Submitted:
30 March 2023
Posted:
11 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Formulation F18-Containing EC16
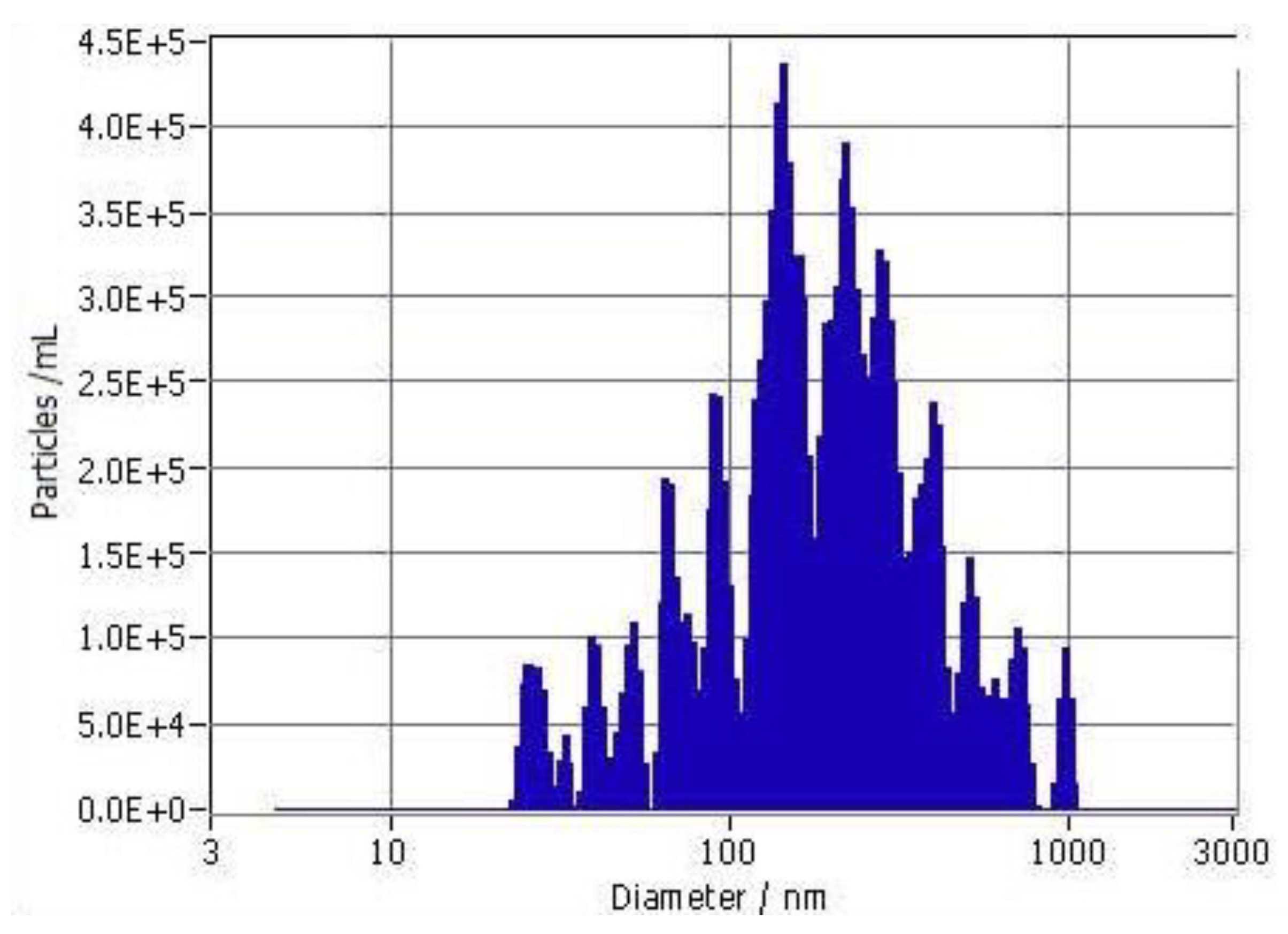
2.2 Size Distribution of Small Particles
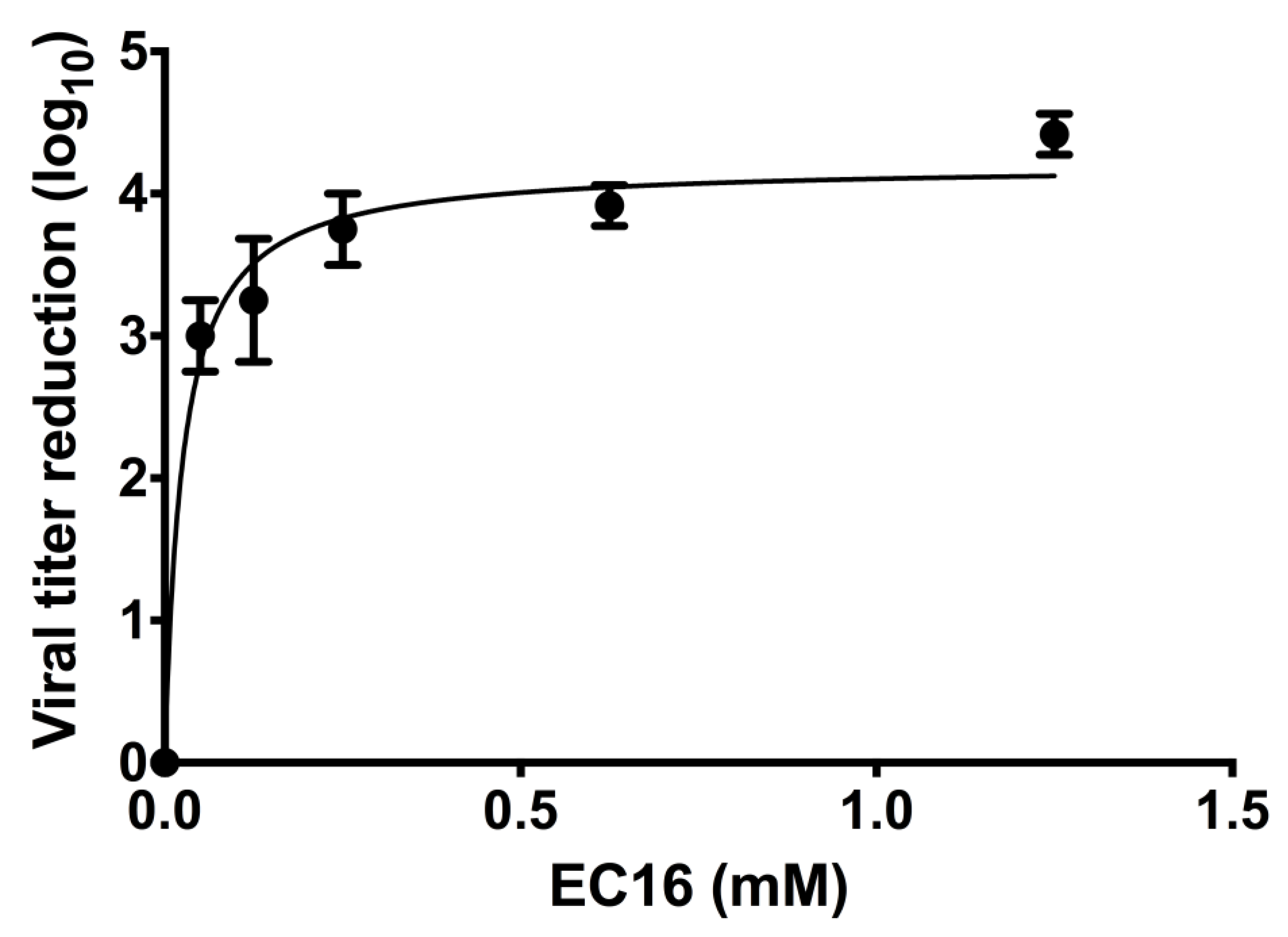
2.3. Dose Effect Results
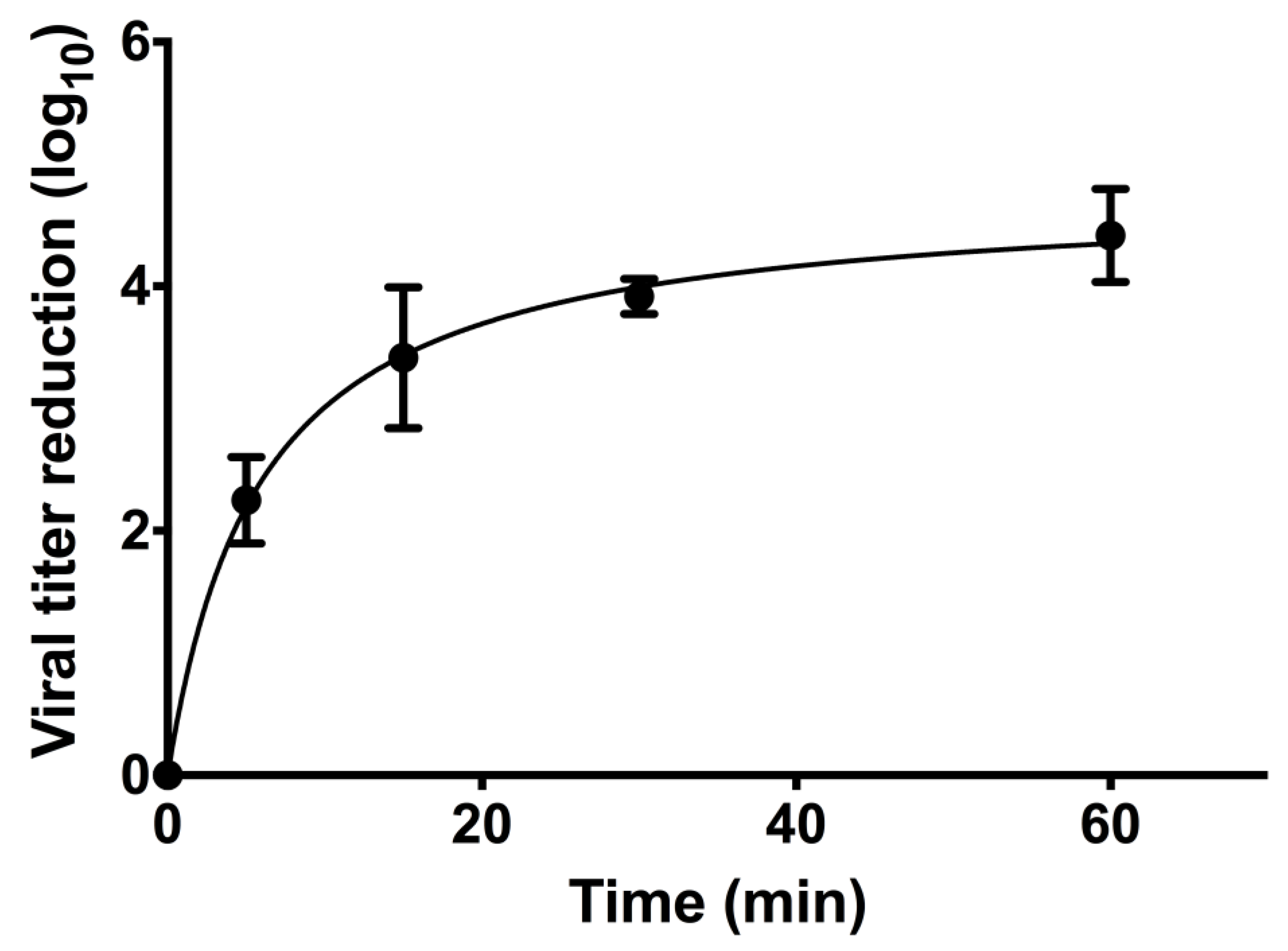
2.4. Time Effect Results
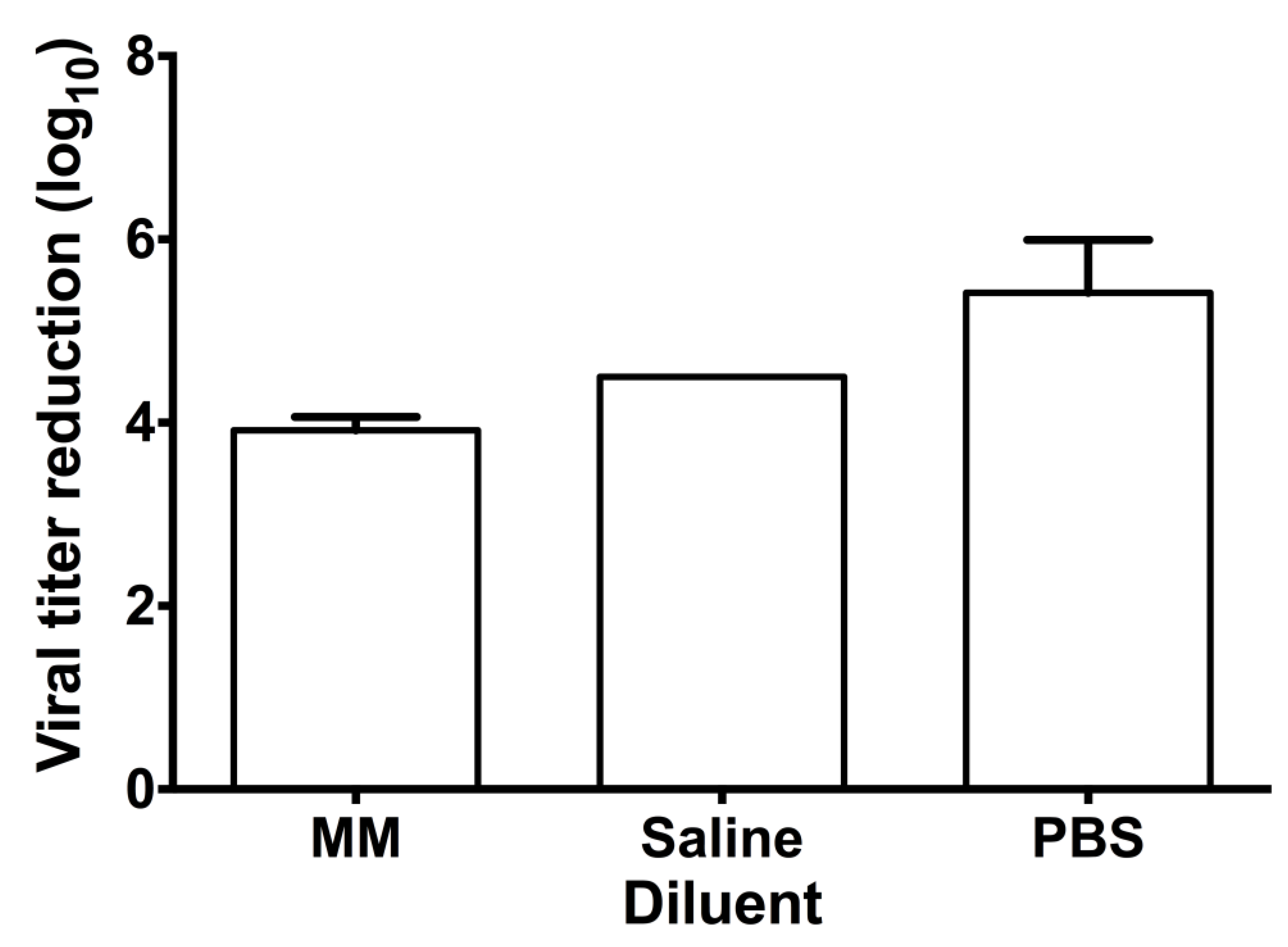
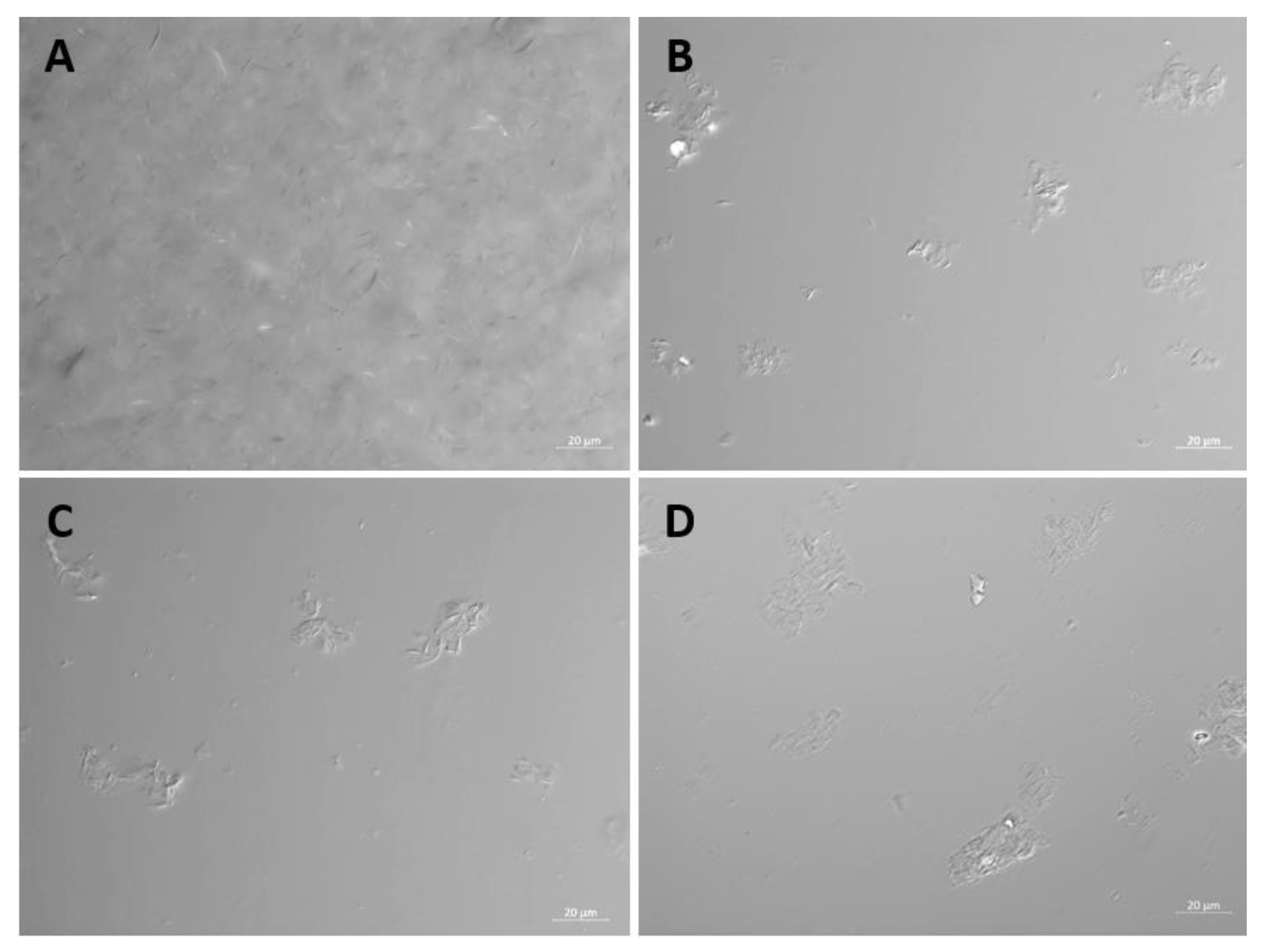
2.5. Contact Inhibition Test of F18 in Different Diluents
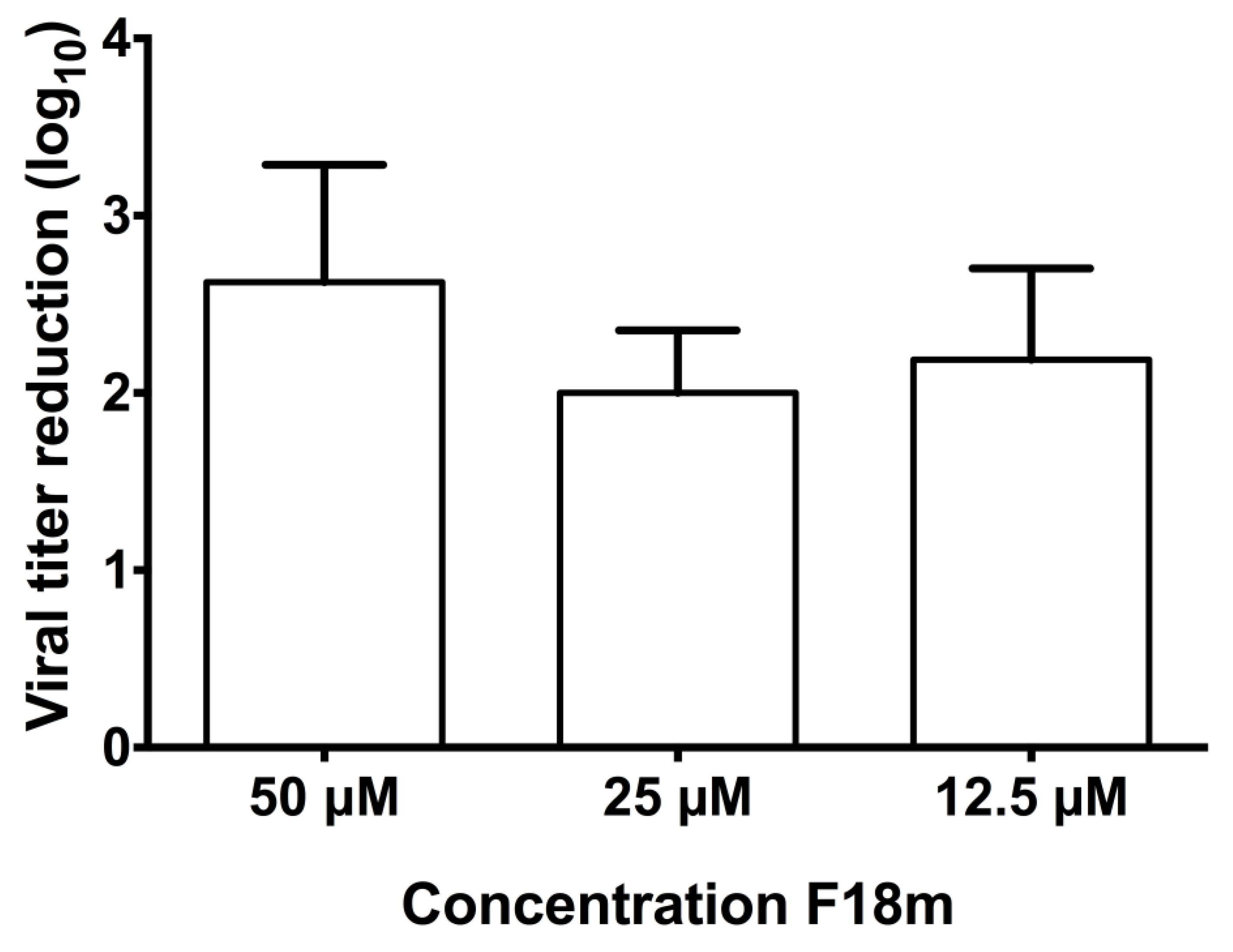
2.6. Contact Inhibition Test of F18m-Containing EC16m
2.7. Pre-Infection Tests
2.8. Post-Infection Tests
3. Discussion
4. Materials and Methods
4.1. Virus and Cell Line
4.2. EC16 and Other Supplies
4.3. EC16 Formulations
4.4. Quantitation of EC16 Polyphenol in Mixtures
4.5. Evaluation of Particle Size Distribution
4.6. Antiviral Activity Tests
4.7. Dose and Time Tests of Direct Contact with Virus
4.8. Pre-Infection Test
4.9. Post-Infection Test
4.10. Microphotography of the Formulations
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Gallo, O.; Locatello, L.G.; Mazzoni, A.; Novelli, L.; Annunziato, F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol 2021, 14, 305–316. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130 e120. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; Dinda, M. Therapeutic potential of green tea catechin, (-)-epigallocatechin-3-O-gallate (EGCG) in SARS-CoV-2 infection: Major interactions with host/virus proteases. Phytomed Plus 2023, 3, 100402. [Google Scholar] [CrossRef]
- Goncalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Hsu, S. Compounds Derived from Epigallocatechin-3-Gallate (EGCG) as a Novel Approach to the Prevention of Viral Infections. Inflamm Allergy Drug Targets 2015, 14, 13–18. [Google Scholar] [CrossRef]
- Kaihatsu, K.; Yamabe, M.; Ebara, Y. Antiviral Mechanism of Action of Epigallocatechin-3-O-gallate and Its Fatty Acid Esters. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int J Mol Sci 2022, 24. [Google Scholar] [CrossRef]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
- de Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem Toxicol 2013, 52, 207–215. [Google Scholar] [CrossRef]
- Dickinson, D.P.; Xayaraj, S.; Dickinson, S.; Shao, X.; Hsu, S. Effect of Novel Formulations using Lipophilic Epigallocatechin-3-Gallate against Influenza Virus Infection. Microbiology & Infectious Diseases. 2018, 14.
- Zhao, M.; Zheng, R.; Jiang, J.; Dickinson, D.; Fu, B.; Chu, T.C.; Lee, L.H.; Pearl, H.; Hsu, S. Topical lipophilic epigallocatechin-3-gallate on herpes labialis: a phase II clinical trial of AverTeaX formula. Oral Surg Oral Med Oral Pathol Oral Radiol 2015, 120, 717–724. [Google Scholar] [CrossRef]
- Zhong, J.; Dickinson, D.; Hsu, S. Effects of Epigallocatechin-3-Gallate-Palmitate (EC16) on In Vitro Norovirus Infection. Microbiol Infect Dis 2021, 5. [Google Scholar] [CrossRef]
- Hurst, B.L.; Dickinson, D.; Hsu, S. Epigallocatechin-3-Gallate (EGCG) Inhibits SARS-CoV-2 Infection in Primate Epithelial Cells: (A Short Communication). Microbiol Infect Dis 2021, 5. [Google Scholar] [CrossRef]
- 772, U.G.N. GRAS Notice for Oil-Soluble Green Tea Extract (Green Tea Catechin Palmitate). 2018.
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 2021, 13. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.; Hong, S.P.; Choi, S.Y.; Yang, M.J.; Ju, Y.S.; Kim, Y.T.; Kim, H.M.; Rahman, M.D.T.; Chung, M.K.; et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Zhou, D.; Chan, J.F.; Zhou, B.; Zhou, R.; Li, S.; Shan, S.; Liu, L.; Zhang, A.J.; Chen, S.J.; Chan, C.C.; et al. Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies. Cell Host Microbe 2021, 29, 551–563 e555. [Google Scholar] [CrossRef]
- Ra, S.H.; Lim, J.S.; Kim, G.U.; Kim, M.J.; Jung, J.; Kim, S.H. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax 2021, 76, 61–63. [Google Scholar] [CrossRef]
- Giunchedi, P.; Gavini, E.; Bonferoni, M.C. Nose-to-Brain Delivery. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J Control Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- de la Torre, R.; de Sola, S.; Farre, M.; Xicota, L.; Cuenca-Royo, A.; Rodriguez, J.; Leon, A.; Langohr, K.; Gomis-Gonzalez, M.; Hernandez, G.; et al. A phase 1, randomized double-blind, placebo controlled trial to evaluate safety and efficacy of epigallocatechin-3-gallate and cognitive training in adults with Fragile X syndrome. Clin Nutr 2020, 39, 378–387. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J 2016, 15, 60. [Google Scholar] [CrossRef]
- Chen, P.; Dickinson, D.; Hsu, S. Lipid-soluble Green Tea Polyphenols: Stabilized for Effective Formulation. In Handbook of Green Tea and Health Research, McKinley, H., Jamieson, M., Eds.; Nova Science Publishers, Inc.: New York, 2009; pp. 45–61. [Google Scholar]
- Hsu, S.; Dickinson, D. Green tea and skin protection: Mechanism of action and practical applications. Household and Personal Care TODAY 2009, n 2, 33–36. [Google Scholar]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (-)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg Med Chem Lett 2008, 18, 4249–4252. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (-)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem 2016, 204, 218–226. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev 1998, 7, 351–354. [Google Scholar]
- Savela, E.S.; Viloria Winnett, A.; Romano, A.E.; Porter, M.K.; Shelby, N.; Akana, R.; Ji, J.; Cooper, M.M.; Schlenker, N.W.; Reyes, J.A.; et al. Quantitative SARS-CoV-2 Viral-Load Curves in Paired Saliva Samples and Nasal Swabs Inform Appropriate Respiratory Sampling Site and Analytical Test Sensitivity Required for Earliest Viral Detection. J Clin Microbiol 2022, 60, e0178521. [Google Scholar] [CrossRef]
- Baxter, A.L.; Schwartz, K.R.; Johnson, R.W.; Kuchinski, A.M.; Swartout, K.M.; Srinivasa Rao, A.S.R.; Gibson, R.W.; Cherian, E.; Giller, T.; Boomer, H.; et al. Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients. Ear Nose Throat J 2022, 1455613221123737. [Google Scholar] [CrossRef]
- Huijghebaert, S.; Hoste, L.; Vanham, G. Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19. Eur J Clin Pharmacol 2021, 77, 1275–1293. [Google Scholar] [CrossRef]
- Panta, P.; Chatti, K.; Andhavarapu, A. Do saline water gargling and nasal irrigation confer protection against COVID-19? Explore (NY) 2021, 17, 127–129. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One 2017, 12, e0170628. [Google Scholar] [CrossRef]
- REED, L.J.; MUENCH, H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS12. American Journal of Epidemiology 1938, 27, 493–497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




