1. Introduction
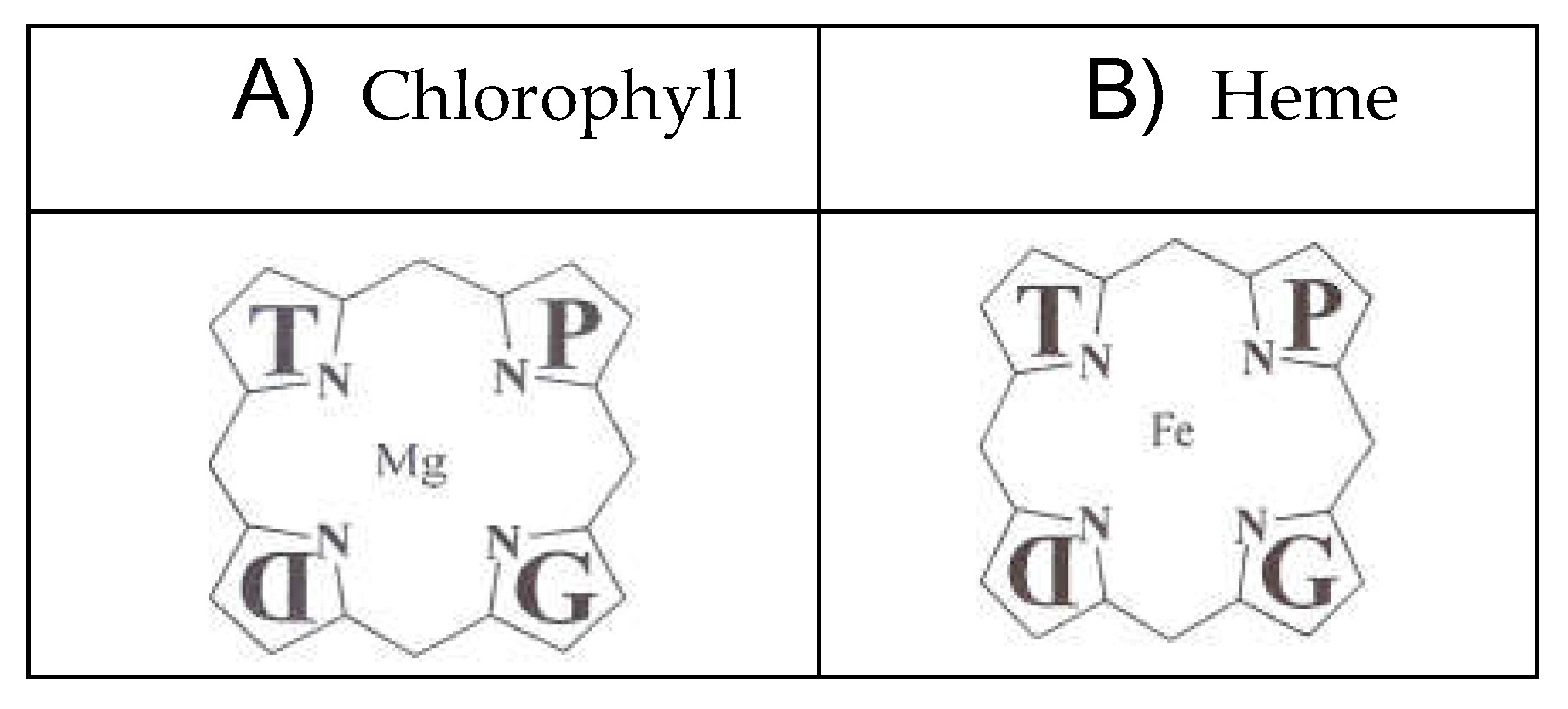
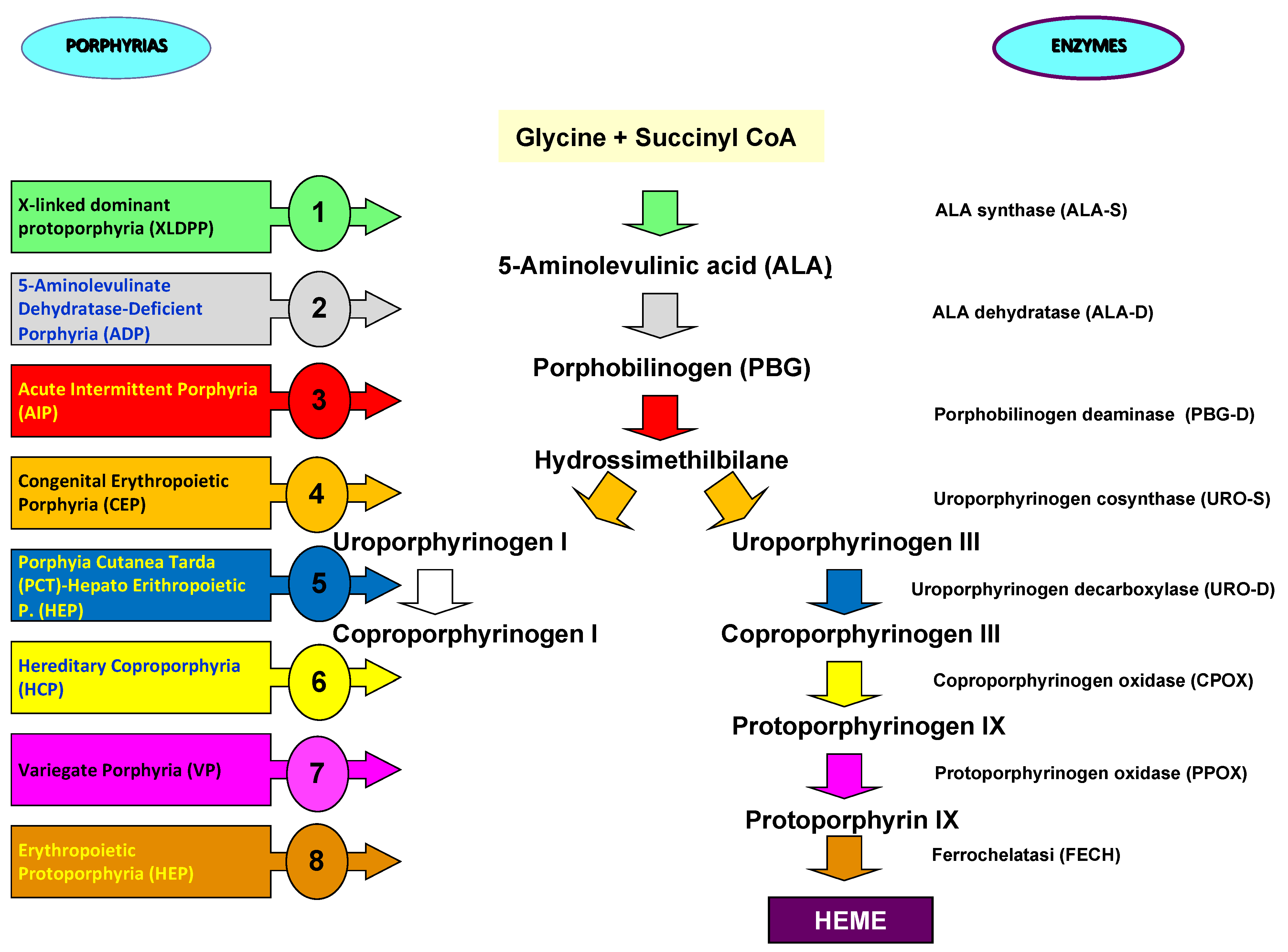
Porphyrias are inherited disorders of heme biosynthesis caused by defects in the metabolism of porphyrins, a group of pigments composed of four pyrrole nuclei connected to each other by methine bridges. Acute hepatic porphyrias have a prevalence of 5 carriers per 100.000 people [
1,
2,
3]. Porphyrins are widespread in the animal and plant world as chlorophyll and, as a heme group, cofactor of hemoproteins such as hemoglobin, cytochromes, catalase and peroxidase [
1,
2,
3] (
Figure 1).
The nitrogen atoms of each pyrrole ring are bonded to a metal atom such as iron in hemoglobin or magnesium in chlorophyll. Porphyrins can be classified into five categories: uroporphyrins, coproporphyrins, erythroporphyrins, protoporphyrins, hematoporphyrins. Their synthesis occurs in the liver and in the cells of the hematopoietic tissue. The inappropriate or excessive production of porphyrins leads to pathologies called porphyrias, characterized by elevated levels of δ-aminolevulinic acid (ALA) and porphobilinogen (PBG). Porphyrias are the result of partial defects in one or more of the eight enzymes involved in heme biosynthesis [
1,
2,
3] (
Figure 2).
Attacks in acute intermittent porphyria (AIP) are characterized by abdominal pain, neurological symptoms and psychiatric disturbances and, in the most severe cases, can lead to respiratory paralysis and coma. In some cases, the metabolic alteration can be acquired. Porphyria has a mortality of 20-25% within the first five years after the first attack. Renal involvement in acute porphyrias includes hyponatremia, urinary retention, tubule-interstitial nephropathy, hypertension, and chronic kidney disease. Most patients suffer from renal colic associated with pallor, nausea, vomiting, fever, acute urine retention and dark urine [
4,
5,
6,
7,
8].
The causes of acute porphyria attacks can be different: drugs, alcohol, stress, fasting, menstrual cycle, infections. An acute attack may be preceded by a period of varying degrees of behavioral changes such as anxiety, irritability, restlessness and insomnia and may rapidly progress to symptoms of severe autonomic neuropathy, and acute sensory and motor neuropathy (similar to Guillain-Barre syndrome) is quite common. It can progress to general paralysis leading to severe respiratory failure up to and including death from cardiorespiratory arrest [
4,
5,
6,
7,
8].
Porphyria patients (mainly patients suffering from acute intermittent porphyria and variegate porphyria) are at risk of developing two types of primary liver tumors, i.e. hepatocellular carcinomas (HCC) or cholangiocarcinomas (CCA), a rare cancer of the biliary tract [
9]. In a study conducted in the United States of America, 1.5% of acute hepatic porphyria patients were diagnosed with HCC in the absence of cirrhosis, differently from other chronic liver diseases, suggesting that acute hepatic porphyria patients aged 50 years and beyond should be screened for HCC [
10]. Anyway, the molecular mechanisms bringing on hepato-carcinogenesis in acute hepatic porphyria have not been identified yet and need further investigation.
Here, we describe a clinical case of a patient affected by acute intermittent porphyria that developed intrahepatic cholangiocarcinoma.
2. Results
The patient under examination was diagnosed as suffering from acute hepatic porphyria in October 1993, at the age of 43 years, with hospitalization at the Molinette Hospital of Turin after double acceptance at the Emergency Department of the Piedmont’s hospital, first with symptoms of medical relevance and subsequently with symptoms of surgical relevance, in two consecutive days. Before hospital discharge, a laboratory evaluation was performed for possible porphyria with evidence of altered urinary values of ALA (15 mg/dl, NV: 1.3-7.0), total porphyrins (249 µg/mL/24h, NV: 50-200), uroporphyrins (76 µg/mL/24h, NV: 15-50), coproporphyrins (173 (µg/mL/24h, NV: 35-150) (
Table 1).
In December 2022, due to weight loss of more than 10 kg and persistent lameness and left groin pain, the patient underwent pelvic MRI with evidence of a skeletal lesion with high osteoblastic activity at the level of the left lesser femoral trochanter (suspected repetitive lesion). Then she was hospitalized in the Orthopedic Unit of the Perrino Hospital, Brindisi, for pathological fracture of the proximal left femur with implantation of a cemented femoral endo-prosthesis and histological evidence of bone localization of poorly differentiated adenocarcinoma of probable origin from the bilio-pancreatic ducts. This diagnosis was corroborated by high metabolic activity in the liver at PET-CT and MRI, indicative of massive solid, heterologous expansive process (mass forming intrahepatic cholangiocarcinoma) in correspondence of the V-VI-VII-VIII segment with satellite nodular lesions, suspected thrombosis of portal branches in the V segment, lymphadenopathy in the hepatic hilum and celiac site and thick-walled gallbladder with thick bile and microstones. No other metastatic localization was identified.
Due to abdominal pain associated with confusion and dyspneic breathing, the patient was admitted to our hospital, in the Interregional Reference Center for the prevention, diagnosis and treatment of Porphyrias, Division of Nephrology, San Giovanni Rotondo. On admission, she presented a globe-shaped abdomen due to fat, painful on deep palpation over the whole area and with slight splenomegaly. The patient was fasted and subjected to total parenteral nutrition by means of a femoral vein catheter considering her precarious health conditions. Laboratory parameters showed progressive clinical and functional deterioration with increase of laboratory features of porphyria (
Table 2).
In consideration of the fact that, on the occasion of the hospitalization at the Molinette Hospital in Turin, no mention was made of any biomolecular examinations which genetically could confirm the diagnosis of acute intermittent porphyria, a genetic test was carried out with Next Generation Sequencing (NGS) for the identification of mutations related to porphyrias with detection of the c.874C>T variant for the
HMBS gene. Interestingly, in a case of porphyria-associated HCC, a second inactivating mutation in the
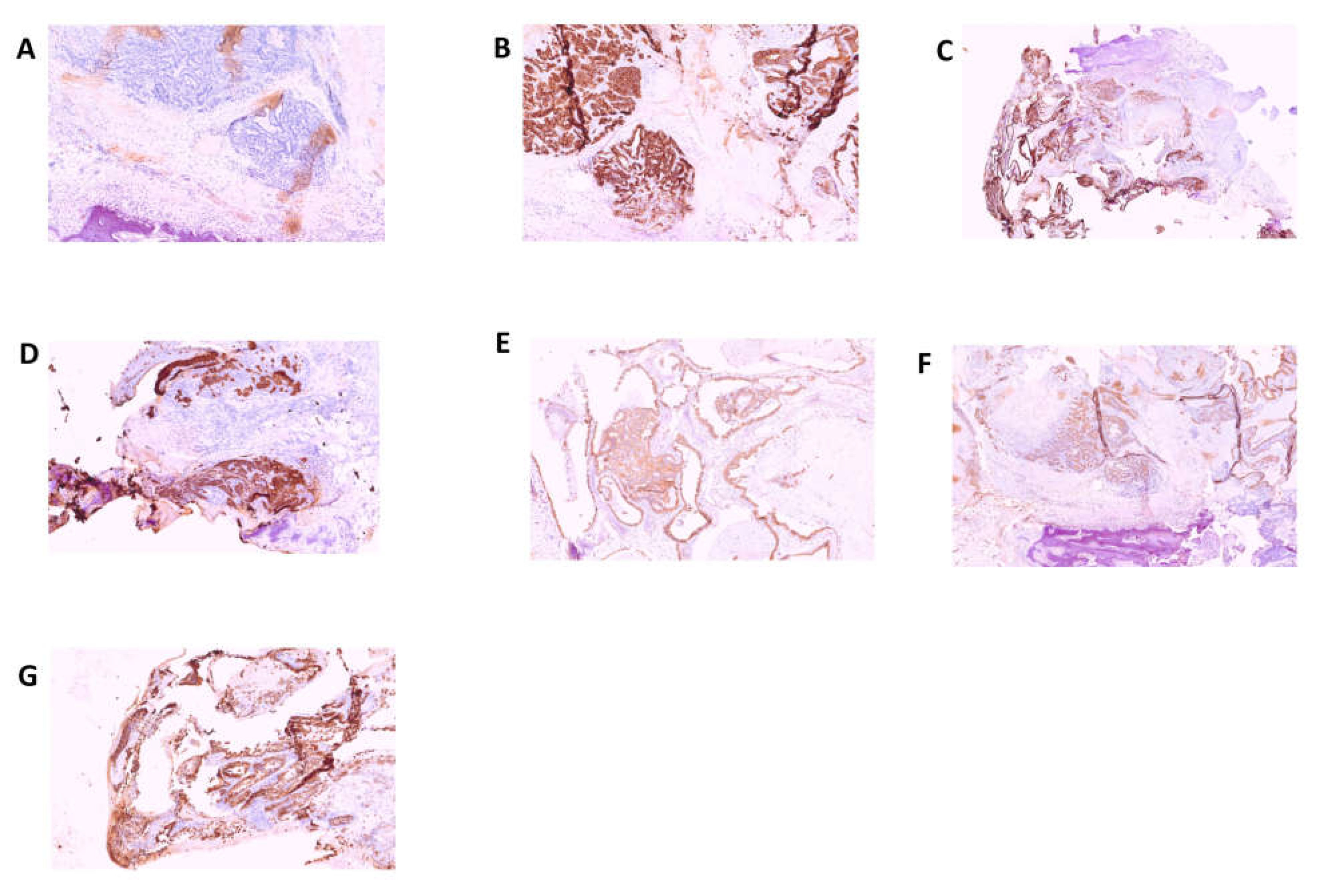
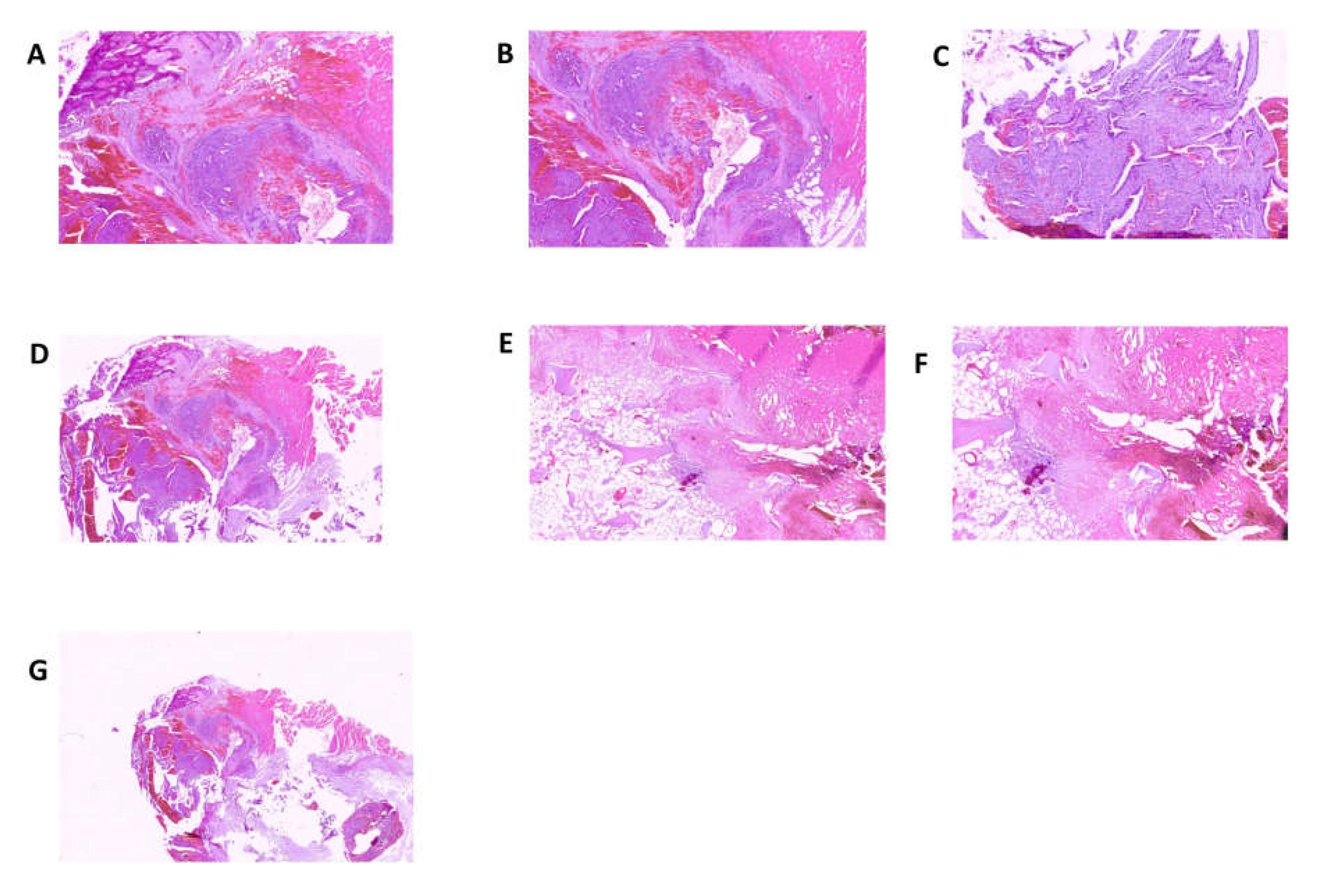
HMBS gene was found in the tumor tissue but not in the matched non-tumorous tissue, whereas in porphyria-associated CCA no forms of mutation were previously found in the liver tissue affected by the neoplastic proliferation. The anatomopathological examination was performed on biopsy specimens. Macroscopic examination took into consideration three fragments of grayish color, irregular shape, with diameters of 3.5 cm, 1.5 cm and 1.3 cm, respectively. The femoral head measuring 4 x 4 x 5.5 cm with a brownish area at the base and a further bone segment 12.5 cm long with a brownish area at one end were examined. The entire sample was subjected to multiple decalcification cycles. The histological examination and immunohistochemistry were performed using tissue sections stained with relevant immunohistochemical panels to support diagnosis of metastatic carcinomas of unknown primary site with antibodies directed to cytokeratins (CK7, CK19, CK20, AE1/AE3), epithelial membrane (EMA), CDX2 (caudal type homeobox 2), TTF1 (thyroid transcription factor 1), GATA3 (GATA binding protein 3), PAX8 (paired box gene 8) and estrogen receptor. The following staining pattern was defined: AE1/AE3+, CK19+, EMA+, CDX2-, CK7+, CK20-/+, estrogen receptor-, TTF1, GATA 3-, PAX 8-. The resulting diagnosis was the following: bone localization of poorly differentiated adenocarcinoma of probable bilio-pancreatic ductal origin (
Figure 3 and 4).
The clinical conditions rapidly further deteriorated with evidence of septicemia caused by Staphylococcus hominis with right subclavian-axillohumeral venous thrombosis, acute renal failure at the 1st K-DIGO stage, radiological evidence of a left thickening and pulmonary nodule and bilateral pleural effusion, marked anemia that was treated with blood transfusions. The very serious conditions of the patient prevented any form of oncological therapy and, in a short time, death occurred.
3. Discussion
Heme manages crucial roles in different bio-systems as a member of the porphyrins family of cyclic tetrapyrroles. All hemes encompass macrocycles composed of four pyrrole-derived rings centered by methine bridges and include a central iron ion joined by the four pyrrole nitrogen atoms [
11]. Heme is the prosthetic group necessary for oxygen transport and storage in hemoproteins, for example hemoglobin and myoglobin, and is required in various cytochromes for electron transport and in cytochrome P450 for mixed function oxidases. Moreover, heme is a cofactor for catalase aimed at hydrogen peroxide decomposition and for peroxidase aimed at hydrogen peroxide production. Likewise, heme upkeeps diatomic gases sensing and signaling, gene transcription/translation, microRNA processing, protein stability, mitochondrial protein import, metabolic pathways, drug detoxification and biological clock functioning. Heme synthesis deficiency can cause porphyrias together with different pathological conditions, including anemias, kidney disease, cerebral hypoperfusion; besides, accumulation of porphyrins triggers toxic effects on hepatocytes, leading to free radical formation, hepatocyte harm and subsequent DNA damage [
11,
12,
13,
14,
15].
As reported above, a cross-sectional multicentric, longitudinal study evaluated patients with HCC in acute hepatic porphyrias confirmed by biochemical and/or genetic testing and reported 1.5% patients with HCC out of 327 acute hepatic porphyria patients, four with acute intermittent porphyria and one with variegate porphyria [
10]. Anyway, we cannot totally exclude the possibility that, in the considered porphyria patient, CCA might be sporadic.
Primary liver cancer is the most reported tumor in patients with acute hepatic porphyria, predominantly HCC and with rare occurrence of CCA. A recent meta-analysis included 7381 patients with porphyria (3476 females) and reported occurrence of primary liver cancer in 4.8% of patients, precisely 3.3% diagnosed with HCC and 0.3% of the total diagnosed with CCA [
16]. Due to the rarity of the disease, screening and follow-up of patients and their relatives must take into account the balance of benefits, costs and harms. Any form of surveillance is potentially harmful, for example by causing anxiety to otherwise asymptomatic individuals and by generating some false positive screening tests. For a pragmatic approach to surveillance, in Sweden annual abdominal ultrasound monitoring is recommended for patients over 50 years of age, whereas α-fetoprotein does not appear to be relevant for monitoring AHP patients [
16,
17,
18,
19].
In other European countries, ultrasound is recommended at least once a year [
3]. The creation of an international consensus between the two main societies dedicated to porphyria (American Porphyria Consortium, APC and EPNET) is necessary [
17,
18,
19,
20].
The possible diagnosis of primary liver tumor must be taken into consideration in any form of acute hepatic porphyria especially in patients with unexplained liver function tests derangement as well as for the presence of elements indicative of incipient liver disease or overt neoplasia. This consideration must be extended to porphyria patients with manifestations of acute liver disease especially after the age of 50 years [
17,
18,
19,
20]. As a therapeutic option of primary liver tumors in patients with acute hepatic porphyria, liver transplantation should be taken into account, especially considering that this therapeutic option will also allow curing acute hepatic porphyria [
21,
22,
23].
4. Conclusions
Acute intermittent porphyria is associated with an increased risk of primary liver cancer, while the risk remains unclear in variegate porphyria and hereditary coproporphyria. At present, the cost-benefit ratio for an adequate surveillance of these pathological models is unknown, even if the annual incidence rate would justify the surveillance as for non-cirrhotic subjects with pathology caused by hepatitis B virus infection. In Europe, ultrasound surveillance is recommended at least every two years as indicated for other liver diseases with an increased risk of neoplastic disease such as HCC and CCA. Our case report strongly suggests that punctual and serial laboratory and instrumental surveillance is necessary in acute hepatic porphyria patients.
Author Contributions
C.C.G., G.M. and M.N. conceived the study; C.C.G., M.N., G.M. T.L., Fr.A., F.A.,T.L., A.C., G.F., F.N., acquired and analyzed data; L.F. performed the anatomopathological examination and the immunohistochemical analyses; G.M. and C.C.G. wrote the manuscript. All authors have read and approved the journal’s authorship agreement. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the “5x1000” voluntary contribution and by a grant to G.M. from the Italian Ministry of Health (RC2022-2024).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
The enrolled subject gave written informed consent to participate.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank our nurses and technicians for procedural support. We would like to thank Chiara Di Giorgio for reviewing and proofreading this manuscript for English language.
Conflicts of Interest
The authors declare that there are no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Bissell, D.M.; Anderson., K.E.; Bonkovsky., H.L. Porphyria. N. Engl. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Bustad, H.J.; Kallio, J.P.; Vorland, M.; Fiorentino, V.; Sandberg, S.; Schmitt, C.; Aarsand, A.K.; Martinez, A. Acute Intermittent Porphyria: An Overview of Therapy Developments and Future Perspectives Focusing on Stabilisation of HMBS and Proteostasis Regulators. Int. J. Mol. Sci. 2021, 22, 675. [Google Scholar] [CrossRef]
- Yasuda, M.; Chen, B.; Desnick, R.J. Recent advances on porphyria genetics: Inheritance, penetrance & molecular heterogeneity, including new modifying/causative genes. Mol. Genet. Metab. 2018, 128, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Wylie, K.; Testai, F.D. Neurological Manifestations of Acute Porphyrias. Curr. Neurol. Neurosci. Rep. 2022, 22, 355–362. [Google Scholar] [CrossRef]
- Puy, H.; Gouya, L.; Deybach, J.-C. Porphyrias. Lancet 2010, 375, 924–937. [Google Scholar] [CrossRef]
- Stein, P.E.; Badminton, M.N.; Rees, D.C. Update review of the acute porphyrias. Br. J. Haematol. 2016, 176, 527–538. [Google Scholar] [CrossRef]
- Baravelli, C.M.; Sandberg, S.; Aarsand, A.K.; Nilsen, R.M.; Tollånes, M.C. Acute hepatic porphyria and cancer risk: a nationwide cohort study. J. Intern. Med. 2017, 282, 229–240. [Google Scholar] [CrossRef]
- Saberi, B.; Naik, H.; Overbey, J.R.; Erwin, A.L.; Anderson, K.E.; Bissell, D.M.; Bonkovsky, H.L.; Phillips, J.D.; Wang, B.; K. Singal, A.; et al. Hepatocellular Carcinoma in Acute Hepatic Porphyrias: Results from the Longitudinal Study of the U.S. Porphyrias Consortium. Hepatology 2020, 73, 1736–1746. [Google Scholar] [CrossRef]
- Ogun., A.S.; Joy., N.V.; Valentine., M. Biochemistry. Heme Synthesis. 2022. [Google Scholar]
- Valle, G.; Guida, C.C.; Nasuto, M.; Totaro, M.; Aucella, F.; Frusciante, V.; Di Mauro, L.; Potenza, A.; Savino, M.; Stanislao, M.; et al. Cerebral Hypoperfusion in Hereditary Coproporphyria (HCP): A Single Photon Emission Computed Tomography (SPECT) Study. Endocrine, Metab. Immune Disord. - Drug Targets 2016, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Guida, C.C.; Manzini, P.; Cuoghi, C.; Ventura, P. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Cappellini, M.D.; Biolcati, G.; Guida, C.C.; Rocchi, E. A challenging diagnosis for potential fatal diseases: Recommendations for diagnosing acute porphyrias. Eur. J. Intern. Med. 2014, 25, 497–505. [Google Scholar] [CrossRef]
- Savino, M.; Guida, C.C.; Nardella, M.; Murgo, E.; Augello, B.; Merla, G.; De Cosmo, S.; Savino, A.F.; Tarquini, R.; Cei, F.; et al. Circadian Genes Expression Patterns in Disorders Due to Enzyme Deficiencies in the Heme Biosynthetic Pathway. Biomedicines 2022, 10, 3198. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Deliwala, S.S.; Chandan, S.; Lester, J.; Singh, J.; Samanta, J.; di Nunzio, S.; Perversi, F.; Cappellini, F.; Shah, A.; et al. Risk of Hepatocellular Carcinoma in Patients with Porphyria: A Systematic Review. Cancers 2022, 14, 2947. [Google Scholar] [CrossRef]
- Balwani, M.; Wang, B.; Anderson, K.E.; Bloomer, J.R.; Bissell, D.M.; Bonkovsky, H.L.; Phillips, J.D.; Desnick, R.J. ; for the Porphyrias Consortium of the Rare Diseases Clinical Research Network Acute hepatic porphyrias: Recommendations for evaluation and long-term management. Hepatology 2017, 66, 1314–1322. [Google Scholar] [CrossRef]
- Innala, E.; Andersson, C. Screening for hepatocellular carcinoma in acute intermittent porphyria: a 15-year follow-up in northern Sweden. J. Intern. Med. 2010, 269, 538–545. [Google Scholar] [CrossRef]
- Neeleman, R.A.; Wagenmakers, M.A.E.M.; Koole-Lesuis, R.H.; Mijnhout, G.S.; Wilson, J.H.P.; Friesema, E.C.H.; Langendonk, J.G. Medical and financial burden of acute intermittent porphyria. J. Inherit. Metab. Dis. 2018, 41, 809–817. [Google Scholar] [CrossRef]
- Lissing, M.; Vassiliou, D.; Floderus, Y.; Harper, P.; Bottai, M.; Kotopouli, M.; Hagström, H.; Sardh, E.; Wahlin, S. Risk of primary liver cancer in acute hepatic porphyria patients: A matched cohort study of 1244 individuals. J. Intern. Med. 2022, 291, 824–836. [Google Scholar] [CrossRef]
- Lissing, M.; Nowak, G.; Adam, R.; Karam, V.; Boyd, A.; Gouya, L.; Meersseman, W.; Melum, E.; Ołdakowska-Jedynak, U.; Reiter, F.P.; et al. Liver Transplantation for Acute Intermittent Porphyria. Liver Transplant. 2020, 27, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, T.; Bronisch, O.; Knösel, T.; Mogler, C.; Weichert, W.; Stauch, T.; Schmid, C.; Rummeny, C.; Beykirch, M.K.; Petrides, P.E. Heterogeneous molecular behavior in liver tumors (HCC and CCA) of two patients with acute intermittent porphyria. J. Cancer Res. Clin. Oncol. 2022, 149, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Peoc'H, K.; Manceau, H.; Karim, Z.; Wahlin, S.; Gouya, L.; Puy, H.; Deybach, J.-C. Hepatocellular carcinoma in acute hepatic porphyrias: A Damocles Sword. Mol. Genet. Metab. 2019, 128, 236–241. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).