1. Introduction: The Burden of Preterm Birth
A major cause of more than 85% of all perinatal complications and neonatal deaths is preterm birth, early preterm birth [
1,
2]. Children born prematurely often suffer from a variety of physical and neurodevelopmental disorders, which may cause substantial consequences [
2]. Among these are for example neurocognitive complications, which can occur early in childhood and manifest as developmental delay, cerebral palsy, hearing, and visual impairments, learning difficulties and psychiatric disorders [
3]. Moreover, preterm birth imposes significant psychological and financial burdens on parents. With estimated costs of
$1.6 billion to
$26 billion annually, preterm birth represents a considerable economic burden and impact on society [
4,
5,
6]. Nevertheless, factors like the country, population size, and preterm birth rate have a strong influence on the range of costs [
4,
5,
6]. Although a large portion of these costs are attributable to neonatal intensive care for infants born very prematurely, further additional costs have been shown to be associated with prematurity. These costs extend beyond the initial hospitalization, even for children born only a few weeks prematurely. However, two thirds of preterm births occur without known biological causes. Hence, strategies for prevention are urgently warranted and still a subject of intense debate [
7,
8].
2. Supplementation with Polyunsaturated Fatty Acids (PUFAs) to Prevent Preterm Birth
Notably, maternal supplementation with omega-3 long chain polyunsaturated fatty acids (long chain PUFAs) might be one of the most promising interventions to prevent preterm (<37 week’s gestation) and early preterm (<34 weeks’ gestation) birth [
9,
10,
11]. Therefore, an assessment of the need for omega-3 long chain PUFAs during pregnancy and their specific role in reducing the risk of preterm birth is needed. Most recommendations and dietary advice recommend omega-3 long chain PUFAs supplementation during pregnancy, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) [
12,
13,
14]. Both fatty acids are thought to play a role in preterm birth. EPA is a precursor of the 3-series prostaglandins. They are homologues and significantly less potent than the 2-series prostaglandins E2 and F2α (derived from arachidonic acid), which are involved in uterine contractions and cervical ripening. Besides this, DHA and EPA might cause the myometrium to relax and thus prevent early onset of labor. Additionally, they may also inhibit the activation of trophoblastic inflammatory pathways. This could lead to a decrease in inflammation-associated preterm birth [
15,
16]. Furthermore, they have been associated with maintaining the fetal supply of omega-3 long chain PUFAs and thus, support brain growth and subsequent neurological development in infants and children [
12,
13,
14].
Overall, published guidelines uniformly recommend that pregnant women should consume about 200 mg of DHA per day. This amount of DHA per day can be achieved via a balanced, omnivorous diet, which comprises fish, seafood, lean red meat, and eggs. Notably, 1-2 portions of fish per week can ensure an intake of 200 mg DHA per day. However, many of the recommendations and guidelines, for example the consensus statement of the Perinatal Lipid Working Group supported by the International Society for the Study of Fatty Acids and Lipids (ISSFAL), are not up-to-date nor do they specifically address the impact of omega-3 long chain PUFAs on prematurity [
12].
The interest in maternal dietary intake of omega-3 long chain PUFAs and perinatal outcomes has increased over the previous years. Until now, several reviews from the Cochrane Collaboration and an abundance of randomised controlled trials have been published on omega-3 long chain PUFAs and preterm birth, highlighting the importance of this subject. The most recent Cochrane review from 2018 has synthesised evidence and included 70 randomised controlled trials (involving 19,927 women at low, mixed or high risk of poor pregnancy outcomes) [
11]. The analysis revealed improvements in several outcomes such as a longer duration of gestation, higher birth weight and reduced risks of preterm birth and early preterm birth associated with omega-3 long chain PUFAs [
11]. In summary, the results suggest that there is high-quality evidence that supplementation with omega-3 long chain PUFAs during pregnancy, particularly with DHA, reduced the risk of having a premature baby by 11% and it also reduced the risk of having a very premature baby, born before 34 weeks, by 42% [
11]. Therefore, the authors concluded that pregnant women with one baby should be advised to take between 500 and 1000 mg of long-chain omega-3 PUFAs every day from the 12th week of pregnancy, in order to increase their chances of having a healthy full-length pregnancy. Following this review, an urgent need to understand the role of omega-3 long chain PUFAs on preterm birth in relation to dose, timing of supplementation and baseline omega-3 status has emerged. In addition to that, the need for the appraisal of the ratio of DHA and EPA has been expressed as well.
3. The Role of Lipid Derived Mediators in Inflammation and Resolution
A microbial infection or injury usually leads to acute inflammation. This reaction is important in order to eliminate pathogens, remove cellular debris and finally restore affected tissue. Eicosanoid lipid mediator molecules are pro-inflammatory signalling molecules. They are synthesized from the omega-6 PUFAs arachidonic acid (AA) and represent characteristic pro-inflammatory signalling molecules. These prostanoids comprise the prostaglandins (PG), leukotrienes (LT) and thromboxanes (TX). They are synthesized via the enzymes cyclooxygenases 1 and 2 (COX-1 and -2) by cells of the innate immune system, i.e. granulocytes or macrophages which are immediately attracted to the localisation of the respective injury or infection [
17]. Mast cells secret further pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNFα) and interleukins 1 and 6 (IL-1 and IL-6). Besides, other pro-inflammatory cytokines such as IL-1β, IL-12 and IL-18 are produced by M1 macrophages [
18]. Thus, they contribute to the inflammatory response and cause the characteristic cardinal symptoms of inflammation such as heat (calor), swelling (tumor), redness (rubor), pain (dolor), and potentially a loss of function (functio laesa). Neutrophils and monocytes are also attracted to the site of infection of injury by leukotriene B4 (LTB4) and cytokines. Following this, they infiltrate the affected tissue, and thus, advance the organisms’ inflammatory response [
17,
19,
20]. As the initiation of inflammation is essential for the organism, it should be triggered rapidly and efficiently. However, on the same time, the cessation of inflammation is equally important and should be initiated rapidly and efficiently as well. Importantly, life-threatening events like cytokine storm or sepsis may occur following excessive inflammation [
21]. Besides this, cardiovascular disease, diabetes or autoimmune disorders are also characteristic examples for inadequate cessation of inflammation [
22,
23]. The severity and consequences of these diseases highlight the importance of the cessation of these processes.
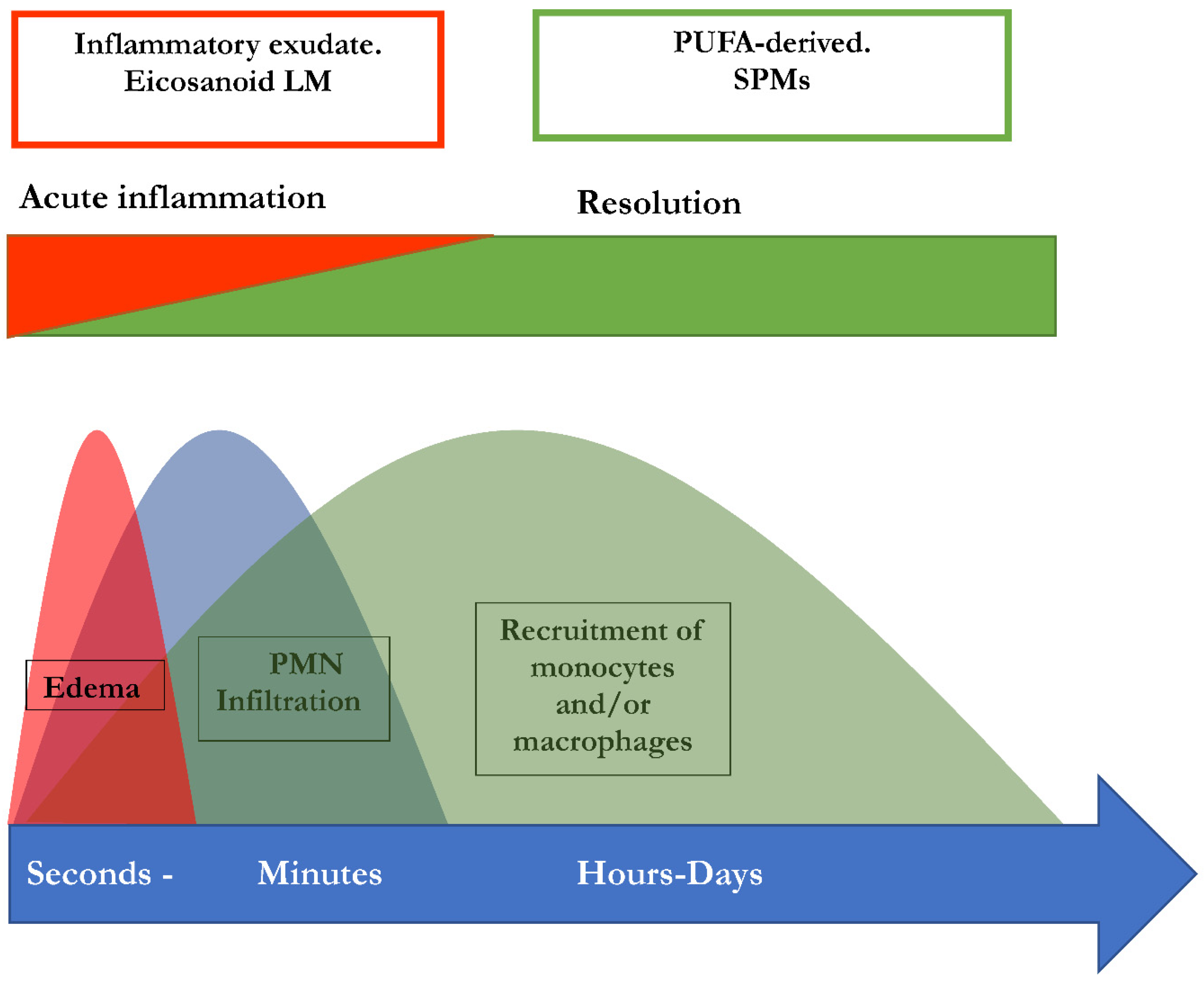
Mediation and resolution of inflammation is an active process triggered by specialized pro-resolving lipid mediator molecules (SPMs), which has been shown in animal models and in different human cells [
24,
25]. They are categorised into four families according to their chemical structure and biosynthetic pathways: resolvins (Rvs), protectins (PD), lipoxins (LXs) and maresins (MaR) [
24,
25,
26]. Rv, PD, and MaR derive from the omega-3 PUFAs DHA, while E-series Rv (RvEs) originate from EPA, and LX stem from the omega-6 AA. Lipoxygenases as well as COX-enzymes are part of the biosynthesis of the SPMs. SPMs are synthesized via the hydroxylated precursors 18-HpETE, 17-HpDHA, and 14-HpDHA (see
Figure 1) [
26,
27]. Aspirin irreversibly binds COX enzymes and thus blocks the synthesis of prostanoids by altering the catalytic domains of COX. However, their capacity to catalyse the synthesis of the SPM precursors 18-HpETE, 17-HpDHA and 14-HpDHA is not abolished. The newly formed SPMs have a different stereochemical structure and are known as aspirin-triggered SPMs (AT-SPMs) [
25,
26,
28,
29].
4. SPMs Are Inevitable for Resolving Inflammation
Several key steps are responsible for the termination of inflammatory processes: dead cells have to be removed, a conversion of macrophages to resolving M2 macrophages must be initiated, and the recruitment of neutrophils must be stopped [
27,
29,
30,
31,
32]. SPMs have a vital role in the termination of neutrophil infiltration and in the initiation of phagocytosis of apoptotic cells. Besides, they are involved in the downregulation of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-8 and IL-12, the reduction of platelet-activating factor and prostaglandin production. In addition to that, SPMs are also involved in in the clearance of the infection site and tissue regeneration, by stimulating efferocytosis and phagocytosis, promoting wound healing. These resolutive processes are triggered simultaneously to inflammation which is evident from the interlinkage of prostaglandin synthesis with the SPM biosynthetic pathways. Both the generation of pro-inflammatory lipid mediators and the subsequent synthesis of inflammation-mediating SPMs are promoted by polymorphonuclear leukocytes (PML) [
33]. This implies a lipid mediator class switch in these cells which is essential for a regulated resolution of inflammation and thus for the prevention of chronification [
33]. PGE2 as well as PGD2 are necessary to induce lipoxygenases which are in turn necessary for the generation of LXs, Rvs, and Protectin D1 [
34]. As a consequence, the switch of the lipid mediator class from a proinflammatory to a pro-resolutive function is disrupted when PG synthesis is inhibited. Hence, this may result in impaired resolution [
33,
35]. In summary, the initiation of inflammation is inseparably linked to its active resolution, thus the beginning of the signaling cascade programs the end [
34]. An illustration of this perception of inflammation is available in
Figure 1.
Although the ability of pro-resolution remains to be the most important activity, SPMs also have other effects related to adaptive immune response. LX also triggers the activation of natural killer cells [
36]. CD4+ T cell differentiation has also been shown to be modulated by the resolvins RvD1, RvD2 and maresin MaR1 [
37]. Notably, 17-HDHA and RvD1 increased IgM and IgG production in human B cells, suggesting SPM activity in humoral response and opening new functions as endogenous non-toxic adjuvants [
38].
5. The Significance of SPMs in Chronic Inflammatory Diseases
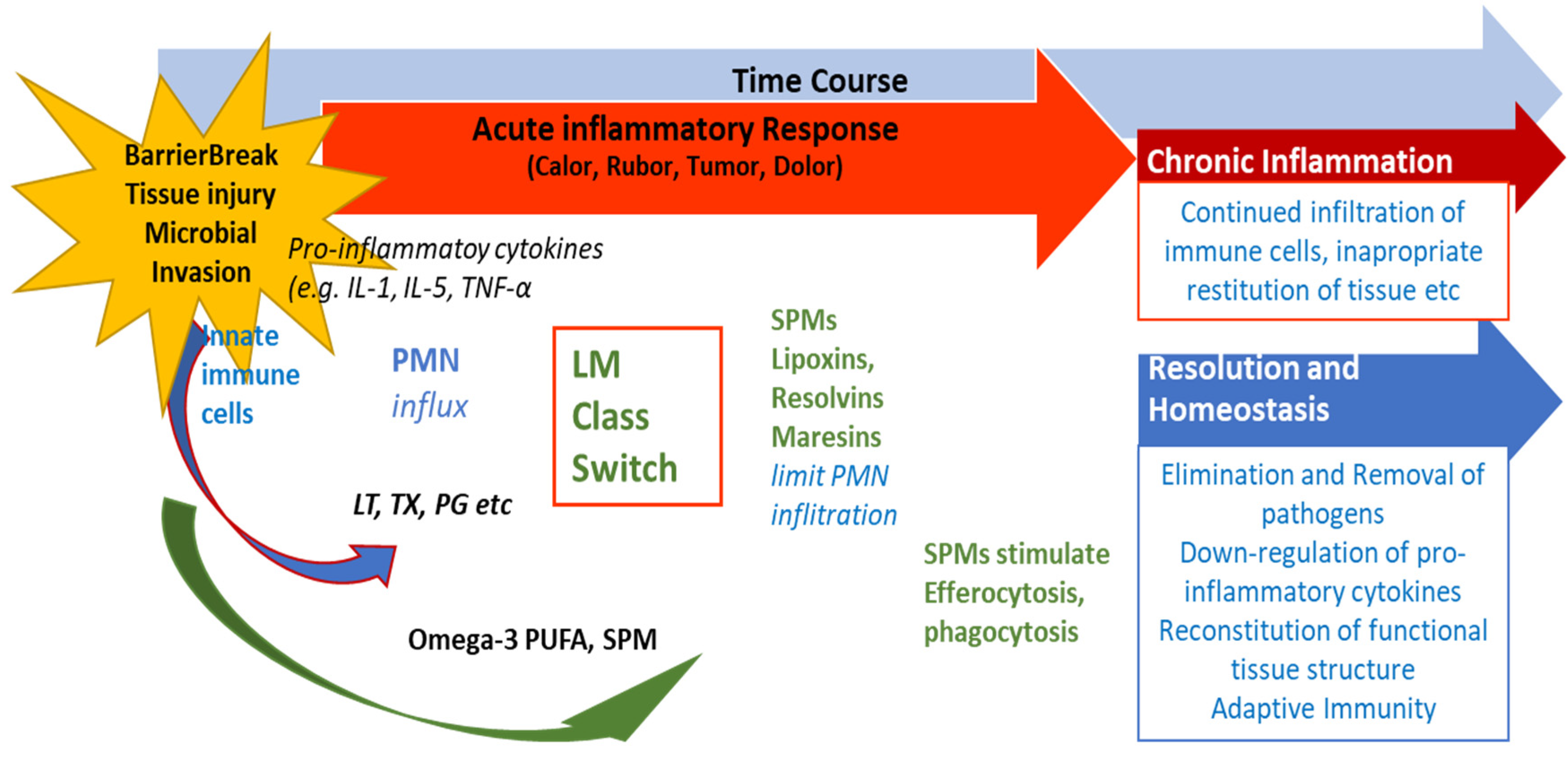
Previous and ongoing research on SPMs has increased the knowledge on their structure and their biosynthetic pathways, receptors and function. Serhan et al. [
24,
26,
27] and Chiang et al. have suggested different options for the outcome of inflammatory responses [
39]. If inflammation does not resolve sufficiently and remains active, i.e., pro-inflammatory signaling molecules are constantly produced, a state of chronic inflammation may develop. This is depicted in
Figure 2.
The critical role of SPMs in the prevention or improvement of chronic inflammatory diseases, has been shown in animal models. One example is the pathogenesis of Periodontitis: An infection with P. gingivalis leads to inflammatory processes. This scenario has been successfully mimicked in air pouch mouse models where increased neutrophil recruitment and upregulation of COX-2 enzymes were reported to be reduced by supplementation with lipoxin LXA analogues [
40]. Another animal model revealed the role of the SPMs LXA4, RvD1 and RvD2 in the pathogenesis of atherosclerosis, a chronic inflammatory disease [
41]. In mice transgenic for 12-/15-lipoxygenase, increased expression of RvD1, PD1, and 17-HpDHA proved to be protective by reducing the development of atherosclerosis compared to wild-type mice. The anti-atherogenic effects of LXA4, PD1, and RvD1 rely on several processes, including decreased expression of endothelial adhesion molecules and decreased secretion of cytokines. Importantly, the influence of nutrition on the pathogenesis of atherosclerosis has been shown in this mouse model as the transgenic mice were as susceptible to atherosclerosis as the wild type animals following a standard high-fat western diet [
41,
42]. Furthermore. experiments in a rat model for arthritis have been conducted which demonstrated that RvD1 and the precursor metabolite 17-HDHA reduced pain and tissue damage more effectively in comparison to steroids [
43].
Fibrosis represents one of the characteristic features of uterine leiomyoma [
1]. Thus, inadequate resolution of inflammation may also be considered as a cause for uterine fibroids (UF). Recent animal models revealed various insights into the role of resolution of inflammation in fibrosis: For example, an animal model in pulmonary fibrosis demonstrated that exogenously administered LX4-epimer (AT-LX4) reduced fibrosis [
44]. Additionally, LXA4 and its analogue benzo-LXA4 reduced the extent of fibrotic changes in kidney in a rat model of early renal fibrosis, as well [
45]. A mouse model of obstructed kidney showed anti-fibrotic effects for RvE1 [
46].
To date, the role of SPMs in the development of UF has not yet been investigated. However, there are some features that UF has in common with other chronic inflammatory diseases where consensus has been reached on the importance of inadequate resolution, including for example the role of SPMs in tumours [
47]. Still, further research in this context is urgently warranted and might lead to novel therapeutic options and insights.
6. Intraamniotic Inflammation and Clinical Chorioamnionitis
In patients in preterm delivery with intact chorioamniotic membranes as well as in patients experiencing prelabor rupture of membranes, intraamniotic inflammation can lead to an intense systemic maternal inflammatory response which is described as clinical chorioamnionitis [
48,
49,
50,
51]. Proceeding subclinically, this condition is one of the most frequent infection-associated diseases worldwide which is predominately diagnosed in young primiparous women [
52]. The prevalence of chorioamnionitis in the United States was 9.7 per 1000 live births in 2008 [
53]. Apart from systemic inflammatory symptoms in the mother, also acute symptoms of histologic chorioamnionitis have been reported [
54] as well as inflammatory responses affecting the fetus which are associated with funisitis or chorionic vasculitis [
55,
56,
57]. Besides being linked to maternal morbidity, neonates which are delivered by mothers suffering from clinical chorioamnionitis at term (TCC) possess an enhanced risk for long-term consequences such as cerebral palsy [
58,
59,
60]. TCC is currently described as a heterogenous disease pattern which is accompanied by symptoms like fever, leukocytosis, foul-smelling amniotic fluid, maternal or fetal tachycardia or uterine tenderness [
61,
62,
63]. A study evaluating patients suffering from clinical chorioamnionitis reported 22% of patients without any intra-amniotic inflammation, 24% showing sterile intra-amniotic inflammation and 54% with microbial-associated intra-amniotic inflammation [
53]. In an effort to unravel the causal mechanisms of a sterile inflammatory response, studies could demonstrate the influence of damage-associated molecular patterns (DAMPs) on sterile inflammation, for example the high mobility gene box-1 [
64]. Furthermore, it could be shown that the amniotic fluid in TCC-patients has high DAMP-levels [
65] which are also associated with induction of labor [
66]. The introduction of clinical tests which are able to assess the differential diagnosis of the three distinct subgroups of chorioamnionitis-patients would be a helpful tool since these patient cohorts need different therapy approaches. Patients diagnosed with microbial-associated intra-amniotic inflammation require antibiotics while patients without any intra-amniotic inflammation do not. The establishment of a clinical biomarker for the detection and identification of chorioamnionitis regardless of the presence of an intra-amniotic infection would contribute to an improved characterization and diagnosis of this disease.
7. SPMs in Amniotic Inflammation
SPMs play a crucial role in the mediation and resolution of microorganism-derived and sterile inflammation [
34,
67,
68,
69]. Since it has been recognized that PGs such as PGE2 and LTs such as LTB4 are elevated in the amniotic fluid in clinical chorioamnionitis, an important contribution of these bioactive lipids in delivery at term can be assumed. Additionally, patients with microbial-associated intra-amniotic inflammation together with clinical chorioamnionitis show significantly high concentrations of arachidonic-acid-derived SPMs compared to those with sterile intra-amniotic inflammation [
70]. Moreover, since TCC is characterized as an acute inflammatory condition, it is assumed that its lipid profile in the amniotic fluid differs from spontaneous labor at term (TLB). In this context, it could be demonstrated that there is no difference between concentrations of proinflammatory lipids in amniotic fluid in TLB and TCC patients. However, in all patients with TCC the presence of SPMs was significantly reduced compared to TLB patients, suggesting a decreased synthesis of SPMs as a characteristic property of TCC as opposed to infection-driven intra-amniotic inflammation where lipid mediators play an essential role [
71].
8. Preeclampsia
Hypertension, in particular preeclampsia (PE) which accounts for the majority of fetal, neonatal and maternal deaths is one of the most common complications worldwide during pregnancy [
72]. Possible symptoms manifest after the 20th week of pregnancy and include de novo hypertension, edema, and proteinuria [
72,
73,
74]. The precise mechanisms causing PE to remain to be elucidated. It is assumed that a shallow placental implantation which develops hypoxia might be responsible for a following immune reaction, leading to a placental upregulation of inflammatory factors, finally evoking an endothelial response of the vasculature [
72].
The main cause of PE is the activation of inflammation provoked through an immunological response against the embryo as an allograft. Its pathology is based on an imbalance of the immunological landscape in the placenta through upregulation of pro-inflammatory immune activators or downregulation of anti-inflammatory immune inhibitors. Aiming to unravel the association of PE and inflammatory responses, the contribution of anti-inflammatory IL-10 has been investigated [
75,
76] as well as the role of pro-inflammatory pattern-recognition receptors [
77]. However, regardless of many theories, it is not fully understood until now how PE is influenced by inflammation.
All pregnant women show a systemic inflammation evoked though clearance of placental debris which is released into the maternal blood circulation. PE occurs simultaneously with a failure of the systemic inflammatory response which is unable to properly compensate the immunogenic burden [
78]. Thus, it can be assumed that an inhibition or downregulation of inflammatory processes is crucial for the development of a PE. In this context, SPMs act as potentially important “braking signals” of inflammation [
79,
80,
81]. LXA4 for example is able to bind to the G-protein-coupled receptor N-formyl peptide receptor 2 (FPR2/ALX) (11) whose downregulatory signalling contributes fundamentally to LXA4-mediated anti-inflammatory response in vivo. Thereby, LXA4 acts as an inhibitor of chemotaxis of eosinophilic and neutrophilic granulocytes [
82,
83], antagonizes peptido-LTs [
84], enhances macrophage-mediated phagocytosis of apoptotic cells [
79], and reduces neutrophile infiltration
in vivo [
83]. Since LXA4 is a crucial regulator of anti-inflammatory response, a contribution to PE can be assumed. Furthermore, anti-angiogenic role of LXA4 has been demonstrated in vitro on human umbilical vein endothelia cells (HUVECS) as well as a LXA4-dependent reduction of lipopolysaccaride (LPS)-induced endothelial hyperpermeability [
85,
86]. Besides, the synthetic analogue of LXA4, 5(S),6(R)-7-trihydroxymthyl heptanoate (BML-111), was proven to reduce systolic blood pressure, 24h urinary albumin excretion, serum TNFα, IL-8 levels, and LPS-dependent morphologic injury of kidney and placenta [
87].
9. First Compounds with an Enriched Marine Oil Supplement on the Market
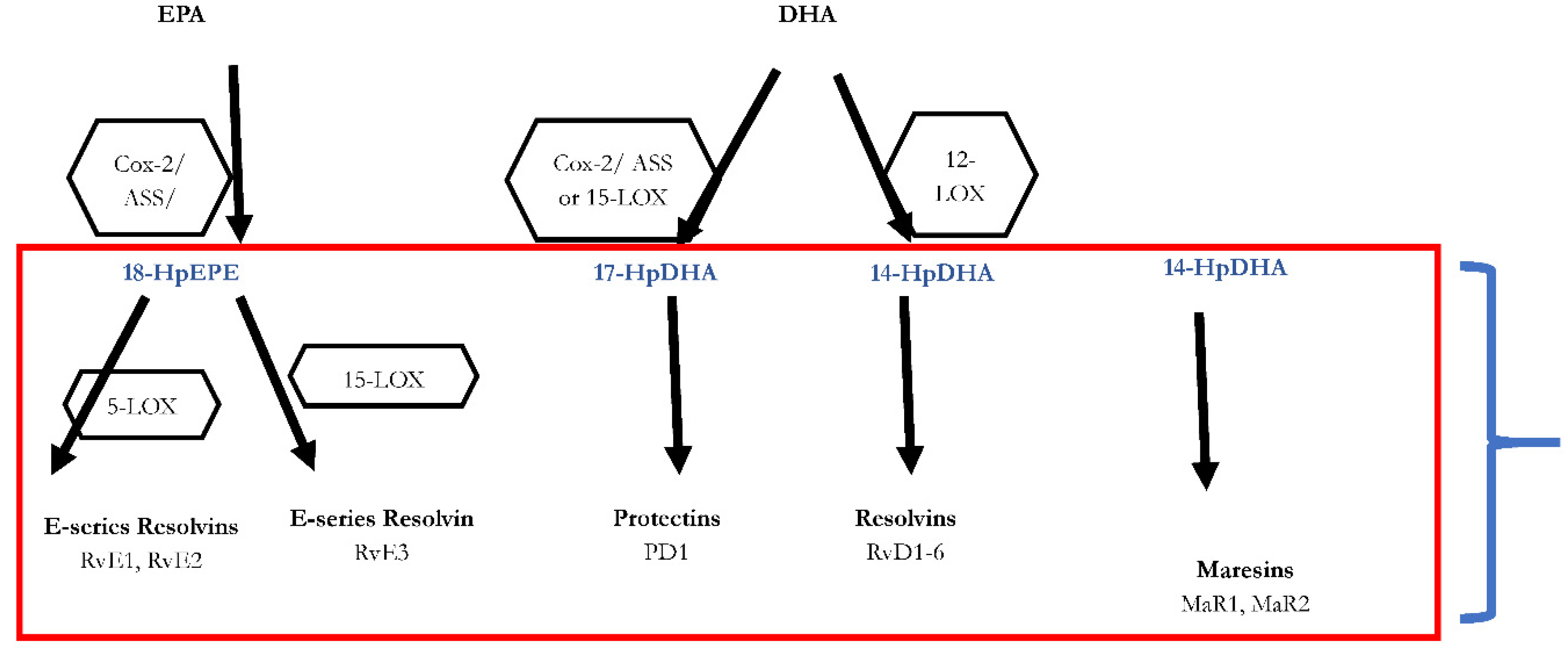
As a result of this research, a formulation with a combined concentration of omega 3 and monohydroxylated (Lipinova ®) appears on the market. This formulation is a set of derivatives of omega 3 fatty acids that includes not only precursor fatty acids such as DHA and EPA, but also their immediate derivatives, los14, 17 and 18 monohydroxylated, 14-HDHA, 17HDHA and 18-HEPE. The advantages are clear. On the one hand we are able to supplement with a more refined product that already has a pre-resolutive effect and we are also able to correct the possible enzymatic deficits in its production.
Figure 3 depicts the metabolic pathway.
Until now we only had generic supplementation and we had to wait for the integrity of the individual's metabolic chain for its resolution in the form of inflammation-solving mediating products. We had drugs that acted on the oxygenase cycle (COX) favoring its action like aspirin, but with side effects and side actions.
We therefore begin to have in our arsenal direct agonist products, which do not block immunity or create side effects for the resolution of inflammation as a therapeutic weapon to correct those pathologies derived from chronic inflammation that complicate our lives, not only during pregnancy, but in the development of autoimmune diseases, brain damage, arteriosclerosis, rheumatological diseases, sports recovery, etc. It opens up a fascinating field of action in a physiological way.
In some multivitamin formulation 30 mg of this marine oil enriched formulation have been introduced to simulate the physiological amount of pro resolving lipid mediators of the placenta.
In this suspension the rate of the following
Selective pro-resolving mediators is found:
17-HDHA 80-400 μg/ml
17-hydroxy-docosahexaenoic acid
18-HEPE 50-400 μg/ml
18-hydroxy-eicosapentaenic acid
14-HDHA 40-200 μg/ml
14-hydroxy-docosahexaenoic acid
The first studies of this nutritional supplement have demonstrated its effectiveness in raising SPMs in plasma in different physiological and pathological conditions.
Having been able to detect a large deficit of SPMs in conditions of inflammation and as described in the protocol, it is estimated that the application of this new formulation will substantially improve both the level of SPMs in plasma and serum and the ratio between SPMs and prostaglandins.
In these studies of Elajami 2016, Conte (not published data) and Souza 2020 it was possible to see that the ideal doses in the intake are between 1500 mg and 3000 mg. Serhan used one formulation for a period of one year while the other studies used the formulation between 1 day and 5 days. Commune to all studies was
(a) zero incidence of side effects, and
(b) the substantial increase in SPMs.
Hence this represents a first step in the creation of a homeostasis system for pregnant women.
10. Conclusions
The use of selective pro resolving mediators including the monohydroxylates are promising substances for the management of different acute and chronic diseases.
In the field of obstetrics many clinical entities like uterine contractions or the occurrence of pre-eclampsia are still serious complications during pregnancy.
The use of enriched marine oil nutritional’s may contribute to an attenuation of these diseases as the containing selective pro resolving mediators exhibit pro resolutive actions that can positively modulate inflammatory diseases and also those leading to serious obstetrical complications.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
Pedro-Antonio Regidor is an employee of Exeltis Healthcare. Johanna Eiblwieser and Theresa Steeb are employees of Exeltis Germany. Jose Miguel Rizo is an employee of Chemo OTC Spain.
References
- D'Apremont, I.; Marshall, G.; Musalem, C.; Mariani, G.; Musante, G.; Bancalari, A.; Fabres, J.; Mena, P.; Zegarra, J.; Tavosnanska, J.; et al. Trends in Perinatal Practices and Neonatal Outcomes of Very Low Birth Weight Infants during a 16-year Period at NEOCOSUR Centers. J Pediatr 2020, 225, 44–50. [Google Scholar] [CrossRef]
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110, 8–16. [Google Scholar] [CrossRef]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Outcomes, I.H. Preterm Birth: Causes, Consequences, and Prevention In Preterm Birth: Causes, Consequences, and Prevention, Behrman, R.E., Butler, A.S., Eds.; The National Academies Collection; Reports funded by National Institutes of Health: Washington, DC, USA, 2007. [Google Scholar]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm birth lifetime costs in the United States in 2016: An update. Semin Perinatol 2021, 45, 151390. [Google Scholar] [CrossRef] [PubMed]
- Newnham, J.P.; Schilling, C.; Petrou, S.; Morris, J.M.; Wallace, E.M.; Brown, K.; Edwards, L.; Skubisz, M.M.; White, S.W.; Rynne, B.; et al. The health and educational costs of preterm birth to 18 years of age in Australia. Aust N Z J Obstet Gynaecol 2022, 62, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.M.; Larson, J.; Jacobsson, B.; Di Renzo, G.C.; Norman, J.E.; Martin, J.N., Jr.; D'Alton, M.; Castelazo, E.; Howson, C.P.; Sengpiel, V.; et al. Cross-Country Individual Participant Analysis of 4.1 Million Singleton Births in 5 Countries with Very High Human Development Index Confirms Known Associations but Provides No Biologic Explanation for 2/3 of All Preterm Births. PLoS ONE 2016, 11, e0162506. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, L.; Care, A.; Alfirevic, Z. Controversies in the prevention of spontaneous preterm birth in asymptomatic women: An evidence summary and expert opinion. BJOG 2021, 128, 177–194. [Google Scholar] [CrossRef]
- Carlson, S.E.; Gajewski, B.J.; Valentine, C.J.; Kerling, E.H.; Weiner, C.P.; Cackovic, M.; Buhimschi, C.S.; Rogers, L.K.; Sands, S.A.; Brown, A.R.; et al. Higher dose docosahexaenoic acid supplementation during pregnancy and early preterm birth: A randomised, double-blind, adaptive-design superiority trial. EClinicalMedicine 2021, 36, 100905. [Google Scholar] [CrossRef]
- Makrides, M.; Best, K.; Yelland, L.; McPhee, A.; Zhou, S.; Quinlivan, J.; Dodd, J.; Atkinson, E.; Safa, H.; van Dam, J.; et al. A Randomized Trial of Prenatal n-3 Fatty Acid Supplementation and Preterm Delivery. N Engl J Med 2019, 381, 1035–1045. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev 2018, 11, CD003402. [Google Scholar] [CrossRef]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Perinatal Lipid Intake Working, G.; Child Health, F.; Diabetic Pregnancy Study, G.; European Association of Perinatal, M.; European Association of Perinatal, M.; European Society for Clinical, N.; et al. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007, 98, 873–877. [Google Scholar] [CrossRef]
- Koletzko, B.; Lien, E.; Agostoni, C.; Bohles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J Perinat Med 2008, 36, 5–14. [Google Scholar] [CrossRef]
- Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap 2010, 91, 1–166. [Google Scholar]
- Frew, L.; Sugiarto, N.U.; Rajagopal, S.P.; He, J.; Leask, R.; Norman, J.E.; Riley, S.C.; Stock, S.J. The effect of omega-3 polyunsaturated fatty acids on the inflammatory response of the amnion. Prostaglandins Leukot Essent Fatty Acids 2013, 89, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–152. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front Immunol 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Galan, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol 2015, 6, 263. [Google Scholar] [CrossRef]
- Flower, R.J. Prostaglandins, bioassay and inflammation. Br J Pharmacol 2006, 147, S182–S192. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B. Role of basic science in the development of new medicines: Examples from the eicosanoid field. J Biol Chem 2012, 287, 10070–10080. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat Rev Dis Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Serhan, C.N.; Fredman, G.; Yang, R.; Karamnov, S.; Belayev, L.S.; Bazan, N.G.; Zhu, M.; Winkler, J.W.; Petasis, N.A. Novel proresolving aspirin-triggered DHA pathway. Chem Biol 2011, 18, 976–987. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr Opin Pharmacol 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat Commun 2018, 9, 3261. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat Immunol 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Bandeira-Melo, C.; Serra, M.F.; Diaz, B.L.; Cordeiro, R.S.; Silva, P.M.; Lenzi, H.L.; Bakhle, Y.S.; Serhan, C.N.; Martins, M.A. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: Relationship with concurrent eosinophilia. J Immunol 2000, 164, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013, 5, 174ra126. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Ramon, S.; Gao, F.; Serhan, C.N.; Phipps, R.P. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol 2012, 189, 1036–1042. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol 2003, 171, 6856–6865. [Google Scholar] [CrossRef] [PubMed]
- Merched, A.J.; Ko, K.; Gotlinger, K.H.; Serhan, C.N.; Chan, L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 2008, 22, 3595–3606. [Google Scholar] [CrossRef]
- Merched, A.J.; Serhan, C.N.; Chan, L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics 2011, 4, 12–24. [Google Scholar] [CrossRef]
- Lima-Garcia, J.F.; Dutra, R.C.; da Silva, K.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol 2011, 164, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Valenca, S.S.; Farias-Filho, F.A.; Molinaro, R.; Simoes, R.L.; Ferreira, T.P.; e Silva, P.M.; Hogaboam, C.M.; Kunkel, S.L.; Fierro, I.M.; et al. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol 2009, 182, 5374–5381. [Google Scholar] [CrossRef]
- Borgeson, E.; Docherty, N.G.; Murphy, M.; Rodgers, K.; Ryan, A.; O'Sullivan, T.P.; Guiry, P.J.; Goldschmeding, R.; Higgins, D.F.; Godson, C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J 2011, 25, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, X.; Yao, J.; Song, J.; Nikolic-Paterson, D.J.; Li, J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol 2012, 228, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol Ther 2021, 218, 107670. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.S.; Romero, R.; Hillier, S.L.; Eschenbach, D.A.; Sweet, R.L. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992, 166, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Gravett, M.G.; Hummel, D.; Eschenbach, D.A.; Holmes, K.K. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol 1986, 67, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaemsaithong, P.; Docheva, N.; Korzeniewski, S.J.; Tarca, A.L.; Bhatti, G.; Xu, Z.; Kusanovic, J.P.; Dong, Z.; Chaiyasit, N.; et al. Clinical chorioamnionitis at term IV: The maternal plasma cytokine profile. J Perinat Med 2016, 44, 77–98. [Google Scholar] [CrossRef]
- Malloy, M.H. Chorioamnionitis: Epidemiology of newborn management and outcome United States 2008. J Perinatol 2014, 34, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Miranda, J.; Kusanovic, J.P.; Chaiworapongsa, T.; Chaemsaithong, P.; Martinez, A.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Shaman, M.; et al. Clinical chorioamnionitis at term I: Microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015, 43, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015, 213, S29–52. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Gotsch, F.; Pineles, B.; Kusanovic, J.P. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007, 65, S194–202. [Google Scholar] [CrossRef]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008, 79, 50–57. [Google Scholar] [CrossRef]
- Becroft, D.M.; Thompson, J.M.; Mitchell, E.A. Placental chorioamnionitis at term: Epidemiology and follow-up in childhood. Pediatr Dev Pathol 2010, 13, 282–290. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Brown, A.G.; Breen, K.; Anton, L.; Maubert, M.; Burd, I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci 2011, 29, 663–671. [Google Scholar] [CrossRef]
- Thomas, W.; Speer, C.P. Chorioamnionitis: Important risk factor or innocent bystander for neonatal outcome? Neonatology 2011, 99, 177–187. [Google Scholar] [CrossRef]
- Tita, A.T.; Andrews, W.W. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010, 37, 339–354. [Google Scholar] [CrossRef]
- Newton, E.R. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol 1993, 36, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaemsaithong, P.; Korzeniewski, S.J.; Kusanovic, J.P.; Docheva, N.; Martinez-Varea, A.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Chaiworapongsa, T.; et al. Clinical chorioamnionitis at term III: How well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med 2016, 44, 23–32. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T.; Savasan, Z.A.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med 2012, 25, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Plazyo, O.; Panaitescu, B.; Furcron, A.E.; Miller, D.; Roumayah, T.; Flom, E.; Hassan, S.S. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016, 75, 3–7. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Harris, S.G.; Padilla, J.; Koumas, L.; Ray, D.; Phipps, R.P. Prostaglandins as modulators of immunity. Trends Immunol 2002, 23, 144–150. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Chaemsaithong, P.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Gomez, R.; Chaiyasit, N.; et al. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. FASEB J 2016, 30, 3296–3307. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Chaemsaithong, P.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Gomez, R.; Docheva, N.; et al. Clinical chorioamnionitis at term: The amniotic fluid fatty acyl lipidome. J Lipid Res 2016, 57, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Saftlas, A.F.; Olson, D.R.; Franks, A.L.; Atrash, H.K.; Pokras, R. Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. Am J Obstet Gynecol 1990, 163, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu Rev Pathol 2010, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, J.H.; South, S.; Chiasson, V.L.; Mitchell, B.M. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 2010, 298, R713–R719. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Nevers, T.; Norris, W.E.; Sharma, S. Vascular IL-10: A protective role in preeclampsia. J Reprod Immunol 2011, 88, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Sado, T.; Naruse, K.; Noguchi, T.; Haruta, S.; Yoshida, S.; Tanase, Y.; Kitanaka, T.; Oi, H.; Kobayashi, H. Inflammatory pattern recognition receptors and their ligands: Factors contributing to the pathogenesis of preeclampsia. Inflamm Res 2011, 60, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil 2001, 29, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Maderna, P.; Godson, C. Lipoxins: Resolutionary road. Br J Pharmacol 2009, 158, 947–959. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol 2008, 3, 279–312. [Google Scholar] [CrossRef]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol 2006, 6, 414–420. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Fiore, S.; Maddox, J.F.; Brady, H.R.; Petasis, N.A.; Serhan, C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J Exp Med 1997, 185, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Badr, K.F.; DeBoer, D.K.; Schwartzberg, M.; Serhan, C.N. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: Evidence for competition at a common receptor. Proc Natl Acad Sci USA 1989, 86, 3438–3442. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, P.; Ye, D.; Huang, Y.; Zhou, X.; Li, Y.; Cai, L. Effects of lipoxin A(4) on CoCl(2)-induced angiogenesis and its possible mechanisms in human umbilical vein endothelial cells. Pharmacology 2009, 84, 17–23. [Google Scholar] [CrossRef]
- Pang, H.; Yi, P.; Wu, P.; Liu, Z.; Liu, Z.; Gong, J.; Hao, H.; Cai, L.; Ye, D.; Huang, Y. Effect of lipoxin A4 on lipopolysaccharide-induced endothelial hyperpermeability. ScientificWorldJournal 2011, 11, 1056–1067. [Google Scholar] [CrossRef]
- Lin, F.; Zeng, P.; Xu, Z.; Ye, D.; Yu, X.; Wang, N.; Tang, J.; Zhou, Y.; Huang, Y. Treatment of Lipoxin A(4) and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reprod Toxicol 2012, 34, 677–685. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).