Submitted:
10 April 2023
Posted:
10 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
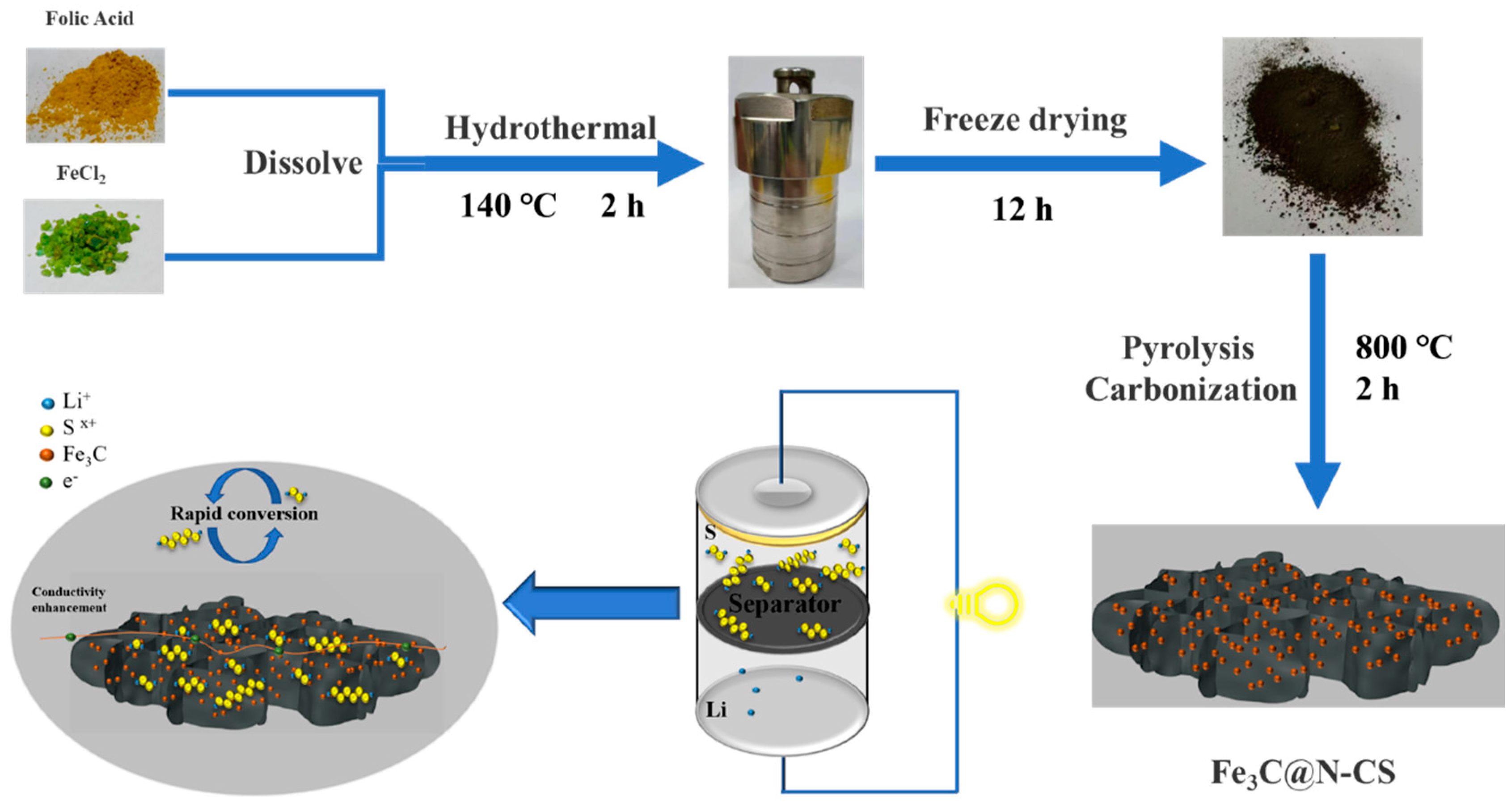
2.1. Preparation of Fe3C@N-CS
2.2. The fabrication of Fe3C@N-CS modified separator
2.3. Synthesis of lithium Li2S6 solution
2.4. Electrochemical measures
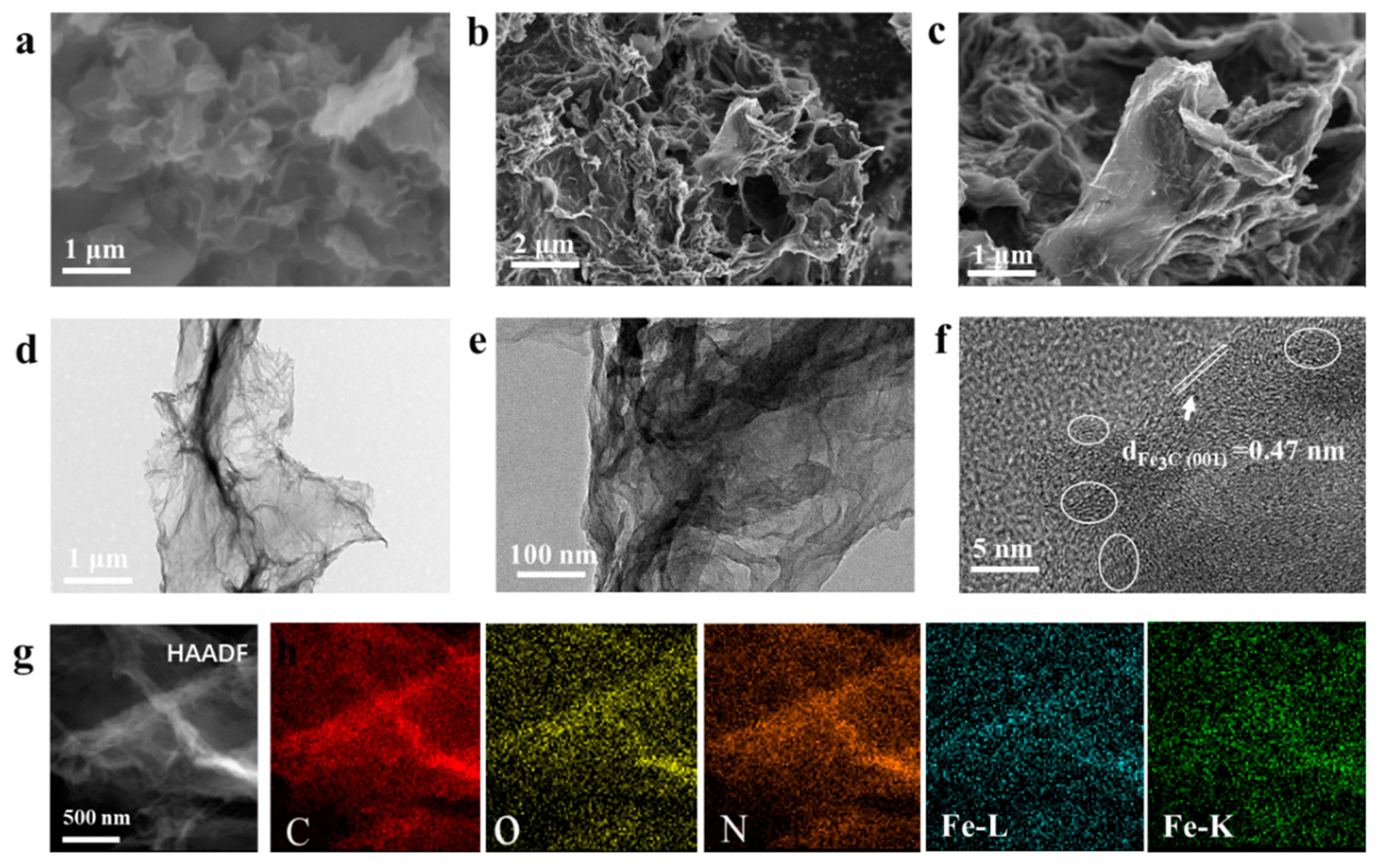
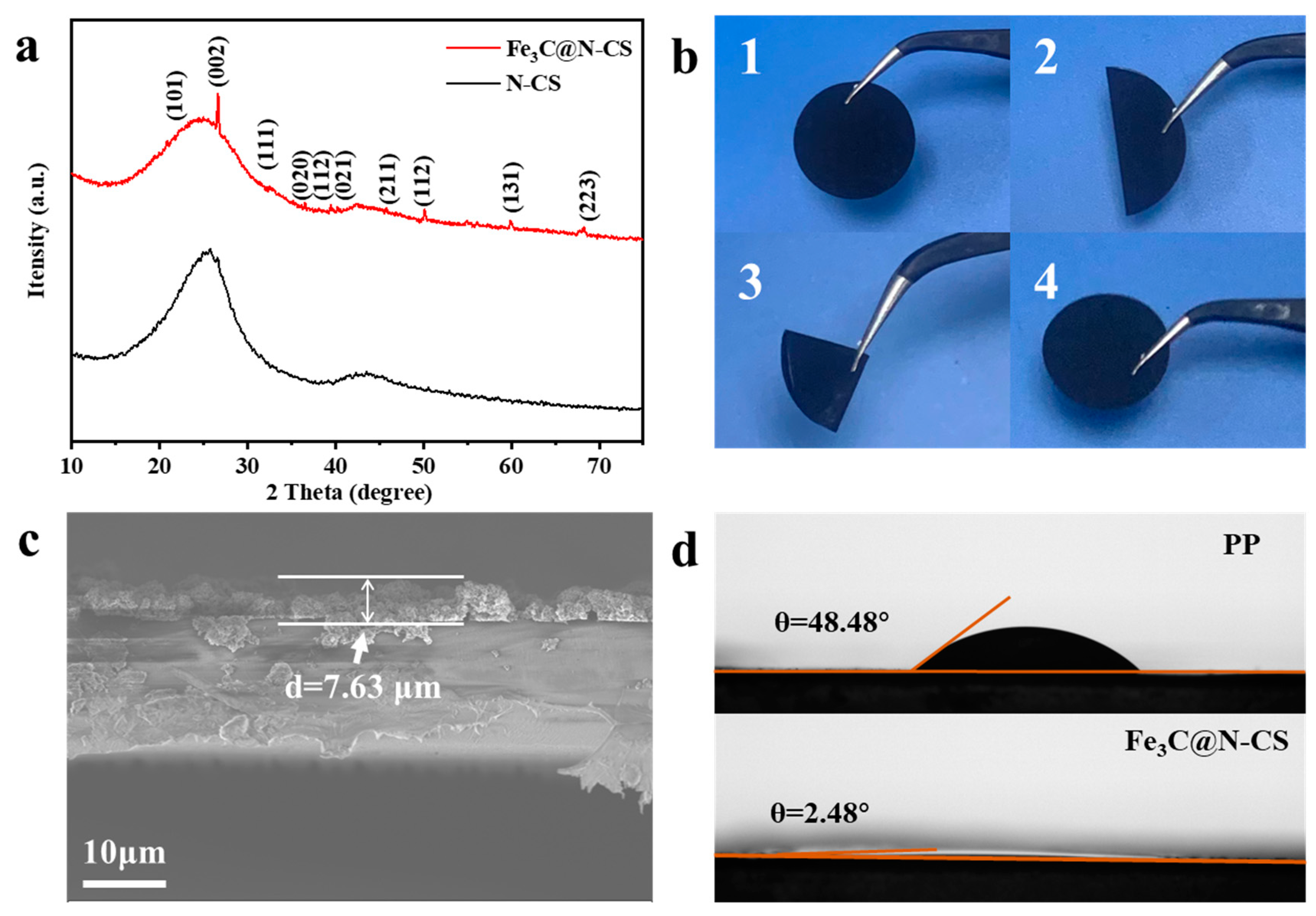
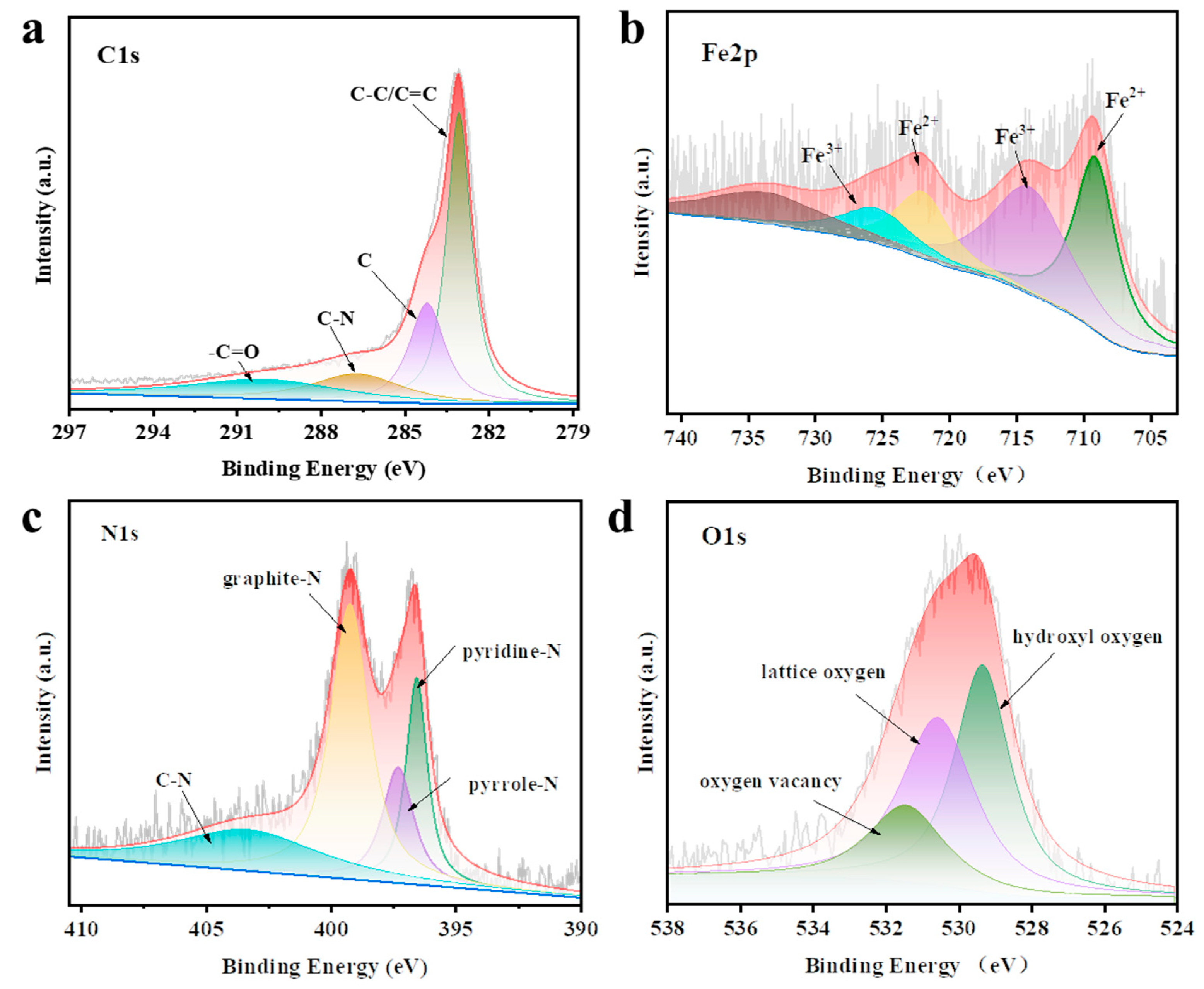
2.5. Material characterization
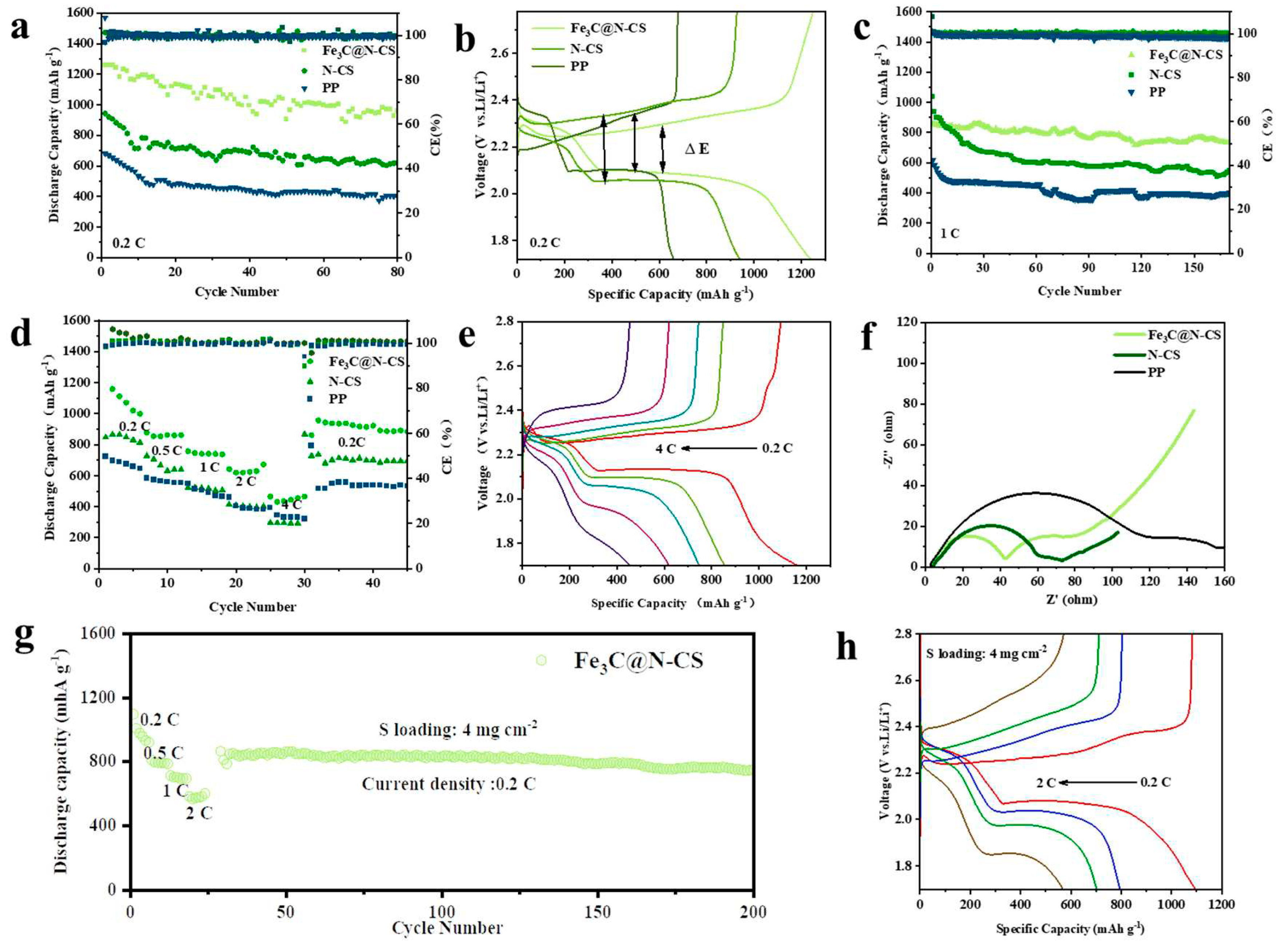
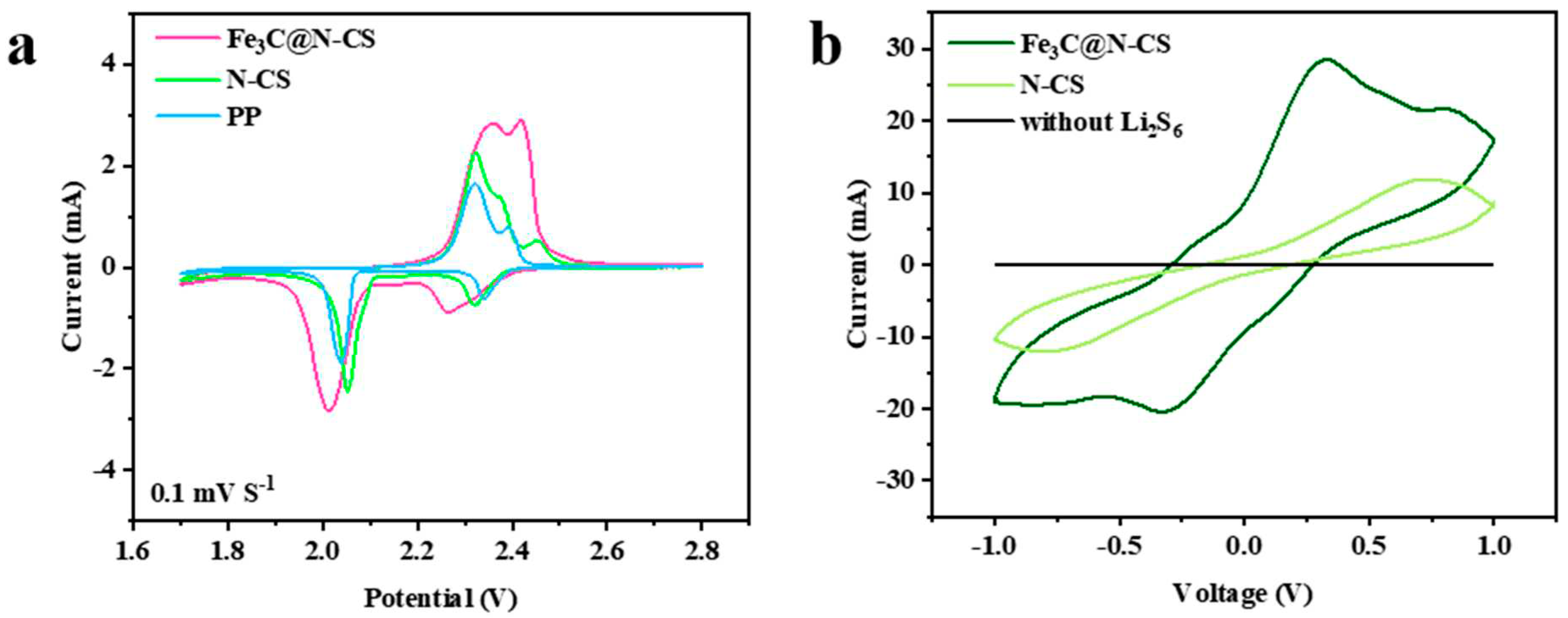
3. Results and discussion
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Qiu, Y.; Jiang, B.; Chen, Z.; Zhao, C.; Zhou, H.; Yang, L.; Fan, L.; Zhang, Y.; Zhang, N. , Isolated Fe-Co heteronuclear diatomic sites as efficient bifunctional catalysts for high-performance lithium-sulfur batteries. Nat Commun 2023, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tian, D.; Song, X.; Jiang, B.; Zhao, C.; Zhang, Y.; Yang, L.; Fan, L.; Yin, X.; Zhang, N. , In situ conversion to construct fast ion transport and high catalytic cathode for high-sulfur loading with lean electrolyte lithium–sulfur battery. Nano Energy 2022, 95, 106979. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L. F. , A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. , Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Jiang, F.-N.; Yang, S.-J.; Chen, Z.-X.; Liu, H.; Yuan, H.; Liu, L.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. , Higher-order polysulfides induced thermal runaway for 1.0 Ah lithium sulfur pouch cells. Particuology 2023, 79, 10–17. [Google Scholar] [CrossRef]
- Wang, S.-M.; Li, H.-N.; Zhao, G.-F.; Xu, L.-F.; Liu, D.-L.; Sun, Y.-J.; Guo, H. , Ni3FeN anchored on porous carbon as electrocatalyst for advanced Li–S batteries. Rare Metals 2022, 42, 515–524. [Google Scholar] [CrossRef]
- Tan, K.; Liu, Y.; Tan, Z.; Zhang, J.; Hou, L.; Yuan, C. , High-yield and in situ fabrication of high-content nitrogen-doped graphene nanoribbons@Co/CoOOH as an integrated sulfur host towards Li–S batteries. J. Mater. Chem. A 2020, 8, 3048–3059. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, J.; Wang, Y.; Xia, Y. , Synergetic Protective Effect of the Ultralight MWCNTs/NCQDs Modified Separator for Highly Stable Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702288. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Q.; Xu, H.; Zhang, Y.; Zhang, B.; Zhang, Z.; Lin, Z.; Zhang, S. , Ni/SiO2/Graphene-modified separator as a multifunctional polysulfide barrier for advanced lithium-sulfur batteries. Nano Energy 2020, 76, 105033. [Google Scholar] [CrossRef]
- Jiang, F.-N.; Yang, S.-J.; Cheng, X.-B.; Shi, P.; Ding, J.-F.; Chen, X.; Yuan, H.; Liu, L.; Huang, J.-Q.; Zhang, Q. , Thermal safety of dendritic lithium against non-aqueous electrolyte in pouch-type lithium metal batteries. J. Energy Chem. 2022, 72, 158–165. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Gu, J.; Li, S.; Yan, T.; Gao, X.-P. , The Fundamental Understanding of Lithium Polysulfides in Ether-Based Electrolyte for Lithium–Sulfur Batteries. ACS Energy Lett. 2021, 6, 537–546. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, X.; Wang, J.; Qian, Z.; Wang, Z.; Guo, H.; Yan, G.; Jiang, D.; Wang, R. , Facile and scalable fabrication of lithiophilic CuO enables stable lithium metal anode. J. Energy Chem. 2022, 75, 285–292. [Google Scholar] [CrossRef]
- Feng, J.; Jiayi, L.; Hongwei, Z.; Wendong, L.; Zenghui, L.; Tianyi, W.; Bing, S.; Xiaoxian, Z.; Fengyun, W.; Jianjun, S. , Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries. Energy Materials 2023, 3, 300001. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Li, W.; Guo, X.; Wang, Y.; Wang, G.; Zhao, D. , Porous Carbon Composites for Next Generation Rechargeable Lithium Batteries. Adv. Energy Mater. 2017, 7, 1700283. [Google Scholar] [CrossRef]
- Xie, C.; Shan, H.; Song, X.; Chen, L.; Wang, J.; Shi, J.-W.; Hu, J.; Zhang, J.; Li, X. , Flexible S@C-CNTs cathodes with robust mechanical strength via blade-coating for lithium-sulfur batteries. Journal of Colloid and Interface Science 2021, 592, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Hu, F.; Zeng, R.; Huang, Y.; Yuan, L.; Xie, J. , Cobalt-embedded carbon nanofiber as electrocatalyst for polysulfide redox reaction in lithium sulfur batteries. Electrochim. Acta 2019, 304, 11–19. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.-Y.; Wang, P.; Wang, S.-M.; Feng, M. , Synthesis of MOF-derived nitrogen-doped carbon microtubules via template self-consumption. Rare Metals 2022, 41, 2582–2587. [Google Scholar] [CrossRef]
- Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q. , Challenges and promises of lithium metal anode by soluble polysulfides in practical lithium–sulfur batteries. Mater Today 2021, 45, 62–76. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. , Emerging Catalysts to Promote Kinetics of Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. , Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, C.; Zeng, P.; Cheng, C.; Yan, T.; Dai, K.; Mao, J.; Zhang, L. , Bidirectionally catalytic polysulfide conversion by high-conductive metal carbides for lithium-sulfur batteries. J. Energy Chem. 2022, 67, 73–81. [Google Scholar] [CrossRef]
- Yang, J.-L.; Cai, D.-Q.; Lin, Q.; Wang, X.-Y.; Fang, Z.-Q.; Huang, L.; Wang, Z.-J.; Hao, X.-G.; Zhao, S.-X.; Li, J.; Cao, G.-Z.; Lv, W. , Regulating the Li2S deposition by grain boundaries in metal nitrides for stable lithium-sulfur batteries. Nano Energy 2022, 91, 106669. [Google Scholar] [CrossRef]
- Zuo, J.-H.; Gong, Y.-J. , Applications of transition-metal sulfides in the cathodes of lithium–sulfur batteries. Tungsten 2020, 2, 134–146. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Luo, L.; Li, H.; He, J.; Zu, H.; Liu, L.; Liu, H.; Wang, F.; Song, J. , Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 2205–2213. [Google Scholar] [CrossRef]
- Song, J.; Guo, X.; Zhang, J.; Chen, Y.; Zhang, C.; Luo, L.; Wang, F.; Wang, G. , Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 6507–6513. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Luo, L.; Zhang, H.; Feng, J.; Zhao, X.; Guo, X.; Dong, H.; Chen, S.; Liu, H.; Shao, G.; Anthopoulos, T. D.; Su, Y.; Wang, F.; Wang, G. , Synergy of MXene with Se Infiltrated Porous N-Doped Carbon Nanofibers as Janus Electrodes for High-Performance Sodium/Lithium–Selenium Batteries. Adv. Energy Mater. 2022, 12, 2200894. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, S.; Zhang, X.; Wei, J.; Liu, Y.; Hou, L.; Yuan, C. , Few-layered V2C MXene derived 3D V3S4 nanocrystal functionalized carbon flakes boosting polysulfide adsorption and catalytic conversion towards Li–S batteries. J. Mater. Chem. A 2022, 10, 18679–18689. [Google Scholar] [CrossRef]
- Ali, T.; Yan, C. , 2 D Materials for Inhibiting the Shuttle Effect in Advanced Lithium–Sulfur Batteries. ChemSusChem 2020, 13, 1447–1479. [Google Scholar] [CrossRef]
- Yu, K.; Wang, X.; Yang, H.; Bai, Y.; Wu, C. , Insight to defects regulation on sugarcane waste-derived hard carbon anode for sodium-ion batteries. J. Energy Chem. 2021, 55, 499–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, R.; Li, Y.; Zhu, X.; Zhang, B.; Lang, X.; Zhao, L.; Jin, B.; Zhu, Y.; Jiang, Q. , Potassium-ion batteries with novel N, O enriched corn silk-derived carbon as anode exhibiting excellent rate performance. J. Power Sources 2021, 481, 228644. [Google Scholar] [CrossRef]
- Wu, R.; Chen, S.; Deng, J.; Huang, X.; Song, Y.; Gan, R.; Wan, X.; Wei, Z. , Hierarchically porous nitrogen-doped carbon as cathode for lithium–sulfur batteries. J. Energy Chem. 2018, 27, 1661–1667. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Li, Y.; Zheng, F.; Li, Y.; Deng, Q.; Zhong, W.; Wang, G.; Liu, T. , FeSe2/nitrogen-doped carbon as anode material for Potassium-ion batteries. Chem. Eng. J. 2020, 393, 124590. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. , Recent progress of emerging cathode materials for sodium ion batteries. Materials Chemistry Frontiers 2021, 5, 3735–3764. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Z.; Lv, H.; Zhao, Y.; Wei, H.; Huai, G.; Xu, R.; Wang, Y. , High-entropy nanoparticle constructed porous honeycomb as a 3D sulfur host for lithium polysulfide adsorption and catalytic conversion in Li–S batteries. J. Mater. Chem. A 2023, 11, 5883–5894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




