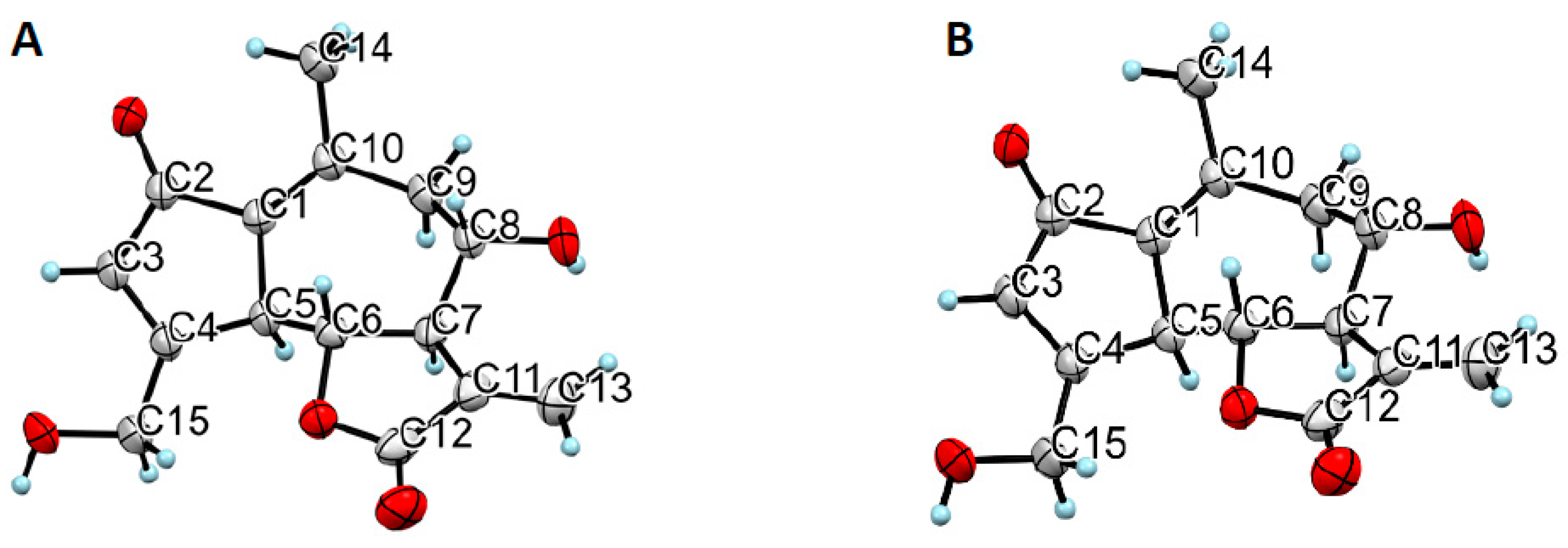
2.5. Isolation of 11β,13-dihydrolactucin-glycoside
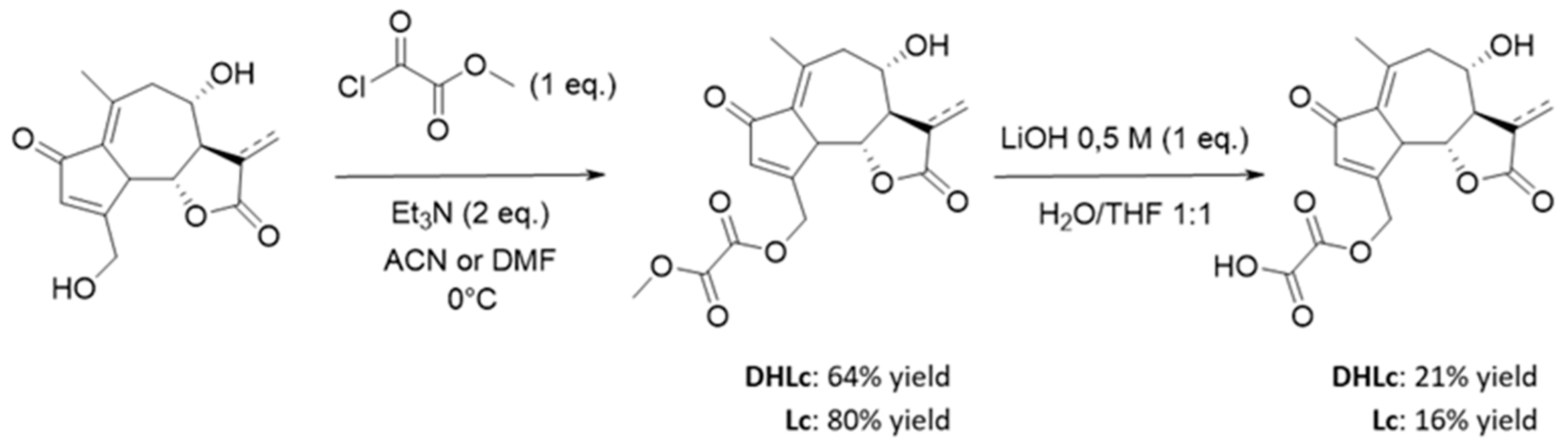
2.6. Synthesis of STLs standards
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Reagents and Chemicals
4.3. Method Development
4.3.1. Extraction of STLs From Freeze-dried Chicory Roots
4.3.2. Scaling-up
4.5. STLs Analysis
4.5.1. Quantification of STLs by UPLC-DAD
4.5.2. LC-QTOF-HRMS Analysis of STLs
4.5.3. NMR analysis
4.5.4. X-ray Structural Determination
4.6. Chemistry
4.6.1. Chemical Synthesis
4.7. Statistical Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
Bibliographic References
- Padilla-Gonzalez:, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene Lactones: More Than Protective Plant Compounds With High Toxicity. Critical Reviews in Plant Sciences 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Maas, P.; King, B.M.; Leclercq, E.; Voragen, A.G.J.; De Groot, A. Bitter Sesquiterpene Lactones from Chicory Roots. J. Agric. Food Chem. 1990, 38, 1035–1038. [Google Scholar] [CrossRef]
- Graziani, G.; Ferracane, R.; Sambo, P.; Santagata, S.; Nicoletto, C.; Fogliano, V. Profiling Chicory Sesquiterpene Lactones by High Resolution Mass Spectrometry. Food Research International 2015, 67, 193–198. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.F. Sesquiterpene Lactones: More Than Protective Plant Compounds With High Toxicity. 21.
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. Journal of Analytical Methods in Chemistry 2015, 2015, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Elsevier, 2006; Vol. 33, pp. 309–392 ISBN 978-0-444-52717-2.
- Häkkinen, S.T.; Nohynek, L.; Ivanov, M.; Tsitko, I.; Matos, M.; Baixinho, J.P.; Ivasiv, V.; Fernández, N. Chicory Extracts and Sesquiterpene Lactones Show Potent Activity against Bacterial and Fungal Pathogens. 2021, 23.
- Wang, X.; Liu, M.; Cai, G.H.; Chen, Y.; Shi, X.C.; Zhang, C.C.; Xia, B.; Xie, B.C.; Liu, H.; Zhang, R.X.; et al. A Potential Nutraceutical Candidate Lactucin Inhibits Adipogenesis through Downregulation of JAK2/STAT3 Signaling Pathway-Mediated Mitotic Clonal Expansion. Cells 2020, 9, 331. [Google Scholar] [CrossRef]
- Imam, K.M.S.U.; Tian, Y.; Xin, F.; Xie, Y.; Wen, B. Lactucin, a Bitter Sesquiterpene from Cichorium Intybus, Inhibits Cancer Cell Proliferation by Downregulating the MAPK and Central Carbon Metabolism Pathway. Molecules 2022, 27, 7358. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial Activity of Lactucin and Lactucopicrin: Sesquiterpene Lactones Isolated from Cichorium Intybus L. Journal of Ethnopharmacology 2004, 95, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and Sedative Activities of Lactucin and Some Lactucin-like Guaianolides in Mice. Journal of Ethnopharmacology 2006, 107, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Silva, P. LEISHMANICIDAL, ANTI-INFLAMMATORY AND ANTI-OBESITY PROPERTIES OF NATURAL PRODUCTS FROM COMMON MEDICINAL AND EDIBLE PLANTS. 135. [CrossRef]
- Jaśkiewicz, A.; Budryn, G.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Evaluation of Activity of Sesquiterpene Lactones and Chicory Extracts as Acetylcholinesterase Inhibitors Assayed in Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 3633. [Google Scholar] [CrossRef]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite Profiling of Sesquiterpene Lactones from Lactuca Species. Journal of Biological Chemistry 2000, 275, 26877–26884. [Google Scholar] [CrossRef]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.-F.; Hilbert, J.-L. A Method for the Simultaneous Determination of Chlorogenic Acid and Sesquiterpene Lactone Content in Industrial Chicory Root Foodstuffs. The Scientific World Journal 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Milala, J.; Grzelak, K.; Król, B.; Juśkiewicz, J.; Zduńczyk, Z. COMPOSITION AND PROPERTIES OF CHICORY EXTRACTS RICH IN FRUCTANS AND POLYPHENOLS.
- Agriculturae Conspectus Scientificus. 2014, 79.
- Deng, Y.; Scott, L.; Swanson, D.; Snyder, J.K.; Sari, N.; Dogan, H. Guaianolide Sesquiterpene Lactones From Cichorium Intybus (Asteraceae) [1]. Zeitschrift für Naturforschung B 2001, 56, 787–796. [Google Scholar] [CrossRef]
- Michalska, K.; Szneler, E.; Kisiel, W. Complete NMR Spectral Assignments of Two Lactucin-Type Sesquiterpene Lactone Glycosides from Picris Conyzoides: Sesquiterpene Lactone Glycosides from Picris Conyzoides. Magn. Reson. Chem. 2011, 49, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Zheng, G.; Zhang, Q.; Jin, P.; Zhang, H.; Su, L.; Qin, D.; Yao, G. Sesquiterpenoids with Diverse Carbon Skeletons from the Roots of Cichorium Glandulosum and Their Anti-Inflammatory Activities. Fitoterapia 2019, 136, 104170. [Google Scholar] [CrossRef] [PubMed]
- Ruban, G.; Zabel, V.; Gensch, K.H.; Smalla, H. The Crystal Structure and Absolute Configuration of Lactucin. Acta Crystallogr B Struct Sci 1978, 34, 1163–1167. [Google Scholar] [CrossRef]
- Escobar-Ledesma, F.R.; Sánchez-Moreno, V.E.; Vera, E.; Ciobotă, V.; Jentzsch, P.V.; Jaramillo, L.I. Extraction of Inulin from Andean Plants: An Approach to Non-Traditional Crops of Ecuador. Molecules 2020, 25, 5067. [Google Scholar] [CrossRef] [PubMed]
- 11beta,13-Dihydrolactucin 3810, CAS 83117-63-9 - SESQUITERPENE. Available online: https://www.extrasynthese.com/sesquiterpene/290-11beta13-dihydrolactucin.html (accessed on 5 March 2023).
- Leclercq, E.; Netjes, J.J. Release of Sesquiterpene Lactones by Enzymatic Liquefaction of Chicory Roots. Z Lebensm Unters Forch 1985, 181, 475–477. [Google Scholar] [CrossRef]
- Baixinho, J.P.; Anastácio, J.D.; Ivasiv, V.; Cankar, K.; Bosch, D.; Menezes, R.; de Roode, M.; dos Santos, C.N.; Matias, A.A.; Fernández, N. Supercritical CO2 Extraction as a Tool to Isolate Anti-Inflammatory Sesquiterpene Lactones from Cichorium Intybus L. Roots. Molecules 2021, 26, 2583. [Google Scholar] [CrossRef]
- Ajiaikebaier, A.; Wei, Z.; Sun, G. Preparation Method of Lactucin, CN111689935A, 2020.
- Preparation Method and Application of Lactucin Extracted from Cichorium Intybus, CN112939912A, 2021.
- Lactucin and Its Preparation Method and Application, CN101099566A, 2008.
- Qin, D.; Zou, N.; Cai, G.; Wang, S.; Su, L.; Dang, T. Lactucin and Application Thereof as Anti-Inflammatory Component, CN112079804A, 2020.
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin Microbiol Rev 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr A Found Adv 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr A Found Crystallogr 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J Appl Crystallogr 2009, 42, 339–341. [Google Scholar] [CrossRef]
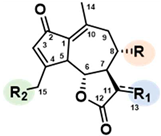 |
R | R1 | R2 | |
| Lactucin | OH | CH2 | OH | |
| Lactucin-15-oxalate | OH | CH2 | OCOCOOH | |
| Lactucin-15-glycoside | OH | CH2 | OGlucose | |
| 11β,13-dihydrolactucin | OH | CH3 | OH | |
| 11β,13-dihydrolactucin-15-oxalate | OH | CH3 | OCOCOOH | |
| 11β,13-dihydrolactucin-15-glycoside | OH | CH3 | OGlucose | |
| 8-deoxylactucin | H | CH2 | OH | |
| 8-deoxylactucin-15-oxalate | H | CH2 | OCOCOOH | |
| 8-deoxylactucin-15-glycoside | H | CH2 | OGlucose | |
| 11β,13-dihydro-8-deoxylactucin | H | CH3 | OH | |
| 11β,13-dihydro-8-deoxylactucin-oxalate | H | CH3 | OCOCOOH | |
| 11β,13-dihydro-8-deoxylactucin-glycoside | H | CH3 | OGlucose | |
| Lactucopicrin |  |
CH2 | OH | |
| Lactucopicrin-15-oxalate | CH2 | OCOCOOH | ||
| Lactucopicrin-15-glycoside | CH2 | OGlucose | ||
| 11β,13-dihydrolactucopicrin | CH3 | OH | ||
| 11β,13-dihydrolactucopicrin-15-oxalate | 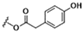 |
CH3 | OCOCOOH | |
| 11β,13-dihydrolactucopicrin-15-glycoside | CH3 | OGlucose |
| Compound | Molecular weight | Retention time (min) | ESI major fragment ions (m/z) |
|---|---|---|---|
| DHLc-gly | 440 | 16.9 | 463 (100) [M+Na]+; 441 (13) [M+H]+; 279 (18) [M-glycosyl+H]+; 261 (9) [M-glycosyl- H2O+H]+; 243 (10) [M-glycosyl-2H2O+H]+; 215 (8) [M-glycosyl- H2O-HCO2H+H]+ |
| DHLc-ox | 350 | 17.7 | 373 (57) [M+Na]+; 351 (100) [M+H]+; 261 (94) [M-oxaloyl- H2O+H]+; 243 (56) [M-oxaloyl-2H2O+H]+; 215 (39) [M-oxaloyl-H2O-HCO2H+H]+ |
| DHLc | 278 | 18.8 | 301 (100) [M+Na]+; 279 (59) [M+H]+; 261 (7) [M- H2O+H]+; 243 (8) [M-2H2O+H]+; 215 (17) [M-HCO2H-H2O+H]+ |
| Lc-ox | 348 | 19.7 | 371 (73) [M+Na]+; 349 (86) [M+H]+; 259 (100) [M-oxaloyl-H2O+H]+; 241 (96) [M-oxaloyl-2H2O+H]+; 213 (60) [M-oxaloyl-H2O-HCO2H+H]+ |
| Lc | 276 | 20.7 | 299 (100) [M+Na]+; 277 (44) [M+H]+; 259 (8) [M– H2O+H]+; 241 (17) [M–2H2O+H]+; 213 (18) [M-HCO2H-H2O+H]+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





