Submitted:
07 April 2023
Posted:
10 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Materials and Methods:
2.1. Setting and ethical consideration:
2.2. Data collection:
2.3. Statistical Analysis:
3. Results:
3.1. Demographic Data and laboratory findings:
3.2. Management Data:
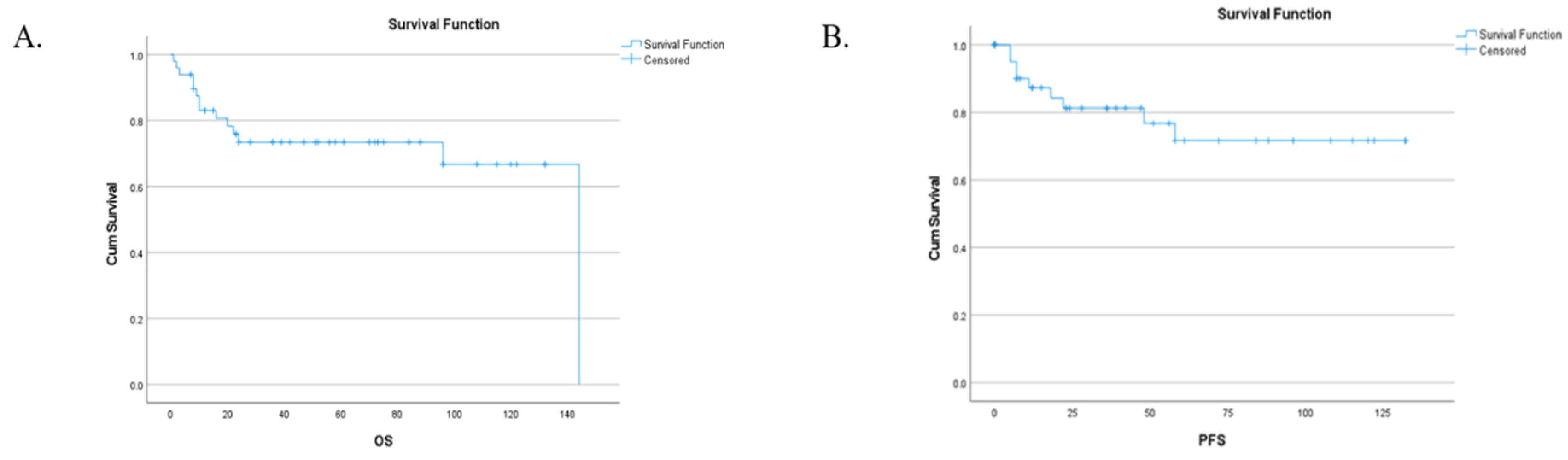
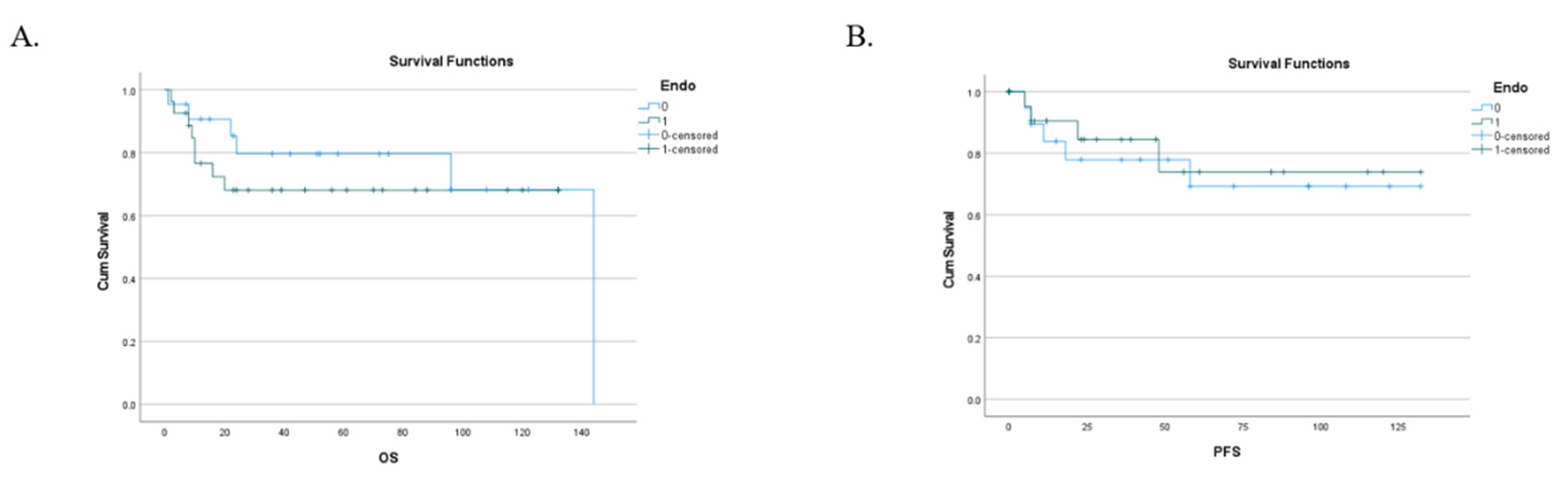
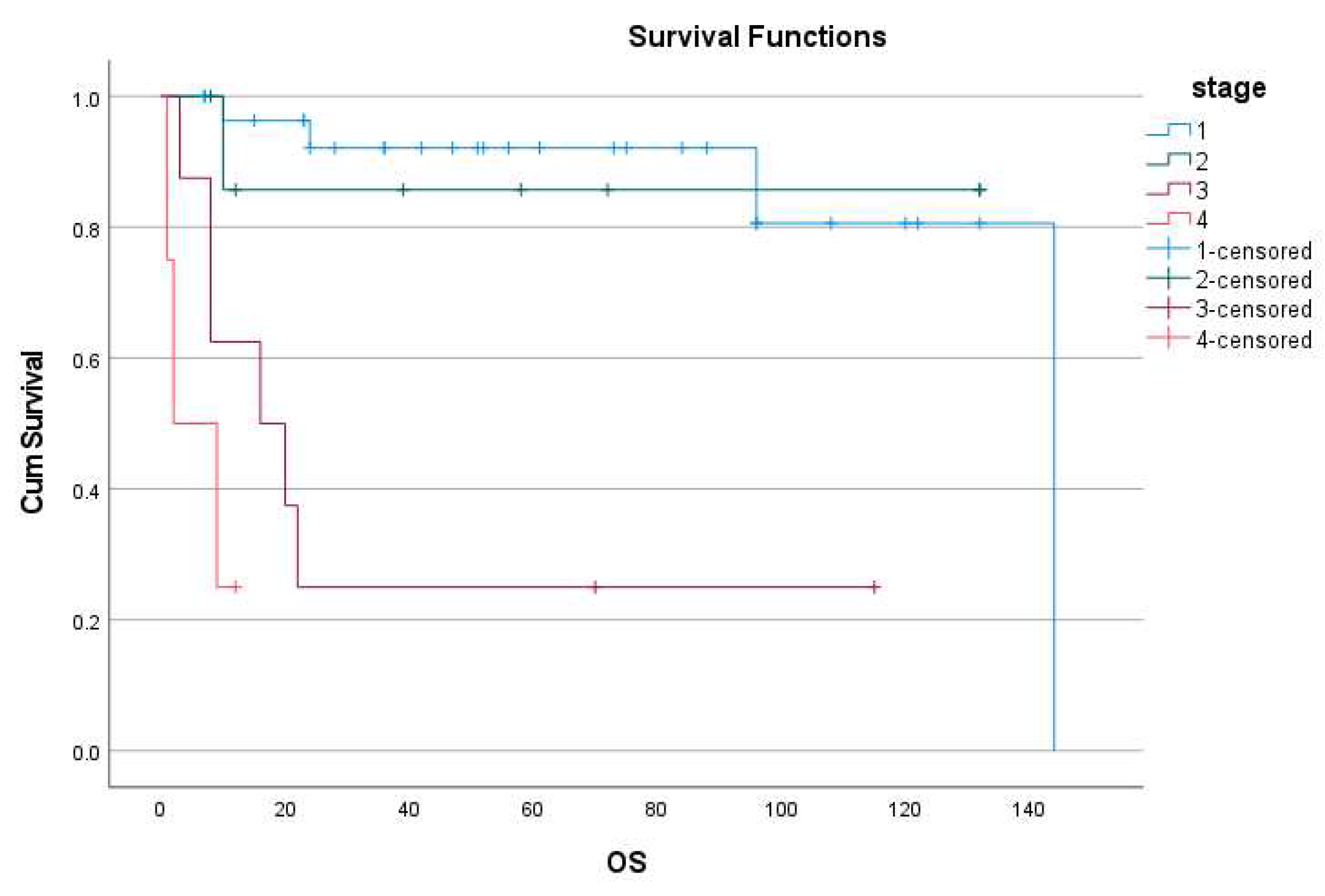
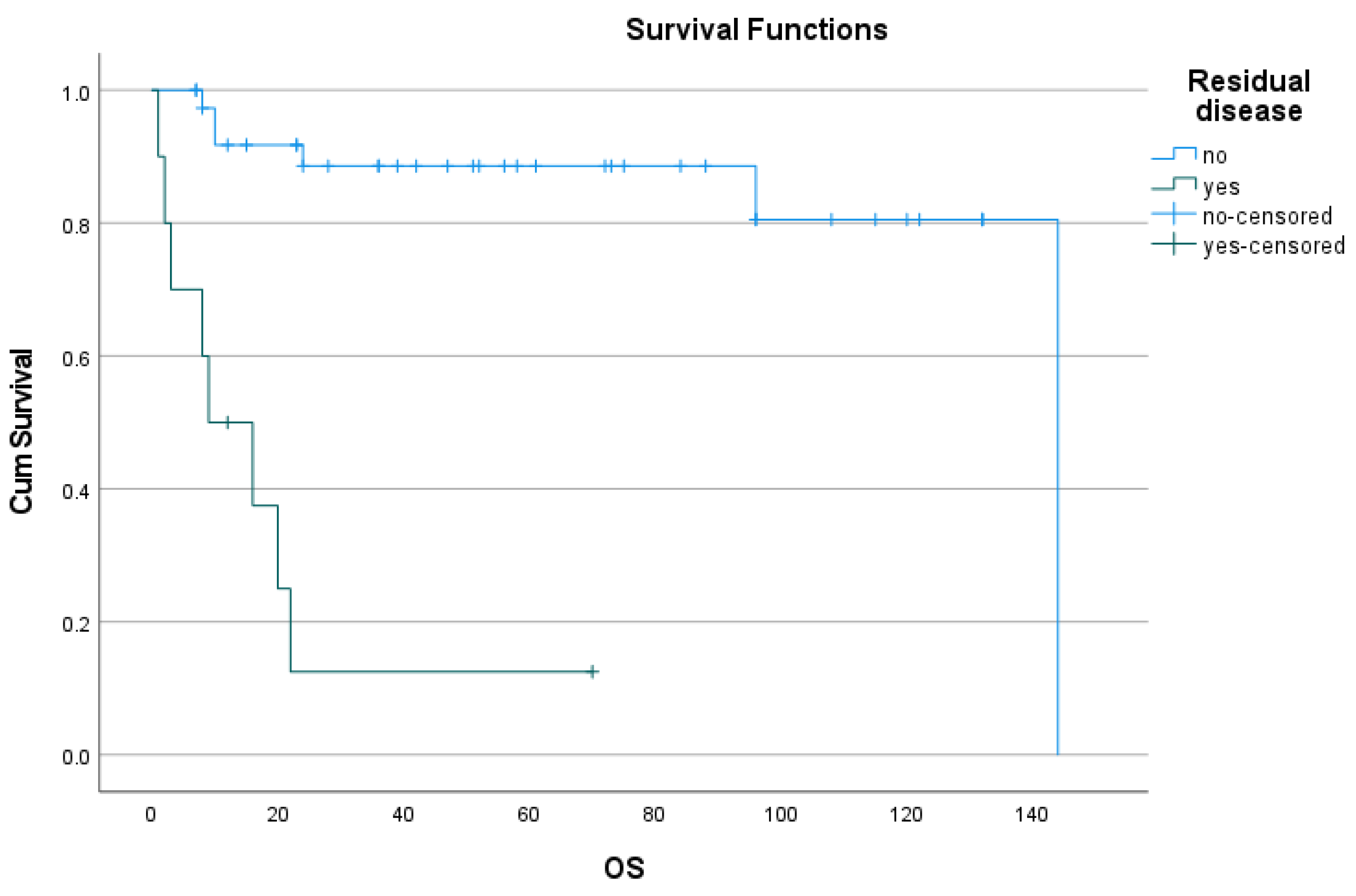
3.3. Follow-up, Disease progression, survival, PFS and recurrence:
4. Discussion:
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, S.I.; Lim, M.C.; Lim, J.; Won, Y.J.; Seo, S.S.; Kang, S.; et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016, 27, e5. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, G.F.; Morassi, F.; Prisco, M.G.; De Stefano, I.; Vellone, V.G.; Arena, V.; Scambia, G.; Gallo, D. Clinicopathologic and immunohistochemical features of ovarian clear cell carcinomas in comparison with type I and type II tumors. Int J Gynecol Pathol 2012, 31, 507–516. [Google Scholar] [CrossRef]
- Keith Y. Terada, Hyeong Jun Ahn, Bruce Kessel. Differences in risk for type 1 and type 2 ovarian cancer in a large cancer screening trial. J Gynecol Oncol. 2016, 27, e25. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sumimoto, K.; Moniwa, N.; et al. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer 2007, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; et al. Clear-cell adenofibroma can be a clonal precursor for clear-cell adenocarcinoma of the ovary: a possible alternative ovarian clear-cell carcinogenic pathway. J Pathol 2008, 216, 103–110. [Google Scholar] [CrossRef]
- Matsuura, Y.; Robertson, G.; Marsden, E.D.; Kim, S.N.; Gebski, V.; Hacker, N.F. Thromboembolic complications in patients with clear cell carcinoma of the ovary. Gynecol Oncol. 2007, 104, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.S.; Walts, A.E.; Karlan, B.Y.; Walsh, C.S. Venous thromboembolism during primary treatment of ovarian clear cell carcinoma is associated with decreased survival. Gynecol. Oncol. 2013, 131, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Yoshihara, K.; Matsuo, K.; Yachida, N.; Miyoshi, A.; Takahashi, K.; et al. Proposing a molecular classification associated with hypercoagulation in ovarian clear cell carcinoma. Gynecologic Oncology 2021, 163, 327–333. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Ji, J.; Dong, R.; Qiu, H.; Dai, X. Prognosis of ovarian clear cell cancer compared with other epithelial cancer types: a population-based analysis. Oncol Lett. 2020, 19, 1947–1957. [Google Scholar] [CrossRef]
- Prat, J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Köbel, M.; Kang, E.Y. The Evolution of Ovarian Carcinoma Subclassification. Cancers 2022, 14, 416. [Google Scholar] [CrossRef]
- Fadare, O.; Zhao, C.; Khabele, D.; Parkash, V.; Quick, C.M.; Gwin, K.; et al. Comparative analysis of Napsin A, alpha-methylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology. 2015, 47, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Aoki, D.; Hattori, K.; Jinushi, M.; Sugiyama, T. The first Japanese nationwide multicenter study of BRCA mutation testing in ovarian cancer: Characterizing the cross-sectionaL approach to ovarian cancer genetic Testing of BRCA (CHARLOTTE). Int J Gynecol Cancer. 2019, 29, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sugimoto, H.; Onishi, S.; Nakano, K. Novel biomarker candidates for the diagnosis of ovarian clear cell carcinoma. Oncol Lett. 2015, 10, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhu, J.; Qian, L.; Liu, H.; Shen, Z.; et al. Clinical characteristics and prognosis of ovarian clear cell carcinoma: a 10-year retrospective study. BMC Cancer 2021, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yao, L.; Dai, L.; Zhu, H.; Ye, X.; et al. Ovarian endometroid carcinoma and clear cell carcinoma: A 21-year retrospective study. Journal of ovarian research 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.A.; Zhou, Q.; Jotwani, A.R.; Iasonos, A.; Leitao, M.M., Jr.; et al. Ovarian clear cell carcinoma, outcomes by stage: The MSK experience. Gynaecologic Oncology 2015, 139, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Kobel, M.; Kalloger, S.E.; Huntsman, D.G.; Santos, J.L.; Swenerton, K.D.; Seidman, J.D.; et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int. J. Gynecol. Pathol. 2010, 29, 203–211. [Google Scholar] [CrossRef] [PubMed]
- www.clinicaltrials.gov ID NCT02718417: Avelumab in previously untreated patients with epithelial ovarian cancer.
- www.clinicaltrials.gov ID NCT00889382: A Study Evaluating Intermittent and Continuous OSI-906 and Weekly Paclitaxel in Patients with Recurrent Epithelial Ovarian Cancer (and Other Solid Tumors).
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomized, controlled, non-inferiority trial. The Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Miller, R.E.; Clamp, A.; Gourley, C.; et al. 2022-RA-1276-ESGO Pembrolizumab monotherapy for advanced clear cell gynaecological cancer: phase II PEACOCC trial. International Journal of Gynecologic Cancer 2022, 32, A316. [Google Scholar]
- Bell, J.; Brady, M.F.; Young, R.C.; Lage, J.; Walker, J.L.; Look, K.Y.; et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2006, 102, 432–439. [Google Scholar] [CrossRef]
- Chan, J.K.; Tian, C.; Fleming, G.F.; Monk, B.J.; Herzog, T.J.; Kapp, D.S.; et al. The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high risk epithelial ovarian cancer: an exploratory analysis of a Gynecologic Oncology Group study. Gynecol. Oncol. 2010, 116, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Trimbos, J.B.; Vergote, I.; Bolis, G.; et al.; EORTC-ACTION Collaborators European organisation for research and treatment of cancer-adjuvant ChemoTherapy in ovarian neoplasm. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for research and treatment of cancer-adjuvant ChemoTherapy in ovarian neoplasmtrial. J Natl Cancer Inst 2003, 95, 113–125. [Google Scholar] [PubMed]
- Mizuno, M.; Kajiyama, H.; Shibata, K.; et al. Adjuvant chemotherapy for stage I ovarian clear cell carcinoma. Is it necessary for stage IA? Int J Gynecol Cancer 2012, 22, 1143–1149. [Google Scholar]
- Takada, T.; Iwase, H.; Iitsuka, C.; et al. Adjuvant chemotherapy for stage I clear cell carcinoma of the ovary. An analysis of fully staged patients. Int J Gynecol Cancer 2012, 22, 573–578. [Google Scholar] [CrossRef]
- Friedlander, M.L.; Russell, K.; Millis, S.; et al. Molecular profiling of clear cell ovarian cancers: identifying potential treatment targets for clinical trials. Int J Gynecol Cancer 2016, 26, 648–654. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef]
- Ye, S.; Yang, J.; You, Y.; Cao, D.; Bai, H.; Lang, J.; et al. Comparative study of ovarian clear cell carcinoma with and without endometriosis in People's Republic of China. Fertil Steril. 2014, 102, 1656–1662. [Google Scholar] [CrossRef]
- Paik, E.S.; Kim, T.J.; Choi, C.H.; Kim, B.G.; Bae, D.S.; Lee, J.W. Clinical outcomes of patients with clear cell and endometrioid ovarian cancer arising from endometriosis. J Gynecol Oncol. 2018, 29, e18. [Google Scholar] [CrossRef]
- King, C.M.; Barbara, C.; Prentice, A.; Brenton, J.D.; Charnock-Jones, D.S. Models of endometriosis and their utility in studying progression to ovarian clear cell carcinoma. J Pathol. 2016, 238, 185–196. [Google Scholar] [CrossRef]
- Orezzoli, J.P.; Russell, A.H.; Oliva, E.; et al. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol. 2008, 110, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Noli, S.; Cipriani, S.; Scarfone, G.; Villa, A.; Grossi, E. Long Term Survival of Ovarian Endometriosis Associated Clear Cell and Endometrioid Ovarian Cancers. Int J Gynecol Cancer 2013, 23, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, F.E.; Tranoulis, A.; Gupta, B.; Balega, J.; Singh, K. The effect of endometriosis on the prognosis of ovarian clear cell carcinoma. The Jury is still out. Int J Gynecol Cancer 2020, 30 (Suppl 4), A1–A142. [Google Scholar]
- Iida, Y.; Okamoto, A.; Hollis, R.L.; Gourley, C.; Herrington, C.S. Clear cell carcinoma of the ovary: a clinical and molecular perspective. Int J Gynecol Cancer 2021, 31, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Manning-Geist, B.; Bregar, A.J.; et al. Associations between residual disease and survival in epithelial ovarian cancer by histologic type. Gynecol Oncol 2017, 147, 250–256. [Google Scholar] [CrossRef]
- Tozzi, R.; Hardern, K.; Gubbala, K.; Garruto Campanile, R.; Soleymani Majd, H. En-bloc resection of the pelvis (EnBRP) in patients with stage IIIC-IV ovarian cancer: A 10 steps standardised technique. Surgical and survival outcomes of primary vs. interval surgery. Gynecol Oncol. 2017, 144, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; Giannice, R.; Cianci, S.; Tardino, S.; Campanile, R.G.; Gubbala, K.; Fachechi, G.; Ferrari, F.; Martinek, I.; Soleymani Majd, H. Neo-adjuvant chemotherapy does not increase the rate of complete resection and does not significantly reduce the morbidity of Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer. Gynecol Oncol. 2015, 138, 252–258. [Google Scholar] [CrossRef]
- Pinelli, C.; Morotti, M.; Casarin, J.; Tozzi, R.; Ghezzi, F.; Mavroeidis, V.K.; Alazzam, M.; Soleymani Majd, H. Interval Debulking Surgery for Advanced Ovarian Cancer in Elderly Patients (≥70 y): Does the Age Matter? J Invest Surg. 2021, 34, 1023–1030. [Google Scholar] [CrossRef]
- Tozzi, R.; Ferrari, F.; Nieuwstad, J.; Campanile, R.G.; Soleymani Majd, H. Tozzi classification of diaphragmatic surgery in patients with stage IIIC-IV ovarian cancer based on surgical findings and complexity. J Gynecol Oncol. 2020. [CrossRef]
- Addley, S.; Morotti, M.; Pinelli, C.; Soleymani Majd, H. Transdiaphragmatic resection of enlarged cardiophrenic lymph node during interval debulking surgery for advanced ovarian cancer. Gynecol Oncol Rep. 2021, 37, 100807. [Google Scholar] [CrossRef]
- Seki, T.; Tate, S.; Nishikimi, K.; Unno, Y.; Itoi, M.; Ikeda, S.; et al. Bevacizumab in first-line chemotherapy to improve the survival outcome for advanced ovarian clear cell carcinoma: A multicenter, retrospective analysis. Journal of Clinical Oncology 2022, 40 (Suppl. 16), 5502–5502. [Google Scholar] [CrossRef]
- Tozzi, R.; et al. Morbidity of multiple bowel resection compared to single bowel resection after debulking surgery for ovarian cancer. European Journal of Obstetrics & Gynecology and Reproductive Biology 2019, 240, 215–219. [Google Scholar]
- Tozzi, R.; et al. Porta hepatis peritonectomy and hepato–celiac lymphadenectomy in patients with stage IIIC–IV ovarian cancer: Diagnostic pathway, surgical technique and outcomes. Gynecol Oncol 2016. [CrossRef]
- Tozzi, R.; et al. Diagnostic flow-chart to identify bowel involvement in patients with stage IIIC-IV ovarian cancer: Can laparoscopy improve the accuracy of CT scan? Gynecologic Oncology 2019, 155, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; et al. Morbidity and reversal rate of ileostomy after bowel resection during Visceral-Peritoneal Debulking (VPD) in patients with stage I. Gynecol Oncol 2017. [CrossRef]
- Majd, H.S.; Ferrari, F.; Manek, S.; Gubbala, K.; Campanile, R.G.; Hardern, K.; et al. Diaphragmatic peritonectomy vs. full thickness resection with pleurectomy during Visceral-Peritoneal Debulking (VPD) in 100 consecutive patients with stage IIIC-IV ovarian cancer: a surgical-histological analysis. Gynecol. Oncol. 2016, 140, 430–435. [Google Scholar] [CrossRef]
- Tozzi, R.; et al. Bowel resection rate but not bowel related morbidity is decreased after interval debulking surgery compared to primary surgery in patients with stage IIIC-IV ovarian cancer. j Gynecol Oncol. 2019, 30, e25. [Google Scholar] [CrossRef]
- Tozzi, R.; et al. Classification of diaphragmatic surgery in patients with stage IIIC–IV ovarian cancer based on surgical findings and complexity. J Gynecol Oncol. 2020, 31, e14. [Google Scholar] [CrossRef]
- Tozzi, R.; Soleymani Majd, H.; Campanile, R.G.; Ferrari, F. Feasibility of laparoscopic diaphragmatic peritonectomy during Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer. J Gynecol Oncol. 2020, 31, e71. [Google Scholar] [CrossRef]
- Gadducci, A.; Tana, R.; Landoni, F.; Ferrari, F.; Peiretti, M.; Perrone, F.; Sartori, E. Analysis of failures and clinical outcome of advanced epithelial ovarian cancer in patients with microscopic residual disease at second-look reassessment following primary cytoreductive surgery and first-line platinum-based chemotherapy. Eur J Gynaecol Oncol. 2013, 34, 213–217. [Google Scholar]
- Soleymani Majd, H.; Ismail, L.; Hardern, K.; Ferrari, F.; Kehoe, S. Comparison of survival outcome of patients with primary peritoneal and fallopian tube carcinoma treated with neoadjuvant chemotherapy versus primary debulking surgery. J Obstet Gynaecol. 2017, 37, 89–92. [Google Scholar] [CrossRef]
- Tozzi, R.; Valenti, G.; Vinti, D.; Campanile, R.G.; Cristaldi, M.; Ferrari, F. Rectosigmoid resection during Visceral-Peritoneal Debulking (VPD) in patients with stage IIIC-IV ovarian cancer: morbidity of gynecologic oncology vs. colorectal team. J Gynecol Oncol. 2021, 32, e42. [Google Scholar] [CrossRef]
- Tozzi, R.; Traill, Z.; Valenti, G.; Ferrari, F.; Gubbala, K.; Campanile, R.G. A prospective study on the diagnostic pathway of patients with stage IIIC-IV ovarian cancer: Exploratory laparoscopy (EXL) + CT scan VS. CT scan. Gynecol Oncol. 2021, 161, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Mendiola, M.; Hernando, B.; Berjon, A.; Cadiz, A.; Chaves-Urbano, B.; Heredia-Soto, V.; Spagnolo, E.; Hernández Gutiérrez, A.; Hardisson, D.; Macintyre, G.; Redondo, A.; Garcia, M.J. Prognostic markers of inflammation in endometrioid and clear cell ovarian cancer. Int J Gynecol Cancer. 2022, 32, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Tranoulis, A.; Buruiana, F.H.; Gupta, B.; Kwong, A.; Lakhiani, A.; Yap, J.; Balega, J.; Singh, K. Friend or foe? The prognostic role of endometriosis in women with clear cell ovarian carcinoma. A UK population-based cohort study. Arch Gynecol Obstet. 2022, 305, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Significance of Ovarian Endometriosis on the Prognosis of Ovarian Clear Cell Carcinoma. Int J Gynecol Cancer. 2018, 28, 11–18. [Google Scholar] [CrossRef]
- Scarfone, G.; Bergamini, A.; Noli, S.; Villa, A.; Cipriani, S.; Taccagni, G.; Vigano', P.; Candiani, M.; Parazzini, F.; Mangili, G. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol. 2014, 133, 480–484. [Google Scholar] [CrossRef]
|
Age Min-Max Mean ± SD <40 40-60 >60 |
Number 49 37-83 62.6 ± 11.04 1 18 30 |
Percentage % 2% 36.7% 61.2% |
|
Menopausal status Pre-menopausal Post-menopausal |
Number 49 11 38 |
Percentage % 22.4% 77.6% |
|
BMI (kg/m2) Min-Max mean Underweight <18.5 Normal 18.5-24.9 Overweight 25-29.9 Obese ≥ 30 |
Number 38 (NA 11) 16.4 – 43 25.86 1 18 10 9 |
Percentage % 2.6% 47.3% 26.3% 23.6% |
|
Laterality Right Left Bilateral |
Numbers 43 (NA 6) 23 19 1 |
Percentage % 53.4% 44.1% 2.3% |
|
Endometriosis associated Yes No |
28 21 |
57.15% 42.85% |
|
Ascites NO Yes |
33 16 |
67.34% 32.65% |
|
FIGO 2014 staging Stage I IA IB IC IC1 IC2 IC3 IC NA Stage II IIA IIB Stage III IIIA IIIB IIIC Stage IV IV A IV B |
Number 49 27 9 0 18 4 4 5 5 9 5 4 9 3 2 4 4 2 2 |
Percentage % 55.1% 18.3% 0% 36.7% 8.1% 8.1% 10.2% 10.2% 18.3% 10.2% 8.1% 18.3% 6.1% 4% 8.1% 8.1% 4% 4% |
|
Pre-operative CA125 Range Mean ± SD <35 IU/ml (normal) 35-99 100-499 500-1000 >1000 |
Number 49 11- 2336 402.4 ± 516.77 10 9 16 9 5 |
Percentage % 20.4% 18.3% 32.6% 18.3% 10.2% |
|
Haemoglobin level g/L Range Mean ± SD <115 ≥115 |
Number 49 89-147 122.12 ± 13.86 13 36 |
Percentage % 26.53% 73.46% |
|
Serum albumin g/L Range Mean ± SD <40 ≥ 40 |
Number 49 14-43 32.04 ± 7.42 40 9 |
Percentage % 81.64% 18.36% |
|
NACT Yes No |
Number 49 4 45 |
Percentage % 8.1% 91.8% |
|
|
Pre-operative laparoscopy Not done Done: Done in separate setting Done in the same setting Done as TLH |
Number 49 36 11 6 5 2 |
Percentage % 73.4% 22.4% 12.2% 10.2% 4.08% |
|
|
Surgical procedure Hysterectomy BSO USO Pelvic lymph node sampling Pelvic lymph node dissection Para aortic lymph node sampling Infracolic omentectomy Appendectomy falciform ligament resection excision of gastric nodule excision of liver nodule bladder peritoneum resection pelvic peritonectomy paracolic peritonectomy SB resection & end to end anastomosis LB resection & end to end anastomosis Colostomy R0 R0 not achieved Intra-operative complications: |
Numbers 47 (NA 2) 40+7 hysterectomy 46 1 8 (positive in 1) 6 (positive) 8 (positive in 1) 47 (positive in 5) 10 (all negative) 3 (all negative) 1 (negative) 1 (positive) 7 (positive in 2) 8 6 2 1 1 39 10 9 |
Percentage % 95.7% 97.8% 2.1% 17% (2.1) 12.7% 17% (2.1) 100% (positive 10.6%) 21.2% 6.3% 2.1% 2.1% 14.8% (positive 4.2) 17% 12.7% 4.2% 2.1% 2.1 % 79.59% 20.41% 19.1% |
|
|
Post operative complications: none Ileus (all managed conservatively) Severe Wound infection Wound dehiscence and return to theatre Pelvic collection (readmission) Peritonitis + PE PE DVT in left subclavian vein Ureteric leaking Lymphocyst Complete heart block and percutaneous pacing Need for ICU admission |
Numbers 40 (NA 9) 24 6 2 1 1 1 1 1 1 1 1 4 |
Percentage % 60% 15% 5% 2.5% 2.5% 2.5% 2.5% 2.5% 2.5% 2.5% 2.5% 10% |
|
Cases involved in trials:
|
5 /49 1 1 1 1 1 |
10.2% 2.04% 2.04% 2.04% 2.04% 2.04% |
|
|
Adjuvant therapy Adjuvant offered:
Adj chemo offered but declined or hold |
Numbers 49 39 37 2 4/39 |
Percentage % 79.5% 75.5% 4% 10.2% |
|
Adjuvant chemotherapy types: Carboplatin± paclitaxel Completed 6 cycles < 6 cycles Data not clear on medical records |
22 7 10 |
| Predictive factor | Independent covariate | ||
|---|---|---|---|
| P value | HR | 95% CI of HR | |
| CA 125 (>200 IU/ml) | 0.253 | 0.3 | 0.04-2.341 |
| Hb (< 115g/L) | 0.098 | 3.2 | 0.804-13.53 |
| Albumin (< 40g/L) | 0.528 | 1.8 | 0.276-12.27 |
| Ascites | 0.669 | 1.471 | 0.250-8.66 |
| Endometriosis-association | 0.427 | 1.980 | 0.367-10.691 |
| FIGO stage I | 0.878 | 1.213 | 0.102-14.462 |
| FIGO stage II | 0.048* | 18.747 | 1.029 -341.687 |
| FIGO stage III | 0.016* | 234.817 | 2.765 - 19940 |
| Residual disease | 0.524 | 0.385 | 0.020 – 7.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





