Submitted:
13 April 2023
Posted:
13 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Eligibility Criteria
Source of Data Used
Data Extraction Methods
Study Design
Quality of studies
3. Results
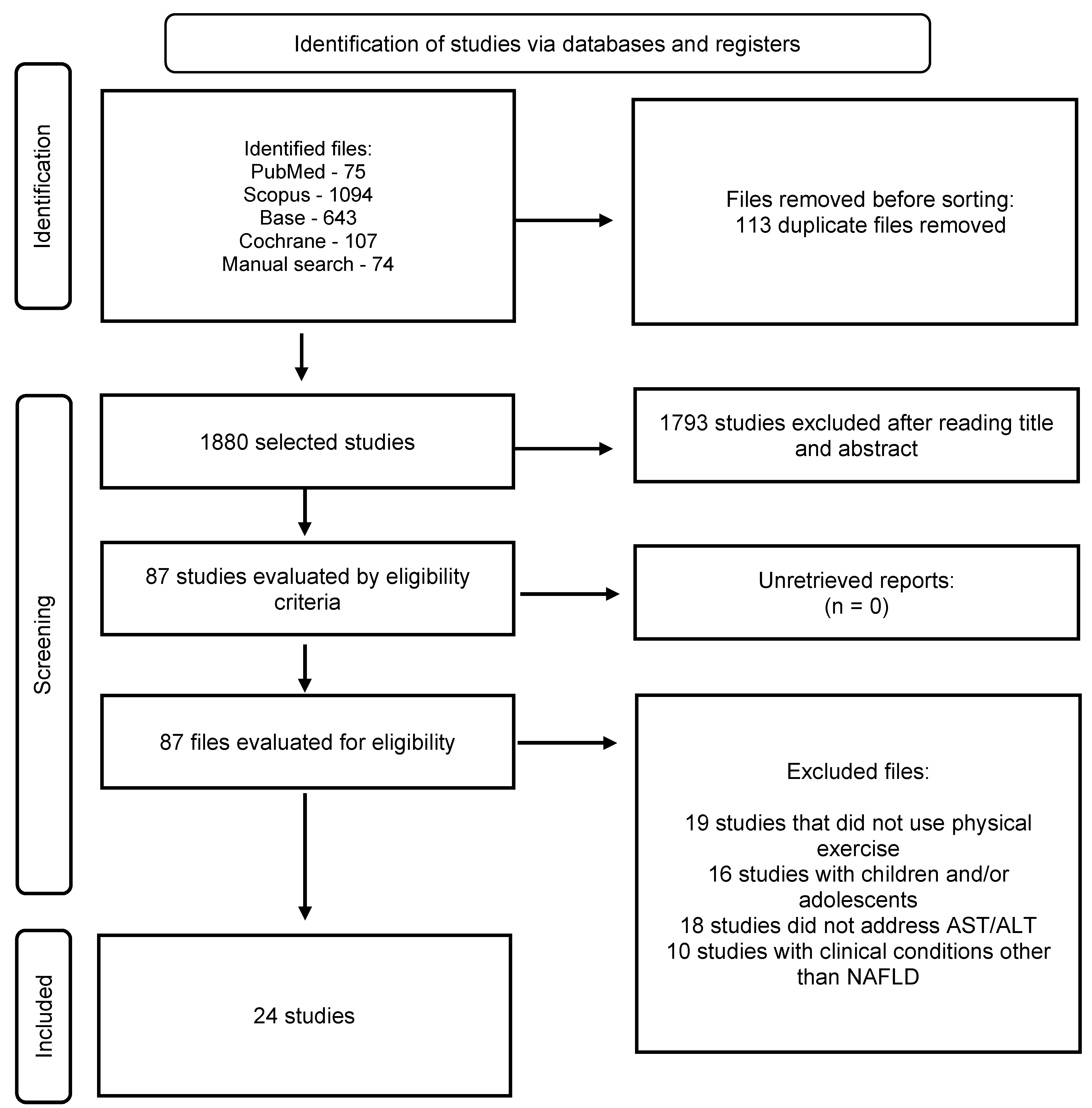
Selection of Studies
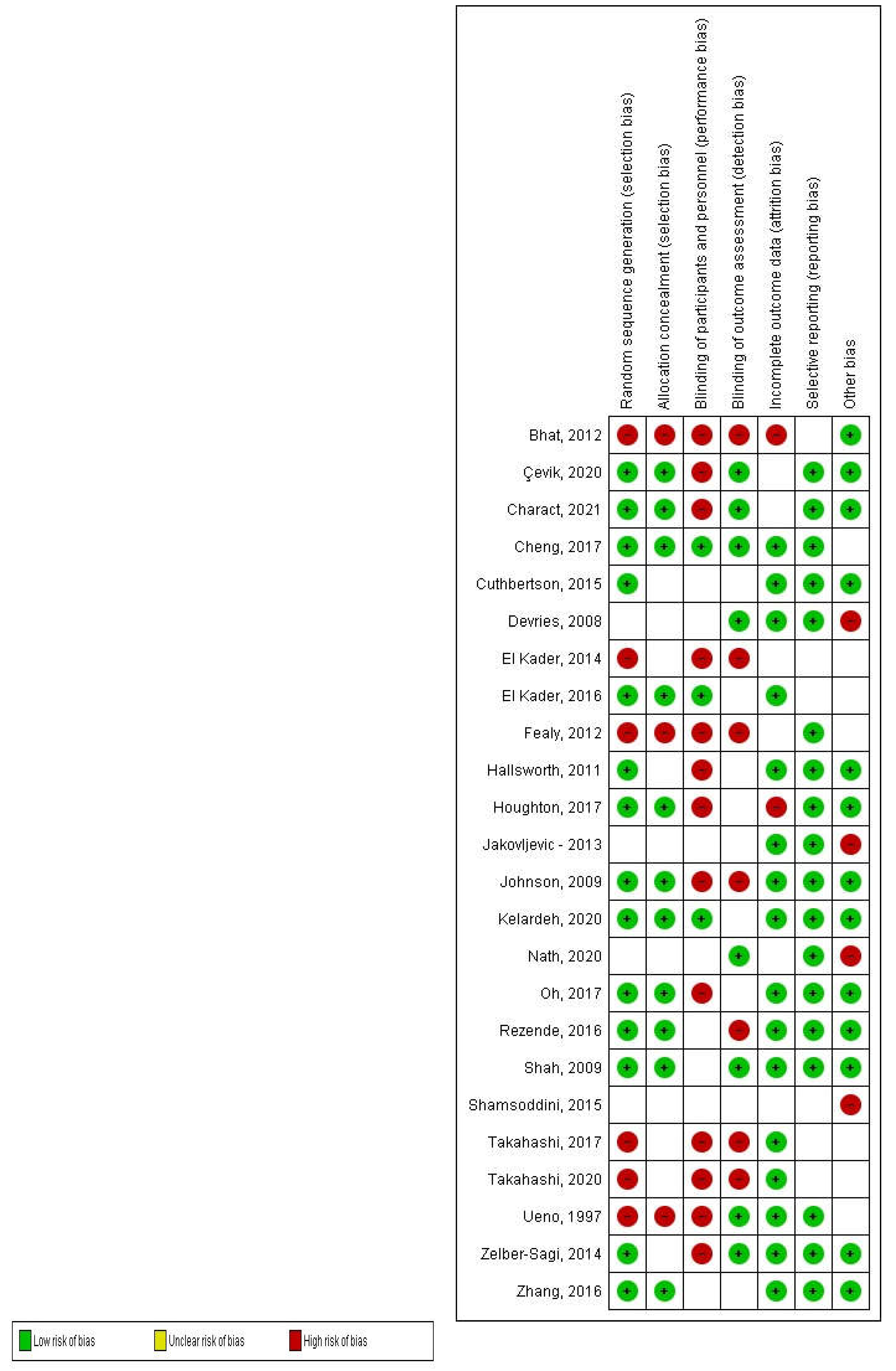
Methodological Quality Assessment and Risk of Bias Rating
Characteristics of the Studies
Types of Intervention
Types of Variables
Physical Activity Protocol
4. Discussion
Financial support and sponsorship
Conflicts of Interest
References
- Hepatologia SB de. Doença Hepática Gordurosa Não Alcoólica Consenso da Sociedade Brasileira de Hepatologia. 2015.
- da Ponte IM, Lima ME de S, Albuquerque MCF, Veloso AF de H, Bachur TPR. Esteato-hepatite não alcoólica: uma síndrome em evidência / Non-Alcoholic Steatohepatitis: a syndrome in evidence. Brazilian Journal of Health Review. 2020;3(1):1077–93.
- Perez PLV, Universidade Federal Fluminense. Instituto de Saúde de Nova Friburgo. Departamento de Ciências Básicas. Nova Friburgo RJ. Doença Hepática Gordurosa Não Alcoólica: Características Clínico-Laboratoriais, Histopatológicas e Seu Estudo em Modelos Animais. 2019.
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–73. [CrossRef]
- Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82–97. [CrossRef]
- Magalhães AJB, Camargo RCT, Castoldi RC, Ozaki GAT, Koike TE, Garcia TA, et al. Qual a melhor conduta terapêutica não-medicamentosa para pacientes com doença hepática gordurosa não-alcoólica? Colloquium Vitae. 2015;6(1):24–33.
- Parente DB. Imaging methods in the assessment of nonalcoholic fatty liver disease. Radiol Bras. 2020;53(2):9–10. [CrossRef]
- Cotrim HP, Parise ER, Figueiredo-Mendes C, Galizzi-Filho J, Porta G, Oliveira CP. Noalcoholic Fatty Liver Disease Brazilian Society Of Hepatology Consensus. Arq Gastroenterol. 2016;53(2):118–22. [CrossRef]
- Ramallo BT, Foschini D, Prestes J, Charro M, Lopes CR, Evangelista AL, et al. Magnitude do dano muscular induzido pelo exercí cio em mulheres treinadas e destreinadas. RBPFEX - Revista Brasileira de Prescrição e Fisiologia do Exercício. 2013;7(40):398–405.
- Varaldo C. Variações nos níveis de transaminases | Hepato. 2005.
- Sergio M, Díaz DV, Marjoris M, Martínez P, Juan M, Sanchez Vega A, et al. Hepatic disease due to fat deposit. MediSan. 2015;19(07):886–96.
- Lisboa QC, Costa SMF, Couto CA. Current management of non-alcoholic fatty liver disease. Rev Assoc Med Bras (1992). 2016;62(9):872–8. [CrossRef]
- Martins FSB, Santos JAR dos. Alterações agudas induzidas por uma prova de triathlon longo em diferentes biomarcadores enzimáticos e da função imune. Revista Brasileira de Fisiologia do Exercício. 2012;11(1):7–12.
- Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring). 2009;17(12):2162–8. [CrossRef]
- Charatcharoenwitthaya P, Kuljiratitikal K, Aksornchanya O, Chaiyasoot K, Bandidniyamanon W, Charatcharoenwitthaya N. Moderate-Intensity Aerobic vs Resistance Exercise and Dietary Modification in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin Transl Gastroenterol. 2021;12(3). [CrossRef]
- Carla Giuliano de Sá P, Marega M, José Antonio Maluf de C, Felipe Gambetta C, Carlos Eduardo Felix L, Fabio Luis C, et al. Physical activity as a protective factor for development of non-alcoholic fatty liver in men. 2015;13(1):34–40. [CrossRef]
- Mariane Pravato M, Tairine Fiorotto S, Jefersnon Colevatti Dos A. Esteatose Hepática Gordurosa Não Alcoólica: Efeitos da Terapia Nutricional e Prática Regular de Exercícios Físicos Como Tratamento Não Medicamentoso. Revista Saúde UniToledo. 2020;4(1):34–40.
- Xiong Y, Peng Q, Cao C, Xu Z, Zhang B. Effect of Different Exercise Methods on Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Meta-Regression. Int J Environ Res Public Health. 2021;18(6):1–18. [CrossRef]
- Kwak MS, Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 2018;33(1):64–74. [CrossRef]
- Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–12. [CrossRef]
- Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJA, Richardson P, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130(2):93–104. [CrossRef]
- Takahashi A, Abe K, Usami K, Imaizumi H, Hayashi M, Okai K, et al. Simple Resistance Exercise helps Patients with Non-alcoholic Fatty Liver Disease. Int J Sports Med. 2015;36(10):848–52. [CrossRef]
- Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients With Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2017;15(1):96-102.e3. [CrossRef]
- Shamsoddini A, Sobhani V, Ghamar Chehreh ME, Alavian SM, Zaree A. Effect of Aerobic and Resistance Exercise Training on Liver Enzymes and Hepatic Fat in Iranian Men With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2015;15(10):31434. [CrossRef]
- El-Kader SMA, Al-Jiffri OH, Al-Shreef FM. Markers of liver function and inflammatory cytokines modulation by aerobic versus resisted exercise training for nonalcoholic steatohepatitis patients. Afr Health Sci. 2014;14(3):551–7. [CrossRef]
- Nath P, Panigrahi MK, Sahu MK, Narayan J, Sahoo RK, Patra AA, et al. Effect of Exercise on NAFLD and Its Risk Factors: Comparison of Moderate versus Low Intensity Exercise. J Clin Transl Hepatol. 2020;8(2):120. [CrossRef]
- El-Kader SMA, Al-Shreef FM, Al-Jiffri OH. Biochemical parameters response to weight loss in patients with non-alcoholic steatohepatitis. Afr Health Sci. 2016;16(1):242–9. [CrossRef]
- Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20(15):4382–92.
- Takahashi A, Imaizumi H, Hayashi M, Okai K, Abe K, Usami K, et al. Simple Resistance Exercise for 24 Weeks Decreases Alanine Aminotransferase Levels in Patients with Non-Alcoholic Fatty Liver Disease. Sports Med Int Open [Internet]. 2017;1(1):E2–7. [CrossRef]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367-378.e5.
- Lucimar Aguiar da S. Efeito do Exercício Físico nos Marcadores Inflamatórios em Mulheres Jovens. Viçosa: Universidade Federal de Viçosa; 2020. [CrossRef]
- Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17(6):510–9.
- Moradi B, Rahmati-Ahmadabad S, Farzanegi P, Helalizadeh M, Azarbayjani MA. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J Bodyw Mov Ther. 2020;24(3):154–60. [CrossRef]
- DH V, Israili Z. Aminotrasferases. In: Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990. [CrossRef]
| Items from the PEDro Scale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Score | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Sulin Cheng - 2017 | 9 | x | x | x | x | x | x | x | x | x | ||
| Kate Hallsworth - 2011 | 7 | x | x | x | x | x | x | x | ||||
| Hui-Jie Zhang - 2016 | 7 | x | x | x | x | x | x | x | ||||
| David Houghton - 2017 | 8 | x | x | x | x | x | x | x | x | |||
| Atsushi Takahashi - 2015 | 7 | x | x | x | x | x | x | x | ||||
| Baharak Moradi - 2020 | 7 | x | x | x | x | x | x | x | ||||
| Tulay Çevik - 2020 | 7 | x | x | x | x | x | x | x | ||||
| Shira Zelber-Sagi - 2014 | 7 | x | x | x | x | x | x | x | ||||
| Atsushi Takahashi - 2017 | 6 | x | x | x | x | x | x | |||||
| Jakovljevic - 2013 | 7 | x | x | x | x | x | x | x | ||||
| Daniel J Cuthbertson - 2015 | 6 | x | x | x | x | x | x | |||||
| Ganesh Bhat - 2012 | 6 | x | x | x | x | x | x | |||||
| Preetam Nath - 2020 | 5 | x | x | x | x | x | ||||||
| Sechang Oh - 2017 | 8 | x | x | x | x | x | x | x | x | |||
| El Kader - 2014 | 5 | x | x | x | x | x | ||||||
| Devries - 2008 | 6 | x | x | x | x | x | x | |||||
| Ciaran E Fealy - 2012 | 5 | x | x | x | x | x | ||||||
| Johnson - 2009 | 10 | x | x | x | x | x | x | x | x | x | x | |
| Rezende - 2016 | 8 | x | x | x | x | x | x | x | x | |||
| Alireza Shamsoddini - 2015 | 7 | x | x | x | x | x | x | x | ||||
| Phunchai Charact. - 2021 | 7 | x | x | x | x | x | x | x | ||||
| Krupa Shah - 2009 | 7 | x | x | x | x | x | x | x | ||||
| El Kader - 2016 | 8 | x | x | x | x | x | x | x | x | |||
| T Ueno - 1997 | 5 | x | x | x | x | x | ||||||
| Author/year | n (sample) | Groups | Training Protocol | Feeding Protocol | Results |
|---|---|---|---|---|---|
| Sulin Cheng et al., 2017 | 85 men and women with a mean age of 60 years | G1 – AEx G2 – DI G3 – AED G4 - CON |
Training with a weekly frequency of 2 to 3 times; duration between 30-60min and progressive intensity between 60-75% VO2max | 38% carbohydrates and 12g/day dietary fiber for 8.6 months | There was a significant difference only in AST in the AED group |
| Kate Hallsworth., 2011 | 19 men and women with an average age of 56 years | G1 – RT G2 - CON |
Training with a weekly frequency of 3x; duration of 45-60min and intensity between 50-70% of 1RM | There was no protocol defined for feeding | There were no significant changes in ALT in either group. |
| Hui-Jie Zhang., 2016 | 220 men and women with a mean age of 53 years | G1 – CON G2 - AEx G3 – JE and AEx |
Training with a weekly frequency of 150 min, being between 45-60 min per session and intensity between 65-80% of HRm for JE and 45-55% of HRm for AEx | There was no protocol defined for feeding | There was no significant difference in ALT between groups during 6 or 12 months of intervention and AST significantly increased in G3 compared to G2 during the 6-month intervention |
| David Houghton., 2017 | 24 men and women with a mean age of 52 years | G1 – CE + RT G2 - CON |
Training with a weekly frequency of 3x; duration of 45-60min and self-reported intensity using the Borg scale between 16 and 18 (TC) and 14-16 (TR) | There was no protocol defined for feeding | There was no significant difference between groups |
| A Takahashi., 2015 | 53 men and women with a mean age of 51 years | G1 – RT G2 - CON |
Training with a weekly frequency of 3x; duration of 20-30min and intensity was not mentioned | Group 2 was educated about food | There was no significant difference between groups |
| Baharak Moradi Kelardeh., 2020 | 45 men and women with a mean age of 66 years | G1 – RT G2 – CS G3 – RT + CS G4 – CON |
Training with a weekly frequency of 3x; duration of 60-70 min and intensity of 40% of 1RM with rest varying from 1-7min | There was no protocol defined for feeding | The decrease in AST was significantly greater in G2 |
| Tulay Çevik., 2020 | 31 men and women with a mean age of 45 years | G1 – AEx with WBV G2 – AEx |
Training with a weekly frequency of 3x; duration of 40-60min and intensity 60-80% of HRr | There was no protocol defined for feeding | The decrease in AST was significantly greater in G2 |
| Shira Zelber Sagi., 2014 | 64 men and women with a mean age of 46 years | G1 – RT G2 – ST |
Training with a weekly frequency of 3x; duration of 40min and the intensity of the load was increased by 2-10% each training | There was no protocol defined for feeding | There was a significant improvement in AST and ALT within the group, but with no difference between the arms. |
| Atsushi Takahashi., 2017 | 59 men and women with a mean age of 52 years | G1 – RT G2 – CON |
Training with a weekly frequency of 3x; 20-30min duration and intensity not mentioned | There was no protocol defined for feeding | ALT and AST levels significantly decreased in G1 |
| Jakovljevic., 2013 | 17 men and women with a mean age of 62 years | G1 – RT G2 – CON |
Training with a weekly frequency of 3x; duration of 45-60 min and intensity of 60% HRm on the cycle ergometer and 50-70% of 1RM | There was no protocol defined for feeding | There was no significant reduction in ALT, while AST was not measured |
| Daniel J Cuthbertson., 2015 | 50 men and women with a mean age of 50 years | G1 – AEx G2 – CON |
Training with a weekly frequency of 3x; duration of 30-45min and intensity 30-60% of HRr | There was no protocol defined for feeding | There was no significant difference in AST and ALT levels |
| Ganesh Bhat., 2020 | 30 men and women with a mean age of 40 years | G1 – AEx G2 – CON |
Training with a weekly frequency of 5x; duration of 45min and intensity of 70% of HRm | Moderate caloric restriction (60% carbohydrate, 20% fat, 20% protein and 200mg of cholesterol for patients with BMI >25kg/m² | There was a significant decrease in ALT |
| Preetam Nath., 2020 | 37 men with a mean age of 53 years | G1 – MIG G2 – LIG |
Training with a weekly frequency of 5-6x; duration of 50-60min and intensity of MET below 3 (G1) and MET between 3-5.9 (G2) | There was no protocol defined for feeding | ALT and AST levels significantly decreased in G1 |
| Sechang Oh., 2017 | 53 men and women with a mean age of 50 years | G1 – RT G2 – HIAT G3 – MICT |
Training with a weekly frequency of 3x; duration of 60-70min and intensity according to the patient's MRI (G1), 80-85% VO2max (G2) and 60-65%VO2max (G3) | There was no protocol defined for feeding | There was a significant difference in ALT in G3 |
| El Kader., 2014 | 50 men and women with a mean age of 31 years | G1 – AEx G2 – RT |
Training with a weekly frequency of 3x; duration of 40min and intensity of 60-80% of HRm (G1) and 60-80% of 1RM (G2) | There was no protocol defined for feeding | ALT and AST levels significantly decreased in G1 and G2, in addition to a significant difference between the two groups after treatment |
| Devries., 2020 | 41 men and women aged 20-55 years | G1 – Obese G2 – Lean |
Training with a weekly frequency of 2-3x; duration of 40min and intensity of 50-70% Vo2peak | There was no protocol defined for feeding | ALT levels were lower in obese women when compared to obese men |
| Ciaran E Fealy., 2012 | 13 men and women with a mean age of 58 years | G1 – AEx | Training for 7 days with duration of 60min and intensity of 80-85% of HRm | Avoid caffeine for 12 hours, alcohol for 48 hours and have a diet with 250g of carbohydrates before the pre-test | There was a significant reduction in ALT, however there was no difference in AST |
| Johnson., 2009 | 19 men and women with a mean age of 47 years | G1 – AEx G2 - CON |
Training for 2-3x; with duration of 30-45min and intensity of 50-70% of Vo2peak | Diet comprising 60% of carbohydrate, 20% as fat and 20% as protein | There was no statistically significant difference in ALT and AST was not cited |
| Rezende., 2016 | 40 women with a mean age of 55 years | G1 – AEx G2 – CON |
Training twice a week; duration of 60min and intensity increasing from 10% of the anaerobic ventilatory threshold to the point of respiratory compensation | All received an energy deficit diet of 500 kcal/d: 35% protein, 25% lipids and 40% carbs |
There was a slight decrease in serum ALT levels in G1 when compared to G2 |
| Alireza Shamsoddini., 2015 | 30 men aged 32-54 years | G1 – AEx G2 – RT G3 – CON |
Training with a weekly frequency of 3x; duration of 40-45min and intensity of 60-75% of HRm (G1) and 60-70% of 1RM (G2) | There was no protocol defined for feeding | ALT and AST levels significantly decreased in G1 and G2 when compared to G3 |
| Phunchai Charact., 2021 | 18 men and women with a mean age of 38 years | G1 – AEx G2 – RT |
Training with a weekly frequency of 5x; duration of 60min and intensity 60-70% of HRm (G1) and 60% of 1RM (G2) | Monthly nutritional advice | There was no statistically significant difference in AST and ALT levels |
| Krupa Shah., 2009 | 18 men and women aged 65-82 years | G1 – DI G2 – AED |
Training with a weekly frequency of 3x; duration of 90min and intensity 65-80% of 1RM | Prescription for a caloric deficit with 30% fat, 50% carbohydrate, and 20% protein (G1) and the same prescription with 130 kcal/day more intake (G2) | There was no statistically significant difference in AST and ALT levels |
| El Kader., 2016 | 100 men and women with a mean age of 45-58 years | G1 – AEx G2 – CON |
Training with a weekly frequency of 3x; duration of 40min and intensity 65-75% of HRm | Diet of 15% protein, 30-35% fat and 50-55% carbohydrate | There was a decrease in AST and ALT in G1 and also a significant difference between G1 and G2 at the end of the study |
| T Ueno., 1997 | 25 men and women with a mean age of 45 years | G1 – AED G2 – CON |
Training with a weekly frequency of 3x; duration of 30-60 min and the intensity was progressive from 3000-10,000 steps/day | 3 meals a day with 20% protein, 30% fat, and 50% carbohydrate, following the ideal weight of 25 cal.kg-1 | ALT levels significantly decreased in G1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





