1. Introduction
Chronic kidney disease (CKD) is a common disease worldwide and a leading cause of mortality and morbidity in older cats. Advanced disease is characterized by incurable kidney function decline and poor prognosis (1, 2). Cardiac function can be impaired by the presence of decreased kidney function in cats, which is known as cardiovascular-renal disorders (CvRD) (3); a subset of cats with CKD can die from heart failure. In addition, the complications of feline CKD include hypertension, proteinuria, hypokalemia, hyperphosphatemia, urinary tract infections, anemia, and CKD-related mineral bone disorders (1, 2). Hyperthyroidism (4) and diabetes mellitus (5) also occur as co-morbidities. All such disorders can also contribute to early mortality. Therefore, the overall survival, based on all-cause death including complications and co-morbidities, is the most important outcome for treatment response.
Vascular endothelial function is systemically impaired from an early stage of CKD. At the same time, endothelial damage causes a progressive loss of renal microvasculature in the tubulointerstitium, which leads to local hypoxia, induction of pre-fibrotic responses, scarring, and deterioration of renal function (6, 7). Although there is no direct evidence in cats with CKD, altered endothelial function in cats with CKD can be predicted given increased plasma asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide (NO) production (8). Beraprost is a prostacyclin analogue that has a protective effect on injured endothelial cells (9). Beraprost induces endothelial NO synthase (eNOS) and increases NO production in vascular endothelial cells (mouse, human and bovine) to improve endothelial function (10), and enhances hepatocyte growth factor expression to provide endothelial cytoprotective effects in a diabetic rat model (11). In preclinical models of CKD, beraprost has been shown to inhibit reduction in renal function and improve survival in nephritic (12) and partially nephrectomized rats (13).
In a randomized, double-blind, placebo-controlled trial, beraprost was reported to suppress the deterioration of renal function, as measured by increased serum creatinine in cats with International Renal Interest Society (IRIS) stages 2 and 3 CKD. Beraprost also showed positive effects on appetite, dehydration, owner-reported quality of life (QOL) evaluation, and overall veterinary evaluation of the treatment effect; however, in that trial, efficacy for delaying disease progression or prolonging survival was not evaluated, standard therapy was partly restricted, and the cats with coexisting disorders were excluded (14).
The purpose of this retrospective cohort study was to describe the relationship between beraprost and overall survival in cats with CKD in real clinical practice.
2. Materials and Methods
2.1. Case Selection
Medical records of the Ichikawa General Hospital, Kariya Animal Hospital Group were searched to identify all cats that had blood and urine collected for serum creatinine analysis (n = 1681) and urinalysis (n = 1399) between April 2017 and December 2020. In this hospital, all test results, death records, and medication records had been archived with an electronic medical record system Ahmics (PetCommunications Co., Ltd.) since before 2017. Cats were included in the study if their medical records confirmed a diagnosis of CKD, based on: elevated serum creatinine, determined on two separate occasions not less than 7 days apart; urine specific gravity < 1.035, with or without persistent proteinuria and other clinical pathology parameters; compatible history (e.g., polyuria polydipsia) and physical examination and/or diagnostic imaging findings (e.g., palpably small kidneys). Staging was undertaken according to the IRIS staging of CKD (modified 2019) (2). Data were collected on baseline characteristics: age, bodyweight, sex, neuter status, breed and therapies being used to manage the CKD. The distribution of IRIS stage and beraprost prescription in the CKD-diagnosed cats was shown in
Table 1. A pilot analysis of all CKD cats was performed on these data and the baseline clinical pathology results, to determine homogeneity of baseline characteristics between those treated with beraprost and those not, within each IRIS stage 1-4 (data not shown). IRIS stage 3 (n = 134, defined as cohort A) cats were well-balanced for most baseline characteristics and therefore only these cats were subjected to further analysis. Additionally, a subcohort of cohort A with baseline phosphate < 6.0 mg/dL was defined as cohort B.
2.2. Study Design
The start date for all time-to-event outcomes was the date of first documented prescription of beraprost (RAPROS®, Toray Industries, Inc.) in the beraprost therapy group, or staging results in no beraprost therapy group. Data on baseline characteristics and coexisting disorders were extracted on or before the start date. Progression of disease was defined in this study as a ≥ 25% increase in serum creatinine, compared to baseline, based on previously published data (15). Progression-free survival was calculated and defined as the time from the start date until the date of progression, death from any cause, euthanasia or last follow-up. Overall survival was measured from the start date until the date of death from any cause, euthanasia or last follow-up. The incidence of chronic disorders developing during the study was also assessed.
2.3. Data Analysis
Baseline characteristics, coexisting disorders and the incidence of chronic disorders were summarized and compared between the groups. Quantitative variables were characterized by their means, medians, 25th and 75th percentile [Q1 and Q3], and standard deviations, while the qualitative variables according to the percentage of cats. The differences between the groups were tested by Mann-Whitney U test or Welch’s t-test for quantitative variables, and Fisher’s exact test for qualitative variables. With regard to main-effect covariates: beraprost, other treatments (subcutaneous fluid therapy, renal diet, phosphate binder, oral activated charcoal, ACEI/ARB, calcium channel blocker, erythrocyte-stimulating agents, and agents used in managing inappetence, nausea and vomiting), and baseline characteristics (age, weight, creatinine, urea, phosphate, calcium, potassium and packed cell volume), multivariable Cox regression models were constructed using forward stepwise selection. Only covariates with a multivariate P < 0.50 for beraprost and other treatments, and P < 0.20 for beraprost and baseline characteristics, respectively, by Wald test were retained. The veracity of the proportional-hazards assumption in the models was assessed by inspecting log-log plots of adjusted survival curves for parallel lines, and no violation of the assumptions was observed. Progression-free survival and overall survival were assessed with the Kaplan-Meier method and compared with the log-rank test. Summary of events (disease progression, treatment discontinuation, death, euthanasia), progression-free survival at 1 year with SE and 95% CI, overall survival at 1 and 3 years with SE and 95% CI, and median progression-free and overall survival with SE and 95% CI were analyzed. In order to demonstrate consistency of the treatment effect in subgroups, progression-free and overall survival analyses were performed in which the hazard ratio and 95% CI were calculated using an unstratified Cox model, p values were determined by Wald test. A commercial statistical software program was used for all analyses (BellCurve for Excel; Social Survey Research Information Co., Ltd.). Statistical significance was set at 2-sided *P < 0.05, **P < 0.01.
3. Results
3.1. Baseline characteristics of all cats in cohort A
One hundred and thirty-four cats with IRIS stage 3 CKD were enrolled in the study (cohort A). Baseline characteristics are presented in
Table 2. The results demonstrate geriatric age (median, 15.3 years), frequently neutered (115 cats, 85.8%), mainly domestic shorthair breed (91 cats, 67.9%), increased creatinine (median, 3.3 mg/dL), urea (median, 42.0 mg/dL), decreased urine specific gravity (median, 1.014) at the time or baseline assessment, mostly receiving subcutaneous fluid therapy (106 cats, 79.1%), and agents for managing inappetence, nausea and vomiting (102 cats, 76.1%) during the study. At the time of the start date, coexisting disorders were present in 38 cats (28.4%), the most observed was hyperthyroidism (13 cats, 9.7%), and the second was congestive heart failure (10 cats, 7.5%). The number of cats prescribed beraprost was 57 cats (42.5%), and the mean dose of beraprost was 15.0 µg/kg twice daily (
Table 2).
3.2. Multivariable analyses of all cats in cohort A
In order to compare treatment effects on survival in the cohort A, multivariable Cox regression models were constructed. When all the treatments during the study were analyzed as covariates, three factors were identified which significantly affect disease progression; the hazard ratio (HR) of beraprost was 0.56 (P = 0.012), phosphate binder was 1.87 (P = 0.010), and erythrocyte-stimulating agents was 1.67 (P = 0.043). Also, two factors significantly influencing overall survival were found; HR of beraprost was 0.57 (P = 0.028) and phosphate binder was 1.91 (P = 0.024). The results indicated that among all the treatments, beraprost only was linked to better survival (HR < 1.00), and all others were associated with poorer prognosis (HR > 1.00) (
Table 3).
Next, to exclude other confounding factors that could influence the treatment effect of beraprost, a multivariable model was constructed from the baseline variables with beraprost as covariates. Three factors were identified which significantly correlate with disease progression; HR of beraprost was 0.59 (P = 0.028), urea was 2.17 (P = 0.002), and packed cell volume was 2.98 (P < 0.001). Likewise, four significant factors for overall survival were found; HR of beraprost was 0.49 (P = 0.008), age was 2.00 (P = 0.008), creatinine was 2.23 (P = 0.007), and phosphate was 3.06 (P = 0.007). HRs of the factors other than beraprost were > 1.00, associated with poorer prognosis. Collectively, these results demonstrated that beraprost was the only study treatment correlated significantly with improvement in progression-free and overall survival, respectively (
Table 4).
3.3. Baseline characteristics of the two groups in cohort A
To address the effects of beraprost on survival in further depth, cohort A (n = 134) was divided into the two groups: the beraprost therapy group (n = 57) and the no beraprost therapy group (n = 77), and a retrospective analysis was carried out. The groups were matched for the distribution over time; the median start date was July 3, 2018 in the beraprost therapy group and June 30, 2018 in the no beraprost therapy group. Baseline characteristics are presented in
Table 5. The results reveal that urea and phosphate were significantly higher in the no beraprost therapy (P < 0.01 by Mann-Whitney U test). In the previous study on the prognostic factors in cats with chronic kidney disease, when the subgroups with phosphate > 4.7 to ≤ 6.8 and ≤ 4.7 mg/dL were compared, higher phosphate was shown to be significantly (P < 0.001) associated with shorter renal survival time (16). Thus, it should be noted in the present study, the difference in baseline phosphate between the two groups could influence the survival analysis results of cohort A.
3.4. Survival analyses of the two groups in cohort A
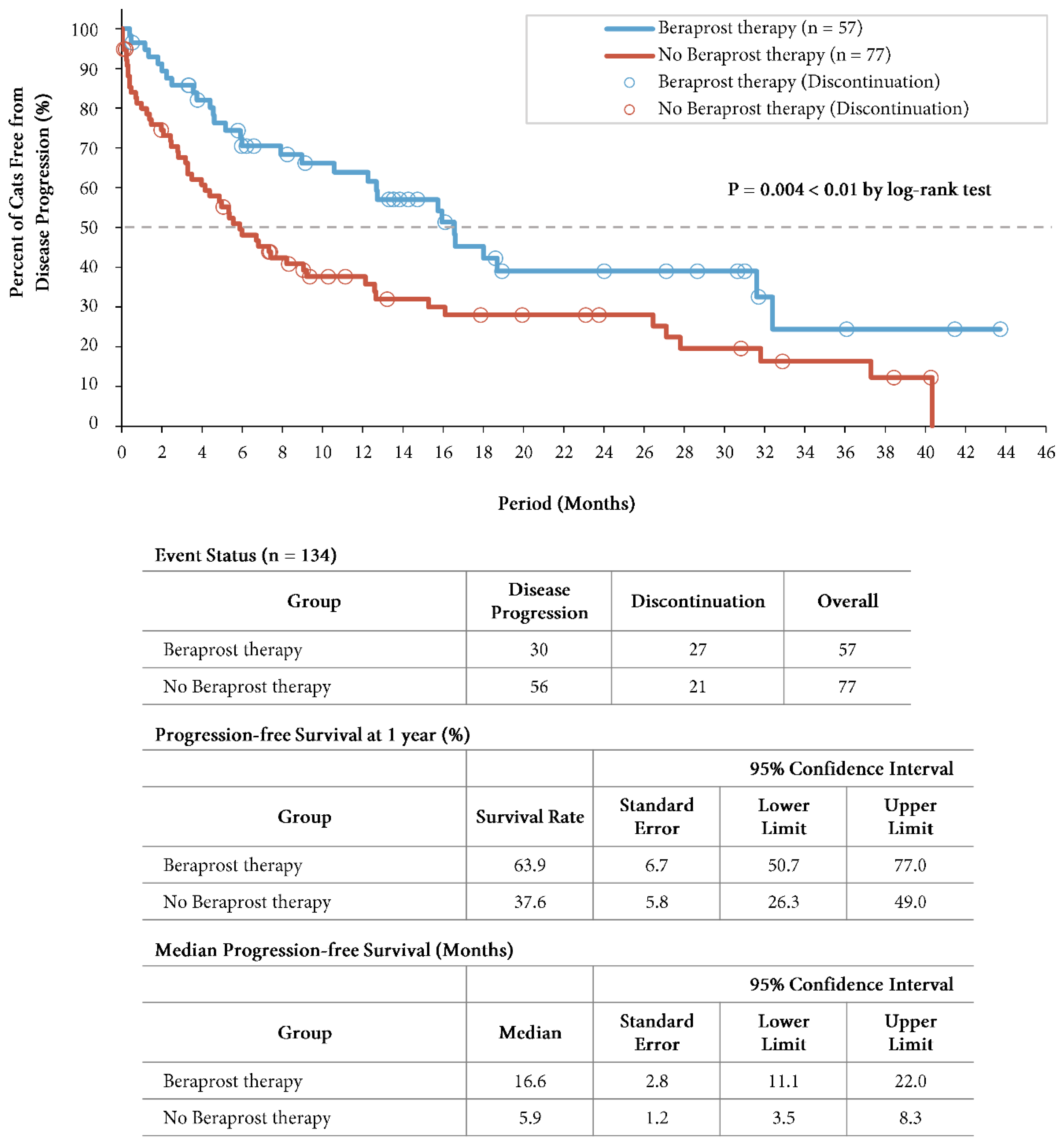
At the time of the final analysis of progression-free survival, the mean duration of follow-up was 10.9 months (13.2 months in the beraprost therapy group and 9.3 months in the no beraprost therapy group). A total of 30 cats (52.6%) in the beraprost therapy group and 56 (72.7%) in the no beraprost therapy group progressed. Progression-free survival was significantly longer with beraprost therapy (P = 0.004 by log-rank test); the estimated rates of progression-free survival at 1 year was 63.9% (SE, 6.7, 95% CI, 50.7 to 77.0) in the beraprost therapy group, and 37.6% (SE, 5.8, 95% CI, 26.3 to 49.0) in the no beraprost therapy group. The median progression-free survival was 16.6 months (SE, 2.8, 95% CI, 11.1 to 22.0) in the beraprost therapy group, and 5.9 months (SE, 1.2, 95% CI, 3.5 to 8.3) in the no beraprost therapy group (
Figure 1).
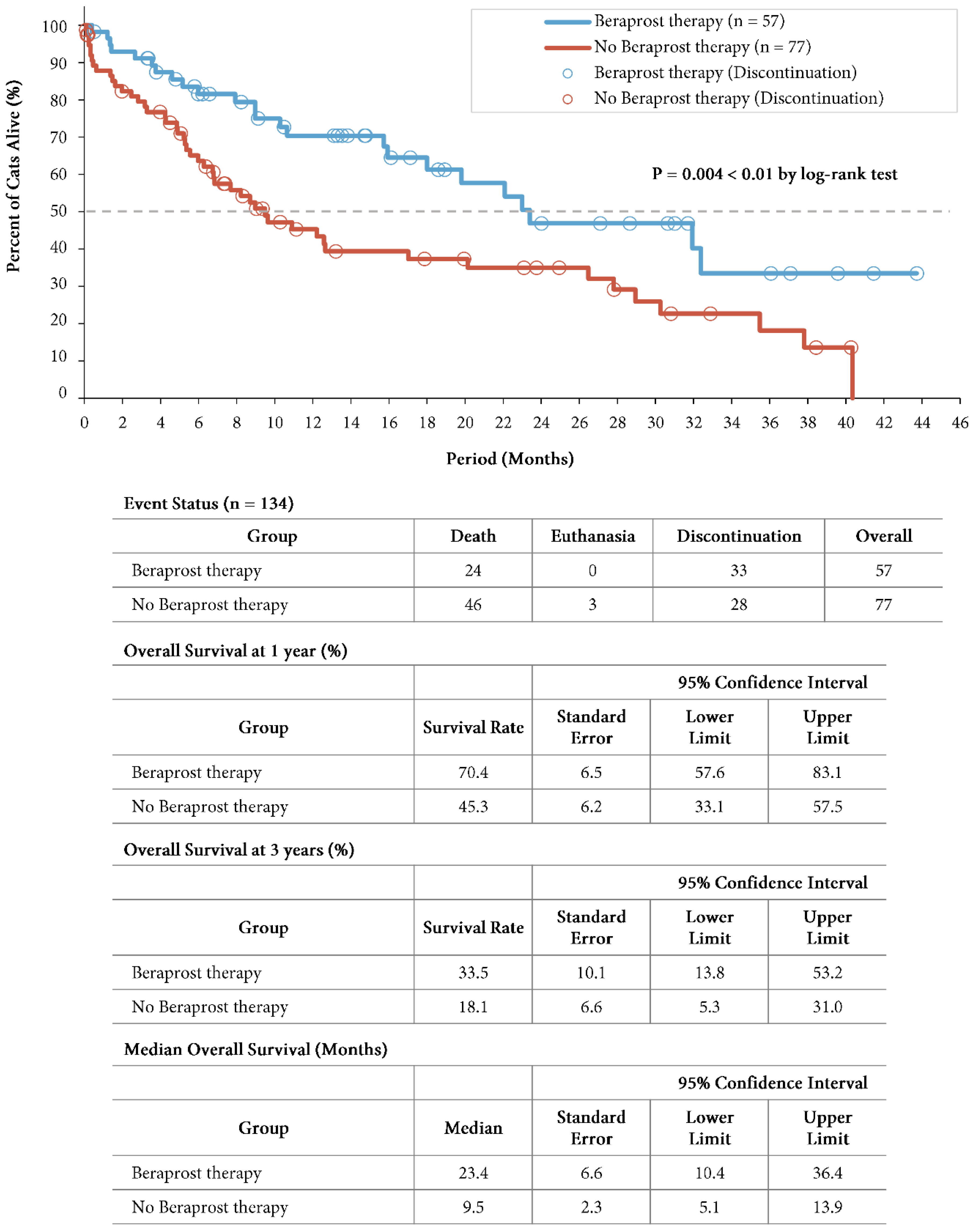
A total of 24 cats (42.1%) in the beraprost therapy group and 49 (63.6%) in the no beraprost therapy group died, which included 3 cats euthanized in the no beraprost therapy group. Overall survival was significantly longer with beraprost therapy; the estimated rates of overall survival at 1 year was 70.4% (SE, 6.5, 95% CI, 57.6 to 83.1) in the beraprost therapy group, and 45.3% (SE, 6.2, 95% CI, 33.1 to 57.5) in the no beraprost therapy group, and at 3 years was 33.5% (SE, 10.1, 95% CI, 13.8 to 53.2) in the beraprost therapy group, and 18.1% (SE, 6.6, 95% CI, 5.3 to 31.0) in the no beraprost therapy group (P = 0.004 by log-rank test). The median overall survival was 23.4 months (SE, 6.6, 95% CI, 10.4 to 36.4) in the beraprost therapy group, and 9.5 months (SE, 2.3, 95% CI, 5.1 to 13.9) in the no beraprost therapy group (
Figure 2).
Further, to examine the robustness of the findings, cohort A was divided into various subgroups based on baseline variables, unstratified Cox regression analyses were carried out for each subgroup. All the HRs of the beraprost therapy group compared to the no beraprost therapy group were significantly better for progression free and overall survival (P = 0.003 to 0.042), and there was no interaction between the treatment effect of beraprost and any of the subgroup variables (
Table 6).
3.5. Baseline characteristics of the two groups in cohort B
There remained a concern with the retrospective analysis of cohort A that baseline phosphate was significantly higher in the no beraprost therapy group, and this is known to be associated with poor prognosis of feline CKD. In order to solve the above concern, cats with phosphate ≥ 6.0 mg/dL were removed from cohort A, another retrospective analysis was conducted. Ninety-seven (97) cats with IRIS stage 3 CKD and phosphate < 6.0 mg/dL were enrolled into cohort B, which was divided into the two groups: the beraprost therapy group (n = 47) and the no beraprost therapy group (n = 50). Baseline characteristics are presented in
Table 7. Phosphate was well balanced between the two groups; median, 4.3 mg/dL in the beraprost therapy group versus to 4.4 mg/dL in the no beraprost therapy group, but urea was significantly higher in the no beraprost therapy group (P < 0.05, Mann-Whitney U test), calcium and renal diet-other (other than Renal support and k/d) were significantly higher in the beraprost therapy group (P < 0.05, Welch T test, P < 0.05, Fisher’s exact test, respectively).
3.6. Survival analyses of the two groups in cohort B
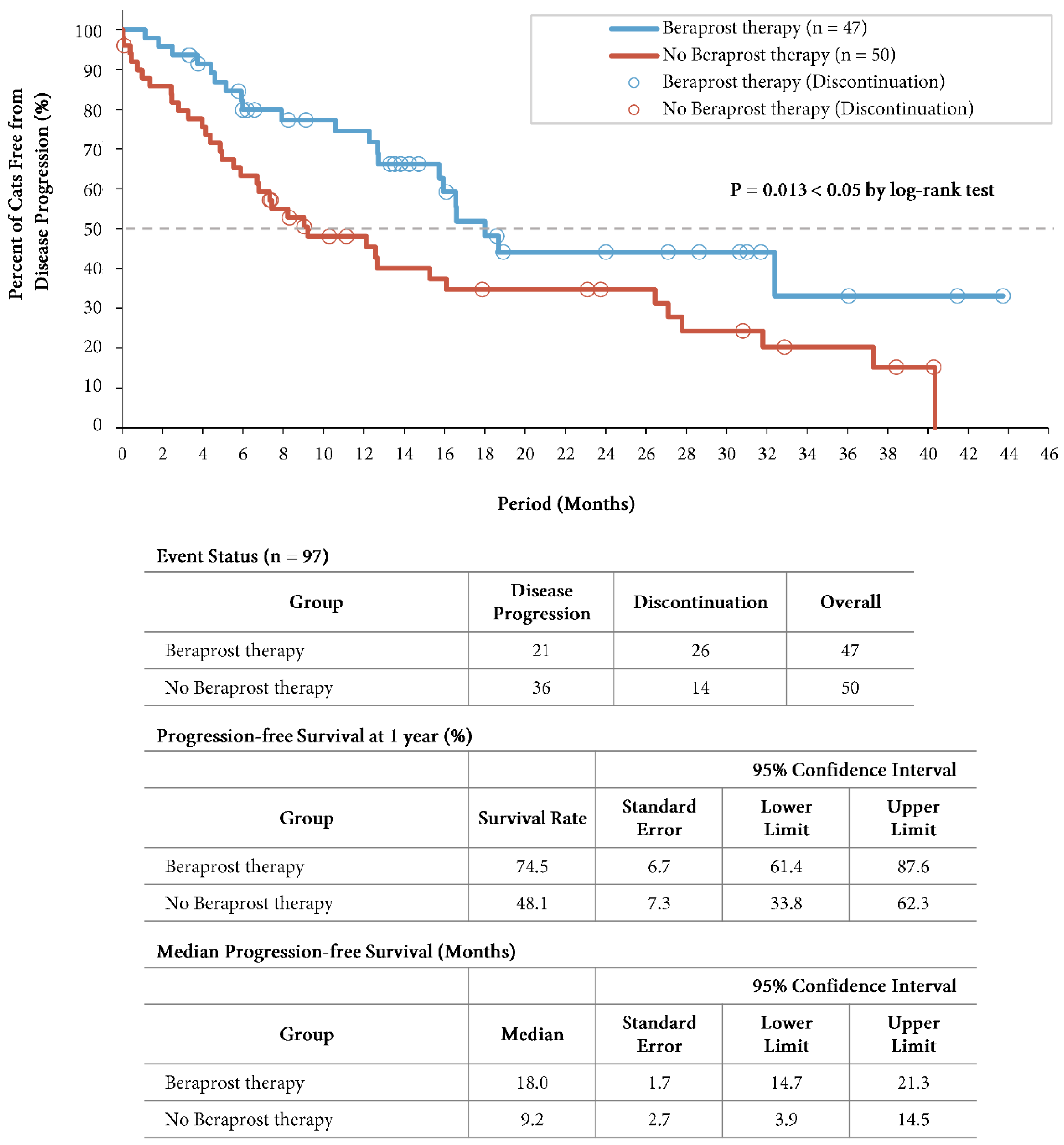
At the time of the final analysis of progression-free survival, the mean duration of follow-up was 13.6 months (14.8 months in the beraprost therapy group and 12.5 months in the no beraprost therapy group). A total of 21 cats (44.7%) in the beraprost therapy group and 36 (72.0%) in the no beraprost therapy group progressed. Progression-free survival was significantly longer in the beraprost therapy group (P = 0.013 by log-rank test); the estimated rates of progression-free survival at 1 year was 74.5% (SE, 6.7, 95% CI, 61.4 to 87.6) in the beraprost therapy group, and 48.1% (SE, 7.3, 95% CI, 33.8 to 62.3) in the no beraprost therapy group. The median progression-free survival was 18.0 months (SE, 1.7, 95% CI, 14.7 to 21.3) in the beraprost therapy group, and 9.2 months (SE, 2.7, 95% CI, 3.9 to 14.5) in the no beraprost therapy group (
Figure 3).
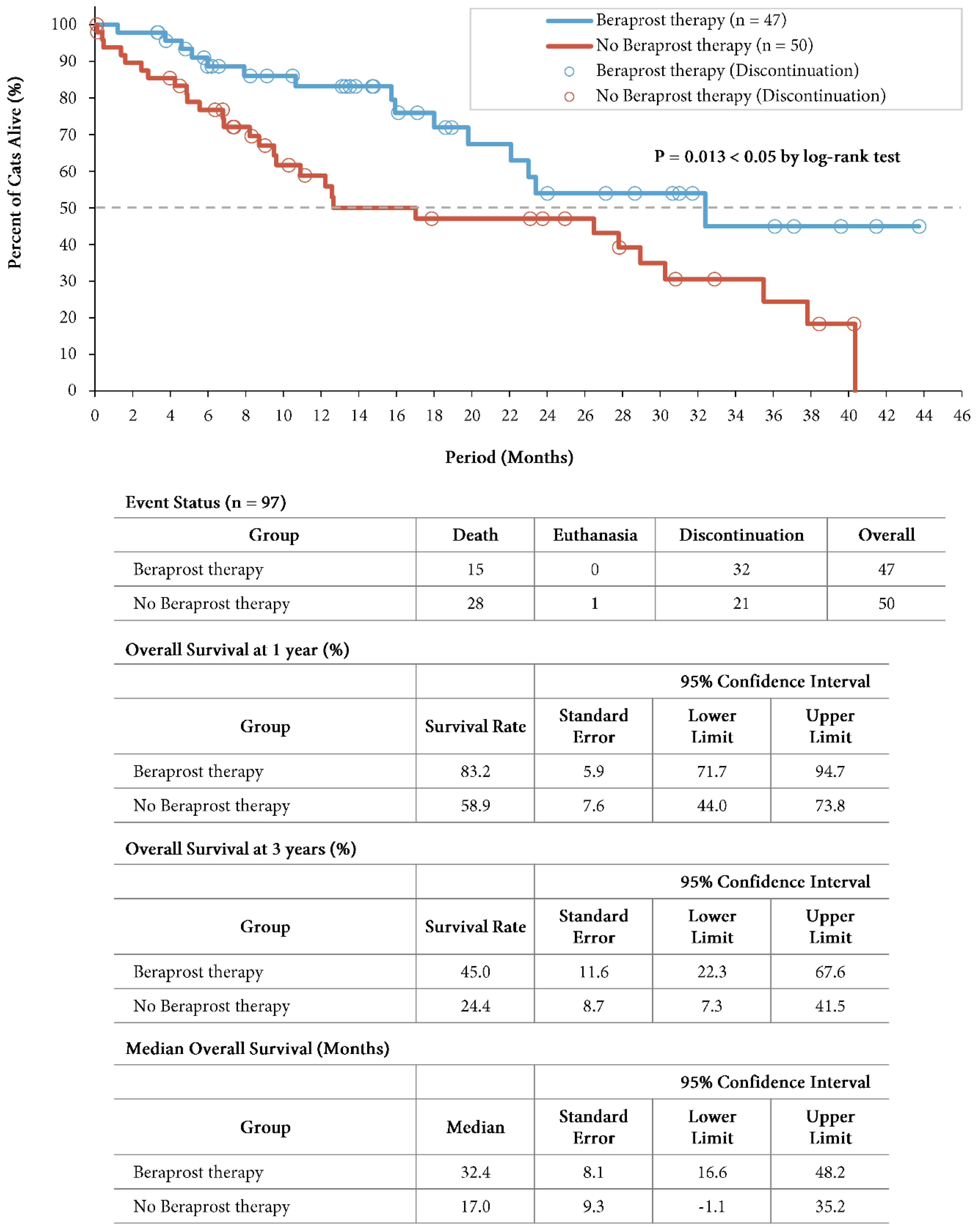
A total of 15 cats (31.9%) in the beraprost therapy group and 29 (58.0%) in the no beraprost therapy group died, which included 1 cat euthanized in the no beraprost therapy group. Overall survival was significantly longer with beraprost therapy; the estimated rates of overall survival at 1 year was 83.2% (SE, 5.9, 95% CI, 71.7 to 94.7) in the beraprost therapy group, and 58.9% (SE, 7.6, 95% CI, 44.0 to 73.8) in the no beraprost therapy group, and at 3 years was 45.0% (SE, 11.6, 95% CI, 22.3 to 67.6) in the beraprost therapy group, and 24.4% (SE, 8.7, 95% CI, 7.3 to 41.5) in the no beraprost therapy group (P = 0.013 by log-rank test). The median overall survival was 32.4 months (SE, 8.1, 95% CI, 16.6 to 48.2) in the beraprost therapy group, and 17.0 months (SE, 9.3, 95% CI, -1.1 to 35.2) in the no beraprost therapy group (
Figure 4). Taken together, these findings indicated that beraprost was an important factor associated with better survival in cohort B, in which any bias from difference in baseline phosphate had been excluded.
3.7. Onset of chronic disorders of the two groups in cohort A
During the study, onset of chronic disorders was found in 21 cats (15.7%) out of 134 cats, the most frequently observed was cardiovascular disorders (10 cats, 7.5%), and the second was neoplasia (6 cats, 4.5%). The overall incidence in the beraprost therapy group was 5 (8.8%), and that of the no beraprost therapy group 16 (20.8%) (
Table 8).
4. Discussion
Retrospective cohort studies have often been considered less valuable than randomized studies in scientifically demonstrating a causal relationship between factors and outcomes. The reasons are as follows: (A) Reporting bias, where outcomes are deliberately not reported or are partially reported, (B) Insufficiency of medical records, especially, shortcomings of paper medical records have been recognized as limited completeness and consistency, (C) Potential confounding variable bias, where non-randomized studies have difficulty avoiding confounding variables over time period. However, non-randomized studies can complement randomized trials about long term outcomes, rare events and populations that are common in real-world practice (17, 18). In this study, all the treatments were compared by multivariable Cox regression analysis for survival in 134 cats with IRIS stage 3 CKD (cohort A). The results showed beraprost only was related to better survival. Furthermore, the cohort was divided into a beraprost therapy group and a no beraprost therapy group, which were analyzed to further evaluate association between beraprost and survival in cats with CKD. This could complement previous information about the efficacy and safety of beraprost in cats with CKD that were demonstrated by a previous randomized study (14). All data regarding test results, diagnoses, prescriptions, and death records were obtained from electronic medical records, so the potential biases were minimized. The evaluated outcomes were clear and the clinical implications straightforward. The robustness of the results was examined with Cox model sensitivity analyses in the various groups and further retrospective analyses.
The baseline profile in the overall study population was mostly consistent with those of previous studies; (A) the median age 15.3 years versus the mean age 14.4 years of cats with uremic chronic renal failure (19), the mean age 10.6 years of cats in IRIS stage 3 (16), and the median age 13.0 years of cats with normotensive IRIS stage 3 CKD (20), (B) the median weight 3.6 kg versus the median weight 3.8 kg of cats with IRIS stage 3 CKD (21), and the mean weight 4.1 kg of cats in IRIS stage 3 (16), (C) the percentages of female and neutered were 46.3% and 85.8% versus the respective percentages 38.5% and 97.4% of cats with uremic chronic renal failure (19), and 36.9% and 93.9% of cats in IRIS stage 3 (16), (D) the most common breed was domestic shorthair 67.9% versus the percentage 74.4% of cats with uremic chronic renal failure (19), and 66.2% of cats in IRIS stage 3 (16), (E) the mean creatinine was 3.4 mg/dL versus the mean 3.6 mg/dL of cats with uremic chronic renal failure (19), and 3.5 mg/dL (16), (F) the mean packed cell volume was 34.5% versus the mean packed cell volume 30% of cats with uremic chronic renal failure (19), and the mean hematocrit 32.3% of cats in IRIS stage 3 (16), and the mostly other parameters were similar. The manifestations of feline CKD vary between individuals, thus there is a need for adjustment of therapy according to individual needs. The international consensus guidelines recommend managing hydration, diet, hypertension, anemia, proteinuria and other treatments (1, 2), which corelates exactly to the follow-up therapies in the present study. The characteristics on the baseline coexisting disorders in the present study were mostly consistent with the previous studies in the literature; (A) the prevalence of hyperthyroidism (9.7%), versus the prevalence in cats over 8 years of age in Germany (11.4%) (22), and in cats older than 9 years in Japan(8.9%) (23) and in the UK (6%) (24), (B) the prevalence of diabetes mellitus (3.0%), versus the prevalence of 2.9% out of 561 cats in Spain (5), (C) the prevalence of congestive heart failure (7.5%), versus the prevalence of hypertrophic cardiomyopathy (the most commonly diagnosed heart disease in cats, as comparative data for were not found congestive heart failure overall), of 14.6% of adult cats (25) and 29.4% of senior cats of 9 years or older (26), and 19.6% in another study (27), (D) the prevalence of neoplasia (6.0%), versus the prevalence in cats with CKD of 4.2% (28), and (E) the prevalence of pancreatitis (2.2%) was similar to 2.4% by following necropsy (29) (comparative data in cats with CKD were not found, most likely because pancreatitis is difficult to diagnose ante mortem). Taken together, all the baseline characteristics in this study suggested the population examined was very consistent with that in previously published studies in this field.
The findings on the progression-free survival analysis in the present study indicate that beraprost therapy will be associated with slower progression. The results of the no beraprost therapy group were very similar to those of a previous study on cats in IRIS stage 3 (15). In the cohort A of this study, the progression-free survival rate at 1 year was 37.6%, disease progression was seen 36 out of 77 cats (46.8%), death in 19 cats (24.7%) and euthanasia in 1 cat (1.3%); in the previous study, disease progression (defined as a 25% increase in creatinine concentration, the same as the present study) was seen in 34 out of 73 cats (46.6%) and death in 19 cats (26.0%). The progression-free survival rate and median progression survival time of the beraprost therapy group were 1.7 and 2.8 times respectively, longer than those of the no beraprost therapy group with a significant difference on Kaplan-Meyer curves (P = 0.004 by log-rank test).
In addition, the data on the overall survival analysis demonstrate that beraprost is associated with prolonged life expectancy in cats with CKD, irrespective of coexisting disorders. The results of the no beraprost therapy group were within the range in the previously published literature, which tended to exclude the cats with coexisting disorders from the scope of research; (A) the overall survival rate at 3 years 18.1% versus the survival rate 12% at 2.6 years of cats with uremic chronic renal failure (19) and 37% at 2.7 years of cats in IRIS stage 3 (16), (B) the median overall survival time 9.5 months versus the survival time 7.8 months of cats with uremic chronic renal failure (19) and 8.8 to 25.9 months of cats in IRIS stage 3 (16, 30, 31). The overall survival rate and median overall survival time of the beraprost therapy group were 1.9 and 2.5 times respectively, longer than those of the no beraprost therapy group with a significant difference on Kaplan-Meyer curves (P = 0.004 by log-rank test). Euthanasia is almost always regarded as a confounding factor in veterinary studies; cats are often euthanized rather than dying naturally (20) and in one study on survival in cats with CKD, 75% of cats were euthanized (31). In the present study, only 3 (2%) out of 134 cats were euthanized, which may be due to geographical variation, but introduces much less bias in the survival analysis.
Proteinuria in cats is often associated with CKD, with prevalence reported to be 16% (30). The severity of proteinuria at the time of diagnosis is also known to indicate a poorer prognosis (15, 16, 20, 32, 33). Benazepril has been clinically tested in cats with CKD, shown to reduce proteinuria assessed by the urine protein-to-creatinine ratio (34, 35) and to decrease serum creatinine (36); however, benazepril efficacy has not been demonstrated for reducing the incidence of death or withdrawal from the trial due to worsening CKD, reaching IRIS stage 4 (34), or the composite endpoint of death, euthanasia or the need for parenteral fluid therapy (35). Similarly, telmisartan has been shown to reduce the severity of proteinuria (37) but its efficacy has not been examined for slowing disease progression or prolonging survival. Weight loss is commonly observed in cats with CKD, prevalence of which is reviewed to be 42 to 82% and is associated with shorter survival (21). Mirtazapine is known to be suppress weight loss in cats with CKD (38, 39), but it will not affect the results of this study, because there was observed no difference in the prescription of mirtazapine between two groups. Collectively, the findings of the present study can be considered the first evidence for a medical therapy associated with an increased life expectancy in cats with CKD.
The onset profile of chronic disorders during the study period in the no beraprost therapy group was mostly consistent with the record of baseline coexisting disorders in the present study; however, there were some differences in that neuromuscular disorders such as epilepsy and seizures were newly observed and that cardiovascular disorders were increased, during the study. Epilepsy and seizures in cats with CKD can be caused by uremic encephalopathy; it is reported that all cats with renal encephalopathy had IRIS stage 3 or 4 CKD with mean creatinine of 2.9 mg/dL (40). Cardiovascular-renal disorders (CvRD) is the pathophysiological relationship between the kidney and heart in disease, and kidney injury is proposed to be able to lead to systemic volume overload that contributes to congestion, especially in animals with coexisting cardiac disease including cardiomyopathy (3); these factors could have influenced the results. Overall, the relative risk of onset of chronic disorders in the beraprost therapy group versus to the no beraprost therapy group was 0.42 (8.8/20.8). Epilepsy and seizures were not seen in the beraprost therapy group but were seen in 3 cats (3.9%) in the no beraprost therapy group. Endothelial dysfunction and reduced NO production are associated with CKD and heart failure and make the link between kidney and cardiac dysfunction (41). Beraprost has a protective effect on endothelial cell damage (9), especially dose-dependently inhibits human aortic endothelial cell injury induced by uremic toxin indoxyl sulfate (13), and stimulates eNOS in human and animal endothelial cells (10). In the present study, the relative risk of incident cardiovascular disorders in the beraprost therapy group comparing the no beraprost therapy group was 0.34 (3.5/10.4). This finding may help to further elucidate the efficacy of beraprost in cats with CvRD. Additionally, there was no difference in the incidence of endocrine disorders and neoplasia between the groups.
While it is true that selection bias was minimized because all cats and their owners were proposed for a treatment of beraprost and there was no special motivation, there may be potential selection bias between the groups. The difference may result from the difference in cats and their owners’ acceptability for the burdens and benefits (e.g., twice daily administration and costs).
In the cohort A with all IRIS stage 3 CKD (n = 134), the baseline phosphate was higher in the no beraprost therapy group, and to remove this difference, a subcohort B was sorted according to phosphate < 6.0 mg/dL (n = 97). This allowed for balanced baseline phosphate between groups, but there remained differences in the level of urea, calcium and the prescription of renal diet-other (other than Renal support and k/d), the potential effects of which are unknown. It was also not possible to analyze a subcohort with phosphate ≥ 6.0 mg/dL due to the low sample size. These remain important limitations of the study. Another limitation of the study is the data used did not include enough information on blood pressure (the implementation rate of blood pressure measurements was 36.6%; most of cats did not undergo the routine assessment early in the study period) and hardly ever on urine protein/creatinine ratio; thus the prevalence of hypertension and proteinuria between the groups could not be accurately examined. In addition, it may be inappropriate to draw conclusions without considering difference of treatment type on coexisting disorders. Also, the effects of beraprost on each coexisting disorder itself were not examined here. Endothelial dysfunction, a main target of beraprost, is known to be a key in the pathogenesis of various diseases such as heart disease and diabetes mellitus. Thus, it would be necessary to verify the current findings by a double blinded randomized controlled clinical trial to address the above limitations.
In conclusion, beraprost therapy was associated with significantly better progression-free survival and overall survival outcomes than no beraprost therapy in cats with CKD. Additionally, the incidence of chronic disorders was not higher in the beraprost therapy group than the no beraprost therapy; this indicates a long-term benefit of beraprost in cats.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This retrospective cohort study was based on data from electronic medical records in clinical practice and was reviewed and approved by Kariya Animal Hospital Group Ethical Review Board (Approval number: IGH2021001). All medical management was carried out with authorized medicinal products (including beraprost: RAPROS), supplements and diets, based on informed consent obtained from the owners of cats, and following manufacturer instruction. No experimental intervention was performed.
Author Contributions
HI and TM participated in hypothesis generation and study design. HI contributed to conducting the study and analyzing the results. TM contributed to visualizing the results and drafting the manuscript. HI, TM, and TS all participated in the interpretation of the data and revision of the manuscript. All the authors endorsed the content of the work.
References
- Sparkes AH, Caney S, Chalhoub S, Elliott J, Finch N, Gajanayake I, et al. ISFM Consensus Guidelines on the Diagnosis and Management of Feline Chronic Kidney Disease. J Feline Med Surg. 2016;18(3):219-39. [CrossRef]
- International Renal Interest Society. IRIS Treatment Recommendations for CKD; 2019 [cited 2021 Mar 28]. Available from: http://iris-kidney.com/guidelines/recommendations.html.
- Pouchelon JL, Atkins CE, Bussadori C, Oyama MA, Vaden SL, Bonagura JD, et al. Cardiovascular-renal axis disorders in the domestic dog and cat: a veterinary consensus statement. J Small Anim Pract. 2015;56(9):537-52. [CrossRef]
- Williams TL, Peak KJ, Brodbelt D, Elliott J, Syme HM. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med. 2010;24(4):863-9. [CrossRef]
- Perez-Lopez L, Boronat M, Melian C, Saavedra P, Brito-Casillas Y, Wagner AM. Assessment of the association between diabetes mellitus and chronic kidney disease in adult cats. J Vet Intern Med. 2019;33(5):1921-5. [CrossRef]
- Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13(3):806-16. [CrossRef]
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983-92. [CrossRef]
- Jepson R, Syme H, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. Journal of veterinary internal medicine. 2008;22(2):317-24. [CrossRef]
- Kainoh M NS, Nakadate T. Cytoprotective Action of Beraprost Sodium against Peroxide-Induced Damage in Vascular Endothelial Cells. Pharmacology. 1992;45:61-70. [CrossRef]
- Niwano K, Arai M, Tomaru K, Uchiyama T, Ohyama Y, Kurabayashi M. Transcriptional stimulation of the eNOS gene by the stable prostacyclin analogue beraprost is mediated through cAMP-responsive element in vascular endothelial cells: close link between PGI2 signal and NO pathways. Circ Res. 2003;93(6):523-30. [CrossRef]
- Matsumoto K, Morishita R, Tomita N, Moriguchi A, Yamasaki K, Aoki M, et al. Impaired endothelial dysfunction in diabetes mellitus rats was restored by oral administration of prostaglandin I2 analogue. J Endocrinol. 2002;175(1):217-23. [CrossRef]
- Goto Y, Yamaguchi S, Tamura M, Mochizuki H, Kurumatani H, Okano K, et al. A prostacyclin analog prevents the regression of renal microvascular network by inhibiting mitochondria-dependent apoptosis in the kidney of rat progressive glomerulonephritis. Prostaglandins Other Lipid Mediat. 2014;112:16-26. [CrossRef]
- Yamaguchi S, Inada C, Tamura M, Sato N, Yamada M, Itaba S, et al. Beraprost sodium improves survival rates in anti-glomerular basement membrane glomerulonephritis and 5/6 nephrectomized chronic kidney disease rats. Eur J Pharmacol. 2013;714(1-3):325-31. [CrossRef]
- Takenaka M, Iio A, Sato R, Sakamoto T, Kurumatani H, Group KTCS. A Double-blind, Placebo-controlled, Multicenter, Prospective, Randomized Study of Beraprost Sodium Treatment for Cats with Chronic Kidney Disease. J Vet Intern Med. 2018;32(1):236-48. [CrossRef]
- Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26(2):275-81. [CrossRef]
- King JN, Tasker S, Gunn-Moore DA, Strehlau G, Group BS. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med. 2007;21(5):906-16. [CrossRef]
- Feinstein AR. An additional basic science for clinical medicine: II. The limitations of randomized trials. Ann Intern Med. 1983;99(4):544-50. [CrossRef]
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312(7040):1215-8. [CrossRef]
- Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39(2):78-85. [CrossRef]
- Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med. 2006;20(3):528-35. [CrossRef]
- Freeman LM, Lachaud MP, Matthews S, Rhodes L, Zollers B. Evaluation of Weight Loss Over Time in Cats with Chronic Kidney Disease. J Vet Intern Med. 2016;30(5):1661-6. [CrossRef]
- Sassnau R. Epidemiological investigation on the prevalence of feline hyperthyroidism in an urban population in Germany. Tierärztliche Praxis, Kleintiere. 2006;34:450–7.
- Miyamoto Y, Miyata, I, Kurobane, K. Prevalence of feline hyperthyroidism in Osaka and the Chugoku region. Journal of the Japanese Veterinary Medical Association. 2002;55:289–92. [CrossRef]
- Wakeling J, Elliott J, Syme H. Evaluation of predictors for the diagnosis of hyperthyroidism in cats. J Vet Intern Med. 2011;25(5):1057-65. [CrossRef]
- Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. 2009;234(11):1398-403. [CrossRef]
- Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol. 2015;17 Suppl 1:S244-57. [CrossRef]
- Gouni V, Chetboul V, Pouchelon JL, Carlos Sampedrano C, Maurey C, Lefebvre HP. Azotemia in cats with feline hypertrophic cardiomyopathy: prevalence and relationships with echocardiographic variables. J Vet Cardiol. 2008;10(2):117-23. [CrossRef]
- Wormser C, Mariano A, Holmes ES, Aronson LR, Volk SW. Post-transplant malignant neoplasia associated with cyclosporine-based immunotherapy: prevalence, risk factors and survival in feline renal transplant recipients. Vet Comp Oncol. 2016;14(4):e126-e34. [CrossRef]
- Owens JM DF, Gilbertson SR. Pancreatic disease in the cat. JAAHA. 1975;11:83-9.
- Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth factor-23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29(6):1494-501. [CrossRef]
- Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000-2002). J Vet Intern Med. 2008;22(5):1111-7. [CrossRef]
- Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med. 2007;21(3):402-9. [CrossRef]
- Syme HM. Proteinuria in cats. Prognostic marker or mediator? J Feline Med Surg. 2009;11(3):211-8. [CrossRef]
- Mizutani H, Koyama H, Watanabe T, Kitagawa H, Nakano M, Kajiwara K, et al. Evaluation of the clinical efficacy of benazepril in the treatment of chronic renal insufficiency in cats. J Vet Intern Med. 2006;20(5):1074-9. [CrossRef]
- King JN, Gunn-Moore DA, Tasker S, Gleadhill A, Strehlau G, Benazepril in Renal Insufficiency in Cats Study G. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med. 2006;20(5):1054-64. [CrossRef]
- Watanabe T, Mishina M. Effects of benazepril hydrochloride in cats with experimentally induced or spontaneously occurring chronic renal failure. J Vet Med Sci. 2007;69(10):1015-23. [CrossRef]
- Sent U, Gossl R, Elliott J, Syme HM, Zimmering T. Comparison of Efficacy of Long-term Oral Treatment with Telmisartan and Benazepril in Cats with Chronic Kidney Disease. J Vet Intern Med. 2015;29(6):1479-87. [CrossRef]
- Quimby J, Lunn KJTVJ. Mirtazapine as an appetite stimulant and anti-emetic in cats with chronic kidney disease: a masked placebo-controlled crossover clinical trial. 2013;197(3):651-5. [CrossRef]
- Quimby JM, Benson KK, Summers SC, Saffire A, Herndon AK, Bai S, et al. Assessment of compounded transdermal mirtazapine as an appetite stimulant in cats with chronic kidney disease. J Feline Med Surg. 2020;22(4):376-83. [CrossRef]
- Kwiatkowska M, Hoppe S, Pomianowski A, Tipold A. Reactive seizures in cats: A retrospective study of 64 cases. Vet J. 2019;244:1-6. [CrossRef]
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9(2):99-111. [CrossRef]
Figure 1.
Progression-free survival in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL). Kaplan-Meier estimates of progression free-survival, summary of events, progression-free survival at 1 year, and median progression-free survival. P value was calculated by two-sided log-rank test. Disease progression events in the beraprost therapy group included death (in 13 cats); disease progression events in the no beraprost therapy group included death (in 19 cats) and euthanasia (in 1 cat).
Figure 1.
Progression-free survival in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL). Kaplan-Meier estimates of progression free-survival, summary of events, progression-free survival at 1 year, and median progression-free survival. P value was calculated by two-sided log-rank test. Disease progression events in the beraprost therapy group included death (in 13 cats); disease progression events in the no beraprost therapy group included death (in 19 cats) and euthanasia (in 1 cat).
Figure 2.
Overall survival in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL). Kaplan-Meier estimates of overall survival, summary of events, overall survival at 1 and 3 years, and median progression-free survival. P value was calculated by two-sided log-rank test.
Figure 2.
Overall survival in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL). Kaplan-Meier estimates of overall survival, summary of events, overall survival at 1 and 3 years, and median progression-free survival. P value was calculated by two-sided log-rank test.
Figure 3.
Progression-free survival in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL. Kaplan-Meier estimates of progression free-survival, summary of events, progression-free survival at 1 year, and median progression-free survival. P value was calculated by two-sided log-rank test. Disease progression events in the beraprost therapy group included death (in 9 cats); disease progression events in the no beraprost therapy group included death (in 12 cats).
Figure 3.
Progression-free survival in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL. Kaplan-Meier estimates of progression free-survival, summary of events, progression-free survival at 1 year, and median progression-free survival. P value was calculated by two-sided log-rank test. Disease progression events in the beraprost therapy group included death (in 9 cats); disease progression events in the no beraprost therapy group included death (in 12 cats).
Figure 4.
Overall survival in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL. Kaplan-Meier estimates of overall survival, summary of events, overall survival at 1 and 3 years, and median progression-free survival. P value was calculated by two-sided log-rank test.
Figure 4.
Overall survival in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL. Kaplan-Meier estimates of overall survival, summary of events, overall survival at 1 and 3 years, and median progression-free survival. P value was calculated by two-sided log-rank test.
Table 1.
Distribution of IRIS stage and beraprost prescription in the CKD-diagnosed cats.
Table 1.
Distribution of IRIS stage and beraprost prescription in the CKD-diagnosed cats.
| |
|
|
Total
(n = 730)
|
Beraprost prescription |
Yes
(n = 124)
|
No
(n = 606)
|
| IRIS |
Stage 1 |
number |
143 |
13 |
130 |
| |
Stage 2 |
number |
369 |
37 |
332 |
| |
Stage 3 |
number |
134 |
57 |
77 |
| |
Stage 4 |
number |
84 |
17 |
67 |
Table 2.
Baseline characteristics of all cats in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 2.
Baseline characteristics of all cats in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| |
|
Total
(n = 134)
|
| Age (years) |
median (25th, 75th percentile) |
15.3 (10.5, 17.3) |
| Weight (kg) |
median (25th, 75th percentile) |
3.6 (3.0, 4.4) |
| Sex |
|
|
| Female |
number (%) |
62 (46.3) |
| Male |
number (%) |
72 (53.7) |
| Neutered |
number (%) |
115 (85.8) |
| Breeds |
|
|
| Domestic Shorthair |
number (%) |
91 (67.9) |
| Japanese Bobtail |
number (%) |
7 (5.2) |
| American Shorthair |
number (%) |
6 (4.5) |
| Other |
number (%) |
30 (22.4) |
| Biochemistry |
|
|
| Creatinine (mg/dL) |
median (25th, 75th percentile) |
3.3 (3.0, 3.7) |
| Urea (mg/dL) |
median (25th, 75th percentile) |
42.0 (35.0, 60.0) |
| Phosphate (mg/dL) |
median (25th, 75th percentile) |
4.9 (4.0, 6.1) |
| Calcium (mg/dL) |
median (25th, 75th percentile) |
9.7 (9.2, 10.1) |
| Potassium (mmol/L) |
median (25th, 75th percentile) |
3.9 (3.5, 4.2) |
| Hematology |
|
|
| Packed cell volume (%) |
median (25th, 75th percentile) |
35.0 (30.0, 40.0) |
| Urinalysis |
|
|
| Urine specific gravity |
median (25th, 75th percentile) |
1.014 (1.012, 1.018) |
| Urine protein (mg/dL) |
mean (SD) |
59.6 (167.9) |
| Blood pressure measurement |
|
|
| Systolic blood pressure (mmHg) |
median (25th, 75th percentile) |
153.0 (132.0, 165.0) |
| Treatment |
|
|
| Beraprost (RAPROS) |
number (%) |
57 (42.5%) |
| Dose (μg/kg twice daily) |
mean (SD, range) |
15.0 (4.0, 7.1-26.2) |
| Subcutaneous fluid therapy (total) |
number (%) |
106 (79.1%) |
| At clinic |
number (%) |
87 (64.9%) |
| At home |
number (%) |
72 (53.7%) |
| Renal Diet (total) |
number (%) |
75 (56.0%) |
| Royal Canin (Renal Support) |
number (%) |
53 (39.6%) |
| Hill's (k/d) |
number (%) |
34 (25.4%) |
| Other |
number (%) |
33 (24.6%) |
| Phosphate binder |
number (%) |
40 (29.9%) |
| Ferric chloride (Lenziaren) |
| Oral activated charcoal |
number (%) |
37 (27.6%) |
| ACEI/ARB (total) |
number (%) |
34 (25.4%) |
| Benazepril (Fortekor) |
number (%) |
27 (20.1%) |
| Telmisartan (Semintra) |
number (%) |
10 (7.5%) |
| Calcium channel blocker |
number (%) |
11 (8.2%) |
| Amlodipine |
| Erythrocyte-stimulating agents |
number (%) |
34 (25.4%) |
| Darbepoetin alfa |
| Managing inappetence, nauseaand vomiting (total) |
number (%) |
102 (76.1%) |
| Maropitant (Cerenia) |
number (%) |
88 (65.7%) |
| Mirtazapine |
number (%) |
44 (32.8%) |
| Famotidine |
number (%) |
27 (20.1%) |
| Omeprazole |
number (%) |
8 (6.0%) |
| Metoclopramide |
number (%) |
11 (8.2%) |
| Coexisting Disorders |
|
|
| Any |
number (%) |
38 (28.4%) |
| Hyperthyroidism |
number (%) |
13 (9.7%) |
| Congestive heart failure |
number (%) |
10 (7.5%) |
| Neoplasia* |
number (%) |
8 (6.0%) |
| Diabetes mellitus |
number (%) |
4 (3.0%) |
| Pancreatitis |
number (%) |
3 (2.2%) |
Table 3.
Multivariable Cox regression analyses of progression-free and overall survival with treatments as covariates in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 3.
Multivariable Cox regression analyses of progression-free and overall survival with treatments as covariates in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| Progression-free survival |
|
|
|
|
|
| Covariate |
Coefficient |
Standard Error |
Hazard
Ratio for Disease Progression
|
95% Confidence Interval |
P value |
| Lower Limit |
Upper Limit |
| Beraprost |
-0.59 |
0.23 |
0.56 |
0.35 |
0.88 |
0.012* |
| Subcutaneous fluid therapy |
-0.32 |
0.31 |
0.72 |
0.40 |
1.32 |
0.289 |
| Phosphate binder |
0.63 |
0.24 |
1.87 |
1.16 |
3.01 |
0.010** |
| ACEI/ARB |
-0.34 |
0.26 |
0.71 |
0.42 |
1.19 |
0.197 |
| Erythrocyte-stimulating agents |
0.51 |
0.25 |
1.67 |
1.02 |
2.75 |
0.043* |
| Overall survival |
|
|
|
|
|
| Covariate |
Coefficient |
Standard Error |
Hazard
Ratio for Death
|
95% Confidence Interval |
P value |
| Lower Limit |
Upper Limit |
| Beraprost |
-0.57 |
0.26 |
0.57 |
0.34 |
0.94 |
0.028* |
| Phosphate binder |
0.65 |
0.29 |
1.91 |
1.09 |
3.37 |
0.024* |
| Oral activated charcoal |
-0.30 |
0.29 |
0.74 |
0.42 |
1.30 |
0.294 |
| ACEI/ARB |
-0.28 |
0.29 |
0.76 |
0.43 |
1.33 |
0.332 |
| Calcium channel blocker |
-0.79 |
0.61 |
0.45 |
0.14 |
1.50 |
0.195 |
| Erythrocyte-stimulating agents |
0.38 |
0.26 |
1.46 |
0.87 |
2.45 |
0.149 |
Table 4.
Multivariable Cox regression analyses of progression-free and overall survival with baseline characteristics as covariates in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 4.
Multivariable Cox regression analyses of progression-free and overall survival with baseline characteristics as covariates in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| Progression-free survival |
|
|
|
|
|
| Covariate |
Coefficient |
Standard Error |
Hazard
Ratio for Disease Progression
|
95% Confidence Interval |
P value |
| Lower Limit |
Upper Limit |
| Beraprost |
-0.52 |
0.24 |
0.59 |
0.37 |
0.95 |
0.028* |
| Weight (kg) < 3.0 |
0.40 |
0.26 |
1.49 |
0.90 |
2.45 |
0.120 |
| Urea (mg/dL) ≥ 60.0 |
0.78 |
0.25 |
2.17 |
1.32 |
3.56 |
0.002** |
| Packed cell volume (%) < 28.0 |
1.09 |
0.30 |
2.98 |
1.66 |
5.33 |
< 0.001** |
| Overall survival |
|
|
|
|
|
| Covariate |
Coefficient |
Standard Error |
Hazard
Ratio for Death
|
95% Confidence Interval |
P value |
| Lower Limit |
Upper Limit |
| Beraprost |
-0.74 |
0.28 |
0.48 |
0.28 |
0.82 |
0.008** |
| Age (years) > 15.0 |
0.69 |
0.26 |
2.00 |
1.20 |
3.34 |
0.008** |
| Creatinine (mg/dL) ≥ 4.0 |
0.80 |
0.29 |
2.23 |
1.25 |
3.96 |
0.007** |
| Phosphate (mg/dL) ≥ 7.0 |
1.12 |
0.42 |
3.06 |
1.34 |
6.97 |
0.008** |
| Calcium (mg/dL) >10.7 |
0.70 |
0.42 |
2.02 |
0.89 |
4.59 |
0.094 |
| Packed cell volume (%) < 28.0 |
0.69 |
0.38 |
2.00 |
0.95 |
4.22 |
0.068 |
Table 5.
Baseline characteristics of the two groups in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 5.
Baseline characteristics of the two groups in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| |
|
Beraprost therapy(n = 57) |
No Beraprost therapy(n = 77) |
| Age (years) |
median (25th, 75th percentile) |
15.9 (13.0, 17.3) |
15.1 (8.6, 17.4) |
| Weight (kg) |
median (25th, 75th percentile) |
3.8 (3.1, 4.4) |
3.4 (2.8, 4.4) |
| Sex |
|
|
|
| Female |
number (%) |
30 (52.6) |
32 (41.6) |
| Male |
number (%) |
27 (47.4) |
45 (58.4) |
| Neutered |
number (%) |
46 (80.7) |
69 (89.6) |
| Breeds |
|
|
|
| Domestic Shorthair |
number (%) |
37 (64.9) |
54 (70.1) |
| Japanese Bobtail |
number (%) |
3 (5.3) |
4 (5.2) |
| American Shorthair |
number (%) |
3 (5.3) |
3 (3.9) |
| Other |
number (%) |
14 (24.5) |
16 (20.8) |
| Biochemistry |
|
|
|
| Creatinine (mg/dL) |
median (25th, 75th percentile) |
3.3 (3.0, 3.7) |
3.3 (3.0, 3.7) |
| Urea (mg/dL) |
median (25th, 75th percentile) |
38.0 (31.0, 52.5) |
46.0 (37.0, 70.0)**
|
| Phosphate (mg/dL) |
median (25th, 75th percentile) |
4.6 (3.9, 5.3) |
5.3 (4.2, 6.8)**
|
| Calcium (mg/dL) |
median (25th, 75th percentile) |
9.8 (9.4, 10.4) |
9.6 (9.0, 10.0) |
| Potassium (mmol/L) |
median (25th, 75th percentile) |
3.9 (3.6, 4.2) |
3.9 (3.5, 4.3) |
| Hematology |
|
|
|
| Packed cell volume (%) |
median (25th, 75th percentile) |
36.0 (31.0, 41.0) |
33.5 (29.5, 40.0) |
| Urinalysis |
|
|
|
| Urine specific gravity |
median (25th, 75th percentile) |
1.014 (1.012, 1.018) |
1.014 (1.012, 1.018) |
| Urine protein (mg/dL) |
mean (SD) |
27.5 (60.1) |
90.0 (223.6) |
| Blood pressure measurement |
|
|
|
| Systolic blood pressure (mmHg) |
median (25th, 75th percentile) |
153.0 (132.0, 165.0) |
151.5 (132.8, 160.3) |
| Treatment |
|
|
|
| Beraprost (RAPROS) |
number (%) |
57 (100.0%)**
|
0 (0.0%) |
| Dose (μg/kg twice daily) |
mean (SD, range) |
15.0 (4.0, 7.1-26.2)**
|
0 (0, 0-0) |
| Subcutaneous fluid therapy (total) |
number (%) |
42 (73.7%) |
64 (83.1%) |
| At clinic |
number (%) |
32 (56.1%) |
55 (71.4%) |
| At home |
number (%) |
28 (49.1%) |
44 (57.1%) |
| Renal Diet (total) |
number (%) |
35 (61.4%) |
40 (51.9%) |
| Royal Canin (Renal Support) |
number (%) |
22 (38.6%) |
31 (40.3%) |
| Hill's (k/d) |
number (%) |
16 (28.1%) |
18 (23.4%) |
| Other |
number (%) |
20 (35.1%) |
13 (16.9%) |
| Phosphate binder |
number (%) |
16 (28.1%) |
24 (31.2%) |
| Ferric chloride (Lenziaren) |
| Oral activated charcoal |
number (%) |
15 (26.3%) |
22 (28.6%) |
| ACEI/ARB (total) |
number (%) |
15 (26.3%) |
19 (24.7%) |
| Benazepril (Fortekor) |
number (%) |
10 (17.5%) |
17 (22.1%) |
| Telmisartan (Semintra) |
number (%) |
5 (8.8%) |
5 (6.5%) |
| Calcium channel blocker |
number (%) |
7 (12.3%) |
4 (5.2%) |
| Amlodipine |
| Erythrocyte-stimulating agents |
number (%) |
11 (19.3%) |
23 (29.9%) |
| Darbepoetin alfa |
| Managing inappetence, nausea and vomiting (total) |
number (%) |
41 (71.9%) |
61 (79.2%) |
| Maropitant (Cerenia) |
number (%) |
34 (59.6%) |
54 (70.1%) |
| Mirtazapine |
number (%) |
21 (36.8%) |
23 (29.9%) |
| Famotidine |
number (%) |
16 (28.1%) |
11 (14.3%) |
| Omeprazole |
number (%) |
2 (3.5%) |
6 (7.8%) |
| Metoclopramide |
number (%) |
5 (8.8%) |
6 (7.8%) |
| Coexisting Disorders |
|
|
|
| Any |
number (%) |
18 (31.6%) |
20 (26.0%) |
| Hyperthyroidism |
number (%) |
7 (12.3%) |
6 (7.8%) |
| Congestive heart failure |
number (%) |
2 (3.5%) |
8 (10.4%) |
| Neoplasia* |
number (%) |
4 (7.0%) |
4 (5.2%) |
| Diabetes mellitus |
number (%) |
3 (5.3%) |
1 (1.3%) |
| Pancreatitis |
number (%) |
2 (3.5%) |
1 (1.3%) |
Table 6.
Subgroup analyses of progression-free and overall survival according to baseline in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 6.
Subgroup analyses of progression-free and overall survival according to baseline in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| Progression-free survival |
|
| |
Beraprost therapy (test) versus
No Beraprost therapy (reference)
|
| Subgroups |
Number of cats |
Hazard Ratio
for Disease Progression
|
95% Confidence Interval |
P value |
| Lower Limit |
Upper Limit |
| All |
134 |
0.53 |
0.34 |
0.83 |
0.005** |
| Age (years) ≤ 15.0 |
61 |
0.39 |
0.18 |
0.83 |
0.015* |
| Weight (kg) ≥ 3.0 |
99 |
0.53 |
0.31 |
0.90 |
0.018* |
| Creatinine (mg/dL) < 4.0 |
109 |
0.53 |
0.32 |
0.88 |
0.015* |
| Urea (mg/dL) < 120.0 |
129 |
0.57 |
0.36 |
0.89 |
0.014* |
| Phosphate (mg/dL) < 6.0 |
97 |
0.51 |
0.30 |
0.88 |
0.015* |
| Calcium (mg/dL) < 10.6 |
116 |
0.48 |
0.30 |
0.78 |
0.003** |
| Potassium (mmol/L) ≥ 3.5 |
104 |
0.59 |
0.35 |
0.98 |
0.042* |
| Packed cell volume (%) ≥ 30.0 |
105 |
0.47 |
0.28 |
0.79 |
0.005** |
| Urine protein (mg/dL) ≤ 300 |
112 |
0.60 |
0.37 |
0.97 |
0.037* |
| Overall survival |
|
| |
Beraprost therapy (test) versus
No Beraprost therapy (reference)
|
| |
Number of cats |
Hazard Ratio
for Death
|
95% Confidence Interval |
P value |
| |
Lower Limit |
Upper Limit |
| All |
134 |
0.49 |
0.30 |
0.81 |
0.005** |
| Age (years) ≤ 15.0 |
61 |
0.25 |
0.10 |
0.67 |
0.006** |
| Weight (kg) ≥ 3.0 |
99 |
0.45 |
0.24 |
0.81 |
0.008** |
| Creatinine (mg/dL) < 4.0 |
109 |
0.45 |
0.25 |
0.80 |
0.007** |
| Urea (mg/dL) < 120.0 |
129 |
0.53 |
0.32 |
0.88 |
0.014* |
| Phosphate (mg/dL) < 6.0 |
97 |
0.46 |
0.25 |
0.86 |
0.015* |
| Calcium (mg/dL) < 10.6 |
116 |
0.44 |
0.26 |
0.76 |
0.003** |
| Potassium (mmol/L) ≥ 3.5 |
104 |
0.55 |
0.31 |
0.97 |
0.039* |
| Packed cell volume (%) ≥ 30.0 |
105 |
0.42 |
0.23 |
0.75 |
0.004** |
| Urine protein (mg/dL) ≤ 300 |
112 |
0.54 |
0.32 |
0.92 |
0.023* |
Table 7.
Baseline characteristics of the two groups in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL.
Table 7.
Baseline characteristics of the two groups in cohort B: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL) and phosphate < 6.0 mg/dL.
| |
|
Beraprost therapy(n = 47) |
No Beraprost therapy(n = 50) |
| Age (years) |
median (25th, 75th percentile) |
15.9 (13.8, 17.1) |
15.1 (9.5, 17.3) |
| Weight (kg) |
median (25th, 75th percentile) |
3.9 (3.4, 4.7) |
3.7 (2.9, 4.8) |
| Sex |
|
|
|
| Female |
number (%) |
27 (57.4) |
26 (52.0) |
| Male |
number (%) |
20 (42.6) |
24 (48.0) |
| Neutered |
number (%) |
38 (80.9) |
47 (94.0) |
| Breeds |
|
|
|
| Domestic Shorthair |
number (%) |
33 (70.2) |
33 (66.0) |
| Japanese Bobtail |
number (%) |
3 (6.4) |
2 (4.0) |
| American Shorthair |
number (%) |
3 (6.4) |
3 (6.0) |
| Other |
number (%) |
8 (17.0) |
12 (24.0) |
| Biochemistry |
|
|
|
| Creatinine (mg/dL) |
median (25th, 75th percentile) |
3.2 (3.0, 3.6) |
3.2 (3.0, 3.4) |
| Urea (mg/dL) |
median (25th, 75th percentile) |
36.0 (30.5, 44.0) |
40.5 (35.3, 48.8)*
|
| Phosphate (mg/dL) |
median (25th, 75th percentile) |
4.3 (3.8, 5.0) |
4.4 (3.9, 5.2) |
| Calcium (mg/dL) |
median (25th, 75th percentile) |
9.8 (9.4, 10.4)*
|
9.5 (9.0, 10.0) |
| Potassium (mmol/L) |
median (25th, 75th percentile) |
4.0 (3.7, 4.2) |
3.9 (3.5, 4.2) |
| Hematology |
|
|
|
| Packed cell volume (%) |
median (25th, 75th percentile) |
37.0 (32.5, 41.0) |
35.0 (31.0, 40.0) |
| Urinalysis |
|
|
|
| Urine specific gravity |
median (25th, 75th percentile) |
1.014 (1.013, 1.017) |
1.014 (1.012, 1.018) |
| Urine protein (mg/dL) |
mean (SD) |
22.8 (51.5) |
52.7 (161.3) |
| Blood pressure measurement |
|
|
|
| Systolic blood pressure (mmHg) |
median (25th, 75th percentile) |
158.0 (140.0, 166.0) |
155.0 (137.5, 160.5) |
| Treatment |
|
|
|
| Beraprost (RAPROS) |
number (%) |
57 (100.0%)**
|
0 (0.0%) |
| Dose (μg/kg twice daily) |
mean (SD, range) |
14.4 (3.6, 7.1-22.2)**
|
0 (0, 0-0) |
| Subcutaneous fluid therapy (total) |
number (%) |
33 (70.2%) |
43 (86.0%) |
| At clinic |
number (%) |
27 (57.4%) |
38 (76.0%) |
| At home |
number (%) |
20 (42.6%) |
28 (56.0%) |
| Renal Diet (total) |
number (%) |
28 (59.6%) |
25 (50.0%) |
| Royal Canin (Renal Support) |
number (%) |
17 (36.2%) |
20 (40.0%) |
| Hill's (k/d) |
number (%) |
13 (27.7%) |
11 (22.0%) |
| Other |
number (%) |
19 (40.4%)*
|
9 (18.0%) |
| Phosphate binder |
number (%) |
9 (19.1%) |
13 (26.0%) |
| Ferric chloride (Lenziaren) |
| Oral activated charcoal |
number (%) |
13 (27.7%) |
13 (26.0%) |
| ACEI/ARB (total) |
number (%) |
12 (25.5%) |
14 (28.0%) |
| Benazepril (Fortekor) |
number (%) |
7 (14.9%) |
12 (24.0%) |
| Telmisartan (Semintra) |
number (%) |
5 (10.6%) |
5 (10.0%) |
| Calcium channel blocker |
number (%) |
7 (14.9%) |
2 (4.0%) |
| Amlodipine |
| Erythrocyte-stimulating agents |
number (%) |
8 (17.0%) |
13 (26.0%) |
| Darbepoetin alfa |
| Managing inappetence, nausea and vomiting (total) |
number (%) |
36 (76.6%) |
41 (82.0%) |
| Maropitant (Cerenia) |
number (%) |
29 (61.7%) |
37 (74.0%) |
| Mirtazapine |
number (%) |
18 (38.3%) |
13 (26.0%) |
| Famotidine |
number (%) |
14 (29.8%) |
8 (16.0%) |
| Omeprazole |
number (%) |
2 (4.3%) |
4 (8.0%) |
| Metoclopramide |
number (%) |
4 (8.5%) |
6 (12.0%) |
| Coexisting Disorders |
|
|
|
| Any |
number (%) |
11 (23.4%) |
13 (26.0%) |
| Hyperthyroidism |
number (%) |
6 (12.8%) |
4 (8.0%) |
| Congestive heart failure |
number (%) |
0 (0.0%) |
4 (8.0%) |
| Neoplasia* |
number (%) |
3 (6.4%) |
3 (6.0%) |
| Diabetes mellitus |
number (%) |
1 (2.1%) |
1 (2.0%) |
| Pancreatitis |
number (%) |
1 (2.1%) |
1 (2.0%) |
Table 8.
Onset of chronic disorders of the two groups in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
Table 8.
Onset of chronic disorders of the two groups in cohort A: IRIS CKD stage 3 (creatinine: 2.9-5.0 mg/dL).
| |
|
Total(n = 134) |
Beraprost therapy(n = 57) |
No Beraprost therapy
(n = 77)
|
| Any |
number (%) |
21 (15.7%) |
5 (8.8%) |
16 (20.8%) |
| Cardiovascular disorders (total) |
number (%) |
10 (7.5%) |
2 (3.5%) |
8 (10.4%) |
| Congestive heart failure |
number (%) |
4 (3.0%) |
0 (0.0%) |
4 (5.2%) |
| Cardiomyopathy |
number (%) |
6 (4.5%) |
2 (3.5%) |
4 (5.2%) |
| Endocrine disorders (total) |
number (%) |
2 (1.5%) |
0 (0.0%) |
2 (2.6%) |
| Hyperthyroidism |
number (%) |
1 (0.7%) |
0 (0.0%) |
1 (1.3%) |
| Diabetes mellitus |
number (%) |
1 (0.7%) |
0 (0.0%) |
1 (1.3%) |
| Neuromuscular disorders (total) |
number (%) |
3 (2.2%) |
0 (0.0%) |
3 (3.9%) |
| Epilepsy & Seizures |
number (%) |
3 (2.2%) |
0 (0.0%) |
3 (3.9%) |
| Neoplasia (total) |
number (%) |
6 (4.5%) |
3 (5.3%) |
3 (3.9%) |
| Mast cell tumor |
number (%) |
1 (0.7%) |
1 (1.8%) |
0 (0.0%) |
| Lymphoma |
number (%) |
1 (0.7%) |
0 (0.0%) |
1 (1.3%) |
| Squamous cell carcinoma |
number (%) |
1 (0.7%) |
1 (1.8%) |
0 (0.0%) |
| Fibrosarcoma |
number (%) |
1 (0.7%) |
0 (0.0%) |
1 (1.3%) |
| Pulmonary adenocarcinoma |
number (%) |
1 (0.7%) |
1 (1.8%) |
0 (0.0%) |
| Mesothelioma |
number (%) |
1 (0.7%) |
0 (0.0%) |
1 (1.3%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).