1. Introduction
Carrageenan (car-), the hydrophilic sulfated-polysaccharides, have innumerable applications in the pharmaceutical and food industry [
1] and are comprised of kappa (κ-), lambda (λ-), and iota (ι-) [
2]. Car- are the polyanionic polymers with varying charge densities which possess highly selective cationic chelating activity that makes them perfect for metal uptake and drug delivery [
3,
4]. Several studies have reported controlled drug release behavior of self blends of κ- and ι- car- [
4,
5] or combination with other polymers such as xanthan gum/gelatin, chitosan, and hydroxypropyl methylcellulose [
6,
7]. Car- may have the potential to be utilized for the delivery of Cu
2+ and Zn
2+ for therapeutic purposes. Complexes of car- with chitosan and of κ- with hydroxyl propyl methylcellulose are used for controlling drug release profile [
6] Chemical structure reveals that only ι- and κ- have 3-6 anhydro bridges while these bridges are not seen in λ-. The presence of these bridges gives rheological characteristics to λ- to have viscosity enhancing properties while κ- and ɩ- shows gel forming properties [
8].
Car- have a role in anticoagulant activity [
9,
10] but their metal loaded blends were never studied. We report the design of CB for multiple purposes such that i) metal ions uptake from aqueous-solutions and ii) to exploit their carrier nature for the therapeutic delivery of Cu
2+ and Zn
2+. CB were studied for their ability to load the metal ions by adsorption and the factors controlling it. Finally, these metal-complexed blends were examined for their anticoagulant and anti-bacterial activities.
Extraction and isolation of active ingredients from plant, algae, and fungi is a common practice. Majority of the products have shown remained unknown for many years [
11,
12]. It is evident that a real need for reliable and specific pharmacological agents should be isolated, developed, and evaluated [
13]. Metal chelates are widely known to have a great potential to improve different diseases [
14]. The biological evaluation of hydrophilic crystalline complexes, in general, are much more efficient than that of the constituent mixtures as plants [
15]. Herein, we report the rheological and mechanical behaviors of CB by varying the temperature conditions ranging from 10 – 40
oC.
2. Materials and Methods
All three car-: κ-, λ-, and ι- were purchased from Sigma Aldrich (Martin Dow Marker Specialties, Karachi, Pakistan). For pH adjustment, HCl and NaOH (BDH Chemicals, Karachi, Pakistan) were used. Millipore-milli-Q deionized water (BDH Chemicals, Karachi, Pakistan) of conductivity of 18.2 mW/cm2 was used to prepare samples.
The potassium chloride (KCl), copper sulfate (CuSO
4) were purchased from MERCK (Kenilworth, NJ, USA). All the glassware was soaked in 0.1% Alconox solution (Fluka-Honeywell Specialty Chemicals, Seelze, Germany) for de-ionized water for 24-hour (h). The brief method of preparation is reported elsewhere [
16].
2.1. Gel Formation
The triple blends such as κ/(ɩ/λ) and their Cu2+ and Zn2+-complexes were mixed with potassium chloride (KCl) [with a potassium concentration (conc.) of 75 or 150 mM] by utilizing MQ-level water at 40 °C with constant stirring for 30 minutes (mins) and then at 90 °C for 120 mins respectively. For letting the air out, the stirring process was ceased for the last 15 mins of the heating procedure. The hot solution of gels was settled in cold water for 60 mins and then moved to the refrigerator at 5 °C for 16-20 hours (h).
2.2. Rheology models
A solution of each car- (10.5 % w/w) was subjected to viscosity shear rate, viscosity-angular frequency, complex shear stress-shear rate with the help of rotational Rheometer (Anton-Paar Physica MCR 301, Germany). For the maintenance of temperature range in between −150 to 1000 °C, and a high pressure, an internal controlling system is present. A computer containing Rheoplus software was attached to Rheometer to obtain the results. For the analysis of CB gels having ratios of 50/50 w/w, with a 3000 mm/min speed, 628 rad/s value of angular frequency, and a 200 mN.m of torque was used.
The gel samples were checked for their microscopic properties. Shear stress & the viscosity measurements were conducted by increasing the shear rate by keeping constant experimental conditions.
Models were employed having i) frequency sweep (complex viscosity, loss/storage modulus) and flow curve (shear rate vs. shear stress/viscosity) and a varying the temperature conditions from 10-50 °C for CB. A specific study at 37 °C was conducted to simulate the rheological properties in the human body. Rheological models such as Ostwald Power rule, Bingham, and Modified Bingham models were used for the interpretation of the results [
17]. Power rule, linear, and nonlinear regression analysis are employed.
where
the viscosity, shear rate is denoted by γ and K is material-based constant. The ‘n’ value defines the dilatant behavior. Eq. 1 demonstrates Power law. The trend of yield stress is analogous to Bingham Model (Eq. 2) as in Modified Bingham Model (Eq. 3). For investigation of flow curve modeling at 37
oC by applying the complete shear rate range (100 s
-1), the Modified Bingham model is applicable to explain shear thinning, yield stress of hydrogels, and overall flow behavior.
3. Results and Discussion of CB
On the other hand, car- has been approved by FDA for the food industry, and is biodegradable and biocompatible and it could be useful for the treatment of metal poisoning in biological fluids. Furthermore, the metal loaded car- topical films could be useful in wound healing applications due to the antimicrobial activities of both Cu2+ and Zn2+.
3.1. Statistical Analysis
Data of the samples were subjected to one-way ANOVA and subsequent Tukey test using GraphPad Prism and SPSS 20.0 in order to determine the statistical meaning behind the observations. P-values < 0.05 indicate significant difference.
3.2. Results
The characterization of the gel samples was according to their flowing behavior. Shear stress and the viscosity measurements were studied by increasing the shear rate by keeping constant experimental conditions. The rotational conditions were changed from 0.0075 to 77.5 rpm for all the experiments. Their rheology was taken at different temperatures i.e. (10, 20, 30, 37, 40 & 50
oC). The results of C2 hydrogels showed increase in shear stress and decrease in viscosity at 40 and 10
oC followed by C1 at 50
oC. (
Figure 1 and
Figure 2) They also demonstrated an irregular pattern of increases as well as decrease in a see-saw manner. Whereas C3 at 40
oC and C4 hydrogels showed highest shear stress at 50
oC. By examining
Figure 1, C3 hydrogels exhibited lowest shear rate which is due to the reason that it contains lowest sulfate concentration. [
18,
19,
20] Hence, it can be concluded that the variation of shear-stress determines the concentration of sulfate content. The hydrogels of κ/ι/λ- are smooth, shiny and opalescent gels which were applied on the back of the hand to check the tactile characteristics and biocompatibility. It is significant to study the macroscopic properties to examine visual quality; odor & tangible properties indicating gel synthesis [
21].
CB were quickly converted to gel after the application on the skin [
18,
22] κ- and ι- exist in two arrangements: (a) an irregular coil that appears to form at high temperature, (b) a regular organized double-helical coil commonly appears at low temperatures or cooling [
23]. An irregular coil shows double-helical structure when a hot solution is in the process of cooling at a particular temperature known as coil helical temperature [
24]. Coil helical transition temperature of ɩ- and κ- is 450 and 38 °C in water [
20]. λ- does not exist in helical arrangement forming any gels [
25].
It is concluded that whether the shear rate is high or low a constant viscosity is observed. Initially, the increase in viscosity occurs for all temperatures and concentration of blends, but as shear rate was increased, the repulsion occurs in polar ends of similar charges and hence the viscosity curves demonstrated a steep. At lower shear rates, the sulfated groups present in the polymer chains become closer and leads to the inter- and intra-polymer H-bonding. While at higher shear rates, the H-bonds are collapsed which results in deformation of hydrogel chains confirming the pseudo-plastic behavior. The viscosity vs. shear rate, shear stress vs. shear rate and complex viscosity vs. angular frequency plots were obtained at a range of temperatures. The solubility and dilatants behavior of the different CB showed that ɩ- form thixotropic solutions when dissolved in hot water and are approximately 32 % substituted by ester sulfate groups while κ- are 25 % substituted with ester sulfate and are hot water-soluble. λ- is partially cold water soluble and completely hot water soluble and is 35 % substituted with ester sulfate groups acquiring the properties like Newtonian fluids.
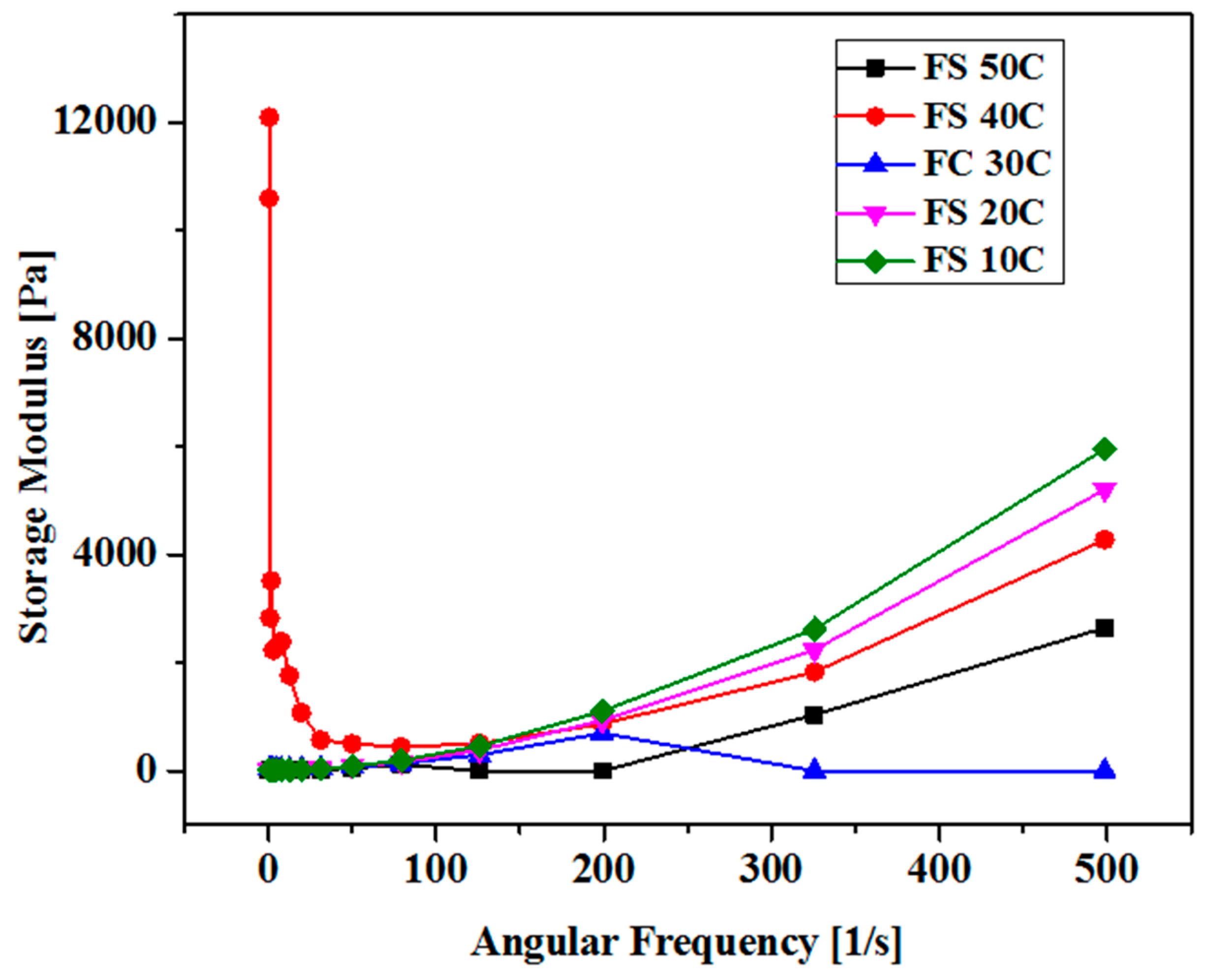
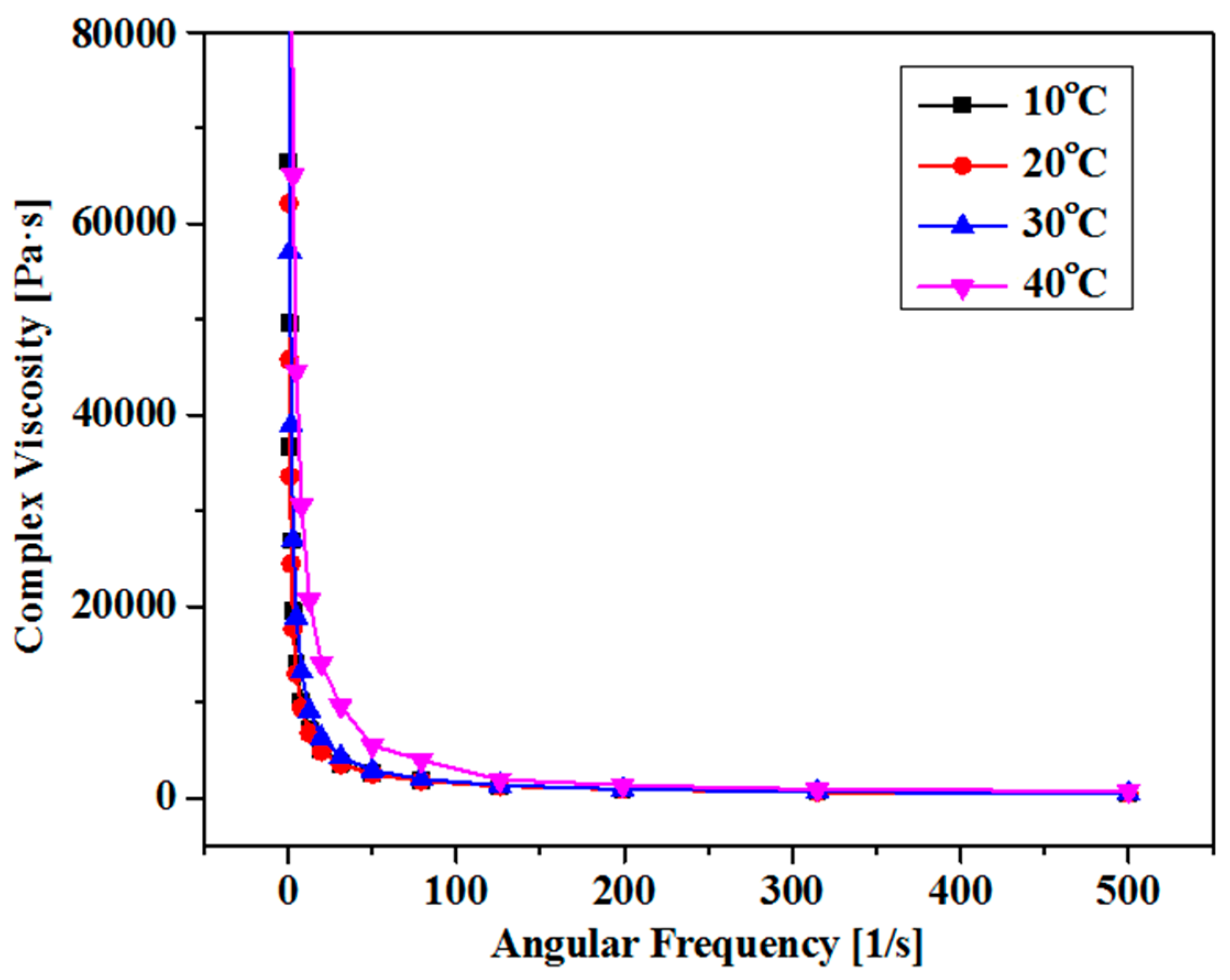
The elastic and viscous nature of these CB is studied through oscillatory rheology by a careful investigation of complex viscosity versus angular frequency. By the temperature elevation, the complex viscosity was increased due to the strong molecular attraction and hence the hydrogel is converted from viscous liquid to semi-solid. (Figure 3) By the temperature elevation, the molecules move apart from each other due to higher kinetic energies, and thus molecular spaces become more and with lesser complex viscosity. This leads to shear thinning behavior of these hydrogels and the molecular interactions among the molecules are undermined significantly. At various temperatures, all the curves converge at one point at higher angular frequencies and demonstrate temperature independent nature, and predominantly a solid material. (Figure 4)
The selected temperatures, in our study, ranged from 10-50 and 37 °C to evaluate the rheological properties of CB. All results are plotted based on Ostwald Power law, Bingham Yield Stress (τB), and Modified Bingham Yield Stress (τMB) [
17] relating viscosity vs. shear rate (
Figure 2). In Table 5.1, ‘τ’ or ‘η’ is plotted against the shear rate (γ) at various temperatures (10, 20, 30, 37, 40
oC). The chemical structure reveals that only ι- and κ- have 3-6 anhydro-bridges while these bridges are not seen in λ-. The presence of these bridges gives rheological characteristics to λ- to have viscosity-enhancing properties while κ- and ι- shows gel-forming properties. The obtained results are in a promising correlation with power-law and τMB. From τB lower shear rates (γ = 0.02 to 20 s
−1) demonstrated the relationship to be stable. By plotting this relationship vs. zero shear rate, τB becomes equal to the intercept. The overall τB values varied from 18.03 to 26.87 Pa, whereas τMB ranged from 28.21 to 42.31 Pa and demonstrated promising fit for the CB (
Table 1). At body temperature, the retention time of the gels was enhanced due to the concentration in the polymeric network which can also aid the drug/ion release. For a specific shear rate, no change in gel viscosity is noticed with temperature variation.
3.3. Discussion
Rheological study was performed by Anton Paar Rheometer and the probe used as a measuring system had a diameter of approx 1 mm. Different rheological parameters were altered by varying the temperature conditions. The rheological tests mainly depend upon the selected temperature. In this study, we have selected different temperatures ranging from 10 - 50
oC for hydrogels storage. A specific study at 37
oC was conducted to evaluate the rheological properties at human body temperature. For hydrogels, the Arrhenius model can well define the temperature dependency. [
1,
20] Several rheological tests explain that by applying greater amount of shear stress vs. the yield stress of the hydrogels, they start to flow [
19]. To estimate the validity, comparison of the flow curves with the simple well-known constitutive models was performed. The chemical structure reveals that only ι- and κ- have 3-6 anhydro-bridges while these bridges are not seen in λ-. The presence of these bridges gives rheological characteristics to λ- to have viscosity-enhancing properties while κ- and ι- shows gel-forming properties [
19]. By applying constitutive models & comparing the results of flow curve models, their validity is estimated. The evaluation of different rheological properties i.e. shear thickening, shear thinning, reasonable yield stress, pseudo-plasticity, temperature dependency & other characteristics shows that by applying high shear rates, the viscosity of these materials decrease & hence they flow readily. On the other hand, by decreasing shear rates, the viscosity of the system increases like spreaded hydrogels. It is significant to study the macroscopic properties to examine visual quality; odor & tangible properties which indicate the gel synthesis. Visually, these gels were transparent smooth & gleaming semisolid materials. No extra macroscopic particles & air bubbles were found. The overall texture of these hydrogels was glossy & smooth. To investigate the tactile properties, these were applied on the skin and after a while the semi-solid hydrogels becomes a solid gel. The biocompatibility was indicated as the gels were applied to the back of the hand skin and gave a smooth and cozy feeling. The flow curves show promising results for CB hydrogels having different sulfate content. Their rheology was taken at different temperatures i.e. (10, 20, 30, 37, 40 & 50
oC). The results of C2 hydrogels showed highest shear stress at 40
oC and 10
oC followed by C1 at 50
oC. They also demonstrated an irregular pattern of increases as well as decrease in a see-saw manner. Whereas C3 at 40
oC and C4 hydrogels showed highest shear stress at 55
oC. By examining figure, C3 hydrogels exhibited lowest shear rate which is due to the reason that it contains lowest sulfate concentration. [
18,
19,
20] Hence, it can be concluded that the concentration of sulfate content is directly dependent on variation of shear stress.
There is a certain threshold polymeric concentration for all the gels, also known as overlap concentration. Beyond this concentration the polymeric networks started to form which lead to gellation. In this case, C2 having an anomalous behavior may be best described in the way that it has the threshold concentration of sulfate content along with other monomers. Whereas, the concentration of co-monomer i.e. blends of κ- and λ- go over the overlap concentration and no polymeric chains formed in hydrogel system. [33,34] These un-polymerized chains coalesce together to form macro-particles which leads to an irregular arrangement of various polymeric chains which definitely define shear stress values and enhance the viscosity which is obvious from viscosity-shear rate graphs. Fig. Shows viscosity profile of four hydrogel-blends. All results are plotted on the basis of Power law relating viscosity (η) vs. shear rate (γ). It is concluded, from these results, that whether the shear rate is high or low a constant viscosity scheme is observed. Initially, the increase in viscosity occurs for all temperatures & concentration of co-monomers as the shear rate is low. [
20] But as shear rate is increased, the repulsion occurs in polar ends of similar charges and hence the viscosity curves show a greater steep. At lower shear rates, the sulfate groups present in the polymer chains becomes closer and leads to the inter- and intra-polymer hydrogen bonding. While at higher shear rates, the hydrogen bonds are collapsed which results in deformation of hydrogels chains. These chains arrange themselves in flow direction which confirms the pseudo-plastic behavior of these gels. At body temperature, the retention time of the gels was enhanced due to the concentration in the polymeric network which can also aid the drug/ion release. For a specific shear rate, no change in gel viscosity is noticed with temperature variation. Yield stress is the minimum stress that should be applied prior to the material really starts to flow [
20]. For the characterization of semi-solid, it is believed as a good indicator, influencing their retention and spreadibility. The closely packed particles in cross linked hydrogels construct yield stress with their neighbor particles. In this study, the rheology of CB was analyzed without any cross-linker. The fact that these have inter and intra-molecular and other valance forces is the reason why the yield stress is revealed by such gels. To calculate the Bingham Yield Stress (τB), Bingham shear stress equation was used. At lower shear rates (γ = 0.01 to 15 s
−1), the relationship (τ-γ) was found to be stable. By plotting this relationship vs. zero shear rate, τB becomes equal to the intercept. The third model is modification of Bingham model which is also called Modified Bingham Model. The Yield Stress (τB) values can be calculated by taking at least two different temperatures (10
°C and 37
°C), and their values shows a gradual increase up to the overlap concentration of co-monomer content. At the body temperature, the retention time of the gels enhanced due to the sulfate concentration in the polymeric network which also aid the specific drug release [
20].
The steady state viscosity measurements were taken at temperature between 10-50
oC for the hydrogel samples. The metallo-chelated gels containing Cu
2+ and Zn
2+ shows significant stability with temperature. For a specific shear rate, no change in gel viscosity is noticed with temperature variation. Temperature dependency of these gels is very weak. By applying Ostwald’s model on flow curves at different temperature i.e. τ=Kγn, whereas “n” is fluidity of power law which represents pseudo plasticity also known as shear thinning of fluidic materials as they show deviation from Newtonian fluids. The value of ‘n’ is also used to interpret the modification of gel structures with shear rate. For weaker hydrogels, the forces of attraction are weak. So, the value of ‘n’ will be higher which decrease the life span of transitory entanglement linkages and vice versa in case of stronger hydrogels. We can safely say that at elevated temperatures hydrogels becomes stronger which helps in drug delivery system to jellify with normal body temperature (37
oC) on the skin. [
20]
The flow curve modeling of the hydrogels can be determined by well-known Ostwald’s law to check their validation. The Ostwald’s model has two forms i.e. τ=K
oγ
n and η=K
oγ, where n-1 is used to calculate the flow behavior & K
o is consistency coefficient which represent viscosity range for the flow curves. In above equation, the exponent ‘n’ is also named as Fluidity index or the power law index or the rate index. [
1,
20] As the product is shear thinning the n value ranges between 0< n< l, and n ≈ 0 for more shear thinning samples. From here the extent of ‘n’ can be calculated. According to the Ostwald’s law, our samples behaved as shear thinning materials. All the samples have been modeled by modified Bingham Model at different temperatures for low shear rates of about 32 s
-1 and shows promising fit in this model.
4. Conclusions
It can be concluded that the concentration of sulfate content is directly dependent on variation of shear stress. CB were quickly converted to gel after the application on the skin. Whether the shear rate is high or low a constant viscosity is observed. Initially, the increase in viscosity occurs for all temperatures and concentration of CB, but as shear rate was increased, the repulsion occurs in polar ends of similar charges and hence the viscosity curves demonstrated a steep. At lower shear rates, the sulfated groups present in the polymer chains become closer and leads to the inter- and intra-polymer H-bonding. While at higher shear rates, the H-bonds are collapsed which results in deformation of hydrogel chains confirming the pseudo-plastic behavior. By the temperature elevation, the complex viscosity was increased due to the strong molecular attraction and hence the hydrogel is converted from viscous liquid to semi-solid. The chemical structure reveals that only ι- and κ- have 3-6 anhydro-bridges while these bridges are not seen in λ-. The presence of these bridges gives rheological characteristics to λ- to have viscosity-enhancing properties while κ- and ι- shows gel-forming properties. Due to the disturbance of weak chemical and physical forces, there is a decrease in viscosity that leads to a pseudo-plastic behavior of the material. At a particular shear rate, CB demonstrates significant temperature dependency. The obtained results are in a promising correlation with power-law and τMB. From τB lower shear rates (γ = 0.02 to 20 s−1) demonstrated the relationship to be stable. The overall τB values varied from 18.03 to 26.87 Pa, whereas τMB ranged from 28.21 to 42.31 Pa and demonstrated promising fit for the CB. At body temperature, the retention time of the gels was enhanced due to the concentration in the polymeric network which can also aid the drug/ion release.
References
- S.R. Derkach, S.O. Ilyin, A.A. Maklakova, V.G. Kulichikhin, A.Y. Malkin, The rheology of gelatin hydrogels modified by κ-carrageenan, LWT - Food Sci. Technol. 63 (2015) 612–619. [CrossRef]
- M. Mahinroosta, Z. Jomeh Farsangi, A. Allahverdi, Z. Shakoori, Hydrogels as intelligent materials: A brief review of synthesis, properties and applications, Mater. Today Chem. 8 (2018) 42–55. [CrossRef]
- Y.S. Khotimchenko, E. V Khozhaenko, M.Y. Khotimchenko, E.A. Kolenchenko, V. V Kovalev, Carrageenans as a new source of drugs with metal binding properties, Mar. Drugs. 8 (2010) 1106–1121. [CrossRef]
- S. Nanaki, E. Karavas, L. Kalantzi, D. Bikiaris, Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers, Carbohydr. Polym. 79 (2010) 1157–1167. [CrossRef]
- E. Karavas, E. Koutris, A.G. Papadopoulos, M.P. Sigalas, S. Nanaki, G.Z. Papageorgiou, D.Z. Achilias, D.N. Bikiaris, Application of density functional theory in combination with FTIR and DSC to characterise polymer drug interactions for the preparation of sustained release formulations between fluvastatin and carrageenans, Int. J. Pharm. 466 (2014) 211–222. [CrossRef]
- L. Zhang, Y. Wang, H. Liu, L. Yu, X. Liu, L. Chen, N. Zhang, Developing hydroxypropyl methylcellulose/hydroxypropyl starch blends for use as capsule materials, Carbohydr. Polym. 98 (2013) 73–79. [CrossRef]
- V Briones, T. Sato, Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan–carrageenan, React. Funct. Polym. 70 (2010) 19–27. [CrossRef]
- N. Rhein-Knudsen, M.T. Ale, F. Ajalloueian, L. Yu, A.S. Meyer, Corrigendum to “Rheological properties of agar and carrageenan from Ghanaian red seaweeds” [Food Hydrocolloids 63 (2017) 50–58], Food Hydrocoll. 81 (2018) 284–285. [CrossRef]
- I.M. Yermak, A.O. Barabanova, D.L. Aminin, V.N. Davydova, E. V Sokolova, T.F. Solov’eva, Y.H. Kim, K.S. Shin, Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities, Carbohydr. Polym. 87 (2012) 713–720. [CrossRef]
- F.R.F. Silva, C.M.P.G. Dore, C.T. Marques, M.S. Nascimento, N.M.B. Benevides, H.A.O. Rocha, S.F. Chavante, E.L. Leite, Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans, Carbohydr. Polym. 79 (2010) 26–33. [CrossRef]
- E. Martino, G. Casamassima, S. Castiglione, E. Cellupica, S. Pantalone, F. Papagni, M. Rui, A.M. Siciliano, S. Collina, Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead, Bioorg. Med. Chem. Lett. 28 (2018) 2816–2826. [CrossRef]
- M.C. Niroumand, M.H. Farzaei, G. Amin, Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: a review, J. Tradit. Chinese Med. 35 (2015) 104–109. [CrossRef]
- J. Xie, A. Zhang, H. Sun, G. Yan, X. Wang, Recent advances and effective strategies in the discovery and applications of natural products, RSC Adv. 8 (2018) 812–824. [CrossRef]
- K. Malczewska-Jaskóła, W. Jankowski, B. Warżajtis, B. Jasiewicz, M. Hoffmann, U. Rychlewska, Chalcogenated (S)-(−)-nicotine derivatives as chiral linkers for 1D coordination polymers, Polyhedron. 100 (2015) 404–411. [CrossRef]
- S. Salman, F. Idrees, S. Pervaiz, F.H. Shah, S. Badshah, M. Usman, S.A. Halimi, J. Idrees, Evaluation of antimicrobial activities of Harmine, Harmaline, Nicotine and their complexes, Pak. J. Pharm. Sci. 29 (2016) 1317–1320.
- S. Salman, S. Asghar, I.U. Khan, S.H. Khalid, F.H. Shah, M. Usman, Equilibrium , kinetics , thermodynamics and docking studies of Cu 2 + ion adsorption over ion-exchange resin and kappa carrageenan blends in blood samples, (2020) 795–803.
- Yahia, K.H. Khayat, Analytical models for estimating yield stress of high-performance pseudoplastic grout, Cem. Concr. Res. 31 (2001) 731–738.
- P. Jyotishkumar, J. Pionteck, C. Özdilek, P. Moldenaers, U. Cvelbar, M. Mozetic, S. Thomas, Rheology and pressure–volume–temperature behavior of the thermoplastic poly(acrylonitrile-butadiene-styrene)-modified epoxy-DDS system during reaction induced phase separation, Soft Matter. 7 (2011) 7248–7256. [CrossRef]
- J. Chen, W. Chen, F. Duan, Q. Tang, X. Li, L. Zeng, J. Zhang, Z. Xing, Y. Dong, L. Jia, H. Gao, The synergistic gelation of okra polysaccharides with kappa-carrageenan and its influence on gel rheology, texture behaviour and microstructures, Food Hydrocoll. 87 (2019) 425–435. [CrossRef]
- T. Brenner, R. Tuvikene, A. Parker, S. Matsukawa, K. Nishinari, Rheology and structure of mixed kappa-carrageenan/iota-carrageenan gels, Food Hydrocoll. 39 (2014) 272–279. [CrossRef]
- S.M.F. Kabir, P.P. Sikdar, B. Haque, M.A.R. Bhuiyan, A. Ali, M.N. Islam, Cellulose-based hydrogel materials: chemistry, properties and their prospective applications, Prog. Biomater. 7 (2018) 153–174. [CrossRef]
- F. Cuomo, M. Cofelice, F. Lopez, Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion, Polymers (Basel). 11 (2019) 259. [CrossRef]
- I.Y. Kim, R. Iwatsuki, K. Kikuta, Y. Morita, T. Miyazaki, C. Ohtsuki, Thermoreversible behavior of κ-carrageenan and its apatite-forming ability in simulated body fluid, Mater. Sci. Eng. C. 31 (2011) 1472–1476. [CrossRef]
- A.L. Daniel-da-Silva, L. Ferreira, A.M. Gil, T. Trindade, Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels, J. Colloid Interface Sci. 355 (2011) 512–517. [CrossRef]
- N. Wu, X. Yang, Z. Teng, S. Yin, J. Zhu, J. Qi, Stabilization of soybean oil body emulsions using κ, ι, λ-carrageenan at different pH values, Food Res. Int. 44 (2011) 1059–1068. [CrossRef]
- M.D. Torres, F. Chenlo, R. Moreira, Thermal reversibility of kappa/iota-hybrid carrageenan gels extracted from Mastocarpus stellatus at different ionic strengths, J. Taiwan Inst. Chem. Eng. 71 (2017). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).