Submitted:
04 April 2023
Posted:
06 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Biological Significance of the SUMO Pathway
1.2. Role of SUMO in Neuroprotection
1.2.1. SUMO and the Ischemia Response
1.2.2. Maintenance of Ion Homeostasis
1.2.3. Preservation of Neural Stem Cell Populations
2. SUMOtherapeutics for Cerebral Ischemia
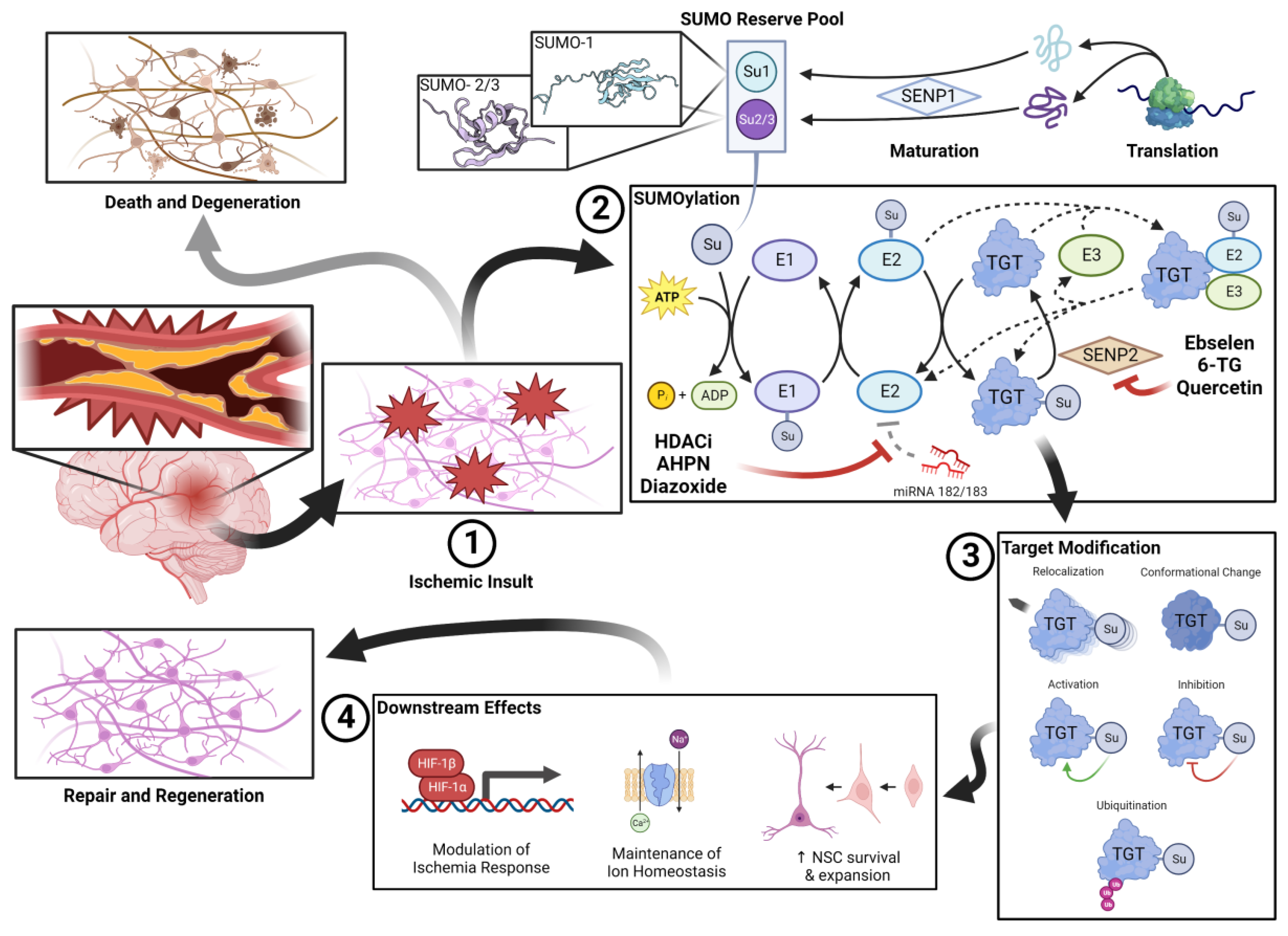
2.1. miRNA-182/183 Inhibitors
2.2. SENP Inhibitors
| Drug | Tissue/ Animal |
Ischemia Model | Intervention | Measured Outcome |
Results Summary | Study | |
|---|---|---|---|---|---|---|---|
| Quercetin | SHSY5Y, B35, E18 PCN | SHSY5Y, B35: 16hr OGD + 5hr recoveryE18 PCN: 5hr OGD + 16hr recovery | Drug treatment ± pre-treatment | SENP activity, SUMOylation, cell survival, LDH release | Decrease in SENP expression*, Increases in SUMOylation*; increase in cell survival (in SHSY5Y and E18 PCN)*; Decrease in LDH release with co-treatment alone and with pre-treatment* | Lee et al., 2016 [84] | |
| Isoprenaline HCl | B35 | 20hr OGD | 4hr pretreatment + treatment | SUMO-1 expression, cell survival | Increase in SUMO-2/3 conjugation*, no significant OGD protection | Bernstock et al., 2018 [82] | |
| Ethyl protocatechuate | |||||||
| 6-thioguanine | SUMO-1 upregulation*, OGD protection* | ||||||
| Ebselen | |||||||
| C57BL6 mice | N/A | 12mg/kg IP bolus treatment | SUMOylation 1hr after bolus | Increases in SUMO-1 and SUMO-2/3 conjugation* | |||
| SHRSP, WKY rat PDN | 24hr OGD + 3hr recovery | treatment + 3hr posttreatment | Cell survival, LDH activity | OGD protection*, no significant difference in LDH activity | Yamagata et al., 2008 [96] | ||
| SHRSP, WKY rat | 0.5 hr BCCO | 30 mg/kg/day pretreatment for 7 days then 30mg/kg/day posttreatment for 3 days | Apoptotic neurons in CA1 subfield of hippocampus | Almost complete inhibition of apoptosis† | |||
| N/A | 60 mg/kg/day treatment for 6 wks | Oxidative stress (via cortical NO and MDA concentrations); iNOS expression | Reduction in NO and MDA concentrations*; reduction in iNOS expression* | Sui et al., 2005 [97] | |||
| Human | MCAO | 150mg PO BID post-treatment within 12 hours of onset for 2 weeks. Placebo controlled, double blind trial. | Infarct volume 1mo post stroke, GOS 3mo post stroke | Reduction in infarct volume*; superior GOS if administered within 6 hours*; no significant difference in GOS vs. negative control overall | Ogawa et al., 1999 [89] | ||
| AIS | 150mg PO BID posttreatment within 48hrs of onset for 2 weeks. Placebo controlled, double blind trial. | GOS (1- and 3-month), neurological status (2wk, modified Mathew Scale), functional status (2 wk, Barthel Index) | Improvement in 1-month GOS* but no significant difference in 3-month GOS; superior GOS if administered ≤ 24 hrs*; reduction in both impairment (Mathew)* and disability (Barthel)* | Yamaguchi et al., 1998 [90] | |||
| SD rats |
Permanent MCAO | 1mg/kg/hr pretreatment 45min pre-stroke to 4hrs post-stroke | Extent of ischemic damage, oxidative stress (via IHC) | 28% reduction in cortical ischemic damage vs. control†; reduction in oxidative stress markers vs. control† | Imai et al., 2003 [98] | ||
| 2hr FCI | 1mg/kg IV bolus + 1mg/kg/hr IV post-treatment for 24hrs | 24 hr neurological deficit, gray matter damage, Axonal damage and oxidative stress (via IHC) | 40.7% reduced neurological deficit at 24hrs vs control*; 53.6% reduction in gray matter damage*; 46.8% reduction in axonal damage IHC markers* | Imai et al., 2001 [99] | |||
| Wistar rats |
45 min BCCO | 30mg/kg PO bolus pretreatment 2hrs prior to stroke | cortical EAA and NO concentrations, 24hr hippocampal CA1 subfield integrity | Increase in intact CA1 neurons*; No difference in EAA or NO concentrations vs control. | Koizumi et al., 2011 [87] | ||
| 2hr FCI | 10mg/kg and 100mg/kg PO bolus pretreatment 1hr prior to FCI | Reduced glutathione concentration; plasma Selenium; 1wk post stroke infarct size | Increase in perfusion with 100mg/kg*; increase in plasma Selenium* | Salom et al., 2004 [100] | |||
| Wistar rat cerebellar neurons | Glutamate exposure | 25min treatment ± posttreatment; posttreatment | 24hr cell survival, 48hr cell survival | Increase in survival with treatment* and posttreatment* comparable to negative control | Porciúncula et al., 2001 [93] | ||
| Wistar rat hippocampal neurons | 45min OGD | Pretreatment and posttreatment | 3hr cell survival | Increase in survival with treatment* and post-treatment* comparable to negative control | Porciúncula et al., 2003 [94] |
2.3. Direct SUMO Upregulators
3. Near Future Innovation in SUMOtherapeutics
4. Conclusions
References
- "Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019." Lancet Neurol 20, no. 10 (2021): 795-820.
- Albers, G. W., M. P. Marks, S. Kemp, S. Christensen, J. P. Tsai, S. Ortega-Gutierrez, R. A. McTaggart, M. T. Torbey, M. Kim-Tenser, T. Leslie-Mazwi, A. Sarraj, S. E. Kasner, S. A. Ansari, S. D. Yeatts, S. Hamilton, M. Mlynash, J. J. Heit, G. Zaharchuk, S. Kim, J. Carrozzella, Y. Y. Palesch, A. M. Demchuk, R. Bammer, P. W. Lavori, J. P. Broderick, M. G. Lansberg, and Defuse Investigators. "Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging." N Engl J Med 378, no. 8 (2018): 708-18. [CrossRef]
- Nogueira, R. G., A. P. Jadhav, D. C. Haussen, A. Bonafe, R. F. Budzik, P. Bhuva, D. R. Yavagal, M. Ribo, C. Cognard, R. A. Hanel, C. A. Sila, A. E. Hassan, M. Millan, E. I. Levy, P. Mitchell, M. Chen, J. D. English, Q. A. Shah, F. L. Silver, V. M. Pereira, B. P. Mehta, B. W. Baxter, M. G. Abraham, P. Cardona, E. Veznedaroglu, F. R. Hellinger, L. Feng, J. F. Kirmani, D. K. Lopes, B. T. Jankowitz, M. R. Frankel, V. Costalat, N. A. Vora, A. J. Yoo, A. M. Malik, A. J. Furlan, M. Rubiera, A. Aghaebrahim, J. M. Olivot, W. G. Tekle, R. Shields, T. Graves, R. J. Lewis, W. S. Smith, D. S. Liebeskind, J. L. Saver, T. G. Jovin, and Dawn Trial Investigators. "Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct." N Engl J Med 378, no. 1 (2018): 11-21. [CrossRef]
- Berkhemer, O. A., P. S. Fransen, D. Beumer, L. A. van den Berg, H. F. Lingsma, A. J. Yoo, W. J. Schonewille, J. A. Vos, P. J. Nederkoorn, M. J. Wermer, M. A. van Walderveen, J. Staals, J. Hofmeijer, J. A. van Oostayen, G. J. Lycklama a Nijeholt, J. Boiten, P. A. Brouwer, B. J. Emmer, S. F. de Bruijn, L. C. van Dijk, L. J. Kappelle, R. H. Lo, E. J. van Dijk, J. de Vries, P. L. de Kort, W. J. van Rooij, J. S. van den Berg, B. A. van Hasselt, L. A. Aerden, R. J. Dallinga, M. C. Visser, J. C. Bot, P. C. Vroomen, O. Eshghi, T. H. Schreuder, R. J. Heijboer, K. Keizer, A. V. Tielbeek, H. M. den Hertog, D. G. Gerrits, R. M. van den Berg-Vos, G. B. Karas, E. W. Steyerberg, H. Z. Flach, H. A. Marquering, M. E. Sprengers, S. F. Jenniskens, L. F. Beenen, R. van den Berg, P. J. Koudstaal, W. H. van Zwam, Y. B. Roos, A. van der Lugt, R. J. van Oostenbrugge, C. B. Majoie, D. W. Dippel, and Mr Clean Investigators. "A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke." N Engl J Med 372, no. 1 (2015): 11-20. [CrossRef]
- Ma, H., B. C. V. Campbell, M. W. Parsons, L. Churilov, C. R. Levi, C. Hsu, T. J. Kleinig, T. Wijeratne, S. Curtze, H. M. Dewey, F. Miteff, C. H. Tsai, J. T. Lee, T. G. Phan, N. Mahant, M. C. Sun, M. Krause, J. Sturm, R. Grimley, C. H. Chen, C. J. Hu, A. A. Wong, D. Field, Y. Sun, P. A. Barber, A. Sabet, J. Jannes, J. S. Jeng, B. Clissold, R. Markus, C. H. Lin, L. M. Lien, C. F. Bladin, S. Christensen, N. Yassi, G. Sharma, A. Bivard, P. M. Desmond, B. Yan, P. J. Mitchell, V. Thijs, L. Carey, A. Meretoja, S. M. Davis, G. A. Donnan, and Extend Investigators. "Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke." N Engl J Med 380, no. 19 (2019): 1795-803. [CrossRef]
- Anand, S. K., W. J. Benjamin, A. R. Adapa, J. V. Park, D. A. Wilkinson, B. J. Daou, J. F. Burke, and A. S. Pandey. "Trends in Acute Ischemic Stroke Treatments and Mortality in the United States from 2012 to 2018." Neurosurg Focus 51, no. 1 (2021): E2. [CrossRef]
- Goyal, M., B. K. Menon, W. H. van Zwam, D. W. Dippel, P. J. Mitchell, A. M. Demchuk, A. Davalos, C. B. Majoie, A. van der Lugt, M. A. de Miquel, G. A. Donnan, Y. B. Roos, A. Bonafe, R. Jahan, H. C. Diener, L. A. van den Berg, E. I. Levy, O. A. Berkhemer, V. M. Pereira, J. Rempel, M. Millan, S. M. Davis, D. Roy, J. Thornton, L. S. Roman, M. Ribo, D. Beumer, B. Stouch, S. Brown, B. C. Campbell, R. J. van Oostenbrugge, J. L. Saver, M. D. Hill, T. G. Jovin, and Hermes collaborators. "Endovascular Thrombectomy after Large-Vessel Ischaemic Stroke: A Meta-Analysis of Individual Patient Data from Five Randomised Trials." Lancet 387, no. 10029 (2016): 1723-31. [CrossRef]
- Yoshimura, S., N. Sakai, H. Yamagami, K. Uchida, M. Beppu, K. Toyoda, Y. Matsumaru, Y. Matsumoto, K. Kimura, M. Takeuchi, Y. Yazawa, N. Kimura, K. Shigeta, H. Imamura, I. Suzuki, Y. Enomoto, S. Tokunaga, K. Morita, F. Sakakibara, N. Kinjo, T. Saito, R. Ishikura, M. Inoue, and T. Morimoto. "Endovascular Therapy for Acute Stroke with a Large Ischemic Region." N Engl J Med 386, no. 14 (2022): 1303-13. [CrossRef]
- Sarraj, A., A. E. Hassan, M. G. Abraham, S. Ortega-Gutierrez, S. E. Kasner, M. S. Hussain, M. Chen, S. Blackburn, C. W. Sitton, L. Churilov, S. Sundararajan, Y. C. Hu, N. A. Herial, P. Jabbour, D. Gibson, A. N. Wallace, J. F. Arenillas, J. P. Tsai, R. F. Budzik, W. J. Hicks, O. Kozak, B. Yan, D. J. Cordato, N. W. Manning, M. W. Parsons, R. A. Hanel, A. N. Aghaebrahim, T. Y. Wu, P. Cardona-Portela, N. Perez de la Ossa, J. D. Schaafsma, J. Blasco, N. Sangha, S. Warach, C. D. Gandhi, T. J. Kleinig, D. Sahlein, L. Elijovich, W. Tekle, E. A. Samaniego, L. Maali, M. A. Abdulrazzak, M. N. Psychogios, A. Shuaib, D. K. Pujara, F. Shaker, H. Johns, G. Sharma, V. Yogendrakumar, F. C. Ng, M. H. Rahbar, C. Cai, P. Lavori, S. Hamilton, T. Nguyen, J. T. Fifi, S. Davis, L. Wechsler, V. M. Pereira, M. G. Lansberg, M. D. Hill, J. C. Grotta, M. Ribo, B. C. Campbell, G. W. Albers, and Select Investigators. "Trial of Endovascular Thrombectomy for Large Ischemic Strokes." N Engl J Med (2023). [CrossRef]
- Huo, X., G. Ma, X. Tong, X. Zhang, Y. Pan, T. N. Nguyen, G. Yuan, H. Han, W. Chen, M. Wei, J. Zhang, Z. Zhou, X. Yao, G. Wang, W. Song, X. Cai, G. Nan, D. Li, A. Y. Wang, W. Ling, C. Cai, C. Wen, E. Wang, L. Zhang, C. Jiang, Y. Liu, G. Liao, X. Chen, T. Li, S. Liu, J. Li, F. Gao, N. Ma, D. Mo, L. Song, X. Sun, X. Li, Y. Deng, G. Luo, M. Lv, H. He, A. Liu, J. Zhang, S. Mu, L. Liu, J. Jing, X. Nie, Z. Ding, W. Du, X. Zhao, P. Yang, L. Liu, Y. Wang, D. S. Liebeskind, V. M. Pereira, Z. Ren, Y. Wang, Z. Miao, and Angel-Aspect Investigators. "Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct." N Engl J Med (2023). [CrossRef]
- Filippopoulou, C., G. Simos, and G. Chachami. "The Role of Sumoylation in the Response to Hypoxia: An Overview." Cells 9, no. 11 (2020). [CrossRef]
- Lee, Y. J., S. Miyake, H. Wakita, D. C. McMullen, Y. Azuma, S. Auh, and J. M. Hallenbeck. "Protein Sumoylation Is Massively Increased in Hibernation Torpor and Is Critical for the Cytoprotection Provided by Ischemic Preconditioning and Hypothermia in Shsy5y Cells." J Cereb Blood Flow Metab 27, no. 5 (2007): 950-62. [CrossRef]
- Lee, Y. J., P. Castri, J. Bembry, D. Maric, S. Auh, and J. M. Hallenbeck. "Sumoylation Participates in Induction of Ischemic Tolerance." J Neurochem 109, no. 1 (2009): 257-67. [CrossRef]
- Oliveira, Frmb, E. S. Soares, C. Harms, H. I. Cimarosti, and R. Sordi. "Sumoylation in Peripheral Tissues under Low Perfusion-Related Pathological States." J Cell Biochem 123, no. 7 (2022): 1133-47. [CrossRef]
- Bernstock, J. D., D. G. Ye, A. Griffin, Y. J. Lee, J. Lynch, L. L. Latour, G. K. Friedman, D. Maric, and J. M. Hallenbeck. "Cerebral Ischemia Increases Small Ubiquitin-Like Modifier Conjugation within Human Penumbral Tissue: Radiological-Pathological Correlation." Front Neurol 8 (2017): 738. [CrossRef]
- Hendriks, I. A., D. Lyon, D. Su, N. H. Skotte, J. A. Daniel, L. J. Jensen, and M. L. Nielsen. "Site-Specific Characterization of Endogenous Sumoylation across Species and Organs." Nat Commun 9, no. 1 (2018): 2456. [CrossRef]
- Sahin, U., H. de The, and V. Lallemand-Breitenbach. "Sumoylation in Physiology, Pathology and Therapy." Cells 11, no. 5 (2022).
- Flotho, A., and F. Melchior. "Sumoylation: A Regulatory Protein Modification in Health and Disease." Annu Rev Biochem 82 (2013): 357-85. [CrossRef]
- Yang, W., H. Sheng, and H. Wang. "Targeting the Sumo Pathway for Neuroprotection in Brain Ischaemia." Stroke Vasc Neurol 1, no. 3 (2016): 101-07. [CrossRef]
- Droescher, M., V. K. Chaugule, and A. Pichler. "Sumo Rules: Regulatory Concepts and Their Implication in Neurologic Functions." Neuromolecular Med 15, no. 4 (2013): 639-60. [CrossRef]
- Zhang, L., X. Liu, H. Sheng, S. Liu, Y. Li, J. Q. Zhao, D. S. Warner, W. Paschen, and W. Yang. "Neuron-Specific Sumo Knockdown Suppresses Global Gene Expression Response and Worsens Functional Outcome after Transient Forebrain Ischemia in Mice." Neuroscience 343 (2017): 190-212. [CrossRef]
- Bernstock, J. D., D. Ye, F. A. Gessler, Y. J. Lee, L. Peruzzotti-Jametti, P. Baumgarten, K. R. Johnson, D. Maric, W. Yang, D. Kogel, S. Pluchino, and J. M. Hallenbeck. "Topotecan Is a Potent Inhibitor of Sumoylation in Glioblastoma Multiforme and Alters Both Cellular Replication and Metabolic Programming." Sci Rep 7, no. 1 (2017): 7425. [CrossRef]
- Yang, W., Q. Ma, G. B. Mackensen, and W. Paschen. "Deep Hypothermia Markedly Activates the Small Ubiquitin-Like Modifier Conjugation Pathway; Implications for the Fate of Cells Exposed to Transient Deep Hypothermic Cardiopulmonary Bypass." J Cereb Blood Flow Metab 29, no. 5 (2009): 886-90. [CrossRef]
- Yang, W., H. Sheng, J. W. Thompson, S. Zhao, L. Wang, P. Miao, X. Liu, M. A. Moseley, and W. Paschen. "Small Ubiquitin-Like Modifier 3-Modified Proteome Regulated by Brain Ischemia in Novel Small Ubiquitin-Like Modifier Transgenic Mice: Putative Protective Proteins/Pathways." Stroke 45, no. 4 (2014): 1115-22.
- Yang, W., H. Sheng, D. S. Warner, and W. Paschen. "Transient Global Cerebral Ischemia Induces a Massive Increase in Protein Sumoylation." J Cereb Blood Flow Metab 28, no. 2 (2008): 269-79. [CrossRef]
- ———. "Transient Focal Cerebral Ischemia Induces a Dramatic Activation of Small Ubiquitin-Like Modifier Conjugation." J Cereb Blood Flow Metab 28, no. 5 (2008): 892-6.
- Chen, Xu, Yuhong Zhang, Qiqi Wang, Yuanyuan Qin, Xinyi Yang, Zhengcao Xing, Yajie Shen, Hongmei Wu, and Yitao Qi. "The Function of Sumoylation and Its Crucial Roles in the Development of Neurological Diseases." The FASEB Journal 35, no. 4 (2021): e21510. [CrossRef]
- Mendler, L., T. Braun, and S. Muller. "The Ubiquitin-Like Sumo System and Heart Function: From Development to Disease." Circ Res 118, no. 1 (2016): 132-44.
- Kho, C., A. Lee, D. Jeong, J. G. Oh, A. H. Chaanine, E. Kizana, W. J. Park, and R. J. Hajjar. "Sumo1-Dependent Modulation of Serca2a in Heart Failure." Nature 477, no. 7366 (2011): 601-5. [CrossRef]
- Guo, C., Q. Wei, Y. Su, and Z. Dong. "Sumoylation Occurs in Acute Kidney Injury and Plays a Cytoprotective Role." Biochim Biophys Acta 1852, no. 3 (2015): 482-9. [CrossRef]
- Zhao, W., X. Zhang, and J. Rong. "Sumoylation as a Therapeutic Target for Myocardial Infarction." Front Cardiovasc Med 8 (2021): 701583. [CrossRef]
- Datwyler, A. L., G. Lattig-Tunnemann, W. Yang, W. Paschen, S. L. Lee, U. Dirnagl, M. Endres, and C. Harms. "Sumo2/3 Conjugation Is an Endogenous Neuroprotective Mechanism." J Cereb Blood Flow Metab 31, no. 11 (2011): 2152-9.
- Cuomo, O., G. Pignataro, R. Sirabella, P. Molinaro, S. Anzilotti, A. Scorziello, M. J. Sisalli, G. Di Renzo, and L. Annunziato. "Sumoylation of Lys590 of Ncx3 F-Loop by Sumo1 Participates in Brain Neuroprotection Induced by Ischemic Preconditioning." Stroke 47, no. 4 (2016): 1085-93. [CrossRef]
- Kunz, K., K. Wagner, L. Mendler, S. Holper, N. Dehne, and S. Muller. "Sumo Signaling by Hypoxic Inactivation of Sumo-Specific Isopeptidases." Cell Rep 16, no. 11 (2016): 3075-86. [CrossRef]
- Dong, P., Q. Li, and H. Han. "Hif-1alpha in Cerebral Ischemia (Review)." Mol Med Rep 25, no. 2 (2022).
- Mabb, A. M., and S. Miyamoto. "Sumo and Nf-Kappab Ties." Cell Mol Life Sci 64, no. 15 (2007): 1979-96.
- Li, J., Y. Xu, X. D. Long, W. Wang, H. K. Jiao, Z. Mei, Q. Q. Yin, L. N. Ma, A. W. Zhou, L. S. Wang, M. Yao, Q. Xia, and G. Q. Chen. "Cbx4 Governs Hif-1alpha to Potentiate Angiogenesis of Hepatocellular Carcinoma by Its Sumo E3 Ligase Activity." Cancer Cell 25, no. 1 (2014): 118-31.
- Pan, Y., Q. Li, Z. Cao, and S. Zhao. "The Sumo E3 Ligase Cbx4 Is Identified as a Poor Prognostic Marker of Gastric Cancer through Multipronged Omic Analyses." Genes Dis 8, no. 6 (2021): 827-37. [CrossRef]
- Nakagawa, K., T. Kohara, Y. Uehata, Y. Miyakawa, M. Sato-Ueshima, N. Okubo, M. Asaka, H. Takeda, and M. Kobayashi. "Pias3 Enhances the Transcriptional Activity of Hif-1alpha by Increasing Its Protein Stability." Biochem Biophys Res Commun 469, no. 3 (2016): 470-6. [CrossRef]
- Tojo, M., K. Matsuzaki, T. Minami, Y. Honda, H. Yasuda, T. Chiba, H. Saya, Y. Fujii-Kuriyama, and M. Nakao. "The Aryl Hydrocarbon Receptor Nuclear Transporter Is Modulated by the Sumo-1 Conjugation System." J Biol Chem 277, no. 48 (2002): 46576-85. [CrossRef]
- Cai, Q., S. C. Verma, P. Kumar, M. Ma, and E. S. Robertson. "Hypoxia Inactivates the Vhl Tumor Suppressor through Piasy-Mediated Sumo Modification." PLoS One 5, no. 3 (2010): e9720. [CrossRef]
- Kang, X., J. Li, Y. Zou, J. Yi, H. Zhang, M. Cao, E. T. Yeh, and J. Cheng. "Piasy Stimulates Hif1alpha Sumoylation and Negatively Regulates Hif1alpha Activity in Response to Hypoxia." Oncogene 29, no. 41 (2010): 5568-78. [CrossRef]
- Nunez-O'Mara, A., A. Gerpe-Pita, S. Pozo, O. Carlevaris, B. Urzelai, F. Lopitz-Otsoa, M. S. Rodriguez, and E. Berra. "Phd3-Sumo Conjugation Represses Hif1 Transcriptional Activity Independently of Phd3 Catalytic Activity." J Cell Sci 128, no. 1 (2015): 40-9.
- Sallais, J., S. Alahari, A. Tagliaferro, J. Bhattacharjee, M. Post, and I. Caniggia. "Factor Inhibiting Hif1-a Novel Target of Sumoylation in the Human Placenta." Oncotarget 8, no. 69 (2017): 114002-18. [CrossRef]
- Chachami, G., N. Stankovic-Valentin, A. Karagiota, A. Basagianni, U. Plessmann, H. Urlaub, F. Melchior, and G. Simos. "Hypoxia-Induced Changes in Sumo Conjugation Affect Transcriptional Regulation under Low Oxygen." Mol Cell Proteomics 18, no. 6 (2019): 1197-209. [CrossRef]
- Zhang, W., I. Potrovita, V. Tarabin, O. Herrmann, V. Beer, F. Weih, A. Schneider, and M. Schwaninger. "Neuronal Activation of Nf-Kappab Contributes to Cell Death in Cerebral Ischemia." J Cereb Blood Flow Metab 25, no. 1 (2005): 30-40.
- Tashiro, K., M. P. Pando, Y. Kanegae, P. M. Wamsley, S. Inoue, and I. M. Verma. "Direct Involvement of the Ubiquitin-Conjugating Enzyme Ubc9/Hus5 in the Degradation of Ikappabalpha." Proc Natl Acad Sci U S A 94, no. 15 (1997): 7862-7.
- Desterro, J. M., M. S. Rodriguez, and R. T. Hay. "Sumo-1 Modification of Ikappabalpha Inhibits Nf-Kappab Activation." Mol Cell 2, no. 2 (1998): 233-9.
- Li, X., Q. Xia, M. Mao, H. Zhou, L. Zheng, Y. Wang, Z. Zeng, L. Yan, Y. Zhao, and J. Shi. "Annexin-A1 Sumoylation Regulates Microglial Polarization after Cerebral Ischemia by Modulating Ikkalpha Stability Via Selective Autophagy." Sci Adv 7, no. 4 (2021).
- Huang, T. T., S. M. Wuerzberger-Davis, Z. H. Wu, and S. Miyamoto. "Sequential Modification of Nemo/Ikkgamma by Sumo-1 and Ubiquitin Mediates Nf-Kappab Activation by Genotoxic Stress." Cell 115, no. 5 (2003): 565-76.
- Yang, T., J. Sun, B. Wei, and S. Liu. "Senp1-Mediated Nemo De-Sumoylation Inhibits Intermittent Hypoxia Induced Inflammatory Response of Microglia in Vitro." J Cell Physiol 235, no. 4 (2020): 3529-38. [CrossRef]
- Lee, J. H., S. M. Park, O. S. Kim, C. S. Lee, J. H. Woo, S. J. Park, E. H. Joe, and I. Jou. "Differential Sumoylation of Lxralpha and Lxrbeta Mediates Transrepression of Stat1 Inflammatory Signaling in Ifn-Gamma-Stimulated Brain Astrocytes." Mol Cell 35, no. 6 (2009): 806-17.
- Cimarosti, H., C. Lindberg, S. F. Bomholt, L. C. Ronn, and J. M. Henley. "Increased Protein Sumoylation Following Focal Cerebral Ischemia." Neuropharmacology 54, no. 2 (2008): 280-9. [CrossRef]
- Guo, C., and J. M. Henley. "Wrestling with Stress: Roles of Protein Sumoylation and Desumoylation in Cell Stress Response." IUBMB Life 66, no. 2 (2014): 71-7. [CrossRef]
- Henley, J. M., T. J. Craig, and K. A. Wilkinson. "Neuronal Sumoylation: Mechanisms, Physiology, and Roles in Neuronal Dysfunction." Physiol Rev 94, no. 4 (2014): 1249-85.
- Guo, C., K. L. Hildick, J. Luo, L. Dearden, K. A. Wilkinson, and J. M. Henley. "Senp3-Mediated Desumoylation of Dynamin-Related Protein 1 Promotes Cell Death Following Ischaemia." EMBO J 32, no. 11 (2013): 1514-28. [CrossRef]
- Neumar, R. W. "Molecular Mechanisms of Ischemic Neuronal Injury." Ann Emerg Med 36, no. 5 (2000): 483-506.
- Feligioni, M., M. P. Mattson, and R. Nistico. "Sumoylation in Neuroplasticity and Neurological Disorders." Neuromolecular Med 15, no. 4 (2013): 637-8. [CrossRef]
- Feligioni, M., A. Nishimune, and J. M. Henley. "Protein Sumoylation Modulates Calcium Influx and Glutamate Release from Presynaptic Terminals." Eur J Neurosci 29, no. 7 (2009): 1348-56. [CrossRef]
- Dustrude, E. T., S. M. Wilson, W. Ju, Y. Xiao, and R. Khanna. "Crmp2 Protein Sumoylation Modulates Nav1.7 Channel Trafficking." J Biol Chem 288, no. 34 (2013): 24316-31.
- Martin, S., A. Nishimune, J. R. Mellor, and J. M. Henley. "Sumoylation Regulates Kainate-Receptor-Mediated Synaptic Transmission." Nature 447, no. 7142 (2007): 321-5. [CrossRef]
- Coelho-Silva, L., G. J. Stephens, and H. Cimarosti. "Sumoylation and Calcium Signalling: Potential Roles in the Brain and Beyond." Neuronal Signal 1, no. 3 (2017): NS20160010. [CrossRef]
- Bernstock, J. D., L. Peruzzotti-Jametti, D. Ye, F. A. Gessler, D. Maric, N. Vicario, Y. J. Lee, S. Pluchino, and J. M. Hallenbeck. "Neural Stem Cell Transplantation in Ischemic Stroke: A Role for Preconditioning and Cellular Engineering." J Cereb Blood Flow Metab 37, no. 7 (2017): 2314-19. [CrossRef]
- Ding, D. C., C. H. Lin, W. C. Shyu, and S. Z. Lin. "Neural Stem Cells and Stroke." Cell Transplant 22, no. 4 (2013): 619-30. [CrossRef]
- Baker, E. W., H. A. Kinder, and F. D. West. "Neural Stem Cell Therapy for Stroke: A Multimechanistic Approach to Restoring Neurological Function." Brain Behav 9, no. 3 (2019): e01214. [CrossRef]
- Tahmasebi, S., M. Ghorbani, P. Savage, G. Gocevski, and X. J. Yang. "The Sumo Conjugating Enzyme Ubc9 Is Required for Inducing and Maintaining Stem Cell Pluripotency." Stem Cells 32, no. 4 (2014): 1012-20. [CrossRef]
- Yamaguchi, T., P. Sharma, M. Athanasiou, A. Kumar, S. Yamada, and M. R. Kuehn. "Mutation of Senp1/Supr-2 Reveals an Essential Role for Desumoylation in Mouse Development." Mol Cell Biol 25, no. 12 (2005): 5171-82.
- Bernstock, J. D., L. Peruzzotti-Jametti, T. Leonardi, N. Vicario, D. Ye, Y. J. Lee, D. Maric, K. R. Johnson, Y. Mou, A. Van Den Bosch, M. Winterbone, G. K. Friedman, R. J. M. Franklin, J. M. Hallenbeck, and S. Pluchino. "Sumoylation Promotes Survival and Integration of Neural Stem Cell Grafts in Ischemic Stroke." EBioMedicine 42 (2019): 214-24. [CrossRef]
- Lee, Y. J., K. R. Johnson, and J. M. Hallenbeck. "Global Protein Conjugation by Ubiquitin-Like-Modifiers During Ischemic Stress Is Regulated by Micrornas and Confers Robust Tolerance to Ischemia." PLoS One 7, no. 10 (2012): e47787. [CrossRef]
- Lee, Y. J., and J. M. Hallenbeck. "Sumo and Ischemic Tolerance." Neuromolecular Med 15, no. 4 (2013): 771-81. [CrossRef]
- Tokarz, P., and K. Wozniak. "Senp Proteases as Potential Targets for Cancer Therapy." Cancers (Basel) 13, no. 9 (2021). [CrossRef]
- Bernstock, J. D., D. G. Ye, Y. J. Lee, F. Gessler, G. K. Friedman, W. Zheng, and J. M. Hallenbeck. "Drugging Sumoylation for Neuroprotection and Oncotherapy." Neural Regen Res 13, no. 3 (2018): 415-16. [CrossRef]
- Bernstock, J. D., Y. J. Lee, L. Peruzzotti-Jametti, N. Southall, K. R. Johnson, D. Maric, G. Volpe, J. Kouznetsova, W. Zheng, S. Pluchino, and J. M. Hallenbeck. "A Novel Quantitative High-Throughput Screen Identifies Drugs That Both Activate Sumo Conjugation Via the Inhibition of Micrornas 182 and 183 and Facilitate Neuroprotection in a Model of Oxygen and Glucose Deprivation." J Cereb Blood Flow Metab 36, no. 2 (2016): 426-41. [CrossRef]
- Nadareishvili, Z., D. Kelley, M. Luby, A. N. Simpkins, R. Leigh, J. K. Lynch, A. W. Hsia, R. T. Benson, K. R. Johnson, J. M. Hallenbeck, and L. L. Latour. "Molecular Signature of Penumbra in Acute Ischemic Stroke: A Pilot Transcriptomics Study." Ann Clin Transl Neurol 6, no. 4 (2019): 817-20. [CrossRef]
- Kim, H. J., M. Rowe, M. Ren, J. S. Hong, P. S. Chen, and D. M. Chuang. "Histone Deacetylase Inhibitors Exhibit Anti-Inflammatory and Neuroprotective Effects in a Rat Permanent Ischemic Model of Stroke: Multiple Mechanisms of Action." J Pharmacol Exp Ther 321, no. 3 (2007): 892-901. [CrossRef]
- Brookes, R. L., S. Crichton, C. D. A. Wolfe, Q. Yi, L. Li, G. J. Hankey, P. M. Rothwell, and H. S. Markus. "Sodium Valproate, a Histone Deacetylase Inhibitor, Is Associated with Reduced Stroke Risk after Previous Ischemic Stroke or Transient Ischemic Attack." Stroke 49, no. 1 (2018): 54-61. [CrossRef]
- Park, M. J., and F. Sohrabji. "The Histone Deacetylase Inhibitor, Sodium Butyrate, Exhibits Neuroprotective Effects for Ischemic Stroke in Middle-Aged Female Rats." J Neuroinflammation 13, no. 1 (2016): 300. [CrossRef]
- Al Shoyaib, A., F. F. Alamri, N. Syeara, S. Jayaraman, S. T. Karamyan, T. V. Arumugam, and V. T. Karamyan. "The Effect of Histone Deacetylase Inhibitors Panobinostat or Entinostat on Motor Recovery in Mice after Ischemic Stroke." Neuromolecular Med 23, no. 4 (2021): 471-84. [CrossRef]
- Bonsack, F., and S. Sukumari-Ramesh. "Entinostat Improves Acute Neurological Outcomes and Attenuates Hematoma Volume after Intracerebral Hemorrhage." Brain Res 1752 (2021): 147222. [CrossRef]
- Shimizu, K., Z. Lacza, N. Rajapakse, T. Horiguchi, J. Snipes, and D. W. Busija. "Mitok(Atp) Opener, Diazoxide, Reduces Neuronal Damage after Middle Cerebral Artery Occlusion in the Rat." Am J Physiol Heart Circ Physiol 283, no. 3 (2002): H1005-11. [CrossRef]
- O'Sullivan, J. C., X. L. Yao, H. Alam, and J. T. McCabe. "Diazoxide, as a Postconditioning and Delayed Preconditioning Trigger, Increases Hsp25 and Hsp70 in the Central Nervous System Following Combined Cerebral Stroke and Hemorrhagic Shock." J Neurotrauma 24, no. 3 (2007): 532-46. [CrossRef]
- Bernstock, J. D., D. Ye, J. A. Smith, Y. J. Lee, F. A. Gessler, A. Yasgar, J. Kouznetsova, A. Jadhav, Z. Wang, S. Pluchino, W. Zheng, A. Simeonov, J. M. Hallenbeck, and W. Yang. "Quantitative High-Throughput Screening Identifies Cytoprotective Molecules That Enhance Sumo Conjugation Via the Inhibition of Sumo-Specific Protease (Senp)2." FASEB J 32, no. 3 (2018): 1677-91. [CrossRef]
- Chojnowski, K., M. Opielka, W. Nazar, P. Kowianski, and R. T. Smolenski. "Neuroprotective Effects of Guanosine in Ischemic Stroke-Small Steps Towards Effective Therapy." Int J Mol Sci 22, no. 13 (2021). [CrossRef]
- Lee, Y. J., J. D. Bernstock, N. Nagaraja, B. Ko, and J. M. Hallenbeck. "Global Sumoylation Facilitates the Multimodal Neuroprotection Afforded by Quercetin against the Deleterious Effects of Oxygen/Glucose Deprivation and the Restoration of Oxygen/Glucose." J Neurochem 138, no. 1 (2016): 101-16. [CrossRef]
- Zhang, L., J. Ma, F. Yang, S. Li, W. Ma, X. Chang, and L. Yang. "Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review." Front Pharmacol 13 (2022): 854249. [CrossRef]
- Guo, C., W. J. Wang, Y. C. Liao, C. Zhao, Y. Yin, M. N. Yao, Y. Ding, and J. W. Wang. "Effect and Mechanisms of Quercetin for Experimental Focal Cerebral Ischemia: A Systematic Review and Meta-Analysis." Oxid Med Cell Longev 2022 (2022): 9749461. [CrossRef]
- Koizumi, H., H. Fujisawa, E. Suehiro, S. Shirao, and M. Suzuki. "Neuroprotective Effects of Ebselen Following Forebrain Ischemia: Involvement of Glutamate and Nitric Oxide." Neurol Med Chir (Tokyo) 51, no. 5 (2011): 337-43. [CrossRef]
- Park, S., S. Kang, D. S. Kim, B. K. Shin, N. R. Moon, and J. W. Daily, 3rd. "Ebselen Pretreatment Attenuates Ischemia/Reperfusion Injury and Prevents Hyperglycemia by Improving Hepatic Insulin Signaling and Beta-Cell Survival in Gerbils." Free Radic Res 48, no. 8 (2014): 864-74.
- Ogawa, A., T. Yoshimoto, H. Kikuchi, K. Sano, I. Saito, T. Yamaguchi, and H. Yasuhara. "Ebselen in Acute Middle Cerebral Artery Occlusion: A Placebo-Controlled, Double-Blind Clinical Trial." Cerebrovasc Dis 9, no. 2 (1999): 112-8.
- Yamaguchi, T., K. Sano, K. Takakura, I. Saito, Y. Shinohara, T. Asano, and H. Yasuhara. "Ebselen in Acute Ischemic Stroke: A Placebo-Controlled, Double-Blind Clinical Trial. Ebselen Study Group." Stroke 29, no. 1 (1998): 12-7.
- Mulder, I. A., E. T. van Bavel, H. E. de Vries, and J. M. Coutinho. "Adjunctive Cytoprotective Therapies in Acute Ischemic Stroke: A Systematic Review." Fluids Barriers CNS 18, no. 1 (2021): 46. [CrossRef]
- Parnham, M. J., and H. Sies. "The Early Research and Development of Ebselen." Biochem Pharmacol 86, no. 9 (2013): 1248-53. [CrossRef]
- Porciuncula, L. O., J. B. Rocha, C. R. Boeck, D. Vendite, and D. O. Souza. "Ebselen Prevents Excitotoxicity Provoked by Glutamate in Rat Cerebellar Granule Neurons." Neurosci Lett 299, no. 3 (2001): 217-20. [CrossRef]
- Porciuncula, L. O., J. B. Rocha, H. Cimarosti, L. Vinade, G. Ghisleni, C. G. Salbego, and D. O. Souza. "Neuroprotective Effect of Ebselen on Rat Hippocampal Slices Submitted to Oxygen-Glucose Deprivation: Correlation with Immunocontent of Inducible Nitric Oxide Synthase." Neurosci Lett 346, no. 1-2 (2003): 101-4. [CrossRef]
- Chen, S., D. Dong, W. Xin, and H. Zhou. "Progress in the Discovery of Small Molecule Modulators of Desumoylation." Curr Issues Mol Biol 35 (2020): 17-34. [CrossRef]
- Yamagata, K., S. Ichinose, A. Miyashita, and M. Tagami. "Protective Effects of Ebselen, a Seleno-Organic Antioxidant on Neurodegeneration Induced by Hypoxia and Reperfusion in Stroke-Prone Spontaneously Hypertensive Rat." Neuroscience 153, no. 2 (2008): 428-35. [CrossRef]
- Sui, H., W. Wang, P. H. Wang, and L. S. Liu. "Protective Effect of Antioxidant Ebselen (Pz51) on the Cerebral Cortex of Stroke-Prone Spontaneously Hypertensive Rats." Hypertens Res 28, no. 3 (2005): 249-54. [CrossRef]
- Imai, H., D. I. Graham, H. Masayasu, and I. M. Macrae. "Antioxidant Ebselen Reduces Oxidative Damage in Focal Cerebral Ischemia." Free Radic Biol Med 34, no. 1 (2003): 56-63. [CrossRef]
- Imai, H., H. Masayasu, D. Dewar, D. I. Graham, and I. M. Macrae. "Ebselen Protects Both Gray and White Matter in a Rodent Model of Focal Cerebral Ischemia." Stroke 32, no. 9 (2001): 2149-54. [CrossRef]
- Salom, J. B., F. J. Perez-Asensio, M. C. Burguete, N. Marin, C. Pitarch, G. Torregrosa, F. J. Romero, and E. Alborch. "Single-Dose Ebselen Does Not Afford Sustained Neuroprotection to Rats Subjected to Severe Focal Cerebral Ischemia." Eur J Pharmacol 495, no. 1 (2004): 55-62. [CrossRef]
- Krajnak, K., and R. Dahl. "Small Molecule Sumoylation Activators Are Novel Neuroprotective Agents." Bioorg Med Chem Lett 28, no. 3 (2018): 405-09. [CrossRef]
- Chang, H. M., and E. T. H. Yeh. "Sumo: From Bench to Bedside." Physiol Rev 100, no. 4 (2020): 1599-619. [CrossRef]
- Melnyk, Oleg, and Jérôme Vicogne. "Total Chemical Synthesis of Sumo Proteins." Tetrahedron Letters 57, no. 39 (2016): 4319-24. [CrossRef]
- Langston, S. P., S. Grossman, D. England, R. Afroze, N. Bence, D. Bowman, N. Bump, R. Chau, B. C. Chuang, C. Claiborne, L. Cohen, K. Connolly, M. Duffey, N. Durvasula, S. Freeze, M. Gallery, K. Galvin, J. Gaulin, R. Gershman, P. Greenspan, J. Grieves, J. Guo, N. Gulavita, S. Hailu, X. He, K. Hoar, Y. Hu, Z. Hu, M. Ito, M. S. Kim, S. W. Lane, D. Lok, A. Lublinsky, W. Mallender, C. McIntyre, J. Minissale, H. Mizutani, M. Mizutani, N. Molchinova, K. Ono, A. Patil, M. Qian, J. Riceberg, V. Shindi, M. D. Sintchak, K. Song, T. Soucy, Y. Wang, H. Xu, X. Yang, A. Zawadzka, J. Zhang, and S. M. Pulukuri. "Discovery of Tak-981, a First-in-Class Inhibitor of Sumo-Activating Enzyme for the Treatment of Cancer." J Med Chem 64, no. 5 (2021): 2501-20. [CrossRef]
- Tymianski, M. "Combining Neuroprotection with Endovascular Treatment of Acute Stroke: Is There Hope?" Stroke 48, no. 6 (2017): 1700-05.
| Drug | Tissue/ Animal |
Ischemia Model | Intervention | Measured Outcome |
Results Summary | Study | |
|---|---|---|---|---|---|---|---|
| Orotic Acid | SHSY5Y, E18 PCN | SHSY5Y: 15hr OGD + 6hr recoveryE18 PCN: 5hr OGD + 16hr recovery | Drug co-treatment | Cell survival, SUMO concentration | SUMO upregulation*, OGD protection* | Bernstock et al., 2016 [73] | |
| AHPN | |||||||
| Telmisartan | |||||||
| TW-37 | |||||||
| Dianiline | |||||||
| Diazoxide | |||||||
| NCGC00185916 | SUMO upregulation* | ||||||
| Romidepsin | |||||||
| VX-702 | |||||||
| Lenalidomide | |||||||
| Belinostat | |||||||
| Pracinostat | |||||||
| Licofelone | |||||||
| Fosmidomycin | |||||||
| JWH-015 | |||||||
| Motesanib | |||||||
| Vatalanib | |||||||
| Entinostat | |||||||
| Panobinostat | |||||||
| CD-1 mice | Photothrombotic stroke at PMCA | Drug post-treatment (post stroke day 5-15) | Motor recovery, infarct volumes | No difference vs. negative control for both measures | Al Shoyaib et al., 2016 [78] | ||
| Entinostat | |||||||
| Collagenase-induced ICH | Drug post-treatment (1 hr post stroke, 10 mg/kg IP in PBS) | Sensorimotor deficit score, CD16/32 expression, neurodegeneration (via TUNEL staining neurons), infarct volume | Reduction in day 1 and day 3 post-ICH sensorimotor deficit*. Reduction in CD16/32 expression*, neurodegeneration, and infarct volume* | Bonsack and Sukumari-Ramesh, 2021 [79] | |||
| Diazoxide |
Wistar rats | 1.5 hr MCAO | Drug pretreatment (15min prior to stroke, 30 uL 0.4mM or 2mM ICV bolus) | 24hr post-stroke neurological score, infarct volume |
Increase in neurological score*; reduction in infarct volume* | Shimizu et al., 2002 [80] | |
| SD rats | 1hr RCCA ligation + hemorrhagic shock | Drug pre- and post-treatment: 5mg/kg IP bolus 24hr pre-stroke; 2.3 mg/kg/10min IV infusion 10min or 60min posttreatment | HSP25 and 70 concentrations | Pretreatment: Upregulation of HSP25 and 70*; 60min Posttreatment: Upregulation of HSP25 and 70* | O'Sullivan et al., 2007 [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





