Submitted:
11 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Lin28B promoter sequence analysis and vector construction
2.4. Vector construction and transfection
2.5. Cell Transfection and Dual-Luciferase Detection of Deletion Fragment Luciferase Reporter Gene Vector
2.6. Transcription factor prediction of highly transcriptionally active fragments
2.7. Construction of Point Mutation Vectors for Transcription Factors SP1 and Egr1
2.8. Construction of transcription factor Egr1 overexpression vector and dual luciferase detection
2.9. Data Analysis
3. Results
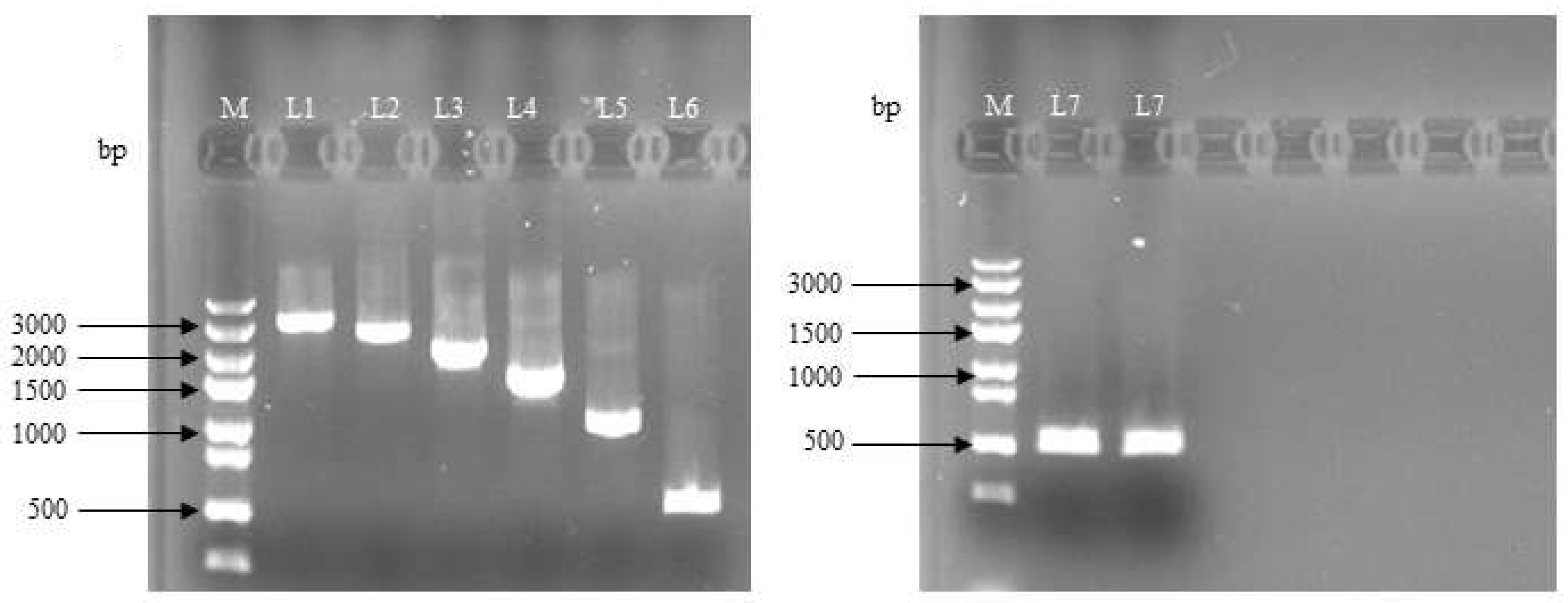
3.1. Construction of the Deletion Vector of Lin28B Gene Promoter Fragment in Dolang Sheep
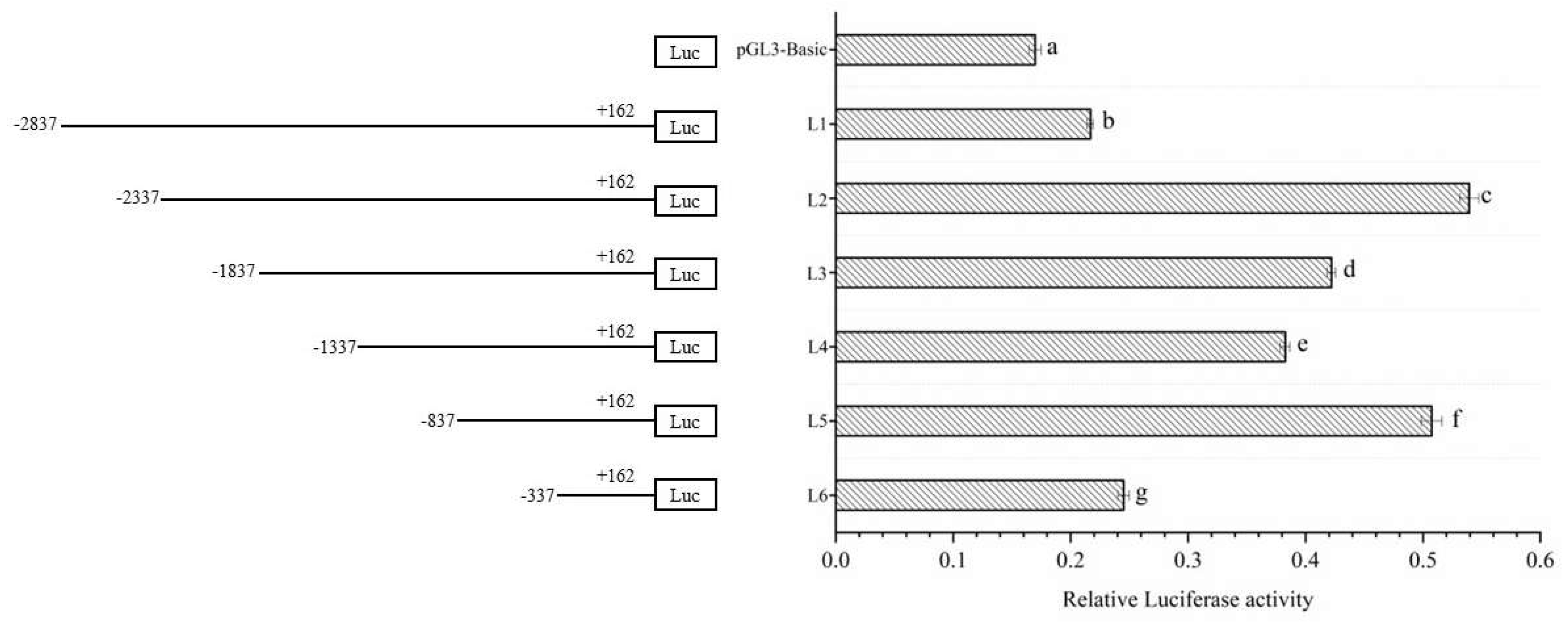
3.2. Detection of dual-luciferase activity in the deletion vector of Lin28B gene promoter fragment in Dolang sheep
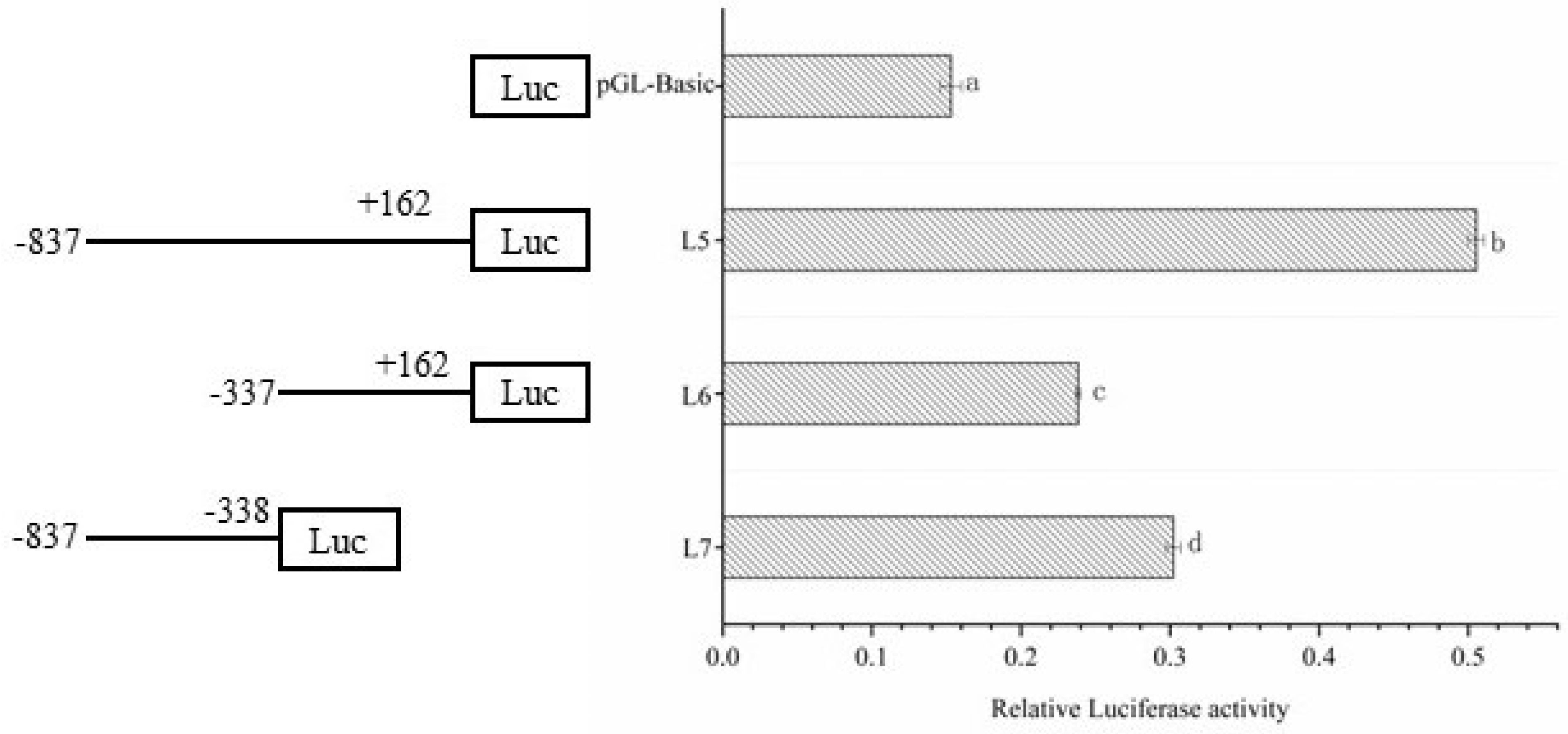
3.3. Dual luciferase activity detection of fragment vectors with high transcriptional activity
3.4. Prediction of transcription factor binding sites in Lin28B promoter region of Dolang sheep
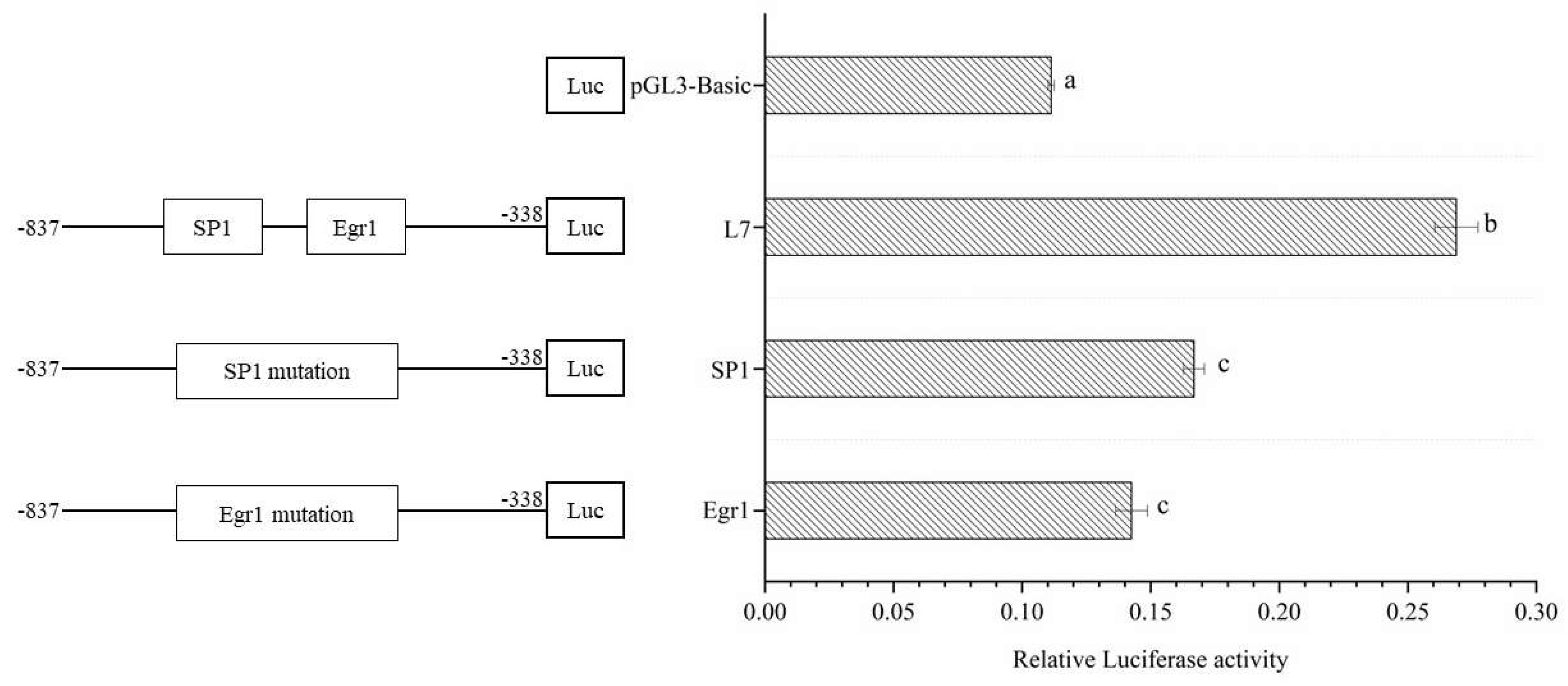
3.5. Verification of the point mutation of the promoter transcription factor in the core region of the Lin28B promoter of Dolang sheep
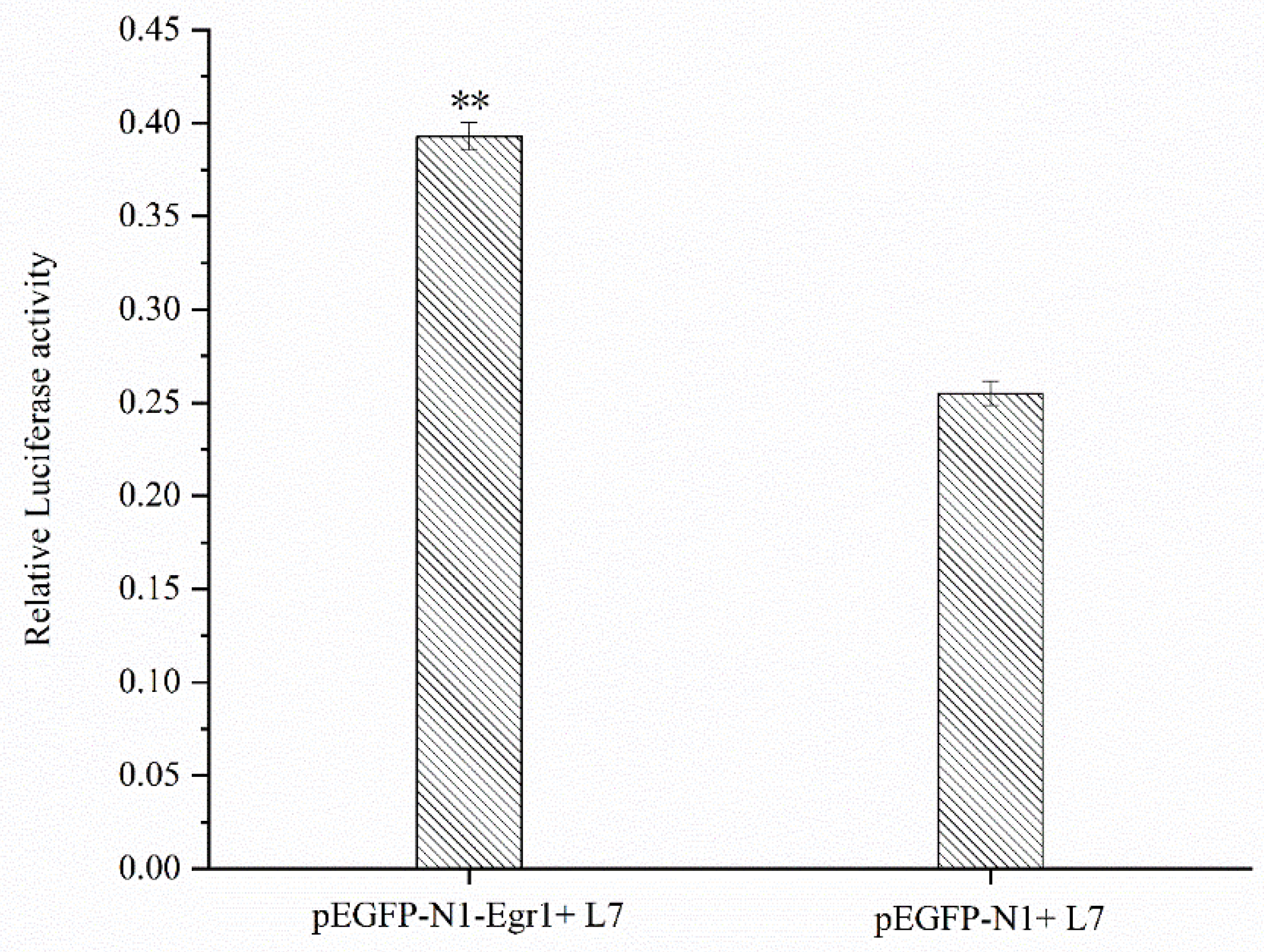
3.6. Effect of Egr1 overexpression on the transcriptional activity of Lin28B promoter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, Z.; Zhang, Z.; Zhang, Y.; Zhang, J.; Li, Q.; Xing, F. Analysis of Methylation and mRNA Expression of Lin28B Gene Promoter Region in the Hypothalamus of Dolang Sheep During Pubertal Initiation. DNA Cell Biol 2023, 42, 130–139. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, C.; Kong, Z. Cloning and expression of lin-28 homolog B gene in the onset of puberty in Duolang sheep. Asian-Australas J Anim Sci 2019, 32, 23–30. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Ito, H.; Watanabe, A.; Ge, X.; Kodama, T.; Aburatani, H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 2006, 384, 51–61. [Google Scholar] [CrossRef]
- Ong, K.K.; Elks, C.E.; Li, S.; Zhao, J.H.; Luan, J.a.; Andersen, L.B.; Bingham, S.A.; Brage, S.; Smith, G.D.; Ekelund, U. Genetic variation in LIN28B is associated with the timing of puberty. Nature Genetics 2009, 41, 729–733. [Google Scholar] [CrossRef]
- Ong, K.K.; Elks, C.E.; Wills, A.K.; Wong, A.; Wareham, N.J.; Loos, R.J.F.; Kuh, D.; Hardy, R. Associations between the Pubertal Timing-Related Variant in LIN28B and BMI Vary Across the Life Course. J. Clin. Endocrinol. Metab. 2011, 96, E125–E129. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Manfredi-Lozano, M.; Ruiz-Pino, F.; Navarro, V.M.; Sanchez-Garrido, M.A.; Leon, S.; Dieguez, C.; Cordido, F.; Matagne, V.; Dissen, G.A.; et al. Changes in Hypothalamic Expression of the Lin28/let-7 System and Related MicroRNAs During Postnatal Maturation and After Experimental Manipulations of Puberty. Endocrinology 2013, 154, 942–955. [Google Scholar] [CrossRef]
- Grieco, A.; Rzeczkowska, P.; Alm, C.; Palmert, M.R. Investigation of peripubertal expression of Lin28a and Lin28b in C57BL/6 female mice. Mol. Cell. Endocrinol. 2013, 365, 241–248. [Google Scholar] [CrossRef]
- Lemon, B.; Tjian, R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev 2000, 14, 2551–2569. [Google Scholar] [CrossRef]
- Dong, W.H.; Yin, X.M.; Sun, L.; Wang, J.; Sun, S.Y.; Zhu, G.Q.; Wu, S.L.; Bao, W.B. Age-associated methylation change of TAP1 promoter in piglet. Gene 2015, 573, 70–74. [Google Scholar] [CrossRef]
- Haberle, V.; Lenhard, B. Promoter architectures and developmental gene regulation. Semin. Cell Dev. Biol. 2016, 57, 11–23. [Google Scholar] [CrossRef]
- Wang, J.M.; Lang, B.; Zhu, H.Y.; Du, H.T.; Tian, Y.M.; Su, Y.H. Cloning and transcriptional activity analysis of the porcine cofilin 2 gene promoter. Gene 2014, 547, 280–287. [Google Scholar] [CrossRef]
- Annicotte, J.; Schoonjans, K.; Haby, C.; Auwerx, J. An E-box in pGL3 reporter vectors precludes their use for the study of sterol regulatory element-binding proteins. Biotechniques 2001, 31, 993–994. [Google Scholar]
- Wei, X.F.; Zhu, Y.C.; Du, J.; Ma, X.J.; Zhao, X.; Ma, Y.Y.; Han, S.; Ma, Y. Analysis of ANGPTL8 promoter activity and screening of related transcription factors in bovine. Gene 2021, 784, 6. [Google Scholar] [CrossRef]
- Xiang, G.M.; Huang, L.; Zhang, X.L.; Wang, N.; Wang, H.; Mu, Y.L.; Li, K.; Liu, Z.G. Molecular Characteristics and Promoter Analysis of Porcine COL1A1. Genes 2022, 13, 14. [Google Scholar] [CrossRef]
- Inukai, S.; Kock, K.H.; Bulyk, M.L. Transcription factor-DNA binding: beyond binding site motifs. Curr. Opin. Genet. Dev. 2017, 43, 110–119. [Google Scholar] [CrossRef]
- Yang, L.; Orenstein, Y.; Jolma, A.; Yin, Y.M.; Taipale, J.; Shamir, R.; Rohs, R. Transcription factor family-specific DNA shape readout revealed by quantitative specificity models. Mol. Syst. Biol. 2017, 13, 14. [Google Scholar] [CrossRef]
- Lee, S.L.; Sadovsky, Y.; Swirnoff, A.H.; Polish, J.A.; Goda, P.; Gavrilina, G.; Milbrandt, J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 1996, 273, 1219–1221. [Google Scholar] [CrossRef]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Trembleau, A.; Gourdji, D.; Driancourt, M.A.; Rao, C.V.; Charnay, P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol 1998, 12, 107–122. [Google Scholar] [CrossRef]
- Slade, J.P.; Carter, D.A. Cyclical expression of egr-1/NGFI-A in the rat anterior pituitary: a molecular signal for ovulation? J Neuroendocrinol 2000, 12, 671–676. [Google Scholar] [CrossRef]
- Wolfe, M.W.; Call, G.B. Early growth response protein 1 binds to the luteinizing hormone-β promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Molecular Endocrinology 1999, 13, 752–763. [Google Scholar]
- Tremblay, J.J.; Drouin, J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Molecular and Cellular Biology 1999, 19, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Lamba, P.; Wang, Y.; Bernard, D.J. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol. Hum. Reprod. 2009, 15, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Tourtellotte, W.G.; Nagarajan, R.; Bartke, A.; Milbrandt, J. Functional compensation by Egr4 in Egr1-dependent luteinizing hormone regulation and Leydig cell steroidogenesis. Molecular and Cellular Biology 2000, 20, 5261–5268. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kimura, A.; Nagai, R.; Horikoshi, M. Regulation of interaction of the acetyltransferase region of p300 and the DNA-binding domain of Sp1 on and through DNA binding. Genes to Cells 2000, 5, 29–41. [Google Scholar] [CrossRef]
- He, S.; Sun, J.-M.; Li, L.; Davie, J.R. Differential intranuclear organization of transcription factors Sp1 and Sp3. Molecular Biology of the Cell 2005, 16, 4073–4083. [Google Scholar] [CrossRef]
- Li, L.; Davie, J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010, 192, 275–283. [Google Scholar] [CrossRef]
- Cai, H.; Liu, B.Y.; Wang, H.R.; Sun, G.H.; Feng, L.Z.; Chen, Z.Q.; Zhou, J.Q.; Zhang, J.W.; Zhang, T.; He, M.N.; et al. SP1 governs primordial folliculogenesis by regulating pregranulosa cell development in mice. J. Mol. Cell Biol. 2020, 12, 230–244. [Google Scholar] [CrossRef]
| Primer name | Primer sequence (5’-3’) | Product length (bp) |
|---|---|---|
| L1 | F: CTAgctagcGATAACCAACGGGCATTTA R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
3012 |
| L2 | F: CTAgctagcCTCCCTCTCCTCTCCCCTCCT CTCCTTTCTTTTACTCTCCACA R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
2512 |
| L3 | F: CTAgctagcTTTATGATAATTTTACATAATGA CATGTCCTCA R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
2012 |
| L4 | F: CTAgctagcTATACATCCATTTATTTC AGATCTGAACTAATTAATTGTCCAT R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
1512 |
| L5 | F: CTAgctagcAGGGACGGTAGGAGCCT AATCCGTTATT R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
1012 |
| L6 | F: CTAgctagcCAGGGCACAATCAGGTACT TGTGT R: CCGctcgagCTGGCAAGAGGAAGAGATAAC |
512 |
| L7 | F: CTAgctagcAGGGACGGTAGGAGCCTAAT CCGTTATT R: CCGctcgagAAATATTTCTCAAATTTAAAAT AAAATCCTACCGGAAAAATCGCT |
512 |
| Primer name | Primer sequence(5’-3’) |
|---|---|
| ETF1 | GCCTGCCTGGCGCTCCCTTCTCCCTCCCTGCCCATACATA |
| ETR1 | R: TATGTATGGGCAGGGAGGGAGAAGGGAGCG CCAGGCAGGC |
| STF2 | F: GGCCCCTCTTTTTCACCTCGCTCCCCAGCCTCGCCCGCTG |
| STR2 | R: CAGCGGGCGAGGCTGGGGAGCGAGGTGAAAAAGAGGGGCC |
| Transcription Factors | Start | Stop | Strand | Matched sequence(5’ - 3’) |
|---|---|---|---|---|
| SP1 | 142 | 151 | + | CCCCTCCCCC |
| SP1 | 219 | 228 | + | CCCCTCCCCC |
| Egr1 | 218 | 231 | + | TCCCCTCCCCCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





