Submitted:
31 March 2023
Posted:
06 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
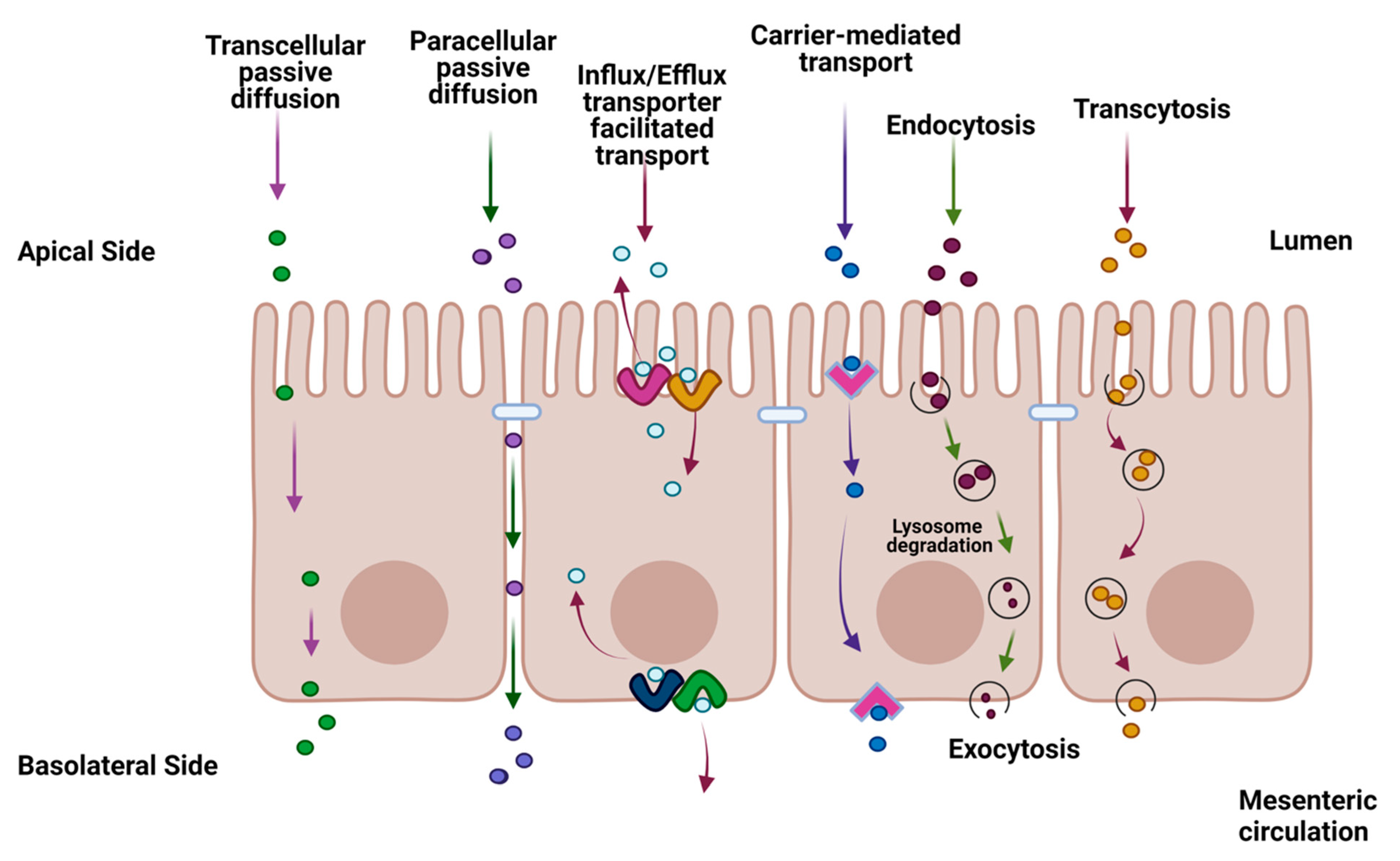
2. Materials and Methods
2.1. Materials
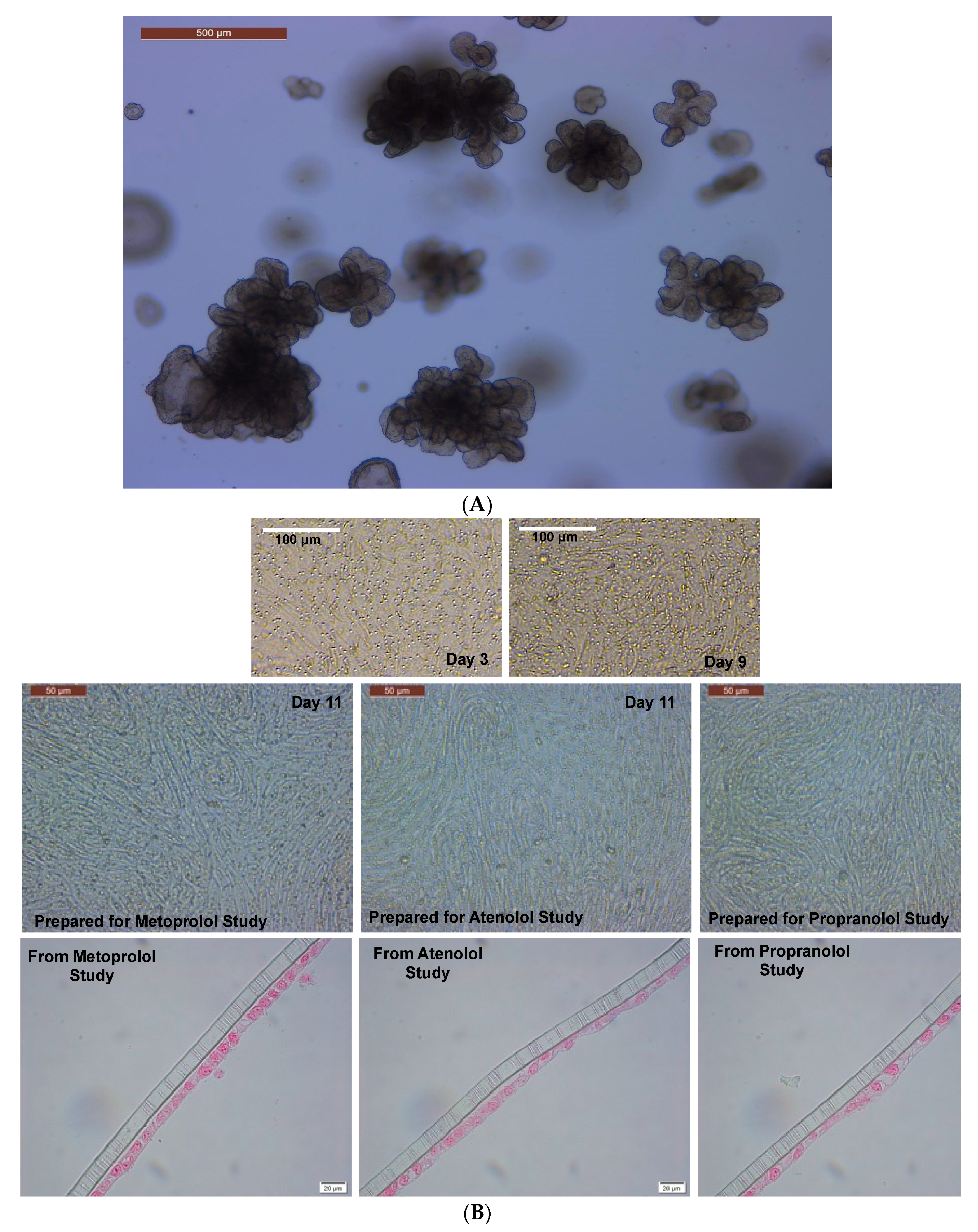
2.2. Caco-2 cell culture and maintenance
2.3. Caco-2 two-dimensional (2D) monolayer preparation and maintenance
2.4. Maintenance of 3D colonoids
2.5. Preparation of 2D canine colonic monolayer and maintenance
2.6. RNA Extraction and quantitative real-time polymerase chain reaction (qPCR) analyses
2.7. Assessment of monolayer integrity by TEER measurement and Fluorescein Isothiocyanate (FITC)-Dextran leakage assay
2.8. Preparation of transport buffer and drug solutions
2.9. Bidirectional transport experiments
2.9.1. Apical-to-basolateral [AP→BL] permeability
2.9.2. Basolateral-to-apical [BL→AP] permeability
2.10. Sample preparation for mass spectrometer analysis
2.10.1. Preparation of standards and solutions
2.10.2. Analytical method
2.11. Papp Calculations
2.12. Statistics
3. Results
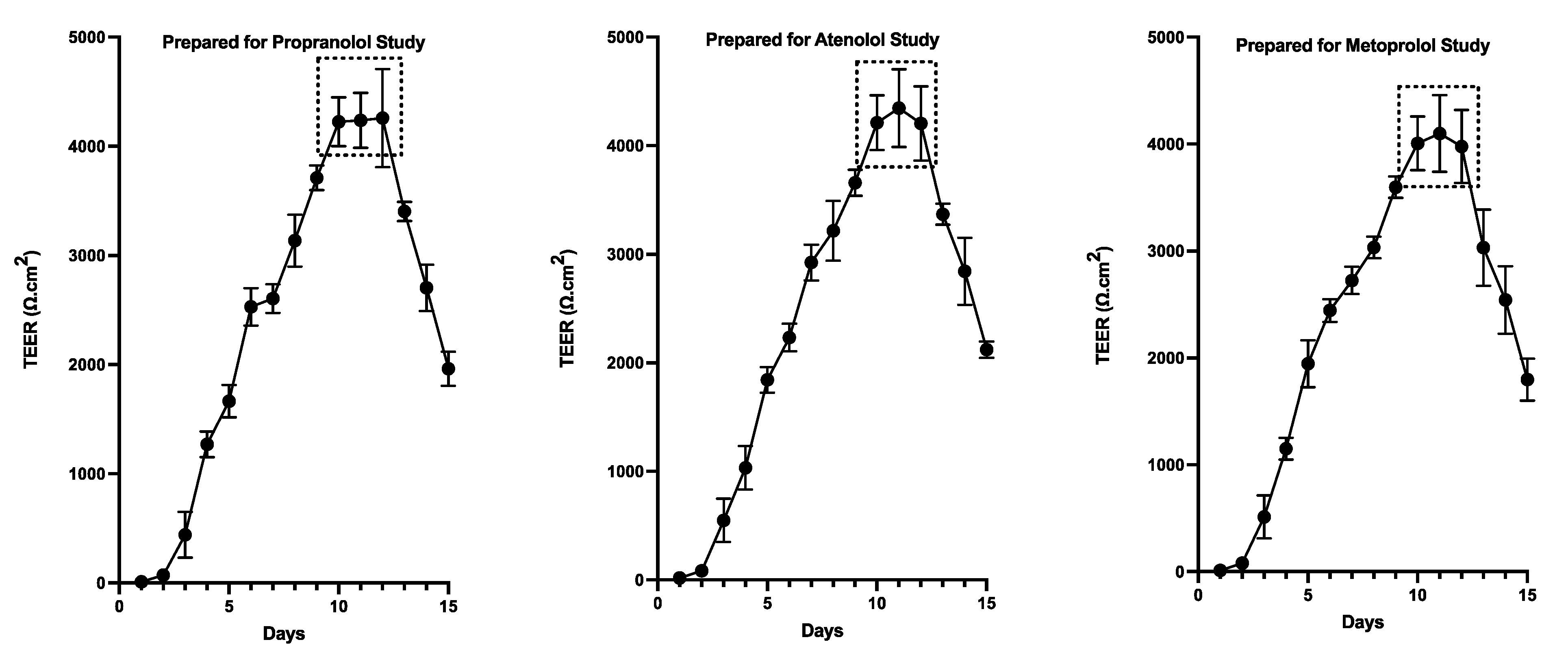
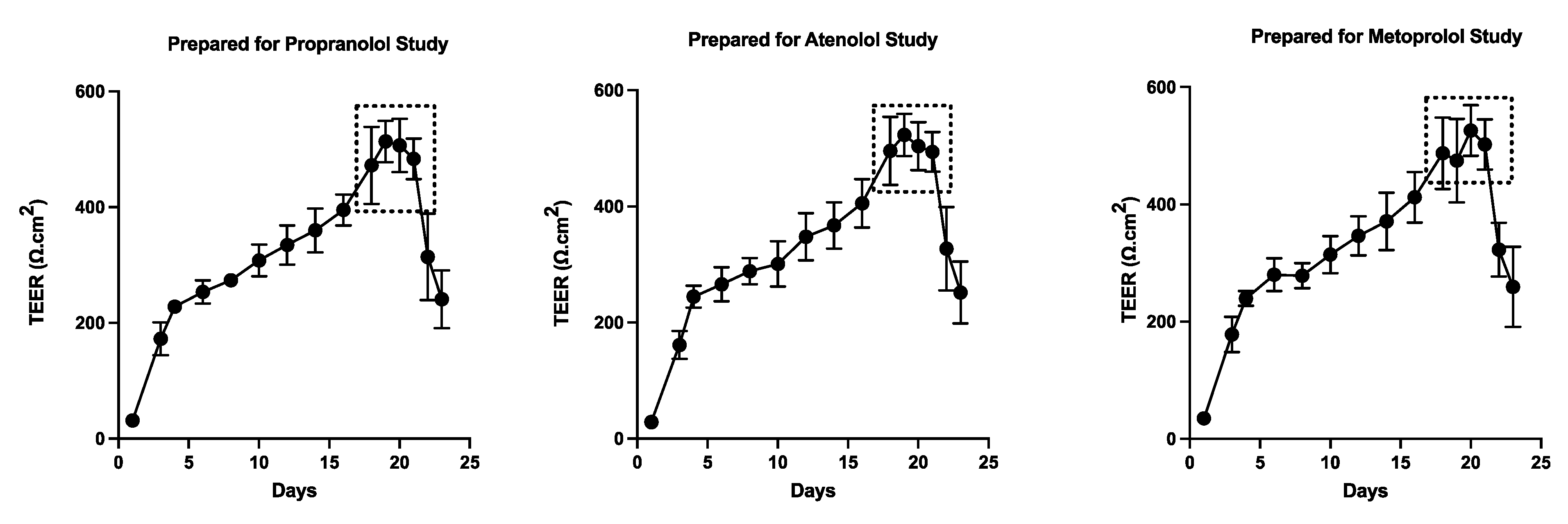
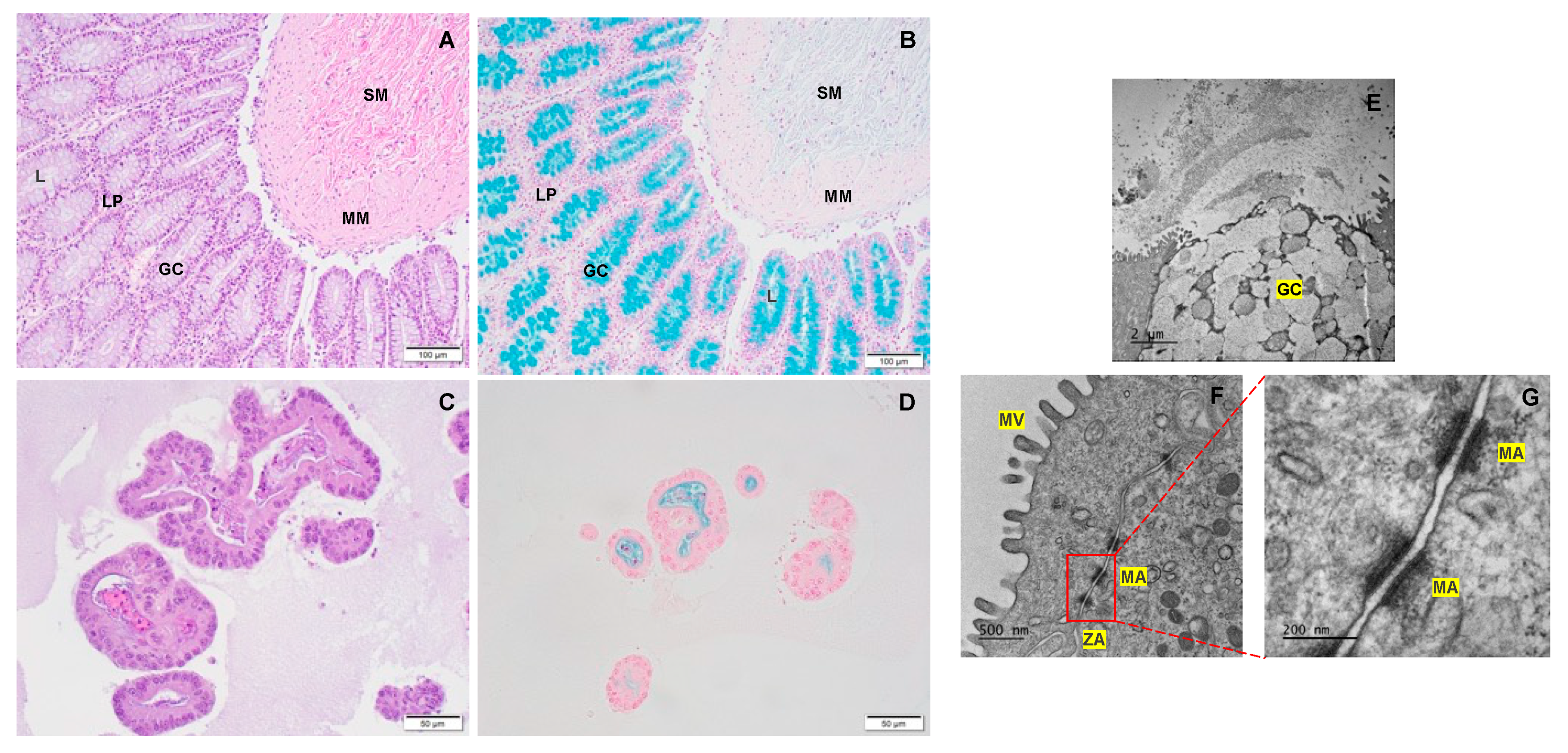
3.1. Assessment of monolayer integrity
3.2. Drug-specific Papp estimates
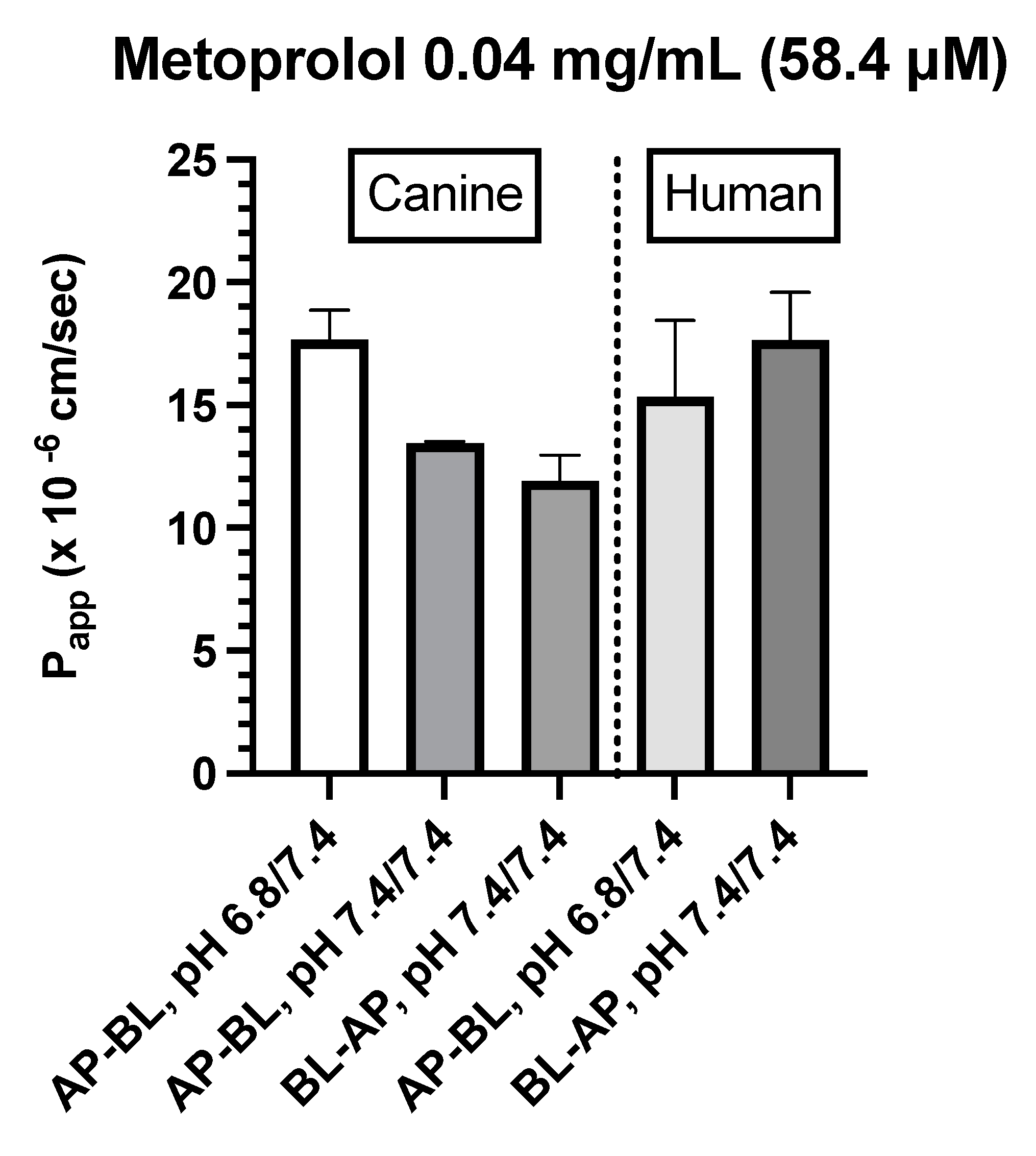
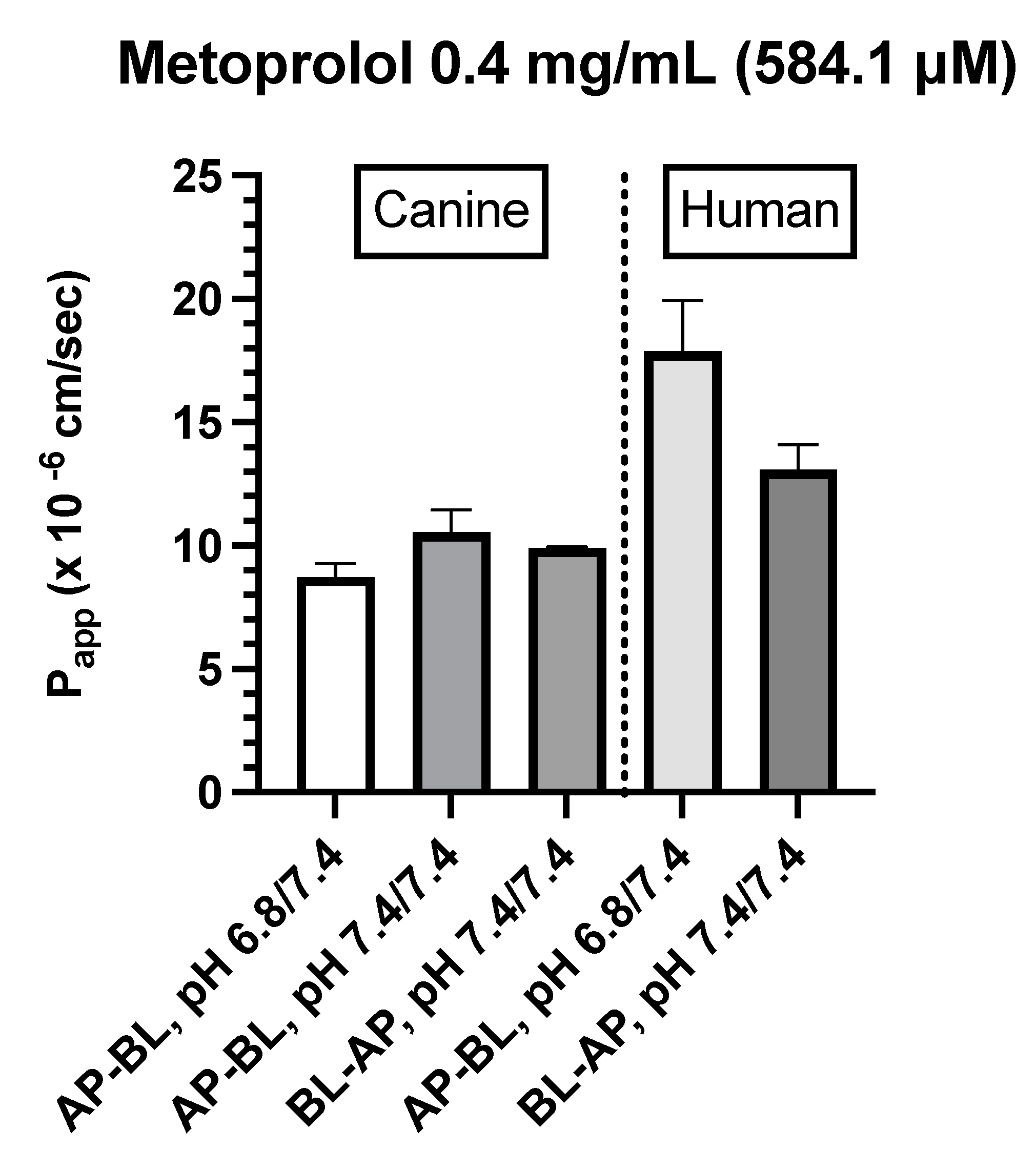
- Canine Colonoids: The AP→BL Papp values were lower in the medium containing 0.4 vs. 0.04 mg/mL metoprolol. Moreover, while at the 0.04 mg/mL concentration, the movement from AP→BL was slightly greater than that from BL→AP. The transport from AP→BL and BL→AP tended to be similar when evaluated at the 0.4 mg/mL concentration. Upon considering the data generated across the two metoprolol concentrations, the pH of the apical chamber did not consistently influence the magnitude of the Papp estimate (Figure 6B,C & Table 4).
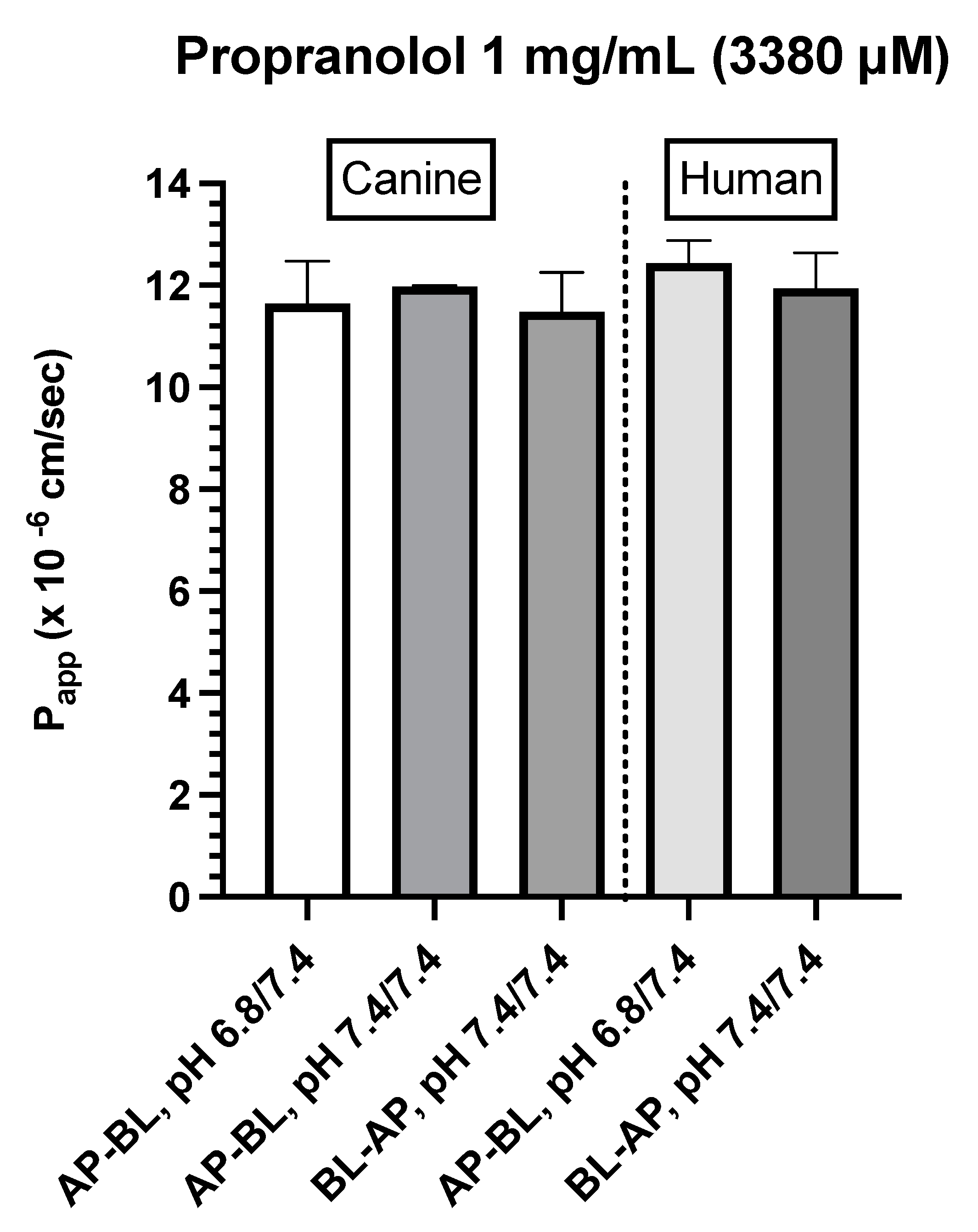
- Caco-2: Unlike canine colonoids, Papp values were not markedly influenced by metoprolol concentration and at both concentrations, the movement from AP→BL and BL→AP were comparable (Figure 6B,C & Table 4). The concentration-associated differences in Papp values seen with the colonoid were not observed with the Caco-2 monolayer. Moreover, although the two cell-line monolayers exhibited similar AP→BL Papp values in the presence of 0.04 mg/mL metoprolol, the AP→BL Papp values for the Caco-2 monolayer tended to be higher than that of the canine colonoid when the donor concentration was increased to 0.4 mg/mL. Although the movement from AP→BL was somewhat greater than that seen in the BL→AP direction in the colonoid (0.04 mg/mL metoprolol but not at the 0.4 mg/mL donor concentration), that difference was not seen with the 0.04 mg/ml concentration or was only minimally appreciated at the 0.4 mg/mL concentration when the Caco-2 monolayer was used. When considering the variability across observations and the small number of wells tested, statistical inferences should not be linked to these outcomes (Figure 6C,D & Table 4). Thus, unlike propranolol, differences in the behavior of metoprolol were seen when comparing the two cell-line systems. However, these preliminary findings should be interpreted cautiously in light of our limited sample size and background variability in our system.
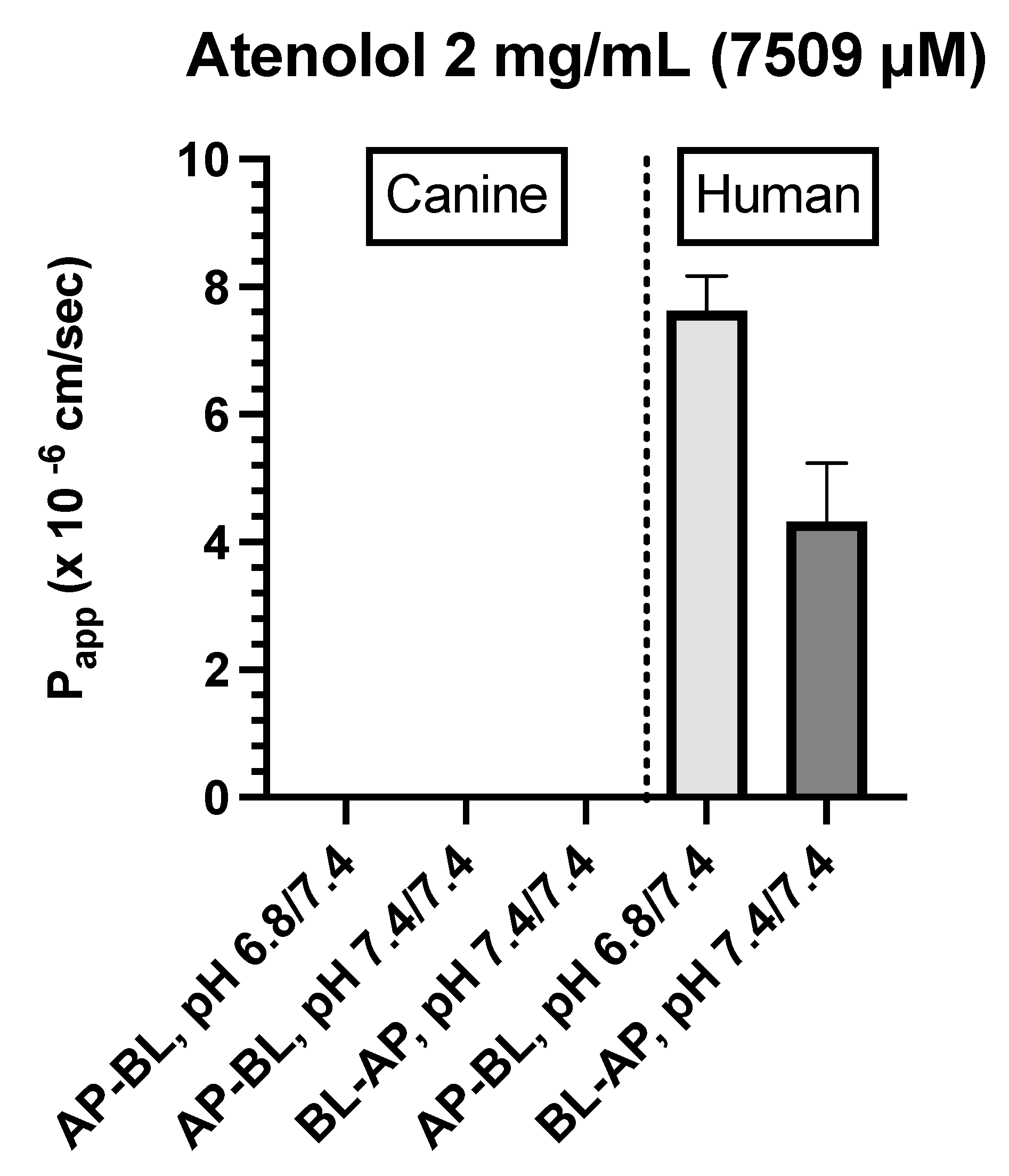
- Caco-2: Incubation of Caco-2 cells with 2.0 mg/mL atenolol resulted in measurable concentrations in the receiver compartment for both AP→BL and BL→AP directions, with higher Papp estimates reported after incubation in the apical chamber (Figure 6D & Table 4). These preliminary findings suggest a somewhat greater ability for atenolol to undergo paracellular transport across the Caco-2 monolayer as compared to that of the dog colonoid. However, at the lower atenolol concentration, neither system was associated with quantifiable movement from donor to receiver compartment (irrespective of direction).
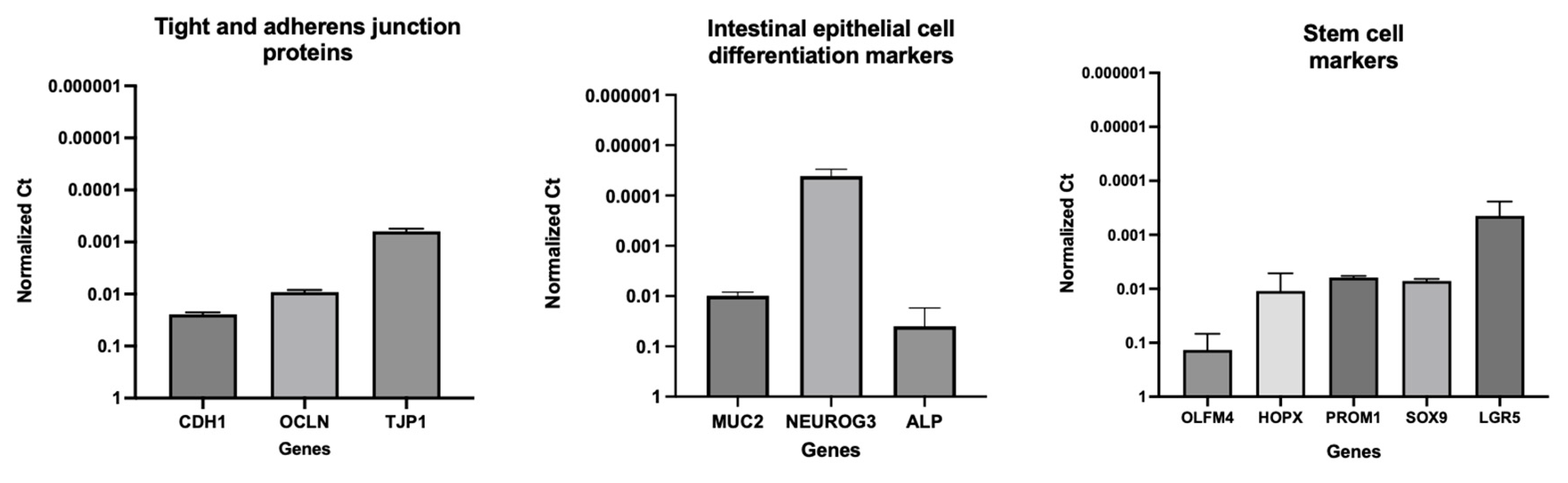
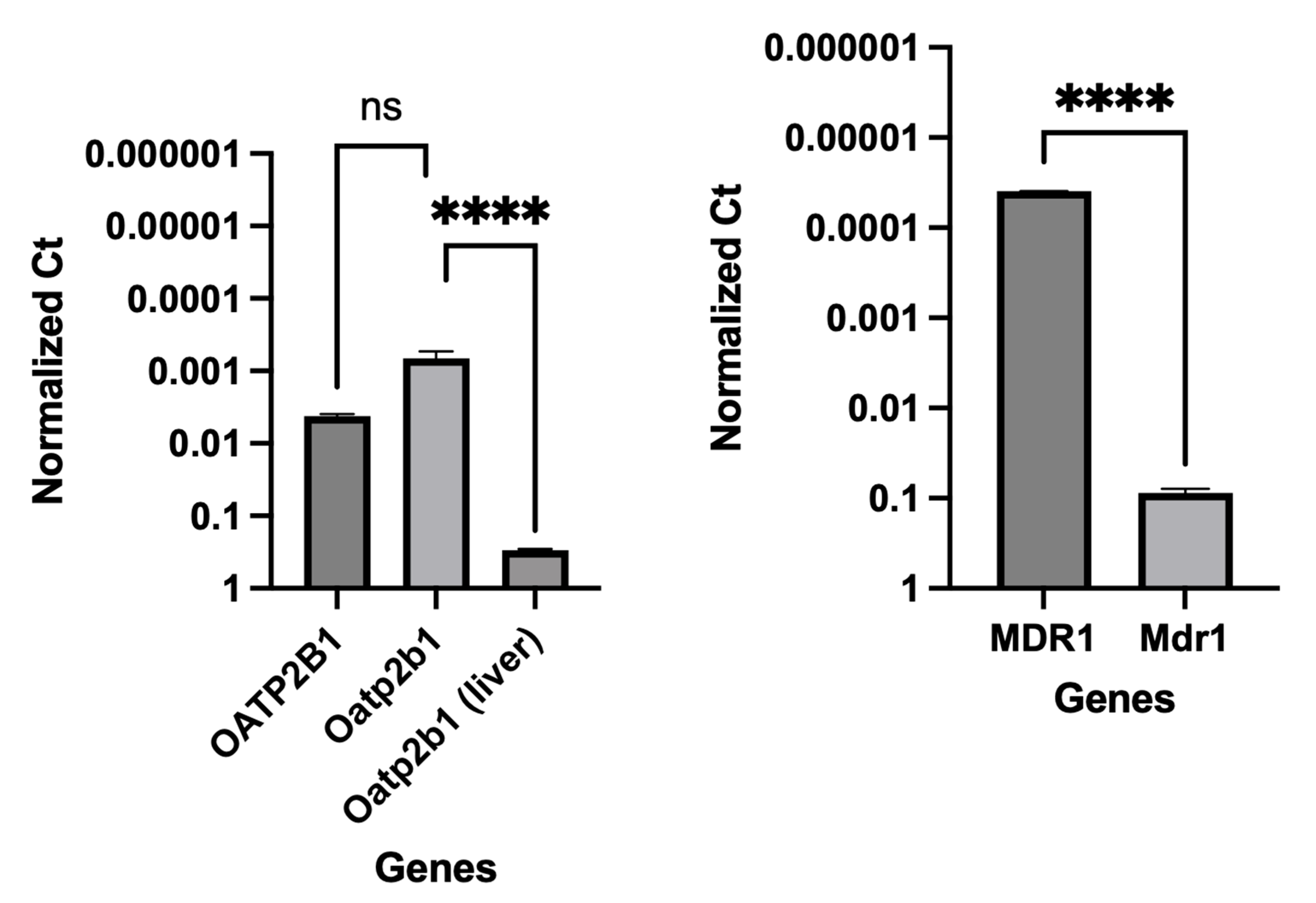
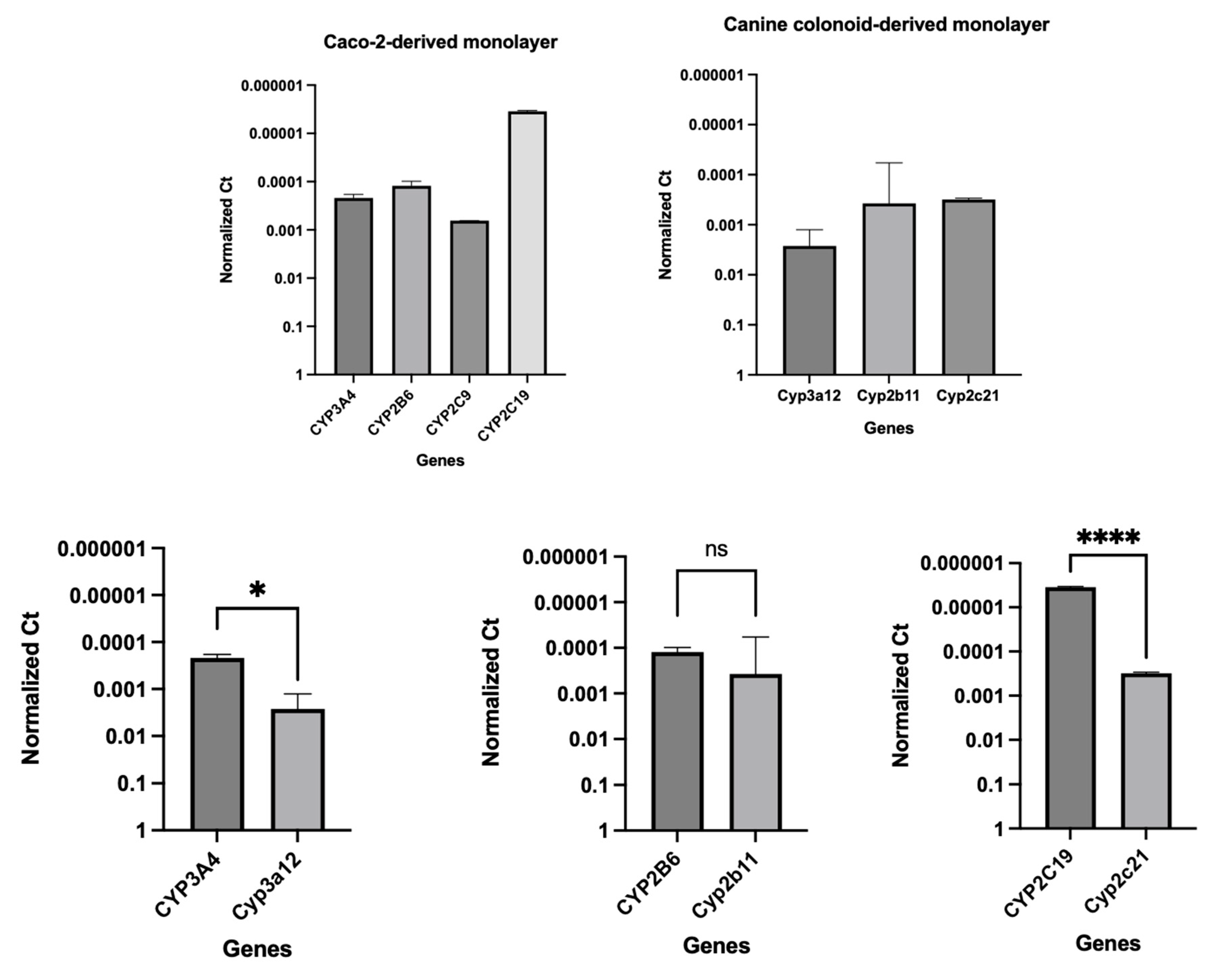
3.3. Gene Expression Analyses
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front Pharmacol 2021, 12, 62. [CrossRef]
- Sugano, K.; Kansy, M.; Artursson, P.; Avdeef, A.; Bendels, S.; Di, L.; Ecker, G.F.; Faller, B.; Fischer, H.; Gerebtzoff, G.; et al. Coexistence of Passive and Carrier-Mediated Processes in Drug Transport. Nat Rev Drug Discov 2010, 9, 597–614.
- Roos, C. The Impact of Regional Permeability, Nanoparticles, and Absorption-Modifying Excipients. 2018.
- Furuse, M. Molecular Basis of the Core Structure of Tight Junctions. Cold Spring Harb Perspect Biol 2010, 2. [CrossRef]
- Fleth-James, J. Suitability of in Vitro, in Silico and in Vivo Methods to Predict Intestinal Absorption in Drug Development. 2018. [CrossRef]
- Teksin, Z.S.; Seo, P.R.; Polli, J.E. Comparison of Drug Permeabilities and BCS Classification: Three Lipid-Component PAMPA System Method versus Caco-2 Monolayers. AAPS J 2010, 12, 238. [CrossRef]
- Volpe, D.A. Variability in Caco-2 and MDCK Cell-Based Intestinal Permeability Assays. J Pharm Sci 2008, 97, 712–725. [CrossRef]
- Volpe, D.A. Drug-Permeability and Transporter Assays in Caco-2 and MDCK Cell Lines. Future Med Chem 2011, 3, 2063–2077. [CrossRef]
- Volpe, D.A. Advances in Cell-Based Permeability Assays to Screen Drugs for Intestinal Absorption. [CrossRef]
- Volpe, D.A. Application of Method Suitability for Drug Permeability Classification. AAPS Journal 2010, 12, 670–678. [CrossRef]
- Hill, D.R.; Huang, S.; Tsai, Y.H.; Spence, J.R.; Young, V.B. Real-Time Measurement of Epithelial Barrier Permeability in Human Intestinal Organoids. J Vis Exp 2017, 2017. [CrossRef]
- Pade, D.; Jamei, M.; Rostami-Hodjegan, A.; Turner, D.B. Application of the MechPeff Model to Predict Passive Effective Intestinal Permeability in the Different Regions of the Rodent Small Intestine and Colon. Biopharm Drug Dispos 2017, 38, 94–114. [CrossRef]
- Macedo, M.H.; Martinez, E.; Barrias, C.C.; Sarmento, B. Development of an Improved 3D in Vitro Intestinal Model to Perform Permeability Studies of Paracellular Compounds. Front Bioeng Biotechnol 2020, 0, 1076. [CrossRef]
- Lea, T. Caco-2 Cell Line. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models 2015, 103–111. [CrossRef]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of Epithelial Cell Culture in Studies of Drug Transport. Methods Mol Biol 2002, 188, 233–272. [CrossRef]
- Sai, Y.; Kaneko, Y.; Ito, S.; Mitsuoka, K.; Kato, Y.; Tamai, I.; Artursson, P.; Tsuji, A. Predominant Contribution of Organic Anion Transporting Polypeptide OATP-B (OATP2B1) to Apical Uptake of Estrone-3-Sulfate by Human Intestinal Caco-2 Cells. Drug Metab Dispos 2006, 34, 1423–1431. [CrossRef]
- Balimane, P. v.; Chong, S. Cell Culture-Based Models for Intestinal Permeability: A Critique. Drug Discov Today 2005, 10, 335–343. [CrossRef]
- Ozawa, T.; Takayama, K.; Okamoto, R.; Negoro, R.; Sakurai, F.; Tachibana, M.; Kawabata, K.; Mizuguchi, H. Generation of Enterocyte-like Cells from Human Induced Pluripotent Stem Cells for Drug Absorption and Metabolism Studies in Human Small Intestine. Scientific Reports 2015 5:1 2015, 5, 1–11. [CrossRef]
- Mochel, J.P.; Jergens, A.E.; Kingsbury, D.; Kim, H.J.; Martín, M.G.; Allenspach, K. Intestinal Stem Cells to Advance Drug Development, Precision, and Regenerative Medicine: A Paradigm Shift in Translational Research. AAPS Journal 2018, 20, 1–9.
- Maubon, N.; Le Vee, M.; Fossati, L.; Audry, M.; Le Ferrec, E.; Bolze, S.; Fardel, O. Analysis of Drug Transporter Expression in Human Intestinal Caco-2 Cells by Real-Time PCR. Fundam Clin Pharmacol 2007, 21, 659–663. [CrossRef]
- Neuhoff, S.; Ungell, A.L.; Zamora, I.; Artursson, P. PH-Dependent Passive and Active Transport of Acidic Drugs across Caco-2 Cell Monolayers. European Journal of Pharmaceutical Sciences 2005, 25, 211–220. [CrossRef]
- Yamashita, T.; Inui, T.; Yokota, J.; Kawakami, K.; Morinaga, G.; Takatani, M.; Hirayama, D.; Nomoto, R.; Ito, K.; Cui, Y.; et al. Monolayer Platform Using Human Biopsy-Derived Duodenal Organoids for Pharmaceutical Research. Mol Ther Methods Clin Dev 2021, 22, 263–278. [CrossRef]
- Ito, K.; Suzuki, H.; Horie, T.; Sugiyama, Y. Apical/Basolateral Surface Expression of Drug Transporters and Its Role in Vectorial Drug Transport. Pharmaceutical Research 2005, 22, 1559–1577. [CrossRef]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T.; et al. Comparison of Drug Transporter Gene Expression and Functionality in Caco-2 Cells from 10 Different Laboratories. European Journal of Pharmaceutical Sciences 2008, 35, 383–396. [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 Cell Culture Practices. Toxicology in Vitro 2012, 26, 1243–1246. [CrossRef]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX Co-Cultured Cells as a Model for Studying Physiological Properties and Toxin-Induced Effects on Intestinal Cells. PLoS One 2021, 16, e0257824. [CrossRef]
- Sun, H.; Chow, E.C.Y.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 Cell Monolayer: Usefulness and Limitations. Expert Opin Drug Metab Toxicol 2008, 4, 395–411. [CrossRef]
- Thummel, K.E.; Brimer, C.; Yasuda, K.; Thottassery, J.; Senn, T.; Lin, Y.; Ishizuka, H.; Kharasch, E.; Schuetz, J.; Schuetz, E. Transcriptional Control of Intestinal Cytochrome P-4503A by 1α,25-Dihydroxy Vitamin D3. Mol Pharmacol 2001, 60, 1399–1406. [CrossRef]
- Minkler, S.; Lucien, F.; Kimber, M.J.; Sahoo, D.K.; Bourgois-Mochel, A.; Musser, M.; Johannes, C.; Frank, I.; Cheville, J.; Allenspach, K.; et al. Emerging Roles of Urine-Derived Components for the Management of Bladder Cancer: One Man’s Trash Is Another Man’s Treasure. Cancers 2021, 13, 422. [CrossRef]
- Shanahan, M.T.; Kanke, M.; Oyesola, O.O.; Hung, Y.H.; Koch-Laskowski, K.; Singh, A.P.; Peck, B.C.E.; Biraud, M.; Sheahan, B.; Cortes, J.E.; et al. Multiomic Analysis Defines the First MicroRNA Atlas across All Small Intestinal Epithelial Lineages and Reveals Novel Markers of Almost All Major Cell Types. Am J Physiol Gastrointest Liver Physiol 2021, 321, G668–G681. [CrossRef]
- Kopper, J.J.; Iennarella-Servantez, C.; Jergens, A.E.; Sahoo, D.K.; Guillot, E.; Bourgois-Mochel, A.; Martinez, M.N.; Allenspach, K.; Mochel, J.P. Harnessing the Biology of Canine Intestinal Organoids to Heighten Understanding of Inflammatory Bowel Disease Pathogenesis and Accelerate Drug Discovery: A One Health Approach. Frontiers in Toxicology 2021, 0, 52. [CrossRef]
- Gabriel, V.; Zdyrski, C.; Sahoo, D.K.; Dao, K.; Bourgois-Mochel, A.; Kopper, J.; Zeng, X.-L.; Estes, M.K.; Mochel, J.P.; Allenspach, K. Standardization and Maintenance of 3D Canine Hepatic and Intestinal Organoid Cultures for Use in Biomedical Research. J Vis Exp 2022. [CrossRef]
- Zdyrski, C.; Iennarella-Servantez, C.A.; Sahoo, D.K.; Ward, J.; Long, E.; Gabriel, V.; Minkler, S.; Mao, S.; Bourgois-Mochel, A.; Jergens, A.; et al. Su124 HOMOLOGY DIRECTED REPAIR IN CANINE DUODENAL ENTEROIDS TO MIMIC THE WILD-TYPE P-GLYCOPROTEIN MUTATION. Gastroenterology 2021, 160, S-625-S-626. [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of Adult Canine Intestinal Organoids for Translational Research in Gastroenterology. BMC Biology 2019, 17, 1–21. [CrossRef]
- Ambrosini, Y.M.; Park, Y.; Jergens, A.E.; Shin, W.; Min, S.; Atherly, T.; Borcherding, D.C.; Jang, J.; Allenspach, K.; Mochel, J.P.; et al. Recapitulation of the Accessible Interface of Biopsy-Derived Canine Intestinal Organoids to Study Epithelial-Luminal Interactions. PLoS One 2020, 15, e0231423. [CrossRef]
- Gabriel, V.; Zdyrski, C.; Sahoo, D.K.; Dao, K.; Bourgois-Mochel, A.; Atherly, T.; Martinez, M.N.; Volpe, D.A.; Kopper, J.; Allenspach, K.; et al. Canine Intestinal Organoids in a Dual-Chamber Permeable Support System. JoVE (Journal of Visualized Experiments) 2022, e63612. [CrossRef]
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers (Basel) 2022, 14, 3525. [CrossRef]
- Bedos, L.; Wickham, H.; Gabriel, V.; Zdyrski, C.; Allbaugh, R.A.; Sahoo, D.K.; Sebbag, L.; Mochel, J.P.; Allenspach, K. Culture and Characterization of Canine and Feline Corneal Epithelial Organoids: A New Tool for the Study and Treatment of Corneal Diseases. Front Vet Sci 2022, 9. [CrossRef]
- Kodama, N.; Iwao, T.; Katano, T.; Ohta, K.; Yuasa, H.; Matsunaga, T. Characteristic Analysis of Intestinal Transport in Enterocyte-Like Cells Differentiated from Human Induced Pluripotent Stem Cells. DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 2016, 44, 1662–1667. [CrossRef]
- Akazawa, T.; Yoshida, S.; Ohnishi, S.; Kanazu, T.; Kawai, M.; Takahashi, K. Application of Intestinal Epithelial Cells Differentiated from Human Induced Pluripotent Stem Cells for Studies of Prodrug Hydrolysis and Drug Absorption in the Small Intestine. Drug Metab Dispos 2018, 46, 1497–1506. [CrossRef]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr Rev 2009, 30, 204–213. [CrossRef]
- Ergün, S.; Wörsdörfer, P. Organoids, Assembloids and Embryoids: New Avenues for Developmental Biology, Disease Modeling, Drug Testing and Toxicity Assessment without Animal Experimentation. Organoids 2022, Vol. 1, Pages 37-40 2022, 1, 37–40. [CrossRef]
- Liebau, S.; Parvin, B.; Ergün, S.; Richiardone, E.; Van Den Bossche, V.; Corbet, C. Metabolic Studies in Organoids: Current Applications, Opportunities and Challenges. Organoids 2022, 1, 85–105. [CrossRef]
- Burgess, T.; Barker, N.; Torresi, J.; Munro, M.J.; Tan, S.T.; Gray, C. Applications for Colon Organoid Models in Cancer Research. Organoids 2023, 2, 37–49. [CrossRef]
- Schutgens, F.; Rookmaaker, M.B.; Margaritis, T.; Rios, A.; Ammerlaan, C.; Jansen, J.; Gijzen, L.; Vormann, M.; Vonk, A.; Viveen, M.; et al. Tubuloids Derived from Human Adult Kidney and Urine for Personalized Disease Modeling. Nat Biotechnol 2019, 37, 303–313. [CrossRef]
- Usui, T.; Sakurai, M.; Nishikawa, S.; Umata, K.; Nemoto, Y.; Haraguchi, T.; Itamoto, K.; Mizuno, T.; Noguchi, S.; Mori, T.; et al. Establishment of a Dog Primary Prostate Cancer Organoid Using the Urine Cancer Stem Cells. Cancer Sci 2017, 108, 2383–2392. [CrossRef]
- Sun, G.; Ding, B.; Wan, M.; Chen, L.; Jackson, J.; Atala, A. Formation and Optimization of Three-Dimensional Organoids Generated from Urine-Derived Stem Cells for Renal Function in Vitro. Stem Cell Res Ther 2020, 11, 1–12. [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine Derived Cells Are a Potential Source for Urological Tissue Reconstruction. Journal of Urology 2008, 180, 2226–2233. [CrossRef]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines (Basel) 2019, 10. [CrossRef]
- Danku, A.E.; Dulf, E.H.; Braicu, C.; Jurj, A.; Berindan-Neagoe, I. Organ-On-A-Chip: A Survey of Technical Results and Problems. Front Bioeng Biotechnol 2022, 10, 94. [CrossRef]
- van Berlo, D.; Nguyen, V.V.T.; Gkouzioti, V.; Leineweber, K.; Verhaar, M.C.; van Balkom, B.W.M. Stem Cells, Organoids, and Organ-on-a-Chip Models for Personalized in Vitro Drug Testing. Curr Opin Toxicol 2021, 28, 7–14. [CrossRef]
- Menon-Andersen, D.; Florian, J.; Madabushi, R. CLINICAL PHARMACOLOGY REVIEW NDA Number 205410 Submission Type; Code. 2013.
- Yang, Y.; Faustino, P.J.; Volpe, D.A.; Ellison, C.D.; Lyon, R.C.; Yu, L.X. Biopharmaceutics Classification of Selected β-Blockers: Solubility and Permeability Class Membership. Mol Pharm 2007, 4, 608–614. [CrossRef]
- Martinez, M.N.; Mochel, J.P.; Neuhoff, S.; Pade, D. Comparison of Canine and Human Physiological Factors: Understanding Interspecies Differences That Impact Drug Pharmacokinetics. AAPS J 2021, 23. [CrossRef]
- Volpe, D.A.; Faustino, P.J.; Ciavarella, A.B.; Asafu-Adjaye, E.B.; Ellison, C.D.; Yu, L.X.; Hussain, A.S. Classification of Drug Permeability with a Caco-2 Cell Monolayer Assay. Ceased 2008, 24, 39–47. [CrossRef]
- Sahoo, D.K.; Roy, A.; Bhanja, S.; Chainy, G.B.N. Hypothyroidism Impairs Antioxidant Defence System and Testicular Physiology during Development and Maturation. Gen Comp Endocrinol 2008, 156. [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol 2015, 111, A3.B.1–A3.B.3. [CrossRef]
- Sahoo, D.K.; Roy, A.; Chainy, G.B.N. Protective Effects of Vitamin E and Curcumin on L-Thyroxine-Induced Rat Testicular Oxidative Stress. Chem Biol Interact 2008, 176. [CrossRef]
- Gabriel, V.; Zdyrski, C.; Sahoo, D.K.; Dao, K.; Bourgois-Mochel, A.; Atherly, T.; Martinez, M.N.; Volpe, D.A.; Kopper, J.; Allenspach, K.; et al. Canine Intestinal Organoids in a Dual-Chamber Permeable Support System. J Vis Exp 2022, 2022. [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J Lab Autom 2015, 20, 107–126.
- Wang, Y.; Bai, X.; Hu, B.; Xing, M.; Cao, Q.; Ji, A.; Song, S. Transport Mechanisms of Polymannuronic Acid and Polyguluronic Acid Across Caco-2 Cell Monolayers. Pharmaceutics 2020, 12. [CrossRef]
- Stockdale, T.P.; Challinor, V.L.; Lehmann, R.P.; de Voss, J.J.; Blanchfield, J.T. Caco-2 Monolayer Permeability and Stability of Chamaelirium Luteum (False Unicorn) Open-Chain Steroidal Saponins. 2019. [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices. Micromachines 2019, 10, 533. [CrossRef]
- PubChem Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 22 December 2022).
- Falavigna, M.; Stein, P.C.; Flaten, G.E.; di Cagno, M.P. Impact of Mucin on Drug Diffusion: Development of a Straightforward In Vitro Method for the Determination of Drug Diffusivity in the Presence of Mucin. Pharmaceutics 2020, 12. [CrossRef]
- Kulkarni, U.D.; Mahalingam, R.; Li, X.; Pather, I.; Jasti, B. Effect of Experimental Temperature on the Permeation of Model Diffusants Across Porcine Buccal Mucosa. AAPS PharmSciTech 2011, 12, 579. [CrossRef]
- Flaten, G.E.; Dhanikula, A.B.; Luthman, K.; Brandl, M. Drug Permeability across a Phospholipid Vesicle Based Barrier: A Novel Approach for Studying Passive Diffusion. Eur J Pharm Sci 2006, 27, 80–90. [CrossRef]
- Caco-2 Permeability Assay Available online: https://www.cyprotex.com/admepk/in-vitro-permeability/caco-2-permeability (accessed on 13 March 2022).
- In Vitro Drug Interaction Studies-Cytochrome P450 Enzyme-and Transporter-Mediated Drug Interactions Guidance for Industry. 2020.
- Katneni, K.; Pham, T.; Saunders, J.; Chen, G.; Patil, R.; White, K.L.; Abla, N.; Chiu, F.C.K.; Shackleford, D.M.; Charman, S.A. Using Human Plasma as an Assay Medium in Caco-2 Studies Improves Mass Balance for Lipophilic Compounds. [CrossRef]
- Heinlein, A.; Metzger, M.; Walles, H.; Buettner, A. Transport of Hop Aroma Compounds across Caco-2 Monolayers. Food Funct 2014, 5, 2719. [CrossRef]
- Quan, Y.; Jin, Y.; Faria, T.N.; Tilford, C.A.; He, A.; Wall, D.A.; Smith, R.L.; Vig, B.S. Expression Profile of Drug and Nutrient Absorption Related Genes in Madin-Darby Canine Kidney (MDCK) Cells Grown under Differentiation Conditions. Pharmaceutics 2012, 4, 314–333. [CrossRef]
- Almeida, L.D.; Quaglio, A.E.V.; De Almeida Costa, C.A.R.; Di Stasi, L.C. Intestinal Anti-Inflammatory Activity of Ground Cherry (Physalis Angulata L.) Standardized CO2 Phytopharmaceutical Preparation. World J Gastroenterol 2017, 23, 4369–4380. [CrossRef]
- Youhanna, S.; Lauschke, V.M. The Past, Present and Future of Intestinal In Vitro Cell Systems for Drug Absorption Studies. J Pharm Sci 2021, 110, 50–65. [CrossRef]
- He, Y.L.; Murby, S.; Warhurst, G.; Gifford, L.; Walker, D.; Ayrton, J.; Eastmond, R.; Rowland, M. Species Differences in Size Discrimination in the Paracellular Pathway Reflected by Oral Bioavailability of Poly(Ethylene Glycol) and D-Peptides. J Pharm Sci 1998, 87, 626–633. [CrossRef]
- Incecayir, T.; Tsume, Y.; Amidon, G.L. Comparison of the Permeability of Metoprolol and Labetalol in Rat, Mouse, and Caco-2 Cells: Use as a Reference Standard for BCS Classification. Mol Pharm 2013, 10, 958–966. [CrossRef]
- Zur, M.; Gasparini, M.; Wolk, O.; Amidon, G.L.; Dahan, A. The Low/High BCS Permeability Class Boundary: Physicochemical Comparison of Metoprolol and Labetalol. Mol Pharm 2014, 11, 1707–1714. [CrossRef]
- Dahlgren, D.; Roos, C.; Sjögren, E.; Lennernäs, H. Direct In Vivo Human Intestinal Permeability (Peff) Determined with Different Clinical Perfusion and Intubation Methods. J Pharm Sci 2015, 104, 2702–2726. [CrossRef]
- Dahlgren, D.; Roos, C.; Johansson, P.; Lundqvist, A.; Tannergren, C.; Abrahamsson, B.; Sjögren, E.; Lennernäs, H. Regional Intestinal Permeability in Dogs: Biopharmaceutical Aspects for Development of Oral Modified-Release Dosage Forms. Mol Pharm 2016, 13, 3022–3033. [CrossRef]
- Machen, T.E.; Erlij, D.; Wooding, F.B.P. PERMEABLE JUNCTIONAL COMPLEXES : The Movement of Lanthanum across Rabbit Gallbladder and Intestine. J Cell Biol 1972, 54, 302. [CrossRef]
- Frömter, E.; Diamond, J. Route of Passive Ion Permeation in Epithelia. Nat New Biol 1972, 235, 9–13. [CrossRef]
- Monaco, A.; Ovryn, B.; Axis, J.; Amsler, K. The Epithelial Cell Leak Pathway. Int J Mol Sci 2021, 22. [CrossRef]
- Amidon, G.; Lee, P.; Topp, E. Transport Processes in Pharmaceutical Systems; 1999;
- Saaby, L.; Helms, H.C.C.; Brodin, B. IPEC-J2 MDR1, a Novel High-Resistance Cell Line with Functional Expression of Human P-Glycoprotein (ABCB1) for Drug Screening Studies. Mol Pharm 2016, 13, 640–652. [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. Pharmaceutics 2019, 11. [CrossRef]
- Li, J.; Volpe, D.A.; Wang, Y.; Zhang, W.; Bode, C.; Owen, A.; Hidalgo, I.J. Use of Transporter Knockdown Caco-2 Cells to Investigate the In Vitro Efflux of Statin Drugs. Drug Metabolism and Disposition 2011, 39, 1196–1202. [CrossRef]
- Meneses, A.M.C.; Schneeberger, K.; Kruitwagen, H.S.; Penning, L.C.; van Steenbeek, F.G.; Burgener, I.A.; Spee, B. Intestinal Organoids—Current and Future Applications. Veterinary Sciences 2016, 3, 31. [CrossRef]
- Reineking, W.; Schauerte, I.E.; Junginger, J.; Hewicker-Trautwein, M. Sox9, Hopx, and Survivin and Tuft Cell Marker DCLK1 Expression in Normal Canine Intestine and in Intestinal Adenoma and Adenocarcinoma. Vet Pathol 2022, 59, 415–426. [CrossRef]
- Karim, B.O.; Rhee, K.J.; Liu, G.; Yun, K.; Brant, S.R. Prom1 Function in Development, Intestinal Inflammation, and Intestinal Tumorigenesis. Front Oncol 2014, 4, 323. [CrossRef]
- Wang, X.Y.; Chen, S.H.; Zhang, Y.N.; Xu, C.F. Olfactomedin-4 in Digestive Diseases: A Mini-Review. World J Gastroenterol 2018, 24, 1881. [CrossRef]

| Species | Category | Gene Full Name | Symbol | Forward Primer Sequence (5'-3') | Reverse Primer Sequence (5'-3') |
|---|---|---|---|---|---|
| Canis lupus familiaris (dog) | Housekeeping gene | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | TCAACGGATTTGGCCGTATTGG | TGAAGGGGTCATTGATGGCG |
| Tight and adherens junction proteins | Cadherin 1 | CDH1 | GACCCAGTAACTAACGACG | CTTCATTCACATCTTCCACG | |
| Occludin | OCLN | CACTACTGTGTGGTGGATCC | CCTTGTCCCACAATATATTCG | ||
| Tight junction protein 1 | TJP1 | GAGGGTGATCAAATTCTCAGG | CTGATTCTACAATGCGACG | ||
| Intestinal epithelial cell differentiation markers | Mucin 2 | MUC2 | CCTGTGCCCCATATTCTGC | GAGATGTTGGAATGGATGCC | |
| Neurogenin 3 | NEUROG3 | GAATGCACAACCTCAACTCG | GTAGAGGCTGTGGTCCGC | ||
| Intestinal alkaline phosphatase | ALP | CGTAGTAAACCGCAACTGG | GGAAACATGTACTTTCGGC | ||
| Stem cell markers | Olfactomedin 4 | OLFM4 | GTATCATGAATGTCAGCAAGC | CTGTAATATTCCAGAATTCTTCC | |
| HOP homeobox | HOPX | GACCAGGTGGAGATTCTGG | GCCAGACGCTGCTTAAACC | ||
| Prominin 1 | PROM1 | GATTATTATTTGTGCTGTCC | GAGACTGTAAAGTATTTCCTC | ||
| SRY-box transcription factor 9 | SOX9 | GTCATCTCCAACATAGAGACC | CTGCTTGGACATCCACACG | ||
| Leucine-rich repeat-containing G protein-coupled receptor 5 | LGR5 | GCTAGATCTGTCTTACAACC | GTTCCAGGCTAAATTCAGC | ||
| Transporters | Organic anion transporting polypeptide | Oatp2b1 | GATGACTTTGCCCACAACAGC | CAGCAGCAGAGATGAGGAAGC | |
| Multidrug resistance p-glycoprotein | Mdr1 | GTAGCTGAAGAAGTCTTAGCAGC | GCGGCACCAATAGAAATGTTGGC | ||
| Cytochrome P450 (CYP) enzymes | Cytochrome P-450 3a12 | Cyp3a12 | GATCATGAACATGAAACTTGC | CTTTTCAGGTTGAATAATCCC | |
| Cytochrome P450 2b11 | Cyp2b11 | CTGAGGGAGTCCTCCAGGACCC | CACATAGAACAAGTTCATCAGG | ||
| Cytochrome P450 2C21 | Cyp2c21 (Cyp2c18) | CAAGCACCTCCTGGATACAGC | CTTCGTGTTCTTTTATTTTTTCC | ||
| Homo sapiens (human) | Housekeeping gene | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Transporters | Organic anion transporting polypeptide 2B1/ solute carrier organic anion transporter family member 2B1 | OATP2B1 (SLCO2B1) | CAAACCTGACTGTGATCCAG | GAGCAGGTTGGCGTATGAGG | |
| ATP binding cassette subfamily B member 1 | ABCB1 (MDR1) | CAGTAGCTGAAGAGGTCTTGGC | CTGTAATAGCTTTCTTTATCCC | ||
| Cytochrome P450 (CYP) enzymes | Cytochrome P450 family 3 subfamily A member 4 | CYP3A4 | GAGATGGTCCCTATCATTGCC | GATGTTCACTCCAAATGATGTGC | |
| Cytochrome P450 family 2 subfamily B member 6 | CYP2B6 | GAAACCGCTGGAAGGTGCTTCG | CTCCTCTATCAGACACTGAGC | ||
| Cytochrome P450 family 2 subfamily C member 9 | CYP2C9 | GAAGGAGATCCGGCGTTTCTCC | CTTGGTTTTTCTCAACTCCTCC | ||
| Cytochrome P450 family 2 subfamily C member 19 | CYP2C19 | GATCTGCTCCATTATTTTCC | GTTTTTAAGTAATTTGTTATGG |
| Drug | Molecular Formula (MF) | Molecular Weight (MW) | Aqueous solubility (25°C) | Log P | Dissociation Constants (Basic pKa) |
|---|---|---|---|---|---|
| Metoprolol | C15H25NO3 | 267.36 g/mol | >1000 mg/mL | 2.15 | 9.56 |
| Atenolol | C14H22N2O3 | 266.34 g/mol | 13.3 mg/mL | 0.16 | 9.58 |
| Propranolol | C16H21NO2 | 259.339 g/mol | 0.0617 mg/L | 3.48 | 9.53 |
| Compound | Time (min) | Donor (%) | Receiver (%) |
|---|---|---|---|
| FITC-Dextran (200 µg/mL) | 0 | 100 ± 0.0 | 0.020 ± 0.001 |
| 15 | 0.021 ± 0.003 | ||
| 30 | 0.024 ± 0.004 | ||
| 45 | 0.022 ± 0.002 | ||
| 60 | 0.024 ± 0.003 | ||
| 90 | 0.023 ± 0.004 | ||
| 120 | 93.7 ± 7.8 | 0.022 ± 0.002 | |
| Metoprolol (0.4 mg/mL) + | 0 | 100 ± 0.0 | 0.021 ± 0.001 |
| FITC-Dextran (200 µg/mL) | 15 | 0.023 ± 0.001 | |
| 30 | 0.020 ± 0.00 | ||
| 45 | 0.020 ± 0.00 | ||
| 60 | 0.022 ± 0.001 | ||
| 90 | 0.023 ± 0.00 | ||
| 120 | 94.6 ± 1.4 | 0.020 ± 0.001 |
| Drug | Species | Direction | Well | Papp × 10-6 | Avg | SD | %CV |
|---|---|---|---|---|---|---|---|
| Metoprolol (0.4 mg/mL or 584.1 μM) |
Human | AP→BL pH 6.8/7.4 | 1 | 19.34 | |||
| 2 | 16.45 | 17.89 | 2.05 | 11.43 | |||
| BL→AP pH 7.4/7.4 | 1 | 13.79 | |||||
| 2 | 12.37 | 13.08 | 1.01 | 7.72 | |||
| Dog* | AP→BL pH 6.8/7.4 | 1 | 8.33 | ||||
| 2 | 9.10 | 8.72 | 0.55 | 6.32 | |||
| AP→BL pH 7.4/7.4 | 1 | 11.19 | |||||
| 2 | 9.90 | 10.54 | 0.91 | 8.66 | |||
| BL→AP pH 7.4/7.4 | 1 | 9.88 | |||||
| 2 | 9.94 | 9.91 | 0.05 | 0.46 | |||
| Metoprolol (0.04 mg/mL or 58.4 μM) |
Human | AP→BL pH 6.8/7.4 | 1 | 17.54 | |||
| 2 | 13.13 | 15.33 | 3.12 | 20.38 | |||
| BL→AP pH 7.4/7.4 | 1 | 16.30 | |||||
| 2 | 19.03 | 17.67 | 1.93 | 10.92 | |||
| Dog | AP→BL pH 6.8/7.4 | 1 | 16.83 | ||||
| 2 | 18.52 | 17.68 | 1.19 | 6.75 | |||
| AP→BL pH 7.4/7.4 | 1 | 13.44 | |||||
| 2 | 13.50 | 13.47 | 0.04 | 0.32 | |||
| BL→AP pH 7.4/7.4 | 1 | 11.19 | |||||
| 2 | 12.67 | 11.93 | 1.04 | 8.76 | |||
| Atenolol (0.2 mg/mL or 750.9 μM) |
Human | AP→BL pH 6.8/7.4 | 1 | BLQ | |||
| 2 | BLQ | ||||||
| BL→AP pH 7.4/7.4 | 1 | BLQ | |||||
| 2 | BLQ | ||||||
| Dog | AP→BL pH 6.8/7.4 | 1 | BLQ | ||||
| 2 | BLQ | ||||||
| AP→BL pH 7.4/7.4 | 1 | BLQ | |||||
| 2 | BLQ | ||||||
| BL→AP pH 7.4/7.4 | 1 | BLQ | |||||
| 2 | BLQ | ||||||
| Atenolol (2 mg/mL or 7,509 μM) |
Human | AP→BL pH 6.8/7.4 | 1 | 7.25 | |||
| 2 | 8.01 | 7.63 | 0.54 | 7.09 | |||
| BL→AP pH 7.4/7.4 | 1 | 3.69 | |||||
| 2 | 4.97 | 4.33 | 0.90 | 20.89 | |||
| Dog | AP→BL pH 6.8/7.4 | 1 | BLQ | ||||
| 2 | BLQ | ||||||
| AP→BL pH 7.4/7.4 | 1 | BLQ | |||||
| 2 | BLQ | ||||||
| BL→AP pH 7.4/7.4 | 1 | BLQ | |||||
| 2 | BLQ | ||||||
| Propranolol (1 mg/mL or 3,380 μM) |
Human | AP→BL pH 6.8/7.4 | 1 | 12.12 | |||
| 2 | 12.75 | 12.44 | 0.45 | 3.59 | |||
| BL→AP pH 7.4/7.4 | 1 | 11.45 | |||||
| 2 | 12.43 | 11.94 | 0.70 | 5.84 | |||
| Dog | AP→BL pH 6.8/7.4 | 1 | 12.23 | ||||
| 2 | 11.05 | 11.64 | 0.83 | 7.15 | |||
| AP→BL pH 7.4/7.4 | 1 | 11.99 | |||||
| 2 | 11.97 | 11.98 | 0.01 | 0.09 | |||
| BL→AP pH 7.4/7.4 | 1 | 10.94 | |||||
| 2 | 12.03 | 11.49 | 0.77 | 6.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





