1. Introduction
Brown seaweeds constitute about 40% of the global seaweed (macroalgae) production, which amounts to approximately 10 million tons annually. In Europe, around 300 000 tons are harvested with
Laminaria hyperborea,
Laminaria digitata, and
Ascophyllum nodosum as the dominating species [
1]. In addition, seaweed cultivation is a growing industry; however, it is still at an early stage in Europe, with total production (of mainly
Saccharina latissima and
Alaria esculenta) below 2000 tons annually [
1,
2]. To advance the transition to a sustainable biobased economy, brown seaweed biomass is of interest as it is abundant in coastal areas of the Atlantic Ocean and can be cultivated and produced in bulk offshore. One area of interest is to expand seaweed's use and applications in developing new European food products.
As a food resource, brown macroalgae contain a number of potential prebiotic and/or bioactive compounds [
3]. Approximately 50% of seaweed's dry weight comprises carbohydrates, including the sugar alcohol mannitol, and the polysaccharides laminarin, alginate, and fucoidan. Mannitol, a storage carbohydrate in brown seaweed, constitutes 3-20% of seaweed's dry weight [
4] with broad applications in the pharmaceutical and food industries. Mannitol is, however, poorly metabolized in the human body and, if consumed in excess, can cause diarrhea, abdominal pain, and flatulence [
5]. The dietary fiber laminarin is another storage carbohydrate of brown seaweed that comprises 1-25% of the seaweed's dry weight, dependent on the harvesting time [
4]. Laminarin is made of a β-(1,3) glucan with occasional β-(1,6) interchain links [
6,
7], which in its oligomeric or polymeric forms have potential biomedical applications [
1]. Alginate, a structural carbohydrate, is available in the seaweed's cell wall. Alginate is a linear polymer made of (1,4)-linked β-
D-mannuronic acid (M) and α-
L-guluronic acid (G) in different sequences with very well-established applications in the pharmaceutical and food industries. Fucoidan, a sulfated polysaccharide, consists of fucose substituted to different degrees with e.g. galactose, mannose, xylose, and uronic acids [
8]. Fucoidan has been reported to have bioactive properties but is not yet in industrial use.
The dietary fibers in brown seaweeds have shown potential benefits to human health in general, and to the gastrointestinal tract in particular [
9,
10]. The gut microbiota consequently plays an important role in human well-being by fermenting indigestible dietary fibers, supplying essential nutrients, synthesizing vitamin K, saving energy, absorbing ions, and improving the immune system [
11]. Human indigenous gut microbes and potentially added probiotics need nutrients to maintain optimal growth and activity. For this purpose, seaweeds are of interest because of their significant number of bioactive compounds. Seaweed dietary fibers, including laminarin, alginate, and fucoidan, have prebiotic properties [
7,
12], meaning they can be utilized by the human gastrointestinal tract and some probiotic bacteria, providing health benefits for the host.
The many reported health benefits of seaweeds and their potential for sustainable cultivation provide the reasons for the recent growing interest in seaweed for food-related applications in Europe. Seaweed biomass has been a food source in Asia for centuries, however, challenges with post-harvest treatment, food safety and consumer acceptance have obstructed the introduction of seaweed and seaweed products to new markets [
13]. One way to overcome these issues is to ferment seaweed and/or its components with lactic acid bacteria (LAB). This not only alters the sensory properties but also improves shelf life and nutritional value by producing lactic acid and short-chain fatty acids (SCFA), thereby lowering the pH of the product [
14,
15].
Another way to utilize this valuable and sustainable biomass is by modification of the seaweed's carbohydrates in the production of value-added ingredients that can be used either as individual health-promoting supplements or as food ingredients that do not alter the taste and the texture of food [
16,
17].
In this study, we evaluated the potential of a small LAB consortium consisting of three strains of Lactiplantibacillus plantarum and one Levilactobacillus brevis strain in fermenting the brown seaweed Alaria esculenta by growing the consortium on fresh frozen seaweed biomass which was either or not pretreated. Furthermore, laminari-oligosaccharides were produced from laminarin as value-added products, and their potential prebiotic properties was evaluated by fermentation implementing the LAB consortium.
2. Materials and Methods
2.1. Materials
The seaweed species Alaria esculenta (L.) Grev. biomass was obtained fresh frozen from Frøya farm (Seaweed Solutions AB, Trondheim, Norway). The LAB consortium, isolated from food, originates from the collections held by ImmuneBiotech AB (Lund, Sweden). All materials were purchased from sigma Aldrich (Merck). Laminari-oligosaccharides from L. digitata were purchased from Megazyme (Neogen).
2.2. Genomic identification of the consortium species and metagenome analysis of the seaweed biomass
The Genomic DNA from the consortium was extracted by GeneJET Genomic Purification Kit (ThermoScientific) and diluted with nuclease-free water before adding it to the PCR mixture. Gene 16S rRNA was amplified in a total volume of 20 µL. The PCR mixture was prepared by mixing 10 µL of IProof HF master mix (2 x) (BioRad), 0.5 µM of universal primer pair 16S-fD1 and 16S-rP2 (Integrated DNA Technologies), 3% (v/v) DMSO and the extracted genomic DNA. The PCR was conducted as follows: initial denaturation at 95 ℃, 3 min; 34 cycles of 95 ℃ denaturation, 30 sec; annealing at 55 ℃, 30 sec; and extension at 72 ℃, 1 min; with a final extension at 72 ℃ for 10 min. The product was purified using GeneJET PCR Purification Kit (ThermoScientific). Obtained PCR product was analysed using agarose electrophoresis and subsequently purified using GeneJET PCR Purification Kit (Thermo Fisher Scientific). The purified PCR product was sequenced by Eurofins Genomics (Solna, Sweden). The resulting sequences were analyzed by blastn integrated into NCBI BLAST suite (blast.ncbi.nlm.nih.gov) against 16S ribosomal RNA sequences (Bacteria and Archaea) database.
For metagenome analysis of the LAB consortium, the LAB culture was grown on de Man, Rogosa, and Sharpe (MRS) broth agar plate under anaerobic condition at 37 °C overnight in order to obtain cells sample. Thereafter, one single colony was inoculated in a liquid MRS medium. Two-milliliter of the consortium culture was harvested by centrifugation at 1700×g for 3 min. The pellet was aseptically washed twice with sterile DNA suspension buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA) and the cells were collected by centrifugation at 1700×g for 3 min. The collected cells were suspended in DNA suspension buffer and then were delivered frozen for metagenome sequencing by CosmosID (Germantown, USA).
The metagenomic analysis of the dried seaweed biomass (for fermented samples with seaweed’s endogenous microbiota) was analyzed using NovaSeq 6000 (Illumina) technology for metagenome sequencing by Eurofins company (Ebersberg, Germany).
2.3. Cultivation of the LAB consortium with different carbohydrates available in brown seaweed
2.3.1. Fermentation on the carbohydrate available in brown seaweed
MRS broth base was prepared by mixing peptone from casein, tryptic digest 10 g/L, meat extract 10 g/L, yeast extract 5 g/L, Tween 80 1 g/L, K2HPO4 2 g/L, Na-acetate 5 g/L, (NH4)3 citrate 2 g/L, MgSO4 x 7 H2O, 0.2 g/L, and MnSO4 x H2O 0.05 g/L. The prepared MRS-base was filled into serum bottles, boiled, and purged with nitrogen gas. The bottles were sealed and autoclaved for 20 min at 1 atm. The carbohydrate sources were prepared and autoclaved separately. Before the cultivation, an amount of the carbohydrate substrate was added to the bottles to reach the desired concentration. Media was 1% inoculated and the bottles were incubated at 37 °C. Optical density at 600 nm (OD600) was measured every 2 h up to 12 h and then after 24 and 48 h.
2.3.2. Seaweed fermentation
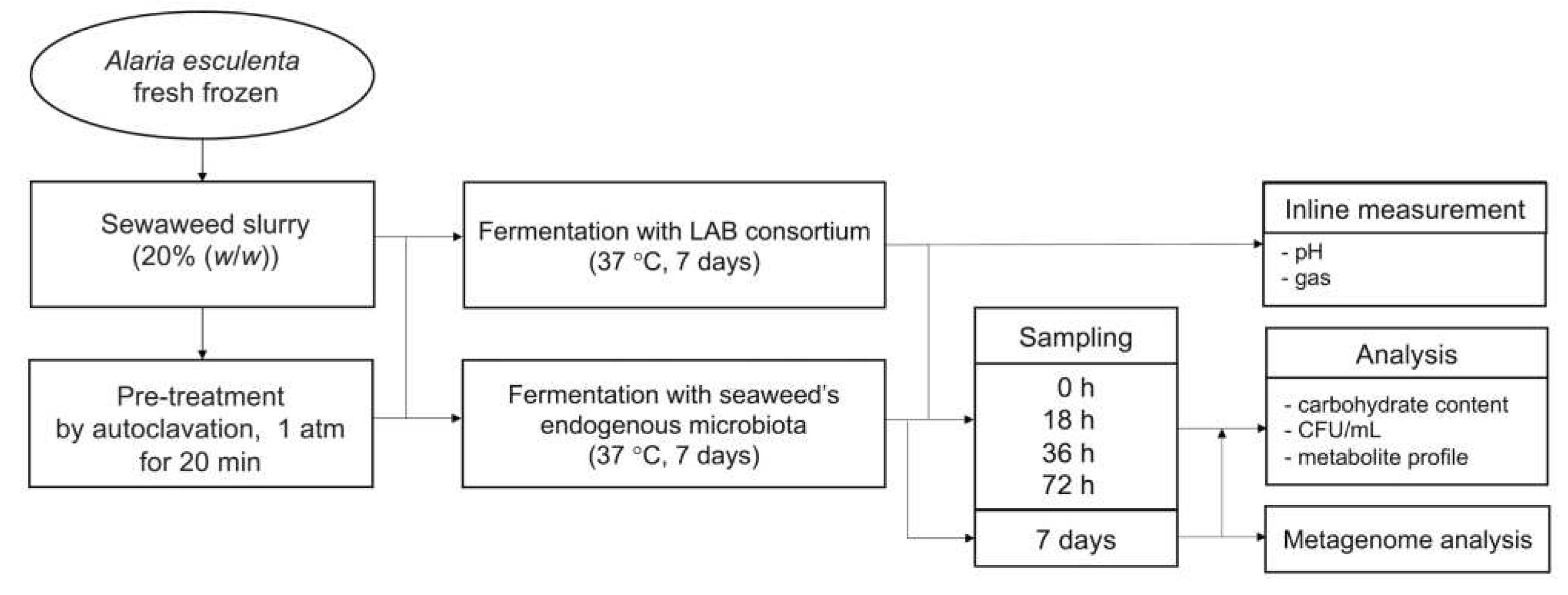
Fresh frozen seaweed biomass was minced by a household mincer while kept ice-cold. Subsequently 20% (wet biomass (g)/ slurry (g)), corresponding to 2% (dried biomass (g)/ slurry (g)), seaweed slurry was prepared by mixing the ice-cold seaweed with distilled water in a 500 mL glass reactor. The seaweed slurry was subjected to fermentation directly or underwent autoclavation at 1 atm for 20 min prior to the fermentation.
The fermentation inoculum was prepared by pre-culturing the LAB consortium from glycerol stock culture in an MRS broth overnight. The bacteria were reinoculated in MRS broth, cultivated and harvested at OD
600 of 2.0 by centrifugation at 3000×g for 20 min at 4 °C. The supernatant was discarded, and the collected cells were washed twice with 0.9%
(w/v) saline solution to completely remove the medium. An amount of cell culture corresponding to 1×10
9 CFU were added to the reactor to reach the concentration around 2×10
6 CFU/g slurry. The fermentation with seaweed’s endogenous microbiota was performed by fermenting seaweed slurry with and without pre-treatment (with or without autoclaving) without added inoculum. All reactors were incubated at 37 °C in a water bath while connected to a gas endeavor system equipped with pH electrodes (Bioprocess Control). The pH and gas production were monitored for 7 days. Duplicate experiments were performed for each cultivation condition. Samples were taken every 18 h to monitor the growth in the seaweed slurry by counting CFU (
Figure 1).
To analyze carbohydrate consumption and organic acids production, the samples were centrifuged at 3000×g for 10 min at 4 °C. The supernatant was collected, diluted with ultrapure water (Milli-Q grade), filtered through PTFE membrane 0.2 μm pore size filters (Pall) and stored at 4 °C for further analysis.
2.4. Laminari-oligosaccharide production
Laminarin was extracted from
L. hyperborea, and laminari-oligosaccharides were produced by enzymatic hydrolysis, using a recombinant in house produced novel glycoside hydrolase (
MlLam17B) classified under glycoside hydrolase family 17, and originating from
Muricauda lutaonensis [
18].
2.4.1. Laminarin extraction
Laminarin was extracted from
L. hyperborea, by a two-step water extraction [
19]. Seaweed slurry (100 g/L) in water was incubated at 30 °C for 2 h followed by 2 h incubation at 70 °C with 160 rpm shaking. The soluble fraction was separated with two-steps centrifugation at 5000×g for 20 min. To precipitate alginate, an equal volume of CaCl
2 (1% (
w/v)) was added to the soluble fraction, and the mixture was incubated at 4 °C overnight. subsequently, alginate was separated by centrifugation at 5000×g for 20 min. The laminarin extract was then separated on a 10 kDa filter using a tangential flow filtration (TFF) to remove high molecular weight carbohydrates and then dialyzed through a 1 kDa filter. Extracted laminarin was lyophilized and stored at 4 °C before further use.
2.4.2. Expression and purification of laminarinase MlLam17B
The recombinant
MlLam17B [
18] was produced as a fusion protein in a modified L-rhamnose inducible dual-tag pJOE vector [
20] in
Escherichia coli NiCo21 (DE3) fused with an N-terminal maltose binding (MBP) domain separated by a
Saccharomyces cerevisiae ubiquitin-like protein motif (Smt3) and C-terminal hexahistidine affinity tag. Recombinant protein was purified from clear cell lysate by maltose affinity chromatography and subsequently by nickel affinity chromatography, after proteolytic cleavage of the MBP domain with ubiquitin-like-specific protease 1 (Ulp1). The integrity and purity of the protein were analyzed by 4–15% glycine-SDS-PAGE. The protein concentration was estimated by measuring A
280 using a NanoDrop 1000 spectrophotometer (Thermo Scientific).
2.4.3. Enzymatic hydrolysis of laminarin
A solution of 20 g/L laminarin was prepared in 50 mM acetate buffer pH 4.5. The mixture was boiled for 30 min while mixing with magnetic stirrer to enhance the solubilization of laminarin. The mixture was cooled down and subsequently centrifuged. The supernatant was subjected to enzymatic hydrolysis with 10 mg of MlLam17B enzyme, performing reaction overnight at 40 °C and 160 rpm. The reaction was terminated by autoclavation at 1 atm for 20 min. Obtained hydrolysate was used for further analysis and fermentation experiments.
2.5. Carbohydrate quantification
Total carbohydrate content of dried
Alaria esculenta was measured using a two-step sulfuric acid hydrolysis method described by Van Wychen and Laurens [
21]. Initially, 2.50 mL of 72% (
w/w) sulfuric acid was added to 0.25 g of seaweed powder and incubated at 30 °C while shaking at 300 rpm for 1 hour. Subsequently, the acid was diluted with distilled water to 4% (
w/w) and the samples were autoclaved at 121 °C for 1 hour. The resulting hydrolysate was then centrifuged at 4000×g for 10 minutes after being cooled to room temperature. The supernatant was then neutralized with 0.1 M Ba(OH)
2.H
2O (0.1 M), and the neutral supernatant was collected after centrifugation at 4000×g for 10 minutes. The hydrolysate was further diluted and filtered through a PTFE membrane 0.2 μm pore size filters (Pall) before analysis of monosaccharides.
Mono- and oligosaccharides were quantified using a high-performance anion exchange chromatography Dionex ICS-5000 (Thermo Fisher Scientific) system equipped with pulsed amperometric detector (HPAEC-PAD). The system was equipped with a Dionex CarboPac PA-20 column coupled with a guard column (Thermo Fisher Scientific) for monosugar analysis and a Dionex CarboPac PA200 column coupled with a guard column (Thermo Fisher Scientific) for oligosaccharide analysis. Eluents were prepared as (A) ultrapure water (Milli-Q grade), (B) 0.2 mM NaOH for PA-20 column or 1 M Na-acetate in 200 mM NaOH for PA-20 column for uonic acid analysis or for PA-200 column for oligosaccharides analysis, and (C) 200 mM NaOH. Monosaccharides were separated by isocratic elution with an eluent mixture of (A) 62.5% and (B) 37.5% for 25 minutes. Afterwards, the column was regenerated using (C) 50% for 5 minutes. Uronic acids were eluted by an eluent mixture of (A) 55%, (B) 15% and (C) 30% for 15 minutes. Oligosaccharides were eluted by a gradient mixture of (B) from 0 to 50% in eluent (C), while eluent (A) was set to 50% for 20 min. Following this the column was regenerated with an eluent mixture of (A) 50% and (B) 50% for 10 minutes. All analysis were performed applying the flow rate at 0.5 mL/min, and the temperature of the column compartment was at 30 °C.
2.6. Metabolite quantification
A high-performance liquid chromatography (HPLC) Dionex UltiMate 3000 RSLC (Thermo Fisher Scientific) system, equipped with an Aminex HPX-87H column (Bio-Rad) was used to quantify short chain fatty acids (SCFA), lactic acid, and ethanol. The cultivation samples were centrifuged at 3000×g for 10 min at RT. One milliliter of the supernatant was mixed with 20 µL of 20% (v/v) sulfuric acid and incubated for 15 min at 4 °C. The samples were filtered through PTFE membrane 0.2 μm pore size filters (Pall) and stored at 4 °C. SCFA were separated using 5 mM sulfuric acid at the flow rate of 0.5 mL/min, and the column compartment was kept at 40 °C. To detect and quantify ethanol, 5 mM sulfuric acid was used as the eluent, and the temperature of the column compartment decreased to 30 °C.
3. Results
3.1. Selection and identification of the bacterial species in the LAB consortium
The LAB consortium used, was chosen based on a prescreening of isolates from food sources for the ability to utilize mannitol as this, if consumed in excess, can cause diarrhea, abdominal pain, and flatulence [
5]. Hence, mannitol was in a first screen used as sole carbohydrate source in MRS-medium (data not shown). The selected mannitol utilizing isolate, was subjected to DNA isolation, 16S rRNA gene sequencing as well as metagenome sequencing. The 16S rRNA sequencing was made after PCR amplification and resulted in a single sequence with 99.93% sequence identity at 100% sequence coverage with a 16S rRNA gene (RefSeq NR_115605.1) from
Lactiplantibacillus plantarum JCM 1149, isolated from pickled cabbage [
22,
23].
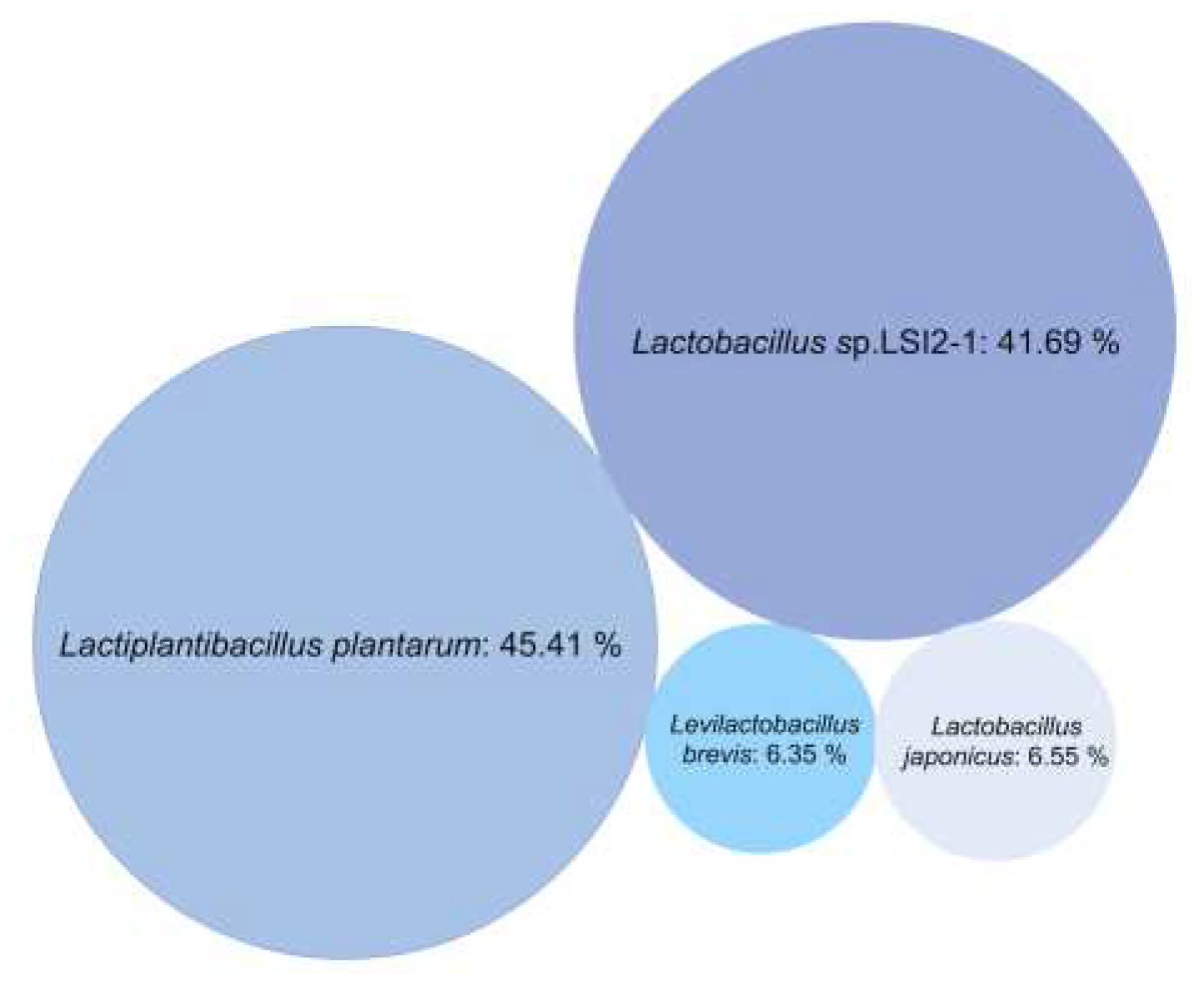
Metagenome analysis, implementing
de novo genome assembly, revealed presence of a microbial consortium composed of three related
L. plantarum strains, and one
L. brevis strain. The
L. plantarum strains dominated the consortium (93.65% abundance) with a relative abundance distribution of 45.41% (closest related deposited strain
L. plantarum subsp
plantarum [
24]), 41.69% (closest related candidate
Lactobacillus sp. LSI2-1 [
25]) and 6.55% (closest related deposited candidate
L. japonicus [
26]), respectively. Moreover,
Lactobacillus sp. LSI2-1, is previously reported to be taxonomically close to
Lactiplantibacillus plantarum subsp
. plantarum ATCC 14917(also deposited as JCM1149) with 100% 16S rRNA sequence similarity at 100% sequence coverage [
25], and
L. japonicus is reclassified as
L. plantarum subsp.
plantarum [
26], explaining the uniform data from 16S rRNA-gene sequencing. The relative abundance of the strain related to
L. brevis [
24] was minor, estimated to 6.35%,(
Figure 2).
3.2. Growth on monosaccharides as sole carbohydrate source in MRS, and relation to brown seaweed’s polysaccharides
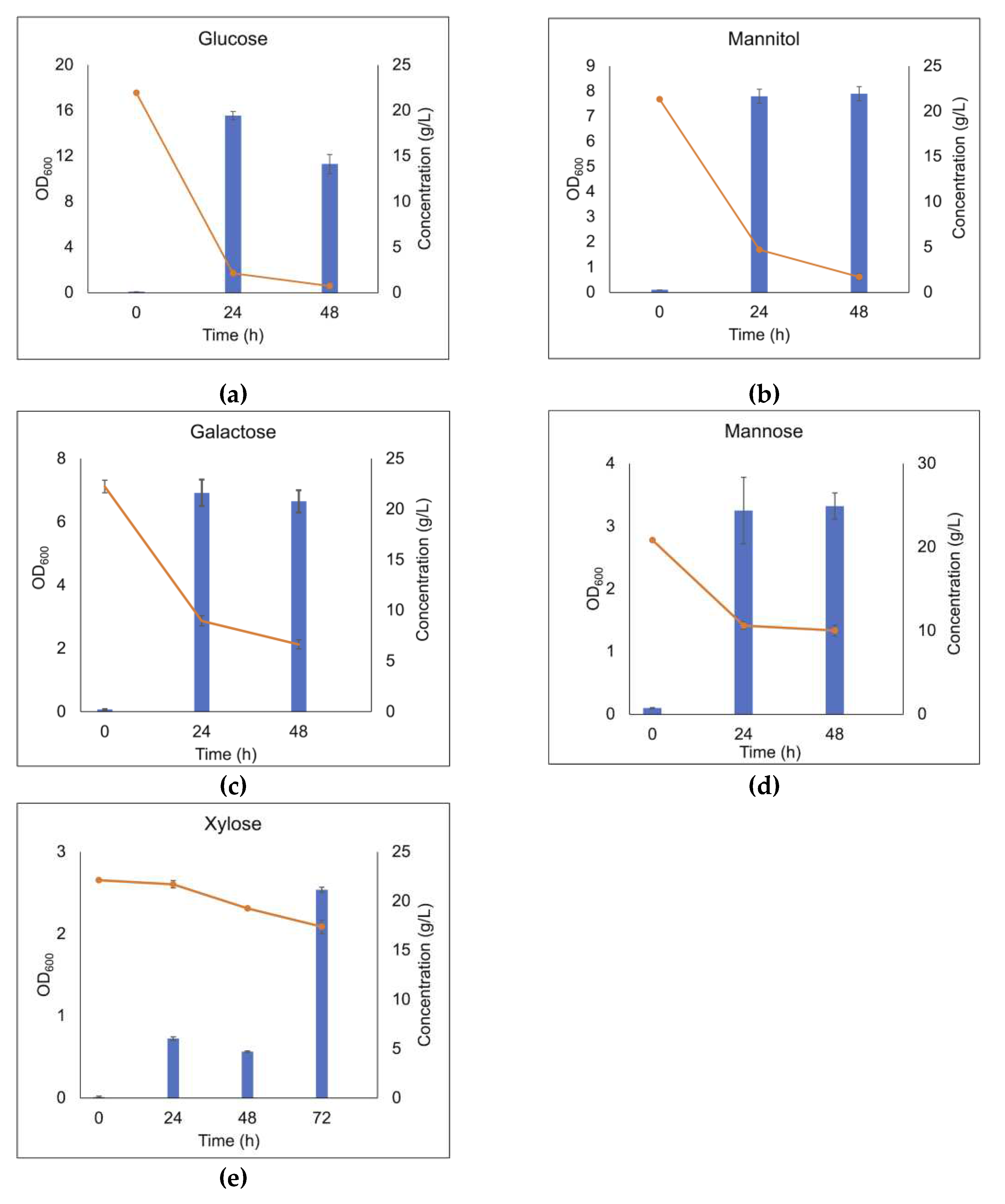
The selected consortium was subjected to growth screening in liquid MRS broth, to verify the consortium's ability to grow on monosaccharides that can be found as building blocks in brown seaweed polysaccharides. The growth was estimated by measuring optical density at 600 nm (OD
600) and carbohydrate consumption. As shown in
Figure 3, the highest cell density was observed when the LAB consortium was grown in MRS supplemented with 20 g/L glucose (the monosugar in the polymer laminarin), reaching a final OD
600 of 15.6 after 24 h (
Figure 3A)
. Somewhat lower cell densities, with a maximum OD
600 of 7.9 was observed in the presence of mannitol (
Figure 3B) which is a free sugar alcohol in the brown seaweeds, as well as the capping sugar-alcohol in a fraction of the laminarin polymers. Moreover, galactose (a monomer component in fucoidan), resulted in an OD
600 of 6.95, in the same range (
Figure 3C). Use of other monosaccharides as carbohydrate source, resulted in lower OD
600 values at the corresponding concentration of the carbohydrate source. For example, mannose resulted in an OD
600 of 3.2 (
Figure 3D), while MRS supplemented with xylose (a minor component in fucoidan) displayed a long lag phase prior to growth reaching an OD
600 of 2.5 (
Figure 3E) after 72 h cultivation. The use of xylose as substrate, may also be a consequence of growth of the
L. brevis strain, present in minor relative abundance in the consortium, as this species has previously been shown to efficiently utilize xylose [
27]. The LAB consortium was not able to utilize fucose (from fucoidan), mannuronic acids or guluronic acids (the building blocks of alginate) (data not shown). In conclusion, glucose and mannitol were almost completely depleted in the cultivations, while incomplete utilization was observed for the other carbohydrates.
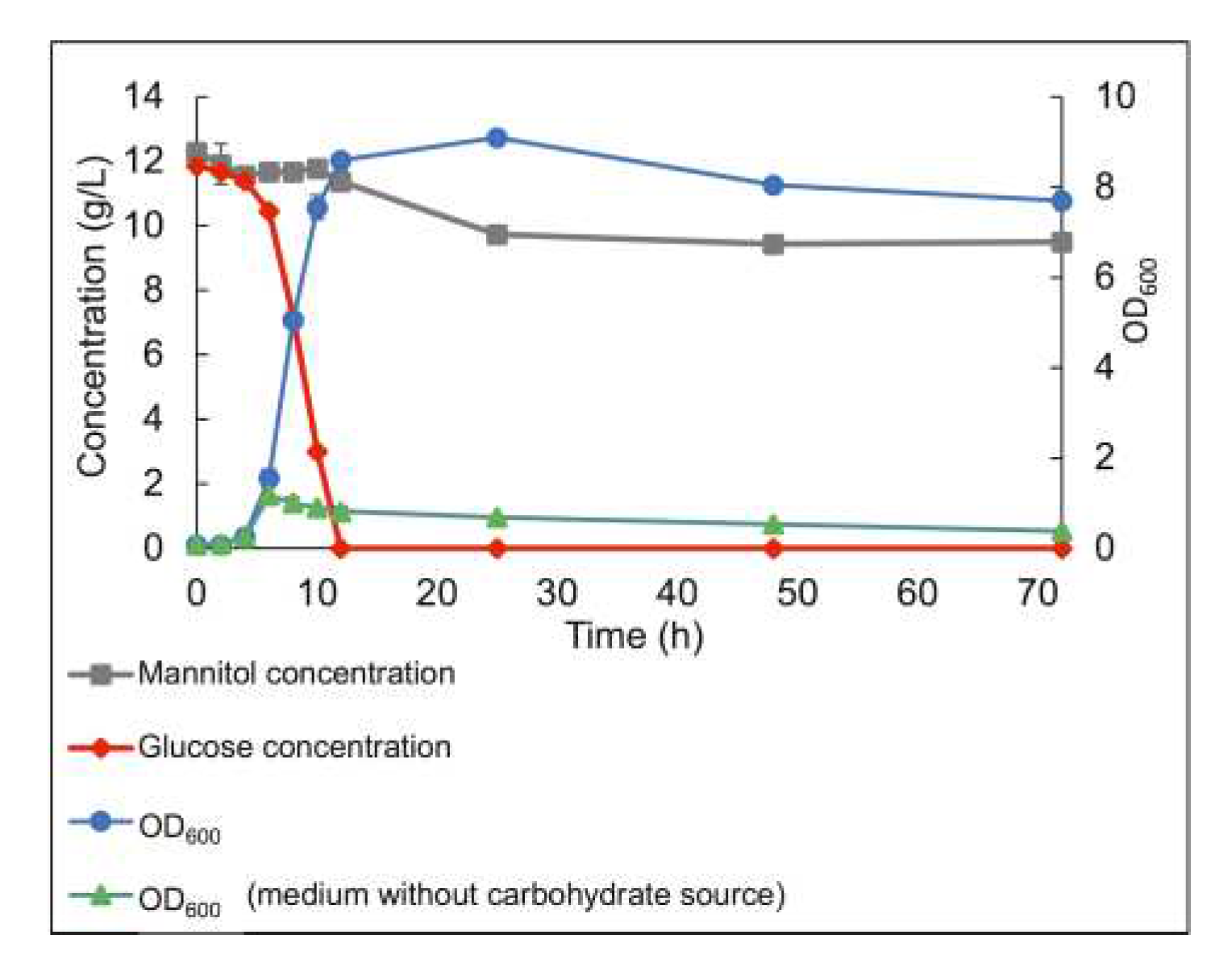
In co-cultivations, with both glucose and mannitol in the medium, the consortium preferably utilized glucose, showing glucose catabolite repression, reaching an OD
600 of 8.60 with an initial glucose concentration of 12 g/L (
Figure 4). Only a small amount of mannitol (0.9 g/L) was used before reaching the stationary phase, and in total, 2.7 g/L of mannitol was consumed over 72 h cultivation. The specific growth rates in the presence of individual substrates (glucose and mannitol) were calculated as 0.66 h
-1 in the presence of glucose and 0.56 h
-1 in the presence of mannitol. However, the specific growth rate throughout the co-fermentation of glucose and mannitol was obtained at 0.65 h
-1, similar to the specific growth rate in the presence of only glucose.
3.3. Growth of the LAB consortium on polysaccharides or oligosaccharides
Utilization of the brown seaweed polysaccharides, alginate, laminarin and fucoidan, were tested, separately, as carbohydrate sources in MRS but the bacteria did not show any growth on any of the polymeric substrates (data not shown).
Hence, the ability of the LAB consortium to grow on a selection of oligosaccharides was investigated with focus on glucose containing oligosaccharides connected by different linkages and of different degree of polymerization (DP).
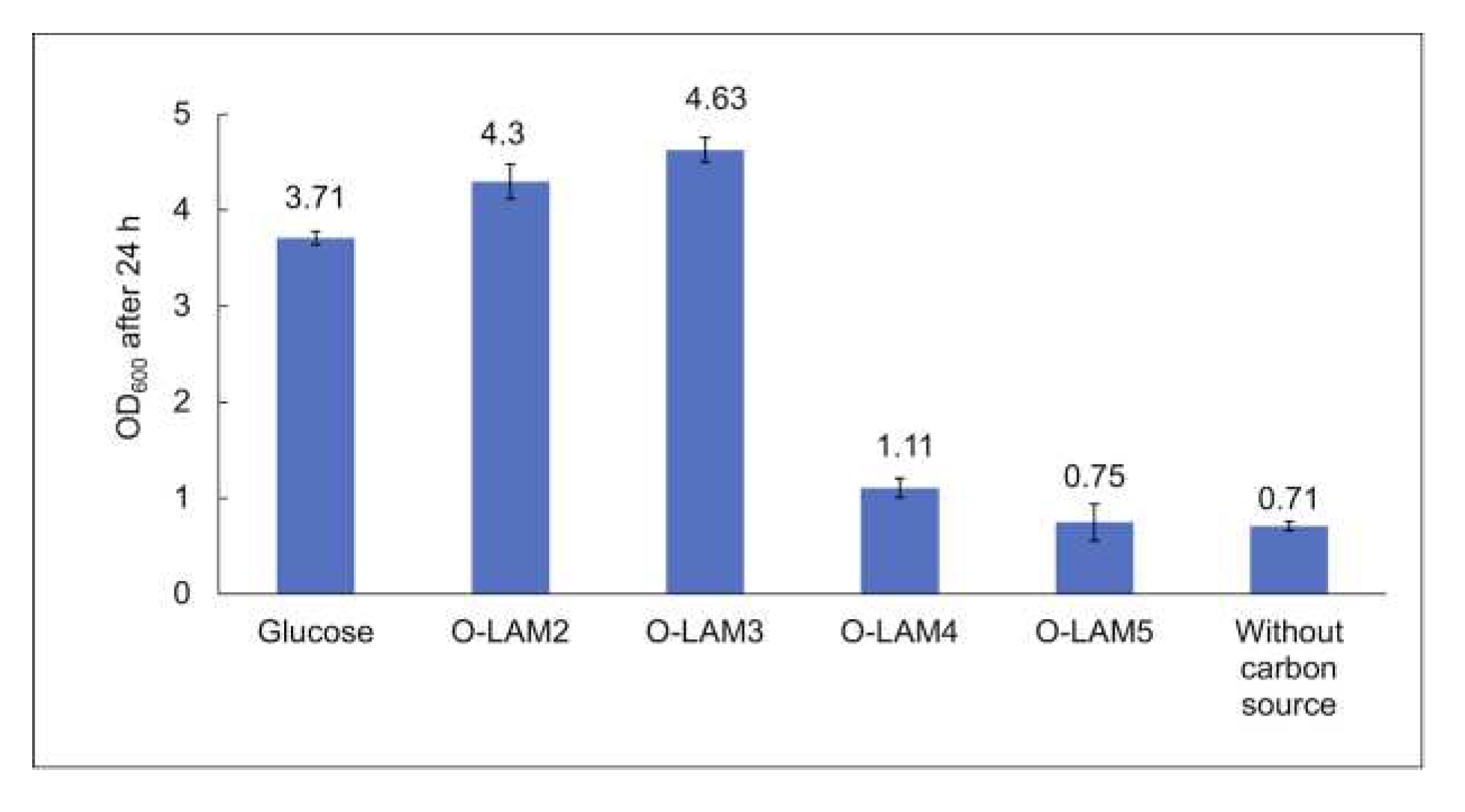
The growth of the LAB consortium on beta-glucan oligosaccharides was tested in MRS broth base individually supplemented with the laminari-oligosaccharides of DP 2-7 (O-LAM2-7). The cell density was monitored after 24 h anaerobic cultivation in the presence of 5 g/L substrate at 37 °C. As illustrated in
Figure 5, in the presence of glucose, the OD
600 increased to 3.7 while slightly higher ODs were reached in the presence of O-LAM2 (OD
600 of 4.3) and O-LAM3 (OD
600 of 4.6), respectively. The pH dropped to almost 4.4 during the cultivation with these substrates. A slight increase in OD
600 was also observed in the presence of O-LAM4 (OD
600 of 1.1) with a pH drop to 5.9. Negligible growth was observed in the presence of O-LAM5 with no pH drop (pH remining at 6.5, which was the initial pH of the medium), and no growth was observed in the presence of O-LAM6, and O-LAM7, indicating a DP4 polymerisation limit for laminari-oligosaccharide utilization of the consortium.
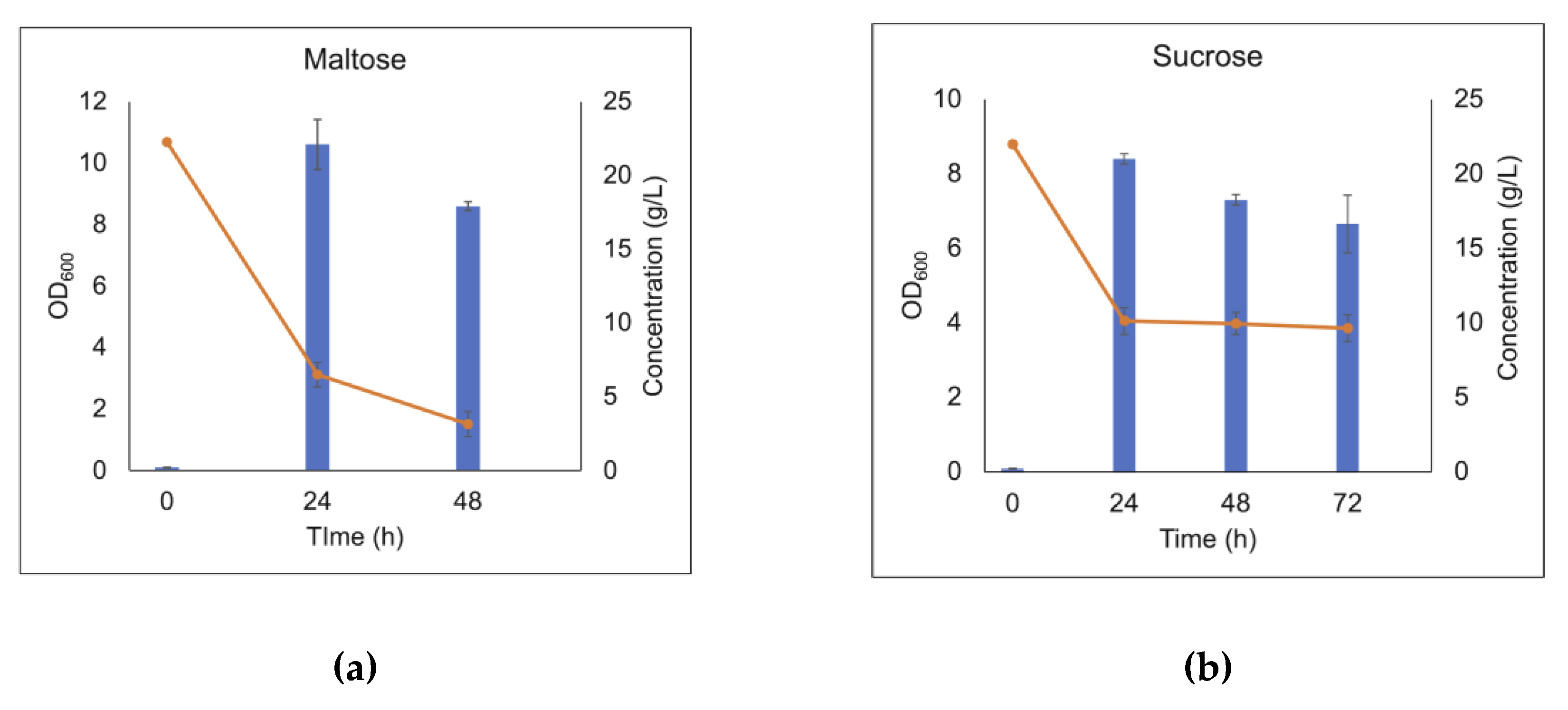
The laminari-oligosaccharides are connected by β-1,3-linkages. To evaluate if the linkage type is of importance, two other glucose containing disaccharides were also tried as carbohydrate sources, in particular maltose (a disaccharide with glucose building blocks connected by an α-1,4 linkage) and sucrose (a disaccharide composed of α-D-glucopyranosyl-(1,2)-β-D-fructo-furanoside). The LAB consortium consumed both maltose and sucrose, reaching an optical density of OD
600 10.61 and 8.4, respectively, after 24 h of cultivation in MRS broth base supplemented with 20 g/L of the respective carbohydrate substrate, showing that disaccharides connected by various linkages could be consumed (
Figure 6). In addition, growth of the LAB consortium on xylo-oligosaccharides was investigated, however, these substrates were not consumed by the consortium within the time frame of the experiments. (data not shown here).
3.4. Co-fermentation of laminari-oligosaccharides by the LAB consortium
3.4.1. Laminari-oligosaccharides production from Laminaria hyperborea extract
A mixture of oligosaccharides was prepared by enzymatic hydrolysis of laminarin extracted from
Laminaria hyperborea using the in house produced laminarinase
MlLam17B (Figure S 2). HPAEC-PAD analysis of the hydrolysate demonstrated that it contained a mixture of laminari-oligosaccharides, glucose, and mannitol (
Table 1). The mannitol in the hydrolysate is likely originating from laminarin chains that contain a terminal mannitol moiety at the reducing end [
1], so called M-chain laminarins.
3.4.2. Co-fermentation of laminari-oligosaccharides
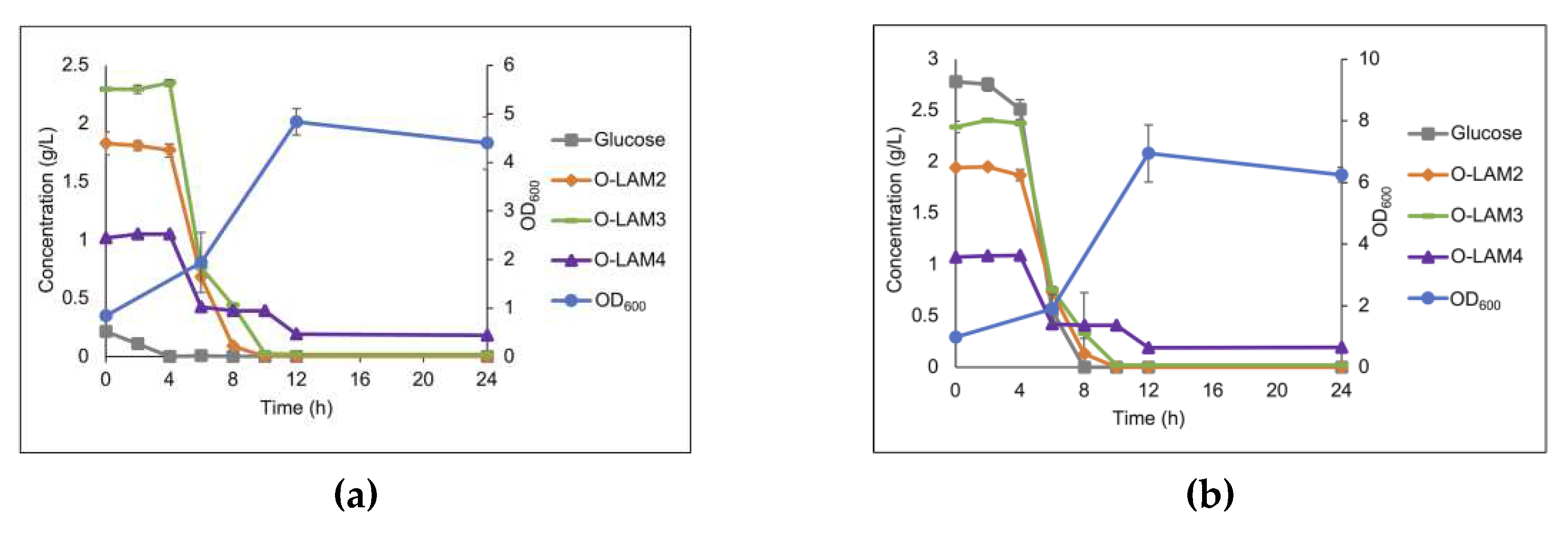
Co-fermentation of the laminari-oligosaccharides present in a hydrolysate by the LAB consortium, in the MRS broth medium, is shown in
Figure 7A. The low amount of glucose present in the hydrolysate was consumed before any decrease in concentration of the oligosaccharides could be observed. Thus, to evaluate if glucose repression was occurring, the hydrolysate was supplemented with additional glucose to a final concentration of 2.5 g/L (
Figure 7B). The supplementation of glucose clearly showed that glucose and the laminari-oligosaccharides were simultaneously utilized during the exponential growth phase, indicative of separate transporter systems. Hence it can be concluded that presence of glucose did not repress utilization (or uptake) of the oligosaccharides in the hydrolysate.
3.5. Direct fermentation of fresh frozen A. esculenta
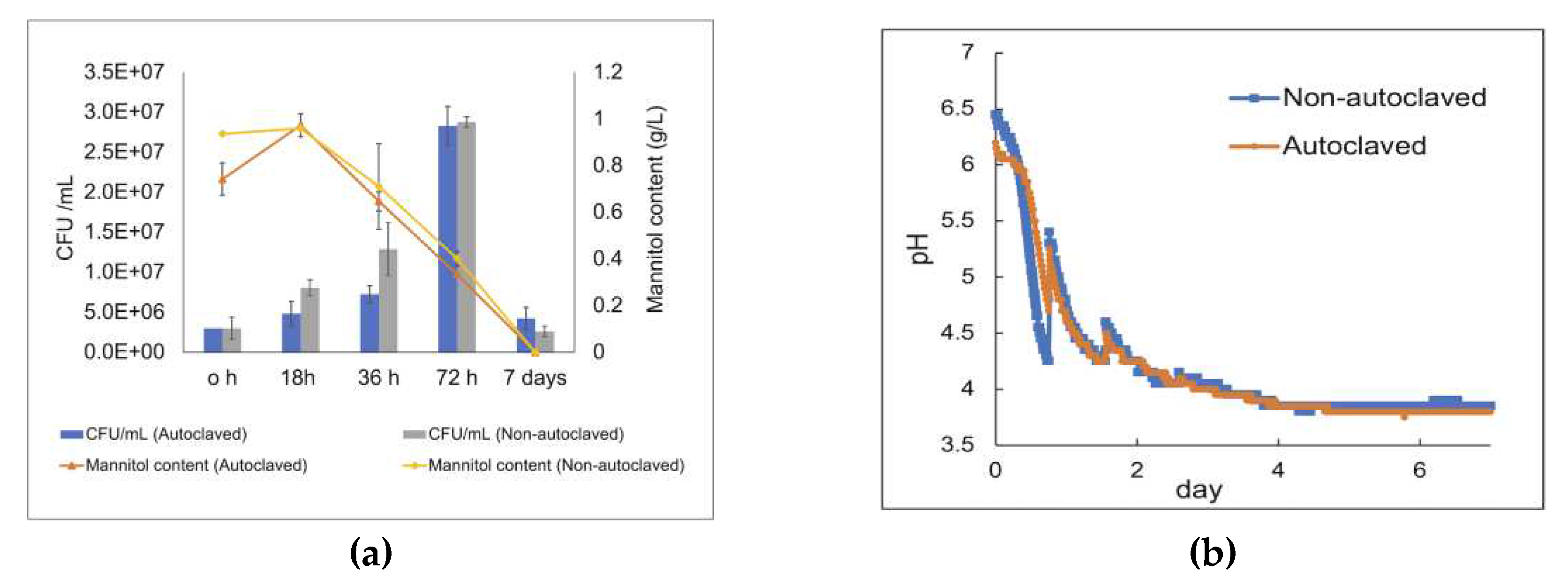
The possibility of direct fermentation of fresh frozen, minced brown seaweed of the species A. esculenta was evaluated, as a potential preservation method of the seaweed. A 20% (wet biomass (w)/slurry (w)), corresponding 2% (dry biomass (w) /slurry(w)), seaweed slurry was prepared by mixing minced fresh frozen seaweed with distilled water in 500 mL reactor bottles, followed by autoclaving to provide sterile condition prior to inoculation with the LAB consortium. In parallel, a corresponding suspension was made without autoclaving to monitor the ability of the LAB consortium to regulate growth of contaminating and/or endogenous bacteria.
To estimate the number of colonies in the starting culture, the relation between optical density and CFU/mL was calculated as CFU/mL= 3×108 OD600 – 1×108 (R2 = 0.9493). An inoculum containing 3×106 cells/g of slurry was added to the reactors prepared for fermentation with the LAB consortium; duplicate reactors were prepared for fermentation of sterilized and non-sterilized seaweed biomass. The inoculum was not added to the reactors (one with sterilized and one with non-sterilized seaweed biomass) prepared to evaluate any possible fermentation by endogenous microbiota.
Carbohydrate quantification of the dried
Alaria esculenta seaweed, which underwent a two-step acid hydrolysis process, revealed that it comprises approximately 29% carbohydrates, consisting of around 12% neutral sugars (with 6% of it being mannitol) and approximately 17% uronic acids that are the primary constituents of alginate (
Table S1). The analysis of carbohydrates in the soluble fraction of the seaweed slurries, including both autoclaved and non-autoclaved reactors, indicated that only mannitol was released into the liquid suspensions. The slight increase in the concentration of mannitol at the beginning is judged to be due to solubilization of mannitol by the heat and pressure applied [
28]. In both inoculated conditions, the CFU increased up to approximately 3x 10
7 CFU/mL after 72 h of fermentation, but the CFUs decreased significantly after 7 days, after depletion of the mannitol that served as carbohydrate source.
The growth behavior after inoculation was very similar in the autoclaved and non-sterilized seaweed slurry reactor, respectively (
Figure 8). The initial pH in the autoclaved reactor was approximately pH 6.2 (
Figure 8b), while the corresponding initial pH in the non-autoclaved reactor was 6.5. This pH difference at the beginning of the fermentation is likely due to release of carbohydrates and minerals into the seaweed suspension during autoclaving. In inoculated reactors, after approximately 3 h lag phase, the pH started to decrease sharply (indicating acid production) reaching pH 4.3 in non-autoclaved reactors and pH 4.7 in autoclaved reactors within 18 h, followed by a gradual decrease until the end of the fermentation, reaching a final pH below pH 4 (pH 3.8 in autoclaved and pH 3.9 in non-autoclaved reactors). Gas production was not observed during the 7-day fermentation of both sterilized and non-sterilized seaweed slurry.
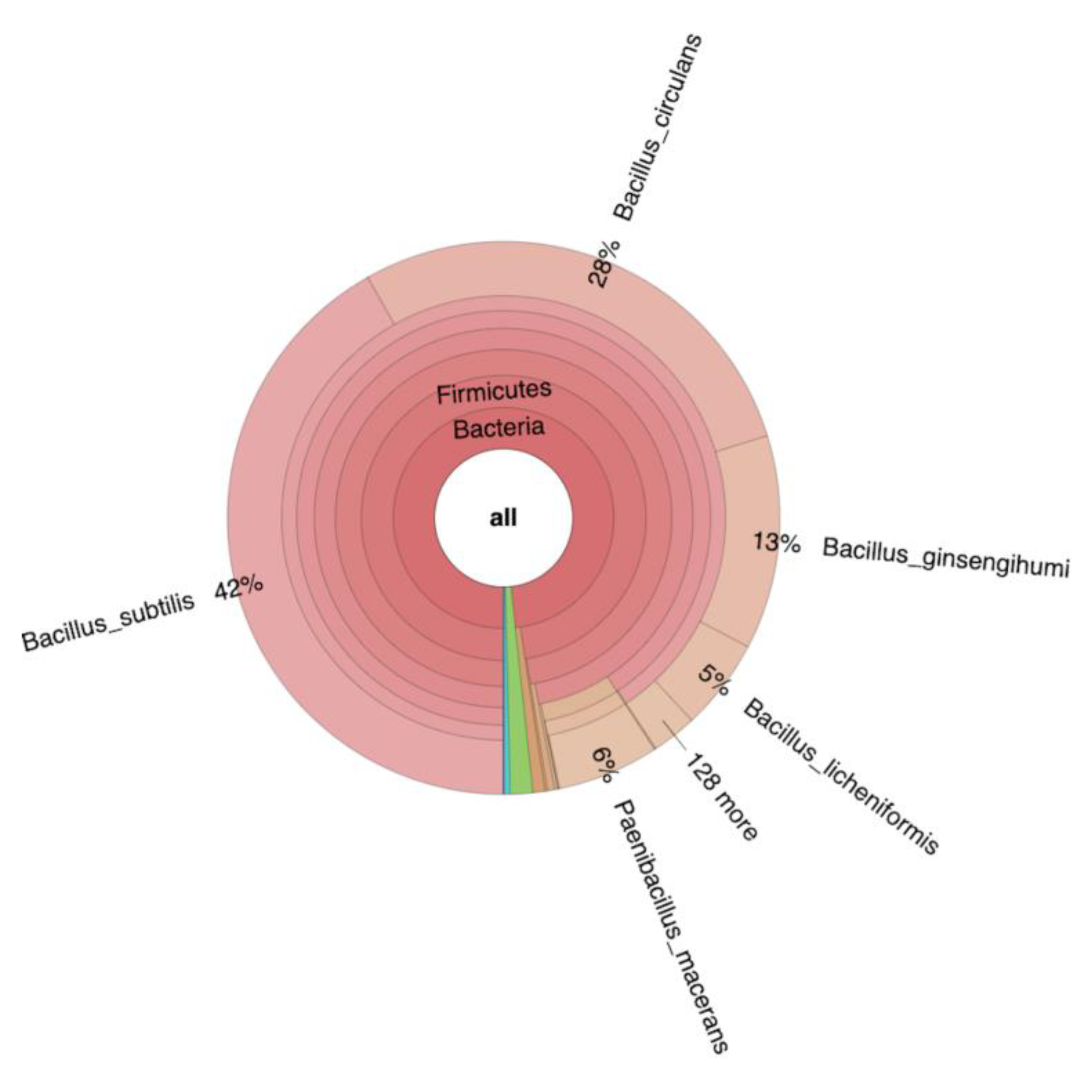
In the non-inoculated reactor with autoclaved seaweed slurry, run to monitor growth of endogenous microbiota, only a slight drop in pH to pH 5.7 was observed while mannitol was completely consumed. In this case, the seaweed slurry became slimy, foamed and an unpleasant smell was noticed, after opening the reactor. Metagenome analysis of the corresponding seaweed sample revealed that the seaweed contained bacteria (relative abundance 96%), fungi (relative abundance 2.8%), and minor amounts of archaea, viruses and eukaryota (relative abundance 1.2%). A mixture of Gram-positive spore forming bacteria strains (93.76% from the genus
Bacillus and 6.23% from the genus
Paenibacillus), were present in the seaweed slurry (
Figure 9) that could not be eliminated by autoclaving, and long incubation at 37 °C of autoclaved seaweed slurry also resulted in their growth and hence spoilage of the seaweed. Similar spoilage was also observed for non-autoclaved seaweed slurry without LAB inoculation. However, metagenome analysis was unsuccessful since it was not possible to generate a library that qualitatively and quantitatively met the requirements for successful sequencing.
3.6. Analysis of metabolite profiles
Lactic acid was the main metabolite produced by the LAB consortium when cultivated in MRS medium with all variants of the carbohydrate sources (monosaccharides and oligosaccharides), as well during seaweed fermentation (
Table 2). Maximum lactate production (21.91 ± 0.32 g/L) was observed in cultivations run in the presence of approximately 22 g/L glucose, after 48 h anaerobic cultivation in MRS (
Figure 10a and
Table 2).
In MRS containing approximately 21.3 g/L mannitol more metabolites were observed. Lactate production was observed from an early stage of the growth and continued throughout the cultivation, reaching a maximum of 21.38 ± 0.12 g/L. However, when the stationary phase was entered, acetate was consumed and ethanol production was observed (
Figure 10b), which was not the case using glucose as sole carbon source. A small peak corresponding to succinate was also observed after 8 h cultivation but was not quantifiable. In the presence of xylose, acetate was also produced with a final concentration of 1.23 ± 0.04 g/L, over 72 h of cultivation.
During co-fermentation of glucose and mannitol in MRS, lactate was the only metabolite detected. In this case, due to the glucose repression, mannitol consumption might be too low for other metabolites (succinate and ethanol) to be produced by the LAB consortium in detectable amounts. The same could be generalized for all substrates that contained mannitol because in most of them such as laminari-oligosaccharides hydrolysate and seaweed slurry the amount of mannitol was very low for the LAB consortium to produce other metabolite in detectable amounts.
The data presented in
Table 2 shows that the production yield of lactic acid (Lactic acid/substrate (
w/w)) is nearly 100% for glucose, mannitol, maltose, and laminari-oligosaccharides. Lower yield was observed when galactose, sucrose, mannose, and a mixture of glucose and mannitol were used as the substrate. The lactate production yield was approximately 86% in the presence of galactose, nearly 50 % in the presence of mannose, about 73% in the presence of sucrose and almost 60% when a mixture of glucose and mannitol was used as the substrate. Lowest yield of approximately 16% was observed in the presence of xylose substrate.
4. Discussion
This study aimed to evaluate the potential of the LAB consortium in fermenting the brown seaweed species A. esculenta as a promising preservation technique for seaweed in new food markets. Production of laminari-oligosaccharides from the brown algae polysaccharide laminarin as value-added products was also performed to evaluate their potential prebiotic properties by fermentation using the LAB consortium.
The genomic analysis of the consortium revealed that the consortium consisted of four strains,
L. plantarum (closely related to
L.plantarum strain
(ATCC 14917 and JCM 1149)),
L. sp. LSI2-1,
L. japonicus, and a minor relative amount of
L. brevis. The strain
Lactobacillus sp. SLI2-1 is closely related to
L. plantarum subsp
. plantarum (ATCC 14917) which according to previous characterization is able to utilize glucose, mannitol and xylose. Moreover, the strain did not show any gas production in the presence of glucose [
22], in accordance with our data.
L. japonicus is closely related to
L. plantarum subsp.
plantarum; this bacterium is a DL-forming mesophilic LAB of plant origin with the ability to ferment pentoses [
26]. Xylose fermentation is also a feature of
L. brevis strains [
27], as was observed xylose was slowly fermented in this study.
This study demonstrates that the investigated LAB consortium can consume most monosugars which are the building blocks of brown seaweed’s polysaccharides including glucose, mannitol, galactose, xylose, and mannose, except for fucose, which is the building blocks of fucoidan.
Glucose and mannitol were the most favorable substrates for the consortium and were completely consumed over 48 h anaerobic cultivation period if either was the sole carbohydrate source and energy source in the medium. However, in co-fermentation, glucose was the preferred substrate and only after depletion of the glucose mannitol was consumed by the bacteria. In co-fermentation of glucose and mannitol at high concentration of the carbohydrate sources, the growth reached the stationary phase by mainly consuming glucose and mannitol was only used by the bacteria for maintenance. The entry of the stationary phase could be due to some limitation in the medium or by the low pH of the medium reached at this stage due to the lactate production. However, at lower initial concentration of glucose, the bacteria consumed mannitol for growth after glucose depletion. The metabolite profile of the culture supernatants indicated that the consortium employed two different pathways in utilization of glucose and mannitol since in the presence of glucose, lactate is the only metabolite while in the presence of mannitol, in addition to lactate, ethanol is produced while acetate is consumed. It appears that in the presence of glucose, the consortium uses the Embden-Meyerhof-Parnas pathway (EMP pathway). In this pathway, pyruvate, the final product of the EMP pathway, is directly reduced to lactate by NAD+-dependent lactate dehydrogenase thereby reoxidizing NADH, which was produced in the early glycolytic steps [
29]. This pathway was used by
Lactiplantibacillus plantarum WCFS1 [
30]. For mannitol utilization, McFeeters and Chen [
31] proposed a pathway for
Lactiplantibacillus plantarum ATCC 14917 and
Lactiplantibacillus plantarum strain C11 in which acetate was converted to ethanol when mannitol was used as substrate in the fermentation. They also showed that in mannitol fermentation, citrate can be converted to succinate and ethanol. Since the MRS medium used in this study contained ammonium citrate, the small amount of succinate in the cultivation broth is likely due to the conversion of acetate to succinate. Yang and co-authors [
32] suggested a pathway for
Lactobacillus plantarum NF92, for mannitol utilization, showing that lactate is the major metabolite in early growth with production of negligible amount of mannitol, while in the late growth phase ethanol is produced. So, it can be concluded that in the presence of glucose and mannitol mostly
Lactiplantibacillus plantarum strains proliferate.
In addition to the ability to consume monosaccharides, the LAB consortium proved an ability to utilize short-chain glucan oligosaccharides with various linkage types. As was demonstrated, it could utilize maltose (a disaccharide made of α-1,4 linked glucose moieties), sucrose (a disaccharide composed of α-
D-glucopyranosyl-(1,2)-β-
D-fructo-furanoside), and β-laminari-oligosaccharides derived from laminarin. There are many studies showing that short oligosaccharides of α- and β- glucans derived from terrestrial biomass can be catabolized by lactic acid bacteria [
33]. However, there are only a few studies showing the utilization of oligosaccharides derived from marine biomass by LAB. In this area Jin et al. [
34] showed that a variety of lactic acid bacteria species including
Lactobacillus plantarum encode specific transporter system for utilization of α-gluco-oligosaccharides derived from brown seaweed
Laminaria japonicus. However, there is a lack of evidence regarding the growth of LAB on β-gluco-oligosaccharides derived from seaweed. In this study it was shown that the LAB consortium under study could utilize β-1,3 linked laminari-oligosaccharides (DP2-4), derived from the brown seaweed
Alaria esculenta. This indicates that short laminari-oligosaccharides has the potential to be utilized as prebiotics. No growth was observed in the presence of polysaccharides available in the
A. esculenta brow seaweed.
Fermentation with LAB is a well-established preservation method and is proven to enhance the taste, texture, and shelf life of the food products by releasing metabolites that lower the pH, hence preventing pathogenic and spoilage microorganisms from growing. In this study, the LAB consortium proved to be able to ferment brown seaweed
A. esculenta, which is the second most cultivated seaweed species in Europe. The pH decreased to approximately pH 3.8 (from pH 6.2 in autoclaved reactors and pH 6.5 in unautoclaved reactors) over the fermentation, while the consortium catabolized mannitol (the only carbohydrate available in the fermentation broth), which if consumed in excess, may have a laxative effect and may cause diarrhea [
35]. The pH drop via fermentation assures that the endogenous spoiling bacteria, pathogens, and fungi are prevented from growing since they only grow in an environment with a pH above pH 4.6 [
36,
37,
38]. This shows the importance of seaweed fermentation since the microbiota of
A. esculenta is yet unknown and could contain potential pathogenic microorganisms. As was shown in this study, fermentation with seaweed’s endogenous microbiota (samples without any added LAB inoculum) for seven days resulted in spoilage, the seaweed became slimy with an unpleasant smell while the pH dropped to pH 5.7. The metagenome analysis of the seaweed fermented with endogenous bacteria showed the presence of spore-forming bacteria and fungi that cannot be sterilized by autoclaving, and fermentation of the seaweed without adding a LAB inoculum resulted in spoilage of the seaweed. However, by adding a LAB inoculum to the samples, the spoilage of the seaweed was prevented.
5. Conclusions
The LAB consortium, investigated in this study, consists of three L. plantarum strains and minor relative abundance of a L. brevis strain. This consortium showed substantial ability in fermenting monosaccharides which are building blocks of seaweed’s carbohydrates, excluding fucose, mannuronic acid and guluronic acid. Additionally, the consortium was also proven to utilize maltose, sucrose, and laminari-oligosaccharides (DP2-4) derived from laminarin. Lactic acid was the major metabolite produced, however, when mannitol was consumed succinate and ethanol were produced while acetate was consumed. In xylose catabolism, in addition to lactate, acetate was also produced. The consortium could successfully directly ferment the brown seaweed Alaria esculenta, lowering pH to pH 3.8 (by producing lactate), while utilizing mannitol, the only carbohydrate released into fermentation broth. Fermentation with seaweed’s endogenous bacteria resulted in seaweed spoilage. This suggests the need for preservation of the seaweed A. esculenta, and fermentation proved to be a promising method to prolong its shelf life and improve its nutritional content.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, G.O.H. and E.N.K. ; methodology, L.A., M.J., A.J., M.D.C, J.A.L.-P., S.L. and M.N.; data curation, validation and visualization, L.A.; formal analysis and investigation, L.A., M.J., A.J., M.D.C, J.A.L.-P., S.L., M.N. and E.N.K.; resources, E.N.K.; writing—original draft preparation, L.A.; writing—review and editing, L.A., M.J., M.D.C., A.J., J.A.L.-P., S.L., G.O.H., and E.N.K.; supervision, E.N.K.; project administration, E.N.K; funding acquisition, E.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Era-net ProSeaFood (Era-net SusFood2, grant agreement No 727473) supported by Formas, and the Eurostars project SeaPro, supported by Vinnova, grant no 201-03368.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is available on request from the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hreggviðsson, G.Ó.; Nordberg-Karlsson, E.M.; Tøndervik, A.; Aachmann, F.L.; Dobruchowska, J.M.; Linares-Pastén, J.; Daugbjerg-Christensen, M.; Moneart, A.; Kristjansdottir, T.; Sletta, H.; et al. Chapter 16 - Biocatalytic refining of polysaccharides from brown seaweeds. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier, 2020; pp. 447–504. [Google Scholar]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; Lucente, D.; Mair, G.; Miao, W.; Potin, P.; Przybyla, C.; Reantaso, M.; Roubach, R.; Tauati, M.; Yuan, X. Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular 2021, 1229, 48. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [CrossRef]
- Yeo, S.-K.; Liong, M.-T. Effect of prebiotics on viability and growth characteristics of probiotics in soymilk. J. Sci. Food Agric. 2010, 90, 267–275. [Google Scholar] [CrossRef]
- Rioux, L.-E.; Turgeon, S.L. Chapter 7 - Seaweed carbohydrates. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, 2015; pp. 141–192. [Google Scholar]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed components as potential modulators of the gut microbiota. Mar. Drugs 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Sardari, R.R.R.; Nordberg Karlsson, E. Marine poly- and oligosaccharides as prebiotics. J. Agric. Food Chem. 2018, 66, 11544–11549. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm J 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.; Li, W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food. Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Noriega Fernández, E. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- Prachyakij, P.; Charernjiratrakul, W.; Kantachote, D. Improvement in the quality of a fermented seaweed beverage using an antiyeast starter of Lactobacillus plantarum DW3 and partial sterilization. World J. Microbiol. Biotechnol. 2008, 24, 1713–1720. [Google Scholar] [CrossRef]
- Reboleira, J.; Silva, S.; Chatzifragkou, A.; Niranjan, K.; Lemos, M.F.L. Seaweed fermentation within the fields of food and natural products. Trends Food Sci. Technol. 2021, 116, 1056–1073. [Google Scholar] [CrossRef]
- Chen, Y.S.; Srionnual, S.; Onda, T.; Yanagida, F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett. Appl. Microbiol. 2007, 45, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation aplications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Allahgholi, L.; Derks, M.G.N.; H. Friðjónsson, Ó.; Óli Hreggviðsson, G.; Nordberg Karlsson, E. Exploring a novel Laminarinase, MlLam17B, from a marine Muricauda lutaonensis strain. 2023.
- Christensen, M.D.; Allahgholi, L.; Freysdóttir, J.; Friðjónsson, Ó.; Moenaert, A.; Karlsson, E.N.; Hreggviðsson, G.Ó. Exploring structural relations of laminarin and laminarin derivatives' immunomodulatory effects on dendritic cells and T cell crosstalk. 2023.
- Motejadded, H.; Altenbuchner, J. Construction of a dual-tag system for gene expression, protein affinity purification and fusion protein processing. Biotechnol. Lett. 2009, 31, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Van Wychen, S.; Laurens, L.M.L. Determination of total carbohydrates in algal biomass; Laboratory NRE: Golden, Colorado. NREL/TP-5100-60957, 2015. [Google Scholar]
- Nurhikmayani, R.; Daryono, B.S.; Retnaningrum, E. The isolation and molecular identification of antimicrobial-producing lactic acid bacteria from chao, South Sulawesi (Indonesia) fermented fish product. Biodiversitas 2019, 20, 1063–1068. [Google Scholar] [CrossRef]
- Horie, M.; Sato, H.; Tada, A.; Nakamura, S.; Sugino, S.; Tabei, Y.; Katoh, M.; Toyotome, T. Regional characteristics of Lactobacillus plantarum group strains isolated from two kinds of Japanese post-fermented teas, Ishizuchi-kurocha and Awa-bancha. Biosci. Microbiota Food Health 2019, 38, 11–22. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Phuengjayaem, S.; Booncharoen, A.; Tanasupawat, S. Characterization and comparative genomic analysis of gamma-aminobutyric acid (GABA)-producing lactic acid bacteria from Thai fermented foods. Biotechnol. Lett. 2021, 43, 1637–1648. [Google Scholar] [CrossRef]
- Kitahara, K.; Obayashi, A. DL-forming lactic acid bacteria. J. Gen. Appl. Microbiol. 1955, 1, 237–245. [Google Scholar] [CrossRef]
- Falck, P.; Precha-Atsawanan, S.; Grey, C.; Immerzeel, P.; Stålbrand, H.; Adlercreutz, P.; Nordberg Karlsson, E. Xylooligosaccharides from hardwood and cereal xylans produced by a thermostable xylanase as carbon sources for Lactobacillus brevis and Bifidobacterium adolescentis. J. Agric. Food Chem. 2013, 61, 7333–7340. [Google Scholar] [CrossRef] [PubMed]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of mannitol extracts From brown macro algae by thermophilic Clostridia. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: a review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 2003, 100, 1990–1995. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Chen, K.-H. Utilization of electron acceptors for anaerobic mannitol metabolism by Lactobacillus plantarum. Compounds which serve as electron acceptors. Food Microbiol. 1986, 3, 73–81. [Google Scholar] [CrossRef]
- Yang, X.; Teng, K.; Su, R.; Li, L.; Zhang, T.; Fan, K.; Zhang, J.; Zhong, J. AcrR and Rex control mannitol and sorbitol utilization through their cross-regulation of aldehyde-alcohol dehydrogenase (AdhE) in Lactobacillus plantarum. Appl Environ Microbiol 2019, 85, e02035–02018. [Google Scholar] [CrossRef]
- Zuniga, M.; Jesus Yebra, M.; Monedero, V. Complex oligosaccharide utilization pathways in Lactobacillus. Curr. Issues Mol. Biol. 2021, 40, 49–80. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, Q.; Chen, X.; Wang, H.; Zhu, J.; Lee, Y.K.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. An α type gluco-oligosaccharide from brown algae Laminaria japonica stimulated the growth of lactic acid bacteria encoding specific ABC transport system components. Food. Funct. 2022, 13, 11153–11168. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K. Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals. Int. J. Dent. 2016, 2016, 5967907. [Google Scholar] [CrossRef]
- Peck, M.W. Bacteria: Clostridium botulinum. In Encyclopedia of Food Safety, Motarjemi, Y., Ed.; Academic Press: Waltham, 2014; pp. 381–394. [Google Scholar]
- Dalgaard, P.; Mejlholm, O. Modeling growth of Listeria and Lactic acid bacteria in food environments. Methods Mol. Biol. 2019, 1918, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Koukou, I.; Mejlholm, O.; Dalgaard, P. Cardinal parameter growth and growth boundary model for non-proteolytic Clostridium botulinum – Effect of eight environmental factors. Int. J. Food Microbiol. 2021, 346, 109–162. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flowchart representing the fermentation experiment. The seaweed slurry (20% (w/w)) was subjected to fermentation with LAB consortium or with seaweed’s endogenous microbiota.
Figure 1.
Flowchart representing the fermentation experiment. The seaweed slurry (20% (w/w)) was subjected to fermentation with LAB consortium or with seaweed’s endogenous microbiota.
Figure 2.
Bubble chart representing relative abundance of bacterial strains in the LAB consortium.
Figure 2.
Bubble chart representing relative abundance of bacterial strains in the LAB consortium.
Figure 3.
Cell density and carbohydrate consumption of the LAB consortium in the presence of glucose (a), mannitol (b), galactose (c), mannose (d), and xylose (e) after 48 h or 72 h batch cultivation in MRS medium supplemented with 20 g/L of the respective substrates. Blue bars represent OD600 value; orange line represents sugar concentration in the medium; error bars show the standard deviation between duplicate measurements.
Figure 3.
Cell density and carbohydrate consumption of the LAB consortium in the presence of glucose (a), mannitol (b), galactose (c), mannose (d), and xylose (e) after 48 h or 72 h batch cultivation in MRS medium supplemented with 20 g/L of the respective substrates. Blue bars represent OD600 value; orange line represents sugar concentration in the medium; error bars show the standard deviation between duplicate measurements.
Figure 4.
Co-fermentation of glucose and mannitol by the LAB consortium throughout anaerobic batch cultivation. Standard deviation of duplicate samples is shown.
Figure 4.
Co-fermentation of glucose and mannitol by the LAB consortium throughout anaerobic batch cultivation. Standard deviation of duplicate samples is shown.
Figure 5.
Growth of the LAB consortium in the presence of glucose and laminari-oligosaccharides O-LAM2-5. Standard deviation between duplicates is shown as error bars.
Figure 5.
Growth of the LAB consortium in the presence of glucose and laminari-oligosaccharides O-LAM2-5. Standard deviation between duplicates is shown as error bars.
Figure 6.
Cell density and carbohydrate consumption of the LAB consortium in the presence of maltose (a), and sucrose (b) after 24 h, 48 h and 72 h batch cultivation in MRS broth base supplemented with 20 g/L of the respective carbohydrate substrate. Error bars represent the standard deviation of duplicates.
Figure 6.
Cell density and carbohydrate consumption of the LAB consortium in the presence of maltose (a), and sucrose (b) after 24 h, 48 h and 72 h batch cultivation in MRS broth base supplemented with 20 g/L of the respective carbohydrate substrate. Error bars represent the standard deviation of duplicates.
Figure 7.
The growth of the LAB consortium in the medium supplemented with laminarin hydrolysate (5 g/L) (a) and hydrolysate (5 g/L) with additional added glucose to final concentration of 2.5 g/L (b).
Figure 7.
The growth of the LAB consortium in the medium supplemented with laminarin hydrolysate (5 g/L) (a) and hydrolysate (5 g/L) with additional added glucose to final concentration of 2.5 g/L (b).
Figure 8.
(a) Carbohydrate content and CFU/mL of the LAB consortium in the autoclaved and non-autoclaved reactors over seven days of fermentation at 37 °C. (b) pH curve from the fermentation of seaweed biomass, obtained from a gas endeavor system. pH curves over the fermentation of raw biomass Alaria esculenta; orange curve represents the autoclaved reactors, and the blue curve indicates the unautoclaved reactors. The experiments were performed in duplicates, and the error bars represent the standard deviation of duplicates.
Figure 8.
(a) Carbohydrate content and CFU/mL of the LAB consortium in the autoclaved and non-autoclaved reactors over seven days of fermentation at 37 °C. (b) pH curve from the fermentation of seaweed biomass, obtained from a gas endeavor system. pH curves over the fermentation of raw biomass Alaria esculenta; orange curve represents the autoclaved reactors, and the blue curve indicates the unautoclaved reactors. The experiments were performed in duplicates, and the error bars represent the standard deviation of duplicates.
Figure 9.
Krona-plot obtained from metagenome analysis of fermented seaweed from autoclaved reactor with endogenous bacteria, performed for 7 days at 37 °C. Green area represents eukaryota (relative abundance 1%), blue and purple areas represent viruses (relative abundance 0.4%) and archaea (relative abundance 0.01%) respectively.
Figure 9.
Krona-plot obtained from metagenome analysis of fermented seaweed from autoclaved reactor with endogenous bacteria, performed for 7 days at 37 °C. Green area represents eukaryota (relative abundance 1%), blue and purple areas represent viruses (relative abundance 0.4%) and archaea (relative abundance 0.01%) respectively.
Figure 10.
Organic acid production in the presence 20 g/L of glucose (a) and 20 g/L of mannitol (b) in MRS under anaerobic condition.
Figure 10.
Organic acid production in the presence 20 g/L of glucose (a) and 20 g/L of mannitol (b) in MRS under anaerobic condition.
Table 1.
Carbohydrate composition of the hydrolysate obtained from enzymatic hydrolysis of laminarin, extracted from Laminaria hyperborean, with MlLam17B.
Table 1.
Carbohydrate composition of the hydrolysate obtained from enzymatic hydrolysis of laminarin, extracted from Laminaria hyperborean, with MlLam17B.
| Carbohydrate composition (% DW) |
|---|
| Glucose |
Mannitol |
Laminariobiose (O-Lam2) |
Laminaritriose(O-Lam3) |
Laminaritetraose(O-Lam4) |
| 0.38 |
2.17 |
16.21 |
29.77 |
11.52 |
Table 2.
Lactate production of the LAB consortium grown in the presence of monosaccharides, a mixture of glucose and mannitol, crude laminari-oligosaccharides with /without added glucose and during fermentation of raw Alaria esculenta.
Table 2.
Lactate production of the LAB consortium grown in the presence of monosaccharides, a mixture of glucose and mannitol, crude laminari-oligosaccharides with /without added glucose and during fermentation of raw Alaria esculenta.
| Substrate |
Lactate (g/L) |
| Glucose (21.93 ± 0.14 g/L) |
21.91 ± 0.32 |
| Mannitol (21.32 ± 0.24 g/L) |
21.38 ± 0.12 |
| Maltose (22.26 ± 0.05 g/L) |
22.21 ± 0.64 |
| Galactose (22.25 ± 0.62 g/L) |
19.22 ± 0.31 |
| Sucrose (21.98 ± 0.23 g/ L) |
16.1 ± 0.24 |
| Glucose + mannitol (12.12 ± 0.49 g/L glucose + 11.87 ± 0.14 g/L mannitol) |
14.21 ± 0.68 |
| Mannose (20.86 ± 0.14 g/L) |
11.13 ± 0.51 |
| Crude laminari-oligosaccharides (5.36 ± 0.1 g/L) + glucose (2.78 ± 0.02 g/L) |
7.65 ± 0.02 |
| Crude laminari-oligosaccharides (5.15 ± 0.1 g/L) |
4.98 ± 0.07 |
| Xylose (21.13 ± 0.1 g/L) |
3.29 ± 0.08 |
| Fermentation, raw A. esculenta, autoclaved (1 g/L mannitol) |
1.03 ± 0.03 |
| Fermentation, raw A. esculenta, non-autoclaved (1 g/L mannitol) |
1.03 ± 0.01 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).